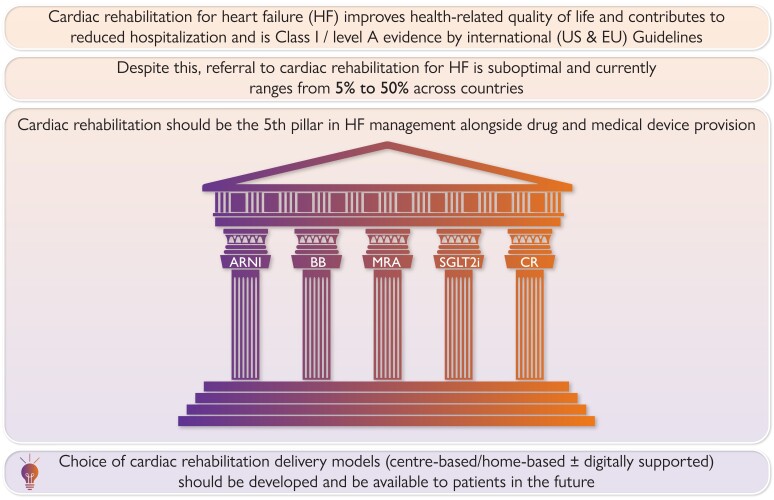
Graphical Abstract
Graphical Abstract.
Graphical abstract summarizing key points of state-of-the-art review.
Keywords: Heart failure, Cardiac rehabilitation, Exercise, Psychosocial support, Health-related quality of life, Rehospitalization
Abstract
Cardiac rehabilitation remains the ‘Cinderella’ of treatments for heart failure. This state-of-the-art review provides a contemporary update on the evidence base, clinical guidance, and status of cardiac rehabilitation delivery for patients with heart failure. Given that cardiac rehabilitation participation results in important improvements in patient outcomes, including health-related quality of life, this review argues that an exercise-based rehabilitation is a key pillar of heart failure management alongside drug and medical device provision. To drive future improvements in access and uptake, health services should offer heart failure patients a choice of evidence-based modes of rehabilitation delivery, including home, supported by digital technology, alongside traditional centre-based programmes (or combinations of modes, ‘hybrid’) and according to stage of disease and patient preference.
Introduction
Despite consistent international clinical guideline recommendations,1–3 cardiac rehabilitation remains the ‘Cinderella’ of heart failure management with less than 20% of patients across Europe and the USA4–6 receiving this evidence-based intervention. Recent authoritative editorials and commentaries have neglected mention of the need, importance, and provision of cardiac rehabilitation for heart failure.7
In this state-of-the-art review, we argue the central place of rehabilitation as a key pillar of heart failure management alongside drug and medical device provision and throughout the patient disease trajectory. Our review (i) discusses the need for contemporary models of cardiac rehabilitation; (ii) provides an update on the current evidence base, clinical guidance, and status of rehabilitation delivery for heart failure; and (iii) closes with implications for clinical practice and future research.
The contemporary face of cardiac rehabilitation
The World Health Organization (WHO) has defined cardiac rehabilitation as: ‘the sum of activities required to influence favourably the underlying cause of the disease, as well as to provide the best possible physical, mental, and social conditions, so that the patients may, by their own efforts, preserve or resume when lost as normal a place as possible in the community’.8 Cardiac rehabilitation has much evolved over the last three decades from focusing only on exercise training to recognition that it needs to be a multicomponent complex intervention that includes ‘patient assessment, education, risk factor modification including dietary recommendations, lifestyle modification, smoking cessation counselling, psychological support, and evaluation and management of barriers to adherence’.9,10 International guidelines today advocate that cardiac rehabilitation programmes also ‘assist people with heart failure to develop the necessary skills to successfully self-manage’ their long-term condition.9 Key aims of rehabilitation in heart failure include improvement in a patient’s exercise capacity and health-related quality of life and complement the impact of drugs and devices in reducing the risk of hospitalizations and mortality.3,9,10
Effective cardiac rehabilitation delivery requires close collaboration between patients, caregivers, and service providers. To ensure quality assurance, national and international guidelines3,9,11–13 recommend involvement of a multidisciplinary team in the optimal delivery of cardiac rehabilitation programmes. Initially, heart failure patients are assessed and referred to rehabilitation by a cardiologist or a physician with a special interest. The multidisciplinary team includes nurse specialists, physiotherapists/exercise therapists, and dietitians who have training in the competencies of delivering the core components of cardiac rehabilitation. Programmes can include other healthcare professionals such as psychologists, behavioural health change specialists, social workers, and pharmacists.
The traditional model of cardiac rehabilitation delivery has been centre-based programmes that typically involve patients attending outpatient rehabilitation service in a hospital-/community-based facility supervised by a multidisciplinary healthcare team. Stimulated by the challenges of the SARS-CoV-2 pandemic cardiac rehabilitation, commentators have called for a ‘root and branch overhaul’ of cardiac rehabilitation provision to better reflect current contemporary clinical practice and patient expectation to include alternative models of delivery: home-based, digitally supported, and hybrid (mix of home and centre) programmes.10,14
The evidence base for cardiac rehabilitation
We address five questions around the evidence base for cardiac rehabilitation in heart failure:
What are the benefits of cardiac rehabilitation for patients with heart failure?
Are the benefits of cardiac rehabilitation consistent across heart failure patient characteristics/subgroups?
Are the benefits of cardiac rehabilitation consistent across delivery settings?
Is cardiac rehabilitation an effective adjuvant intervention to drug therapy for heart failure?
Are there any safety concerns when applying cardiac rehabilitation in a heart failure population?
What are the benefits of cardiac rehabilitation for heart failure?
Several meta-analyses published over the last decade have reported the benefits of cardiac rehabilitation for several cardiac indications, including heart failure.15–17 The 2022 update of the Cochrane systematic review and meta-analysis provides a comprehensive and contemporary review of randomized trial evidence. This Cochrane review17 and its latest version (unpublished, in review) identified a total of 60 trials comparing exercise-based cardiac rehabilitation to no exercise control in 8728 patients with median of 6-month follow-up, the majority in patients with heart failure with reduced ejection fraction (HFrEF) (mean left ventricular ejection fraction: 32%) with a mean age of 63 years, and the majority of patients with New York Heart Association (NYHA) Class II or III. The pooled patient outcome results of 2022 Cochrane review and recently published meta-analyses17–20 are summarized in Supplementary material online, Table S1.
Although this latest analysis shows that participation in cardiac rehabilitation has no clear benefit in terms of overall mortality, it confirms the patient-reported outcome benefits cited in the previous meta-analyses, i.e. 25%–30% relative reduction in the risk of all-cause and heart failure hospitalization and concomitant reduction in healthcare costs and improvement in health-related quality of life. Meta-analysis showed an improvement in disease-specific health-related quality of life assessed by the total Minnesota Living with Heart Failure Questionnaire (MLWHF) score [mean −7.4, 95% confidence interval (CI) −10.3 to −4.5]. Given that change in MLWHF total score of ≥5 points is considered clinically meaningful,21 this effect is not only statistically significant but important to patients. These gains in patient-related outcomes are underpinned by improvements in mechanistic endpoints with cardiac rehabilitation including improved endothelial function, reduced catecholamine spillover, increased peripheral oxygen extraction, and improvement in peak oxygen consumption.22,23
The Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF) study, like the Cochrane review above, included randomized trials of exercise-based cardiac rehabilitation but focused on the subgroup of studies with a more precise definition of HFrEF (left ventricular ejection fraction <40%) and published after 1999 (therefore receiving contemporary drug and secondary prevention of heart failure) with 6-month or more follow-up.24 Whilst the CROS-HF reported no clear improvement in either mortality or hospitalization, it confirms the improvement in exercise capacity and health-related quality of life with participation in exercise-based cardiac rehabilitation. Beyond the scope of this present review, detailed recommendations of exercise prescription for heart failure patients are presented elsewhere.25,26
Are the benefits of cardiac rehabilitation consistent across heart failure patients?
As outlined above, most of the evidence for cardiac rehabilitation has been reported in HFrEF. However, there is a growing randomized trial literature demonstrating the potential benefits in heart failure with preserved ejection fraction (HFpEF) in terms of improvement in health-related quality of life, exercise capacity, and mechanistic echocardiographic measures (see Supplementary material online, Table S1). However, whilst exercise capacity and health-related quality of life are strong prognostic predictors in HFpEF,27,28 given the small number of recruited patients and relatively short follow-up of trials of exercise training interventions, there are insufficient data at this time to fully determine the impact of cardiac rehabilitation on clinical events, including mortality and hospital admission in HFpEF.29 The 2022 American College of Cardiology/American Heart Association joint committee guidelines reflect this evidence gap and prioritize the need for appropriately powered randomized trials assessing the efficacy and safety of cardiac rehabilitation in HFpEF and heart failure with mildly reduced ejection fraction (HFmrEF) patients.2 Although very few data exist, a small number of studies have investigated the impact of exercise training/cardiac rehabilitation on HFmrEF.30,31
Except for the large multicentre HF-ACTION trial (2331 patients),32 individual trials of exercise training or more comprehensive cardiac rehabilitation interventions for heart failure are too small (mean sample size: 131 patients) to have adequate statistical power to assess the impact of cardiac rehabilitation across patient characteristics or subgroups, e.g. males vs. females, NYHA Class I/II vs. Class III/IV. However, the ExTraMATCH II study, an individual participant data meta-analysis, was specifically designed to address this question, curating and reanalysing outcome data from 3990 patients and 13 randomized trials (including HF-ACTION).33 The authors concluded that the benefits of reduced hospitalization and improved health-related quality of life were consistent across HFrEF patient groups that included age, sex, ethnicity, NYHA functional class, ischaemic aetiology, ejection fraction, and baseline exercise capacity. This finding supports current international guidance that exercise-based cardiac rehabilitation should be offered to all patients with a diagnosis of HFrEF. However, further research is needed to determine efficacy and safety in HFpEF, where the majority of evidence is in small trials focusing on exercise training.29
Frailty is common among the growing number of older people with HFrEF and HFpEF and is associated with worse outcomes.34 A substudy from the HF-ACTION trial found that baseline frailty modified the treatment effect of aerobic exercise training and a greater reduction in the risk of all-cause mortality was demonstrated.35 Another substudy from the EJECTION-HF trial36 underpins the potential modifiable nature of frailty of cardiac rehabilitation, but physical frailty as a modifiable treatment target of multidomain physical function intervention is still to be fully documented.34
Are the benefits of cardiac rehabilitation consistent across delivery settings?
The traditional mode of delivery for cardiac rehabilitation has been centre-based exercise programme. As discussed above, given the relatively poor uptake of cardiac rehabilitation across Europe and more globally4–6 and accentuated by the learnings during the pandemic,10,37 there have been increasing calls for other more innovative delivery models to improve patient access, including home-based, digitally supported, and hybrid (mix of home and centre).38,39 Reflective of this, 10 of 15 of the new randomized trials identified in the updated 2022 Cochrane review were either home-based (5 trials) or hybrid (5 trials) and showed similar outcome impacts to centre-based trials. This finding is supported by a small number of head-to-head trials and recent network meta-analysis40 that show similar improvements in patient-reported outcomes between centre- and home-based cardiac rehabilitation (with or without digital technology support) in heart failure populations.17,41,42
Is there evidence of additional benefit of cardiac rehabilitation as adjuvant intervention to standard of care drug therapy for heart failure?
Current heart failure management guidelines consistently recommend a medical model of the use of drug and devices. Latest guidelines recommend concomitant drug treatment [angiotensin-converting enzyme inhibitor or angiotensin receptor–neprilysin inhibitor (ARNI) and beta-blocker and mineralocorticoid receptor antagonists and sodium–glucose co-transporter-2 (SGLT2) inhibitors] for HFrEF patients1,2—the so-called ‘four pillars’ of heart failure treatment.43 Cardiac rehabilitation is the ideal time and place for implementation and titration of these drugs. However, given that there is growing evidence base demonstrating that heart failure patients have a strong preference for either improving their health-related quality of life or prolonging survival or both,44,45 it is of central importance that patients, clinicians, and policymakers understand the impact of heart failure interventions so that they can make decisions that are congruent with patient-specific goals.
The impact of pharmacotherapy on health-related quality of life in patients with HFrEF was recently reported in systematic review/meta-analysis of placebo-controlled trials.46 Given the range of health-related quality-of-life measures reported, the authors pooled results across trials in each drug class using standardized mean differences but to aid interpretation, back-transformed results to mean difference in two commonly used disease-specific health-related quality-of-life measures [MLWHF and Kansas City Cardiomyopathy Questionnaire (KCCQ)] (see Supplementary material online, Table S2A). These analyses show that quality-of-life impact of drugs is highly variable with most key drugs classes either having no quality-of-life benefit or mean improvement that falls below what might be considered a patient relevant effect (i.e. <5 for both KCCQ and MLWHF). A similar conclusion can be drawn from key contemporary pivotal drug trials in HFpEF47–52 (see Supplementary material online, Table S2B). In summary, for HFrEF, only intravenous iron and ARNI (valsartan/sacubitril) at 3 months produced a mean health-related quality-of-life improvement that surpassed the 5-point threshold on KCCQ or MLWHF reflecting a clinically important difference.46 In HFpEF only, the use of the SGLT2 dapagliflozin surpassed the 5-point threshold50 although a smaller improvement in KCCQ vs. placebo has been seen in other trials including the larger EMPEROR-Preserved study with empagliflozin.49,51
The substantial gain in health-related quality of life with cardiac rehabilitation emphasizes the importance of its combination with current pharmacological therapy for patients with HF41,52–54 as the ‘fifth pillar’ of heart failure management, and cardiac rehabilitation allows us to address patients’ preference for improving their morbidity, survival, and quality of life.
Are there any safety concerns?
Evidence regarding exercise-based rehabilitation in heart failure is derived from studies implementing exercise training programmes that are considered safe. No major adverse effects of exercise training were reported in the included studies of above systematic review/meta-analyses.17,19,52 There is a general agreement across guidelines1,2,26 that exercise intervention should only be initiated in stable individuals after medical therapy has been initiated and the clinical status is stable. The 2020 European Society of Cardiology guidelines on sports cardiology and exercise in patients with cardiovascular diseases have pointed out key components of risk stratification and preliminary evaluation ahead of exercise initiation.26 When these recommendations are followed, the overall risk of exercise is considered low, even during higher-intensity exercises, in patients with more severe heart failure,55,56 and in home-based settings.57
Clinical guidance/guidelines for cardiac rehabilitation for heart failure
International heart failure guidelines1,2 recommend rehabilitation (including patient education, self-care, and exercise training as a Class IA recommendation) for patients with chronic heart failure regardless of left ventricular ejection fraction which may also include those with cardiac implantable electronic or ventricular assist devices.3 Detailed guidance on providing the core components of cardiac rehabilitation in chronic heart failure has been prepared by the European Association of Preventive Cardiology.3 A rehabilitation programme duration can run from 8- to 36-week duration for up to 7 days/week with an emphasis on supervised exercise training.3
How to deliver cardiac rehabilitation in heart failure?
Alternatives to centre-based programmes include home-based models and digital modes of delivery that allow patients to access cardiac rehabilitation virtually.17,38,57–60 Digital delivery embraces cardiac tele-rehabilitation (which includes use of mobile or internet-based communication and social media platforms) that have been advocated by several international sources60–62 including the European Association of Preventive Cardiology who provided a practical guide to deliver a comprehensive rehabilitation programme within the constraints of the SARS-CoV-2 public health measures.37 Hybrid cardiac rehabilitation and home-based and remotely delivered virtual/tele-rehabilitation programmes are emerging to overcome access, especially in rural areas.62–65 Lower levels of digital literacy and access to the internet in certain groups such as ethnic minorities, the elderly, and the socioeconomically deprived could worsen inequalities by further limiting their participation in cardiac tele-rehabilitation.66–68 Whilst there is suboptimal uptake and inequities in access to rehabilitation and a growing body evidence of effectiveness and safety of tele-rehabilitation,42,69 there remain some important practical considerations in the widespread application of alternative modes of cardiac rehabilitation delivery to clinical practice. To date, patients included in tele-rehabilitation studies are mostly low-risk patients without a diagnosis of heart failure.69 Most tele-rehabilitation studies performed exercise training only (the precise amount of exercise undertaken often being unclear), a more comprehensive rehabilitation approach (i.e. inclusion of education and psychological support) not being delivered.70 At this time, tele-rehabilitation might be most appropriate for a selected heart failure population (stable NYHA Class II, without cardiac arrhythmias, or implantable cardioverter defibrillator) with less need for psychosocial support or education in whom adherence to cardiac rehabilitation is less likely to be problematic.71
Cardiac rehabilitation as part of integrated heart failure care
Cardiac rehabilitation as defined in guidelines is an important part of heart failure management that can be introduced and re-introduced throughout the whole heart failure trajectory irrespective of disease stage: from onset, through critical events, periods of apparent stability, up until the terminal stages.1
Along the heart failure disease trajectory, cardiac rehabilitation services have overlapping focus and content with nurse-led disease management programmes, self-management strategies, as well as palliative care interventions72,73—separate approaches which are all rooted in the biopsychosocial model.74
The evidence on how to combine and coordinate these different and interconnected approaches and their best timing is not yet well established. The 2021 European Society of Cardiology guidance recommends that the organization of follow-up interventions should be tailored to the individual patient’s needs and stage of disease.1 Many patients with heart failure would derive benefit from the integration of nurse-led disease management programmes, self-management strategies, cardiac rehabilitation, and a palliative and supportive care approach from onset until death. However, there is absence of evidence on how to specifically combine these approaches; thus, adaptation to national healthcare systems, available resources (infrastructure, facilities, staff, and finances), and administrative policies will be necessary. Evidence-based home-based self-management rehabilitation interventions, such as the REACH-HF programme,75 can be used throughout the heart failure disease trajectory, especially at times when there is deterioration in the physical status.
Current status of cardiac rehabilitation for heart failure
Despite strong guideline recommendations for cardiac rehabilitation, poor participation of patients with heart failure was evident even before the SARS-CoV-2 pandemic. Referral and attendance rates are <20% in Europe4,5 and the USA6 over the last 15 years, with reported rates of participation as low as 5%.76 These rates of cardiac rehabilitation participation remain consistently lower than those typically seen for patients with a coronary heart disease diagnosis (including following acute myocardial infraction and revascularization).77
Suboptimal participation in cardiac rehabilitation has been attributed to several key contributory factors related to (i) healthcare professionals (clinicians), (ii) patients, and (iii) system-level issues/healthcare policy.9
Barriers caused by healthcare professionals
Low referral rates with the associated lack of endorsement of cardiac rehabilitation by clinicians have been put down to a lack of awareness on the evidence of effectiveness and inadequate education in clinician training curricula.9 Extending education on the benefits of cardiac rehabilitation beyond clinicians within cardiology could help to provide more trained staff to deliver programmes and improve the uptake.
Patient-level barriers
Nearly 80% of patients with heart failure have three or more comorbidities78 and are prone to higher levels of disability, as disease progression leads to increasing incapacity and deconditioning.9 These physical factors coupled with psychosocial and economic factors can influence participation in cardiac rehabilitation. Groups such as women, ethnic minorities, and the elderly and those from areas of high socioeconomic deprivation and rural locations are particularly underrepresented in cardiac rehabilitation programmes.9,79,80 Other patient-related factors that may limit participation to centre-based programmes include the affordability and co-payments for insurance coverage in some countries (e.g. the USA), the inconvenience of travel, a dislike of group-based activities, ‘time lost from work, poor social support, and the lack of perceived benefit’.9,81
System-level barriers
System-level issues such as the availability of cardiac rehabilitation programmes and the payment structures affect the number of patients who participate.80 In countries where cardiac rehabilitation services are well organized and state funded, such as Denmark, referral rates of around 50% in patients with heart failure are reported.82 However, in most high- and low-income countries, the availability and utilization of cardiac rehabilitation remain very limited.79,83–85 Even in the USA, where referral rates to cardiac rehabilitation are highest in those with medical insurance,86 it is only authorized for patients with stable chronic heart failure with a left ventricular ejection fraction of <35% and NYHA Classes II–IV and not to patients with HFpEF.9
Healthcare policies have an impact on the rates of participation in cardiac rehabilitation.67 Some countries have introduced policies to increase the overall uptake of cardiac rehabilitation. For example, the National Health Service in the UK has a ‘long-term plan’ which aims to increase the national uptake of cardiac rehabilitation to 85% for all eligible patients with cardiovascular disease by 2028.87 Given the very low rates of participation (<10%) in heart failure, the target set for heart failure is more modest: 33% by 2023.86 In 2017, the US ‘Million Hearts Cardiac Rehabilitation Collaborative’ published a proposal to achieve >70% participation by 202288—aiming to save 25 000 lives and 180 000 hospitalizations per year. With the negative impact of SARS-CoV-2 on health service staffing/capacity over the last 2 years, there are likely to be delays in meeting these ambitious pre-pandemic timelines and targets.
Disruption of many non-essential hospital services, through staff redeployment and sickness, and the advice to vulnerable patients to shield and self-isolate during the SARS-CoV-2 pandemic have had a significant impact on rates of participation in cardiac rehabilitation.35,89 Thus, the pandemic has accentuated the pre-existing barriers to accessing traditional centre-based programmes and has led to a renewed call for alternatives to improve the uptake of cardiac rehabilitation.38,39
Conclusions
Cardiac rehabilitation is a multicomponent evidence-based complex intervention that provides important benefits to the patients with heart failure, including health-related quality of life. In addition to its Class I recommendation by the current European and international clinical guidelines, the clinical community needs to embrace and implement cardiac rehabilitation as a key pillar of heart failure management alongside drug and medical device provision (Graphical Abstract). The SARS-CoV-2 pandemic has provided a unique opportunity to fast-track a reframing of the traditional model of cardiac rehabilitation with alternative home-based programme and digitally supported models of delivery to improve access and uptake of cardiac rehabilitation, especially in hard-to-reach patient populations. Cardiac rehabilitation needs to be delivered across the disease trajectory and appropriately integrated with services, including the provision heart failure nurse and self-management strategies.
We provide a list of some key areas for future research for cardiac rehabilitation in heart failure in Table 1.
Table 1.
Cardiac rehabilitation for heart failure: key areas for future research/evidence generation and health policy
|
Author contributions
R.S.T., H.D., and A.D.Z. each drafted sections of this paper. All the authors agreed the final version.
Rod S. Taylor, MSc, PhD, Ann-Dorthe Zwisler, and Hasnain Dalal
Supplementary Material
Acknowledgements
None.
Contributor Information
Rod S Taylor, MRC/CSO Social and Public Health Sciences Unit & Robertson Centre for Biostatistics, School of Health & Well Being, Clarice Pears Building, University of Glasgow, Byres Rd, Glasgow G12 8TA, UK; Health Service Research, College of Medicine and Health, University of Exeter, Heavitree Rd, Exeter, EX2 4TH, UK; Faculty of Health Sciences and National Institute of Public Health, University of Southern Denmark, Studiestræde 6, 1455, Copenhagen, Denmark.
Hasnain M Dalal, University of Exeter Medical School, Royal Cornwall Hospital, Truro, UK; Primary Care Research Group, University of Exeter Medical School, St Luke’s Campus, Exeter, UK.
Ann-Dorthe Zwisler, Faculty of Health Sciences and National Institute of Public Health, University of Southern Denmark, Studiestræde 6, 1455, Copenhagen, Denmark; Department of Cardiology, Odense University Hospital, J. B. Winsløws Vej 4, 5000, Odense C, Denmark; REHPA, Vestergade 17, 5800, Nyborg, Denmark; Department of Clinical Research, University of Southern Denmark, Campusvej 55, DK-5230 Odense M, Denmark.
Supplementary data
Supplementary data are available at European Heart Journal online.
Data availability
No new data were generated or analysed in support of this research.
Funding
This review has been prepared without funding.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. . 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895–e1032. [DOI] [PubMed] [Google Scholar]
- 3. Ambrosetti M, Abreu A, Corra U, Davos CH, Hansen D, Frederix I, et al. . Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2021;28:460–495. [DOI] [PubMed] [Google Scholar]
- 4. Bjarnason-Wehrens B, McGee H, Zwisler AD, Piepoli MF, Benzer W, Schmid JP, et al. . Cardiac rehabilitation in Europe: results from the European Cardiac Rehabilitation Inventory Survey. Eur J Cardiovasc Prev Rehabil 2010;17:410–418. [DOI] [PubMed] [Google Scholar]
- 5.https://www.hqip.org.uk/resource/national-heart-failure-audit-nhfa-2021-summary-report/#.Y4DnOXbP3fs National Heart Failure Audit (NHFA) - Summary Report. 2021. Available from:
- 6. Golwala H, Pandey A, Ju C, Butler J, Yancy C, Bhatt DL, et al. . Temporal trends and factors associated with cardiac rehabilitation referral among patients hospitalized with heart failure: findings from Get With The Guidelines-Heart Failure Registry. J Am Coll Cardiol 2015;66:917–926. [DOI] [PubMed] [Google Scholar]
- 7. Adamo M, Gardner RS, McDonagh TA, Metra M. The ‘Ten Commandments’ of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2022;43:440–441. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization . Needs and action priorities in cardiac rehabilitation and secondary prevention in patients with coronary heart disease. Geneva. Switzerland 1993.
- 9. Bozkurt B, Fonarow GC, Goldberg LR, Guglin M, Josephson RA, Forman DE, et al. . Cardiac rehabilitation for patients with heart failure: JACC Expert Panel. J Am Coll Cardiol 2021;77:1454–1469. [DOI] [PubMed] [Google Scholar]
- 10. Redfern J, Gallagher R, O’Neil A, Grace SL, Bauman A, Jennings G, et al. . Historical context of cardiac rehabilitation: learning from the past to move to the future. Front Cardiovasc Med 2022;9:842567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.https://www.bacpr.org/__data/assets/pdf_file/0026/39437/BACPR_Standards_and_Core_Components_2017.pdf BACPR Standards and Core Components for Cardiovascular Disease Prevention and Rehabilitation 2017.
- 12. Rauch B, Salzwedel A, Bjarnason-Wehrens B, Albus C, Meng K, Schmid JP, et al. On Behalf of the Cardiac Rehabilitation Guideline Group . Cardiac rehabilitation in German speaking countries of Europe-rvidence-based guidelines from Germany, Austria and Switzerland LLKardReha-DACH-part 1. J Clin Med 2021;10:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwaab B, Bjarnason-Wehrens B, Meng K, Albus C, Salzwedel A, Schmid JP, et al. . Cardiac rehabilitation in German speaking countries of Europe - evidence-based guidelines from Germany, Austria and Switzerland LLKardReha-DACH-part 2. J Clin Med 2021;10:3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laoutaris ID, Dritsas A, Adamopoulos S. Cardiovascular rehabilitation in the COVID-19 era: ‘a phoenix arising from the ashes?’. Eur J Prev Cardiol 2022;29:1372–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abraham LN, Sibilitz KL, Berg SK, Tang LH, Risom SS, Lindschou J, et al. . Exercise-based cardiac rehabilitation for adults after heart valve surgery. Cochrane Database Syst Rev 2021;5:CD010876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dibben G, Faulkner J, Oldridge N, Rees K, Thompson DR, Zwisler AD, et al. . Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2021;11:CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, et al. . Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev 2019;1:CD003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uddin J, Zwisler AD, Lewinter C, Moniruzzaman M, Lund K, Tang LH, et al. . Predictors of exercise capacity following exercise-based rehabilitation in patients with coronary heart disease and heart failure: a meta-regression analysis. Eur J Prev Cardiol 2016;23:683–693. [DOI] [PubMed] [Google Scholar]
- 19. Fukuta H, Goto T, Wakami K, Kamiya T, Ohte N. Effects of exercise training on cardiac function, exercise capacity, and quality of life in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Heart Fail Rev 2019;24:535–547. [DOI] [PubMed] [Google Scholar]
- 20. Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, et al. . Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 2015;8:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.https://qol.thoracic.org/sections/instruments/ko/pages/mlwhfq.html Minnesota Living with Heart Failure Questionnaire 2004. Available from:
- 22. Santos FV, Chiappa GR, Ramalho SHR, de Lima A, de Souza FSJ, Cahalin LP, et al. . Resistance exercise enhances oxygen uptake without worsening cardiac function in patients with systolic heart failure: a systematic review and meta-analysis. Heart Fail Rev 2018;23:73–89. [DOI] [PubMed] [Google Scholar]
- 23. Haykowsky MJ, Timmons MP, Kruger C, McNeely M, Taylor DA, Clark AM. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. Am J Cardiol 2013;111:1466–1469. [DOI] [PubMed] [Google Scholar]
- 24. Bjarnason-Wehrens B, Nebel R, Jensen K, Hackbusch M, Grilli M, Gielen S, et al. . Exercise-based cardiac rehabilitation in patients with reduced left ventricular ejection fraction: the Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF): a systematic review and meta-analysis. Eur J Prev Cardiol 2020;27:929–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piepoli MF, Conraads V, Corra U, Dickstein K, Francis DP, Jaarsma T, et al. . Exercise training in heart failure: from theory to practice. a consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 2011;13:347–357. [DOI] [PubMed] [Google Scholar]
- 26. Pelliccia A, Sharma S, Gati S, Back M, Borjesson M, Caselli S, et al. . 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021;42:17–96. [DOI] [PubMed] [Google Scholar]
- 27. Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, et al. . Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol 2017;69:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pandey A, Patel KV, Bahnson JL, Gaussoin SA, Martin CK, Balasubramanyam A, et al. . Association of intensive lifestyle intervention, fitness, and body mass index with risk of heart failure in overweight or obese adults with type 2 diabetes mellitus: an analysis from the Look AHEAD trial. Circulation 2020;141:1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edwards JJ, O’Driscoll JM. Exercise training in heart failure with preserved and reduced ejection fraction: a systematic review and meta-analysis. Sports Med Open 2022;8:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kitagawa T, Hidaka T, Watanabe N, Naka M, Yamaguchi M, Kanai K, et al. . Current conditions and significance of outpatient cardiac rehabilitation and home nursing-care services in heart failure patients with mid-range or preserved ejection fraction: post-hoc analysis of the REAL-HF registry. Heart Vessels 2022;37:745–754. [DOI] [PubMed] [Google Scholar]
- 31. Schürmann J, Noack F, Bethge S, Heinze V, Schlitt A. Patients with heart failure during and after inpatient cardiac rehabilitation. Vasc Health Risk Manag 2021;17:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. . Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O, et al. . Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure: individual participant meta-analysis. J Am Coll Cardiol 2019;73:1430–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pandey A, Kitzman D, Reeves G. Frailty is intertwined with heart failure: mechanisms, prevalence, prognosis, assessment, and management. JACC Heart Fail 2019;7:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pandey A, Segar MW, Singh S, Reeves GR, O'Connor C, Pina I, et al. . Frailty status modifies the efficacy of exercise training among patients with chronic heart failure and reduced ejection fraction: an analysis from the HF-ACTION Trial. Circulation 2022;146:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mudge AM, Pelecanos A, Adsett JA. Frailty implications for exercise participation and outcomes in patients with heart failure. J Am Geriatr Soc 2021;69:2476–2485. [DOI] [PubMed] [Google Scholar]
- 37. Scherrenberg M, Wilhelm M, Hansen D, Voller H, Cornelissen V, Frederix I, et al. . The future is now: a call for action for cardiac telerehabilitation in the COVID-19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2021;28:524–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, et al. . Home-Based Cardiac Rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation 2019;140:e69–e89. [DOI] [PubMed] [Google Scholar]
- 39. Clark RA, Conway A, Poulsen V, Keech W, Tirimacco R, Tideman P. Alternative models of cardiac rehabilitation: a systematic review. Eur J Prev Cardiol 2015;22:35–74. [DOI] [PubMed] [Google Scholar]
- 40. Tegegne TK, Rawstorn JC, Nourse RA, Kibret KT, Ahmed KY, Maddison R. Effects of exercise-based cardiac rehabilitation delivery modes on exercise capacity and health-related quality of life in heart failure: a systematic review and network meta-analysis. Open Heart 2022;9:e001949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, et al. . Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017;6:CD007130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Owen O, O'Carroll V. The effectiveness of cardiac telerehabilitation in comparison to centre-based cardiac rehabilitation programmes: a literature review. J Telemed Telecare 2022:1357633X2210858. 10.1177/1357633X221085865. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Straw S, McGinlay M, Witte KK. Four pillars of heart failure: contemporary pharmacological therapy for heart failure with reduced ejection fraction. Open Heart 2021;8:e001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kraai IH, Vermeulen KM, Luttik ML, Hoekstra T, Jaarsma T, Hillege HL. Preferences of heart failure patients in daily clinical practice: quality of life or longevity? Eur J Heart Fail 2013;15:1113–1121. [DOI] [PubMed] [Google Scholar]
- 45. Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant 2001;20:1016–1024. [DOI] [PubMed] [Google Scholar]
- 46. Turgeon RD, Barry AR, Hawkins NM, Ellis UM. Pharmacotherapy for heart failure with reduced ejection fraction and health-related quality of life: a systematic review and meta-analysis. Eur J Heart Fail 2021;23:578–589. [DOI] [PubMed] [Google Scholar]
- 47. Fukuta H, Goto T, Wakami K, Kamiya T, Ohte N. Effects of mineralocorticoid receptor antagonists on left ventricular diastolic function, exercise capacity, and quality of life in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Heart Vessels 2019;34:597–606. [DOI] [PubMed] [Google Scholar]
- 48. Pieske B, Wachter R, Shah SJ, Baldridge A, Szeczoedy P, Ibram G, et al. . Effect of sacubitril/valsartan vs standard medical therapies on plasma NT-proBNP concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction: the PARALLAX randomized clinical trial. JAMA 2021;326:1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 50. Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, et al. . The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med 2021;27:1954–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. doi: 10.1093/eurheartj/ehab715. Volpe M, Patrono C. The EMPEROR-Preserved study: end of the search for the “Phoenix” or beginning of a new season for trials in heart failure with preserved ejection fraction. Eur Heart J 2021;42:4621–4623. [DOI] [PubMed] [Google Scholar]
- 52. Zhuang C, Luo X, Wang Q, Wang W, Sun R, Zhang X, et al. . The effect of exercise training and physiotherapy on diastolic function, exercise capacity and quality of life in patients with heart failure with preserved ejection fraction: a systematic review and meta-analysis. Kardiol Pol 2021;79:1107–1115. [DOI] [PubMed] [Google Scholar]
- 53. Redfield MM, Borlaug BA. Quality of life and exercise ability in heart failure with preserved ejection fraction: no time for therapeutic complacency. JAMA 2021;326:1913–1915. [DOI] [PubMed] [Google Scholar]
- 54. Pandey A, Butler J. Improving exercise tolerance and quality of life in heart failure with preserved ejection fraction - time to think outside the heart. Eur J Heart Fail 2021;23:1552–1554. [DOI] [PubMed] [Google Scholar]
- 55. Ellingsen O, Halle M, Conraads V, Stoylen A, Dalen H, Delagardelle C, et al. . High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation 2017;135:839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rognmo O, Moholdt T, Bakken H, Hole T, Molstad P, Myhr NE, et al. . Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 2012;126:1436–1440. [DOI] [PubMed] [Google Scholar]
- 57. Stefanakis M, Batalik L, Antoniou V, Pepera G. Safety of home-based cardiac rehabilitation: a systematic review. Heart Lung 2022;55:117–126. [DOI] [PubMed] [Google Scholar]
- 58. Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart 2016;102:1183–1192. [DOI] [PubMed] [Google Scholar]
- 59. Taylor RS, Dalal HM, McDonagh STJ. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat Rev Cardiol 2022;19:180–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dalal HM, Doherty P, McDonagh ST, Paul K, Taylor RS. Virtual and in-person cardiac rehabilitation. BMJ 2021;373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vishwanath V, Beckman AL, Kazi DS. Reimagining cardiac rehabilitation in the era of coronavirus disease 2019. JAMA Health Forum 2020;1:e201346. [DOI] [PubMed] [Google Scholar]
- 62. Thomas EG, Grace SL. Future-proofing cardiac rehabilitation: transitioning services to telehealth during COVID-19. Eur J Prev Cardiol 2021;28:e35–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brahmbhatt DH, Cowie MR. Remote management of heart failure: an overview of telemonitoring technologies. Card Fail Rev 2019;5:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Imran HM, Baig M, Erqou S, Taveira TH, Shah NR, Morrison A, et al. . Home-based cardiac rehabilitation alone and hybrid with center-based cardiac rehabilitation in heart failure: a systematic review and meta-analysis. J Am Heart Assoc 2019;8:e012779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Forman DE, LaFond K, Panch T, Allsup K, Manning K, Sattelmair J. Utility and efficacy of a smartphone application to enhance the learning and behavior goals of traditional cardiac rehabilitation: a feasibility study. J Cardiopulm Rehabil Prev 2014;34:327–334. [DOI] [PubMed] [Google Scholar]
- 66. Eberly LA, Khatana SAM, Nathan AS, Snider C, Julien HM, Deleener ME, et al. . Telemedicine outpatient cardiovascular care during the COVID-19 pandemic: bridging or opening the digital divide? Circulation 2020;142:510–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, et al. . Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation 2011;124:2951–2960. [DOI] [PubMed] [Google Scholar]
- 68. Vogels EA. Digital divide persists even as Americans with lower incomes make gains in tech adoption. Pew Research Center 2021.https://www.pewresearch.org/fact-tank/2021/06/22/digital-divide-persists-even-as-americans-with-lower-incomes-make-gains-in-tech-adoption/.
- 69. Hwang R, Bruning J, Morris NR, Mandrusiak A, Russell T. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: a randomised trial. J Physiother 2017;63:101–107. [DOI] [PubMed] [Google Scholar]
- 70. Salvi D, Ottaviano M, Muuraiskangas S, Martinez-Romero A, Vera-Munoz C, Triantafyllidis A, et al. . An m-Health system for education and motivation in cardiac rehabilitation: the experience of HeartCycle guided exercise. J Telemed Telecare 2018;24:303–316. [DOI] [PubMed] [Google Scholar]
- 71. Thomas RJ, Balady G, Banka G, Beckie TM, Chiu J, Gokak S, et al. . 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol 2018;71:1814–1837. [DOI] [PubMed] [Google Scholar]
- 72. Timm H, Thuesen J, Clark D. Rehabilitation and palliative care: histories, dialectics and challenges. Wellcome Open Res 2021;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol 2009;54:386–396. [DOI] [PubMed] [Google Scholar]
- 74. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–136. [DOI] [PubMed] [Google Scholar]
- 75. Dalal HM, Taylor RS, Jolly K, Davis RC, Doherty P, Miles J, et al. . The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: the REACH-HF multicentre randomized controlled trial. Eur J Prev Cardiol 2019;26:262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pandey A, Keshvani N, Zhong L, Mentz RJ, Pina IL, DeVore AD, et al. . Temporal trends and factors associated with cardiac rehabilitation participation among Medicare beneficiaries with heart failure. JACC Heart Fail 2021;9:471–481. [DOI] [PubMed] [Google Scholar]
- 77. Ogmundsdottir Michelsen H, Sjolin I, Schlyter M, Hagstrom E, Kiessling A, Henriksson P, et al. . Cardiac rehabilitation after acute myocardial infarction in Sweden - evaluation of programme characteristics and adherence to European guidelines: the Perfect Cardiac Rehabilitation (Perfect-CR) study. Eur J Prev Cardiol 2020;27:18–27. [DOI] [PubMed] [Google Scholar]
- 78. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. . Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ruano-Ravina A, Pena-Gil C, Abu-Assi E, Raposeiras S, van ‘t Hof A, Meindersma E, et al. . Participation and adherence to cardiac rehabilitation programs. A systematic review. Int J Cardiol. 2016;223:436–443. [DOI] [PubMed] [Google Scholar]
- 80. Ragupathi L, Stribling J, Yakunina Y, Fuster V, McLaughlin MA, Vedanthan R. Availability, use, and barriers to cardiac rehabilitation in LMIC. Glob Heart 2017;12:323–334. [DOI] [PubMed] [Google Scholar]
- 81. Valencia HE, Savage PD, Ades PA. Cardiac rehabilitation participation in underserved populations. Minorities, low socioeconomic, and rural residents. J Cardiopulm Rehabil Prev 2011;31:203–210. [DOI] [PubMed] [Google Scholar]
- 82. Thygesen LC, Zinckernagel L, Dalal H, Egstrup K, Glumer C, Grønbæk M, et al. . Cardiac rehabilitation for patients with heart failure: association with readmission and mortality risk. Eur Heart J Qual Care Clin Outcomes 2022;8:830–839. [DOI] [PubMed] [Google Scholar]
- 83. Pesah E, Turk-Adawi K, Supervia M, Lopez-Jimenez F, Britto R, Ding R, et al. . Cardiac rehabilitation delivery in low/middle-income countries. Heart 2019;105:1806–1812. [DOI] [PubMed] [Google Scholar]
- 84. Babu AS, Turk-Adawi K, Supervia M, Jimenez FL, Contractor A, Grace SL. Cardiac rehabilitation in India: results from the International Council of Cardiovascular Prevention and Rehabilitation's Global Audit of Cardiac Rehabilitation. Glob Heart 2020;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lima de Melo Ghisi G, Pesah E, Turk-Adawi K, Supervia M, Lopez Jimenez F, Grace SL. Cardiac rehabilitation models around the globe. J Clin Med. 2018;7:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Aragam KG, Dai D, Neely ML, Bhatt DL, Roe MT, Rumsfeld JS, et al. . Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol 2015;65:2079–2088. [DOI] [PubMed] [Google Scholar]
- 87. NHS England . The NHS long term plan London: NHS England; 2019. Available from:https://longtermplan.nhs.uk
- 88. Ades PA, Keteyian SJ, Wright JS, Hamm LF, Lui K, Newlin K, et al. . Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc 2017;92:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Babu AS, Arena R, Ozemek C, Lavie CJ. COVID-19: a time for alternate models in cardiac rehabilitation to take centre stage. Can J Cardiol 2020;36:792–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.