Abstract
Organophosphate esters (OPEs), widely used as flame retardants and plasticizers for commercial and residential purposes, are suspected of being neurotoxic. We aimed to assess exposure to an OPE mixture in early life and its relationship to parent-reported child behavior. We measured urinary concentrations of three OPE metabolites, bis-2-chloroethyl phosphate (BCEP), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), and diphenyl phosphate (DPHP), at pregnancy (16 and 26 weeks of gestation and delivery) and postnatal time points (ages 1, 2, 3, and 5 years) in the Health Outcomes and Measures of the Environment Study, a longitudinal pregnancy and birth cohort in Cincinnati, Ohio, USA (enrolled 2003–2006, n = 219). We used latent variable analysis in structural equations models and quantile g-computation to investigate associations of a mixture of the three OPE metabolites with parent-reported child behaviors at 3 and 8 years, measured using the Behavioral Assessment System for Children, Second Edition. Higher log-transformed urinary OPE latent variable values at 16 weeks were associated with fewer externalizing problem behaviors (ß = −5.74; 95% CI = −11.24, −0.24) and fewer overall behavioral problems at age 3 years (ß = −5.26; 95% CI = −10.33, −0.19), whereas having higher OPEs at delivery was associated with poorer overall behavioral problems at age 3 years (ß = 2.87; 95% CI = 0.13, 5.61). OPE latent variable values at 16 weeks, 26 weeks, and delivery were not associated with child behavior at 8 years. However, higher OPE latent variable values at 3 years were associated with fewer externalizing behaviors at 8 years (ß = −2.62; 95% CI = −5.13, −0.12). The quantile g-computation estimates had directions largely consistent with the latent variable analysis results. Pregnancy and postnatal urinary OPE metabolite mixtures were associated with child internalizing, externalizing, and overall negative behaviors at 3 and 8 years, but we did not identify a consistent pattern in terms of the direction of the effects or a particularly sensitive time point.
Keywords: organophosphate esters, cohort, children, gestation, behavior
INTRODUCTION
In the first 5 years of life, the brain undergoes about 90% of its total development (1). Poor early-life developmental trajectories in the brain can result in poorer overall functioning in adulthood and can significantly impact one’s risk of psychiatric disorders and lifetime earning potential (2–4). Brain function is highly complex, with various regions controlling different processes, and stimuli such as hormones, stress, and lived experiences able to affect neural circuitry. However, some observable metrics that are derived from higher-level processes and the integration of multiple brain domains can be used to measure brain development broadly. Behavior is one of these constructs that can give clinicians and researchers information about overall brain maturity and function in children.
Environmental toxicants, including endocrine-disrupting chemicals, can negatively alter brain development and behavior (5). Organophosphate esters (OPEs) are suspected of disrupting thyroid hormones, the stress response system, and sex hormones, all of which are necessary for proper brain development (6–10). OPE exposure may cause alterations in neurotransmitters, gene expression, inflammation, synaptogenesis, and neural network activity (11). OPEs have been added to consumer products as flame retardant and plasticizing chemicals since the 1970s, but a large increase in OPE production volume in recent years has prompted a closer investigation into these chemicals to determine the extent of their potential effects on human health (12).
OPEs can be released from consumer products and settle into homes, offices, and cars (13–15). Once released into the environment, humans are exposed to OPEs through various pathways, including accidental ingestion and inhalation of dust, dermal sorption, and food and water contamination (16–20). In recent years, work has been done to characterize the toxicity profile of OPEs to determine adverse exposure-related health outcomes in humans. Endocrine disruption may occur via altered gene regulation and hormone receptor activity in the presence of OPEs, which can induce neurotoxicity through changes in neuronal growth and differentiation and inflammation (21). In epidemiologic studies, exposure to OPEs has been associated with alterations in child neurobehavior. Still, the evidence is inconsistent concerning which developmental windows are most sensitive, which OPEs are associated with adverse effects, and which neurobehavioral domains are affected.
Only one study of OPEs and child behavior so far has examined postnatal exposures; however, it had a cross-sectional design and no internal biomarkers of exposure (22). Other studies have focused only on OPE exposures during pregnancy, but all assessed exposure at a single time point, which increases measurement error and eliminates the ability to assess potential sensitive windows to exposure during pregnancy, especially with short-half life chemicals that are measured in urine (23–26). Further, although OPEs are applied in chemical mixtures, and humans experience exposure to a combination of chemicals in their daily lives, much of the current literature on the health effects of OPEs has studied associations as single chemicals. This approach, however, does not adequately capture the total and combined effects of OPE mixtures in the environment. Further, studying chemical mixtures allows researchers to model how reducing overall OPE exposure might affect health, which approximates how a theoretical public health intervention of restricting or eliminating OPEs from consumer goods might impact the population.
This study aims to assess exposure to an OPE mixture in early life and its relationship to parent-reported child behavior. We used data from a well-established pregnancy and birth cohort to estimate the association of pregnancy and postnatal exposure to OPEs with child behavior at ages 3 and 8 years.
MATERIALS AND METHODS
Study Population
All participants in the present study were recruited from March 2003 to February 2006 for the Health Outcomes and Measures of the Environment (HOME) Study, a pregnancy and birth cohort in Cincinnati, Ohio (27,28). The HOME Study was established to investigate the role of common environmental exposures on child health and development in a population targeted to mimic the demographics of the Cincinnati area. Pregnant women were eligible to participate if they were at least 18 years of age; at 16 ± 3 weeks’ gestation; living in a home built before 1978 (relating to the original study goal of examining lead exposure); fluent in English; not diagnosed with bipolar disorder, schizophrenia, diabetes, or cancer that required radiation or chemotherapy; not taking medication for thyroid disorders or seizures; HIV negative; and not planning to move outside of the Greater Cincinnati Area.
For the present study, we included pregnant women and their singleton children if they had at least one measurement of urinary OPE metabolites during pregnancy (16 weeks, 26 weeks, or delivery) or early childhood (ages 1, 2, 3, or 5 years), and a parent completed the Behavioral Assessment System for Children, 2nd Edition (BASC-2) at either the 3- or 8-year study visits. The 3- and 8-year time points for behavioral assessments were chosen to maximize available sample sizes and avoid cross-sectionality with urinary OPE metabolite measurements. All participants provided written informed consent for themselves and their children, and the Institutional Review Board (IRB) at Cincinnati Children’s Hospital Medical Center (CCHMC) approved the study protocol. The Centers for Disease Control and Prevention (CDC) deferred to the CCHMC IRB as the IRB of record.
Urine Collection and Analysis
Pregnant study participants provided urine samples in polypropylene specimen cups at approximately 16 and 26 weeks of pregnancy during prenatal care appointments and during the hospital visit for delivery (may have taken place before or after delivery). The time of day for urine collection was not standardized. The samples were aliquoted and stored at −20 °C until overnight shipment to the Centers for Disease Control and Prevention (CDC) laboratory. Child study participants who were not yet toilet-trained provided urine samples via a surgical diaper insert provided by the study team during a study visit. Child study participants who were in the process of being toilet-trained provided urine samples using a training potty lined with surgical inserts during a study visit, and toilet-trained children provided samples in polypropylene specimen cups during a study visit. Surgical inserts were expressed by laboratory technicians in vials and aliquoted. Child urine samples were also aliquoted; all urine aliquots were stored at −20 °C until overnight shipment to the laboratory. Trained laboratory technicians measured the specific gravity in the urine samples using the ATAGO PAL-10S pocket refractometer (ATAGO CO., Tokyo, Japan).
The CDC’s National Center for Environmental Health Laboratory received all study urine samples and quantified concentrations of three OPE metabolites: bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), a metabolite of tris(1,3-dichloro-2-propyl) phosphate (TDCIPP); bis-2-chloroethyl phosphate (BCEP), a metabolite of tris(2-chloroethyl) phosphate (TCEP); and diphenyl phosphate (DPHP), a metabolite of triphenyl phosphate (TPHP) and 2-ethylhexyl diphenyl phosphate (EHDPP), among other OPEs, in 200μL of urine. Following enzymatic hydrolysis, technicians used automated off-line solid-phase extraction, reversed-phase high-performance liquid chromatography, and isotope dilution-electrospray ionization tandem mass spectrometry to quantify total (free and conjugated) concentrations of OPE metabolites (29,30). Please see elsewhere for quality control and other analytic details (31).
The limit of detection (LOD) was 0.1 μg/L for all three urinary OPE metabolites. The percent of samples with detectable OPE concentrations ranged from 83.9 – 95.5% for BCEP, 88.6 – 100% for BDCIPP, and 97.6 – 100% for DPHP (Supplemental Table 1). For concentrations below the LOD, we substituted a value of LOD/√2 (32). Then, OPE metabolite concentration values were standardized by urinary specific gravity to account for dilution using the following formula (33,34):
where is the observed OPE metabolite concentration, is the median specific gravity for the cohort at the appropriate time point, and is the specific gravity of the urine sample.
Child Behavior Measurements
The BASC-2 is a 160-item parent-report assessment of a child’s behavior in public and home settings (35). BASC-2 yields four composite scales: Internalizing Problems, Externalizing Problems, Behavioral Symptom Index (BSI), and Adaptive Skills (not examined in this study). The Internalizing Problems scale measures harmful behaviors directed toward oneself, including anxiety, depression, and somatization. The Externalizing Problems scale measures harmful behaviors directed toward others, including aggression and hyperactivity. The BSI composite is an overall measurement of maladaptive behaviors, including aggression, atypicality, attention problems, depression, hyperactivity, and withdrawal. BASC-2 scores are age normalized to a population mean of 50 and a standard deviation of 10, with higher scores indicating more problem behavior. The internal consistency of the BASC-2 ranges from r = .87-.95 for the subscales across ages 3 and 8 years, and correlations with other measures of child behavior range from r = .65-.84 depending on the subscale (35). Caregivers of study participants, usually mothers (98%), completed the BASC-2 at the 3- and 8-year study visits after receiving instructions from trained study staff.
Covariate Measurements
We considered the following as potential covariates for adjusted models: breastfeeding status (ever vs. never), maternal race, household income at baseline, maternal depression at the time of BASC-2 measurement, maternal education at baseline, maternal age at baseline, marital status at baseline, and the caregiving environment. We then used a Directed Acyclic Graph to determine the final covariate set: maternal depression, breastfeeding status, maternal race (white or non-white), household income, and caregiving environment (Supplemental Figure 1). All covariate information was collected via questionnaire at study visits except for caregiving environment. This variable was assessed using the Home Observation and Measurement of the Environment, a semi-structured questionnaire and interview that captures the quality and quantity of caregiving in the child’s home environment based on observations by trained research assistants in study participants’ homes at age 12 months (36). The internal consistency of the HOME score is r = .89, and the test-retest reliability from 12 to 24 months is r = .77. In addition, maternal depression was measured by the Beck’s Depression Inventory (37). We did not have any missing covariate data.
Statistical Analysis
We completed descriptive analyses and then natural-log transformed the specific-gravity standardized urinary OPE metabolite concentrations before statistical analyses to reduce the influence of outliers. We calculated the intraclass correlation coefficients (ICCs) using a two-way mixed effects model of log-transformed OPE metabolites at pregnancy and postnatal time points to determine the correlation of repeated measurements. ICC values reflect the strength of the correlations: ≤0.4 (poor), 0.4–0.75 (fair to good), and ≥0.75 (excellent) (38).
To explore the associations between urinary OPE metabolites and child behavior, we used two statistical methods chosen to provide a summary of how multiple OPEs are jointly associated with our outcomes of interest, as this conceptualization reflects real-world human exposures and can provide insight into the potential effects of a public health policy to reduce OPEs. First, we used structural equations modeling (SEM) to create and model latent variables of total OPE exposure at each time point based on the three urinary OPE metabolites BCEP, BDCIPP, and DPHP. Then we used quantile g-computation to complement our latent variable SEMs, as both methods estimate the incremental effects of exposure to multiple chemicals on an outcome.
We used Mplus for SEM (39). The outcome variables were the Internalizing Problems, Externalizing Problems, and BSI composite scores from the BASC-2 at ages 3 and 8 years, which are analyzed separately. We constructed latent variables to represent OPE exposure at a single time point, including the observed BCEP, BDCIPP, and DPHP urinary concentrations (Supplemental Figures 2–4). Latent variable analysis allows the measurement of latent or unobserved variables (i.e., joint OPE exposure) based on measured variables (i.e., multiple urinary OPE metabolite concentrations). We first used confirmatory factor analysis (CFA) to ensure that our latent variable measurement models fit appropriately. The fit was considered excellent if the comparative fit index (CFI) was above 0.95, the Tucker Lewis Index (TLI) was above 0.95, and the root mean square error of approximation (RMSEA) was below 0.07 (40). Next, we used SEM to model associations of latent OPE exposure variables with BASC-2 composite scores after adjustment for covariates (Supplemental Figures 2–4). As our outcome variables were all continuous, we used the maximum likelihood estimator for our models. Parameter estimates can be interpreted as the average change in BASC-2 composite score for a one-unit increase in total log-OPE metabolite concentration at the given time point.
We used R version 4.1.2 and the qgcomp package for quantile g-computation (41,42). This method models how a simultaneous one-quantile increase of all chemicals in a defined mixture affects the specified outcome. It is based on a joint marginal structural model and allows individual chemicals in the mixture to have either positive or negative weights (42). We used four quantiles in our analysis, and effect estimates can be interpreted as the difference in BASC-2 scores for a simultaneous one-quartile increase in BCEP, BDCIPP, and DPHP urinary concentrations. We created two different models: 1) an estimate of a linear dose-response parameter for a single time point (16 weeks, 26 weeks, delivery, 1 year, 2 years, 3 years, and 5 years) of combined OPE exposure biomarkers (i.e., combined BCEP, BDCIPP, and DPHP at 16 weeks), and 2) an estimate of the linear dose-response parameter for longitudinal combined pregnancy (16 weeks, 26 weeks, and delivery) and combined postnatal (1 year, 2 years, 3 years, and 5 years) OPE exposure biomarkers (i.e., combined BCEP, BDCIPP, and DPHP across pregnancy or across the postnatal period) using cluster weighting and 5000 bootstrapped estimates for standard errors.
RESULTS
Of the 389 women who gave birth to singleton infants and participated in the HOME Study, 170 were excluded because they either did not have at least one measurement of OPEs for themselves or their child or had not completed a BASC-2 questionnaire at either ages 3 or 8 years. Our final analytical sample was 219 mother-child dyads. On average, the pregnant study participants were aged 29.2 years at delivery, 61.2% were non-Hispanic white persons, and the majority had some college education. Children were 56.2% female, and 80.8% were ever breastfed (Table 1).
Table 1:
Maternal and child characteristics and specific gravity-corrected urinary OPE metabolite concentrations (n = 219), the HOME Study (2003–2006).
| Maternal Characteristic | N | % |
|
| ||
| Race | ||
| Non-Hispanic White | 134 | 61.2 |
| Other race | 85 | 38.8 |
| Education | ||
| High school or less | 55 | 25.1 |
| Any college or trade school | 125 | 57.1 |
| Graduate school | 39 | 17.8 |
| Age at delivery [median, IQR] | 29.3 | 24.9, 33.2 |
|
| ||
| Child Characteristic | N | % |
|
| ||
| Sex | ||
| Male | 96 | 43.8 |
| Female | 123 | 56.2 |
| Ever breastfed | ||
| Yes | 177 | 80.8 |
| No | 42 | 19.2 |
|
| ||
| OPE Metabolite Concentrations (pg/L)* | Median | IQR |
|
| ||
| BCEP | ||
| 16 weeks | 0.60 | 0.34, 1.07 |
| 26 weeks | 0.50 | 0.23, 1.09 |
| Delivery | 0.53 | 0.28, 1.10 |
| 1 year | 1.13 | 0.47, 2.73 |
| 2 years | 0.95 | 0.49, 2.54 |
| 3 years | 1.21 | 0.50, 3.04 |
| 5 years | 0.80 | 0.39, 1.87 |
| BDCIPP | ||
| 16 weeks | 0.81 | 0.49, 1.49 |
| 26 weeks | 0.61 | 0.29, 1.13 |
| Delivery | 0.64 | 0.62, 1.32 |
| 1 year | 1.60 | 0.79, 3.40 |
| 2 years | 2.04 | 1.03, 4.30 |
| 3 years | 3.65 | 1.50, 7.12 |
| 5 years | 3.36 | 1.59, 8.15 |
| DPHP | ||
| 16 weeks | 1.63 | 0.94, 3.13 |
| 26 weeks | 1.32 | 0.79, 2.33 |
| Delivery | 1.68 | 0.93, 3.35 |
| 1 year | 2.28 | 1.59, 4.00 |
| 2 years | 2.70 | 1.65, 4.57 |
| 3 years | 2.68 | 1.70, 4.81 |
| 5 years | 2.69 | 1.60, 6.42 |
Abbreviations: HOME Study, Health Outcomes and Measures of the Environment Study; IQR, interquartile range; OPE, organophosphate ester; BCEP, bis-2-chloroethyl phosphate; BDCIPP, bis(1,3-dichloro-2-propyl) phosphate; DPHP, diphenyl phosphate.
The limit of detection for all OPE metabolites was 0.1 μg/L.
Specific gravity-standardized BCEP concentrations were the lowest at all time points, ranging from a median of 0.50 μg/L at 26 weeks of gestation to 1.21 μg/L at 3 years of age. DPHP concentrations, which were the highest during pregnancy and ages 1 and 2 years, ranged from a median of 1.32 μg/L at 26 weeks of gestation to 2.70 μg/L at 2 years of age. BDCIPP concentrations were highest at ages 3 and 5 years (medians of 3.65 and 3.36 μg/L, respectively, see Table 1). ICC values were < 0.4 for all OPE metabolites during pregnancy and childhood, indicating poor correlation of the urinary OPE concentrations over time (Supplemental Table 1).
The fit statistics for all SEMs were considered very good, with CFIs ≥ 0.917, TLIs ≥ 0.933, and RMSEA ≤ 0.054 (Supplemental Table 2). The sample size for analyses of prenatal OPEs and behavior at age 3 years was n = 206; the sample size for analyses of prenatal OPEs and behavior at age 8 years was n = 210; the sample size for analyses of postnatal OPEs and behavior at age 8 years was n = 196. For all latent variables except the 2-year variable, the OPEs loaded as BDCIPP>BCEP>DPHP. In the 2-year variable, the loadings were BCEP>BDCIPP>DPHP (Supplemental Table 3).
In SEMs, higher urinary OPE latent variable values at 16 weeks of gestation were associated with fewer Externalizing Problems (ß = −5.74; 95% CI = −11.24, −0.24) and better BSI scores (ß = −5.26; 95% CI = −10.33, −0.19) at age 3 years. However, higher urinary OPE latent variable values at delivery were associated with more Internalizing Problems (ß = 2.93; 95% CI = −0.07, 5.94) and poorer BSI scores (ß = 2.87; 95% CI = 0.13, 5.61) at age 3 years. In addition, the urinary OPE latent variable at age 3 years was associated with fewer Externalizing Problems at age 8 (ß = −2.62; 95% CI = −5.13, −0.12). Confidence intervals for estimates of pregnancy urinary OPE latent variables and child behavior at age 8 years included the null, as did all latent variable models at ages 26 weeks, 1 year, 2 years, and 5 years (Figure 2, Supplemental Table 4).
Figure 2:
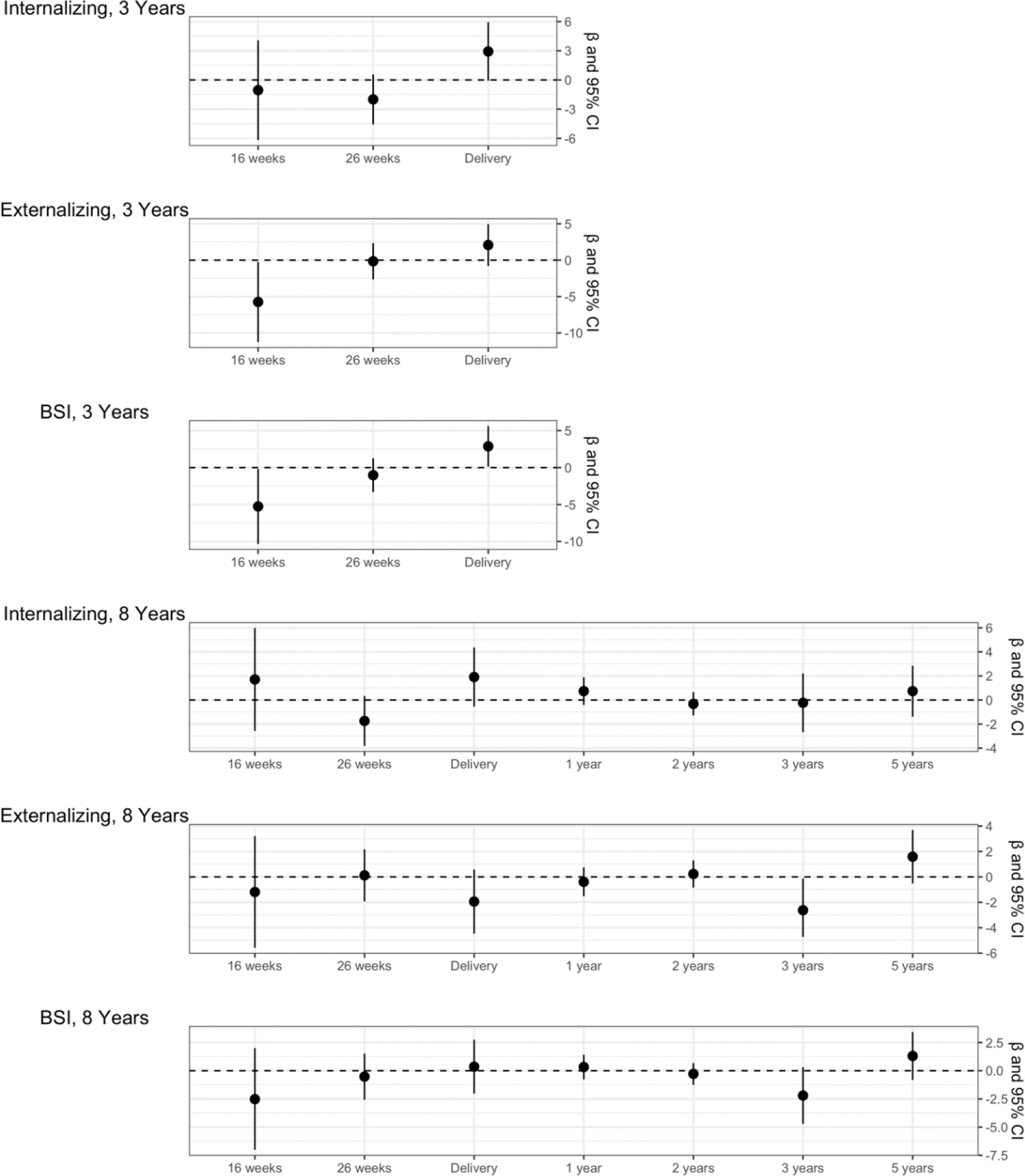
SEM models for natural-log transformed OPE latent variables and BASC-2 outcome t-scores (n = 219). All models are adjusted for maternal depression, breastfeeding status, maternal race, income, and HOME score.
Abbreviations: CI, confidence interval; BSI, Behavioral Symptom Index.
In quantile g-computation models for individual exposure time points, the relative magnitudes and directions of estimates were similar to SEM results, but all confidence intervals included the null (Table 2). However, in the longitudinal quantile g-computation models, pregnancy concentrations of OPEs were associated with fewer Externalizing Problems at age 8 years (ß = −0.96, 95% CI = −1.93, 0.01), a result not observed with the other model types (Table 3).
Table 2:
Quantile g-computation models for OPEs and BASC-2 outcomes, estimates for increasing urine exposure biomarkers by one quartile (n = 219). All models are adjusted for maternal depression, breastfeeding status, maternal race, income, and HOME score.
| Exposure assessment | Outcome | Estimate | 95% CI |
|---|---|---|---|
|
| |||
| 16 Weeks | Internalizing, 3 Years | −0.36 | −2.39, 1.67 |
| Externalizing, 3 Years | −1.02 | −2.93, 0.88 | |
| BSI, 3 Years | −0.64 | −2.36, 1.08 | |
| 26 Weeks | Internalizing, 3 Years | −0.61 | −2.35, 1.13 |
| Externalizing, 3 Years | −0.49 | −2.13, 1.14 | |
| BSI, 3 Years | −0.72 | −2.24, 0.80 | |
| Delivery | Internalizing, 3 Years | 0.34 | −1.66, 2.34 |
| Externalizing, 3 Years | 1.08 | −0.79, 2.95 | |
| BSI, 3 Years | 0.58 | −1.10, 2.26 | |
|
| |||
| 16 Weeks | Internalizing, 8 Years | 0.52 | −1.07, 2.11 |
| Externalizing, 8 Years | −1.14 | −2.68, 0.41 | |
| BSI, 8 Years | −1.01 | −2.53, 0.52 | |
| 26 Weeks | Internalizing, 8 Years | −0.07 | −1.43, 1.30 |
| Externalizing, 8 Years | −0.36 | −1.73, 1.01 | |
| BSI, 8 Years | −0.37 | −1.72, 0.98 | |
| Delivery | Internalizing, 8 Years | 0.41 | −1.16, 1.98 |
| Externalizing, 8 Years | −0.64 | −2.15, 0.87 | |
| BSI, 8 Years | −0.27 | −1.82, 1.27 | |
|
| |||
| 1 Year | Internalizing, 8 Years | 0.21 | −1.36, 1.77 |
| Externalizing, 8 Years | −0.65 | −2.33, 1.04 | |
| BSI, 8 Years | −0.43 | −2.05, 1.20 | |
| 2 Years | Internalizing, 8 Years | −0.59 | −2.32, 1.13 |
| Externalizing, 8 Years | −0.67 | −2.24, 0.90 | |
| BSI, 8 Years | −1.14 | −2.83, 0.54 | |
| 3 Years | Internalizing, 8 Years | 0.18 | −1.41, 1.77 |
| Externalizing, 8 Years | −1.17 | −2.70, 0.37 | |
| BSI, 8 Years | −1.30 | −2.78, 0.18 | |
| 5 Years | Internalizing, 8 Years | 0.56 | −0.91, 2.03 |
| Externalizing, 8 Years | 0.37 | −1.18, 1.91 | |
| BSI, 8 Years | 0.19 | −1.28, 1.67 | |
Abbreviations: CI, confidence interval; BSI, Behavioral Symptom Index.
Table 3:
Longitudinal OPE exposure and BASC-2 outcome t-scores quantile g-computation models, estimates for increasing exposure biomarker concentrations by one quartile (n = 187). All models are adjusted for maternal depression, breastfeeding status, maternal race, income, and HOME score.
| Exposure assessment | Outcome | Estimate | 95% CI |
|---|---|---|---|
|
| |||
| Pregnancy | Internalizing, 3 years | −0.22 | −1.34, 0.89 |
| Externalizing, 3 years | −0.12 | −1.34, 1.10 | |
| BSI, 3 years | −0.27 | −1.38, 0.84 | |
| Pregnancy | Internalizing, 8 years | 0.21 | −0.67, 1.10 |
| Externalizing, 8 years | −0.96 | −1.93, 0.01 | |
| BSI, 8 years | −0.60 | −1.55, 0.36 | |
| Postnatal | Internalizing, 8 years | 0.30 | −0.54, 1.14 |
| Externalizing, 8 years | −0.22 | −1.10, 0.65 | |
| BSI, 8 years | −0.37 | −1.25, 0.51 | |
Abbreviations: CI, confidence interval; BSI, Behavioral Symptom Index.
DISCUSSION
In this longitudinal cohort study of OPE exposures during early brain development and child behavior at ages 3 and 8 years, we found that in utero exposures were more frequently and significantly associated with internalizing problems, externalizing problems, and overall maladaptive behavior at age 3 years compared with childhood exposures in latent variable analyses. However, the direction of associations was inconsistent. OPE exposure at delivery was associated with more behavior problems, while OPE exposure at 16 weeks of gestation and age 3 years was associated with fewer behavior problems. In single-timepoint quantile g-computation models, the magnitude and direction of the null associations were similar to the latent variable results. We did observe a small and significant decrease in externalizing behavior problems at age 8 years with in utero exposure longitudinal quantile g-computation models.
The associations we observed showing decreased behavioral issues at age 8 years with higher OPEs earlier in life could be partly due to remedial actions taken by parents and schools that would not yet have been accomplished at age 3 years. Externalizing behaviors in particular are likely to be intervened upon in school-age children. Although we do not have the data to test this hypothesis, it could be an area of future study for other researchers.
Others have also reported associations between OPE exposures and child behavior measured by the BASC-2. Doherty et al. measured urinary OPE metabolites once during pregnancy, around 27 weeks, in a cohort of North Carolina women and reported associations of BDCIPP and DPHP concentrations with higher scores on the Externalizing Problems and BSI composite scores at age 3 years (26). However, urinary concentrations of isopropyl-phenyl phenyl phosphate (ip-PPP), an OPE not measured in our study, were associated with lower Internalizing Problems and BSI composite scores. The observation of mixed results concerning the direction of the effects is consistent with our study. Castorina et al. also measured urinary OPE metabolites once during pregnancy, around 26 weeks, in a cohort of California women (mainly Hispanic, living in farm-working communities) and assessed child behavior at age 7 years with the BASC-2. They did not report associations with composite scales but did observe that BDCIPP and ip-PPP concentrations were associated with higher scores on the attention and hyperactivity subscales, respectively, which contribute to the Externalizing and BSI composite scores (23). We observed some evidence of an association between pregnancy OPEs and externalizing behaviors at 8 years in our quantile g-computation analysis, but in the opposite direction to what Castorina et al. reported. Neither of these cited studies reported multiple time points of OPE exposure or associations with repeated measurements of child behavior, which makes them more susceptible to measurement error.
We are only aware of one study that reported associations between childhood OPE exposure and behavior. In 3- to 5-year-old children, Lipscomb et al. measured levels of OPEs parent compounds in silicone wristbands worn for 7 days and observed that higher OPEs were associated with less responsible behavior and more externalizing problems based on a teacher-reported rating scale of social skills (22). They did not observe any associations with internalizing behaviors. However, this study was limited by its cross-sectional design and lack of internal biomarkers of exposure.
While our mixture-based analysis—which more closely approximated real-world exposures—was a strength of the study, our results should be interpreted cautiously. Our findings were inconsistent with respect to which exposure periods during brain development were sensitive to OPEs and the effects on behavioral domains. This variability could result from spurious findings, uncontrolled confounding, or our inability to account for the effects of other chemical exposures from different classes in our mixtures analysis. A limitation to our mixture approach is that we were unable to include more than three OPEs in our models. We previously attempted to quantify the concentrations of additional OPE metabolites in our participants’ urine, but the number of samples that were below the limit of detection made the inclusion of additional metabolites unfeasible for our statistical methods (31). This is likely due to the time period in which our samples were collected, during the phase-out of polybrominated diphenyl ethers from use as flame retardants, when environmental concentrations of OPEs were lower than they are today. Other cohorts might be better able to include a broader group of OPEs in future mixtures analyses.
A limitation of our study is that we relied on a parent-report questionnaire to assess child behavior instead of utilizing a direct assessment or a self-report method. We also did not specifically exclude intellectually disabled mothers from the study, which may have impacted the reliability of a parent-report questionnaire. However, the BASC-2 is useful for this study in that it is logistically convenient and repeatable at ages 3 and 8 years. Other assessment options, such as the Bayley Scales of Infant and Child Development (43) or a child-reported questionnaire, would have only been appropriate for one age group and would have limited our ability to directly compare our results between ages. It cannot be entirely ruled out that higher exposed women might have completed the BASC-2 with a systematic bias, but we attempted to control for this type of confounding by adjusting for maternal depression. However, we cannot rule out residual confounding in our analyses.
However, the study had several strengths. OPEs have a short biological half-life and poor reliability between measurements (31,44), so frequent quantification of OPE metabolite concentrations is critical to reducing exposure misclassification as the chemicals do not accumulate for long in the body. We had three urinary OPE metabolite measurements during pregnancy and four during early childhood; this is more than any other study currently in the literature. We also assessed child behavior at two different time points, at age 3 years during early childhood and at age 8 years, when child behavioral measures tend to be more reliable. Finally, we utilized two methods to quantify associations between OPE mixtures and child behavior, and the results were largely consistent.
This study is the first that utilized a chemical mixtures approach to study pregnancy and postnatal OPE exposure and behavior in children. Therefore, additional research will provide helpful information to determine the direction and magnitude of the associations and the public health relevancy of the results. Given our findings and the findings of others, we would urge decision-makers to inform the public about ways to reduce exposure to OPEs. Chlorinated OPEs, like the parent compounds of BCEP and BDCIPP, are typically used as flame retardants, while the parent compound of DPHP is used as a plasticizing chemical and as a component of commercial flame retardant mixtures, including FM550 (15). Therefore, recommendations that include frequent cleaning in rooms containing electronics or furniture that may have been treated with OPEs as flame retardant chemicals, the use of indoor air purification to keep down dust levels, and reducing dependance on plastic products may be appropriate.
CONCLUSIONS
Pregnancy and early childhood mixtures of urinary OPE metabolites were associated with some measures of child behavior in this pregnancy and birth cohort. We did not observe consistent patterns concerning the direction of the association, vulnerable time points, or the type of behaviors affected. However, the results were similar across two different statistical methods. The heterogeneity may be actual or due to measurement error, and further research on other populations can help inform public health decisions.
Supplementary Material
Figure 1:
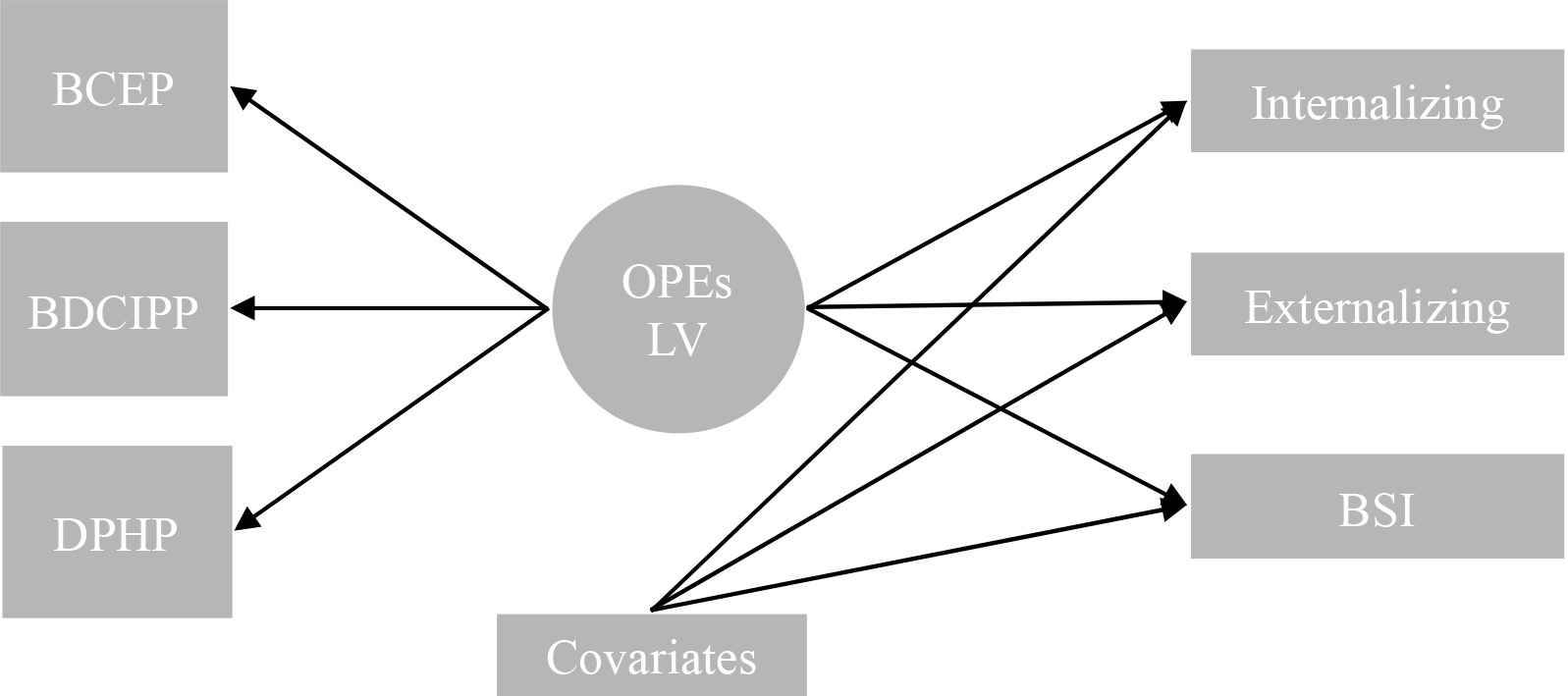
Simplified SEM diagram for modeling strategy.
Abbreviations: BCEP, bis-2-chloroethyl phosphate; BDCIPP, bis(1,3-dichloro-2-propyl) phosphate; DPHP, diphenyl phosphate; OPEs, organophosphate esters; LV, latent variable; BSI, Behavioral Symptom Index.
HIGHLIGHTS.
We studied pregnancy and postnatal OPEs and child behavior in a longitudinal cohort.
We used latent variable analysis and quantile g-computation to assess OPE mixtures.
Pregnancy OPEs were associated with child behavior at 3 years.
The direction of associations and vulnerable time points were inconsistent.
Acknowledgments:
We acknowledge Nayana Jayatilaka, Paula Restrepo, Zack Davis, and Meghan Vidal (CDC) for providing the OPE metabolites measurements. We also thank Dr. Alex Keil (University of North Carolina Chapel Hill) for his feedback regarding our statistical methods.
Funding:
This work was supported by grants from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (NIEHS F30 ES033086, P01 ES011261, R01 ES014575, R01 ES014575, R01 ES020349, R01 ES027224, R01 ES028277; EPA P01 R829389), and the University of Cincinnati Medical Scientist Training Program Grant 2T32GM063483-1. We also thank the LB Research and Education Foundation for their support of this work.
Footnotes
Conflicts of interest: Dr. Braun was financially compensated for serving as an expert witness in litigation related to perfluorooctanonic acid contamination in drinking water.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). The use of trade names is for identification only and does not imply endorsement by the CDC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brown TT, Jernigan TL. Brain development during the preschool years. Neuropsychol Rev. 2012;22(4):313–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caspi A, Moffitt TE, Newman DL, et al. Behavioral Observations at Age 3 Years Predict Adult Psychiatric Disorders. Arch Gen Psychiatry. 1996;53:1033–9. [DOI] [PubMed] [Google Scholar]
- 3.Loth AK, Drabick DAG, Leibenluft E, et al. Do childhood externalizing disorders predict adult depression? A meta-analysis. J Abnorm Child Psychol. 2014;42(7):1103–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergunst F, Tremblay RE, Nagin D, et al. Association of Behavior in Boys from Low Socioeconomic Neighborhoods with Employment Earnings in Adulthood. JAMA Pediatr. 2019;173(4):334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurology. 2015;13(3):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo K, Liu J, Wang Y, et al. Associations between organophosphate esters and sex hormones among 6–19-year old children and adolescents in NHANES 2013–2014. Environ Int. 2020;136(January):105461. [DOI] [PubMed] [Google Scholar]
- 7.Ren X, Cao L, Yang Y, et al. In vitro assessment of thyroid hormone receptor activity of four organophosphate esters. J Environ Sci (China) [electronic article]. 2015;45:185–190. ( 10.1016/j.jes.2015.12.021) [DOI] [PubMed] [Google Scholar]
- 8.Tao Y, Hu L, Liu L, et al. Prenatal exposure to organophosphate esters and neonatal thyroid-stimulating hormone levels: A birth cohort study in Wuhan, China. Environ Int [electronic article]. 2021;156:106640. ( 10.1016/j.envint.2021.106640) [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Jung D, Jo A, et al. Long-term exposure to triphenylphosphate alters hormone balance and HPG, HPI, and HPT gene expression in zebrafish (Danio rerio). Environ Toxicol Chem. 2016;35(9):2288–2296. [DOI] [PubMed] [Google Scholar]
- 10.Percy Z, Vuong AM, Xu Y, et al. Maternal Urinary Organophosphate Esters and Alterations in Maternal and Neonatal Thyroid Hormones. Am J Epidemiol. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patisaul HB, Behl M, Birnbaum LS, et al. Beyond Cholinesterase Inhibition: Developmental Neurotoxicity of Organophosphate Ester Flame Retardants and Plasticizers. Environ Health Perspect. 2021;129(10):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty BT, Hammel SC, Daniels JL, et al. Organophosphate Esters: Are These Flame Retardants and Plasticizers Affecting Children’s Health? Curr Environ Health Rep. 2019;6(4):201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergh C, Torgrip R, Emenius G, et al. Organophosphate and phthalate esters in air and settled dust - a multi-location indoor study. Indoor Air. 2011;21(1):67–76. [DOI] [PubMed] [Google Scholar]
- 14.Wei GL, Li DQ, Zhuo MN, et al. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environmental Pollution [electronic article]. 2015;196:29–46. ( 10.1016/j.envpol.2014.09.012) [DOI] [PubMed] [Google Scholar]
- 15.van der Veen I, de Boer J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere [electronic article]. 2012;88(10):1119–1153. ( 10.1016/j.chemosphere.2012.03.067) [DOI] [PubMed] [Google Scholar]
- 16.He C, Wang X, Tang S, et al. Concentrations of Organophosphate Esters and Their Specific Metabolites in Food in Southeast Queensland, Australia: Is Dietary Exposure an Important Pathway of Organophosphate Esters and Their Metabolites? Environ Sci Technol. 2018;52(21):12765–12773. [DOI] [PubMed] [Google Scholar]
- 17.Han L, Sapozhnikova Y, Nuñez A. Analysis and Occurrence of Organophosphate Esters in Meats and Fish Consumed in the United States. J Agric Food Chem. 2019;67(46):12652–12662. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Zhao L, Letcher RJ, et al. A review on organophosphate Ester (OPE) flame retardants and plasticizers in foodstuffs: Levels, distribution, human dietary exposure, and future directions. Environ Int [electronic article]. 2019;127(March):35–51. ( 10.1016/j.envint.2019.03.009) [DOI] [PubMed] [Google Scholar]
- 19.Kim UJ, Kannan K. Occurrence and Distribution of Organophosphate Flame Retardants/Plasticizers in Surface Waters, Tap Water, and Rainwater: Implications for Human Exposure. Environ Sci Technol. 2018;52(10):5625–5633. [DOI] [PubMed] [Google Scholar]
- 20.Kim U-J, Wang Y, Li W, et al. Occurrence of and human exposure to organophosphate flame retardants/plasticizers in indoor air and dust from various microenvironments in the United States. Environ Int [electronic article]. 2019;125(January):342–349. (https://linkinghub.elsevier.com/retrieve/pii/S0160412018327296) [DOI] [PubMed] [Google Scholar]
- 21.Yan Z, Jin X, Liu D, et al. The potential connections of adverse outcome pathways with the hazard identifications of typical organophosphate esters based on toxicity mechanisms. Chemosphere. 2021;266:128989. [DOI] [PubMed] [Google Scholar]
- 22.Lipscomb ST, McClelland MM, MacDonald M, et al. Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ Health. 2017;16(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castorina R, Bradman A, Stapleton HM, et al. Current-use flame retardants: Maternal exposure and neurodevelopment in children of the CHAMACOS cohort. Chemosphere [electronic article]. 2017;189:574–580. ( 10.1016/j.chemosphere.2017.09.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi G, Keil AP, Richardson DB, et al. Pregnancy exposure to organophosphate esters and the risk of attention-deficit hyperactivity disorder in the Norwegian mother, father and child cohort study. Environ Int [electronic article]. 2021;154:106549. ( 10.1016/j.envint.2021.106549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Luo D, Xia W, et al. Prenatal exposure to halogenated, aryl, and alkyl organophosphate esters and child neurodevelopment at two years of age. J Hazard Mater [electronic article]. 2021;408(December 2020):124856. ( 10.1016/j.jhazmat.2020.124856) [DOI] [PubMed] [Google Scholar]
- 26.Doherty BT, Hoffman K, Keil AP, et al. Prenatal exposure to organophosphate esters and behavioral development in young children in the Pregnancy, Infection, and Nutrition Study. Neurotoxicology. 2019;73(March):150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun JM, Buckley JP, Cecil KM, et al. Adolescent follow-up in the Health Outcomes and Measures of the Environment (HOME) Study: Cohort profile. BMJ Open. 2020;10(5):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun JM, Kalloo G, Chen A, et al. Cohort profile: The Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol. 2017;46(1):24–24i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayatilaka NK, Restrepo P, Williams L, et al. Quantification of three chlorinated dialkyl phosphates, diphenyl phosphate, 2,3,4,5-tetrabromobenzoic acid, and four other organophosphates in human urine by solid phase extraction- high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2017;409(5):1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayatilaka NK, Restrepo P, Davis Z, et al. Quantification of 16 urinary biomarkers of exposure to flame retardants, plasticizers, and organophosphate insecticides for biomonitoring studies. Chemosphere. 2019;235:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Percy Z, Vuong AM, Ospina M, et al. Organophosphate esters in a cohort of pregnant women : Variability and predictors of exposure. Environ Res [electronic article]. 2020;184(February):109255. ( 10.1016/j.envres.2020.109255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 33.MacPherson S, Arbuckle TE, Fisher M. Adjusting urinary chemical biomarkers for hydration status during pregnancy. J Expo Sci Environ Epidemiol. 2018;28(5):481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duty SM, Ackerman RM, Calafat AM, et al. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect. 2005;113(11):1530–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds C, Kamphaus R. Behavior Assessment System for Children-Second Edition. Circle Pines, MN: American Guidance Services Publishing; 2004. [Google Scholar]
- 36.Caldwell B, Bradley R. Home Observation for Measurement of the Environment. 1984;
- 37.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. 1996;
- 38.Rosner B Fundamentals of Biostatistics. Boston, MA: Cengage Learning; 2011. [Google Scholar]
- 39.Muthen L, Muthen B. Mplus User’s Guide. Eighth Edition. 2017; [Google Scholar]
- 40.Hooper D, Coughlan J, Mullen MR. Structural equation modeling: Guidelines for determining model fit. Electronic Journal of Business Research Methods. 2008;6(1):53–60. [Google Scholar]
- 41.R Core Team. R: A language and environment for statistical computing. 2019;
- 42.Keil AP, Buckley JP, O’Brien KM, et al. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect. 2020;128(4):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bayley N. Bayley Scales of Infant Devlopment: manual. 2nd ed. San Antonio: The Psychological Corporation; 1993. [Google Scholar]
- 44.Wang X, Liu Q, Zhong W, et al. Estimating renal and hepatic clearance rates of organophosphate esters in humans: Impacts of intrinsic metabolism and binding affinity with plasma proteins. Environ Int [electronic article]. 2020;134(November 2019):105321. ( 10.1016/j.envint.2019.105321) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


