Abstract
Background:
Preterm birth is the leading cause of infant morbidity and mortality worldwide. Elevated levels of oxidative stress have been associated with an increased risk of delivering preterm. However, most studies testing this hypothesis have been conducted in racially and demographically homogenous study populations, which do not reflect the diversity within the United States.
Objective:
We leveraged four cohorts participating in the Environmental influences on Child Health Outcomes Program to conduct the largest study to date examining biomarkers of oxidative stress and preterm birth (N=1,916). We hypothesized that elevated oxidative stress would be associated with higher odds of preterm birth, particularly preterm birth of spontaneous origin.
Study Design:
We conducted a pooled and meta-analysis of four birth cohorts spanning multiple geographic regions in the mainland United States and Puerto Rico (N=208 preterm births; N=1,708 full-term births). 8-iso-prostaglandin-F2α, 2,3-dinor-5,6-dihydro-8-iso-prostaglandin-F2α (F2-IsoP-M; the major 8-iso-prostaglandin-F2α metabolite), and prostaglandin-F2α were measured in urine samples obtained during the second and third trimesters. Logistic regression was used to calculate adjusted odds ratios and 95% confidence intervals for the associations between averaged biomarker concentrations for each participant and all preterm births, spontaneous preterm births, non-spontaneous preterm births (births of medically indicated or unknown origin), and categories of preterm birth (early, moderate, late). Individual oxidative stress biomarkers were examined in separate models.
Results:
Approximately 11% of our analytic sample was born preterm. Relative to full-term births, an interquartile range increase in averaged concentrations of F2-IsoP-M was associated with higher odds of all preterm births (odds ratio=1.29, 95% confidence interval=1.11, 1.51), with a stronger association observed for spontaneous preterm birth (odds ratio= 1.47, 95% confidence interval=1.16, 1.90). An interquartile range increase in averaged concentrations of 8-iso-prostaglandin-F2α was similarly associated with higher odds of all preterm births (odds ratio=1.19, 95% confidence interval=0.94, 1.50). The results from our meta-analysis were similar to those from the pooled combined cohort analysis.
Conclusions:
We observed that oxidative stress, as measured by 8-iso-prostaglandin-F2α, F2-IsoP-M, and prostaglandin-F2α in urine, was associated with increased odds of preterm birth, particularly preterm birth of spontaneous origin and delivery prior to 34 completed weeks of gestation.
Keywords: isoprostanes, preterm birth, oxidative stress, adverse pregnancy outcomes, preterm labor, preterm PROM, spontaneous preterm birth, 8-iso-prostaglandin-F2α, prostaglandin F2α, F2-IsoP-M, oxylipins, lipid peroxidation
Condensation:
Among 1,916 pregnant people in the United States, we observed that elevated oxidative stress was associated with increased odds of preterm birth.
INTRODUCTION
Oxidative stress, defined as an imbalance between the body’s antioxidant system and reactive oxygen species (ROS), has been identified as a key mechanistic pathway linking chemical and non-chemical stress exposures to adverse pregnancy outcomes, including preterm birth (PTB).1 Disease states such as preterm labor, preeclampsia, and preterm premature rupture of the membranes (PROM), may in part be caused by the release of ROS and other mediators in the placenta,2-8 which can exert immune effects and contribute to a heighted inflammatory state during pregnancy.9 Intravascular inflammation is also a mechanism of disease in preeclampsia, fetal growth restriction, chorioamnionitis, preterm labor and preterm PROM.10-12 These inflammatory processes, as well as an increase in inflammatory cytokines, can trigger oxidative stress,13 highlighting that oxidative stress and inflammation are closely tied, and regulation of both is necessary for a healthy pregnancy.
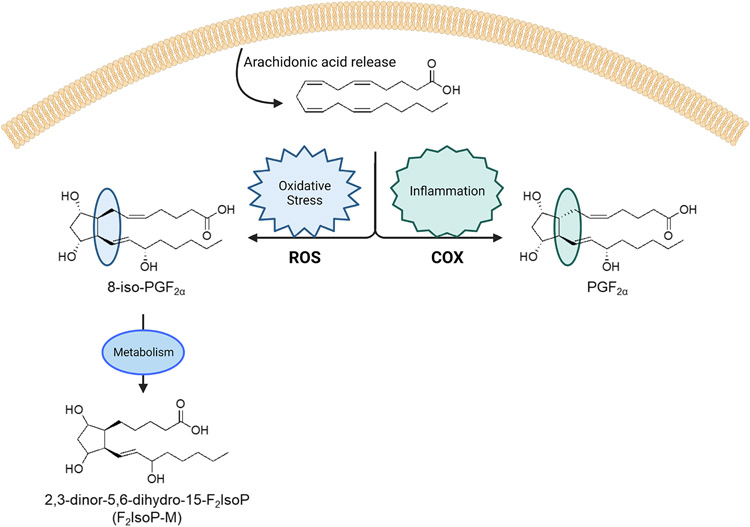
Studies in pregnant and non-pregnant populations find that oxidative stress levels are associated with biological, behavioral, and social factors reflecting socioeconomic disadvantage, and are elevated in response to environmental chemical and psychosocial stress exposures.1-14-20 Oxidative stress biomarkers are stable throughout the day, are excreted in urine and easily detected using mass-spectrometry. Of these, F2-isoprostanes (F2-IsoPs) are considered to be the ‘gold standard’ indicators of lipid peroxidation, and reflect different biologic process compared to other oxidative stress biomarkers, such as 8-hydroxyguanosine, which reflects oxidative DNA damage, and 3-nitrotyrosine, 3-chlorotyrosine, and o,o′-dityrosine which reflect protein oxidation. F2-IsoPs are formed from the non-enzymatic free radical oxidation of arachidonic acid, and have been identified as excellent biomarkers of endogenous oxidative stress.21-25 Numerous F2-IsoP isomers can be generated during this oxidative process (Figure 1). 8-iso-prostaglandin-F2α (8-iso-PGF2α) is formed in abundance and is the most commonly quantified F2-IsoP in biological samples. Elevated levels of 8-iso-PGF2α, particularly those measured during mid-pregnancy, are associated with higher odds of delivering preterm.2-26-31 Importantly, 8-iso-PGF2α differs from prostaglandin-F2α (PGF2α), a product of the enzymatic oxidation of arachidonic acid via cyclooxygenase (COX-1 and COX-2),32 only by the stereochemistry at carbon-8. PGF2α may also independently increase the risk of PTB, as PGF2α is part of the inflammatory response and stimulates uterine contractions, leading to spontaneous labor.33-34 In addition to F2-IsoPs, other enzymatically produced oxylipins, including those biosynthesized from arachidonic acid via cyclooxygenase, lipoxygenase, and cytochrome p450s have been associated with adverse pregnancy outcomes, including overall and spontaneous PTB, fetal growth restriction, and preeclampsia.1
Figure 1.
Biosynthetic pathways showing how oxidative stress biomarkers measured in the present study (8-iso-PGF2α, F2-IsoP-M, PGF2α) are generated.
Studies suggest that 8-iso-PGF2α is not exclusively formed from non-enzymatic lipid peroxidation; it can also be generated as a side product of arachidonic acid oxidation via COX. We can distinguish the portion of 8-iso-PGF2α that is generated from non-enzymatic (or chemical) vs. enzymatic (or inflammatory) oxidation using the ratio of 8-iso-PGF2α to PGF2α.35 In addition to quantification of 8-iso-PGF2α and PGF2α, quantification of the major urinary metabolite of 8-iso-PGF2α, 2,3-dinor-5,6-dihydro-8-iso-PGF2α (F2-IsoP-M), yields further insight into the endogenous formation of this biomolecule. F2-IsoP-M may be a more sensitive biomarker than its parent compound when measured in urine, as it is not subject to autoxidation and renal production.36 Prior work has additionally shown that higher concentrations of F2-IsoP-M are associated with higher odds of spontaneous PTB.37
In the present study, we leveraged data from four U.S. birth cohorts with detailed information on gestational age and perinatal outcomes to test the hypothesis that elevated oxidative stress biomarkers would be associated with higher odds of PTB, specifically spontaneous PTB. A better understanding of these different pathways may aid in identifying high-risk populations and thus help in planning targeted interventions. Our analytic sample, comprised of nearly 2,000 births from racially and ethnically diverse study populations, addresses important gaps in the literature related to: (1) diversity, (2) timing and type of PTB, and (3) small sample size.
MATERIALS AND METHODS
Study Population
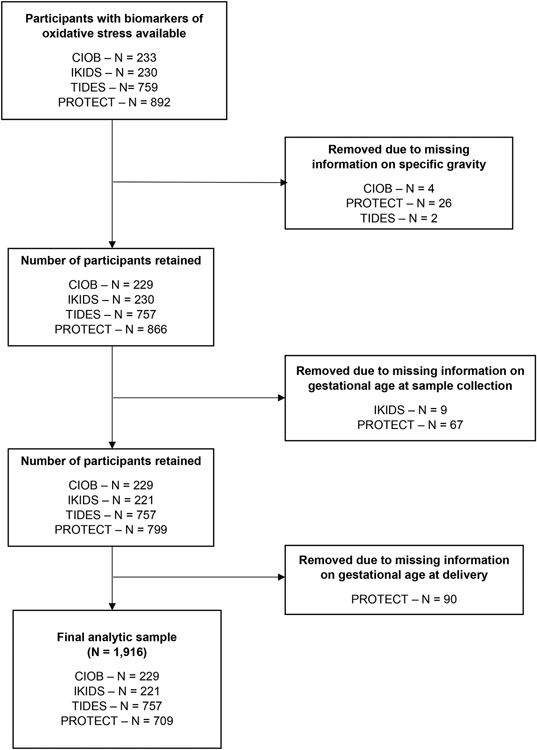
Our study population included four cohorts participating in the National Institutes of Health Environmental influences on Child Health Outcomes (ECHO) consortium: Chemicals in Our Bodies (CIOB– San Francisco, CA),38 Illinois Kids Development Study (IKIDS–Champaign-Urbana, IL),38 Puerto Rico Testsite for Exploring Contamination Threats (PROTECT),39 and The Infant Development and Environment Study (TIDES – San Francisco, CA, Rochester, NY, Seattle, WA, and Minneapolis, MN).40 Detailed information regarding study recruitment, data collection and inclusion criteria is provided elsewhere.38-40 Across all cohorts, English or Spanish speaking participants were recruited during pregnancy and were eligible for inclusion if they were at least 18 years of age and not pregnant with multiples. Recruitment for CIOB, IKIDS, and PROTECT began in 2014, 2013, and 2010, respectively, and is ongoing. Recruitment for TIDES occurred between 2010-2012.38-40 Each cohort was reviewed and approved by the local Institutional Review Board, and all cohort participants provided written informed consent. In the present analysis, we restricted to those with information on oxidative stress and gestational age at delivery and included 1,916 participants (Figure 2), of which 229 were enrolled in CIOB, 221 in IKIDS, 709 in PROTECT, and 757 in TIDES. In CIOB and IKIDS, oxidative stress biomarkers were obtained for all PTBs and a random sample of full-term births. Among the full- term births, we measured oxidative stress among those who had complete covariate data and who were demographically similar to those included in the larger study.38
Figure 2. Flowchart indicating participant selection into final analytic sample.
CIOB, Chemicals in Our Bodies; IKIDS, Illinois Kids Development Study; PROTECT, Puerto Rico Testsite for Exploring Contamination Threats; TIDES, The Infant Development and Environment Study.
Oxidative Stress Biomarkers
Urine samples were collected at three time points in PROTECT (mean 18.2, 23.6, and 26.9 weeks gestation), two time points in both CIOB (mean 20.2 and 31.0 weeks gestation) and IKIDS (mean 16.2 and 23.3 weeks gestation), and once in TIDES (mean 32.7 weeks of gestation). Samples were frozen at −80°C prior to analysis of oxidative stress biomarkers. The Eicosanoid Core Laboratory at Vanderbilt University Medical Center quantified the levels of 8-iso-PGF2α, F2-IsoP-M, and PGF2α using stable isotope dilution gas chromatography-negative ion chemical ionization–mass spectrometry (GC/MS) or liquid chromatography-mass spectrometry (LC/MS).22 Briefly, for the GC/MS analysis, urine was extracted via C-18 Sep-Pak cartridges (Waters Corporation, Milford, MA, USA) prior to derivatization to the pentafluorobenzyl-ester, as previously described.22 The LC/MS procedure is detailed in supplementary material.
The ratio of 8-iso-PGF2α/PGF2α was used to estimate non-enzymatic vs. enzymatic formation of this biomarker.35 The chemical fraction captures non-enzymatic lipid peroxidation as a result of oxidative stress while the enzymatic fraction is generated from prostaglandin-endoperoxide synthases (or COX) and is reflective of inflammation.35
To account for urinary dilution, oxidative stress biomarkers were corrected for specific gravity (SpG), as previously described.18 Oxidative stress biomarker concentrations below the limit of detection (LOD; 0.05 ng/mL) were imputed using . All SpG-corrected biomarker concentrations were right skewed and natural log transformed for analyses.
Preterm Birth
Gestational age at birth was ascertained through a combination of self-reported date of last menstrual period and first ultrasound estimates in PROTECT,39 best estimated gestational age at delivery from the medical record in CIOB,38 a combination of maternal ultrasound and infant date of birth in IKIDS,38 and from the first available ultrasound or physician’s best estimate of gestational age at birth in TIDES.37 PTB subtypes included early preterm (<32 completed weeks gestation), moderate preterm (32 to <34 completed weeks gestation), and late preterm (34 to <37 completed weeks gestation).41 Births were classified as being of spontaneous origin if the medical record indicated spontaneous labor (CIOB) or if PROM, spontaneous preterm labor, or both were present (PROTECT and TIDES).37-39 Mothers self-reported whether the birth was spontaneous in IKIDS.38 PTBs that were medically indicated or of unknown origin were classified as being non-spontaneous.
Statistical Analysis
We examined the distribution of demographic characteristics among the overall study population. The distribution of measured and derived oxidative stress biomarkers was assessed using geometric means, geometric standard deviations (SDs), and selected percentiles. Spearman correlation coefficients were used to examine correlations between oxidative stress biomarkers and gestational age. Generalized additive mixed models with a random intercept for participant ID and a smoothing term for gestational age at sample collection were used to graphically depict levels of oxidative stress biomarkers across gestation.
We calculated geometric mean biomarker concentrations for participants with repeated measurements available. If only one measurement was available for the participant, we used only that measure. For participants with multiple exposure measurements, we calculated intraclass correlation coefficients (ICC) to examine variability in biomarker concentrations across gestation.42
Logistic regression was used to examine the relationship between oxidative stress biomarkers and PTB. Oxidative stress biomarkers were treated as continious measures, natural log transformed, and standardized to the population’s interquartile range (IQR), which corresponds to an increase from the 25th to 75th percentile, equating to an increase of 1.03, 0.71, and 2.1 ng/mL for 8-iso-PGF2α, F2-IsoP-M, and PGF2α, respectively. This was done for consistency with prior studies and to increase interperatblity,26-27-37 as we felt that an IQR increase repeated a more meaningful change as opposed to a one ng/mL increase. We additionally examined spontaneous PTB, non-spontaneous PTB, and PTB categories (early/moderate vs. late) as separate outcomes. Early and moderate PTBs were combined due to the small number of early PTBs (N=19 early PTBs). Covariates included an indicator for cohort, average gestational weeks at sample collection, maternal age, maternal educational attainment, marital status, and pre-pregnancy body mass index (BMI). These confounders were chosen based on their known associations with oxidative stress and PTB in the literature and in our study population.16-43-44
We conducted several sensitivity analyses. To assess non-linear relationships, we examined quartiles of oxidative stress in relation to all PTB outcomes. We also assessed the relationship between oxidative stress biomarkers and continuous gestational age using linear regression. We additionally examined the relationship between oxidative stress and all PTBs stratified by infant sex, as prior work suggests that antioxidant status and oxidative stress levels may differ by fetal sex.45-46 Given that our study design involved heterogenous birth cohorts, we applied four complimentary approaches to determine whether any of our results could be attributable to underlying differences in individual cohorts. First, we examined adjusted associations between oxidative stress biomarkers and all PTBs stratified by cohort. Second, we conducted a meta-analysis of the individual cohort-specific estimates to compare with the combined sample analysis.47-48 Third, to determine if associations were driven by specific cohorts, we included an interaction term for cohort*oxidative stress biomarkers in the models that included all PTBs as an outcome. Finally, to address the influence of cohort heterogeneity on our results, we conducted a leave-one-out analysis removing one cohort at a time.
RESULTS
Most participants in our analytic sample had normal pre-pregnancy BMI (52%) and self-identified as White (40%) or Latina (44%) (Table 1). IKIDS had the highest percentage of White participants (81%) while PROTECT had the highest percentage of Latinas (100%) (Table 1). In the overall sample, 11% of births were preterm and 6% of births were spontaneous preterm. Mothers who delivered preterm compared with term were more likely to have completed some college or less and be unmarried (Supplemental Table 1).
Table 1.
Distribution of demographic characteristics and pregnancy outcomes in the overall study population and stratified by individual cohort.
| Overall (N=1,916) |
CIOB (N=229) |
IKIDS (N=221) |
PROTECT (N=709) |
TIDES (N=757) |
|
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Preterm birth | |||||
| No | 1708 (89%) | 189 (83%) | 196 (89%) | 627 (88%) | 696 (92%) |
| Yes | 208 (11%) | 40 (17%) | 25 (11%) | 82 (12%) | 61 (8%) |
| Maternal age (years) | |||||
| 18-24 | 405 (21%) | 23 (10%) | 14 (6%) | 269 (37%) | 99 (13%) |
| 25-29 | 476 (25%) | 26 (11%) | 73 (33%) | 220 (31%) | 157 (21%) |
| 30-34 | 580 (30%) | 86 (38%) | 105 (48%) | 142 (21%) | 247 (33%) |
| ≥35 | 412 (22%) | 94 (41%) | 29 (13%) | 78 (11%) | 211 (28%) |
| Missing | 43 (2.2%) | 0 (0%) | 0 (0%) | 0 (0%) | 43 (5.7%) |
| Pre-pregnancy BMI (kg/m2) | |||||
| Underweight | 75 (4%) | 6 (3%) | 6 (3%) | 47 (7%) | 16 (2%) |
| Normal | 991 (52%) | 116 (51%) | 120 (54%) | 331 (47%) | 424 (56%) |
| Overweight | 436 (23%) | 59 (26%) | 43 (19%) | 174 (25%) | 160 (21%) |
| Obese | 360 (19%) | 39 (17%) | 48 (22%) | 125 (18%) | 148 (20%) |
| Missing | 54 (2.8%) | 9 (3.9%) | 4 (1.8%) | 32 (4.5%) | 9 (1.2%) |
| Maternal race/ethnicity | |||||
| White | 772 (40%) | 102 (45%) | 179 (81%) | <5 | 490 (65%) |
| Black | 114 (6%) | 13 (6%) | 11 (5%) | <5 | 90 (12%) |
| Asian/Pacific Islander | 108 (6%) | 36 (16%) | 14 (6%) | <5 | 58 (8%) |
| Latina | 849 (44%) | 70 (31%) | 4 (2%) | 707 (100%) | 68 (9%) |
| Other/Multi-Racial | 49 (3%) | 8 (3%) | 13 (6%) | <5 | 28 (4%) |
| Missing | 24 (1.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 23 (3.0%) |
| Maternal education | |||||
| <High school | 126 (7%) | 24 (10%) | <5 | 42 (6%) | 59 (8%) |
| High school or some college | 417 (22%) | 55 (24%) | 30 (14%) | 193 (27%) | 139 (18%) |
| ≥College degree | 752 (39%) | 55 (24%) | 81 (37%) | 381 (54%) | 235 (31%) |
| Graduate | 610 (32%) | 93 (41%) | 109 (49%) | 90 (13%) | 318 (42%) |
| Missing | 11 (0.6%) | 2 (0.9%) | 0 (0%) | 3 (0.4%) | 6 (0.8%) |
| Marital status | |||||
| Married | 1266 (66%) | 167 (73%) | 201 (91%) | 371 (52%) | 527 (70%) |
| Living together | 351 (18%) | 40 (17%) | 10 (5%) | 198 (28%) | 103 (14%) |
| Single | 292 (15%) | 20 (9%) | 10 (5%) | 137 (19%) | 125 (17%) |
| Missing | 7 (0.4%) | 2 (0.9%) | 0 (0%) | 3 (0.4%) | 2 (0.3%) |
| Parity | |||||
| 0 | 862 (45%) | 126 (55%) | 85 (38%) | 261 (37%) | 390 (52%) |
| 1+ | 896 (47%) | 98 (43%) | 136 (62%) | 342 (48%) | 320 (42%) |
| Missing | 158 (8.2%) | 5 (2.2%) | 0 (0%) | 106 (15.0%) | 47 (6.2%) |
| Current smoker | |||||
| No | 1772 (92%) | 227 (99%) | 217 (98%) | 694 (98%) | 634 (84%) |
| Yes | 73 (4%) | 0 (0%) | <5 | 13 (2%) | 56 (7%) |
| Missing | 71 (3.7%) | 2 (0.9%) | 0 (0%) | 2 (0.3%) | 67 (8.9%) |
Preterm birth includes all births occurring prior to 37 weeks of gestation. Non-spontaneous preterm b includes all preterm births that were of non-spontaneous origin (medically indicated or unknown). Early/moderate preterm birth includes birth occurring prior to 34 completed weeks of gestation. Late preterm birth includes births occurring between 34 and 36 completed weeks of gestation. BMI, body mass index. CIOB, Chemicals in Our Bodies; IKIDS, Illinois Kids Development Study; LOD, limit of detection; PROTECT, Puerto Rico Testsite for Exploring Contamination Threats; SD, standard deviation; TIDES, The Infant Development and Environment Study.
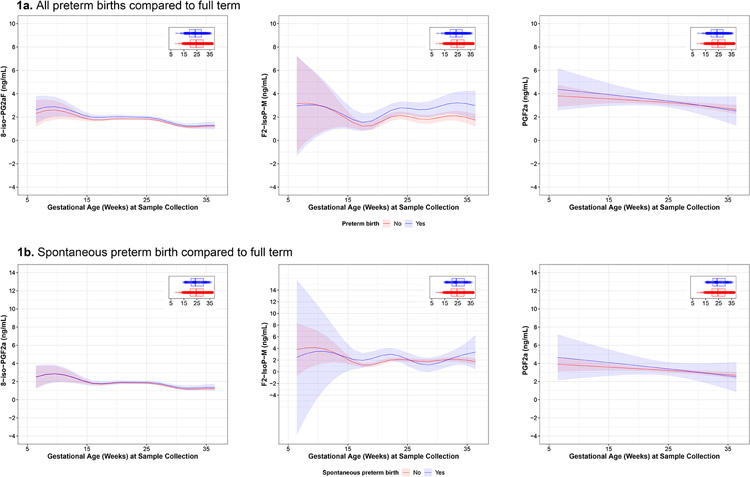
The geometric mean biomarker concentration was highest for PGF2α (geometric mean=2.19 ng/mL [geometric SD=2.15]), followed by 8-iso-PGF2α (geometric mean=1.19 ng/mL [geometric SD=1.95]) and F2-IsoP-M (geometric mean=0.81 ng/mL [geometric SD=2.69]) (Supplemental Table 2). The concentration of F2-IsoP-M was highest among CIOB participants (1.80 ng/mL), whereas the concentrations of 8-iso-PGF2α and PGF2α were highest among PROTECT participants (1.81 ng/mL and 2.78 ng/mL, respectively) (Supplemental Table 2). The ICC value for 8-iso-PGF2α was 0.54 (95% confidence interval [CI]=0.50, 0.58). ICC values for F2-IsoP-M and PGF2α indicated greater variability (ICC=0.23, 95% CI=0.17, 0.29 and ICC=0.11, 95% CI= 0.05, 0.17, respectively). When visually examining the levels of 8-iso-PGF2α and PGF2α across gestation, we observed slight decreases towards the end of pregnancy. Compared with those who delivered full term, the levels of F2-IsoP-M in those with PTBs were elevated beginning in mid-pregnancy (Figure 3). Gestational age at delivery was negatively correlated with all oxidative stress biomarkers (Supplemental Figure 1). The strongest correlation observed between measured oxidative stress biomarkers was between 8-iso-PGF2α and PGF2α (Spearman ρ=0.59).
Figure 3.
Predicted values (95% confidence intervals) of specific gravity–corrected urinary oxidative stress biomarker concentrations by gestational age at sample collection in all preterm, spontaneous preterm only, and full-term births obtained from generalized additive mixed models with a random intercept for participant ID.
IQR increases in 8-iso-PGF2α and F2-IsoP-M were associated with higher odds of all PTBs after adjustment for confounders (odds ratio [OR]=1.19, 95% CI=0.94, 1.50; OR=1.29, 95% CI 0=1.11, 1.51, respectively) (Table 2). When spontaneous PTB was the outcome, the effect of F2-IsoP-M was greater in magnitude (OR=1.47, 95% CI 0=1.16, 1.90). When examining categories of PTB, an IQR increase in any of the biomarkers was associated with higher odds of early or moderately preterm delivery, with PGF2α and the enzymatic fraction, which reflect inflammatory pathways, being the greatest in magnitude (Table 2). Additional adjustment for smoking status or alcohol consumption did not meaningfully change effect estimates.
Table 2.
Odds ratios (95% confidence intervals) for preterm birth, spontaneous preterm birth, non-spontaneous preterm birth, and preterm birth subtypes in association with an interquartile range increase in averaged concentrations of urinary specific gravity–corrected oxidative stress biomarkers (ng/mL) within the overall sample and in a meta-analysis of cohort-specific estimates.
| Unadjusted | Adjusted | Meta-analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| N (PTB, FTB) |
OR | (95% CI) | N (PTB, FTB) |
OR | (95% CI) | OR | (95% CI) | |
| All preterm births | ||||||||
| Measured | ||||||||
| 8-iso-PGF2α | (208, 1708) | 1.32 | (1.07, 1.64) | (190, 1623) | 1.19 | (0.94, 1.5) | 1.16 | (0.92, 1.40) |
| F2-IsoP-M | (208, 1708) | 1.25 | (1.10, 1.44) | (190, 1623) | 1.29 | (1.11, 1.51) | 1.22 | (1.07, 1.37) |
| PGF2α | (208, 1708) | 1.13 | (0.95, 1.35) | (190, 1623) | 1.04 | (0.86, 1.25) | 1.06 | (0.88, 1.25) |
| Derived | ||||||||
| 8-iso-PGF2α chemical | (208, 1708) | 1.22 | (0.98, 1.51) | (190, 1623) | 1.11 | (0.88, 1.41) | 1.08 | (0.84, 1.32) |
| 8-iso-PGF2α enzymatic | (208, 1708) | 1.02 | (0.86, 1.20) | (190, 1623) | 0.98 | (0.83, 1.16) | 1.00 | (0.82, 1.18) |
| Spontaneous preterm birth | ||||||||
| Measured | ||||||||
| 8-iso-PGF2α | (112, 1708) | 1.21 | (0.91, 1.61) | (99, 1623) | 1.17 | (0.86, 1.61) | 1.13 | (0.80, 1.45) |
| F2-IsoP-M | (112, 1708) | 1.24 | (1.03, 1.51) | (99, 1623) | 1.47 | (1.16, 1.90) | 1.38 | (1.14, 1.63) |
| PGF2α | (112, 1708) | 1.19 | (0.94, 1.51) | (99, 1623) | 1.13 | (0.88, 1.45) | 1.12 | (0.86, 1.38) |
| Derived | ||||||||
| 8-iso-PGF2α chemical | (112, 1708) | 1.04 | (0.79, 1.38) | (99, 1623) | 1.02 | (0.75, 1.40) | 0.98 | (0.67, 1.29) |
| 8-iso-PGF2α enzymatic | (112, 1708) | 1.14 | (0.91, 1.46) | (99, 1623) | 1.13 | (0.89, 1.46) | 1.13 | (0.88, 1.39) |
| Non-spontaneous preterm birth | ||||||||
| Measured | ||||||||
| 8-iso-PGF2α | (96, 1708) | 1.48 | (1.09, 1.99) | (91, 1623) | 1.22 | (0.88, 1.69) | 1.19 | (0.86, 1.52) |
| F2-IsoP-M | (96, 1708) | 1.27 | (1.06, 1.53) | (91, 1623) | 1.18 | (0.98, 1.42) | 1.10 | (0.91, 1.29) |
| PGF2α | (96, 1708) | 1.08 | (0.85, 1.39) | (91, 1623) | 0.96 | (0.74, 1.25) | 1.01 | (0.74, 1.27) |
| Derived | ||||||||
| 8-iso-PGF2α chemical | (96, 1708) | 1.49 | (1.09, 2.05) | (91, 1623) | 1.24 | (0.89, 1.74) | 1.20 | (0.85, 1.55) |
| 8-iso-PGF2α enzymatic | (96, 1708) | 0.90 | (0.72, 1.14) | (91, 1623) | 0.85 | (0.68, 1.07) | 0.87 | (0.63, 1.11) |
| Early/Moderate preterm | ||||||||
| Measured | ||||||||
| 8-iso-PGF2α | (42, 1708) | 1.61 | (1.06, 2.44) | (34, 1623) | 1.36 | (0.81,2.28) | 1.22 | (0.72, 1.72) |
| F2-IsoP-M | (42, 1708) | 1.17 | (0.95, 1.48) | (34, 1623) | 1.37 | (1.03, 1.9) | 1.21 | (0.91, 1.51) |
| PGF2α | (42, 1708) | 1.83 | (1.25, 2.68) | (34, 1623) | 1.48 | (0.96, 2.31) | 1.60 | (1.16, 2.05) |
| Derived | ||||||||
| 8-iso-PGF2α chemical | (42, 1708) | 1.33 | (0.86, 2.07) | (34, 1623) | 1.19 | (0.71, 2.03) | 0.98 | (0.47, 1.49) |
| 8-iso-PGF2α enzymatic | (42, 1708) | 1.83 | (1.17, 3.09) | (34, 1623) | 1.47 | (0.94, 2.49) | 1.65 | (1.13, 2.17) |
| Late preterm birth | ||||||||
| Measured | ||||||||
| 8-iso-PGF2α | (165, 1708) | 1.25 | (0.98, 1.59) | (155, 1623) | 1.16 | (0.89, 1.5) | 1.13 | (0.86, 1.39) |
| F2-IsoP-M | (165, 1708) | 1.31 | (1.11, 1.55) | (155, 1623) | 1.26 | (1.07, 1.51) | 1.21 | (1.03, 1.39) |
| PGF2α | (165, 1708) | 1.01 | (0.83, 1.22) | (155, 1623) | 0.97 | (0.79, 1.19) | 0.97 | (0.76, 1.18) |
| Derived | ||||||||
| 8-iso-PGF2α chemical | (165, 1708) | 1.18 | (0.93, 1.51) | (155, 1623) | 1.09 | (0.85, 1.42) | 1.08 | (0.81, 1.34) |
| 8-iso-PGF2α enzymatic | (165, 1708) | 0.91 | (0.77, 1.09) | (155, 1623) | 0.91 | (0.76, 1.1) | 0.92 | (0.73, 1.11) |
Note: Unadjusted models are minimally adjusted for cohort and averaged gestational age (weeks) at sample collection. Adjusted models include maternal age, education, marital status, pre-pregnancy body mass index, averaged gestational age (weeks) at sample collection, and cohort. Early/moderate preterm birth models were not adjusted for smoking status due to a small number of smokers in this group. Preterm birth includes all births occurring prior to 37 weeks of gestation. Non-spontaneous preterm birth includes all preterm births that were of non-spontaneous origin (medically indicated or unknown). Early/moderate preterm birth includes birth occurring prior to 34 completed weeks of gestation. Late preterm birth includes births occurring between 34 and 36 completed weeks of gestation. CI, confidence interval; FTB, full-term birth; OR, odds ratio; PTB, preterm birth.
When continuous gestational age at delivery was the outcome, an IQR increase in 8-iso-PGF2α and F2-IsoP-M was associated with shorter gestational age (Supplemental Table 3). When stratifying by infant sex, the association between 8-iso-PGF2α and all PTBs persisted only among females, although CIs were wide (Supplemental Table 4). When examining quartiles of oxidative stress biomarkers in relation to all and spontaneous PTBs, associations were generally greater in magnitude when comparing the upper quartile to the lowest quartile, suggesting evidence of a dose response (Supplemental Table 5).
In our sensitivity analysis allowing for interaction between cohort and oxidative stress, we observed no evidence of interaction (Supplemental Table 6). In analyses stratified by cohort, the relationship between 8-iso-PGF2α and all PTBs was strongest for the PROTECT cohort (OR=1.57, 95% CI 0=0.95, 2.61), whereas the relationship between all PTBs and F2-IsoP-M was strongest for the TIDES cohort (OR=1.65, 95% CI= 0.99, 2.71) (Supplemental Table 7).26-37 Results from our meta-analysis were similar to the pooled combined cohort analysis (Table 2). The OR estimates were similar in the leave-one-out analysis, although CIs were less precise (Supplemental Table 8).
COMMENT
Principal Findings
Our pooled cohort study examined the association between oxidative stress and PTB. We observed that F2-IsoP-M concentrations, averaged across pregnancy, were positively associated with odds of PTB. The association was greater in magnitude for PTB of spontaneous origin. 8-iso-PGF2α, the parent compound, was similarly associated with modestly higher odds of delivering preterm, particularly delivering prior to 34 weeks of completed gestation. A growing body of evidence links 8-iso-PGF2α to socioeconomic disadvantage, environmental chemical exposures, and psychosocial stress.1-14-16,18,30,49,50 Qur findings support the hypothesis that oxidative stress is one pathway by which chemical and non-chemical exposures may lead to PTB.15,51-53
Results in the Context of What is Known
In preliminary analyses within a subset of the PROTECT cohort, as well as within the TIDES cohort, we observed that higher concentrations of 8-iso-PGF2α and F2-IsoP-M were associated with higher odds of all and spontaneous PTBs.26-37 The present study confirms these findings with a much larger sample size. Our findings are also supported by studies conducted in other research groups. A Chicago-based study of 237 preterm infants without congenital anomalies found that cord blood levels of 8-iso-PGF2α were elevated among those who delivered prior to 28 weeks completed gestation relative to those who were bom between 34 and 36 weeks.54 This suggests that 8-iso-PGF2α may cross the placenta, which is supported by our finding that levels of 8-iso-PGF2α decreased slightly across gestation. Additionally, a study conducted among infants suspected of having patent ductus arteriosus found that plasma 8-iso-PGF2α levels measured 24-48 hours post-birth were higher among infants born prior to 32 weeks gestation relative to those bom at term.55 In prior work in LIFECODES, a predominately white and high socioeconomic status birth cohort in Boston, higher concentrations of urinary 8-iso-PGF2α, averaged across the first and second trimesters, were associated with higher odds of all PTBs and spontaneous PTB.27 In that study, ORs were greater in magnitude when 8-iso-PGF2α was measured at median 18 and 26 weeks, relative to 10 weeks.27 OR estimates for averaged oxidative stress were also larger in that study than what we observed (for all PTBs, OR=2.22 [95% CI 0=1.47, 3.36] vs. 0R=1.19 [95% CI 0=0.94, 1.5]).27 Differences may be due to statistical power, as the CIs in our study were more precise and we had a larger number of cases (126 versus 208 cases of PTBs).27 While the effect size in our study was smaller, our findings indicate that the positive association between elevated oxidative stress and PTB persists across racial, ethnic, and socioeconomic groups. Other studies have also suggested that oxidative stress biomarkers are associated with a higher risk of PTB or shorter gestational duration.2,56-58
A novel aspect of our study is that we used a ratio formula of 8-iso-PGF2α to PGF2α to determine the amount of 8-iso-PGF2α derived from oxidative stress and inflammation pathways (i.e., the chemical and enzymatic fractions, respectively).35 This represents an advancement over prior studies that have interpreted 8-iso-PGF2α solely as an indicator of oxidative stress. We observed that elevated levels of the enzymatic fraction were associated with higher odds of early or moderate PTB and lower odds of non-spontaneous PTB. This may suggest differential mechanisms linking oxidative stress and inflammation to PTB subtypes, including early versus late preterm delivery.
Clinical Implications
We observed that an IQR increase in 8-iso-PGF2α and F2-IsoP-M were associated with a 17% and 47% increase in odds of spontaneous PTB, respectively. These estimates are similar in magnitude to what has been observed for other known risk factors for spontaneous PTB, including infection (OR=1.23, 95% CI= 0.96, 1.57)59 and prior preterm delivery (absolute risk= 25%, 95% CI= 24%, 26%).60 In addition to acting as a precursor to spontaneous labor,1 oxidative stress also leads to apoptosis, DNA damage, and shorted telomere length, potentially leading to PROM.56-61 Elevated oxidative stress can also increase the risk of infection and placental dysfunction.56 Early indicators of PTB, such as intrauterine infection, may lead to the production of pro-inflammatory cytokines and ultimately the release of free radicals, inducing oxidative stress.62 PGF2α, which is more reflective of underlying inflammatory processes, may also lead to PTB through increased cytokine production, as PGF2α is stimulated in response to proinflammatory cytokines, which promote cervical ripening and stimulate uterine contractions.1,33,34,63
While the goal of our study was to examine associations between continious measures of oxidative stress biomarkers and PTB, an important next step of this work is to determine cut points for high and low oxidative stress, which would allow us to evaluate the diagnostic performance of these biomarkers. Prior work assessing the prediction of eicosanoids and oxidative stress biomarkers within the LIFECODES cohort that found many lipid biomarkers (53 eicosanoids) were needed to have modest prediction accuracy (area under the receiver operating curve (AUC)> 0.7).64 In comparison, AUC values for individual oxidative stress biomarkers were low (AUC = 0.52 for 8-OH-dG and AUC = 0.64 for protein oxidation markers).64 In light of this prior work,64 and the limited number of oxidative stress biomarkers used in our analysis, the implications of our results for clinical and upstream diagnostic applications remain uncertain. Therefore, future analyses of a broader scope of oxidative stress biomarkers should assess how their combined diagnostic performance varies across trimesters, which may enable clinicians to identify high risk individuals early in pregnancy and allocate them to potential treatments, such as supplemental progesterone.65
Strengths and Limitations
An important strength of our study is that we leveraged four prospective cohorts, increasing the generalizability of our findings. Our sample was composed of nearly 2,000 births (including 60% non-White) spanning a wide array of socioeconomic strata. We were also able to examine different categories of PTB and assess spontaneous and non-spontaneous PTB as separate outcomes. This is particularly important, as we observed the strongest effects for spontaneous and early or moderate PTB. Additionally, F2-IsoPs are considered to be the “gold standard” biomarkers of oxidative stress, as they indicate endogenous lipid peroxidation, are not confounded by dietary lipid intake, and measurement does not require fasting.66-67 On a population level, 8-iso-PGF2α levels in a spot urine sample are similar to what is observed with a 24-hour fasting sample.68 Finally, in addition to 8-iso-PGF2α, we examined two other biomarkers of oxidative stress and inflammation, F2-IsoP-M and PGF2α, which we observed to be strongly associated with spontaneous and early or moderate PTB, respectively.
We also acknowledge this study’s limitations. First, for participants with repeated biomarker measurements, the geometric mean biomarker concentration was used as our primary independent variable. While this does allow us to create a more stable estimate of oxidative stress across pregnancy, it limits our ability to look at windows of susceptibility. Additionally, our results may be subject to selection bias, as those who delivered preterm were missing an oxidative stress measurement at later study visits. However, any bias is likely to be minimal and would likely drive results toward the null value, as only those who delivered early preterm (N=19) could possibly have missed subsequent study visits. For those participants who delivered moderate and late preterm, none were missing oxidative stress measurements. While our study population spanned a wide array geographic groups, our analytic sample included few participants who were not college-educated and a small proportion of African Americans, which may limit generalizability to these groups. Additionally, assessment of spontaneous PTB varied across cohorts, which may have resulted in outcome misclassification. Lastly, information on prior PTB, cervical insufficiency, and use of supplemental progesterone was unavailable in our study population. Thus, we were unable to assess their associations with oxidative stress biomarkers and PTB.
Research Implications and Conclusions
We observed that elevated oxidative stress biomarkers were positively associated with PTB. Particularly, we found levels of F2-IsoP-M to be higher among those who delivered spontaneously preterm. PGF2α, which is more reflective of underlying inflammatory processes, was most strongly associated with early and moderate PTB, suggesting that the underlying mechanisms linking oxidative stress and inflammation to PTB differ by preterm clinical subtype. While randomized trials assessing the impact of antioxidant supplementation and subsequent birth outcomes have produced negative results,69-70 it is possible that antioxidant supplementation is non-specific in the sense that it might not affect F2-IsoPS. Importantly, upstream systems level changes (e.g. increased access to healthy foods and to safe and walkable neighborhoods) may also reduce the burden of stressors and promote healthy behaviors, such as achieving a healthy weight prior to and during pregnancy, undertaking physical activity, and limiting alcohol consumption, which in turn may reduce chronic inflammation and oxidative stress7,18-43-71 Future research should focus on examining oxidative stress as a mediating pathway linking environmental chemical and social stressor exposures to PTB and identifying modifiable risk factors for elevated oxidative stress, which could inform clinical practice, including the early identification of at-risk pregnancies.
Supplementary Material
AJOG at a Glance.
A. Elevated oxidative stress has been associated with preterm birth. However, prior studies have been limited by small sample size and were conducted in primarily White populations, which does not reflect the diversity in the United States.
B. Oxidative stress, as measured by 8-iso-prostaglandin-F2α, 2,3-dinor-5,6-dihydro-8-iso-prostaglandin-F2α, and prostaglandin F2α in urine, is associated with increased odds of preterm birth, particularly preterm birth of spontaneous origin and delivery prior to 34 completed weeks of gestation.
C. Among a racially, ethnically, and demographically diverse study population spanning the mainland United States and Puerto Rico, our study supports prior findings that elevated oxidative stress is on the causal pathway to preterm birth. Results from our study indicate that underlying mechanisms linking oxidative stress to preterm birth differ by preterm clinical subtype.
ACKNOWLEDGEMENTS
The authors wish to thank our ECHO colleagues, the medical, nursing, and program staff, as well as the children and families participating in the ECHO cohorts. We also acknowledge the contribution of the following ECHO program collaborators: ECHO Components – Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Smith PB, Newby KL.
Source of Funding
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) Program, Office of The Director, National Institutes of Health, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), and 5U2COD023375-05 (Opportunities and Infrastructure Fund), UG3OD023251, UH3OD023251, UG3OD023272, UH3OD023272, UG3OD023271, UH3OD023271, UG3023305, and UH3OD023305. This work was additionally supported by the Children’s Environmental Health and Disease Prevention Research Center (ES022848 and RD83543401), T32 National Institutes of Health Institution Training Grant Predoctoral Traineeship in Endocrine, Developmental, and Reproductive Toxicology (ES007326), United States Environmental Protection Agency (RD83543301), National Institute of Environmental Health Sciences (NIEHS) (P42ES017198, P50ES026049, P01ES022841, R01ES02705, R01ES025169, R01ES016863, P30ES019776, P30ES030284, and P30ES005022), and the Intramural Research Program, NIEHS (ZIAES103313). G.L.M. is supported by the Vanderbilt Diabetes Research Center with funding from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-20593). L.G.K. is supported by NIH grant R00ES030403.
Role of the Funding Source
The sponsor had no role in the study design; the collection, analysis, and interpretation of the data; the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors report no conflicts of interest.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
See Acknowledgments for full listing of collaborators
Contributor Information
Stephanie M. Eick, Gangarosa Department of Environmental Health, Emory University Rollins School of Public Health, Atlanta, GA.
Sarah D. Geiger, Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Champaign, IL; Department of Kinesiology and Community Health, University of Illinois at Urbana-Champaign, Champaign, IL.
Akram Alshawabkeh, College of Engineering, Northeastern University, Boston, MA.
Max Aung, Department of Population and Public Health Sciences, Keck School of Medicine, University of Southern California, Los Angeles, CA.
Emily S. Barrett, Environmental and Occupational Health Sciences Institute, Rutgers School of Public Health, Rutgers University, Piscataway, NJ.
Nicole Bush, Department of Psychiatry and Behavioral Sciences, University of California, San Francisco, San Francisco, CA.
Kecia N. Carroll, Departments of Pediatrics and Environmental Medicine and Public Health, The Icahn School of Medicine at Mount Sinai, New York, NY.
José F. Cordero, Department of Epidemiology and Biostatistics, College of Public Health, University of Georgia, Athens, GA.
Dana E. Goin, Program on Reproductive Health and the Environment, University of California, San Francisco, San Francisco, CA.
Kelly K. Ferguson, Epidemiology Branch, National Institute of Environmental Health Sciences, Durham, NC.
Linda G. Kahn, Departments of Pediatrics and Population Health, New York University Grossman School of Medicine, New York, NY.
Donghai Liang, Gangarosa Department of Environmental Health, Emory University Rollins School of Public Health, Atlanta, GA.
John D. Meeker, Department of Environmental Health Sciences, University of Michigan School of Public Health, Ann Arbor, MI.
Ginger L. Milne, Division of Clinical Pharmacology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN.
Ruby H.N. Nguyen, Department of Epidemiology & Community Health, University of Minnesota, Minneapolis, MN.
Amy M. Padula, Program on Reproductive Health and the Environment, University of California, San Francisco, San Francisco, CA.
Sheela Sathyanarayana, Department of Pediatrics, University of Washington, Seattle, WA; Seattle Children’s Research Institute, Seattle, WA.
Kaitlin R. Taibl, Gangarosa Department of Environmental Health, Emory University Rollins School of Public Health, Atlanta, GA.
Susan L. Schantz, Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Champaign, IL; Department of Comparative Biosciences, University of Illinois at Urbana-Champaign, Champaign, IL.
Tracey J. Woodruff, Program on Reproductive Health and the Environment, University of California, San Francisco, San Francisco, CA.
Rachel Morello-Frosch, Program on Reproductive Health and the Environment, University of California, San Francisco, San Francisco, CA; Department of Environmental Science, Policy and Management and School of Public Health, University of California, Berkeley, Berkeley, CA.
Data Availability Statement
The datasets for this manuscript are not publicly available because, per the NIH-approved ECHO Data Sharing Policy, ECHO-wide data have not yet been made available to the public for review/analysis. Requests to access the datasets should be directed to the ECHO Data Analysis Center, ECHO-DAC@rti.org.
REFERENCES
- 1.Welch BM, McNeil EE, Edin ML, Ferguson KK. Inflammation and oxidative stress as mediators of the impacts of environmental exposures on human pregnancy: Evidence from oxylipins. Pharmacology & Therapeutics. Published online April 1, 2022:108181. doi: 10.1016/j.pharmthera.2022.108181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longini M, Perrone S, Vezzosi P, et al. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clinical Biochemistry. 2007;40(11):793–797. doi: 10.1016/j.clinbiochem.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 3.Ferguson KK, Meeker JD, McElrath TF, Mukherjee B, Cantonwine DE. Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies. Am J Obstet Gynecol. 2017;216(5):527.e1–527.e9. doi: 10.1016/j.ajog.2016.12.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joo EH, Kim YR, Kim N, Jung JE, Han SH, Cho HY. Effect of Endogenic and Exogenic Oxidative Stress Triggers on Adverse Pregnancy Outcomes: Preeclampsia, Fetal Growth Restriction, Gestational Diabetes Mellitus and Preterm Birth. International Journal of Molecular Sciences. 2021;22(18). doi: 10.3390/ijms221810122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon R, Fortunato SJ, Milne GL, et al. Amniotic fluid eicosanoids in preterm and term births: effects of risk factors for spontaneous preterm labor. Obstet Gynecol. 2011;118(1):121–134. doi: 10.1097/AOG.0b013e3182204eaa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher JJ, Bartho LA, Perkins AV, Holland OJ. Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy. Clinical and Experimental Pharmacology and Physiology. 2020;47(1):176–184. doi: 10.1111/1440-1681.13172 [DOI] [PubMed] [Google Scholar]
- 7.Zavalza-Gomez AB. Obesity and oxidative stress: a direct link to preeclampsia? Archives of Gynecology and Obstetrics. 2011;283(3):415–422. doi: 10.1007/s00404-010-1753-1 [DOI] [PubMed] [Google Scholar]
- 8.Wang CN, Chen JYS, Sabu S, et al. Elevated amniotic fluid F2-isoprostane: A potential predictive marker for preeclampsia. Free Radical Biology and Medicine. 2011;50(9):1124–1130. doi: 10.1016/j.freeradbiomed.2011.01.022 [DOI] [PubMed] [Google Scholar]
- 9.Hussain T, Murtaza G, Metwally E, et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Jin M, ed. Mediators of Inflammation. 2021;2021:9962860. doi: 10.1155/2021/9962860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erez O, Romero R, Jung E, et al. Preeclampsia and eclampsia: the conceptual evolution of a syndrome. American Journal of Obstetrics and Gynecology. 2022;226(2, Supplement):S786–S803. doi: 10.1016/j.ajog.2021.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cappelletti M, Presicce P, Kallapur SG. Immunobiology of Acute Chorioamnionitis. Frontiers in Immunology. 2020; 11. https://www.frontiersin.org/articles/10.3389/fimmu.2020.00649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redline RW, Roberts DJ, Parast MM, et al. Placental pathology is necessary to understand common pregnancy complications and achieve an improved taxonomy of obstetrical disease. American Journal of Obstetrics and Gynecology. Published online August 13, 2022. doi: 10.1016/j.ajog.2022.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Trejo M, Montoya-Estrada A, Torres-Ramos Y, et al. Oxidative stress biomarkers and their relationship with cytokine concentrations in overweight/obese pregnant women and their neonates. BMC Immunology. 2017;18(1):3. doi: 10.1186/s12865-016-0184-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cathey AL, Eaton JL, Ashrap P, et al. Individual and joint effects of phthalate metabolites on biomarkers of oxidative stress among pregnant women in Puerto Rico. Environment International. 2021; 154:106565. doi: 10.1016/j.envint.2021.106565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson KK, Chin HB. Environmental chemicals and preterm birth: Biological mechanisms and the state of the science. Curr Epidemiol Rep. 2017;4(1):56–71. doi: 10.1007/s40471-017-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eick SM, Meeker JD, Brown P, et al. Associations between socioeconomic status, psychosocial stress, and urinary levels of 8-iso-prostaglandin-F(2a) during pregnancy in Puerto Rico. Free Radio Biol Med. 2019;143:95–100. doi: 10.1016/j.freeradbiomed.2019.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irie M, Asami S, Nagata S, Ikeda M, Miyata M, Kasai H. Psychosocial factors as a potential trigger of oxidative DNA damage in human leukocytes. Jpn J Cancer Res. 2001;92(3):367–376. doi: 10.1111/j.1349-7006.2001.tb01104.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eick SM, Geiger SD, Alshawabkeh A, et al. Associations between social, biologic, and behavioral factors and biomarkers of oxidative stress during pregnancy: Findings from four ECHO cohorts. Science of The Total Environment. Published online April 29, 2022:155596. doi: 10.1016/j.scitotenv.2022.155596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skoczynska A, Skoczynska M, Turczyn B, Wojakowska A, Gruszczynski L, Scieszka M. Exposure to Arsenic in the Air and 15-F2t-lsoprostane in Urine in a Sub-population of Inhabitants of a Copper Smelter Region. Exposure and Health. 2021;13(3):403–418. doi: 10.1007/s12403-021-00392-x [DOI] [Google Scholar]
- 20.Palta P, Szanton SL, Semba RD, Thorpe RJ, Varadhan R, Fried LP. Financial strain is associated with increased oxidative stress levels: The Women’s Health and Aging Studies. Geriatric Nursing. 2015;36(2, Supplement):S33–S37. doi: 10.1016/j.gerinurse.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts U 2nd. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87(23):9383–9387. doi: 10.1073/pnas.87.23.9383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2(l):221–226. doi: 10.1038/nprot.2006.375 [DOI] [PubMed] [Google Scholar]
- 23.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of Oxidative Stress Study II: Are oxidation products of lipids, proteins, and DNA markers of CCI4 poisoning? Free Radical Biology and Medicine. 2005;38(6):698–710. doi: 10.1016/j.freeradbiomed.2004.09.017 [DOI] [PubMed] [Google Scholar]
- 24.Milne GL. Classifying oxidative stress by F2-Isoprostane levels in human disease: The re-imagining of a biomarker. Redox Biology. 2017;12:897–898. doi: 10.1016/j.redox.2017.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radio Biol Med. 2000;28(4):505–513. doi: 10.1016/s0891-5849(99)00264-6 [DOI] [PubMed] [Google Scholar]
- 26.Eick SM, Ferguson KK, Milne GL, et al. Repeated measures of urinary oxidative stress biomarkers and preterm birth in Puerto Rico. Free Radic Biol Med. 2020;146:299–305. doi: 10.1016/j.freeradbiomed.2019.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferguson KK, McElrath TF, Chen YH, Loch-Caruso R, Mukherjee B, Meeker JD. Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. Am J Obstet Gynecol. 2015;212(2):208.el–8. doi: 10.1016/j.ajog.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peter Stein T, Scholl TO, Schluter MD, et al. Oxidative stress early in pregnancy and pregnancy outcome, null. 2008;42(10):841–848. doi: 10.1080/10715760802510069 [DOI] [PubMed] [Google Scholar]
- 29.Venkatesh KK, Meeker JD, Cantonwine DE, McElrath TF, Ferguson KK. Association of antenatal depression with oxidative stress and impact on spontaneous preterm birth. J Perinatol. 2019;39(4):554–562. doi: 10.1038/s41372-019-0317-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferguson KK, Chen YFI, VanderWeele TJ, McElrath TF, Meeker JD, Mukherjee B. Mediation of the Relationship between Maternal Phthalate Exposure and Preterm Birth by Oxidative Stress with Repeated Measurements across Pregnancy. Environ Health Perspect. 2017;125(3):488–494. doi: 10.1289/ehp282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin A, Faes C, Debevec T, Rytz C, Millet G, Pialoux V. Preterm birth and oxidative stress: Effects of acute physical exercise and hypoxia physiological responses. Redox Biology. 2018;17:315–322. doi: 10.1016/j.redox.2018.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nature Reviews Immunology. 2015;15(8):511–523. doi: 10.1038/nri3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peiris FIN, Romero R, Vaswani K, et al. Prostaglandin and prostamide concentrations in amniotic fluid of women with spontaneous labor at term with and without clinical chorioamnionitis. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2020;163:102195. doi: 10.1016/j.plefa.2020.102195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddipati KR, Romero R, Chaiworapongsa T, et al. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. The FASEB Journal. 2014;28(ll):4835–4846. doi: 10.1096/fj.14-254383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van‘t Erve TJ, Lih FB, Kadiiska MB, Deterding LJ, Eling TE, Mason RP. Reinterpreting the best biomarker of oxidative stress: The 8-iso-PGF2α/PGF2α ratio distinguishes chemical from enzymatic lipid peroxidation. Free Radical Biology and Medicine. 2015;83:245–251. doi: 10.1016/j.freeradbiomed.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorjgochoo T, Gao YT, Chow WFI, et al. Major metabolite of F2-isoprostane in urine may be a more sensitive biomarker of oxidative stress than isoprostane itself. Am J Clin Nutr. 2012;96(2):405–414. doi: 10.3945/ajcn.112.034918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen EM, van ’t Erve TJ, Boss J, et al. Urinary oxidative stress biomarkers and accelerated time to spontaneous delivery. Free Radic Biol Med. 2019;130:419–425. doi: 10.1016/j.freeradbiomed.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eick SM, Enright EA, Geiger SD, et al. Associations of Maternal Stress, Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFAS), and Demographic Risk Factors with Birth Outcomes and Offspring Neurodevelopment: An Overview of the ECFIO.CA.IL Prospective Birth Cohorts. International Journal of Environmental Research and Public Health. 2021;18(2):742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferguson KK, Rosario Z, McElrath TF, et al. Demographic risk factors for adverse birth outcomes in Puerto Rico in the PROTECT cohort. PLoS One. 2019;14(6):e0217770–e0217770. doi: 10.1371/journal.pone.0217770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swan SFI, Sathyanarayana S, Barrett ES, et al. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod. 2015;30(4):963–972. doi: 10.1093/humrep/deu363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10 Suppl 1(Suppl 1):S2–S2. doi: 10.1186/1742-4755-10-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosner B (Bernard A). Fundamentals of Biostatistics. Seventh edition. Boston: Brooks/Cole, Cengage Learning, [2011] ©2011; 2011. https://search.library.wisc.edu/catalog/9910098055302121 [Google Scholar]
- 43.Eick SM, Barrett ES, van ’t Erve TJ, et al. Association between prenatal psychological stress and oxidative stress during pregnancy. Paediatr Perinat Epidemiol. 2018;32(4):318–326. doi: 10.1111/ppe.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marseglia L, Manti S, D’Angelo G, et al. Oxidative stress in obesity: a critical component in human diseases. IntJMolSci. 2014;16(l):378–400. doi: 10.3390/ijms16010378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Jacobis IT, Vona R, Straface E, et al. Sex differences in blood pro-oxidant status and platelet activation in children admitted with respiratory syncytial virus bronchiolitis: a pilot study. Italian Journal of Pediatrics. 2020;46(1):29. doi: 10.1186/s13052-020-0792-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lien YC, Zhang Z, Cheng Y, et al. Human Placental Transcriptome Reveals Critical Alterations in Inflammation and Energy Metabolism with Fetal Sex Differences in Spontaneous Preterm Birth. International Journal of Molecular Sciences. 2021;22(15). doi: 10.3390/ijms22157899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilier CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–2191. doi: 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmerman E, Aguiar A, Aung MT, et al. Examining the association between prenatal maternal stress and infant non-nutritive suck. Pediatric Research. Published online December 16, 2021. doi: 10.1038/s41390-021-01894-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashrap P, Watkins DJ, Milne GL, et al. Maternal Urinary Metal and Metalloid Concentrations in Association with Oxidative Stress Biomarkers. Antioxidants. 2021;10(1). doi: 10.3390/antiox10010114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eaton JL, Cathey AL, Fernandez JA, et al. The association between urinary glyphosate and aminomethyl phosphonic acid with biomarkers of oxidative stress among pregnant women in the PROTECT birth cohort study. Ecotoxicology and Environmental Safety. 2022;233:113300. doi: 10.1016/j.ecoenv.2022.113300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welch BM, Keil AP, Buckley JP, et al. Associations Between Prenatal Urinary Biomarkers of Phthalate Exposure and Preterm Birth: A Pooled Study of 16 US Cohorts. JAMA Pediatrics. Published online July 11, 2022. doi: 10.1001/jamapediatrics.2022.2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bekkar B, Pacheco S, Basu R, DeNicola N. Association of Air Pollution and Heat Exposure With Preterm Birth, Low Birth Weight, and Stillbirth in the US: A Systematic Review. JAMA Network Open. 2020;3(6):e208243–e208243. doi: 10.1001/jamanetworkopen.2020.8243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goin DE, Gomez AM, Farkas K, et al. Occurrence of fatal police violence during pregnancy and hazard of preterm birth in California. Paediatric and Perinatal Epidemiology. 2021;n/a(n/a). doi: 10.1111/ppe.12753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mestan K, Matoba N, Arguelles L, et al. Cord blood 8-isoprostane in the preterm infant. Early Hum Dev. 2012;88(8):683–689. doi: 10.1016/j.earlhumdev.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inayat M, Bany-Mohammed F, Valencia A, et al. Antioxidants and Biomarkers of Oxidative Stress in Preterm Infants with Symptomatic Patent Ductus Arteriosus. Am J Perinatol. 2015;32(09):895–904. [DOI] [PubMed] [Google Scholar]
- 56.Menon R Oxidative Stress Damage as a Detrimental Factor in Preterm Birth Pathology. Frontiers in Immunology. 2014;5. https://www.frontiersin.org/article/10.3389/fimmu.2014.00567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber D, Stuetz W, Bernhard W, et al. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. European Journal of Clinical Nutrition. 2014;68(2):215–222. doi: 10.1038/ejcn.2013.263 [DOI] [PubMed] [Google Scholar]
- 58.Dhobale M, Joshi S. Altered maternal micronutrients (folic acid, vitamin B-12) and omega 3 fatty acids through oxidative stress may reduce neurotrophic factors in preterm pregnancy. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2011;25:317–323. doi: 10.3109/14767058.2011.579209 [DOI] [PubMed] [Google Scholar]
- 59.Tedesco RP, Galvão RB, Guida JP, et al. The role of maternal infection in preterm birth: evidence from the Brazilian Multicentre Study on Preterm Birth (EMIP). Clinics. 2020;75:e1508. doi: 10.6061/clinics/2020/e1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kazemier B, Buijs P, Mignini L, et al. Impact of obstetric history on the risk of spontaneous preterm birth in singleton and multiple pregnancies: a systematic review. BJOG: An International Journal of Obstetrics & Gynaecology. 2014;121(10):1197–1208. doi: 10.1111/1471-0528.12896 [DOI] [PubMed] [Google Scholar]
- 61.Gavia-García G, Rosado-Pérez J, Arista-Ugalde TL, Aguiñiga-Sánchez I, Santiago-Osorio E, Mendoza-Núñez VM. Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging. Biology. 2021;10(4). doi: 10.3390/biology10040253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stefanovic V, Andersson S, Vento M. Oxidative stress - Related spontaneous preterm delivery challenges in causality determination, prevention and novel strategies in reduction of the sequelae. Free Radical Biology and Medicine. 2019;142:52–60. doi: 10.1016/j.freeradbiomed.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 63.Xu C, Liu W, You X, et al. PGF2α modulates the output of chemokines and pro-inflammatory cytokines in myometrial cells from term pregnant women through divergent signaling pathways. Molecular Human Reproduction. 2015;21(7):603–614. doi: 10.1093/molehr/gav018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aung MT, Yu Y, Ferguson KK, et al. Prediction and associations of preterm birth and its subtypes with eicosanoid enzymatic pathways and inflammatory markers. Sci Rep. 2019;9(1):17049–17049. doi: 10.1038/s41598-019-53448-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Norwitz ER, Caughey AB. Progesterone supplementation and the prevention of preterm birth. Reviews in Obstetrics and Gynecology. 2011;4(2):60. [PMC free article] [PubMed] [Google Scholar]
- 66.Gopaul NK, Halliwell B, Änggård EE. Measurement of plasma F2-isoprostanes as an index of lipid peroxidation does not appear to be confounded by diet. null. 2000;33(2):115–127. doi: 10.1080/10715760000300671 [DOI] [PubMed] [Google Scholar]
- 67.Richelle M, Turini ME, Guidoux R, Tavazzi I, Métairon S, Fay LB. Urinary isoprostane excretion is not confounded by the lipid content of the diet. FEBS Letters. 1999;459(2):259–262. doi: 10.1016/S0014-5793(99)01259-4 [DOI] [PubMed] [Google Scholar]
- 68.Helmersson J, Basu S. F2-isoprostane and prostaglandin F2αmetabolite excretion rate and day to day variation in healthy humans. Prostaglandins, Leukotrlenes and Essential Fatty Acids (PLEFA). 2001;65(2):99–102. doi: 10.1054/plef.2001.0295 [DOI] [PubMed] [Google Scholar]
- 69.Catov JM, Bodnar LM, Olsen J, Olsen S, Nohr EA. Periconceptional multivitamin use and risk of preterm or small-for-gestational-age births in the Danish National Birth Cohort1–4. The American Journal of Clinical Nutrition. 2011;94(3):906–912. doi: 10.3945/ajcn.111.012393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harville EW, Lewis CE, Catov JM, Jacobs DR Jr, Gross MD, Gunderson EP. A longitudinal study of pre-pregnancy antioxidant levels and subsequent perinatal outcomes in black and white women: The CARDIA Study. PLOS ONE. 2020;15(2):e0229002. doi: 10.1371/journal.pone.0229002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sánchez-Polán M, Silva-Jose C, Franco E, et al. Prenatal Anxiety and Exercise. Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021;10(23). doi: 10.3390/jcm10235501 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets for this manuscript are not publicly available because, per the NIH-approved ECHO Data Sharing Policy, ECHO-wide data have not yet been made available to the public for review/analysis. Requests to access the datasets should be directed to the ECHO Data Analysis Center, ECHO-DAC@rti.org.