Abstract
Objective:
We sought to assess the prevalence and clinical predictors of satellite nodules in patients undergoing lobectomy for clinical stage Ia disease.
Methods:
The National Cancer Database was queried for patients who underwent lobectomy for clinical stage cT1N0 NSCLC. Collaborative staging information was used to identify patients who were pathologically upstaged based on having separate tumor nodules in the same lobe as trhe primary tumor. Multivariable logistic regression was used to assess the association of clinical factors with the detection of separate nodules.
Results:
A separate tumor nodule was recorded in 2.8% (n=1,284) of 45,842 clinical stage Ia patients treated with lobectomy or bilobectomy. Female gender (3.1% vs. male 2.5%; p=0.002) and non-squamous histology (adenocarcinoma 3.2% and large cell neuroendocrine 3.0% vs. squamous cell 1.9% tumors; p<0.001) were associated with the presence of separate nodules. The frequency increased significantly for tumors larger than 3 cm (≤3cm, 3.7% vs. >3cm, 3.8%; p<0.001). Other factors associated with separate nodules were upper lobe location, pleural and/or lymphovascular invasion and occult lymph node disease. The best predictive model for separate nodules based on the available clinical variables resulted in an area under the curve of 0.645 (95% CI 0.629–0.660).
Conclusion:
Separate tumor nodules may be detected with a low but relatively consistent frequency across the spectrum of patients with clinical stage Ia NSCLC. The predictive ability using basic clinical factors in the database is limited.
Keywords: lung cancer, satellite nodule, upstaging, lobectomy
Micro Abstract:
Awareness of satellite lung cancer nodules in the same lobe is important for clinicians considering local treatments for NSCLC. The results of this large national cancer database study show that in clinical stage Ia patients, occult satellite nodules may be encountered at a low frequency across the disease spectrum and few clinical characteristics may aid in their prediction.
1. INTRODUCTION
Intrapulmonary metastases have been reported 8–10% of all patients with operable non-small cell lung cancer (NSCLC), which may change the TNM status and influence therapeutic decisions [1, 2]. Separate tumor nodules with the same histopathological and molecular features as the co-existing dominant lesion are considered satellite nodules. Starting in the seventh and currently in the eighth edition of the TNM staging manual, additional nodules in the same lobe are classified as T3 and ipsilateral nodules in different lobes as T4 [3]. In the absence of N2 disease, their prognosis after resection for T3/T4 tumors with satellite lesions is still comparable to stage IIb or IIIa disease based on tumor size and invasion or lymph node disease, and significantly better that of pulmonary metastases to the contralateral lung (M1a).
Advances in lung imaging with computed tomography (CT) and positron emission tomography (PET-FDG) have undoubtedly improved the accuracy of clinical staging. However, up to half or more (49–89%) of satellite nodules in modern surgical series are still discovered at time of surgery or on surgical pathology [1, 4, 5]. Patients with stage Ia NSCLC are also increasingly considered for sublobar resection which may be oncologically equivalent to lobectomy for well selected small peripheral lesions [6, 7]. Understanding the prevalence of occult satellite nodules in patients with clinical stage Ia disease may therefore be helpful to surgeons when planning the extent of an operation.
This current study was designed to examine the prevalence and predictability of unsuspected separate tumor nodules in patients diagnosed with clinical stage Ia NSCLC undergoing lobectomy using the National Cancer Database (NCDB).
2. PATIENTS AND METHODS
Data Source and Study Population
This study utilized the NCDB, which is the largest nationwide cancer registry. The NCDB is jointly sponsored by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society and captures approximately 70% of all newly diagnosed cancers in the United States from over 1,500 reporting institutions. Due to the de-identified nature of the NCDB, this study was deemed exempt from review by the Institutional Review Board at the Ohio State University.
The NCDB was queried for all patients with clinical stage Ia non-small cell lung cancer diagnosed between years 2004 and 2018. We selected patients with pathologically confirmed invasive non-small cell lung cancer based on ICD-9 histology codes. The study cohort was limited to patients with clinical stage Ia (cT1N0) disease, who had upfront surgery with a pulmonary resection by either lobectomy or bilobectomy. The Collaborative Staging (CS) site-specific factor 1 information was used to identify patients who were found to have a separate tumor nodule in the same lobe of the primary tumor (CS site specific-factor 1: 010 or 030), as previously described [8].
We further excluded patients with incomplete pathologic staging information, unknown CS- Factor 1, and/or N3 disease on pathology. The selection process is summarized in the CONSORT-diagram (Figure 1). Clinical characteristics for analysis included demographics, Charlson-Deyo comorbidity scores, socioeconomic status, and tumor descriptors. Clinical and pathologic stage was recorded using the AJCC 6th and 7th edition TNM-staging manual.
Figure 1.
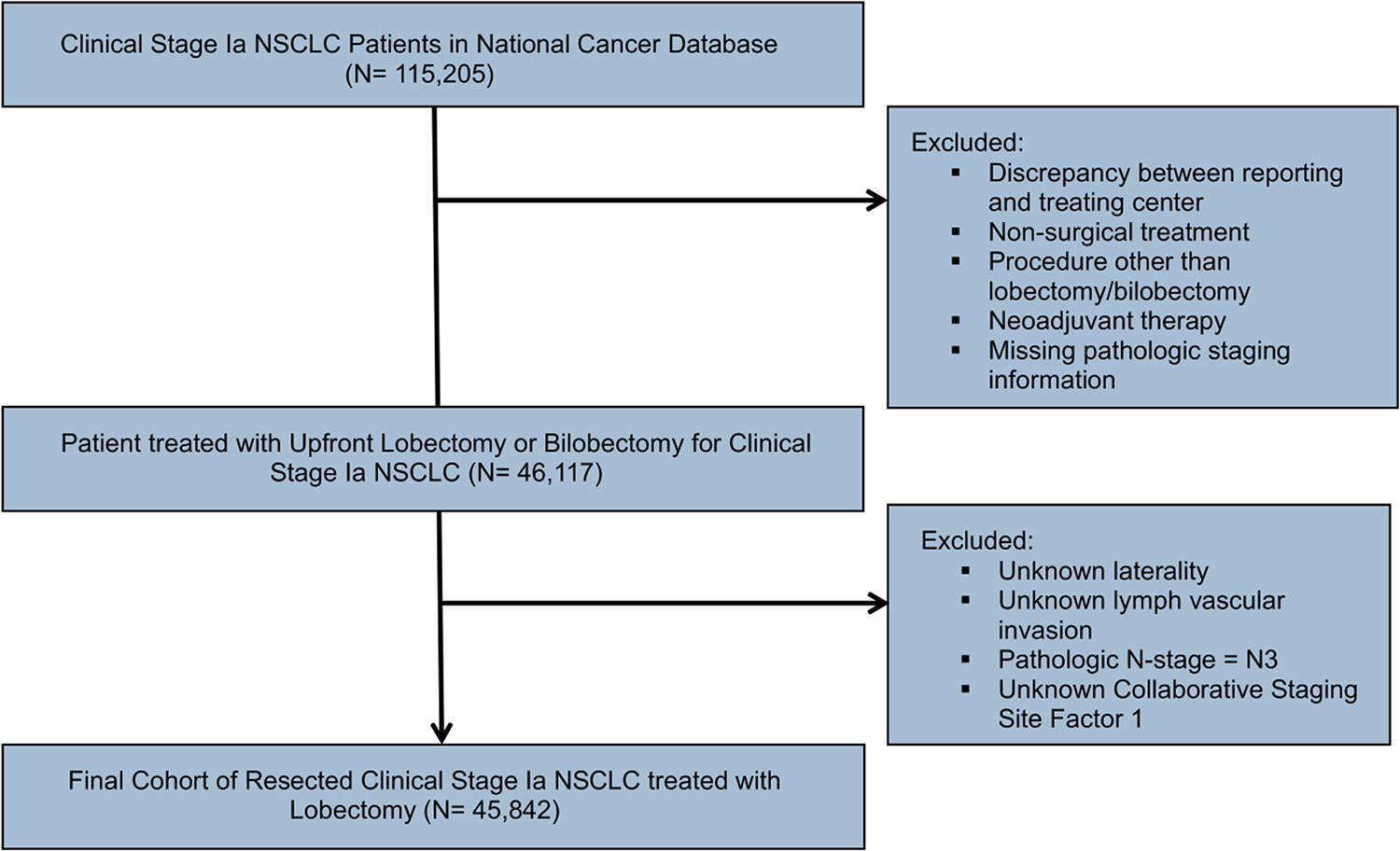
CONSORT flow diagram outlining the patient selection process
Central Image: CT image of a biopsy proven lung adenocarcinoma in the right upper lobe with an indeterminate separate apical small nodule. CONSORT, Consolidated Standards of Reporting Trials; CT, Computed tomography
Study Objective
The primary outcome was presence of a separate ipsilateral lung nodule, in the same lobe of the primary tumor, which was resected.
Statistical Analysis
Summary statistics for demographic, clinical, and surgical outcomes are reported as frequencies (percentage) and tested for association with separate tumor nodules using chi-squared or Fisher’s exact tests where relevant. An informed parsimonious multivariable logistic regression model was fit to predict separate tumor nodules. Model results are reported as odds ratios (95% confidence intervals). Hypothesis testing was conducted at a 5% type I error rate (alpha = 0.05). The area under the receiver operating characteristic curve (AUC) was calculated to measure the aggregate predictive performance of the regression model. The statistical analysis was designed and conducted by an experienced biostatistician (MA) using SAS version 9.4 (SAS Institute, Cary NC).
3. RESULTS
Of 45,842 patients in the NCDB with clinical stage Ia NSCLC who underwent either lobectomy or bilobectomy that met the selection criteria, a total of 1,284 (2.8%) patients were found to have a separate tumor nodule in the same lobe. A summary of demographics and socioeconomic patient characteristics is presented in Table 1. The occurrence of separate tumor nodules varied only slightly based on patient factors. Separate nodules were found more often amongst patients 65+ years of age (3.0% vs. 2.5% <65 years, p=0.010), in female patients (3.1% vs. male 2.5%, p=0.0002), and those with higher Charlson-Deyo comorbidity score of 3 (Table 1).
Table 1:
Demographics of clinical stage Ia NSCLC patients undergoing lobectomy by presence of separate tumor nodules
| Variable | Level | No separate tumor nodules (n=44,558) | Separate tumor nodules in ipsilateral lung (n=1,284) | Total (n=45,842) | p-value |
|---|---|---|---|---|---|
|
| |||||
| Age at Diagnosis | 65+ Years | 27859 (97.05%) | 848 (2.95%) | 28707 (62.62%) | 0.0101 |
| <65 Years | 16699 (97.46%) | 436 (2.54%) | 17135 (37.38%) | ||
|
| |||||
| Sex | Female | 25527 (96.95%) | 802 (3.05%) | 26329 (57.43%) | 0.0002 |
| Male | 19031 (97.53%) | 482 (2.47%) | 19513 (42.57%) | ||
|
| |||||
| Race | Black | 3900 (97.23%) | 111 (2.77%) | 4011 (8.75%) | 0.3747 |
| Other/Unknown | 2104 (97.68%) | 50 (2.32%) | 2154 (4.7%) | ||
| White | 38554 (97.17%) | 1123 (2.83%) | 39677 (86.55%) | ||
|
| |||||
| Charlson-Deyo Score | 0 | 22476 (97.34%) | 614 (2.66%) | 23090 (50.37%) | 0.1598 |
| 1 | 14589 (97.09%) | 437 (2.91%) | 15026 (32.78%) | ||
| 2 | 5225 (97.14%) | 154 (2.86%) | 5379 (11.73%) | ||
| 3 | 2268 (96.63%) | 79 (3.37%) | 2347 (5.12%) | ||
|
| |||||
| Percent No High School Degree | ≥ 21.0% | 6278 (97.48%) | 162 (2.52%) | 6440 (16.15%) | 0.2532 |
| 13.0% – 20.9% | 10404 (97.08%) | 313 (2.92%) | 10717 (26.87%) | ||
| 7.0% – 12.9% | 13156 (97.23%) | 375 (2.77%) | 13531 (33.93%) | ||
| < 7.0% | 8912 (96.97%) | 278 (3.03%) | 9190 (23.05%) | ||
|
| |||||
| Insurance Status | Government/Other | 31016 (97.09%) | 931 (2.91%) | 31947 (69.69%) | 0.0258 |
| Private | 13542 (97.46%) | 353 (2.54%) | 13895 (30.31%) | ||
|
| |||||
| Census Median Income Quartiles (2008–2012) | <$38,000 | 6774 (97.4%) | 181 (2.6%) | 6955 (17.45%) | 0.2136 |
| >=$38,000 | 31964 (97.13%) | 946 (2.87%) | 32910 (82.55%) | ||
|
| |||||
| Residence Area | Metro | 36212 (97.21%) | 1038 (2.79%) | 37250 (83.48%) | |
| Rural | 847 (96.91%) | 27 (3.09%) | 874 (1.96%) | 0.8394 | |
| Urban | 6321 (97.26%) | 178 (2.74%) | 6499 (14.56%) | ||
|
| |||||
| Treating Facility Type | Academic/Research Program | 15022 (97.06%) | 455 (2.94%) | 15477 (33.86%) | 0.1749 |
| Community/Comprehensive Community/Integrated | 29411 (97.28%) | 822 (2.72%) | 30233 (66.14%) | ||
Disease Factors Associated with Separate Tumor Nodules
The association of disease factors is presented in Table 2. Separate tumor nodules were similarly often found between right and left sided tumors and slightly more often for upper tumors as compared to middle and lower lobe tumors (Table 2). The highest frequency of separate nodules were documented for patients with tumors involving overlapping lobes, or those with an unspecified primary site (12.1%, Table 2). Adenocarcinoma and large cell histology was associated with a higher frequency of separate nodules as compared to squamous cell carcinomas (3.2% vs. 3.0% vs. 1.7% respectively, p<0.0001). The frequency of separate tumor nodules increased for tumors larger than 3 cm (<1cm 2.8% vs. 1-<3cm 2.7% vs. >3cm 3.8%; p<0.001, Figure 3). Similarly, separate tumor nodules were more often found amongst patients with unsuspected pathologic lymph node disease (N=4,833) and the frequency increased with higher nodal stage (pN0, 2.5% vs. pN1, 4.7%, vs, pN2, 6.8%; p<0.0001; Figure 2). Presence of pleural and/or lymphovascular invasion was also associated with a higher frequency of separate tumor nodules in 4.8 % and 4.7% of patients, respectively (both, p<0.0001).
Table 2.
Disease characteristics stratified by presence of separate tumor nodules
| Variable | Level | No separate tumor nodules (n=44,558) |
Separate tumor nodules in ipsilateral lung (n=1,284) |
Total (n=45,842) |
p-value |
|---|---|---|---|---|---|
|
| |||||
| Primary Site | Lower lobe, lung | 13942 (97.44%) | 366 (2.56%) | 14308 (31.21%) | |
| Main | 590 (95.16%) | 30 (4.84%) | 620 (1.35%) | ||
| bronchus\Overlapping\NOS | 0.0004 | ||||
| Middle lobe, lung | 2589 (97.81%) | 58 (2.19%) | 2647 (5.77%) | ||
| Upper lobe, lung | 27437 (97.06%) | 830 (2.94%) | 28267 (61.66%) | ||
|
| |||||
| Laterality | Origin of primary is left | 17463 (97.22%) | 500 (2.78%) | 17963 (39.18%) | 0.8560 |
| Origin of primary is right | 27095 (97.19%) | 784 (2.81%) | 27879 (60.82%) | ||
|
| |||||
| Tumor Size | < 1 cm | 1371 (97.17%) | 40 (2.83%) | 1411 (3.08%) | 0.0001 |
| >= 1 cm, < 3 cm | 26540 (97.31%) | 733 (2.69%) | 27273 (59.49%) | ||
| >= 3 cm | 4746 (96.19%) | 188 (3.81%) | 4934 (10.76%) | ||
| Unknown, size not stated | 11901 (97.36%) | 323 (2.64%) | 12224 (26.67%) | ||
|
| |||||
| Grade | Well differentiated, NOS | 8259 (97.41%) | 220 (2.59%) | 8479 (18.5%) | 0.5405 |
| Moderately differentiated | 21926 (97.13%) | 647 (2.87%) | 22573 (49.24%) | ||
| Poorly differentiated | 11555 (97.19%) | 334 (2.81%) | 11889 (25.93%) | ||
| Undifferentiated | 154 (95.65%) | 7 (4.35%) | 161 (0.35%) | ||
| Unknown | 2664 (97.23%) | 76 (2.77%) | 2740 (5.98%) | ||
|
| |||||
| Histology Category | Adenocarcinoma | 33028 (96.84%) | 1076 (3.16%) | 34104 (74.39%) | <0.0001 |
| Large cell | 962 (96.98%) | 30 (3.02%) | 992 (2.16%) | ||
| Squamous cell carcinoma | 10568 (98.34%) | 178 (1.66%) | 10746 (23.44%) | ||
|
| |||||
| Pathologic T-Stage | Unknown | 582 (97.82%) | 13 (2.18%) | 595 (1.3%) | <0.0001 |
| pT0/p1 | 33724 (99.6%) | 134 (0.4%) | 33858 (73.86%) | ||
| pT2 | 9728 (99.42%) | 57 (0.58%) | 9785 (21.35%) | ||
| pT3 | 489 (32.13%) | 1033 (67.87%) | 1522 (3.32%) | ||
| pT4 | 35 (42.68%) | 47 (57.32%) | 82 (0.18%) | ||
|
| |||||
| Pathologic N-Stage | Unknown | 1008 (97.3%) | 28 (2.7%) | 1036 (2.26%) | <0.0001 |
| pN0 | 38985 (97.53%) | 988 (2.47%) | 39973 (87.2%) | ||
| pN1 | 2836 (95.23%) | 142 (4.77%) | 2978 (6.5%) | ||
| pN2 | 1729 (93.21%) | 126 (6.79%) | 1855 (4.05%) | ||
|
| |||||
| Pleural Invasion | Unknown | 5778 (97.95%) | 121 (2.05%) | 5899 (12.87%) | <0.0001 |
| No | 32397 (97.46%) | 843 (2.54%) | 33240 (72.51%) | ||
| Yes | 6382 (95.23%) | 320 (4.77%) | 6702 (14.62%) | ||
|
| |||||
| Lymph Vascular Invasion | Not present | 35629 (97.53%) | 902 (2.47%) | 36531 (80.41%) | <0.0001 |
| Present | 5770 (95.26%) | 287 (4.74%) | 6057 (13.33%) | ||
| Unknown | 2753 (96.9%) | 88 (3.1%) | 2841 (6.25%) | ||
Figure 3.
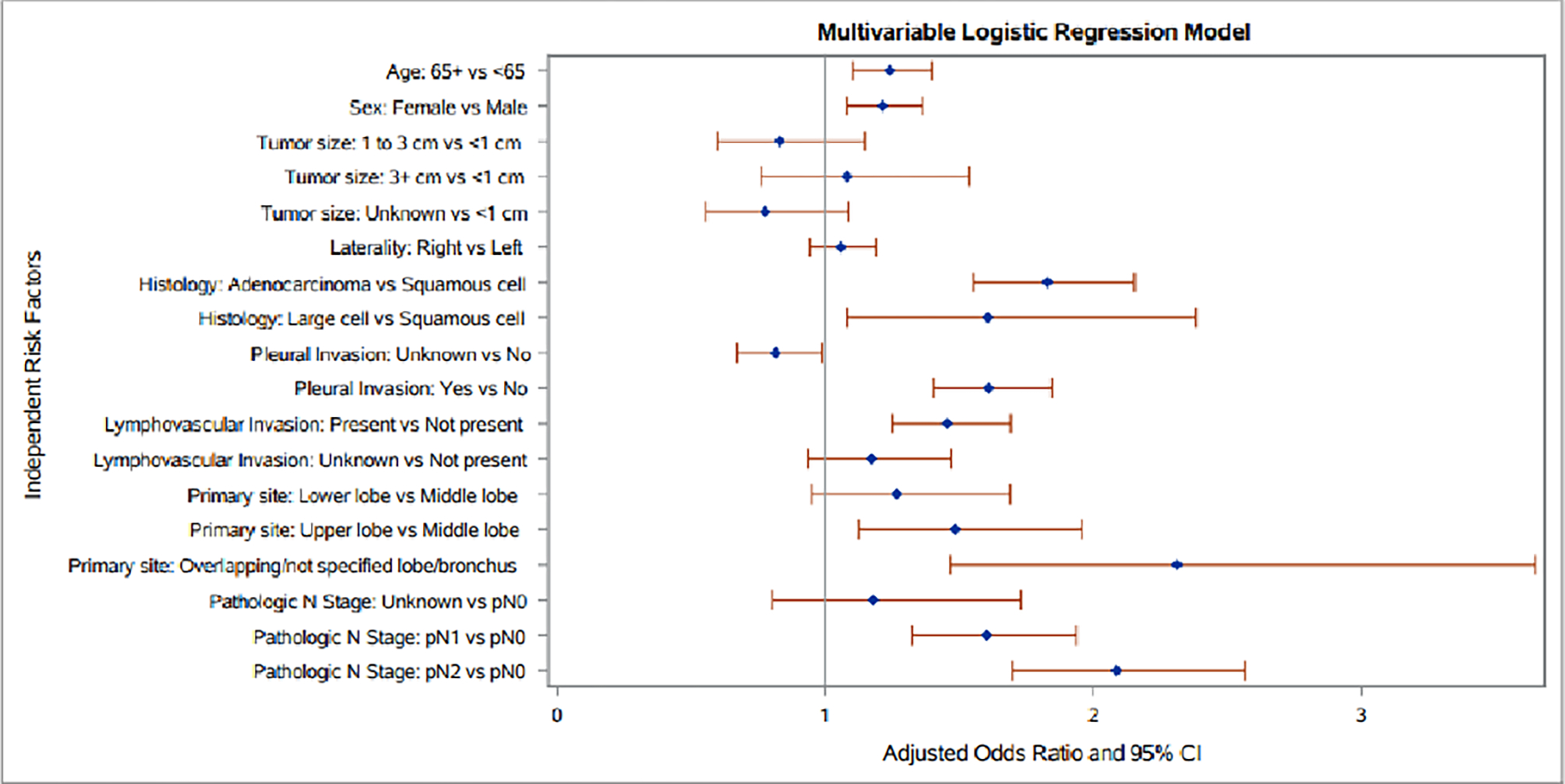
Forrest plot of a best predictive model for separate tumor nodules in clinical stage Ia patients undergoing lobectomy using of patient and disease factors. Estimates derived from multivariable logistic regression model.
Figure 2:
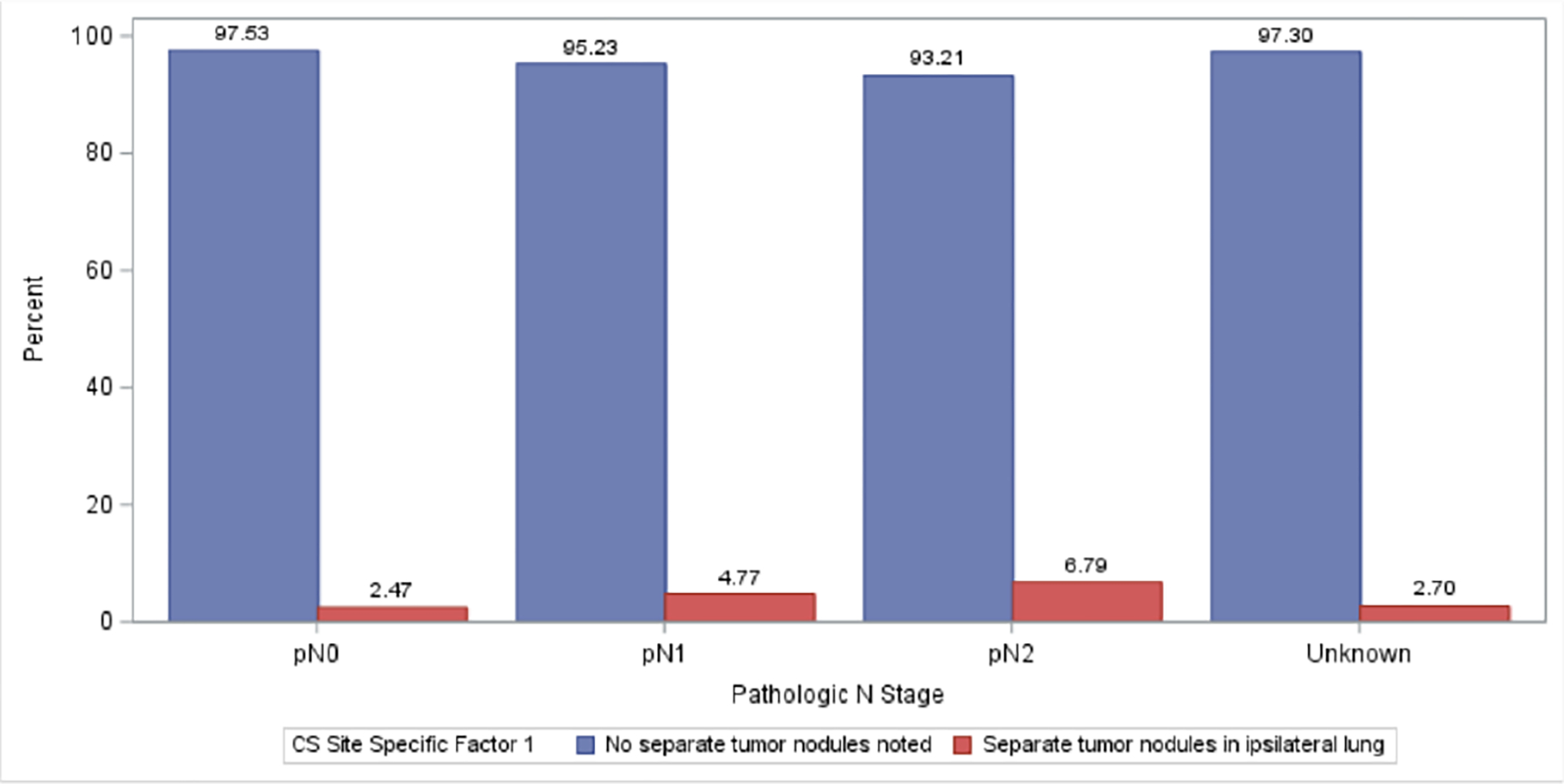
Frequency of separate ipsilateral tumor nodules in NSCLC increased with pathologic lymph node stage.
Predictive Model for Separate Tumor Nodules
A best predictive multivariable logistic regression model for separate ipsilateral tumor nodules was developed which included both clinical patient and disease factors (Table 3). As shown in Figure 3, age and sex remained independently associated with separate tumor nodules. Disease factors independently associated with separate tumor nodules were adenocarcinoma and large cell histology, upper lobe tumor location, pathologic N1 and N2 lymph node upstaging, and presence of lymphovascular and pleural invasion (Figure 3). The model showed limited predictive ability with an AUC of 0.645 (95% confidence interval 0.639–0.660) and good model fit with a Hosmer-Lemeshow Goodness-of-Fit test p-value=0.593. When the prediction model was using only clinical factors that would be available preoperatively, the predictive ability was further reduced, with an AUC 0.595 (95% confidence interval 0.560–0.610) (Supplemental Figure).
Table 3.
Multivariable model of clinicopathologic factors associated with presence of separate tumor nodules.
| Effect Variable | OR (95% CI) | p-value |
|---|---|---|
| Age: 65+ Years vs <65 Years | 1.24 (1.10, 1.40) | 0.0004 |
| Tumor Size: ≥ 1 cm, < 3 cm vs < 1 cm | 0.83 (0.60, 1.15) | 0.2595 |
| Tumor Size: ≥ 3 cm vs < 1 cm | 1.08 (0.76, 1.54) | 0.6661 |
| Tumor Size: Unknown, size not stated vs < 1 cm | 0.78 (0.56, 1.09) | 0.1401 |
| Primary Site: Lower lobe, lung vs Middle lobe, lung | 1.27 (0.95, 1.69) | 0.1085 |
| Primary Site: Main bronchus\Overlapping\NOS vs Middle lobe, lung | 2.31 (1.47, 3.65) | 0.0003 |
| Primary Site: Upper lobe, lung vs Middle lobe, lung | 1.48 (1.13, 1.96) | 0.0052 |
| Laterality: Origin of primary is right vs Origin of primary is left | 1.06 (0.94, 1.19) | 0.3407 |
| Sex: Female vs Male | 1.21 (1.08, 1.36) | 0.0011 |
| Histology Category: Adenocarcinoma vs Squamous cell carcinoma | 1.83 (1.55, 2.15) | <.0001 |
| Histology Category: Large Cell Carcinoma vs Squamous cell carcinoma | 1.61 (1.08, 2.38) | 0.0188 |
| Pleural Invasion Unknown vs Pleural Invasion: No | 0.82 (0.67, 0.99) | 0.0379 |
| Pleural Invasion: Yes vs Pleural Invasion: No | 1.61 (1.40, 1.85) | <.0001 |
| Lymph Vascular Invasion: Present vs Not present | 1.45 (1.25, 1.69) | <.0001 |
| Lymph Vascular Invasion: Unknown vs Not present | 1.17 (0.94, 1.47) | 0.1651 |
| Pathologic N Stage: Unknown vs pN0 | 1.18 (0.80, 1.73) | 0.4009 |
| Pathologic N Stage: pN1 vs pN0 | 1.60 (1.32, 1.94) | <.0001 |
| Pathologic N Stage: pN2 vs pN0 | 2.09 (1.70, 2.57) | <.0001 |
4. DISCUSSION
In this study we demonstrate that the overall frequency of occult separate tumor nodules in the same lobe as clinical stage Ia NSCLC is a rare finding in approximately 2.8% of patients after lobectomy. Importantly, we demonstrate that the frequency of separate nodules showed a narrow range amongst most clinical and pathological variables in this large database. Few pathological factors were associated with the highest rates of separate tumor nodules, which included adenocarcinoma or large cell histology, tumor size over 3cm, lymphovascular and visceral pleural invasion and co-existing occult lymph node disease. Using a combination of patient and disease factors, the best predictive regression model of satellite nodules however had only limited predictive ability (AUC 0.645). Therefore, satellite nodules may be encountered with a low frequency across the disease spectrum and should be borne in mind when planning resection or alternative local treatments for all clinical stage Ia patients.
In contrast to occult malignant satellite nodules, separate co-existing lung nodules are a common finding amongst patients undergoing lung cancer surgery. Several studies have reported on the prevalence of lung nodules in operable NSCLC and showed that the majority of these are most often benign. Stiles and colleagues reviewed the CT imaging of 155 consecutive patients after lung cancer resection and found at least one additional nodule in over half of patients [9]. Most nodules were followed and were determined to be benign during follow-up and only a 15% of nodules that were resected for suspicion of cancer were intrapulmonary metastases and 25% were a second primary tumor [9]. Similarly, Ruppert and colleagues identified additional lung nodules in 24% of 239 patients undergoing resection for NSCLC and only 27% were determined to be malignant [10]. Yuan et al. found additional pulmonary nodules in 26% in operable NSCLC of which malignancy 25% were malignant [11]. Carretta et al. reported a malignancy rate of 32% percent of additional nodules in their series of 29 patients [12]. A Korean study by Kim et al. found a total of 138 small < 10 mm additional nodules in 62 (44%) of patients, of which 93% were found to be benign [13]. Differences in the rate of malignancy rates may be attributed to the variable study populations (i.e. rate of smokers), prevalence of granulomatous disease and imaging methodology and biopsy approaches.
This current study is the first to analyze factors associated with unsuspected separate tumor nodules specifically in the ipsilateral lung. Our results however corroborate several predictive factors found in in previous studies that have examined the malignant potential of preoperatively detected co-existing lung nodules. Factors that have been associated with malignancy in lung nodules in surgical lung cancer patients include adenocarcinoma histology, larger tumor size, location in the upper lobe, and co-localization in the same lobe as the primary tumor [10, 14]. In addition, radiographic characteristics may inform on the nature of separate tumor nodules. Nodules greater than 5mm were are more likely malignant [15]. Spiculated appearance on CT is associated with a malignancy rate of >50% in additional lung nodules [10, 14, 15]. Ruppert et al. showed that lobulated shape additional lesions harbored malignancy in over a third of patients, and the lowest rate of malignancy was seen in well-defined and ground glass lesions [10]. Molecular characterization of separate tumor nodules have been used to distinguish intrapulmonary metastases from separate unrelated primary [16]. However, little is known on how molecular features of a primary tumor may aid in estimating the risk of satellite nodules.
The present study has several important limitations. The clinical staging modalities are not captured in the NCDB and we are not able to discern how many separate nodules may have been noted on preoperative imaging studies. Importantly, we could not confirm the histology and molecular features of separate tumor nodules to distinguish between intrapumonary metastases and separate primary tumors. Furthermore, the rate of separate tumor nodules in a different ipsilateral lobe was not included in the analysis, as this requires either bilobectomy or additional sublobar resection of ipsilateral lobes. Lastly, the NCDB does not capture long term data on recurrence, and hence we are not able to discern missed intrapulmonary nodules that may have led to a recurrence. Nonetheless, the present study contained a large number of patients from a data base with high accuracy and used robust statistical techniques to provide a realistic assessment of the rare endpoint of separate lung nodules in this patient population.
5. CONCLUSION
The current study may help surgeons and clinicians estimate the rate of unsuspected satellite lung nodules in the same lobe as clinical stage Ia NSCLC. A heightened awareness of separate tumor nodules by careful review of preoperative imaging and intraoperative exam is emphasized given that satellite nodules may occur across the early disease spectrum. However, the surgical approach to clinical stage Ia NSCLC is unlikely affected in light of the overall low frequency of separate tumor nodules. Clinical features of the primary tumor such adenocarcinoma histology, upper lobe location, and size, and lymph node status may guide surgeons on the need for resection of separate nodules.
Supplementary Material
Supplemental Figure: Forrest plot for prediction of separate tumor nodules in clinical stage Ia patients undergoing lobectomy based on preoperatively available patient and disease factors. AUC 0.5949 (95%CI: 0.5796, 0.6101). The Hosmer-Lemeshow test p-value was 0.9202.
Clinical Practice Points.
What is already known about this subject?
Satellite nodules in early stage non-small cell lung cancer (NSCLC) patients may harbor in intrapulmonary pulmonary metastasis in the ipsilateral lung.
Satellite nodules are often detected incidentally on surgical pathology after anatomic lung resection and leading to pathologic T3 or T4 upstaging.
Knowledge of satellite nodules is important for clinicians planning local treatments for clinical stage Ia NSCLC.
What are the new findings?
This large national cancer database study of patients with clinical stage Ia NSCLC revealed that clinically occult satellite tumor nodules in the same or ipsilateral lobe can be detected at a low but consistent rate along the disease spectrum.
The regression analysis shows that few clinical factors may help predict occult satellite nodules.
The overall predictability of satellite nodules base on clinical information in the database is limited.
How might it impact on clinical practice in the future?
The results of this study provide an estimate of the prevalence of occult satellite nodules in the ipsilateral lung in patients.
The poor predictability based on clinical information emphasizes that surgeons and clinicians need to be vigilant about detection of satellite tumor nodules even in patients with clinical stage Ia disease when planning the extent of resection or other local treatments.
ACKNOWLEDGEMENTS
The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Mahmoud Abdel-Rasoul is supported by the CTSA grant number (UL1TR002733) from the National Center for Advancing Translational Sciences.
Glossary of Abbreviations
- AJCC
American Joint Committee on Cancer
- AUC
area under the receiver operating characteristic curve
- COC
Commission on Cancer
- CONSORT
Consolidated Standards of Reporting Trials
- CT
Computed tomography
- NCDB
National Cancer Database
- NSCLC
Non-small cell lung cancer
- PET-FDG
Fluorodeoxyglucose positron emission tomography
Footnotes
Disclosures: Dr. Merritt is a speaker for Surgical Intuitive Inc. Dr. D’Souza is a proctor for Surgical Intuitive Inc. The lead author and none of the authors have a financial or personal conflict of interest to disclose.
Presented at the 48th Annual Western Thoracic Surgical Association Meeting, June 25th, 2022, in Koloa, Hawaii
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rao J, Sayeed RA, Tomaszek S, Fischer S, Keshavjee S, and Darling GE, Prognostic factors in resected satellite-nodule T4 non-small cell lung cancer. Ann Thorac Surg, 2007. 84(3): p. 934–8; discussion 939. [DOI] [PubMed] [Google Scholar]
- 2.Deslauriers J, Brisson J, Cartier R, Fournier M, Gagnon D, Piraux M, and Beaulieu M, Carcinoma of the lung. Evaluation of satellite nodules as a factor influencing prognosis after resection. J Thorac Cardiovasc Surg, 1989. 97(4): p. 504–12. [PubMed] [Google Scholar]
- 3.Detterbeck FC, Boffa DJ, Kim AW, and Tanoue LT, The Eighth Edition Lung Cancer Stage Classification. Chest, 2017. 151(1): p. 193–203. [DOI] [PubMed] [Google Scholar]
- 4.Port JL, Korst RJ, Lee PC, Kansler AL, Kerem Y, and Altorki NK, Surgical resection for multifocal (T4) non-small cell lung cancer: is the T4 designation valid? Ann Thorac Surg, 2007. 83(2): p. 397–400. [DOI] [PubMed] [Google Scholar]
- 5.Okubo K, Bando T, Miyahara R, Sakai H, Shoji T, Sonobe M, Fujinaga T, Sato K, Wada H, and Tanaka T, Resection of pulmonary metastasis of non-small cell lung cancer. J Thorac Oncol, 2009. 4(2): p. 203–7. [DOI] [PubMed] [Google Scholar]
- 6.Kneuertz PJ, Abdel-Rasoul M, D’Souza DM, Zhao J, and Merritt RE, Segmentectomy for clinical stage I non-small cell lung cancer: National benchmarks for nodal staging and outcomes by operative approach. Cancer, 2022. [DOI] [PubMed] [Google Scholar]
- 7.Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, Aoki T, Okami J, Yoshino I, Ito H, Okumura N, Yamaguchi M, Ikeda N, Wakabayashi M, Nakamura K, Fukuda H, Nakamura S, Mitsudomi T, Watanabe SI, Asamura H, West Japan Oncology G, and Japan Clinical Oncology G, Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet, 2022. 399(10335): p. 1607–1617. [DOI] [PubMed] [Google Scholar]
- 8.Salazar MC, Rosen JE, Arnold BN, Thomas DC, Kim AW, Detterbeck FC, Blasberg JD, and Boffa DJ, Adjuvant Chemotherapy for T3 Non-Small Cell Lung Cancer with Additional Tumor Nodules in the Same Lobe. J Thorac Oncol, 2016. 11(7): p. 1090–100. [DOI] [PubMed] [Google Scholar]
- 9.Stiles BM, Schulster M, Nasar A, Paul S, Lee PC, Port JL, and Altorki NK, Characteristics and outcomes of secondary nodules identified on initial computed tomography scan for patients undergoing resection for primary non-small cell lung cancer. J Thorac Cardiovasc Surg, 2015. 149(1): p. 19–24. [DOI] [PubMed] [Google Scholar]
- 10.Ruppert AM, Lerolle U, Carette MF, Lavole A, Khalil A, Bazelly B, Antoine M, Cadranel J, and Milleron B, Coexisting pulmonary nodules in operable lung cancer: prevalence and probability of malignancy. Lung Cancer, 2011. 74(2): p. 233–8. [DOI] [PubMed] [Google Scholar]
- 11.Yuan Y, Matsumoto T, Hiyama A, Miura G, Tanaka N, Emoto T, Kawamura T, and Matsunaga N, The probability of malignancy in small pulmonary nodules coexisting with potentially operable lung cancer detected by CT. Eur Radiol, 2003. 13(11): p. 2447–53. [DOI] [PubMed] [Google Scholar]
- 12.Carretta A, Ciriaco P, Canneto B, Nicoletti R, Del Maschio A, and Zannini P, Therapeutic strategy in patients with non-small cell lung cancer associated to satellite pulmonary nodules. Eur J Cardiothorac Surg, 2002. 21(6): p. 1100–4. [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Lee KS, Primack SL, Kim H, Kwon OJ, Kim TS, Kim EA, Kim J, and Shim YM, Small pulmonary nodules on CT accompanying surgically resectable lung cancer: likelihood of malignancy. J Thorac Imaging, 2002. 17(1): p. 40–6. [DOI] [PubMed] [Google Scholar]
- 14.Guo J, Chu X, Liu Y, and Liang C, Small satellite pulmonary nodules in operable lung cancer: Diagnosis and therapeutic strategy. Journal of Cancer Research & Therapy, 2014. 2(8): p. 111–115. [Google Scholar]
- 15.Ginsberg MS, Akin O, Berger DM, Zakowski MF, and Panicek DM, Pulmonary tumorlets: CT findings. AJR Am J Roentgenol, 2004. 183(2): p. 293–6. [DOI] [PubMed] [Google Scholar]
- 16.Schneider F and Dacic S, Histopathologic and molecular approach to staging of multiple lung nodules. Transl Lung Cancer Res, 2017. 6(5): p. 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: Forrest plot for prediction of separate tumor nodules in clinical stage Ia patients undergoing lobectomy based on preoperatively available patient and disease factors. AUC 0.5949 (95%CI: 0.5796, 0.6101). The Hosmer-Lemeshow test p-value was 0.9202.


