Introduction
Gastrointestinal pH is crucial for physiological gut functioning. Variations in pH along the gastrointestinal tract in health are designed for nutrient digestion and absorption and provide innate defense against ingested microorganisms (1). Small intestinal pH is influenced by the expression and function of mucosal acid transporters (e.g., SLC9A3) and base transporters (e.g., CFTR, SLC26A3, SLC26A6) along with alkaline-rich pancreatic and biliary secretions (2, 3). Colon pH is thought to be regulated by many of the same acid-base transporters but may also be influenced by colonic bacterial fermentation, production of carbon dioxide, and short-chain fatty acids that are transported in a proton-coupled manner via SLC16A1 (4, 5).
Small intestinal pH is decreased in cystic fibrosis (CF) due, at least in part, to decreased CFTR-mediated bicarbonate secretion (6). It remains unknown if colonic pH is altered in CF. Given the observed base transport disturbances and dysmotility in other regions of the gastrointestinal tract in CF, we hypothesized that the colon may also display altered intestinal pH and this may contribute to chronic abdominal pain, distal intestinal obstruction syndrome, bloating, and chronic constipation experienced by many persons with CF.
To address this hypothesis, we re-analyzed prior wireless motility capsule (WMC) studies in CF and non-CF subjects (7) to determine pH, estimated bicarbonate concentrations, and motility profile of the distal colon. We also re-analyzed prior single-cell RNA sequencing (scRNA-seq) studies of human non-CF colon and rectum to characterize acid-base transporters of the colon and rectum. This combination of WMC and scRNA-seq studies provide us new insights into colonic pathophysiology in CF.
Methods
Human Subjects:
We conducted an expanded analysis of WMC data previously collected from individuals with CF and non-CF matched control subjects (7). All data was de-identified and Institutional Review Board exempt. Data was obtained from 10 CF subjects, ≥18 years-old with exocrine pancreatic insufficiency, and were at baseline health with no hospitalization within the 2 months preceding the WMC. No subjects were on CFTR modulator therapies, gastric acid inhibitors, or inhaled antibiotics during the study period. Each CF subject underwent two independent WMC studies, generally 8 weeks apart. Healthy control subjects were matched by age ±3 years, sex, and body mass index ±2 kg/m2.
pH Measurements.
The WMC (Medtronic Corporation, Minneapolis MN) possesses sensors to measure pH, pressure and temperature for approximately 72 hours, as previously described (7). All subjects passed the WMC within 72 hours. Data was extracted using GIMS software (Gastrointestinal Motility Software [GIMS™] Medtronic Corporation, Minneapolis MN). Anal exit was defined as an acute temperature drop and permanent loss of signal on the receiver. We measured pH and pressure changes from the distal colon, divided into 4 segments, each 30-min intervals before the anal exit of the WMC. Mean pH values over 30-min increments were calculated. For comparison, gastric measurements were taken prior to gastric emptying, defined as an abrupt rise in the pH by 3 or more units above the stomach pH.
Calculation of Bicarbonate Concentration.
To estimate the colonic bicarbonate concentration ([HCO3−]), we utilized the Henderson-Hasselbach equation, which considers the proton acceptor (HCO3−) and the proton donor (CO2) to determine the pH, [HCO3−] = 0.03 × pCO2 × 10 (pH – 6.1). Due to the absence of human colonic pCO2 data, previously published porcine sigmoid colon pCO2 values were used (8).
Single-Cell RNA Sequencing Analysis.
The R toolkit Seurat (9) was used to perform quality control and analysis of human colonic and rectal single-cell RNA sequencing (scRNA-seq) data from a previously published study on non-CF subjects (10). In brief, we embedded the cells through a K-nearest neighbor graph and reduced the data by principal component analysis. We visualized single-cell gene expressions per cell type as annotated by Wang et al. (10) with Violin plots in Seurat.
Statistics:
Means and standard deviations were computed for all measurements. Non-parametric Mann-Whitney t-test or analysis of variance was used to evaluate the differences between CF and control data using GraphPad Prism 9.4 (San Diego, CA). Significance was set to P<0.05.
Results
Colonic pH and bicarbonate concentration.
Colonic pH from WMC measurements in control subjects showed similar median pH values of 7.46, 7.60, 7.53 at the proximal time points (2, 1:30, and 1 hours prior to exit, respectively), with the most distal measurements having a generally higher pH of 7.88, although this was not significant (P=0.280, Figure 1A). In comparison, median colonic pH measurements in CF subjects were similar across all time points at: 6.58, 6.54, 6.58, 6.53 (proximal to distal) and were significantly less than control subjects, most notably in the most distal measurements (Figure 1A). Calculation of [HCO3−] showed similar trends in control (median proximal to distal [HCO3−] = 55.1, 63.8, 46.2, and 121.9 mM, respectively) and CF subjects (median proximal to distal [HCO3−] = 6.2, 5.6, 6.1, and 5.5 mM, respectively) (Figure 1B).
Figure 1. Decreased distal colonic pH and bicarbonate concentration in CF compared to controls based on WMC.
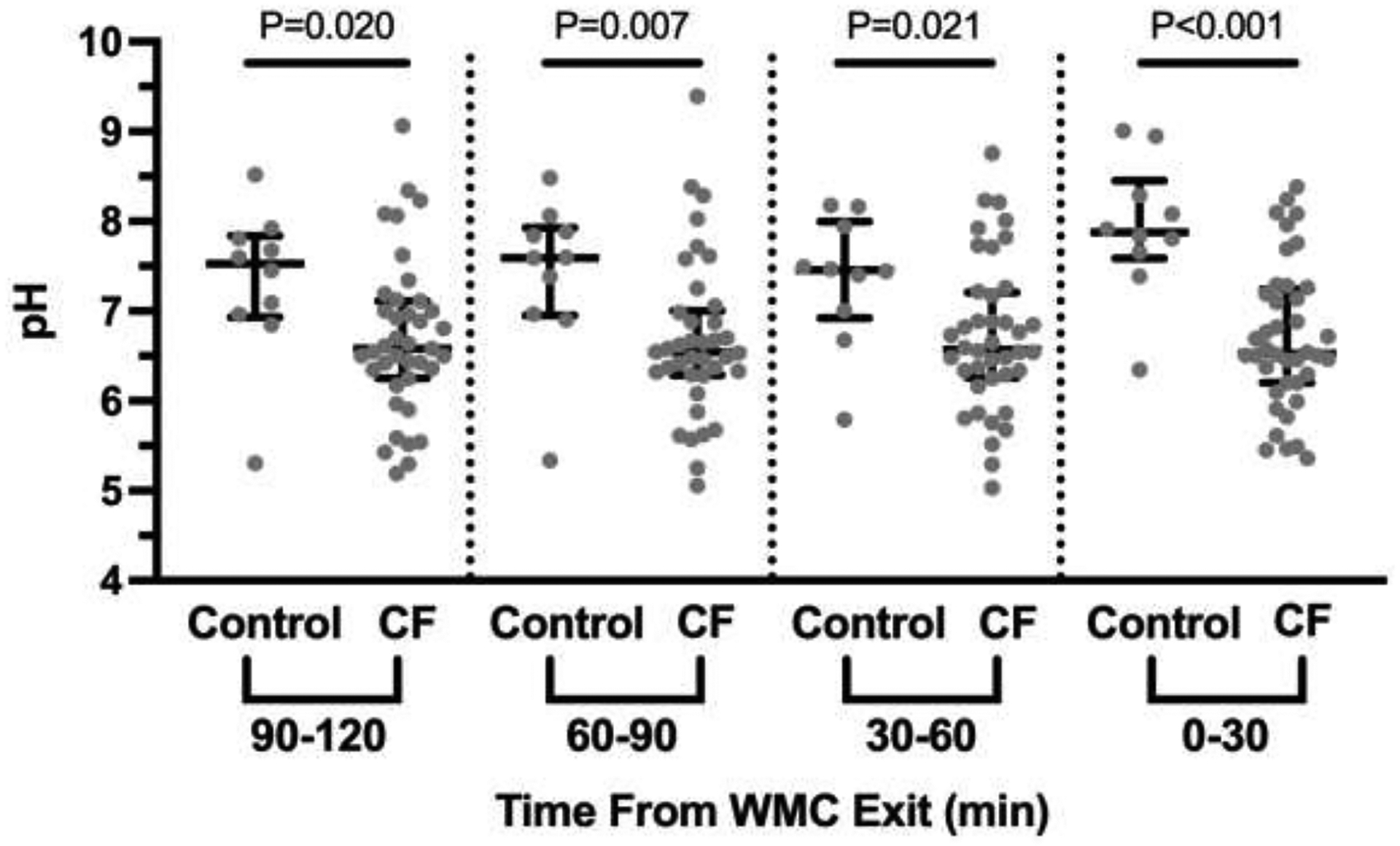
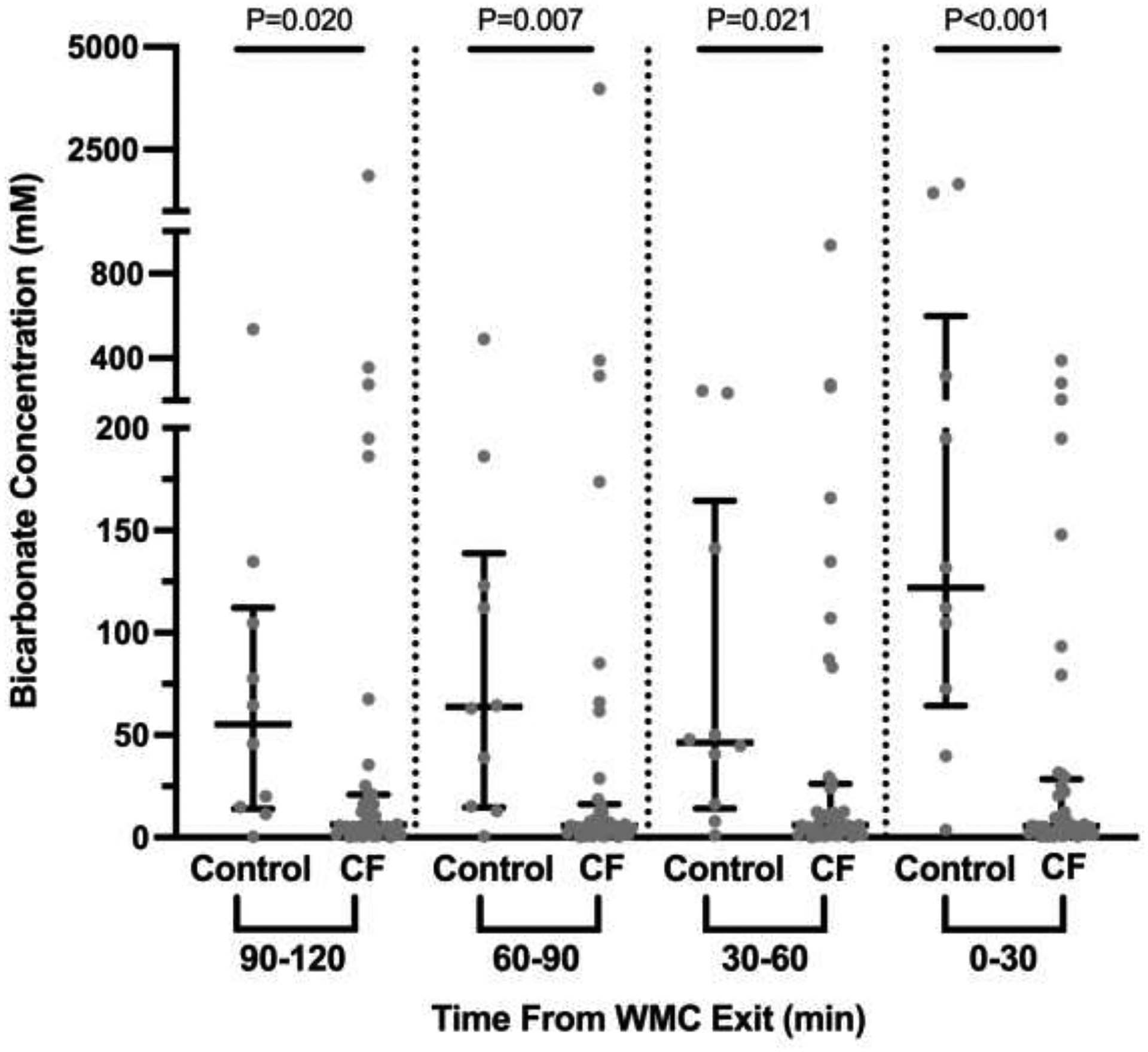
A. The average pH recordings over 30 minutes for each interval was calculated and is shown for each of the 4 intervals, with proximal indicating 2 hours prior to exit and distal the last 30 minutes prior to exit. Dots indicate results from each subject. Median with 95% confidence intervals is indicated by black bars. Significance was determined by non-parametric Mann-Whitney t-test. B. pH recordings from Figure 1A were used to calculate bicarbonate concentrations and are presented in the same manner as Figure 1A.
Colonic motility.
To determine if colonic dysmotility may contribute to decreased colonic pH in CF, we examined the contractions and motility parameters at the same timepoints that pH were measured. As seen in Table 1, there were no significant differences in the number of contractions, contractions per minute, the Camilleri or Ouyang motility indices, or the area under the pressure curve between control and CF subjects. To verify WMC’s ability to identify dysmotility, we examined stomach motility, given the high prevalence of gastroparesis in CF (11). As seen in Table 1, we found low stomach motility indices in CF and significant differences compared to control subjects.
Table 1.
No evidence of distal colonic dysmotility in CF with WMC, despite decreased gastric motility profiles
| Controls (n=10) | CF (n=39) | P Value | |
|---|---|---|---|
| Number of Contractions | 86.7 (114.0) | 111 (121.8) | 0.5619 |
| Contractions Per Minute | 0.09 (0.10) | 0.13 (0.14) | 0.2867 |
| Motility Index - Camilleri | 12.3 (2.2) | 12.64 (2.59) | 0.6785 |
| Motility Index - Ouyang | 24.83 (27.88) | 28.97 (48.09) | 0.7270 |
| Area Under Pressure Curve | 22,068 (29,561.2) | 26,867.3 (48,438.4) | 0.6964 |
| Number of Contractions | 184.7 (137.8) | 82.6 (59.17) | 0.0011 |
| Contractions Per Minute | 0.86 (0.72) | 0.4 (0.26) | 0.0019 |
| Motility Index - Camilleri | 13.86 (1.33) | 12.28 (1.22) | 0.0026 |
| Motility Index - Ouyang | 71.87 (63.09) | 33.31 (14.04) | 0.0009 |
| Area Under Pressure Curve | 14,213.5 (11,064.2) | 6,684.5 (2,618.5) | 0.0003 |
Expression of colonic acid-base transporters.
With dysmotility likely not playing a significant role in decreased colonic and rectal pH in CF subjects, we next examined whether the decreased colonic pH and [HCO3−] observed in CF might be due primarily to decreased CFTR expression or potentially to other acid-base transporters. ScRNA-seq of 4,472 colonic cells and 3,898 rectal cells from non-CF subjects (n=4) showed colonic expression profiles of acid-base transporters in enterocytes (greatest to least) as: SLC26A3>>CFTR≈SLC16A1>SLC26A6. For rectum: SLC26A3>>SLC16A1≈SLC26A6>CFTR. Acid transporter SLC9A3 was not detected in either colon or rectum (Figure 2).
Figure 2. Single cell RNA sequencing of human colon and rectum.
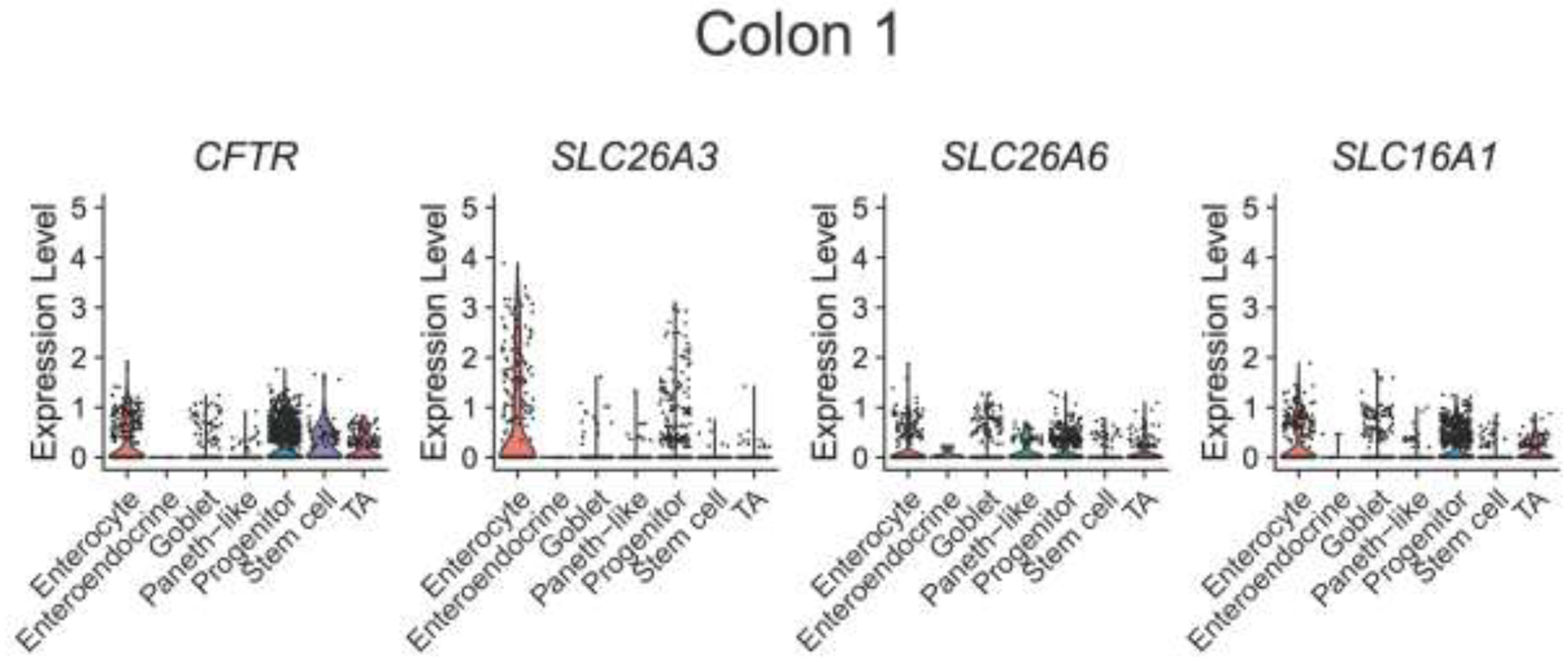
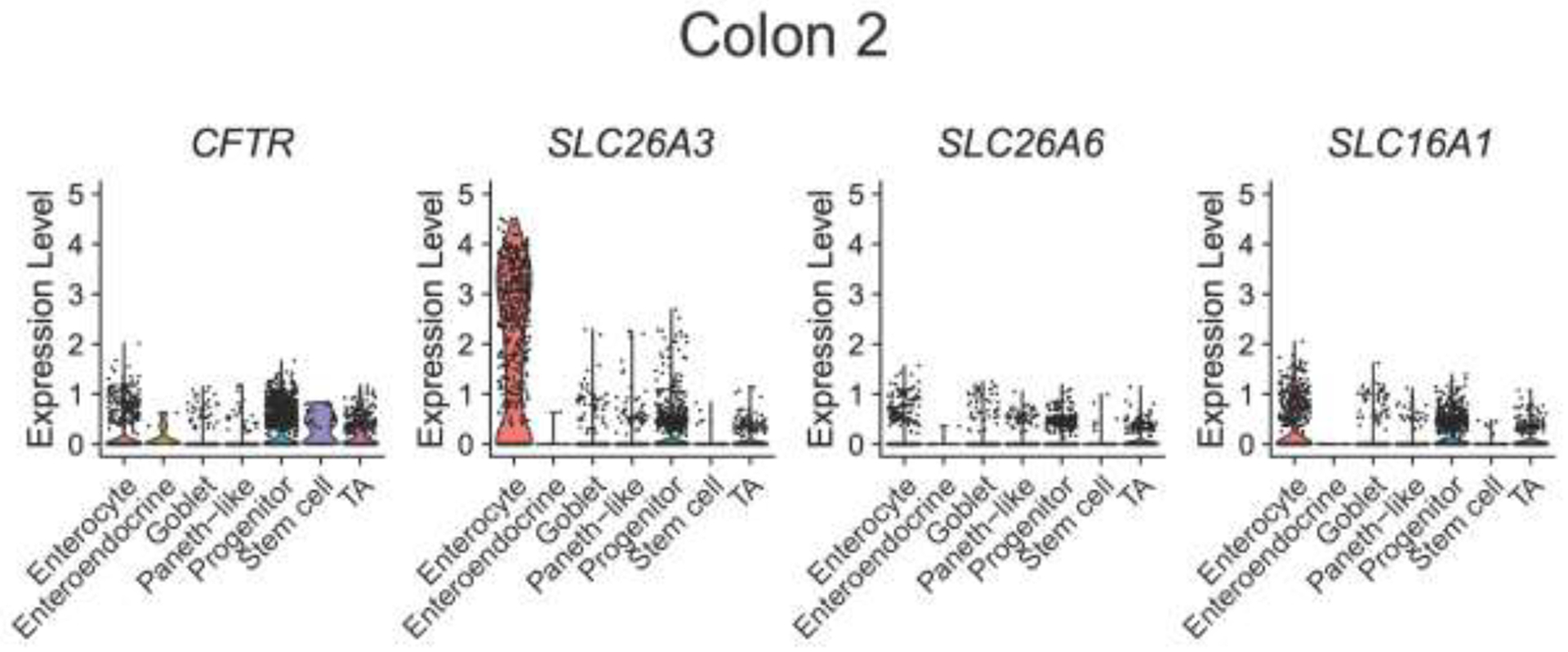
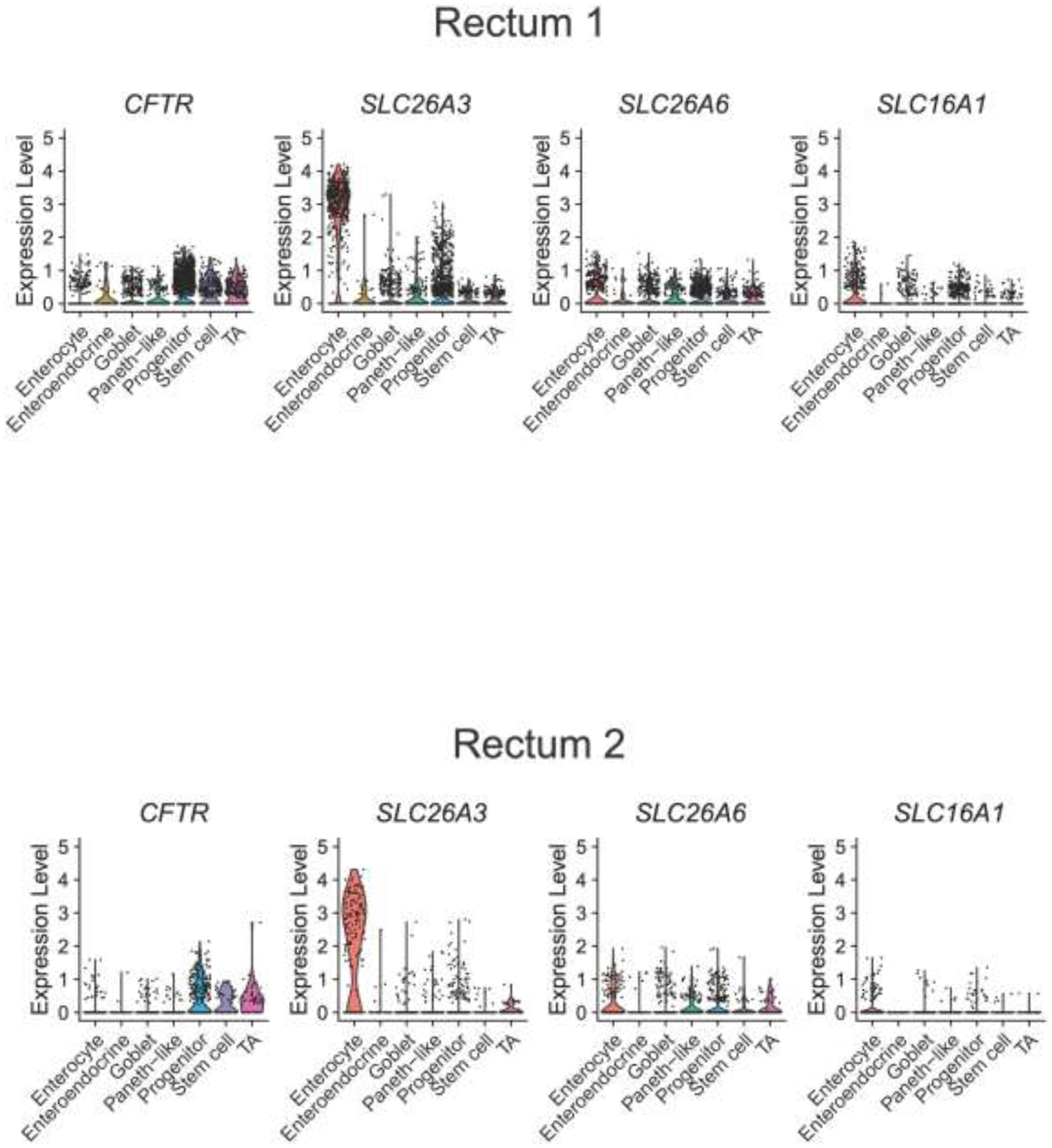
Prior scRNA-seq data from Wang et al (10) was used to perform analysis on the mRNA expression of CFTR, SLC26A3 (down-regulated in adenoma, DRA), SLC26A6 (putative anion transporter-1, PAT-1), and SLC16A1 (monocarocylate transporter 1, MCT1) from 4,472 colonic cells (A) and 3,898 rectal cells (B) from four non-CF subjects. Violin plots represent expression relative to all cells of that type, with each dot representing the expression of individual cells within each cell type.
Discussion
Gastrointestinal pH is physiologically variable along the small and large intestine. In mouse and human studies, including with the WMC, loss of CFTR function in CF decreases proximal small intestine bicarbonate secretion and pH (7, 12, 13). In health, intestinal pH decreases in the cecum and then gradually increases distally towards the rectum. Decreased colonic pH has been noted in irritable bowel syndrome (14) and inflammatory bowel disease (15), however, the colonic pH profile in CF has not been investigated.
In our current study we found that WMC-measured distal colonic pH was markedly lower in CF subjects than healthy control subjects. Colonic pH may be influenced by mucosal bicarbonate and proton transport, lactate production, bacterial fermentation of carbohydrates, absorption of short chain fatty acids (coupled with proton transport), and intestinal transit. Due to the retrospective nature of our analysis, we did not have data on the presence or absence of subclinical intestinal inflammation or the colonic microbiome so we cannot ascertain to what degree, if any, these may have contributed to our findings. However, with the WMC we were able to evaluate colonic motility through contraction measurements. Our findings that distal colon and rectal contractions in CF subjects were similar to healthy control subjects argues against impaired colonic motility being responsible for our findings of decreased intraluminal pH. While manometry is the gold-standard for motility, this data is lacking in CF. In non-CF subjects, WMC detected 86% of contractility events (compared to simultaneous manometry) with a negative predictive value of 99.9% (16, 17). Therefore, WMC may serve as a good surrogate marker for noninvasive measure of GI motility in CF. Additionally, magnetic resonance imaging-based colonic transit measurements in CF identified proximal, but not distal, colonic dysmotility, similar to our findings (18).
Rectal biopsies from CF patients have been used to identify loss of CFTR mediated transport by short-circuit and organoid swelling (19–21). However, there is no data on intraluminal bicarbonate concentration, bicarbonate transport processes, or even the expression profile of acid-base transporters in the distal colon and/or rectum. Re-analyzing existing scRNA-seq data we identified that DRA (SLC26A3) is highly expressed bicarbonate transporter in the colon and rectum, even more so than CFTR. In human proximal colonoids, Tse et al. found that DRA activity was influenced by the expression of CFTR (22). It is unclear if the profound decrease in rectal pH and bicarbonate concentration we observed was due to loss of CFTR-mediated bicarbonate secretion alone or to a combination of CFTR- and DRA-mediated bicarbonate transport. Further research into how loss of CFTR affects other acid-base transporters in the colon will be important.
Our current study has several limitations. The WMC has only one pressure sensor, it cannot measure contraction related propagation between two points. Hence, we were not able to define details of high amplitude propagating contractions (HAPC) which are considered hallmark of normal neuromuscular integrity of the colon. WMC also does not provide information on exact location. We attempted to overcome this by using predefined windows from anal exit. Bicarbonate concentration values are estimates rather than actual values since intraluminal pCO2 measurements were not obtained at the time of WMC. ScRNA-seq data was only available from non-CF patients and mRNA and protein membrane expression may be discordant. Finally, our scRNA-seq data involved analysis only of non-CF subjects. We did not compare transcriptomic analysis of acid-base transporter expression in subjects with CF, although this would be an important next step to deepen our understanding how loss of CFTR expression and/or function impacts intestinal pH. Despite these limitations, our study uses a combination of clinical and laboratory techniques to bring new insight into colonic pH in CF. We hope that the data generated in this pilot study will help spur further research in the clinical utility of measuring colorectal pH in CF, pathogenesis of CF colonic disease, and potential new targets to ameliorate GI complications in CF.
Highlights.
We provide the first study attempting to understand colonic pH, bicarbonate, and motility profiles from patients with cystic fibrosis.
Colonic pH and bicarbonate concentration is impaired in PwCF, particularly in the distal rectosigmoid.
These changes likely originate from alterations in intestinal ion transport rather than colonic dysmotility.
SLC26A3 is abundantly expressed in the human colon and rectum and may be a potential therapeutic target for restoration of bicarbonate transport in PwCF.
Our findings may help better understand the gastrointestinal symptoms in PwCF and provides the framework for future studies in this area.
ACKNOWLEDGMENTS
Drs. Patel and Sellers are former Cystic Fibrosis Foundation DIGEST awardees (SLUPATEL19GE0, SELLER19GE0). Dr. Sellers is also supported by the National Institute of Diabetes, Digestive, and Kidney Diseases (K08DK124684), the Cystic Fibrosis Foundation (SELLER20-KB), and Stanford University.
Funding:
Funds for personnel to perform the study was provided by the Cystic Fibrosis Foundation (SLUPATEL19GE0 to DP, SELLER19GE0 and SELLER20-KB to ZMS) and the National Institute of Diabetes, Digestive, and Kidney Diseases (K08DK124684 to ZMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors report no conflict of interest related to this study.
REFERENCES
- 1.Howden CW, Hunt RH. Relationship between gastric secretion and infection. Gut. 1987;28(1):96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishiguro H, Steward M, Naruse S. Cystic fibrosis transmembrane conductance regulator and SLC26 transporters in HCO(3)(−) secretion by pancreatic duct cells. Sheng Li Xue Bao. 2007;59(4):465–76. [PubMed] [Google Scholar]
- 3.Seidler U, Nikolovska K. Slc26 Family of Anion Transporters in the Gastrointestinal Tract: Expression, Function, Regulation, and Role in Disease. 92019. p. 839–72. [DOI] [PubMed] [Google Scholar]
- 4.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr Clin Pract. 2006;21(4):351–66. [DOI] [PubMed] [Google Scholar]
- 6.Borowitz D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr Pulmonol. 2015;50 Suppl 40:S24–S30. [DOI] [PubMed] [Google Scholar]
- 7.Gelfond D, Ma C, Semler J, Borowitz D. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig Dis Sci. 2013;58(8):2275–81. [DOI] [PubMed] [Google Scholar]
- 8.Koga I, Stiernstrom H, Christiansson L, Wiklund L. Intraperitoneal and sigmoid colon tonometry in porcine hypoperfusion and endotoxin shock models. Acta Anaesthesiol Scand. 1999;43(7):702–7. [DOI] [PubMed] [Google Scholar]
- 9.Hao Y, Hao S, Andersen-Nissen E, Mauck WM 3rd, Zheng S, Butler A, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573–87 e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Song W, Wang J, Wang T, Xiong X, Qi Z, et al. Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine. J Exp Med. 2020;217(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corral JE, Dye CW, Mascarenhas MR, Barkin JS, Salathe M, Moshiree B. Is Gastroparesis Found More Frequently in Patients with Cystic Fibrosis? A Systematic Review. Scientifica (Cairo). 2016;2016:2918139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA. Acid-stimulated duodenal bicarbonate secretion involves a CFTR-mediated transport pathway in mice. Gastroenterology. 1997;113(2):533–41. [DOI] [PubMed] [Google Scholar]
- 13.Pratha VS, Thompson SM, Hogan DL, Paulus P, Dreilinger AD, Barrett KE, et al. Utility of endoscopic biopsy samples to quantitate human duodenal ion transport. J Lab Clin Med. 1998;132(6):512–8. [DOI] [PubMed] [Google Scholar]
- 14.Farmer AD, Mohammed SD, Dukes GE, Scott SM, Hobson AR. Caecal pH is a biomarker of excessive colonic fermentation. World J Gastroenterol. 2014;20(17):5000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nugent SG, Kumar D, Rampton DS, Evans DF. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48(4):571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brun R, Michalek W, Surjanhata BC, Parkman HP, Semler JR, Kuo B. Comparative analysis of phase III migrating motor complexes in stomach and small bowel using wireless motility capsule and antroduodenal manometry. Neurogastroent Motil. 2012;24(4). [DOI] [PubMed] [Google Scholar]
- 17.Kloetzer L, Chey WD, McCallum RW, Koch KL, Wo JM, Sitrin M, et al. Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule. Neurogastroenterol Motil. 2010;22(5):527–33, e117. [DOI] [PubMed] [Google Scholar]
- 18.Malagelada C, Bendezu RA, Segui S, Vitria J, Merino X, Nieto A, et al. Motor dysfunction of the gut in cystic fibrosis. Neurogastroenterol Motil. 2020;32(9):e13883. [DOI] [PubMed] [Google Scholar]
- 19.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19(7):939–45. [DOI] [PubMed] [Google Scholar]
- 20.Silva IAL, Duarte A, Marson FAL, Centeio R, Dousova T, Kunzelmann K, et al. Assessment of Distinct Electrophysiological Parameters in Rectal Biopsies for the Choice of the Best Diagnosis/Prognosis Biomarkers for Cystic Fibrosis. Front Physiol. 2020;11:604580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veeze HJ, Sinaasappel M, Bijman J, Bouquet J, de Jonge HR. Ion transport abnormalities in rectal suction biopsies from children with cystic fibrosis. Gastroenterology. 1991;101(2):398–403. [DOI] [PubMed] [Google Scholar]
- 22.Tse CM, Yin J, Singh V, Sarker R, Lin R, Verkman AS, et al. cAMP Stimulates SLC26A3 Activity in Human Colon by a CFTR-Dependent Mechanism That Does Not Require CFTR Activity. Cell Mol Gastroenterol Hepatol. 2019;7(3):641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


