Abstract
Background:
This study sought to investigate health and healthcare disparities in the management of severe mitral regurgitation with transcatheter edge-to-edge repair using MitraClip and how racial differences impact resource utilization and costs.
Methods:
We retrospectively analyzed the National Inpatient Sample (NIS) for patients who underwent Transcatheter Edge-to-Edge Repair (TEER) using MitraClip between 2016 and 2018. The patients were stratified into four racial cohorts and study outcomes included high resource utilization (HRU), periprocedural complications, and total procedural costs. High resource utilization (HRU) was defined as length of stay (LOS) ≥7 days or a nonhome disposition at discharge. Multivariate logistic regression models were utilized to determine independent predictors of HRU.
Results:
17,100 weighted TEER patients were segregated by race: Caucasian (n = 13,270), others (n = 1510), African Americans, AA (n = 1245) and Hispanics (n = 1075). More African Americans and Hispanics had TEER at Urban facilities (P < 0.001), which were teaching hospitals as well (P < 0.001) but were less likely to be covered by public insurance options -Medicare or Medicaid (P < 0.001). More AA (52.2 %) and Hispanics (27.6 %) were likely to be in the lowest median annual income quartile versus Caucasians (19.2 %) (P = 0.003). AA and Hispanics had higher resource utilization (HRU), prolonged length of stay, nonhome disposition at discharge, higher procedural costs and periprocedural complications versus Caucasians. The logistic regression model revealed acute kidney injury (AKI) and actual procedural costs as independent predictors of HRU in both African American and Hispanic groups.
Conclusion:
Significant Health and healthcare disparities do exist among underrepresented, racial minority patients undergoing transcatheter edge-to-edge repair in the US. These disparities were associated with higher resource utilization and actual costs in patients with mitral regurgitation treated with TEER.
Keywords: Transcatheter, Healthcare resource utilization, Disparity
1. Introduction
Mitral regurgitation (MR) is the most common valvular heart disease in the United States, affecting >2 million people [1]. Cardiac surgery is considered the standard of care in the treatment of symptomatic severe primary mitral regurgitation [2,3]. Recently, Transcatheter-edge-to-edge repair (TEER) using MitraClip was approved and incorporated into the practice guidelines as a reasonable option for patients with primary severe MR who are at high or prohibitive surgical risk with at least 1-year life expectancy [4]. TEER has also found utility as a reasonable consideration in the management of patients with secondary severe mitral regurgitation with left ventricular ejection fraction of 20–50 % on maximally tolerated Goal Directed Medical Therapy (GDMT) with clinical benefits sustained through 3 years after percutaneous MitraClip placement [4,5].
Racial disparities in the utilization and mortality outcomes of TEER have been suggested in a previous analysis of a nationwide database which showed Caucasian patients had higher in-hospital mortality compared with African Americans [6]. However, a recent Cohort-based observational study using the National Inpatient Sample showed that African American patients experienced a higher rate of in-hospital death, but a similar overall rate of post-procedural adverse events when compared to Caucasian patients. It also alluded to the negative impact of Lower income levels on clinical outcomes in underrepresented minority TEER patients [7]. While comparative clinical outcomes following TEER in different ethnicities and racial groups have been shown, the impact of race on resource utilization and procedural cost has not been well established. We analyzed racial disparities among severe mitral regurgitation patients treated with TEER using MitraClip and identified how these dissimilarities led to high resource utilization (HRU). HRU was defined as a prolonged length of hospitalization greater or equal to 7 days or nonhome disposition at discharge [8]. Results from this analysis may have far-reaching implications in recent efforts to reduce overall costs in the current healthcare economy.
2. Methods
The National inpatient sample (NIS) was the data source utilized for this retrospective observational analysis. The NIS is the largest widely accessible registry of inpatients in the United States. This registry is maintained through a Federal-State-industry partnership under the auspices of the Agency for Healthcare Research and Quality (AHRQ). It contains clinical data from >7 million admissions annually representing over 35 million actual hospitalizations [9]. Institutional Review Board approval was not required as the registry contained de-identified patient information and is publicly accessible.
We analyzed the NIS from 2016 to 2018 for patients who underwent Transcatheter Edge-to-Edge Repair (TEER) with MitraClip using the ICD-10 procedure code 02UG3JZ. 17,100-weighted study subjects who underwent TEER were included in the analysis while 67 patients with missing data were excluded from the study. The final study subjects were stratified into four Ethnic/Racial cohorts: Caucasians, African Americans (AA), Hispanics, and others (Asians, Pacific Islanders, and Native Americans). The baseline features extracted included median age, sex, race, associated comorbidities (Diabetes mellitus, hypertension, obesity, coronary artery disease, chronic heart failure, chronic lung disease, and chronic renal failure), Charlson co-morbidity categories, median household income according to patient’s zip code extrapolated from census data, insurance type, hospital characteristics.
The principal outcome of interest was HRU, a surrogate for the composite of prolonged hospital stay greater or equal to 7 days or nonhome disposition at discharge [10]. Secondary endpoints were actual procedural costs, length of stay, non-home discharge location, periprocedural complications including acute coronary syndrome, vascular injury, stroke, acute kidney injury, stroke, acute kidney injury, acute kidney injury requiring dialysis, use of mechanic circulatory supportive devices, pacemaker placement and a composite of any of these procedural complications.
3. Statistical analysis
We described baseline characteristics and outcomes within each ethnic/racial cohort, using descriptive statistics and analyzed utilizing the Kruskal Wallis H method. A multivariate logistic regression model was utilized to determine independent variables which successfully predicted HRU within each cohort. A stepwise regression evaluation was used to select the variables.
Variables used for the multivariate logistic regression model, included Age, female gender, associated comorbidities (Diabetes mellitus, hypertension, obesity, coronary artery disease, chronic heart failure, chronic lung disease, and chronic renal failure), insurance status, median annual income, hospital bed size, teaching hospital status, hospital regions, hospital location, perioperative acute coronary syndrome, vascular injury, acute kidney injury, acute kidney injury requiring renal replacement therapy, mechanical circulatory support, and pacemaker placement. The reference cohort was the Caucasian group. Associations were deemed significant if the p-value was <0.05. Statistical analyses were performed using STATA software: version 17 (Stata/BE 17, StataCorp College Station, TX, USA).
4. Results
Among 17,100 study subjects who underwent TEER between 2016 and 2018, 13,270 were Caucasians (77.6 %), 1510 were others (8.8 %), 1245 were African Americans (7.3 %), and 1075 were Hispanics (6.3 %). The racial minority cohort was significantly younger when compared with the Caucasian cohort. The gender distribution showed that the African American and Hispanic categories had female majorities of 57 % and 50.7 % while the others and Caucasians had female minorities of 49.9 % and 46.3 %. The comorbidity burden was heterogeneously distributed across the racial groups. Of note, the minority groups had a higher comorbidity burden of Charlson comorbidity index of 3 or greater with African Americans having the highest percentage of 74.1, followed by Hispanics (64 %), others (56.8 %), and Caucasians (52.0 %). Comorbidities that were significantly different across the groups, included diabetes mellitus (P < 0.001), obesity (P < 0.001), coronary artery disease (P < 0.001), chronic lung disease (P = 0.025), and chronic renal failure (P < 0.001).
The Hispanic cohort had the highest proportions with a history of diabetes mellitus (44.2 %). African Americans had the highest proportion of obesity (19 %), while Caucasians had the highest percentage of patients with a history of coronary artery disease (72.2 %). African Americans had the highest percentage of chronic lung disease (29.3 %) and chronic renal failure (47.3 %). This study revealed a heterogenous distribution of median annual income, a surrogate of socioeconomic status, across the four racial groups. Essentially, the ethnic minorities were in the lowest median annual income level when they were compared with the Caucasian cohort. The African American group had 52.2 % in the lowest socio-economic group, followed by Hispanics (27.6 %), Caucasians (19.2 %), and others (18.5 %) (Table 1). Furthermore, the minority cohorts largely, underwent TEER in large bed-sized, urban hospitals, when compared with the Caucasian group. 84.7 % of Hispanics, 80.1 % of others, and 77.5 % of African Americas patronized hospitals with large bed sizes for the management of severe mitral regurgitation via TEER, compared with 74.3 % of Caucasians.
Table 1.
Baseline demographic and clinical characteristics of TEER patients in the United States stratified by race.
| Variable | Caucasians (n = 13,270) | African American (n = 1245) | Hispanics (n = 1075) | Others (n = 1510) | P value |
|---|---|---|---|---|---|
| Age | 79.1 ± 8 | 70.8 ± 10 | 76.1 ± 9 | 76.8 ± | <0.001 |
| Sex | |||||
| Female | 6146 (46.3) | 710 (57.0) | 545 (50.7) | 754 (49.9) | <0.001 |
| Male | 7124 (53.7) | 535 (43.0) | 530 (49.3) | 756 (50.1) | <0.001 |
| History of | |||||
| DM | 4486 (33.8) | 523 (42.0) | 475 (44.2) | 604 (40.0) | <0.001 |
| Hypertension | 8028 (60.5) | 748 (60.1) | 664 (61.8) | 939 (62.2) | 0.542 |
| Obesity | 1991 (15.1) | 237 (19.0) | 169 (15.7) | 183 (12.1) | <0.001 |
| CAD | 9581 (72.2) | 830 (66.7) | 741 (68.9) | 1060 (70.2) | <0.001 |
| CHF | 438 (3.3) | 54 (4.3) | 35 (3.3) | 50 (3.3) | 0.365 |
| Chronic lung disease | 3875 (29.2) | 365 (29.3) | 283 (26.3) | 387 (25.6) | 0.025 |
| Chronic renal failure | 4127 (31.1) | 589 (47.3) | 366 (34.0) | 521 (34.5) | <0.001 |
| Charlson co-morbidity | 0.004 | ||||
| 0 | 860 (6.5) | 35 (2.8) | 25 (2.3) | 80 (5.3) | |
| 1 | 2970 (22.4) | 120 (9.6) | 205 (14.0) | 105 (20.6) | |
| 2 | 2540 (19.1) | 165 (13.2) | 230 (19.0) | 85 (16.7) | |
| ≥3 | 6900 (52.0) | 925 (74.1) | 615 (64.0) | 290 (56.8) | |
| Insurance status | 0.569 | ||||
| Public | 12,119 (91.3) | 1101 (88.4) | 953 (88.7) | 1358 (89.9) | |
| Private | 1115 (8.4) | 144 (11.6) | 112 (10.4) | 152 (10.1) | |
| Uninsured or self-pay | 36 (0.3) | 0 | 10 (0.9) | 0 | |
| Median Annual income (US dollars) | 0.003 | ||||
| 1–45,999 | 2550 (19.2) | 650 (52.2) | 328 (27.6) | 280 (18.5) | |
| 46,000–58,999 | 3198 (24.1) | 274 (22.0) | 282 (28.6) | 346 (22.9) | |
| 59,000 - 78,999 | 3781 (28.5) | 213 (17.1) | 261 (21.4) | 437 (29.0) | |
| ≥79,000 | 3741 (28.2) | 108 (8.6) | 204 (19.1) | 447 (29.6) | |
| Hospital bed size | <0.001 | ||||
| Small | 786 (5.9) | 60 (4.8) | 75 (7.0) | 115 (10.3) | |
| Medium | 2630 (19.8) | 220 (17.7) | 90 (8.3) | 145 (9.6) | |
| Large | 9854 (74.3) | 965 (77.5) | 910 (84.7) | 1210 (80.1) | |
| Hospital location | <0.001 | ||||
| Urban | 13,210 (99.6) | 1245 (100.0) | 1075 (100.0) | 1510 (100.0) | |
| Rural | 60 (0.4) | 0 | 0 | 0 | |
| Hospital teaching status | <0.001 | ||||
| Non-teaching | 1080 (8.1) | 90 (7.2) | 100 (9.3) | 215 (14.2) | |
| Teaching | 12,190 (91.9) | 1155 (92.8) | 975 (90.7) | 1295 (85.8) | |
| Hospital region | <0.001 | ||||
| Northeast | 2385 (18.0) | 160 (12.9) | 120 (11.2) | 215 (14.2) | |
| Midwest | 2730 (20.5) | 225 (18.1) | 70 (6.5) | 470 (31.1) | |
| South | 4751 (35.8) | 630 (50.6) | 490 (45.6) | 265 (17.6) | |
| West | 3404 (25.7) | 230 (18.4) | 395 (36.7) | 560 (37.1) |
Abbreviations: CAD, coronary artery disease; CHF, chronic heart failure; DM, diabetes mellitus; TEER, transcatheter edge-to-edge repair; US, United States.
Data presented as mean ± standard deviation or n (%).
All the minority groupings underwent TEER in urban hospitals (100 %) compared with Caucasians (99.6 %). Caucasians (0.4 %) were the only racial group that was seen in rural settings. African Americans were largely concentrated in the South (50.6 %), followed by Hispanics (45.9 %), and Caucasians (35.8 %). Others were more likely to present to hospitals in the West (37.1 %).
The primary outcome of interest, HRU, was significantly different across the groups (P = 0.003). African Americans (22.5 %), Hispanics (20.5 %), and others (15.9) were more likely to have high resource utilization when compared with Caucasians (14.9 %). The secondary outcomes of our study were also statistically different in length of stay (P < 0.001), nonhome disposition at discharge (P < 0.001), actual procedural costs (P < 0.001), periprocedural acute coronary syndrome (P < 0.001), AKI (P < 0.001), AKI requiring dialysis (P < 0.001), and composite perioperative complications (P < 0.001). The racial minority patients (African Americans, Hispanics, and others) had higher rates of these periprocedural complications. Hispanics and African Americans were more likely to be discharged to nonhome locations (24.5 % and 20.9 %) versus Caucasians (16.9 %). Racial minority cohorts, African Americans, Hispanics, and others had higher actual procedural costs ($25,218 ± 15,743, $25,415 ± 16,816, and $27,443 ± 14,952 respectively), when compared to Caucasians ($22,516 ± 12,683) Additionally, Hispanics, African Americans, and others had longer length of stay (7.1, 5.5 and 4.8 days respectively), while the Caucasians had 4.3 days of hospitalization. Pacemaker placement (P = 0.055), mechanical circulatory support (P = 0.091), stroke (P = 0.064), and vascular injuries (P = 0.072) were not different among the groups (Table 2).
Table 2.
In hospital outcomes of study population stratified by race.
| Variable | Caucasians (n = 13,270) | African Americans (n = 1245) | Hispanics (n = 1075) | Others (n = 1510) | P value |
|---|---|---|---|---|---|
| Length of stay (days) | 4.3 | 5.5 | 7.1 | 4.8 | <0.001 |
| Nonhome disposition at discharge | 2245 (16.9) | 305 (24.5) | 225 (20.9) | 235 (15.6) | <0.001 |
| Actual costs (US dollars) | 22,516 ± 12,683 | 25,218 ± 15,743 | 25,415 ± 16,816 | 27,443 ± 14,952 | <0.001 |
| HRU | 1970 (14.9) | 280 (22.5) | 220 (20.5) | 240 (15.9) | 0.003 |
| Periprocedural ACS | 795 (6.0) | 90 (7.1) | 100 (9.3) | 155 (10.3) | <0.001 |
| Vascular injury | 170 (1.3) | 25 (2.0) | 15 (1.4) | 20 (1.3) | 0.072 |
| Stroke | 25 (0.2) | 0 | 0 | 5 (0.3) | 0.064 |
| AKI | 1920 (14.5) | 230 (18.5) | 200 (18.6) | 280 (18.5) | <0.001 |
| AKI requiring dialysis | 165 (1.2) | 80 (6.4) | 70 (6.5) | 40 (2.7) | <0.001 |
| MCS | 60 (0.5) | 5 (0.4) | 0 | 15 (1.0) | 0.091 |
| PPM placement | 60 (0.5) | 5 (0.4) | 5 (0.5) | 0 | 0.055 |
| Composite complications | 2370 (17.9) | 295 (23.7) | 270 (25.1) | 335 (22.2) | <0.001 |
Abbreviations: ACS, acute coronary syndrome; AKI, acute kidney injury; HRU, high resource utilization; MCS, mechanical circulatory support; PPM, permanent pacemaker.
Data presented as mean ± standard deviation, median (IQR), or n (%).
The multivariate logistic regression model used, showed that AKI and actual procedural costs were independent predictors of HRU following TEER in all patients in the study. When the model was applied to the TEER Caucasian patients, AKI and actual procedural costs predicted HRU. Similarly, the logistic regression analyses applied to the African American patients also showed that AKI and actual procedural costs were strongly associated with HRU (Fig. I). The multivariate logistic regression model applied to both the Hispanic and other cohorts did not yield any associations (Fig. 2).
Fig. I.
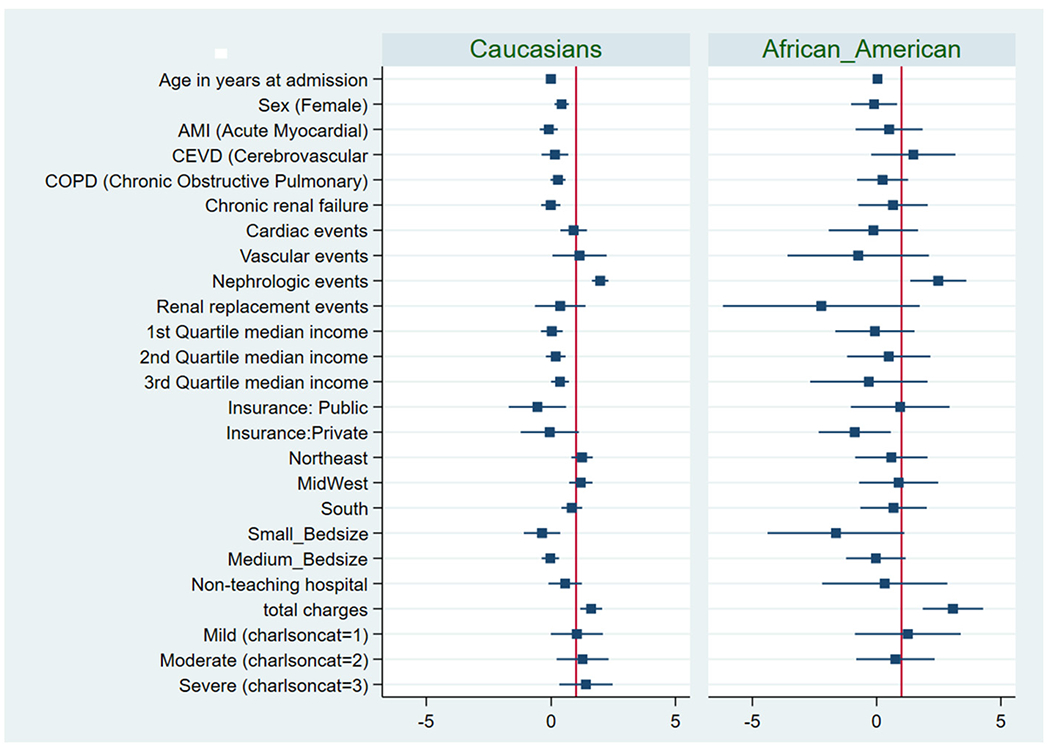
Forest plot showing the multivariate logistic regression analyses for variables associated with HRU including independent predictors of high resource utilization among TEER patient in Caucasian and African American cohorts.
Fig. 2.
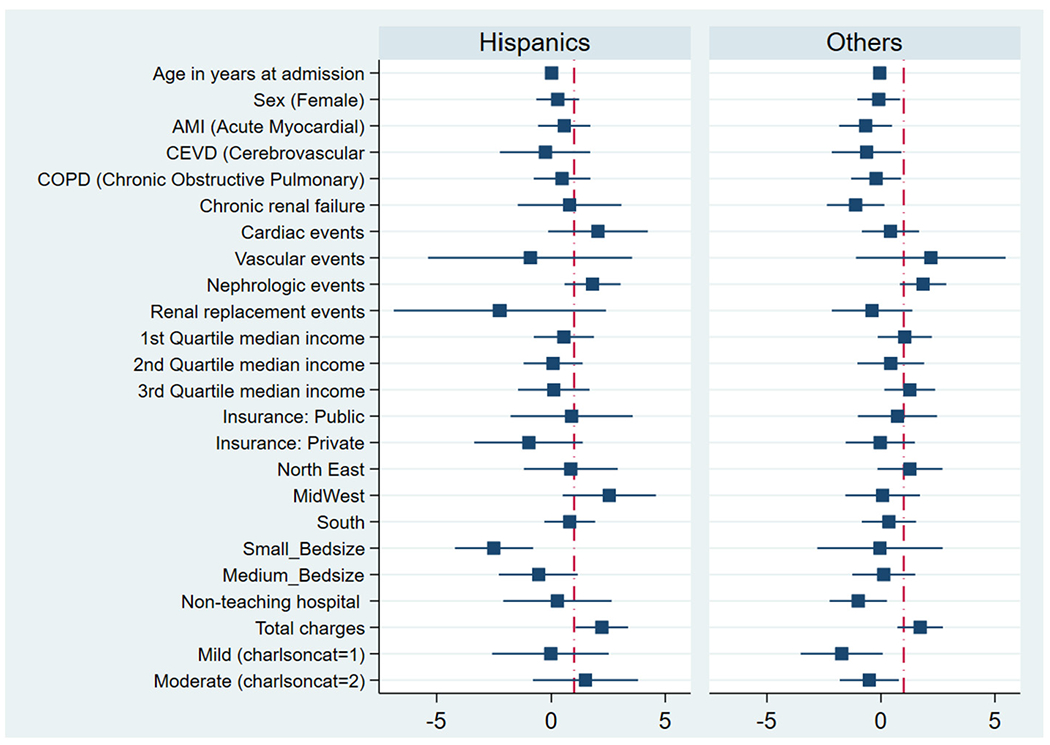
Forest plot showing the multivariate logistic regression analyses for variables associated with HRU including independent predictors of high resource utilization among TEER patient in Hispanic and other groups.
5. Discussion
Our study showed that there exist health and healthcare disparities among patients with severe mitral regurgitation who underwent TEER in the United States. Essentially, racial minority TEER patients reported a higher rate of perioperative complications which translated into higher resource utilization and actual procedural costs.
There was a substantial underrepresentation in access of these minority patients who underwent TEER with MitraClips for the management of patients with both primary and secondary severe mitral regurgitation. This disparity in access to innovative technologies in the management of structural heart disease has been reported in previous studies [6,7,11,12]. Furthermore, this study demonstrated that minority patients were significantly younger with higher baseline comorbidities when compared with Caucasian patients. This was further corroborated by a previous observational analysis which showed that among 7940 transcatheter mitral valve repair (TMVR) from 2012 to 2016, Caucasians were significantly older (77.7 ± 10.8 vs. 67.2 ± 14.28, p < 0.001) when compared their African American counterparts [6]. Elbadawi et al. also showed higher rates of chronic illnesses, such as diabetes, CKD, chronic lung disease, chronic anemia, and prior ICD placement [6]. Considering this high burden of comorbidities in underrepresented racial minority patients, it is safe to state that these are the patients who are more likely to benefit from TEER as their overall profile may increase their surgical risks as compared with Caucasian patients.
We also showed that minority TEER patients had disproportionately high resource utilization (HRU) and procedure costs, with worse periprocedural complication rates when juxtaposed with their Caucasian colleagues. These attributions are supported by previous reports from the American Heart Association, which forecasted that the total costs of cardiovascular diseases in minority patients are anticipated to exceed those of Caucasians in the next thirteen years [13]. As the focus on heart care begins to shift efforts from curative measures to preventive strategies, this healthcare cost can be alleviated as the burden to curb to rising prevalence of cardiovascular disease including valvular heart disease such as mitral regurgitation. Even though some of these racial disparities may be attributed to genetic differences and other social determinants of health, these preventive efforts are monumental in the management of the cardiovascular disease [14].
In a cohort-based observational study of the national inpatient sample, Sparrow et al. showed racial and ethnic disparities in TEER outcomes, aged-adjusted race and ethnic minorities were less underrepresented in clinical events as compared to Caucasian patients, with lower income levels having a negative impact on in-hospital outcomes [7]. Additionally, in another retrospective analysis, Alkhouli et al. observed no significant differences in the adjusted hospital mortality or key complications between patients of Caucasian, African American, and Hispanic ethnicity following three structural heart disease interventions, transcatheter aortic valve replacement, transcatheter aortic valve replacement, transcatheter mitral valve repair or left atrial appendage occlusion. They also showed no difference in cost between Caucasian and African American patients [11]. Our study is one of few analyses to show that racial disparities may have an impact on healthcare resource utilization and costs. This is the first study that assessed the impact of race on increased healthcare resource utilization by looking at the length of stay and non-home discharge disposition, including actual operative costs, which is a significant component of overall healthcare cost, in the realm of transcatheter edge-to-edge repair with MitraClips.
6. Limitations
This analysis, as with all other observational studies, is not without limitations. Unmeasured variables and other confounders may affect some of the associations described in this study. An important limitation of this study stems from the nature of the NIS database, as results from this study may be subject to coding and documentation errors. Data used for this analysis was without granular echocardiographic information, including left ventricular systolic and diastolic, regurgitant fraction and volume, regurgitant orifice area, and quantitation of the severity of regurgitant jet. All of which may potentially have an impact on in-hospital clinical outcomes in studies such as our analysis. Despite the unavailability of these parameters, analysis of large registries such as the National inpatient sample (NIS) database provide key insights into the understanding of the current real-world practice and serves as hypothesis-generating tools in the field of structural interventional cardiology and other areas of important research.
Lastly, our study primarily reported in-hospital outcomes without long-term follow-up data. The strength of this analysis is highlighted in the large size of the study population, including the nationally representative nature of the subjects included in the study.
7. Conclusion
In conclusion, we demonstrated that racial minority groups undergoing TEER for severe mitral regurgitation, even though, were younger had a higher burden of comorbid maladies at baseline. These minority groups also had low socio-economic status. These disparities were associated with poor clinical outcomes, periprocedural complications rates, and ultimately HRU. Further research is needed to uncover the factors that underpin these disparities in health and healthcare resource utilization in the realm of transcatheter edge-to-edge repair and more importantly, strategies to bridge these gaps in healthcare delivery in the United States.
Funding
The authors received no monetary support for the conduct of this research and its subsequent publication.
Footnotes
CRediT authorship contribution statement
Sheriff N Dodoo: conceptualization, methodology, data curation, writing - original draft, visualization, investigation, writing- review & editing.
Alexis Okoh: writing- review and editing.
Tanya Aggarwal: writing - review and editing.
Abdul- Fatawu Osman: writing- review and editing.
Emmanuel Nkansah: conceptualization, metrology, data curation, writing.
Abdullahi Oseni: writing- review and editing.
Oghenerukevwe Odiete: writing- review and editing.
Ugochukwu Egolum: writing - review and editing.
Declaration of competing interest
The authors declare no actual or potential conflicts of interest relevant to the conduct of this research and its subsequent publication.
References
- [1].Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006. Sept 16;368:1005–11. [DOI] [PubMed] [Google Scholar]
- [2].Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology /American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
- [3].Dodoo SN, Okoh AK, Oseni A, Odiete O, Okafor HE. Adverse events following transcatheter edge-to-edge repair (TEER) using MitraClip: lessons learned from the manufacturer and user facility device experience (MAUDE) registry. Cardiovasc Revasc Med. 2022. Jun;39:101–5. 10.1016/j.carrev.2021.09.007. Epub 2021 Sep 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021. Feb 2;143:e72–227. [DOI] [PubMed] [Google Scholar]
- [5].Mack MJ, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant BK, Grayburn PA, Rinaldi MJ, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Rogers JH, Marx SO, Cohen DJ, Weissman NJ, Stone GW, Investigators COAPT. 3-Year outcomes of transcatheter mitral valve repair in patients with heart failure. J Am Coll Cardiol. 2021. Mar 2;77(8):1029–40. 10.1016/j.jacc.2020.12.047. [DOI] [PubMed] [Google Scholar]
- [6].Elbadawi A, Mahmoud K, Elgendy IY, Elzeneini M, Megaly M, Ogunbayo G, Omer MA, Albert M, Kapadia S, Jneid H. Racial disparities in the utilization and outcomes of transcatheter mitral valve repair: insights from a national database. Cardiovasc Revasc Med. 2020. Nov;21(11):1425–30. 10.1016/j.carrev.2020.04.034. Epub 2020 Apr 30 [DOI] [PubMed] [Google Scholar]
- [7].Sparrow RT, Sanjoy SS, Lindman BR, Tang GHL, Kaneko T, Wasfy JH, Pershad A, Villablanca PA, Guerrero M, Alraies MC, Choi YH, Sposato LA, Mamas MA, Bagur R. Racial, ethnic and socioeconomic disparities in patients undergoing transcatheter mitral edge-to-edge repair. Int J Cardiol. 2021. Dec 1;344:73–81. 10.1016/j.ijcard.2021.09.037. Epub 2021 Sep 21 [DOI] [PubMed] [Google Scholar]
- [8].Okoh AK, Dhaduk N, Shah AM, Gold J, Fugar S, Kassotis J, Chen C, Lee LY, Russo MJ. Health and healthcare disparities: impact on resource utilization and costs after transcatheter aortic valve replacement. (Phila). Innovations 2021. May-Jun;16(3):262–6. 10.1177/1556984521996694. Epub 2021 Mar 18. [DOI] [PubMed] [Google Scholar]
- [9].Healthcare Cost and Utilization project (HCUP): Agency for Healthcare Research and Quality. Accessed in July 14th, 2020 at. www.hcup-us.ahrq.gov/nisoverview.jsp; December 2019.
- [10].Okoh AK, Dhaduk N, Shah AM, Gold J, Fugar S, Kassotis J, Chen C, Lee LY, Russo MJ. Health and healthcare disparities: impact on resource utilization and costs after transcatheter aortic valve replacement. (Phila). Innovations 2021. May-Jun;16(3): 262–42. 10.1177/1556984521996694. Epub 2021 Mar 18. [DOI] [PubMed] [Google Scholar]
- [11].Alkhouli M, Alqahtani F, Holmes DR, Berzingi C. Racial disparities in the utilization and outcomes of structural heart disease interventions in the United States. J Am Heart Assoc. 2019. Aug 6;8(15):e012125. 10.1161/JAHA.119.012125. Epub 2019 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Spring AM, Catalano MA, Rutkin B, Hartman A, Yu PJ. Racial and socioeconomic disparities in urgent transcatheter mitral valve repair: a National Inpatient Sample analysis. J Card Surg. 2021. Sep;36(9):3224–9. 10.1111/jocs.15735. Epub 2021 Jun 10 [DOI] [PubMed] [Google Scholar]
- [13].American Heart Association. Cardiovascular disease: a costly burden in America, projections through 2035. https://healthmetrics.heart.org/-/media/files/get-involved/advocacy/burden-report-consumer-report.pdf. (accessed June 13th, 2022).
- [14].Mensah GA. Cardiovascular diseases in African Americans: fostering community partnerships to stem the tide. Am J Kidney Dis. 2018;72(5 Suppl 1):S37–42. 10.1053/j.ajkd.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]


