Graphical Abstract
Graphical abstract.
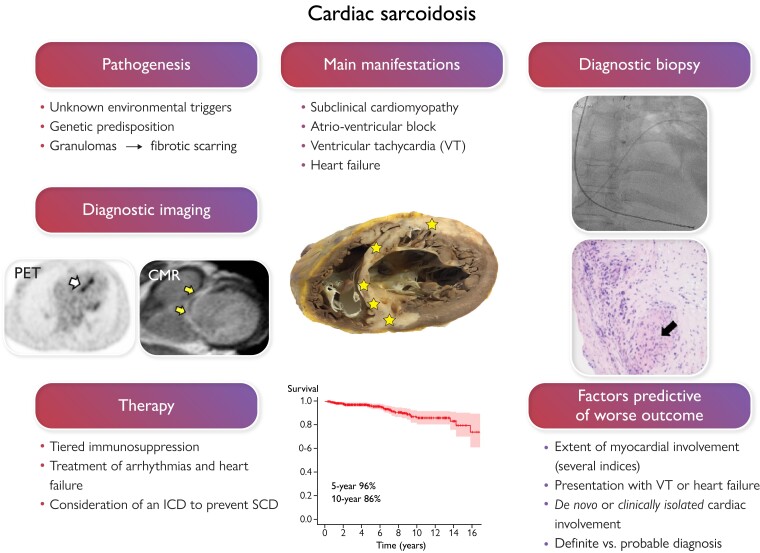
In cardiac sarcoidosis (CS), inflammatory granulomas invade the heart leading to injury and fibrosis (yellow stars in the section of a sarcoidotic heart in the middle of the graph). CS is often subclinical, but, when clinically manifest, presents commonly with slow or fast arrhythmias or heart failure. On the left of the figure, positron emission tomography (PET) exposes focal septal uptake of 18-F fluorodeoxyglucose suggesting active inflammation (white arrow), and contrast-enhanced cardiac magnetic resonance (CMR) shows septal late gadolinium enhancement (arrows) indicating replacement fibrosis. Both constitute major diagnostic criteria for CS and entitle probable CS diagnosis if accompanied by confirmed extracardiac histology of sarcoidosis. Yet, the only way to definite diagnosis, demonstrated on the right, is myocardial biopsy showing non-necrotic granulomas (black arrow). The therapy of CS is based on immunosuppression and management of heart block, ventricular arrhythmias, and heart failure. The risk of sudden cardiac death (SCD) needs assessment and consideration of an implantable cardioverter-defibrillator (ICD). With current therapy, expected 5-year survival is well above 90% as shown by the Kaplan–Meier graph of a 398-patient Finnish CS cohort.
Keywords: Cardiac sarcoidosis, Inflammatory heart disease, Heart failure, Pacemaker, Implantable cardioverter-defibrillator
Abstract
Cardiac sarcoidosis (CS) results from epithelioid cell granulomas infiltrating the myocardium and predisposing to conduction disturbances, ventricular tachyarrhythmias, and heart failure. Manifest CS, however, constitutes only the top of an iceberg as advanced imaging uncovers cardiac involvement 4 to 5 times more commonly than what is clinically detectable. Definite diagnosis of CS requires myocardial biopsy and histopathology, but a sufficient diagnostic likelihood can be achieved by combining extracardiac histology of sarcoidosis with clinical manifestations and findings on cardiac imaging. CS can appear as the first or only organ manifestation of sarcoidosis or on top of pre-existing extracardiac disease. Due to the lack of controlled trials, the care of CS is based on observational evidence of low quality. Currently, the treatment involves corticosteroid-based, tiered immunosuppression to control myocardial inflammation with medical and device-based therapy for symptomatic atrioventricular block, ventricular tachyarrhythmias, and heart failure. Recent outcome data indicate 90% to 96% 5-year survival in manifest CS with the 10-year figures ranging from 80% to 90%. Major progress in the care of CS awaits the key to its molecular–genetic pathogenesis and large-scale controlled clinical trials.
Introduction
Sarcoidosis is an enigmatic disease for many reasons, not least due to its exact cause and pathogenesis remaining hidden despite decades of focused research. Hypotheses abound, however, the overarching one being that the disease results from environmental antigens—infectious, occupational, or other—triggering a dysregulated T cell-driven immunologic response in a genetically predisposed individual.1 The response generates non-necrotic inflammatory granulomas that may appear anywhere in the body leading to local injury and fibrosis—or resolving spontaneously.1 Cardiac sarcoidosis (CS) usually presents in tandem with extracardiac involvement but can be the first or even isolated sign of sarcoidosis.2 No cardiac structure is safe from granulomas, but myocardial infiltration does most of the harm. The clinical spectrum of CS extends from silence to sudden cardiac death (SCD),2 the predominant manifestations being impaired conduction, ventricular arrhythmias (VAs), and heart failure. The main challenges of CS are—no less than—diagnosis and treatment (Graphical Abstract). As confirming the presence of myocardial granulomas is challenging, different sets of criteria3–5 are used for clinical CS diagnosis, yet none are validated or universally adopted. Further, CS being rare and unknown for pathogenesis, precision therapy has not been possible and no controlled trial data exist. The many unknowns and uncertainties about CS puzzle even the most astute clinician. The present review updates the current knowledge of CS focusing on clinical aspects. Readers wishing deeper insight into its possible molecular–genetic mechanisms are referred to recent reviews elsewhere.1,6
Phenotypes
CS hidden until autopsy
A perceptible segment of CS presents as an unexpected SCD and is diagnosed only at autopsy.7,8 These individuals have been free of both known sarcoidosis and cardiac manifestations while alive, or their symptoms and signs of heart disease have been misdiagnosed on lifetime examinations. Among the 351 cases of CS detected in Finland from 1998 to the end of 2015, 62 were diagnosed postmortem, and in 38 (11% of all cases) SCD was the first and only manifestation of CS.8
CS with dominant extracardiac sarcoidosis and no or minimal cardiac symptoms
The typical patient is one with known sarcoidosis found to have cardiac involvement on routine screening or in examinations for mild symptoms and/or abnormalities on a 12-lead electrocardiogram (ECG). Postmortem9 and cardiac magnetic resonance (CMR) studies10–12 have uncovered myocardial involvement in 25% to 30% of all sarcoidosis and even in 9% of patients without symptoms or ECG abnormalities.12 Yet, in a screening study of 2163 patients with extracardiac sarcoidosis, only 3.2% had CS detectable by clinical means alone.13
Clinically manifest CS
These patients are admitted for often acute and serious cardiac symptoms, undergo diagnostic assessment, and are found to have CS either on admission or during subsequent examinations. Although only a minority,2,8 or one-half at most,14,15 give a history of sarcoidosis, most are ultimately found to have multi-organ disease. The prevalence of isolated CS16 has varied from 3%17to 43%,18 the most common approximate being 20% to 25%.16,19–21 Cardiac involvement as the first or only organ manifestation, i.e. de novo or clinically isolated CS, implies more serious disease than CS appearing on top of extracardiac disease.2,14,15
The manifestations of CS (Table 1) depend on the location and extent of granulomas, with high-grade atrioventricular block (AVB) and VAs being the most common initial signs.8,22–25 Sustained ventricular tachycardia (VT) results from re-entry circuits in inflamed and scarred myocardial areas, but automatic and triggered arrhythmias are also possible.26 Multiple VT morphologies are common. Heart failure reflects widespread left ventricular (LV) infiltration and systolic dysfunction, but restricted filling due to edematous or fibrotic LV walls can contribute. Mitral regurgitation results from LV or mitral annular dilatation, scarred LV wall restricting valve closure, or from granulomas invading the valve leaflets.27 Infiltration of the right ventricle may masquerade as arrhythmogenic right ventricular cardiomyopathy.28 Atrial fibrillation is rare at presentation but has a considerable later incidence.29 Angina-like chest pain30 can occur and is usually attributed to impaired coronary flow reserve31 from compression of the myocardial microvasculature. However, granulomatous coronary arteritis is also possible and, rarely, CS presents as a full disguise of an acute myocardial infarction with angiography showing normal findings or either dissection or total occlusion of a single coronary artery.27,32,33 Effusive and constrictive pericarditis are exceptional manifestations.34
Table 1.
Symptoms at presentation and main manifestations of cardiac sarcoidosis (CS) in two large cohorts
| Symptoms in 383 Americans with CSa | % | Main manifestations in 289 Finns with CSb | % |
|---|---|---|---|
| Dyspnoea | 50–70 | High-grade atrioventricular block | 46 |
| Palpitation | 40–60 | Heart failure with LV dysfunction | 18 |
| Fatigue | 30–45 | Sustained VT | 17 |
| Chest pain | 20–30 | NSVT or frequent ventricular premature beats | 7 |
| Presyncope | 15–30 | Aborted sudden cardiac death | 4 |
| Syncope | 15–20 | Syndrome mimicking acute myocardial infarctionc | 4 |
| Edema | 5–10 | Atrial tachyarrhythmia | 1 |
| Cardiac arrest | 2–10 | Other | 3 |
NSVT indicates non-sustained ventricular tachycardia; VT, ventricular tachycardia.
From the study of Rosenbaum et al.22 involving a predominantly white (88%) male (63%) population aged on average 54 years. The diagnosis of cardiac sarcoidosis was based on the Heart Rhythm Society’s (HRS) criteria4 in 73% of the cohort, the rest having presumed CS without proof of sarcoidosis histology.
From the study of Ekström et al.8 involving a nationwide cohort with female predominance (74%) and a mean age of 50 years. All patients fulfilled the HRS diagnostic criteria.
acute chest pain, ischemic ECG changes, and elevated cardiac biomarkers with a normal coronary arteriogram.
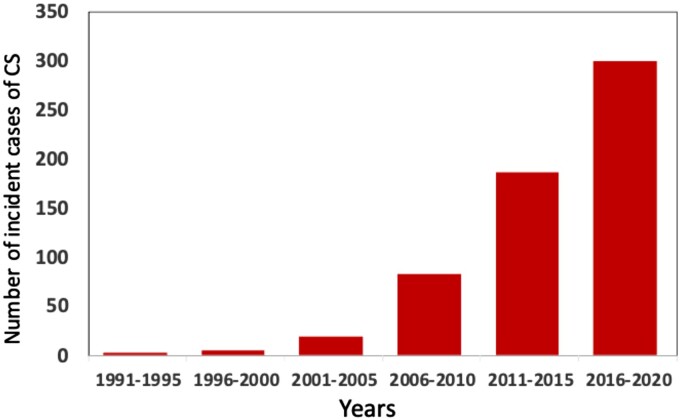
The nationwide registry of Myocardial Inflammatory Diseases in Finland (MIDFIN) includes data on adult patients diagnosed with clinically manifest CS from the late 1980s onwards.2,8,24Figure 1 shows an exponential rise in the 5-year detection rate of new cases ever since. At the end of 2021, according to confirmed but partly unpublished entries, the registry included 703 cases, of which 641 were survivors giving a crude prevalence of clinically manifest CS at 14/100 000 population aged >18 y. Chow et al.35 recently reported a CS prevalence of 4.4/100 000 for a small district in South Island, New Zealand, yet one-half of their patients did not have CS meeting the current diagnostic criteria.3–5 No other CS-specific prevalence data exist, but the figures are likely to differ since the all-inclusive prevalence of sarcoidosis depends on geography and race, among other factors, being highest in the northern countries (Canada and Sweden, 140–160/100 000) and lowest in the East (Taiwan and Japan, 2-4/100 000).36 The majority (≈70%) of Finns with CS are women in accord with recent observations from Europe and Japan.11,12,25
Figure 1.
Incident cases of clinically manifest cardiac sarcoidosis (CS) in adults (>18 years) diagnosed in Finnish hospitals from 1991 through 2020. Curiously, 3 vs. 300 new cases were detected over the first and last 5-year periods, respectively. The population of Finland is 5.5 million with 4.5 million adults. The figure is based on Kandolin et al.2 and unpublished data.
Diagnosis
Initial approach
The diagnosis of sarcoidosis rests on the triad of compatible clinical characteristics, proof of histology, and exclusion of other diseases. Suspicion of CS should arise, first, in patients with prevalent sarcoidosis presenting with cardiac signs or symptoms (Table 1 and Table 2)2,4,8,22,37–41 and, second, in all patients with initially unexplainable 2nd or 3rd degree AVB, sustained VAs, or heart failure. Though rare, CS has been shown to cause 20% to 34% of idiopathic high-grade AVB in middle-aged individuals,42,43 a nearly similar proportion of idiopathic sustained VT,44,45 and 5% of mixed VAs including frequent premature beats.46 In pre-existing sarcoidosis, elevated cardiac troponins,47 natriuretic peptides,11,48 and, as newcomers, anti-heart and anti-intercalated disk antibodies49 support the suspicion of CS. Echocardiography has limited sensitivity but can provide confirmatory evidence for the presence of CS (see Table 2 and Supplementary Figures 1 and 2). Strain imaging improves its ability to detect myocardial involvement.40,50,51 Still, both cardiac ultrasound and 12-lead ECG can look fully normal in CS.52 In patients without known sarcoidosis, circulating lysozyme, angiotensin-converting enzyme, and soluble interleukin-2 receptor may help,2,48,53 but only when abnormally elevated. Computed tomography of the chest can suggest intrathoracic sarcoidosis but, ultimately, the key studies involve advanced imaging with CMR and/or 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET).
Table 2.
Possible abnormalities on 12-lead electrocardiogram and echocardiography in patients with cardiac sarcoidosis
| Twelve-lead electrocardiogram | Echocardiogram |
|---|---|
| AVB, any degree | LV dysfunction (EF < 50%) |
| Fragmented or prolonged QRS complex | LV wall thickening or thinning |
| Complete bundle branch block | Local akinesia or dyskinesia |
| Abnormal Q-waves | LV aneurysm |
| T-wave inversions | Reduced global LV longitudinal strain |
| Frequent premature ventricular beats or NSVT | RV enlargement and reduced RV free wall strain |
| Epsilon-wave | Pericardial effusion |
Advanced imaging
18F-FDG-PET
The rationale of 18F-FDG-PET in suspected CS is that active inflammatory cells in sarcoid granulomas avidly take up glucose and its analogs.37 Cardiac PET is usually combined with whole-body imaging to uncover extracardiac involvement. For diagnostic imaging, physiologic cardiac glucose metabolism is switched off by a low-carbohydrate/high-fat diet followed by fasting and, in some centers, by additional intravenous unfractionated heparin to raise the availability of fatty acids, although the contribution of heparin is unclear.54 Regardless, 10%–15% of cardiac PET studies are diagnostic failures due to poor suppression of physiologic glucose uptake.55 For assessment of LV scarring, parallel scanning with either PET or single-photon emission computed tomography is done using their respective perfusion tracers. PET is currently combined with chest computed tomography for co-localization and attenuation correction. Effective radiation doses approximate 7 mSv for cardiac and 10 mSv for whole-body 18F-FDG-PET. In rest perfusion imaging, the doses are notably smaller.
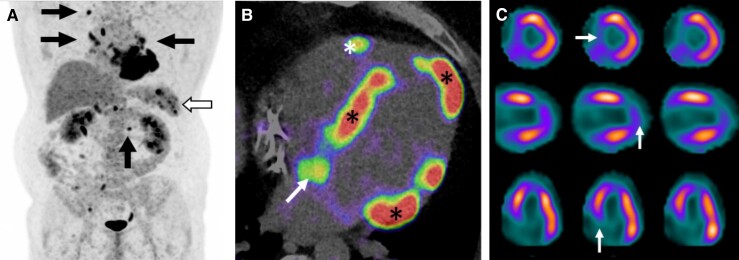
Abnormal cardiac PET is a key criterion in the diagnostic rubrics for CS3–5 and typically involves one or more spots of increased 18F-FDG uptake on suppressed or diffuse myocardial uptake (Figure 2). A ‘hot spot’ of 18F-FDG overlapping a perfusion defect is a characteristic finding (‘mismatch pattern’). Perfusion defects result from either LV scarring or reversible impairment of microcirculation.33 In addition to a visual review of PET images, quantification of inflammation is possible. ‘Standardized uptake value’ is calculated as radioactivity concentration in the region of interest relative to the injected dose and body weight.37 Several metrics thereof exist describing either intensity and heterogeneity of 18F-FDG uptake or myocardial metabolic volume and activity.37,54 In a meta-analysis of 17 studies involving 891 patients with suspected CS, the sensitivity and specificity of PET were 84% and 83%, respectively.56 However, the reference was not the gold standard (myocardial histology) but CS diagnosis by criteria57 suffering from significant limitations.10,58 Hibernating myocardium, other forms of myocarditis, rheumatologic diseases with cardiac involvement, and some genetic cardiomyopathies may also cause abnormal cardiac 18F-FDG uptake. Absence of extracardiac uptake decreases the specificity of PET for CS.59
Figure 2.
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) scans of a patient with tri-fascicular block and depressed left ventricular (LV) function; cardiac sarcoidosis was verified by endomyocardial biopsy. (A) whole-body PET with 18F-FDG positive lymph nodes (arrows) and splenic radiotracer accumulation (hollow arrow). (B) 4-chamber PET/CT image showing 18F-FDG uptake on LV septum, apex, and basal lateral wall (asterisks), on right ventricular free wall (arrow), and on interatrial septum (arrow). (C) Single-photon emission computed tomography 99mTc-tetrofosmin scans showing perfusion defects (white arrows) on LV septum and apex overlapping areas of 18F-FDG uptake on PET (mismatch pattern). From top to bottom, the rows represent short-axis, vertical, and horizontal views of the heart.
18F-FDG-PET scanning has therapeutic and prognostic implications, too. Initiating immunosuppression for CS presupposes proof of inflammatory activity, and repeat scans may help identify response to and relapse after therapy.60,61 The prognostic value of PET was confirmed in a recent meta-analysis of pertinent studies,62 though not all works are supportive.63,64 A ‘mismatch pattern’ (see Figure 2) and RV uptake are the key predictors of cardiac events.58,65,66 Atrial 18F-FDG uptake portends atrial tachyarrhythmias.29 In the future, cardiac PET studies can involve tracers that work without dietary preparation, such as somatostatin analogs,54 and hybrid PET/CMR imaging may improve diagnostic accuracy.66,67
CMR
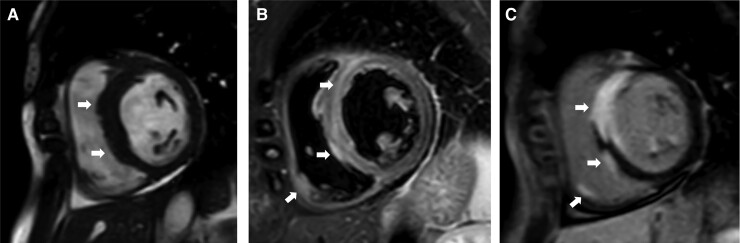
In suspected CS, multimodal CMR imaging visualizes not only anatomy and function but also myocardial edema, necrosis, and scarring (Figure 3). Besides volumetric measurements, cine CMR enables identification of abnormalities in myocardial thickness and motion, such as septal thinning (Figure 4A), local dyskinesia, and ventricular aneurysms.68–70 Myocardial edema is detectable on T2-weighted imaging,71 and inflammation can also be seen as myocardial gadolinium enhancement 3–5 min after contrast administration.72 Delayed postcontrast (15 min) imaging, however, is the key CMR modality in CS. Late gadolinium enhancement (LGE) reflects extracellular expansion and delayed contrast wash-out related to necrosis and edema in the acute phase and replacement fibrosis in the chronic setting.73 Typically, LGE involves basal LV segments and the RV side of the septum, but any part of the heart can be affected (Figure 4).69,70,74 LGE is most often distributed in patchy, non-ischemic pattern, but subendocardial and even transmural involvement is possible.75 A ‘hook sign’ (or ‘hug sign’) of septal LGE continuing into the RV free wall has been coined as an imaging biomarker for CS (Figure 5),76 but an identical pattern can be seen in giant cell myocarditis.77
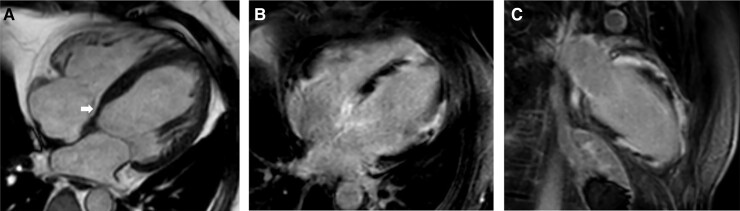
Figure 3.
Magnetic resonance images of a patient with cardiac sarcoidosis, 3rd degree atrioventricular block, and normal left and right ventricular ejection fraction. The arrows highlight key findings. (A) short-axis cardiac cine image showing thickened ventricular septum. (B) T2-weighted image showing septal and local right ventricular edema indicating active inflammation. (C) late gadolinium enhancement image showing transmural septal, papillary muscle, and local right ventricular free wall involvement.
Figure 4.
Magnetic resonance images of a patient with 3rd degree atrioventricular block and depressed left ventricular function; cardiac sarcoidosis was verified by endomyocardial biopsy. (A) apical 4-chamber cine image showing basal septal thinning (arrow) and thickened mid-septum. (B and C) apical 4- and 2-chamber images, respectively, of late gadolinium enhancement showing patchy left ventricular involvement.
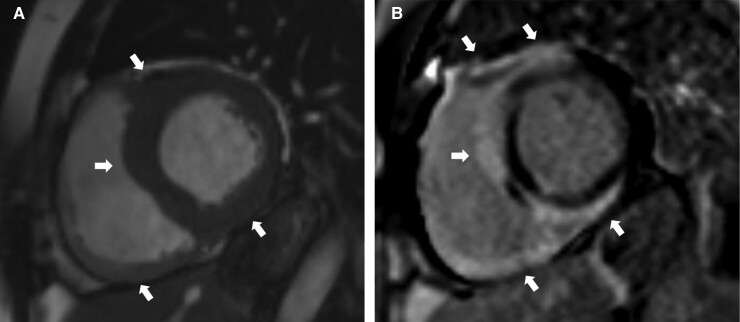
Figure 5.
Magnetic resonance images of a patient with right bundle branch block, ventricular tachycardia, and depressed left and right ventricular function; cardiac sarcoidosis was verified by endomyocardial biopsy. The arrows point at key findings. (A) Short-axis cardiac cine image showing thickened left ventricular myocardium and inferior right ventricular wall. (B) A ‘hook sign’ pattern of cardiac sarcoidosis76 characterized by late gadolinium enhancement in the septum continuing to ventricular insertion points and right ventricular free wall.
The presence of myocardial LGE constitutes a major diagnostic criterion for CS.3–5 Studies on the performance of CMR69,70,78 have yielded sensitivities between 75% and 100% and specificities from 77% to 85% with clinical CS diagnosis57 surrogated for myocardial histology as the reference. On the other hand, Divakaran et al.59 found that only 1 of 8 cases likely to have CS by pretransplant CMR imaging ultimately had CS in the study of the explanted heart. The repeatability of CMR appears fair in suspected CS. In a recent work, 2 experts agreed on myocardial LGE in 80% of cases, and Cohen’s kappa was 0.59.79
In addition to visual assessment, quantification of the CMR findings is possible. Myocardial T1 and T2 relaxation times enable the detection of subclinical CS and quantification of diffuse interstitial fibrosis.80–82 Their clinical utility remains unsettled, though. More importantly, the extent of LGE can be determined as the percentage of LV mass83 or simply as the number of involved segments. The presence and extent of LGE predict serious events in suspected CS.84
Confirmation of sarcoidosis histology
Biopsies
For proof of sarcoidosis histology, the HRS guideline4 recommends, and many centers prefer, extracardiac over endomyocardial biopsy (EMB) with arguments of better sensitivity and safety. The much-criticized sensitivity of only 19%–25% for EMB is not current, however, as it represents the obsolete technique of non-targeted RV biopsies.85,86 Selecting the ventricle and the myocardial area for biopsy with help of cardiac imaging87 and/or intracardiac voltage mapping88 improves EMB’s sensitivity. Its yield is higher if LVEF is impaired,85,89 PET shows metabolism-perfusion mismatch,58 or signs of RV involvement are present.58,90,91 The risk of serious complications is <1%.92,93 EMB also enables myocardial immunohistochemistry and transcriptomics that may help distinguish CS from other cardiomyopathies.94–97 At our center, prior histology of extracardiac sarcoidosis is considered diagnostically sufficient but otherwise EMB is the procedure of choice (Figure 6). Exceptionally, if confirmation of isolated CS is considered imperative but EMBs fail, LV biopsies can be taken under direct visual control using video-assisted thoracoscopy.98 Diagnosing isolated CS without histology5 is questionable16,99 because no cardiac manifestations or imaging findings are specific for myocardial granulomas.59,88,100 Admittedly, views differ about the importance of histology,22,33,101 some experts even considering emphasis on tissue diagnosis ‘the largest limitation of the current guidelines’.101
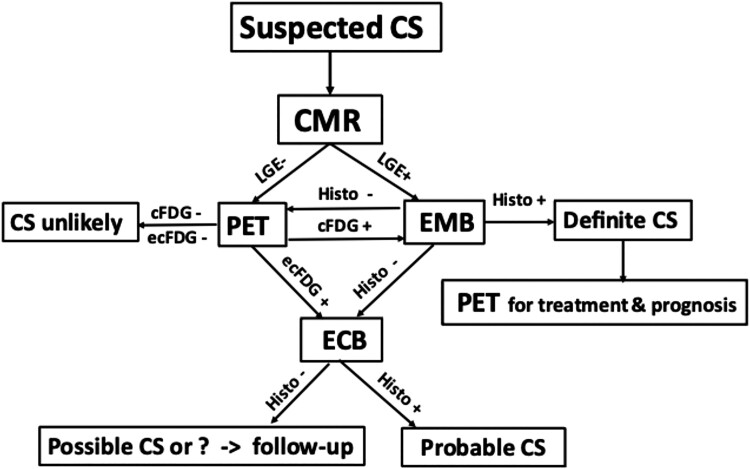
Figure 6.
Flowchart for cardiac imaging and biopsies at Helsinki University Hospital for suspected cardiac sarcoidosis (CS) after exclusion of ischemic heart disease and in the absence of histologically verified extracardiac sarcoidosis. If cardiac magnetic resonance (CMR) shows late gadolinium enhancement (LGE), imaging-guided endomyocardial biopsy (EMB) is performed first. If either CMR or EMB is negative, whole-body positron emission tomography (PET) is done. PET being positive, either EMB or extracardiac biopsy (ECB) is performed depending on PET and CMR findings and patient’s preferences. cFDG and ecFDG indicate cardiac and extracardiac uptake of fluorodeoxyglucose, respectively; histo, histology of sarcoidosis.
Myocardial histopathology
In our unit, typically 10 heart muscle samples are taken in a diagnostic EMB session for suspected CS. Histopathology involves an examination of ca. 50–60 myocardial sections for routine histochemical stains and additional sections for immunohistochemistry (Figure 7). Findings in CS include non-necrotic granulomas and isolated giant cells with or without surrounding lymphocytic/granulocytic infiltration combined with myocardial fibrosis, sharply demarcated areas of involvement, and no extensive eosinophilia or myocyte necrosis. Non-necrotic granulomas per se signify sarcoidosis only if other causes are excluded.4,5 Recently, indirect EMB signs of CS have been suggested,102,103 including small collections of histiocytes (‘microgranulomas’), lymphangiogenesis, and confluent fibrosis with fatty change.
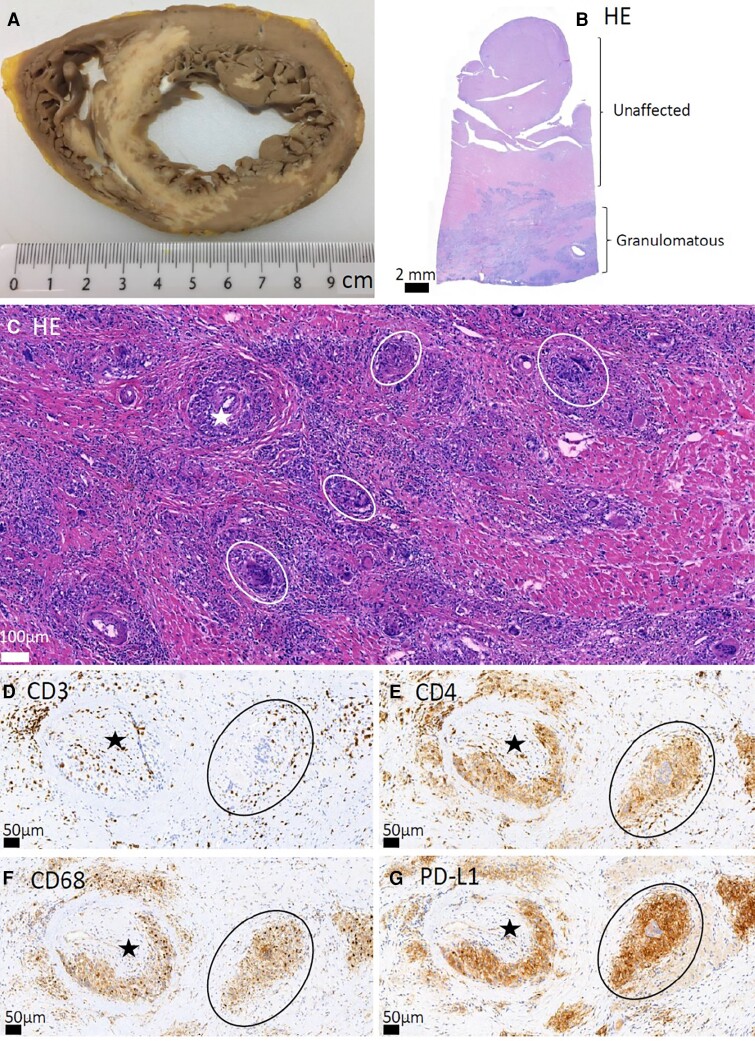
Figure 7.
Histopathology of cardiac sarcoidosis. Sharply demarcated (‘geographical’) inflammatory lesions in a gross photo of an explanted heart (A) and in low-magnification hematoxylin-eosin (HE) staining (B). In panels (C–G), stars mark small coronary artery branches with vascular wall granulomas and circles encompass non-caseating granulomas. In panel D, CD3 immunostaining highlights T cells, and in panel E, CD4 antibody stains T cells intensely and macrophages and giant cells less strongly. Macrophages and giant cells can also be highlighted with CD68 (F) and PD-L1 (G) antibody staining. Scalebars are shown in each panel.
Diagnostic criteria in societal guidelines
There exist three current sets of diagnostic criteria for CS.3–5 The ones from the World Association for Sarcoidosis and Other Granulomatous Disorders3 and HRS4 are nearly identical and straightforward. The updated criteria of the Japanese Circulation Society5 are more complex but do not, unlike the other two,3,4 require proof of histology. Table 3 details the HRS criteria4 that many centers are using including ours. Although probable CS (Table 3) is widely considered sufficient for clinical practice, the certainty of diagnosis is not indifferent as definite diagnosis has predicted worse outcome in many studies,25,86,104–107 though not in all.22 The differences across the diagnostic guidelines have resulted in CS cohorts that are not entirely comparable across countries or institutions.108,109
Table 3.
The heart rhythm society’s criteria for the diagnosis of cardiac sarcoidosis4
| 1. Histological diagnosis from myocardial tissue, definite cardiac sarcoidosis requires presence of non-necrotizing granulomas with no alternative cause |
| 2. Clinical diagnosis from noninvasive and invasive studies, probable cardiac sarcoidosis requires histologic diagnosis of extracardiac sarcoidosis and presence of one or more of the following: |
|
|
|
|
|
|
|
| and exclusion of other causes for the cardiac manifestations |
CMR indicates cardiac magnetic resonance; PET, positron emission tomography.
in a pattern consistent with cardiac sarcoidosis.
The challenging diagnosis of CS has led some centers to set up formal multidisciplinary diagnostic teams,109 while others, emphatic about imaging, have generated a tandem analysis of CMR and PET images to classify CS diagnosis into categories of increasing likelihood.110,111 Yet, imaging-based diagnosis of CS (probable-to-high likelihood) has shown limited specificity for definite CS.59,100 Diagnostic collaboration across specialties is indispensable regardless of whether cardiologists or a multidisciplinary team are responsible for the diagnosis of CS.
Differential diagnosis
Lymphocytic, eosinophilic, and giant cell myocarditis with acquired and genetic cardiomyopathies and granulomatous infections constitute differential diagnostic alternatives to CS beyond ischemic heart disease. The distinction between CS and giant cell myocarditis defies the skills of clinicians and pathologists alike,24,97 and whether they represent a one-disease continuum is debated. Although CS can disguise as a phenocopy of arrhythmogenic right ventricular cardiomyopathy, impaired AV conduction, LV dysfunction, septal LGE, and mediastinal lymphadenopathy are more common in the former.112 Desmoplakin cardiomyopathy113 can also imitate CS, and gene tests with pedigree analysis may be needed to distinguish CS from genetic cardiomyopathies. RV sarcoidosis must be distinguished from RV dysfunction due to sarcoidosis-associated pulmonary hypertension.114 Advanced CS can be mistaken for dilated cardiomyopathy. Several transplant centers115–117 have identically reported that all their cases of CS in the explanted heart had a pretransplant misdiagnosis of idiopathic dilated cardiomyopathy!
Screening
Given the notoriety of CS, cardiac screening of patients with extracardiac sarcoidosis appears desirable or even imperative.118 Yet, whether detailed screening ultimately is beneficial, considering the conceivable harms and biases,119 has not been thoroughly debated in the case of sarcoidosis. The American Thoracic Society120 recommends symptom history and 12-lead ECG for routine screening, the HRS4 advising echocardiography in addition; CMR and/or FDG-PET are recommended should abnormalities be noted. Dedicated screening studies, small as they are, suggest a low risk of serious events given absent cardiac symptoms and ECG abnormalities.12,121–124 In a Danish epidemiologic study125 of 11 834 sarcoidosis patients free of cardiac history, the 10-year risks were higher than in the background population but still only 3.18% for heart failure, 0.96% for VAs or implantation of a cardioverter-defibrillator, and 0.94% for slow arrhythmias or pacemaker implantation. All things considered, assessment of cardiac symptoms and 12-lead ECG on scheduled surveillance visits, followed by CMR in case of abnormalities, appears a sensible approach today.
Treatment and follow-up
The care of clinically manifest CS involves immunosuppression to control the underlying myocardial inflammation and medical and device therapy for the consequences of cardiac injury and scarring. Although there exist societal guidelines for treatment,4,5,126 their recommendations are based on evidence of low or very low quality. The care of CS should be handled in tertiary referral centers.
Immunosuppression
Initial therapy
In CS, unequivocal clinical manifestations with evidence of active inflammation on EMB or PET indicate initiation of immunosuppression.4,5,126 In subclinical disease with absent LV dysfunction, the benefit of immunosuppression is unknown, and treatment decisions must be individualized in consideration of the extent of myocardial inflammation, other organ involvement, and risks of therapy.
Nonspecific immunosuppression with corticosteroids constitutes the mainstay of treatment.4,5,126,127 A review of 34 clinical reports involving >1000 patients concluded that corticosteroids improve AV conduction in 40% of patients and may prevent the deterioration of LV function, whereas their effects on arrhythmias and mortality remain ambiguous due to poor data quality.127 Observations contradict whether corticosteroids benefit in severe LV dysfunction.2,25,128,129
In general, treatment is initiated with solo prednisone at a dose of 0.5 mg/kg/day. Yet, it is prudent to adjust the initial immunosuppression to the seriousness of the clinical manifestations. Rapidly progressive heart failure, life-threatening arrhythmias, and extensive inflammation on cardiac PET should prompt an upfront addition of another immunomodulator or intravenous pulses of methylprednisolone (500–1000 mg/day in 2–3 successive days).130 Although there is no robust agreement on the detailed treatment protocol, prednisone is usually titrated down every 4 weeks in decrements of 5–10 mg until a maintenance dose of 10 mg/day is reached. The effect of treatment is checked initially at 3– 6 months intervals by assessing symptoms, LV function, 12-lead ECG, arrhythmia burden, and circulating cardiac biomarkers. ECG changes raising suspicion of active inflammation include worsening atrioventricular or intraventricular conduction, increased ventricular ectopy, non-sustained VT, and new ST-T changes; whereas decreasing EF, new wall-motion abnormalities, and increasing mitral regurgitation suggest persisting disease activity on echocardiography. In clinical follow-up, the toxic effects of steroids are also addressed with all other concerns the patient raises. Here the assistance of a nurse specialist is important. Regarding advanced imaging, follow-up CMR studies have a limited role due intracardiac devices causing troublesome image artifacts.131,132 Instead, many centers repeat FDG-PET studies routinely to follow the activity of CS and to tailor treatment accordingly.61,76,130,133 Sensible as it sounds, the ‘routine PET strategy’ has not been shown to improve either the quality of life or event-free survival, yet it exposes patients to cumulative ionizing radiation and may lead to treatment of images instead of patients. In a recent study of immunosuppression for suspected active CS, the rate of major cardiac events did not differ statistically significantly between patients showing a complete clearance of 18FDG uptake vs. no response on early follow-up PET.134 We prefer a ‘selective PET strategy’ where repeat studies are done if there are discrepant clinical observations or if either insufficient treatment response or relapse is suspected. In our practice, corticosteroids are discontinued after 12 to 16 months of therapy supposing absent signs of disease inactivity. Follow-up visits continue annually for 3–5 years and every other year thereafter. Late relapses are possible.
Second-line therapy
Second-line immunosuppressive agents, including methotrexate, azathioprine, mycophenolate mofetil, leflunomide, and cyclophosphamide, are initiated in case corticosteroids have insufficient efficacy or their dose needs a reduction to spare the patient their toxic effects. Although small studies135,136 and some expert opinions137 support combination therapy from the beginning, no good evidence for improved outcome exists.126 Methotrexate in weekly doses of 10–20 mg is used most, while our preference has been azathioprine 1–2 mg/kg body weight per day. Both need precautions and careful follow-up for adverse effects including leukopenia, hepatotoxicity, and gastrointestinal complications.138 Genetic defects in the activity enzymes degrading thioguanine increase the toxicity of azathioprine and need to be identified by genotyping before therapy or when problems appear.
Third-line therapy
Biologic anti-tumor necrosis factor (TNF) agents can prevent granuloma formation, and observational data support their efficacy in CS when other therapies have failed.139–142 Infliximab is a chimeric TNF antibody which in doses <10 mg/kg is well tolerated even in patients with impaired LV function.143 Before the start of therapy, comprehensive screening for tuberculosis and viral infections is needed, and vaccination status must be updated. In our practice, 5 mg/kg of infliximab is administered at weeks 0, 2, and 4, and every 8th week thereafter for one year or until signs of inflammation abide. Adjunct therapy with low-dose methotrexate or azathioprine reduces the production of neutralizing antibodies to infliximab. Adalimumab, a human monoclonal TNF antibody, is a subcutaneously administered alternative.141,142 Although B lymphocyte-targeted therapy with rituximab has had some success in CS,144 the experience remains small for conclusions. In biologic therapy, surveillance for infectious and other complications is critical.
Ongoing trials
A few prospective controlled studies, long overdue in CS, are underway on medical therapy. The CHASM-CS trial probes the hypothesis that a low-dose prednisone-methotrexate combination is as effective as a standard dose of prednisone.145 The MAGIC-ART trial tests the influence of anakinra, an interleukin-1 receptor antagonist, on biomarkers of the activity of CS.146 The J-ACNES trial compares the therapeutic effects of corticosteroids given alone or together with antibiotics based on the assumed pathogenetic role of Propionibacterium acnes.147 The RESOLVE-Heart is an industry-driven trial focusing on the safety of namilumab, a monoclonal antibody targeting the granulocyte-macrophage colony stimulating factor, in active CS (https://clinicaltrials.gov/ct2/show/NCT05351554).
Control and prevention of symptomatic VAs and SCD
Symptomatic VAs
The observed effects of immunosuppression on symptomatic VAs in CS are unpredictable and partly confusing.26,127,148 Still, corticosteroids are recommended if there is proof of inflammatory activity.4 Antiarrhythmic drugs, mainly amiodarone or sotalol for VT, are started concomitant with immunosuppression or following an insufficient response. If medical therapy fails, catheter ablation can be considered.4 In a meta-analysis of 15 studies involving >400 patients with refractory VT, freedom from recurrences was 45% after the first ablation and 63% after repeated procedures.149 A varying proportion of patients, 13% to 40%, needed epicardial ablation. Importantly, VT ablation helps control stormy or incessant VTs.150 Preceding ablation, intravenous methylprednisolone 40–80 mg/day is worth a trial if VT storm associates with active inflammation.151 In cases refractory to medical and ablative therapy, bilateral cardiac sympathectomy may be considered.152
Prevention of SCD
Patients presenting with clinically manifest CS have a 10% risk of SCD over 5 years of follow-up.107 In subclinical CS, the risk is unknown but likely much lower. As there is no good evidence for the preventive efficacy of any medical therapy, the question of when to recommend an implantable cardioverter-defibrillator (ICD) attains crucial importance. Table 4 summarizes the pertinent recommendations given by the HRS,4 the ACC/AHA/HRS consortium,153 and the European Society of Cardiology (ESC).154 All replicate the general ICD indications for secondary prevention and recommend implantation when LVEF is ≤35% or permanent pacing is needed. Of note, permanent pacing is recommended for high-grade AVB even despite improvement of conduction with steroid therapy.4,155 History of syncope is an ICD indication by the North American guidelines4,153 but not by the European one,154 which also recommends programmed electrical stimulation (PES) and an ICD for inducible sustained VAs only in the presence of LV dysfunction (EF 35%–50%).154 Both the AHA/ACC/HRS consortium and the ESC recommend ICD implantation if advanced cardiac imaging reveals signs of ‘extensive’ or ‘significant’ LV scarring, but, unfortunately, what quantities these qualifiers stand for remains undefined.153,154 In our experience,107 85% of patients with clinically manifest CS meet the HRS indications, and practically all meet the ACC/AHA/HRS indications for an ICD at disease presentation. Those 15% judged not to benefit from the device by the HRS statement,4 still have a combined risk of SCD, sustained VAs, and de novo ICD indications exceeding 50% at 5 years from presentation.107 In such patients, long-term arrhythmia monitoring using an implantable loop recorder may help early detection of serious arrhythmias, but its prognostic impact remains unknown.156
Table 4.
Current recommendations by expert societies for an implantable cardioverter-defibrillator in patients with cardiac sarcoidosis
| Classa | 2014 HRS Consensus Statement on Management of Arrhythmias in Cardiac Sarcoidosis4 | 2017 AHA/ACC/HRS Guideline for Management of Ventricular Arrhythmias and Prevention of Sudden Cardiac Death153 | 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death154 |
|---|---|---|---|
| I | Prior aborted cardiac arrest, documented spontaneous sustained ventricular tachycardia, or LVEF ≤ 35%b,c | ||
| IIa | LVEF > 35% with an indication for permanent pacemaker | ||
| History of syncope compatible with arrhythmogenic etiology | |||
| Inducible sustained ventricular arrhythmia at PES | Inducible sustained monomorphic ventricular arrhythmia at PES in a patient with LVEF 35%–50% and minor LGE at CMRI | ||
| LVEF > 35% with evidence of myocardial scar (or ‘extensive scar’) by CMRI or PETc | LVEF >35% with significant myocardial LGE at CMRI after resolution of acute inflammation | ||
| IIb | LVEF 36%–49% or RVEF < 40%b | ||
ACC indicates American College of Cardiology; AHA, American Heart Association; CMRI, cardiac magnetic resonance imaging; ESC, European Society of Cardiology; HRS, Heart Rhythm Society; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; PES, programmed electrical stimulation; PET, positron emission tomography; RVEF, right ventricular ejection fraction.
Class I is recommended (‘is useful/indicated/beneficial’, ‘should be performed’); Class IIa, modest recommendation (‘can be useful/beneficial’, ‘should be considered’); and Class IIb, weak recommendation (‘usefulness is unknown/uncertain’, ‘may/might be considered’).
2014 HRS guidance presupposes optimal medical therapy and a period of immunosuppression in the presence of active inflammation.
2017 ACC/AHA/HRS guideline presupposes meaningful expected survival ≥1 year.
The use of PES to evaluate the risk of SCD is generally recommended in CS without severe LV dysfunction or other ICD indications for primary prevention.4,153 The 2022 ESC guideline,154 however, does not recommend PES if LVEF is >50% and there is no LGE on CMR imaging. A recent meta-analysis157 found that non-inducibility is strongly associated with absence of future VAs. Yet, CS can progress, and further scarring may increase the initially low arrhythmogenicity. More research is needed focusing on the risk and predictors of SCD. Quantitative data from analyses of MRI, FDG-PET, and circulating biomarkers may help,58,62,63,84,106,158,159 and involvement of the right ventricle needs more emphasis.58,65,66,160 Whether the diagnosis of CS is definite or probable also deserves consideration.107 In the future, artificial intelligence may help predict the SCD risk in CS.161
In Finland, the cumulative rate of ICD implantations in clinically manifest CS has been 75% over the last three decades.107 Due to our recent observations,107 and pending sharper risk assessment, we currently discuss implantation of an ICD with every patient having clinically manifest CS. Whether to ultimately implant or not rests on a shared decision with a patient fully informed of the risk of fatal arrhythmias, the conceivable benefits and harms of an ICD, and the uncertainties involved.
The complications following ICD implantations appear to be more common in CS than in the general ICD population.162 Inappropriate therapies have been recorded in 15% to 24% of patients,162,163 and the combined incidence of other complications, including lead problems and infections, has exceeded 15%.162 It is prudent to start immunosuppression post-implantation to reduce the risk of device infections.
Treatment of heart failure
All guideline-directed medical therapies164 can be used to treat CS-related congestive heart failure. Corticosteroids are indicated if there is proof of active myocardial inflammation. Aggressive immunosuppression and mechanical support may be needed in the rare cases of CS-related fulminant myocarditis.165 Observations on the use of cardiac resynchronization thereapy (CRT) devices in CS have been somewhat disappointing.166,167 Widespread scarring, suboptimal CRT pacing, and false CS diagnoses constitute possible causes for the considerable proportion of non-responders.168
LV assist devices and cardiac transplantation can be considered for CS-related terminal heart failure. Large registry studies have shown that patients with CS have as good post-transplant survival and a similar risk of late complications as the non-CS transplant recipients.169,170 Recurrence of CS in the allograft is rare and has not resulted in graft failure.171
Long-term outcome and prognostic factors
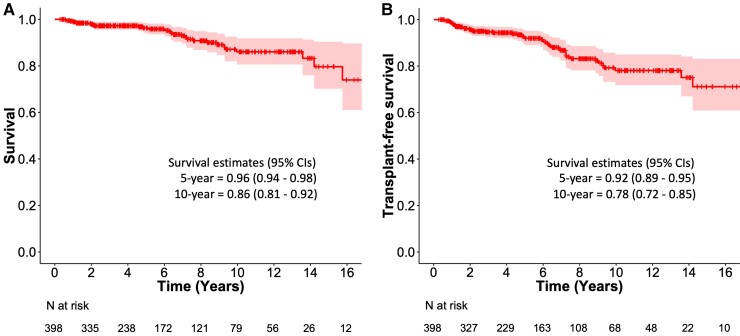
Table 5 shows the 5-year and 10-year survival rates in recent CS cohorts with a comparative summary of the cohorts’ characteristics.25,38,105–107 Although the study populations differ in several key aspects, their 5-year survival prospects are consistently 90% or higher. In our 398-patient cohort of clinically manifest CS followed for a median of 5 years,107 eight patients suffered a SCD, seven died of heart failure, nine suffered post-transplant deaths (of 25 undergoing transplantation), and nine died of non-cardiac causes. The overall survival estimate was 96% at 5 years and 86% at 10 years from presentation (Figure 8A). For transplant-free survival, the 5-year and 10-year estimates were 92% and 78%, respectively (Figure 8B).
Table 5.
Contemporary cohort studies reporting long-term survival in cardiac sarcoidosis
| Cacoub et al38 | Kusano et al25 | Kitai et al105 Nabeta et al106 |
Nordenswan et al107 | |
|---|---|---|---|---|
| Size of cohort, n | 157 | 422 | 512 | 398 |
| Nationality | French | Japanese | Japanese | Finnish |
| Time span of diagnoses | 1980–2016 | NA | 2001–2017 | 1988–2017 |
| Mean age at diagnosis, y | 40 | 60 | 62 | 51 |
| Female sex, % | 41 | 68 | 64 | 72 |
| Histology of sarcoidosis, % | ||||
| myocardial | 2 | 18 | 11 | 48 |
| extracardiac | 98 | 39 | 52 | 52 |
| missing | 0 | 43 | 37 | 0 |
| Presenting manifestation, % | ||||
| ȃhigh-grade AVB | 10 | 40 | 43 | 54 |
| ȃVT or VF | 8 | 18 | 20 | 18 |
| ȃheart failure | 10 | NA | 21 | 14 |
| Impaired LVEF (<50%), % | 26 | 48 | 52 | 44 |
| Positive FDG-PET, n (%)a | 12/37 (32) | 273/406 (67) | 324/342 (95) | 236/265 (89) |
| LGE on CMRI (%), n (%) | 39/91 (44) | 184/216 (85) | 282/307 (92) | 201/208 (98) |
| Immunosuppressive treatment, % | 96 | 84 | 88 | 96 |
| ICD implantation rate, % | 3 | 33 | 28 | 74 |
| Heart transplantation rate, % | 1.3 | 0.5 | 0 | 6.3 |
| Median follow-up, y | 8 | 5 | 2.9 | 5.0 |
| Overall survival, % b | ||||
| ȃ5-year | 94 | 90 | 90 | 96 |
| ȃ10-year | 90 | 81 | 82 | 86 |
AVB, indicates atrioventricular block; CMRI, cardiac magnetic resonance imaging; FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; ICD, implantable cardioverter-defibrillator; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; NA, not available; VF, ventricular fibrillation; VT, ventricular tachycardia.
focal or focal on diffuse myocardial uptake of 18F-FDG suggestive of active inflammation.
survival percentages are Kaplan–Meier estimates.
Figure 8.
Kaplan–Meier curves of overall (A) and transplant-free (B) survival in a cohort of 398 patients with clinically manifest cardiac sarcoidosis diagnosed in Finland from 1988 through 2017 and followed for a median of 5.0 years. The figures are based on the data reported by Nordenswan et al.107 and summarized in Table 5. The survival graphs reflect the care of cardiac sarcoidosis based on the following principles: requirement of diagnostic histology and pursuit of definite diagnosis, consistent use of corticosteroids with azathioprine and infliximab as the main additional immunomodulators, clinical follow-up with selective instead of routine repeats of positron emission tomography, frequent use of implantable cardioverter-defibrillators, and no sarcoidosis-specific restrictions to heart transplantation.
The prognostic factors in CS fall into three main categories. One is the extent of myocardial involvement. The predictive values of LVEF,2,25,63,106,172 quantity of LGE on CMRI,173–175 summed rest score of segments with perfusion defects63 or segments with perfusion-metabolism mismatch,158 circulating natriuretic peptides,106 LV global longitudinal strain,51 and right ventricular EF160 all reflect aspects of the extent of cardiac involvement. The second category relates to how CS shows itself. Presentations with sustained VT25,63,106,172 or heart failure2 imply poor outcome, while lone AVB is prognostically less ominous.176De novo and clinically isolated presentation also predict worse outcome, likely due to delayed diagnosis and more advanced disease.2,14,15,18,21 The third prognostic category concerns the certainty of diagnosis: definite, myocardial histology-based CS diagnosis portends poorer outcome than probable diagnosis supported by extracardiac histology.25,86,104–107 Whether the current treatment improves prognosis cannot be concluded from the data available today.
Challenges for future research
The prevailing challenges of CS relate to the unknown molecular–genetic etiopathogenesis with its diagnostic and therapeutic ramifications, the need and forms of cardiac screening, the prognosis of subclinical cardiac involvement and whether watchful waiting is safe, how to diagnose CS leaving as little room for doubt as possible, how to tailor immunosuppression and when to discontinue, and how to best assess the risk of SCD and identify patients benefiting from an ICD. As the first step, we would welcome universal adoption of a single set of diagnostic criteria. That would facilitate the much-needed larger prospective clinical trials.
Supplementary Material
Acknowledgements
The authors want to thank the members of the nationwide MIDFIN research network (Drs P. Pietilä-Effati, A. Alatalo, T. T. Rissanen, P. Haataja, T. Vihinen, K. Kaikkonen, T. Kerola, V. Vepsäläinen, R. Kandolin, K. Ekström, P. Simonen, A. Räisänen-Sokolowski, and H.K. Nordenswan) for their work with CS in their respective hospitals.
Contributor Information
Jukka Lehtonen, Heart and Lung Center, Helsinki University Central Hospital and University of Helsinki, Haartmaninkatu 4, 00290 Helsinki, Finland.
Valtteri Uusitalo, Clinical Physiology and Nuclear Medicine, Helsinki University Central Hospital and University of Helsinki, Haartmaninkatu 4, 00290 Helsinki, Finland.
Pauli Pöyhönen, Heart and Lung Center, Helsinki University Central Hospital and University of Helsinki, Haartmaninkatu 4, 00290 Helsinki, Finland.
Mikko I Mäyränpää, Department of Pathology, University of Helsinki and Helsinki University Hospital, Haartmaninkatu 3C, 00290 Helsinki, Finland.
Markku Kupari, Heart and Lung Center, Helsinki University Central Hospital and University of Helsinki, Haartmaninkatu 4, 00290 Helsinki, Finland.
Author contributions
J.L., V.U., P.P., M.I.M., and M.K. conceived and designed the contents of the review, drafted the manuscript, and made critical revision of the manuscript for key intellectual content.
Supplementary data
Supplementary data is available at European Heart Journal Online.
Data availability
No new data were generated or analysed in support of this review.
Funding
The Finnish Medical Foundation (M.I.M.). This work was supported by a Finnish government grant for Medical Research for medical research (J.L.), Aarne Koskelo’s foundation (J.L.), and the Finnish Foundation for Cardiovascular Research (J.L.).
References
- 1. Drent M, Crouser ED, Grunewald J. Challenges of Sarcoidosis and Its Management. N Engl J Med 2021;385:1018–1032. [DOI] [PubMed] [Google Scholar]
- 2. Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 2015;131:624–632. [DOI] [PubMed] [Google Scholar]
- 3. Judson M, Costabel U, Drent Met al. The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis 2014;31:19–27. [PubMed] [Google Scholar]
- 4. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1305–1323. [DOI] [PubMed] [Google Scholar]
- 5. Terasaki F, Azuma A, Anzai T, Ishizaka N, Ishida Y, Isobe M, et al. Japanese Circulation Society Joint Working Group. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis. Circ J 2019;83:2329–2388. [DOI] [PubMed] [Google Scholar]
- 6. Lee S, Birnie D, Dwivedi G. Current perspectives on the immunopathogenesis of sarcoidosis. Respir Med 2020;173:106–161. [DOI] [PubMed] [Google Scholar]
- 7. Hu X, Carmona E, Yi E, Pellikka P, Ryu J. Causes of death in patients with chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2016;33:275–280. [PubMed] [Google Scholar]
- 8. Ekström K, Lehtonen J, Nordenswan HK, Mäyränpää MI, Räisänen-Sokolowski A, Kandolin R, et al. Sudden death in cardiac sarcoidosis: an analysis of nationwide clinical and cause-of-death registries. Eur Heart J 2019;40:3121–3128. [DOI] [PubMed] [Google Scholar]
- 9. Silverman K, Hutchins G, Bulkley B. Cardiac sarcoid a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978;58:1204–1211. [DOI] [PubMed] [Google Scholar]
- 10. Patel M, Cawley P, Heitner J, Klem I, Parker MA, Jaroudi WA, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009;120:1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martusewicz-Boros MM, Boros PW, Wiatr E, , Zych J, Piotrowska-Kownacka D, Roszkowski-Śliż K. Prevalence of cardiac sarcoidosis in white population: a case-control study: proposal for a novel risk index based on commonly available tests. Medicine (Baltimore) 2016;95:e4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kouranos V, Tzelepis GE, Rapti Aet al. Complementary Role of CMR to Conventional Screening in the Diagnosis and Prognosis of Cardiac Sarcoidosis. JACC Cardiovasc Imaging 2017;10:1437–1447. [DOI] [PubMed] [Google Scholar]
- 13. Schupp JC, Freitag-Wolf S, Bargagli E, Mihailović-Vučinić V, Rottoli P, Grubanovic A, et al. Phenotypes of organ involvement in sarcoidosis. Eur Respir J 2018;51:1700991. [DOI] [PubMed] [Google Scholar]
- 14. Rosen NS, Pavlovic N, Duvall C, Wand AL, Griffin JM, Okada DR, et al. Cardiac sarcoidosis outcome differences: a comparison of patients with de novo cardiac versus known extracardiac sarcoidosis at presentation. Respir Med 2022;198:106864. [DOI] [PubMed] [Google Scholar]
- 15. Eldhagen P, Bobbio E, Darlington P, Grunewald J, Eklund A, Polte CL, et al. Phenotypic and HLA-DRB1 allele characterization of Swedish cardiac sarcoidosis patients. Int J Cardiol 2022;359:108–112. [DOI] [PubMed] [Google Scholar]
- 16. Kupari M, Lehtonen J. POINT: should isolated cardiac sarcoidosis be considered a significant manifestation of sarcoidosis? Yes. Chest 2021;160:36–38. [DOI] [PubMed] [Google Scholar]
- 17. Juneau D, Nery P, Russo J, de Kemp RA, Leung E, Beanlands RSB, et al. How common is isolated cardiac sarcoidosis? Extra-cardiac and cardiac findings on clinical examination and whole-body (18)F-fluorodeoxyglucose positron emission tomography. Int J Cardiol 2018;253:189–193. [DOI] [PubMed] [Google Scholar]
- 18. Sato K, Kawamatsu N, Yamamoto M, Machino-Ohtsuka T, Ishizu T, et al. Utility of Updated Japanese Circulation Society Guidelines to Diagnose Isolated Cardiac Sarcoidosis. J Am Heart Assoc 2022;11:e025565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okada DR, Bravo PE, Vita T, Agarwal V, Osborne MT, Taqueti VR, et al. Isolated cardiac sarcoidosis: a focused review of an under-recognized entity. J Nucl Cardiol 2018;25:1136–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawai H, Sarai M, Kato Y, Naruse H, Watanabe A, Matsuyama T, et al. Diagnosis of isolated cardiac sarcoidosis based on new guidelines. ESC Heart Fail 2020;7:2662–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takaya Y, Nakamura K, Nishii N, Ito H. Clinical outcomes of patients with isolated cardiac sarcoidosis confirmed by clinical diagnostic criteria. Int J Cardiol 2021;345:49–53. [DOI] [PubMed] [Google Scholar]
- 22. Rosenbaum AN, Kolluri N, Elwazir MY, Kapa S, Abou Ezzeddine OF, Bois JP, et al. Identification of a novel presumed cardiac sarcoidosis category for patients at high risk of disease. Int J Cardiol 2021;335:66–72. [DOI] [PubMed] [Google Scholar]
- 23. Fussner LA, Karlstedt E, Hodge DO, Fine NM, Kalra S, Carmona EM, et al. Management and outcomes of cardiac sarcoidosis: a 20-year experience in two tertiary care centres. Eur J Heart Fail 2018;20:1713–1720. [DOI] [PubMed] [Google Scholar]
- 24. Nordenswan HK, Lehtonen J, Ekstrom K, Räisänen-Sokolowski A, Mäyränpää MI, Vihinen T, et al. Manifestations and Outcome of Cardiac Sarcoidosis and Idiopathic Giant Cell Myocarditis by 25-Year Nationwide Cohorts. J Am Heart Assoc 2021;10:e019415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kusano K, Noda T, Nakajima K, Nakasuka K, Terasaki S, Hattori Y, et al. Prognosis and Outcomes of Clinically Diagnosed Cardiac Sarcoidosis Without Positive Endomyocardial Biopsy Findings. JACC Asia 2021;1:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okada DR, Smith J, Derakhshan A, Gowani Z, Misra S, Berger RD, et al. Ventricular Arrhythmias in Cardiac Sarcoidosis. Circulation 2018;138:1253–1264. [DOI] [PubMed] [Google Scholar]
- 27. Barton JH, Tavora F, Farb A, Li L, Burke AP. Unusual cardiovascular manifestations of sarcoidosis, a report of three cases: coronary artery aneurysm with myocardial infarction, symptomatic mitral valvular disease, and sudden death from ruptured splenic artery. Cardiovasc Pathol 2010;19:e119–123. [DOI] [PubMed] [Google Scholar]
- 28. Vasaiwala SC, Finn C, Delpriore J, Leya F, Gagermeier J, Akar JG, et al. Prospective study of cardiac sarcoid mimicking arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol 2009;20:473–476. [DOI] [PubMed] [Google Scholar]
- 29. Niemelä M, Uusitalo V, Pöyhönen P, Schildt J, Lehtonen J, Kupari M. Incidence and Predictors of Atrial Fibrillation in Cardiac Sarcoidosis. JACC Cardiovasc Imaging 2022;15:1622–1631. [DOI] [PubMed] [Google Scholar]
- 30. Wait JL, Movahed A. Anginal chest pain in sarcoidosis. Thorax 1989;44:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kruse MJ, Kovell L, Kasper EK, Pomper MG, Moller DR, Solnes L, et al. Myocardial Blood Flow and Inflammatory Cardiac Sarcoidosis. JACC Cardiovasc Imaging 2017;10:157–167. [DOI] [PubMed] [Google Scholar]
- 32. Lam CS, Tolep KA, Metke MP, Glockner J, Cooper LT Jr. Coronary sarcoidosis presenting as acute coronary syndrome. Clin Cardiol 2009;32:E68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kandolin R, Ekström K, Simard T, Hibbert B, Nery P, Lehtonen Jet al. Spontaneous coronary artery dissection in cardiac sarcoidosis. Oxf Med Case Reports 2019;2019:omz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Darda S, Zughaib ME, Alexander PB, Machado CE, David SW, Saba S. Cardiac sarcoidosis presenting as constrictive pericarditis. Tex Heart Inst J 2014;41:319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chow KL, O'Donnell JL, Crozier I. Prevalence, incidence and survival outcomes of cardiac sarcoidosis in the South Island, New Zealand. Int J Cardiol 2022;357:128–133. [DOI] [PubMed] [Google Scholar]
- 36. Arkema EV, Cozier YC. Sarcoidosis epidemiology: recent estimates of incidence, prevalence and risk factors. Curr Opin Pulm Med 2020;26:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Slart RHJA, Glaudemans AWJM, Lancellotti P, Hyafil F, Blankstein R, Schwartz RGet al. A joint procedural position statement on imaging in cardiac sarcoidosis: from the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. J Nucl Cardiol 2018;25:298–319. [DOI] [PubMed] [Google Scholar]
- 38. Cacoub P, Chapelon-Abric C, Resche-Rigon M, Saadoun D, Desbois AC, Biard L. Cardiac sarcoidosis: a long term follow up study. PLoS One 2020;15:e0238391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Willy K, Dechering DG, Reinke F, Bögeholz N, Frommeyer G, Eckardt L. The ECG in sarcoidosis - a marker of cardiac involvement? Current evidence and clinical implications. J Cardiol 2021;77:154–159. [DOI] [PubMed] [Google Scholar]
- 40. Kusunose K, Fujiwara M, Yamada H, Nishio S, Saijo Y, Yamada Net al. Deterioration of biventricular strain is an early marker of cardiac involvement in confirmed sarcoidosis. Eur Heart J Cardiovasc Imaging 2020;21:796–804. [DOI] [PubMed] [Google Scholar]
- 41. Kurmann R, Mankad SV, Mankad R. Echocardiography in Sarcoidosis. Curr Cardiol Rep 2018;20:118. [DOI] [PubMed] [Google Scholar]
- 42. Kandolin R, Lehtonen J, Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol 2011;4:303–309. [DOI] [PubMed] [Google Scholar]
- 43. Nery PB, Beanlands RS, Nair GM, Green M, Yang J, McArdle BAet al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol 2014;25:875–881. [DOI] [PubMed] [Google Scholar]
- 44. Tung R, Bauer B, Schelbert H, Lynch JP, Auerbach M, Gupta P, et al. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: the potential role of occult inflammation in arrhythmogenesis. Heart Rhythm 2015;12:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nery PB, Mc Ardle BA, Redpath CJ, Leung E, Lemery R, Dekemp R, et al. Prevalence of cardiac sarcoidosis in patients presenting with monomorphic ventricular tachycardia. Pacing Clin Electrophysiol 2014;37:364–374. [DOI] [PubMed] [Google Scholar]
- 46. Kebed KY, Carter SV, Flatley E, Ward RP, Moss JD, Appelbaum DE, et al. Prevalence of newly diagnosed sarcoidosis in patients with ventricular arrhythmias: a cardiac magnetic resonance and 18F-FDG cardiac PET study. Int J Cardiovasc Imaging 2021;37:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Kaikkonen K, et al. Usefulness of Cardiac Troponins as Markers of Early Treatment Response in Cardiac Sarcoidosis. Am J Cardiol 2015;116:960–964. [DOI] [PubMed] [Google Scholar]
- 48. Kiko T, Yoshihisa A, Kanno Y, Yokokawa T, Abe S, Miyata-Tatsumi M, et al. A Multiple Biomarker Approach in Patients with Cardiac Sarcoidosis. Int Heart J 2018;59:996–1001. [DOI] [PubMed] [Google Scholar]
- 49. Caforio ALP, Baritussio A, Marcolongo R, Cheng C-Y, Pontara E, Bison E, et al. Serum anti-heart and anti-intercalated disk autoantibodies: novel autoimmune markers in cardiac sarcoidosis. J Clin Med 2021;10:2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barssoum K, Altibi AM, Rai D, Kumar A, Kharsa A, Chowdhury M, et al. Speckle tracking echocardiography can predict subclinical myocardial involvement in patients with sarcoidosis: a meta-analysis. Echocardiography 2020;37:2061–2070. [DOI] [PubMed] [Google Scholar]
- 51. Di Stefano C, Bruno G, Arciniegas Calle MC, Acharya GA, Fussner LM, Ungprasert P, et al. Diagnostic and predictive value of speckle tracking echocardiography in cardiac sarcoidosis. BMC Cardiovasc Disord. 2020;20:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ohira H, Sato T, Manabe O, Oyama-Manabe N, Hayashishita A, Nakaya T, et al. Underdiagnosis of cardiac sarcoidosis by ECG and echocardiography in cases of extracardiac sarcoidosis. ERJ Open Res 2022;8:00516–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eurelings LEM, Miedema JR, Dalm VASH, van Daele PLA, van Hagen PM, van Laar JAM, et al. Sensitivity and specificity of serum soluble interleukin-2 receptor for diagnosing sarcoidosis in a population of patients suspected of sarcoidosis. PLoS One 2019;14:e0223897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chareonthaitawee P, Beanlands RS, Chen W, Dorbala S, Miller EJ, Murthy VL, et al. Joint SNMMI–ASNC Expert Consensus Document on the Role of 18F-FDG PET/CT in Cardiac Sarcoid Detection and Therapy Monitoring. J Nucl Med 2017;58:1341–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saric P, Young KA, Rodriguez-Porcel M, Chareonthaitawee P. PET imaging in cardiac sarcoidosis: a narrative review with focus on novel PET tracers. Pharmaceuticals (Basel )2021;14:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim SJ, Pak K, Kim K. Diagnostic performance of F-18 FDG PET for detection of cardiac sarcoidosis; A systematic review and meta-analysis. J Nucl Cardiol 2020;27:2103–2115. [DOI] [PubMed] [Google Scholar]
- 57. The Japan Society of Sarcoidosis and Other Granulomatous Disorders, Committee for revision of the diagnostic standard for sarcoidosis. Diagnostic standard and guideline for sarcoidosis - 2006. The Japanese Journal of Sarcoidosis and Other Granulomatous Disorders 2007:89–102.
- 58. Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Divakaran S, Stewart GC, Lakdawala NK, Padera RF, Zhou W, Desai AS, et al. Diagnostic Accuracy of Advanced Imaging in Cardiac Sarcoidosis. Circ Cardiovasc Imaging 2019;12:e008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC, et al. Reduction in (1)(8)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol 2014;21:166–174. [DOI] [PubMed] [Google Scholar]
- 61. Giblin GT, Murphy L, Stewart GC, Desai AS, Di Carli MF, Blankstein R, et al. Cardiac sarcoidosis: when and how to treat inflammation. Card Fail Rev 2021;7:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ahmed AI, Abebe AT, Han Y, Alnabelsi T, Agrawal T, Kassi M, et al. The prognostic role of cardiac positron emission tomography imaging in patients with sarcoidosis: a systematic review. J Nucl Cardiol 2021;28:1545–1552. [DOI] [PubMed] [Google Scholar]
- 63. Patel VN, Pieper JA, Poitrasson-Riviere A, Kopin D, Cascino T, Aaronson K, et al. The prognostic value of positron emission tomography in the evaluation of suspected cardiac sarcoidosis. J Nucl Cardiol 2022;29:2460–2470. [DOI] [PubMed] [Google Scholar]
- 64. Gowani Z, Habibi M, Okada DR, Smith J, Derakhshan A, Zimmerman SL, et al. Utility of Cardiac Magnetic Resonance Imaging Versus Cardiac Positron Emission Tomography for Risk Stratification for Ventricular Arrhythmias in Patients With Cardiac Sarcoidosis. Am J Cardiol 2020;134:123–129. [DOI] [PubMed] [Google Scholar]
- 65. Bekki M, Tahara N, Tahara A, Sugiyama Y, Maeda-Ogata S, Honda A, et al. Localization of myocardial FDG uptake for prognostic risk stratification in corticosteroid-naive cardiac sarcoidosis. J Nucl Cardiol 2022;29:132–2144. [DOI] [PubMed] [Google Scholar]
- 66. Wicks EC, Menezes LJ, Barnes A, Mohiddin SA, Sekhri N, Porter JC, et al. Diagnostic accuracy and prognostic value of simultaneous hybrid 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging 2018;19:757–767. [DOI] [PubMed] [Google Scholar]
- 67. Greulich S, Gatidis S, Grani C, Blankstein R, Glatthaar A, Mezger K, et al. Hybrid Cardiac Magnetic Resonance/Fluorodeoxyglucose Positron Emission Tomography to Differentiate Active From Chronic Cardiac Sarcoidosis. JACC Cardiovasc Imaging 2022;15:445–456. [DOI] [PubMed] [Google Scholar]
- 68. Riedy K, Fisher MR, Belic N, Koenigsberg DI. MR imaging of myocardial sarcoidosis. AJR Am J Roentgenol 1988;151:915–916. [DOI] [PubMed] [Google Scholar]
- 69. Vignaux O, Dhote R, Duboc D, Blanche Philippe, Devaux J-Y, Weber S, et al. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrast-enhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr 2002;26:762–767. [DOI] [PubMed] [Google Scholar]
- 70. Smedema JP, Snoep G, van Kroonenburgh MP, Dassen WRM, Gorgels APM, Crijns HJGM. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005;45:1683–1690. [DOI] [PubMed] [Google Scholar]
- 71. Abdel-Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 2005;45:1815–1822. [DOI] [PubMed] [Google Scholar]
- 72. Schulz-Menger J, Wassmuth R, Abdel-Aty H, Siegel I, Franke A, Dietz Ret al. Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart 2006;92:399–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 2005;26:1461–1474. [DOI] [PubMed] [Google Scholar]
- 74. Tadamura E, Yamamuro M, Kubo S, Kanao S, Saga T, Harada M, et al. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol 2005;185:110–115. [DOI] [PubMed] [Google Scholar]
- 75. Watanabe E, Kimura F, Nakajima T, Hiroe M, Kasai Y, Nagata M, et al. Late gadolinium enhancement in cardiac sarcoidosis: characteristic magnetic resonance findings and relationship with left ventricular function. J Thorac Imaging 2013;28:60–66. [DOI] [PubMed] [Google Scholar]
- 76. Trivieri MG, Spagnolo P, Birnie Det al. Challenges in cardiac and pulmonary sarcoidosis: JACC state-of-the-art review. J Am Coll Cardiol 2020;76:1878–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang S, Chen X, Li J, Sun Y, Song J, Wang H, et al. Late gadolinium enhancement characteristics in giant cell myocarditis. ESC Heart Fail 2021;8:2320–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Aitken M, Chan MV, Urzua Fresno C, Farrell A, Islam N, McInnes MDFet al. Diagnostic accuracy of cardiac MRI versus FDG PET for cardiac sarcoidosis: a systematic review and meta-analysis. Radiology 2022;304:566–579. [DOI] [PubMed] [Google Scholar]
- 79. Juneau D, Nery PB, Pena E, Inácio JR, Beanlands RSB, deKemp RA, et al. Reproducibility of cardiac magnetic resonance imaging in patients referred for the assessment of cardiac sarcoidosis; implications for clinical practice. Int J Cardiovasc Imaging 2020;36:2199–2207. [DOI] [PubMed] [Google Scholar]
- 80. Greulich S, Kitterer D, Latus J, Aguor E, Steubing H, Kaesemann P, et al. Comprehensive Cardiovascular Magnetic Resonance Assessment in Patients With Sarcoidosis and Preserved Left Ventricular Ejection Fraction. Circ Cardiovasc Imaging 2016;9:e005022. [DOI] [PubMed] [Google Scholar]
- 81. Puntmann VO, Isted A, Hinojar R, Foote L, Carr-White G, Nagel E. T1 and T2 Mapping in Recognition of Early Cardiac Involvement in Systemic Sarcoidosis. Radiology 2017;285:63–72. [DOI] [PubMed] [Google Scholar]
- 82. Crouser ED, Ruden E, Julian MW, Raman SV. Resolution of abnormal cardiac MRI T2 signal following immune suppression for cardiac sarcoidosis. J Investig Med 2016;64:1148–1150. [DOI] [PubMed] [Google Scholar]
- 83. Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti Cet al. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging 2011;4:150–156. [DOI] [PubMed] [Google Scholar]
- 84. Hulten E, Agarwal V, Cahill M, Cole G, Vita T, Parrish S, et al. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: a systematic review and meta-analysis. Circ Cardiovasc Imaging 2016;9:e005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J 1999;138:299–302. [DOI] [PubMed] [Google Scholar]
- 86. Ardehali H, Howard DL, Hariri A, Qasim A, Hare JM, Baughman KL, et al. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J 2005;150:459–463. [DOI] [PubMed] [Google Scholar]
- 87. Kandolin R, Lehtonen J, Graner M, Schildt J, Salmenkivi K, Kivistö SM, et al. Diagnosing isolated cardiac sarcoidosis. J Intern Med 2011;270:461–468. [DOI] [PubMed] [Google Scholar]
- 88. Ezzeddine FM, Kapa S, Rosenbaum A, Blauwet L, Deshmukh AJ, AbouEzzeddine OF, et al. Electrogram-guided endomyocardial biopsy yield in patients with suspected cardiac sarcoidosis and relation to outcomes. J Cardiovasc Electrophysiol 2021;32:2486–2495. [DOI] [PubMed] [Google Scholar]
- 89. Komoriyama H, Omote K, Nagai T, Kato Y, Nagano N, Koyanagawa K, et al. Lower left ventricular ejection fraction and higher serum angiotensin-converting enzyme activity are associated with histopathological diagnosis by endomyocardial biopsy in patients with cardiac sarcoidosis. Int J Cardiol 2020;321:113–117. [DOI] [PubMed] [Google Scholar]
- 90. Vakil K, Minami E, Fishbein DP. Right ventricular sarcoidosis: is it time for updated diagnostic criteria? Tex Heart Inst J 2014;41:203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Omote K, Naya M, Koyanagawa K, Aikawa T, Manabe O, Nagai T, et al. (18)F-FDG uptake of the right ventricle is an important predictor of histopathologic diagnosis by endomyocardial biopsy in patients with cardiac sarcoidosis. J Nucl Cardiol 2020;27:2135–2143. [DOI] [PubMed] [Google Scholar]
- 92. Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A, et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation 2010;122:900–909. [DOI] [PubMed] [Google Scholar]
- 93. Chimenti C, Frustaci A. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies: a retrospective study over a 28-year period. Circulation 2013;128:1531–1541. [DOI] [PubMed] [Google Scholar]
- 94. Honda Y, Nagai T, Ikeda Y, Sakakibara M, Asakawa N, Nagano N, et al. Myocardial Immunocompetent Cells and Macrophage Phenotypes as Histopathological Surrogates for Diagnosis of Cardiac Sarcoidosis in Japanese. J Am Heart Assoc 2016;5:e004019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu J, Ma P, Lai L, Villanueva A, Koenig A, Bean GR, et al. Transcriptional and immune landscape of cardiac sarcoidosis. Circ Res 2022;131:654–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lassner D, Kuhl U, Siegismund CS, Rohde M, Elezkurtaj S, Escher F, et al. Improved diagnosis of idiopathic giant cell myocarditis and cardiac sarcoidosis by myocardial gene expression profiling. Eur Heart J 2014;35:2186–2195. [DOI] [PubMed] [Google Scholar]
- 97. Ekstrom K, Raisanen-Sokolowski A, Lehtonen J, Nordenswan H-K, Mäyränpää MI, Kupari M. Idiopathic giant cell myocarditis or cardiac sarcoidosis? A retrospective audit of a nationwide case series. ESC Heart Fail 2020;7:1362–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lehtonen JY, Jokinen JJ, Holmstrom M, Kupari M. Open chest core needle biopsy of left ventricle in the evaluation of suspected focal myocardial inflammation. J Thorac Cardiovasc Surg 2015;149:e99–102. [DOI] [PubMed] [Google Scholar]
- 99. Birnie DH, Nery PB, Beanlands RS. COUNTERPOINT: should isolated cardiac sarcoidosis be considered a significant manifestation of sarcoidosis? No. Chest 2021;160:38–42. [DOI] [PubMed] [Google Scholar]
- 100. Ueberham L, Jahnke C, Paetsch I, Klingel K, Kuehl M, Hindricks G, et al. Current Diagnostic Criteria Show a Substantial Disagreement in Classification of Patients With Suspected Cardiac Sarcoidosis. JACC Clin Electrophysiol 2021;7:538–539. [DOI] [PubMed] [Google Scholar]
- 101. Gilotra N, Okada D, Sharma A, Chrispin J. Management of Cardiac Sarcoidosis in 2020. Arrhythm Electrophysiol Rev 2020;9:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cha MJ, Seo JW, Oh S, Park E-A, Lee S-H, Kim MY, et al. Indirect pathological indicators for cardiac sarcoidosis on endomyocardial biopsy. J Pathol Transl Med 2020;54:396–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kato S, Sakai Y, Okabe A, Kawashima Y, Kuwahara K, Shiogama K, et al. Histology of Cardiac Sarcoidosis with Novel Considerations Arranged upon a Pathologic Basis. J Clin Med 2022;11:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kupari M, Nordenswan H-K, Kandolin R, Ekström K, Lehtonen J. Long-Term Survival and Adverse Events in Cardiac Sarcoidosis. JACC Asia 2022;2:212–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kitai T, Nabeta T, Naruse Y, Naruse Y, Taniguchi T, Yoshioka K, et al. Comparisons between biopsy-proven versus clinically diagnosed cardiac sarcoidosis. Heart 2022;108:1887–1894. [DOI] [PubMed] [Google Scholar]
- 106. Nabeta T, Kitai T, Naruse Y, Taniguchi T, Yoshioka K, Tanaka H, et al. Risk stratification of patients with cardiac sarcoidosis: the ILLUMINATE-CS registry. Eur Heart J 2022;43:3450–3459. [DOI] [PubMed] [Google Scholar]
- 107. Nordenswan HK, Pöyhönen P, Lehtonen J, Ekström K, Uusitalo V, Niemelä M, et al. Incidence of Sudden Cardiac Death and Life-Threatening Arrhythmias in Clinically Manifest Cardiac Sarcoidosis With and Without Current Indications for an Implantable Cardioverter-Defibrillator. Circulation 2022;146:964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ribeiro Neto ML, Jellis C, Hachamovitch R, Wimer A, Highland KB, Sahoo D, et al. Performance of diagnostic criteria in patients clinically judged to have cardiac sarcoidosis: is it time to regroup? Am Heart J 2020;223:106–109. [DOI] [PubMed] [Google Scholar]
- 109. Kouranos V, Sharma R. Cardiac sarcoidosis: state-of-the-art review. Heart 2021;107:1591–1599. [DOI] [PubMed] [Google Scholar]
- 110. Vita T, Okada DR, Veillet-Chowdhury M, Bravo PE, Mullins E, Hulten E, et al. Complementary Value of Cardiac Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Assessment of Cardiac Sarcoidosis. Circ Cardiovasc Imaging 2018;11:e007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Miller EJ, Culver DA. Establishing an evidence-based method to diagnose cardiac sarcoidosis: the complementary use of cardiac magnetic resonance imaging and FDG-PET. Circ Cardiovasc Imaging 2018;11:e007408. [DOI] [PubMed] [Google Scholar]
- 112. Philips B, Madhavan S, James CA, te Riele ASJM, Murray B, Tichnell C, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy and cardiac sarcoidosis: distinguishing features when the diagnosis is unclear. Circ Arrhythm Electrophysiol 2014;7:230–236. [DOI] [PubMed] [Google Scholar]
- 113. Smith ED, Lakdawala NK, Papoutsidakis N, Aubert G, Mazzanti A, McCanta ACet al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct From typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation 2020;141:1872–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. daSilva-deAbreu A, Mandras SA. Sarcoidosis-associated pulmonary hypertension: an updated review and discussion of the clinical conundrum. Curr Probl Cardiol 2021;46:100506. [DOI] [PubMed] [Google Scholar]
- 115. Roberts WC, Roberts CC, Ko JM, Filardo G, Capehart JE, Hall SA. Morphologic features of the recipient heart in patients having cardiac transplantation and analysis of the congruence or incongruence between the clinical and morphologic diagnoses. Medicine (Baltimore) 2014;93:211–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Luk A, Metawee M, Ahn E, Gustafsson F, Ross H, Butany J. Do clinical diagnoses correlate with pathological diagnoses in cardiac transplant patients? The importance of endomyocardial biopsy. Can J Cardiol 2009;25:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chazal T, Varnous S, Guihaire J, Goeminne C, Launay D, Boignard A, et al. Sarcoidosis diagnosed on granulomas in the explanted heart after transplantation: results of a French nationwide study. Int J Cardiol 2020;307:94–100. [DOI] [PubMed] [Google Scholar]
- 118. Judson MA. The three tiers of screening for sarcoidosis organ involvement. Respir Med 2016;113:42–49. [DOI] [PubMed] [Google Scholar]
- 119. Grimes DA, Schulz KF. Uses and abuses of screening tests. Lancet 2002;359:881–884. [DOI] [PubMed] [Google Scholar]
- 120. Crouser ED, Maier LA, Wilson KC, Bonham CA, Morgenthau AS, Patterson KC, et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2020;201:e26–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Holtzclaw AW, Mrsic Z, Church TL, Shumar JN, Liotta RA, Aslam SN, et al. Optimizing routine screening for cardiac sarcoidosis through use of commonly available studies. Respir Med 2021;178:106331. [DOI] [PubMed] [Google Scholar]
- 122. Mehta D, Lubitz SA, Frankel Z, Wisnivesky JP, Einstein AJ, Goldman M, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest 2008;133:1426–1435. [DOI] [PubMed] [Google Scholar]
- 123. Nagao S, Watanabe H, Sobue Y, Kodama M, Tanaka J, Tanabe N, et al. Electrocardiographic abnormalities and risk of developing cardiac events in extracardiac sarcoidosis. Int J Cardiol 2015;189:1–5. [DOI] [PubMed] [Google Scholar]
- 124. Nagai T, Kohsaka S, Okuda S, , Anzai T, Asano K, Fukuda K. Incidence and prognostic significance of myocardial late gadolinium enhancement in patients with sarcoidosis without cardiac manifestation. Chest 2014;146:1064–1072. [DOI] [PubMed] [Google Scholar]
- 125. Yafasova A, Fosbol EL, Schou M, Gustafsson F, Rossing K, Bundgaard H, et al. Long-Term Adverse Cardiac Outcomes in Patients With Sarcoidosis. J Am Coll Cardiol 2020;76:767–777. [DOI] [PubMed] [Google Scholar]
- 126. Baughman RP, Valeyre D, Korsten P, Mathioudakis AG, Wuyts WA, Wells A, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J 2021;58:2004079. [DOI] [PubMed] [Google Scholar]
- 127. Fazelpour S, Sadek MM, Nery PB, Beanlands RS, Tzemos N, Toma M, et al. Corticosteroid and immunosuppressant therapy for cardiac sarcoidosis: a systematic review. J Am Heart Assoc 2021;10:e021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chiu CZ, Nakatani S, Zhang Get al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Card 2005;95:143–146. [DOI] [PubMed] [Google Scholar]
- 129. Wand AL, Pavlovic N, Duvall Cet al. Effect of corticosteroids on left ventricular function in patients with cardiac sarcoidosis. Am J Cardiol 2022;177:108–115. [DOI] [PubMed] [Google Scholar]
- 130. Gilotra NA, Griffin JM, Pavlovic N, Houston BA, Chasler J, Goetz C, et al. Sarcoidosis-related cardiomyopathy: current knowledge, challenges, and future perspectives state-of-the-art review. J Card Fail 2022;28:113–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Symons R, Zimmerman SL, Bluemke DA. CMR and CT of the patient with cardiac devices. JACC Cardiovasc Imaging 2019;12:890–903. [DOI] [PubMed] [Google Scholar]
- 132. Mesubi O, Ahmad G, Jeudy J, Jimenez A, Kuk R, Saliaris A, et al. Impact of ICD artifact burden on late gadolinium enhancement cardiac MR imaging in patients undergoing ventricular tachycardia ablation. Pacing Clin Electrophysiol. 2014;37:1274–1283. [DOI] [PubMed] [Google Scholar]
- 133. Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac Sarcoidosis. J Am Coll Cardiol 2016;68:411–421. [DOI] [PubMed] [Google Scholar]
- 134. Rojulpote C, Bhattaru A, Jean C, Adams SL, Patel V, Vidula MK, et al. Effect of immunosuppressive therapy and biopsy status in monitoring therapy response in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 2022;15:1944–1955. [DOI] [PubMed] [Google Scholar]
- 135. Nagai S, Yokomatsu T, Tanizawa K, Ikezoe K, Handa T, Ito Y, et al. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med 2014;53:427–433. [DOI] [PubMed] [Google Scholar]
- 136. Ballul T, Borie R, Crestani B, Daugas E, Descamps V, Dieude P, et al. Treatment of cardiac sarcoidosis: a comparative study of steroids and steroids plus immunosuppressive drugs. Int J Cardiol 2019;276:208–211. [DOI] [PubMed] [Google Scholar]
- 137. Vorselaars ADM, Culver DA. Hit-hard and early versus step-up treatment in severe sarcoidosis. Curr Opin Pulm Med 2022;28:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Vorselaars ADM, Wuyts WA, Vorselaars VMM, Zanen P, Deneer VHM, Veltkamp M, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest 2013;144:805–812. [DOI] [PubMed] [Google Scholar]
- 139. Bakker ALM, Mathijssen H, Azzahhafi J, Swaans MJ, Veltkamp M, Keijsers RGM, et al. Effectiveness and safety of infliximab in cardiac Sarcoidosis. Int J Cardiol 2021;330:179–185. [DOI] [PubMed] [Google Scholar]
- 140. Harper LJ, McCarthy M, Ribeiro Neto ML, Hachamovitch R, Pearson K, Bonanno B, et al. Infliximab for Refractory Cardiac Sarcoidosis. Am J Cardiol 2019;124:1630–1635. [DOI] [PubMed] [Google Scholar]
- 141. Rosenthal DG, Parwani P, Murray TO, Petek BJ, Benn BS, De Marco T, et al. Long-Term Corticosteroid-Sparing Immunosuppression for Cardiac Sarcoidosis. J Am Heart Assoc 2019;8:e010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gilotra NA, Wand AL, Pillarisetty A, Devraj M, Pavlovic N, Ahmed S, et al. Clinical and imaging response to tumor necrosis factor alpha inhibitors in treatment of cardiac sarcoidosis: a multicenter experience. J Card Fail 2021;27:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chung ES, Packer M, Lo KH, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators . Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003;107:3133–3140. [DOI] [PubMed] [Google Scholar]
- 144. Elwazir M, Krause ML, Bois JP, Christopoulos G, Kendi AT, Cooper LT Jr, et al. Rituximab for the treatment of refractory cardiac sarcoidosis: a single-center experience. J Card Fail 2022;28:247–258. [DOI] [PubMed] [Google Scholar]
- 145. Birnie D, Beanlands RSB, Nery P, Aaron SD, Culver DA, DeKemp RA, et al. Cardiac Sarcoidosis multi-center randomized controlled trial (CHASM CS- RCT). Am Heart J 2020;220:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kron J, Crawford T, Mihalick V, Bogun F, Jordan JH, Koelling T, et al. Interleukin-1 blockade in cardiac sarcoidosis: study design of the multimodality assessment of granulomas in cardiac sarcoidosis: anakinra randomized trial (MAGiC-ART). J Transl Med 2021;19:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ishibashi K, Eishi Y, Tahara N, Asakura M, Sakamoto N, Nakamura K, et al. Japanese Antibacterial Drug Management for Cardiac Sarcoidosis (J-ACNES): a multicenter, open-label, randomized, controlled study. J Arrhythm 2018;34:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Viwe M, Nery P, Birnie DH. Management of ventricular tachycardia in patients with cardiac sarcoidosis. Heart Rhythm O2 2021;2:412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Adhaduk M, Paudel B, Liu K, Ashwath M, Giudici M. Meta-Analysis of Catheter Ablation Outcomes in Patients With Cardiac Sarcoidosis Refractory Ventricular Tachycardia. Am J Cardiol 2022;174:136–142. [DOI] [PubMed] [Google Scholar]
- 150. Siontis KC, Santangeli P, Muser D, Marchlinski FE, Zeppenfeld K, Hoogendoorn JC, et al. Outcomes Associated With Catheter Ablation of Ventricular Tachycardia in Patients With Cardiac Sarcoidosis. JAMA Cardiol 2022;7:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kafil TS, Sparrow R, Khan HR, Manian U, Elrayes M, Bagur R, et al. Intravenous methylprednisolone for ventricular tachycardia electrical storm in cardiac sarcoidosis: case series and 1-year follow-up. JACC Clin Electrophysiol 2021;7:536–537. [DOI] [PubMed] [Google Scholar]
- 152. Okada DR, Assis FR, Gilotra NA, Ha JS, Berger RD, Calkins H, et al. Cardiac sympathectomy for refractory ventricular arrhythmias in cardiac sarcoidosis. Heart Rhythm 2019;16:1408–1413. [DOI] [PubMed] [Google Scholar]
- 153. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2018;72:e91–e220. [DOI] [PubMed] [Google Scholar]
- 154. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Eur Heart J 2022;43:3997–4126.36017572 [Google Scholar]
- 155. Glikson M, Nielsen JC J, Kronborg, MBet al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–3520. [DOI] [PubMed] [Google Scholar]
- 156. Bakker A, Mathijssen H, Dorland G, Balt JC, van Dijk VF, Veltkamp Met al. Long-term monitoring of arrhythmias with cardiovascular implantable electronic devices in patients with cardiac sarcoidosis. Heart Rhythm 2022;19:352–360. [DOI] [PubMed] [Google Scholar]
- 157. Adhaduk M, Paudel B, Liu K, Ashwath M, Giudici M. The role of electrophysiology study in risk stratification of cardiac sarcoidosis patients: meta-analyses and systemic review. Int J Cardiol 2022;349:55–61. [DOI] [PubMed] [Google Scholar]
- 158. Sperry BW, Tamarappoo BK, Oldan JD, Javed O, Culver DA, Brunken R, et al. Prognostic Impact of Extent, Severity, and Heterogeneity of Abnormalities on (18)F-FDG PET Scans for Suspected Cardiac Sarcoidosis. JACC Cardiovasc Imaging 2018;11:336–345. [DOI] [PubMed] [Google Scholar]
- 159. Yoshitomi R, Kobayashi S, Yano Y, Nakashima Y, Fujii S, Nanno T, et al. Enhanced oxidative stress and presence of ventricular aneurysm for risk prediction in cardiac sarcoidosis. Heart 2022; 108:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kagioka Y, Yasuda M, Okune M, Kakehi K, Kawamura T, Kobuke Ket al. Right ventricular involvement is an important prognostic factor and risk stratification tool in suspected cardiac sarcoidosis: analysis by cardiac magnetic resonance imaging. Clin Res Cardiol 2020;109:988–998. [DOI] [PubMed] [Google Scholar]
- 161. Shade JK, Prakosa A, Popescu DM, Yu R, Okada DR, Chrispin J, et al. Predicting risk of sudden cardiac death in patients with cardiac sarcoidosis using multimodality imaging and personalized heart modeling in a multivariable classifier. Sci Adv 2021;7:eabi8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kron J, Sauer W, Schuller J, Bogun F, Crawford T, Sarsam S, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace 2013;15:347–354. [DOI] [PubMed] [Google Scholar]
- 163. Halawa A, Jain R, Turagam MK, Kusumoto FM, Woldu HG, Gautam S. Outcome of implantable cardioverter-defibrillator in cardiac sarcoidosis: a systematic review and meta-analysis. J Interv Card Electrophysiol 2020;58:233–242. [DOI] [PubMed] [Google Scholar]
- 164. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 165. Bogabathina H, Olson P, Rathi VK, Biederman RW. Cardiac sarcoidosis or giant cell myocarditis? On treatment improvement of fulminant myocarditis as demonstrated by cardiovascular magnetic resonance imaging. Case Rep Cardiol 2012;2012:647041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Yufu K, Kondo H, Shinohara T, Kawano K, Ishii Y, Miyoshi M, et al. Patients with cardiac sarcoidosis who received cardiac resynchronization therapy: comparison with dilated cardiomyopathy patients. J Cardiovasc Electrophysiol 2017;28:177–181. [DOI] [PubMed] [Google Scholar]
- 167. Shabtaie SA, Sehrawat O, Lee JZ, Cha YM, Mulpuru SK, Kowlgi NG, et al. Cardiac resynchronization therapy response in cardiac sarcoidosis. J Cardiovasc Electrophysiol 2022;33:2072–2080. [DOI] [PubMed] [Google Scholar]
- 168. Chicos AB. Does cardiac resynchronization help patients with cardiac sarcoidosis? J Cardiovasc Electrophysiol 2022;33:2081–2082. [DOI] [PubMed] [Google Scholar]
- 169. McGoldrick MT, Giuliano K, Etchill EW, Barbur I, Yenokyan G, Whitman G, et al. Long-term survival after heart transplantation for cardiac sarcoidosis. J Card Surg 2021;36:4247–4255. [DOI] [PubMed] [Google Scholar]
- 170. Jackson KC, Youmans QR, Wu T, Harap R, Anderson AS, Chicos A, et al. Heart transplantation outcomes in cardiac sarcoidosis. J Heart Lung Transplant 2022;41:113–122. [DOI] [PubMed] [Google Scholar]
- 171. Rosenthal DG, Anderson ME, Petek BJ, Arnett DM, Bravo PE, Raghu G, et al. Invasive Hemodynamics and Rejection Rates in Patients With Cardiac Sarcoidosis After Heart Transplantation. Can J Cardiol 2018;34:978–982. [DOI] [PubMed] [Google Scholar]
- 172. Yazaki Y, Isobe M, Hiroe Met al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001;88:1006–10. [DOI] [PubMed] [Google Scholar]
- 173. Ise T, Hasegawa T, Morita Y, Yamada N, Funada A, Takahama H, et al. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart 2014;100:1165–72. [DOI] [PubMed] [Google Scholar]
- 174. Crawford T, Mueller G, Sarsam S, Prasitdumrong H, Chaiyen N, Gu X, et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol 2014;7:1109–15. [DOI] [PubMed] [Google Scholar]
- 175. Ekström K, Lehtonen J, Hänninen H, Kandolin R, Kivistö S, Kupari M. Magnetic Resonance Imaging as a Predictor of Survival Free of Life-Threatening Arrhythmias and Transplantation in Cardiac Sarcoidosis. J Am Heart Assoc 2016;5:e003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Nordenswan HK, Lehtonen J, Ekstrom K, Kandolin R, Simonen P, Mäyränpää M, et al. Outcome of Cardiac Sarcoidosis Presenting With High-Grade Atrioventricular Block. Circ Arrhythm Electrophysiol 2018;11:e006145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this review.