Abstract
Objective:
We evaluated the pharmacokinetics of double-dose levonorgestrel implants to overcome the drug-drug interaction with efavirenz-based antiretroviral therapy (ART).
Study Design:
We conducted a non-randomized, open-label, parallel-group, longitudinal pharmacokinetic study among Ugandan women ages 18 to 45 years. Participants with HIV on ART containing efavirenz 600mg received 300mg LNG implants (Jadelle®, Bayer, New Zealand): 300LNG+ART group. We compared our outcomes with women without HIV using standard dose, 150mg LNG implants: 150LNG group. The implant was placed on day 0 in both groups, and we quantified plasma LNG concentrations over 48 weeks post-implant insertion. LNG pharmacokinetic parameters were estimated using noncompartmental techniques. Our primary outcome was the geometric mean ratio (GMR) with 90% confidence intervals (CI) of LNG area under the concentration-time curve over 24 weeks (AUC0–24w) between groups. Demographic data were described as median (interquartile range). A secondary outcome compared between-group percent of levonorgestrel concentrations ≥300 pg/mL, a minimum threshold selected a priori based on observed pregnancies in Ugandan women on standard-dose levonorgestrel implants plus efavirenz.
Results:
We enrolled 27 women in the 300LNG+ART group [34 (28.0–40.5) years and 61.0 (49.8–66.0) kg] and 19 women in the 150LNG group [33 (30.0–34.5) years and 64.9 (59.0–74.5) kg]. LNG AUC0–24w was 34% lower for 300LNG+ART vs. 150LNG [geometric mean 9998 vs. 15,231 pg*week/mL, respectively (GMR 0.66 (90% CI: 0.54–0.80)]. The percentage of participants with LNG concentrations ≥300 pg/mL was not statistically different between groups at week 24 (300LNG+ART: 74.1%; 150LNG: 89.5%; P=0.27).
Conclusion:
Double-dose LNG implant did not completely overcome the drug-drug interaction with efavirenz.
1. Objectives
More than 222 million women face an unmet need for effective contraception in low- and middle-income countries [1]. In sub-Saharan Africa, women and girls comprised 63% of new HIV infections in 2020 [2]. Because long-acting reversible contraceptives offer high contraceptive effectiveness following a single medical intervention [3], modern contraceptive methods are critical for preventing unplanned pregnancies in these settings.
In sub-Saharan African countries, more than 60% of integrated family planning and HIV care sites offer contraceptive implants [4]. Clinically significant drug-drug interactions may occur between certain antiretrovirals and progestins released from implants [5–10]. We previously reported a 57% decrease in levonorgestrel (LNG) concentrations and three unintended pregnancies among Ugandan women using the 150mg LNG implant (2-rod subdermal implant, LNG 75mg/rod) plus efavirenz 600mg-based antiretroviral therapy (ART) over 48 weeks [6]. Efavirenz is a known inducer of the cytochrome P450 3A4 enzyme (CYP3A4), the enzyme primarily responsible for levonorgestrel metabolism. Thus, managing drug-drug interactions between contraceptive implants and efavirenz is a pressing public health need.
We investigated dose-adjustment of the LNG implant to overcome the established drug-drug interaction with efavirenz-based ART. Our primary objective was to determine the LNG plasma concentrations over 24 weeks of double-dose LNG implant in women with HIV taking efavirenz-based ART. We hypothesized that double-dose LNG would result in similar LNG exposure compared with a control group using a standard-dose implant.
2. Materials and Methods
We conducted a non-randomized, open-label, longitudinal pharmacokinetic study to assess the safety and pharmacokinetics of 300mg LNG subdermal implants (2 × 2-rod 75mg LNG/rod; Jadelle®; Bayer, New Zealand) among Ugandan women taking efavirenz-based ART (300LNG+ART group, n=27) [11]. We compared outcomes with a contemporaneous cohort of 19 adult Ugandan women without HIV and not on efavirenz-based ART receiving standard dose LNG implant (1 × 2-rod 75mg LNG/rod; 150LNG group) [12]. Investigators performed study-related procedures at the Infectious Diseases Institute (IDI), Makerere University College of Health Sciences, Kampala, Uganda, in accordance with the Declaration of Helsinki. Ethics boards at the Joint Clinical Research Centre Kampala, Uganda National Council for Science and Technology, and the University of Nebraska Medical Center approved all study procedures. All volunteers provided written informed consent. This study is registered in ClinicalTrials.gov (Identifier: NCT02722421).
2.1. Study population
We included women ages 18 and 45 years who desired the LNG implant based on clinical need [13]. Participants in the 300LNG+ART group were all living with HIV, taking once-daily efavirenz 600mg-based ART for at least 3 months before enrollment who had an undetectable viral load at screening (HIV-1 RNA <50 copies/mL) [14]. All 150LNG group volunteers had a negative HIV test at entry and received HIV prevention counseling at each visit. We excluded volunteers within 30 days postpartum, those breastfeeding within 6 months of delivery, those with contraindications to the LNG subdermal implant, and those taking products known to interact with LNG or efavirenz [11, 13, 15].
2.2. Intervention
All 300LNG+ART participants agreed to have a copper intrauterine device placed at entry to avoid the risk of undesired pregnancy. On day 0, after confirming a negative pregnancy test and reviewing participants’ self-reported sexual activity before entry, a trained clinician inserted the LNG subdermal implants according to product labeling [11]. In the 300LNG+ART group, the clinician inserted one system into each arm to avoid impacting LNG absorption from two systems at a single implant site. In the 150LNG group, clinicians inserted one implant system.
2.3. Sampling strategy and clinical assessments
All participants had whole blood collected on day 0 and weeks 1, 4, 12, 24, 36, and 48 to determine plasma LNG concentrations, a urine pregnancy test, and laboratory safety assessments. For participants taking efavirenz, we quantified mid-dose efavirenz concentrations (12–14 hours post-dose) from the same plasma sample to assess ART adherence. The 150LNG group completed follow-up at week 48. The 300LNG+ART group had extended follow-up every 24 weeks through week 144 (Figure 1). The 300LNG+ART group had optional visits on days 1 to 4 to characterize LNG maximum concentrations (Cmax) and the time to Cmax (Tmax).
Figure 1.
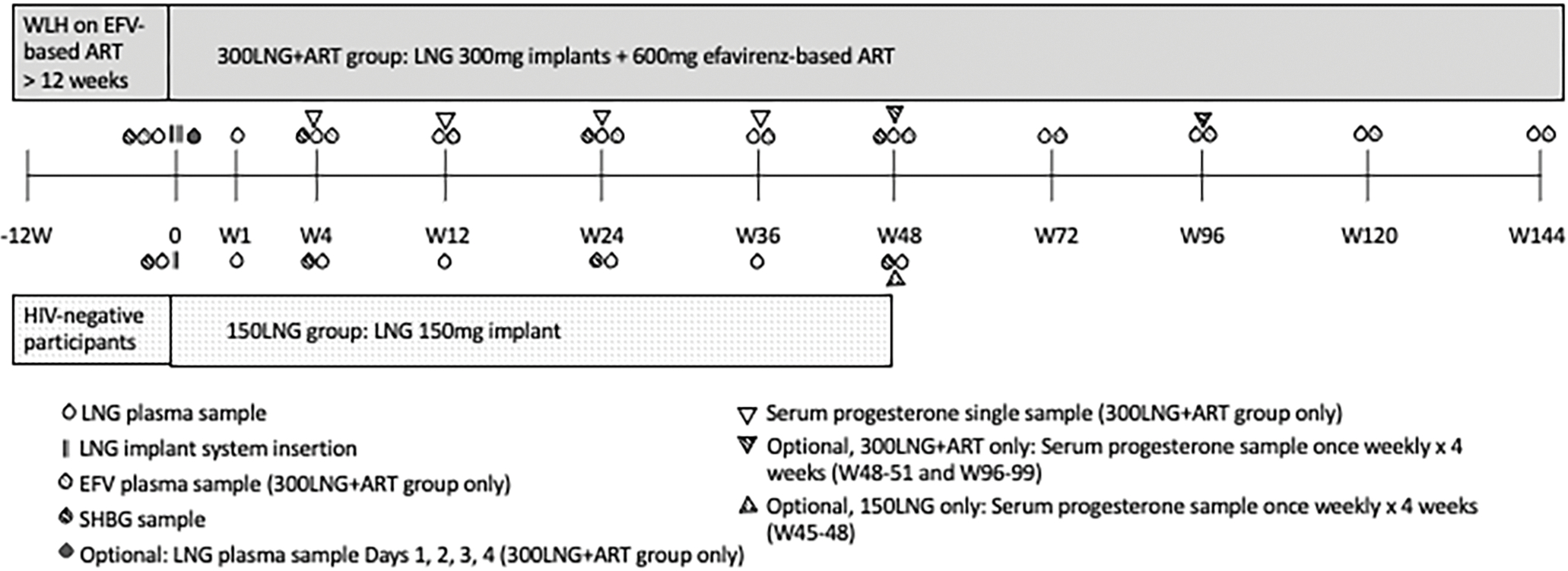
Study schema. Abbreviations: ART, antiretroviral therapy; EFV, efavirenz; LNG, levonorgestrel; SHBG, sex hormone binding globulin; WLH, women living with HIV.
To characterize possible ovulatory activity, we sampled endogenous serum progesterone concentrations and summarized the number of samples with progesterone concentrations ≥3 ng/mL [9.5 nmol/L] and ≥5 ng/mL [16 nmol/L] [16, 17]. In the 300LNG+ART group, we sampled progesterone at individual study visits in all participants, and for 4 weeks around week 48 and 96 study visits for those who consented to return weekly (Figure 1). Because the 150LNG group had serum progesterone concentrations sampled for 4 weeks around the week 48 visit only, we statistically compared the percent of participants with at least one progesterone sample ≥3 ng/mL and ≥5 ng/mL between both groups at week 48. We measured sex hormone binding globulin (SHBG) levels at regular intervals over 48 weeks.
Site investigators assessed study related clinical and laboratory adverse effects at all visits using the Division of AIDS Adverse Event Grading Tables [18]. Investigators assessed HIV treatment response and immune function by determining plasma HIV-1 RNA and CD4 T lymphocyte cell counts every 6 months during the first year then yearly thereafter.
2.4. Assay characteristics
Plasma was separated by centrifugation and stored at −80 degrees Celsius until sample shipment. LNG concentrations were determined by a validated liquid chromatography tandem mass spectrometry assay at the University of Nebraska Medical Center Antiviral Pharmacology Laboratory, Omaha, Nebraska (limit of quantification: 25.0 pg/mL; linear calibration range: 25.0–30,000 pg/mL); the interday coefficient of variation was between 4.4% and 5.0%; accuracy was between −6.3% and −0.7%) [19]. We determined plasma efavirenz concentrations using a high-performance liquid chromatography assay with ultraviolet detection at the Makerere University College of Health Sciences Translational Research Laboratory, IDI, Kampala, Uganda (linear calibration range: 0.20–10 mg/L); the interday coefficient of variation was between 2.8% and 5.3%; accuracy was between −6.4% and 7.0%) [6]. Endogenous progesterone and SHBG concentrations were quantified using Roche cobas (Roche Diagnostics; assay range: 0.03–60 ng/mL) and Abbott Architect immunoassay instruments (Abbott Laboratories; assay range: 0.0–250 nmol/L), respectively, at Lancet Lab, Kampala, Uganda.
2.5. Pharmacokinetic and statistical analyses
Based on LNG implant concentration inter-patient variability (30–50%) [6, 20], 25 participants in the 300LNG+ART group and 17 participants in the 150LNG group provided 83% power to test bioequivalence of the natural log-transformed LNG area under the plasma concentration-time curve from weeks 0 to 24 (AUC0–24w) relative to our 150LNG group using a no-effect boundary of 0.8–1.25 [21]. We calculated the plasma LNG AUC0–24w using the linear trapezoidal rule and compared between groups using geometric mean ratios (90% confidence intervals, CI) and an unpaired t test, based on normality of the result distribution. A two-sided alpha level of 0.05 was considered significant. We summarized and compared LNG concentrations at each visit using geometric means (95% CI) and medians (interquartile ranges) and Wilcoxon rank sum test. A two-sided alpha level of 0.008 (Bonferroni correction) was considered significant.
As a proposed pharmacokinetic threshold for contraceptive effectiveness, we compared between-group LNG concentrations ≥300 pg/mL at weeks 24 and 48 using Fisher’s exact test with two-sided alpha level of 0.025 (Bonferroni correction). The threshold of 300 pg/mL was based upon the last measured plasma LNG concentration before unintended pregnancies in a similar Ugandan cohort receiving standard dose LNG implants and efavirenz-based ART (303 pg/mL) [6]. We summarized the percent of participants with efavirenz concentrations ≥1 mg/L at all study visits; this threshold was defined a priori based on antiviral effectiveness [22]. We compared SHBG concentrations between groups using unpaired t tests with two-sided alpha level of 0.017 (Bonferroni correction). We compared data between groups using Wilcoxon rank sum tests or unpaired t tests, and Chi-square or Fisher exact tests as appropriate based on data distribution (SAS software, v9.2, SAS Institute Inc., Cary, North Carolina).
3. Results
3.1. Baseline Characteristics
We enrolled 28 women with HIV and 23 women without HIV between March 2017 and October 2019; 27 300LNG+ART participants and 19 150LNG participants reached the primary endpoint (Figure 2). Twenty (74.0%) participants in the 300LNG+ART group completed study procedures through week 144. Except for weight and body mass index (BMI), which were slightly lower in the 300LNG+ART group, and SHBG, which was significantly higher in the 300LNG+ART group, participants had similar baseline characteristics (Table 1). SHBG levels were higher in 300LNG+ART vs. 150LNG at all time points (Supplementary Table 1).
Figure 2.
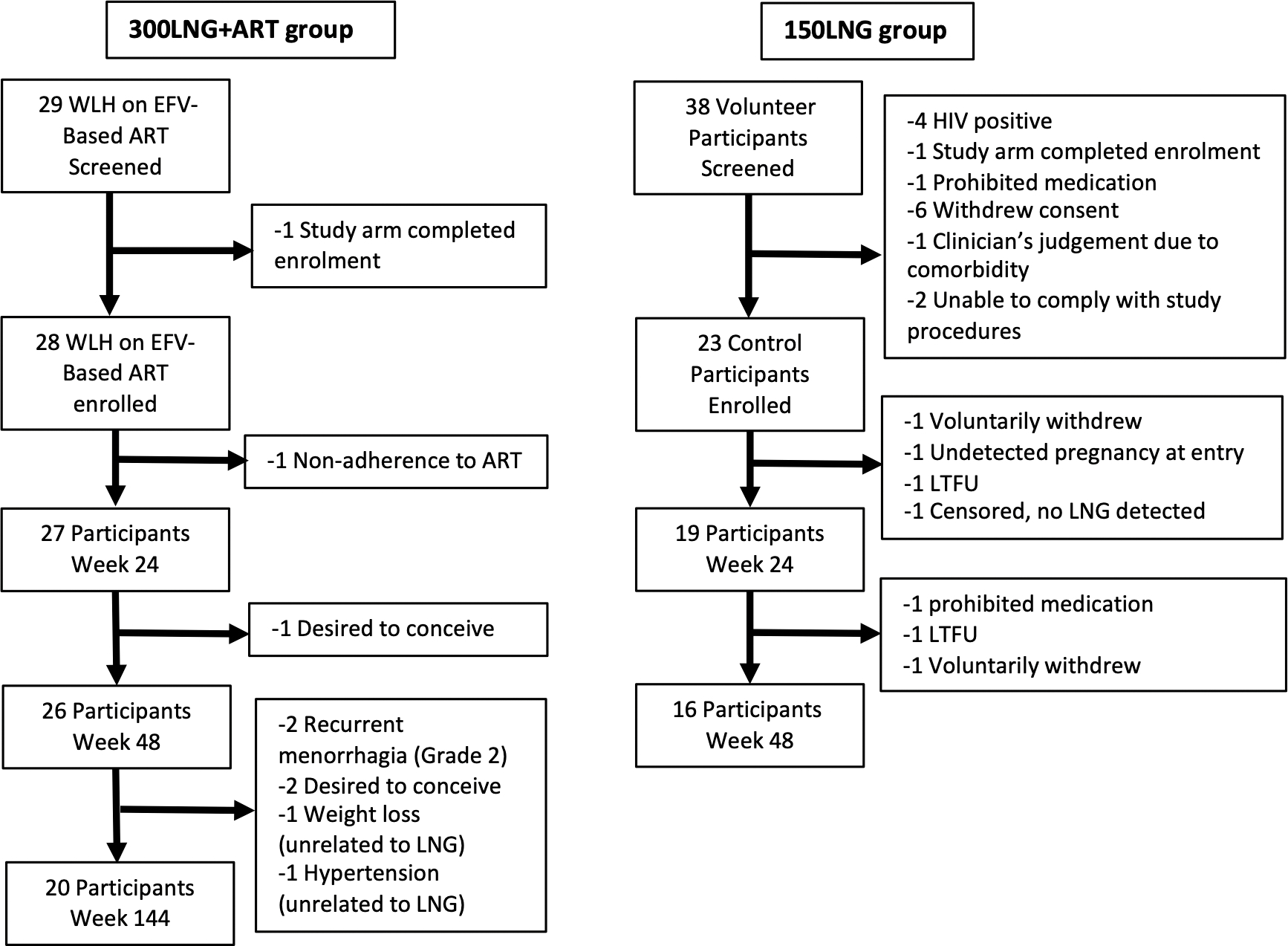
Participant disposition. Abbreviations: ART, antiretroviral therapy; EFV, efavirenz; LNG, levonorgestrel; LTFU, lost to follow-up; WLH, women living with HIV.
Table 1.
Baseline demographics and clinical characteristics of cis-gender Ugandan female participants with HIV on efavirenz 600mg-based ART who received double-dose LNG implant (300LNG+ART) and participants without HIV who received standard-dose LNG implant (150LNG).
| Characteristic | 300LNG + ART n=27 |
150LNG n=19 |
P value |
|---|---|---|---|
|
| |||
| Age in years | 34.0 (28.0–40.5) | 33.0 (30.0–34.5) | 0.35 |
| Weight, kga | 61.0 (49.8–66.0) | 64.9 (59.0–74.5) | <0.05 |
| BMI, kg/m2a | 23.1 (20.9–25.1) | 26.5 (23.4–29.9) | 0.02 |
| SHBG, nmol/La | 135.2 (88.0–170.4) b | 54.8 (40.8–89.3) | <0.001 |
| Cohabitating or married | 18 (66.6) | 12 (63.2) | 1.00 |
| Prior live births | 4.0 (2.0–4.0) | 3.0 (2.0–3.5) | 0.27 |
| CD4 cell count, cells/microliter | 649 (475–846) | NA | - |
| HIV-1 RNA <50 copies/mLa | 27 (100%) | NA | - |
| Efavirenz-based ART duration, monthsa | 44 (28–51) | NA | - |
Continuous data presented as median (interquartile range); categorical data presented as n (%).
ART = antiretroviral therapy; BMI = body mass index; LNG = levonorgestrel; NA = not applicable; SHBG = sex hormone binding globulin
All data were collected at screening, unless indicated. Body weight, and BMI, SHBG, CD4 count, and ART duration were measured or assessed at enrollment.
n=22; 5 (of 27) participants in the 300LNG + ART group had baseline SHBG concentrations >250 nmol/L, the assay upper limit of quantification, and were excluded from the SHBG summary statistics.
3.2. Study outcomes
The geometric mean LNG AUC0–24w was 9998 pg*week/mL (95% CI: 8492–11,771 pg*week/mL) in the 300LNG+ART group compared with 15,231 pg*week/mL (95% CI: 12,969–17,887 pg*week/mL) in the 150LNG group (geometric mean ratio: 0.66 [90% CI: 0.54–0.80], P<0.001). During day 1–4 blood sampling visits in the 300LNG+ART group (n=14), the median LNG Cmax was 806 pg/mL (interquartile range: 595–866) pg/mL and median Tmax was 3 days. Over 48 weeks, LNG concentrations were up to 40% lower in the 300LNG+ART group relative to the 150LNG group (Figure 3; Table 2). At week 144, 300LNG+ART median LNG concentrations were 471 pg/mL (interquartile range: 353–617 pg/mL) (Table 2). When considering our proposed LNG threshold for effectiveness, the percent of participants with LNG concentrations ≥300 pg/mL in 300LNG+ART vs. 150LNG were 74.1% vs. 89.5%, respectively, at week 24 (P=0.27) and 80.8% vs. 100%, respectively, at week 48 (P=0.14).
Figure 3.
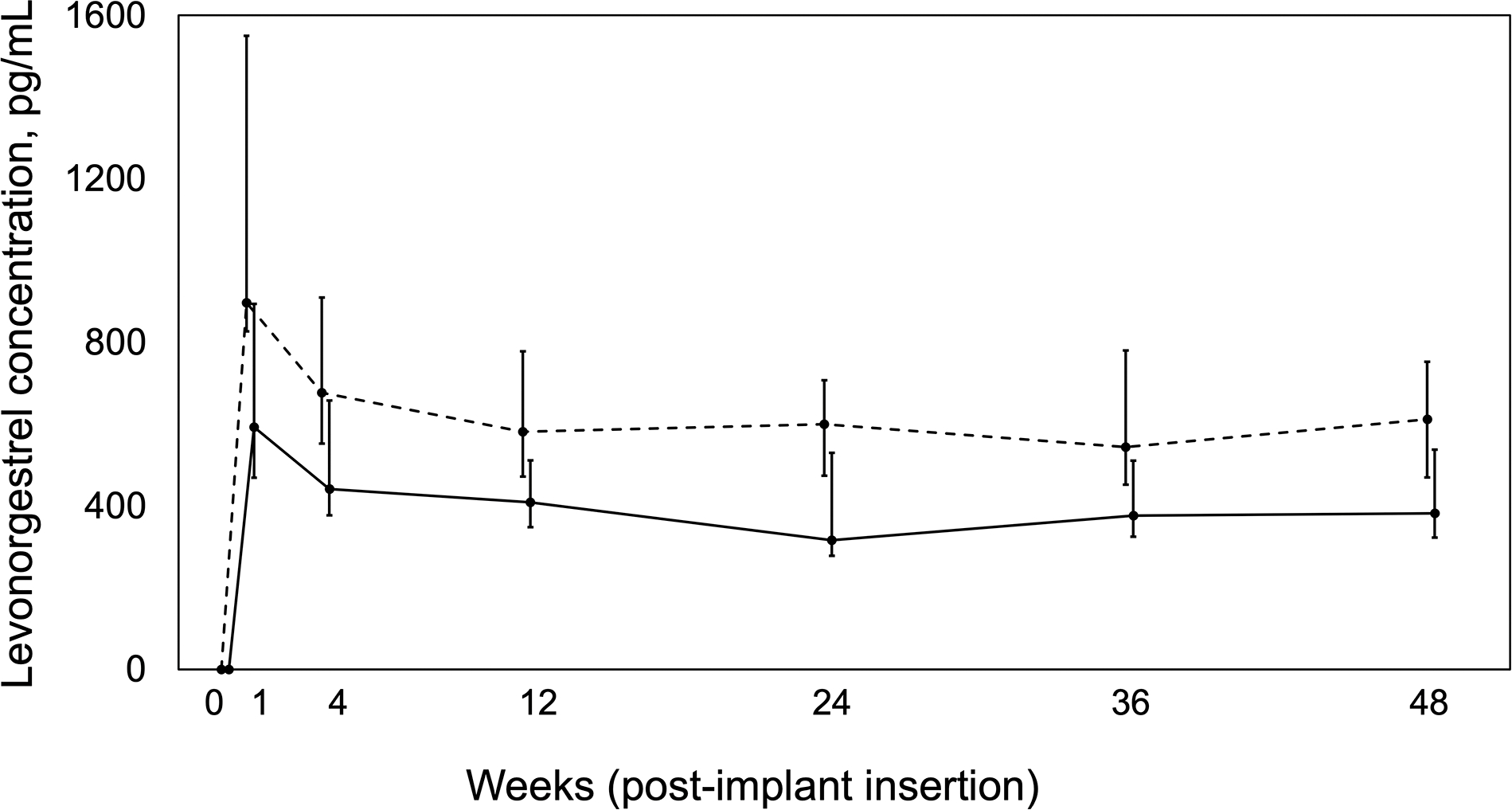
Plasma levonorgestrel concentration versus time profile over 48 weeks post-implant insertion for women with HIV receiving double-dose levonorgestrel implants (300 mg) plus efavirenz-based antiretroviral therapy (n=27, solid line) compared with women without HIV taking standard-dose levonorgestrel implant (150 mg) alone (n=19, dashed line) [12]. Data presented as medians and interquartile ranges.
Table 2.
Plasma LNG concentrations over 144-weeks post-implant placement among study participants with HIV on efavirenz 600mg-based ART who received double-dose LNG implant (300LNG+ART) and women without HIV who received standard-dose LNG implant (150LNG).
| LNG concentration (pg/mL) | Group | N | Median (IQR) | Geometric Mean (95% CI) | Geometric Mean Ratio (90% CI) | Wilcoxon rank-sum P value |
|---|---|---|---|---|---|---|
|
| ||||||
| Day 1 | 300LNG + ART | 14 | 795 (527–960) | 739 (595–917) | - | - |
| Day 2 | 14 | 763 (597–995) | 791 (630–993) | |||
| Day 3 | 14 | 806 (595–866) | 765 (613–956) | |||
| Day 4 | 14 | 647 (447–738) | 658 (504–859) | |||
|
| ||||||
| Week 1 | 150LNG | 18* | 897 (827–1550) | 1026 (845–1245) | ||
| 300LNG + ART | 27 | 593 (469–894) | 615 (501–754) | 0.60 (0.47–0.76) | 0.003 | |
| Week 4 | 150LNG | 19 | 677 (552–910) | 714 (592–860) | ||
| 300LNG + ART | 27 | 441 (377–658) | 461 (380–560) | 0.65 (0.51–0.81) | 0.005 | |
| Week 12 | 150LNG | 19 | 581 (472–778) | 577 (488–683) | ||
| 300LNG + ART | 27 | 409 (348–512) | 411 (355–477) | 0.71 (0.59–0.86) | 0.007 | |
| Week 24 | 150LNG | 19 | 600 (474–707) | 578 (481–695) | ||
| 300LNG + ART | 27 | 316 (278–530) | 345 (286–417) | 0.60 (0.48–0.74) | 0.002 | |
| Week 36 | 150LNG | 19 | 544 (452–780) | 594 (494–716) | ||
| 300LNG + ART | 26 | 376 (325–511) | 386 (327–455) | 0.65 (0.53–0.80) | 0.002 | |
| Week 48 | 150LNG | 16 | 612 (469–753) | 602 (513–707) | ||
| 300LNG + ART | 26 | 382 (322–538) | 401 (341–471) | 0.67 (0.55–0.81) | 0.003 | |
|
| ||||||
| Week 72 | 300LNG + ART | 24 | 466 (336–547) | 429 (354–521) | - | - |
| Week 96 | 22 | 471 (353–617) | 471 (381–581) | |||
| Week 120 | 20 | 328 (279–477) | 343 (278–422) | |||
| Week 144 | 20 | 416 (327–637) | 438 (339–566) | |||
Control group volunteer censored at Week 1 due to LNG concentrations suggestive of additional oral exposure.
ART = antiretroviral therapy; CI = confidence interval; IQR = interquartile range; LNG = levonorgestrel
Figure 4 describes all serum progesterone samples collected through week 51 and Table 3 describes participant characteristics of those with possible ovulatory activity. Among participants who consented to the optional four weekly progesterone measurements around week 48, 5 of 24 (20.8%) 300LNG+ART participants had possible ovulatory activity (progesterone ≥3 ng/mL); 4 of these participants (16.7% of 24) had progesterone concentrations ≥5 ng/mL. No 150LNG group participants had ovulatory activity (0 of 9 participants; 0.0%, P=0.29; Figure 4) [12]. Over weeks 96 through 99, 4 of 12 (33.3%) 300LNG+ART group participants had progesterone samples ≥5ng/mL. The LNG concentrations measured around the time of possible ovulatory activity ranged from 130–751 pg/mL (Table 3).
Figure 4.
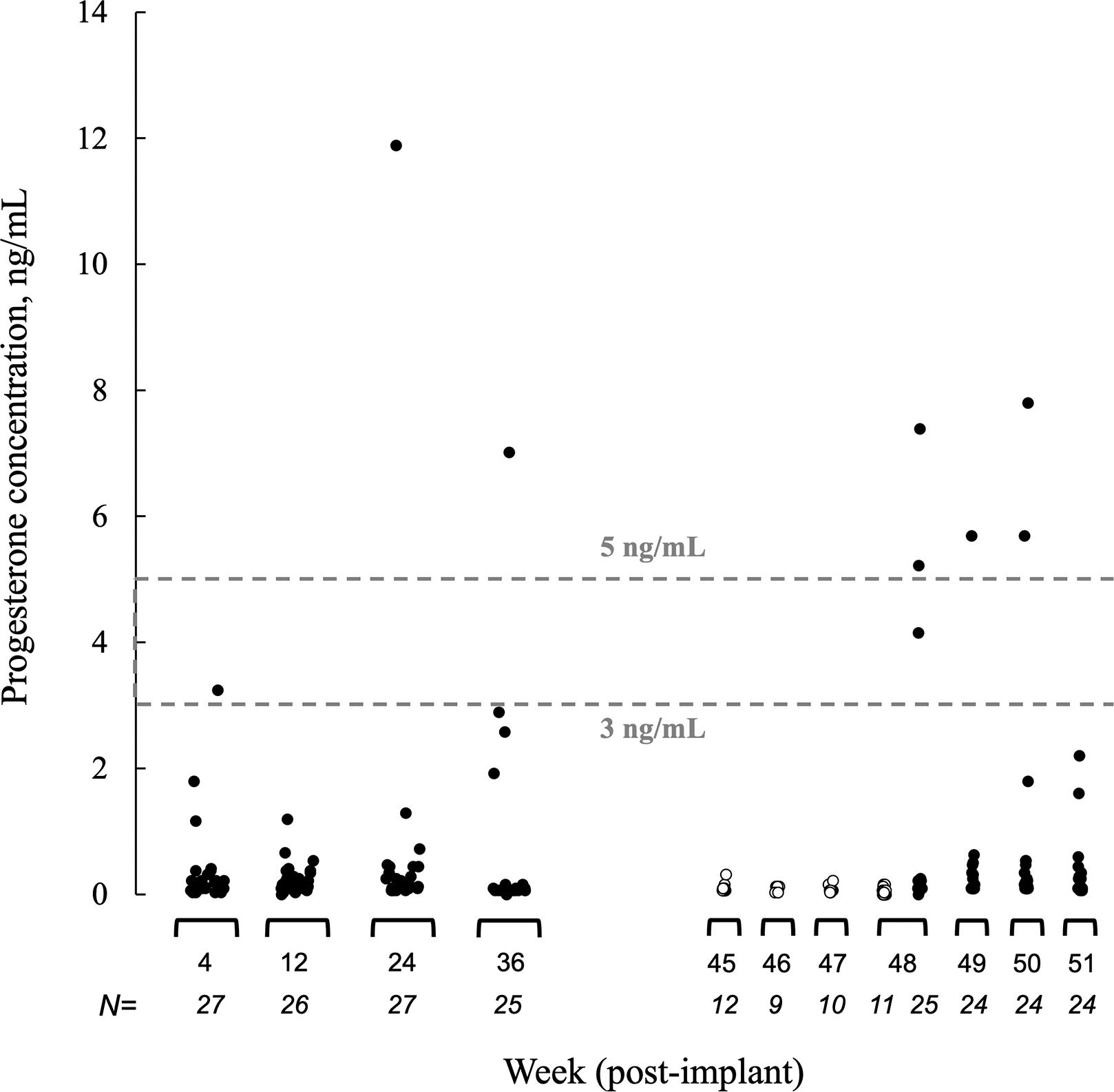
Endogenous serum progesterone concentrations through 51 weeks post-implant insertion. Dashed lines indicate two serum progesterone concentration thresholds associated with recent possible ovulatory activity (3 ng/mL and 5 ng/mL) [16, 17]. The 300LNG+ART group (300mg levonorgestrel plus efavirenz-based antiretroviral therapy; closed circles) progesterone concentrations were sampled at weeks 48, 49, 50, 51 post-insertion. 150LNG group (150 mg levonorgestrel alone; open circle) progesterone concentrations were sampled at weeks 45, 46, 47, 48 post insertion [12]. To convert progesterone to SI units multiply by 3.179.
Table 3.
Selected characteristics of study participants with HIV on efavirenz 600mg-based ART who received double-dose LNG implant (300LNG+ART) and had possible ovulatory activity over 99-weeks post-implant placement.a
| Participant ID | Progesterone, ng/mLb | Progesterone measurement, week post-implant | Age, years | Weight, kg | Plasma LNG, pg/ml | LNG measurement, Week post-implant |
|---|---|---|---|---|---|---|
|
| ||||||
| 3 | 7.0 | 36 | 44 | 65.0 | 318 | 36 |
| 7.8 | 50 | 69.0 | 256 | 48 | ||
| 16 | 7.4 | 48 | 34 | 54.0 | 373 | 48 |
| 5.0 | 99 | 55.0 | 643 | 96 | ||
| 17 | 3.8 | 98 | 32 | 50.0 | 353 | 96 |
| 5.3 | 99 | - | - | |||
| 18 | 4.2 | 48 | 35 | 81.0 | 319 | 48 |
| 19 | 3.2 | 4 | 28 | 44.0 | 655 | 4 |
| 20 | 6.0 | 98 | 44 | 68.0 | 177 | 96 |
| 23 | 5.2 | 48 | 28 | 53.0 | 130 | 48 |
| 29 | 11.9 | 24 | 35 | 49.0 | 530 | 24 |
| 5.7 | 49 | 49.0 | 540 | 48 | ||
| 5.7 | 50 | - | - | |||
| 8.5 | 97 | 50.0 | 751 | 96 | ||
3.3. HIV-related clinical assessments
All 300LNG+ART group participants had undetectable viral loads and stable CD4 counts through week 144 (726 [546–895] cells/microliter vs. baseline [Table 1], P=0.36). Eighteen (66.6%) participants had efavirenz concentrations ≥1 mg/L at all study visits.
3.4. Safety and tolerability related to study procedures
Over 24 weeks post-implant placement, 300LNG+ART participants had higher frequencies of menorrhagia (2 moderate, remainder mild intensity; P=0.02), dysmenorrhea (1 moderate, remainder mild intensity; P<0.001) and weight gain (all mild intensity [≤5% body weight increase from enrollment], P=0.001) compared with the 150LNG group. All other adverse effect frequencies were similar between groups over 48 weeks and most were mild intensity (Supplementary Table 2). No participants discontinued the study prior to week 48 due to adverse events. Among 300LNG+ART participants remaining in the study, 7 did not complete study procedures through week 144. Two (7.4%) of these participants discontinued due to a LNG-related adverse effect (persistent moderate intensity menorrhagia, weeks 51 and 72; Supplementary Table 3).
4. Discussion
We observed 34% lower plasma LNG exposure (AUC) over 24 weeks among women with HIV taking efavirenz-based ART and using double-dose LNG implant compared with a control group of women without HIV on standard-dose LNG implant. We also observed higher, but not statistically different, ovulatory activity at 1 year in the 300LNG+ART group compared with the 150LNG group. Double-dose LNG was well-tolerated among study participants.
Although double-dose LNG concentrations were higher than historical concentrations of standard-dose LNG in combination with efavirenz [6], the higher concentrations were not proportional to the increased dose. LNG concentrations were 29–40% lower in the 300LNG+ART group compared with the 150LNG group (Table 2). Although we did not enroll a concurrent group of women receiving efavirenz-based ART with standard-dose LNG, we previously observed 45–57% lower LNG exposure with a standard-dose LNG implant in Ugandan women with HIV receiving efavirenz compared with women with HIV not on ART [6]. Progestin dose-adjustment overcame physiological and pharmacological decreases in systemic progestins in other studies. For example, among women with BMI ≥30 kg/m2, maximum plasma LNG concentrations were ~90% higher among obese participants who received double-dose (3.0mg) versus standard-dose (1.5mg) oral LNG emergency contraception (EC) [23]. In a recent study of women with HIV, giving 3mg LNG EC plus efavirenz-based ART resulted in similar LNG maximum concentration and AUC over 8 hours compared with a control group of participants receiving 1.5mg LNG in the absence of a drug-drug interaction [24]. However, women receiving efavirenz had 30–40% lower AUCs by 24 and 48 hours after the EC dose and reflected an elimination half-life of 12 hours in participants receiving efavirenz compared with 24 hours in the control group. Similar to our findings with LNG implants, these oral LNG results illustrate the effect of efavirenz-related CYP3A4 induction on LNG exposure.
Other factors may influence LNG concentrations. Body weight ≥70 kg is associated with lower LNG plasma concentrations compared with body weight ≤50 kg [11]. However, fewer 300LNG+ART participants had body weights ≥70 kg through week 48 relative to the 150LNG group (15.4% vs. 50.0%, respectively). LNG binds with high affinity to SHBG, and differences in SHBG concentrations by LNG [11] or HIV status [25] may have influenced the free concentrations of LNG. However, our SHBG results do not support this hypothesis, as SHBG levels were higher in the 300LNG+ART group throughout the study. Further, 300LNG+ART participants had a smaller percent change from baseline after LNG initiation (Supplementary Table 1). Finally, pharmacogenetic characteristics of participants in the 300LNG+ART group may influence our findings. Slow metabolizers of efavirenz have higher systemic efavirenz concentrations relative to people with normal or intermediate metabolizer status, resulting in greater CYP enzyme induction, leading to lower exogenous progestin concentrations [26].
At 1-year post-implant insertion, 20.8% of the 300LNG+ART group had ovulatory activity compared with no participants in the 150LNG group (P=0.29). Although this finding was not statistically significant, the number of participants with evaluable serial progesterone data was small. In two cohorts of women without HIV using contraceptive implants (200mg LNG), investigators reported 11.1% to 18.0% of cycles were ovulatory over the first year of implant use (progesterone threshold: 4.7 ng/mL and 3.0 ng/mL, respectively) [27, 28]. Despite possible ovulatory activity observed in our study, progestins have additional mechanisms of contraceptive effectiveness, specifically cervical mucus changes that maintain effectiveness despite ongoing ovulation.
We used a LNG efficacy threshold (300 pg/mL) established by observed pregnancies in a similar cohort of Ugandan women and a similar LC-MS/MS assay to quantify plasma LNG between cohorts. However, the pharmacodynamic threshold for contraceptive efficacy is not well defined. If using an often proposed 200 pg/mL plasma LNG efficacy threshold [29], more than 93% of 300LNG+ART participants had concentrations ≥200 pg/mL at weeks 24 and 48. However, we observed elevated progesterone levels in some participants despite LNG concentrations measured at the nearest visit well above either proposed threshold (Table 3). These results highlight uncertainty around the pharmacokinetic-pharmacodynamic relationship for LNG, and the desired threshold may have high inter-individual variation [29].
This study has certain limitations. We cannot rule out potential physiological differences between the 300LNG+ART and 150LNG groups. Reassuringly, our HIV-negative control group had similar LNG exposure over 24 weeks to Ugandan women with HIV not yet receiving ART in a separate study: 15,231 pg*week/mL in 150LNG vs. 15,168 pg*wk/mL in women with HIV not on ART [6]. We did not design this study to assess contraceptive effectiveness, and questions remain regarding the clinical interpretation of hormone pharmacokinetic studies without a clear pharmacodynamic measure of contraceptive effectiveness. We cannot evaluate effects of lower dose efavirenz on double-dose LNG pharmacokinetics [14]. However, effects of lower efavirenz dosing on systemic LNG exposure relative to 600mg dosing are likely modest [30].
Double-dose LNG implant improved LNG exposure compared with the standard dose [6], but it did not overcome the drug-drug interaction with efavirenz-based ART. For women desiring the LNG contraceptive implant and taking efavirenz-based ART, double-dose LNG implants may offer an alternative to other less effective forms of contraception. Because updated international HIV treatment guidelines recommended dolutegravir-based ART as the preferred first-line treatment of HIV [14], these antiretroviral regimens should be prioritized for women with HIV desiring hormonal contraception for family planning to avoid detrimental efavirenz-related drug-drug interactions.
Supplementary Material
Implication.
In women using antiretroviral therapy (ART) containing efavirenz, placing two implant systems (300mg) did not normalize LNG pharmacokinetics compared with the standard-dose implant (150mg), and some women had evidence of ovulatory activity. Alternative ART without drug-drug interactions, such as dolutegravir, are recommended with contraceptive implants.
Acknowledgements
The authors thank the members of the clinical study team at IDI, the staff of the IDI Core Laboratory, and the staff of the Antiviral Pharmacology Laboratory at the University of Nebraska Medical Center, including Leah Mbabazi, Ian Musinguzi, Johnson Magoola, Emmanuel Sempijja, and Jeff Jeppson. We thank members of the Safety Monitoring Committee for oversight of this study, including Concepta Merry, Robert Murphy, and Heather Watts. Finally, we are grateful to David Back for his guidance and collaboration.
Funding
This work was supported by the Eunice Kennedy Shrive National Institute of Child Health and Human Development [grant number R01 HD085887 to KS]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the funding source:
The funder had no role in the conduct, analyses, and conclusions of the study. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Footnotes
Declaration of Competing Interest
KKS has received investigator-initiated research support from Organon, LLC, paid to her institution. CAC has received investigator-initiated research support from Organon, LLC and Gilead Sciences, paid to her institution. ML has received investigator-initiated research support from Janssen, paid to his institution. All other authors declare no conflicts of interest.
References
- [1].Singh S and Darroch JE. Adding it up: costs and benefits of contraceptive services - estimates for 2012, New York: Guttmacher Institute and United Nations Population Fund (UNFPA), 2012. Available at: http://www.guttmacher.org/pubs/AIU-201-estimates.pdf. Accessed: 12 December 2014. [Google Scholar]
- [2].The Joint United Nations Programme on HIV/AIDS (UNAIDS). Fact sheet 2022. UNAIDS 2022 epidemiological estimates; 2022. [cited 2022 Aug 1]; Available from: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. [Google Scholar]
- [3].Bahamondes L, Fernandes A, Monteiro I, Bahamondes MV. Long-acting reversible contraceptive (LARCs) methods. Best Practice & Research Clinical Obstetrics & Gynaecology. 2020;66:28–40. [DOI] [PubMed] [Google Scholar]
- [4].Kanyangarara M, Sakyi K, Laar A. Availability of integrated family planning services in HIV care and support sites in sub-Saharan Africa: a secondary analysis of national health facility surveys. Reproductive Health. 2019;16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kreitchmann R, Stek A, Best BM, Capparelli E, Wang J, Shapiro D, et al. Interactions between etonogestrel-releasing contraceptive implant and 3 antiretroviral regimens. Contraception. 2022;105:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Scarsi KK, Darin KM, Nakalema S, Back DJ, Byakika-Kibwika P, Else LJ, et al. Unintended pregnancies observed with combined use of the levonorgestrel contraceptive implant and efavirenz-based antiretroviral therapy: a three-arm pharmacokinetic evaluation over 48 weeks. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62:675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vieira CS, Bahamondes MV, de Souza RM, Brito MB, Rocha Prandini TR, Amaral E, et al. Effect of antiretroviral therapy including lopinavir/ritonavir or efavirenz on etonogestrel-releasing implant pharmacokinetics in HIV-positive women. J Acquir Immune Defic Syndr. 2014;66:378–85. [DOI] [PubMed] [Google Scholar]
- [8].Tang JH, Davis NL, Corbett AH, Chinula L, Cottrell ML, Zia Y, et al. Effect of efavirenz on levonorgestrel concentrations among Malawian levonorgestrel implant users for up to 30 months of concomitant use: a subanalysis of a randomized clinical trial. Contracept X. 2020;2:100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Patel RC, Stalter RM, Thomas KK, Tamraz B, Blue SW, Erikson DW, et al. A pharmacokinetic and pharmacogenetic evaluation of contraceptive implants and antiretroviral therapy among women in Kenya and Uganda. AIDS. 2019;33:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chappell CA, Lamorde M, Nakalema S, Chen BA, Mackline H, Riddler SA, et al. Efavirenz decreases etonogestrel exposure: a pharmacokinetic evaluation of implantable contraception with antiretroviral therapy. AIDS. 2017;31:1965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jadelle. [package insert]. Auckland, New Zealand: Bayer New Zealand Limited Company. Sept 2020. https://www.medsafe.govt.nz/profs/datasheet/j/jadelleimplant.pdf. [Google Scholar]
- [12].Nakalema S, Chappell CA, Pham M, Byakika-Kibwika P, Kaboggoza J, Walimbwa SI, et al. Pharmacokinetics of levonorgestrel and etonogestrel contraceptive implants over 48 weeks with rilpivirine- or darunavir-based antiretroviral therapy. J Antimicrob Chemother. 2022;77:3144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].World Health Organization. Medical eligibility criteria for contraceptive use. 5th ed. World Health Organization; 2015. [cited 2022 Aug 1]; Available from: https://apps.who.int/iris/rest/bitstreams/1243459/retrieve. [Google Scholar]
- [14].World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. World Health Organization; Geneva; 2021. [cited 2022 July 4]; Available from: https://apps.who.int/iris/rest/bitstreams/1357089/retrieve [PubMed] [Google Scholar]
- [15].Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. U.S. Department of Health & Human Services; [cited 2022 July 4]; Available from: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf. [Google Scholar]
- [16].Makarainen L, van Beek A, Tuomivaara L, Asplund B, Coelingh Bennink H. Ovarian function during the use of a single contraceptive implant: Implanon compared with Norplant. Fertility and sterility. 1998;69:714–21. [DOI] [PubMed] [Google Scholar]
- [17].Israel R, Mishell DR, Stone SC, Thorneycroft IH, Moyer DL. Single luteal phase serum progesterone assay as an indicator of ovulation. American Journal of Obstetrics & Gynecology. 1997;176:490–1. [DOI] [PubMed] [Google Scholar]
- [18].Division of AIDS, National Institute of Allergy and Infectious Diseases. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, corrected version 2.1. National Institutes of Health, U.S. Department of Health and Human Services; 2017. [cited 2022 July 4]; Available from: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. [Google Scholar]
- [19].Cirrincione LR, Penchala SD, Scarsi KK, Podany AT, Winchester LC, Back DJ, et al. Development, validation and utilization of a highly sensitive LC-MS/MS method for quantification of levonorgestrel released from a subdermal implant in human plasma. Journal of Chromatography B. 2018;1084:106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sivin I, Lähteenmäki P, Ranta S, Darney P, Klaisle C, Wan L, et al. Levonorgestrel concentrations during use of levonorgestrel rod (LNG ROD) implants. Contraception. 1997;55:81–5. [DOI] [PubMed] [Google Scholar]
- [21].Center for Drug Evaluation and Research (CDER). Clinical drug interaction studies—cytochrome P450 enzyme- and transporter-mediated drug interactions. U.S. Department of Health and Human Services Food and Drug Administration; 2020. [cited 2022 July 4]; Available from: https://www.fda.gov/media/134581/download. [Google Scholar]
- [22].Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–5. [DOI] [PubMed] [Google Scholar]
- [23].Edelman AB, Cherala G, Blue SW, Erikson DW, Jensen JT. Impact of obesity on the pharmacokinetics of levonorgestrel-based emergency contraception: single and double dosing. Contraception. 2016;94:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Scarsi KK, Smeaton LM, Podany AT, Olefsky M, Woolley E, Barr E, et al. Pharmacokinetics of dose-adjusted levonorgestrel emergency contraception combined with efavirenz-based antiretroviral therapy or rifampicin-containing tuberculosis regimens. Contraception. 2023:109951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Karim R, Mack WJ, Kono N, Tien PC, Anastos K, Lazar J, et al. Gonadotropin and sex steroid levels in HIV-infected premenopausal women and their association with subclinical atherosclerosis in HIV-infected and -uninfected women in the women’s interagency HIV study (WIHS). J Clin Endocrinol Metab. 2013;98:E610–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Neary M, Lamorde M, Olagunju A, Darin KM, Merry C, Byakika-Kibwika P, et al. The Effect of Gene Variants on Levonorgestrel Pharmacokinetics When Combined With Antiretroviral Therapy Containing Efavirenz or Nevirapine. Clinical pharmacology and therapeutics. 2017;102:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Croxatto HB, Diaz S, Pavez M, Miranda P, Brandeis A. Plasma progesterone levels during long-term treatment with levonorgestrel silastic implants. Acta endocrinologica. 1982;101:307–11. [DOI] [PubMed] [Google Scholar]
- [28].Brache V, Alvarez-Sanchez F, Faundes A, Tejada AS, Cochon L. Ovarian endocrine function through five years of continuous treatment with NORPLANT® subdermal contraceptive implants. Contraception. 1990;41:169–77. [DOI] [PubMed] [Google Scholar]
- [29].Cherala G, Edelman A, Dorflinger L, Stanczyk FZ. The elusive minimum threshold concentration of levonorgestrel for contraceptive efficacy. Contraception. 2016;94:104–8. [DOI] [PubMed] [Google Scholar]
- [30].Roberts O, Rajoli RKR, Back DJ, Owen A, Darin KM, Fletcher CV, et al. Physiologically based pharmacokinetic modelling prediction of the effects of dose adjustment in drug-drug interactions between levonorgestrel contraceptive implants and efavirenz-based ART. J Antimicrob Chemother. 2018;73:1004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


