Abstract
A recent environmental theory of negative symptoms posits that environmental contexts (e.g., location, social partner) play a significant—yet often unaccounted for—role in negative symptoms of schizophrenia (SZ). “Gold-standard” clinical rating scales offer limited precision for evaluating how contexts impact symptoms. To overcome some of these limitations, Ecological Momentary Assessment (EMA) was used to determine whether there were state fluctuations in experiential negative symptoms (anhedonia, avolition, and asociality) in SZ across contexts (locations, activities, social interaction partner, social interaction method). Outpatients with SZ (n = 52) and healthy controls (CN: n = 55) completed 8 daily EMA surveys for 6 days assessing negative symptom domains (anhedonia, avolition, and asociality) and contexts. Multilevel modeling demonstrated that negative symptoms varied across location, activity, social interaction partner, and social interaction method. For the majority of contexts, SZ and CN did not report significantly different levels of negative symptoms, with SZ only reporting higher negative symptoms than CN while eating, resting, interacting with a significant other, or being at home. Further, there were several contexts where negative symptoms were similarly reduced (e.g., recreation, most social interactions) or elevated (e.g., using the computer, working, running errands) in each group. Results demonstrate that experiential negative symptoms dynamically change across contexts in SZ. Some contexts may “normalize” experiential negative symptoms in SZ, while other contexts, notably some used to promote functional recovery, may increase experiential negative symptoms.
Keywords: Environment, Anhedonia, Avolition, Asociality, Psychosis
Introduction
Many with schizophrenia (SZ) experience significant occupational and social functioning impairments (Pinkham et al., 2012). Negative symptoms such as avolition and anhedonia are critical contributors to these functional impairments (Luther et al., 2020b; Strauss et al., 2021). However, extant psychosocial and pharmacological interventions have largely failed to produce clinically significant negative symptom improvements (Fusar-Poli et al., 2015), creating a critical yet unmet need in SZ therapeutics.
One reason for the lack of progress in treating negative symptoms is a limited understanding of the environmental or contextual factors that may influence these symptoms. The recent Bioecosystem Theory of Negative Symptoms (Strauss, 2021) posits that interactive environmental systems of varying proximity to an individual may influence the emergence and maintenance of negative symptoms. The most immediate environmental contexts, known as the microsystem, include factors that a person has direct contact with, such as activities (e.g., work, recreation), locations (e.g., home, work), and social partners (e.g., family, friends). These immediate environmental contexts and their interactions are thought to directly influence negative symptoms. For example, locations such as work or school may lead to greater avolition reductions, while a friend’s house may lead to asociality reductions. Similarly, social partner type (e.g., friend versus a stranger) may differentially influence one’s social drive and pleasure. However, little empirical work has examined the influence of context on negative symptoms in daily life.
Early descriptions of negative symptoms noted a progressive worsening over the course of illness (Bleuler, 1950; Kraepelin et al., 1919). However, these observations were primarily made in psychiatric hospitals where environmental resources and social stimulation were limited, potentially exacerbating symptom decline. More recent longitudinal studies using clinical rating scales with outpatients have shown that negative symptoms are largely stable across weeks to even years during non-acute illness phases and when treatment is relatively constant (Harvey et al., 1990; Kirkpatrick et al., 2011; Kring et al., 2013; Pogue-Geile and Harrow, 1985). This work has resulted in a long-standing belief that negative symptoms are highly stable trait-like features that are contextually invariant. However, it is unclear whether this holds when negative symptoms are assessed across dynamic contexts. Identifying whether certain contexts exacerbate or reduce negative symptoms may inform interventions such as activity scheduling and ecological momentary interventions delivered using context-based just-in-time adaptive approaches (Nahum-Shani et al., 2018).
Extant negative symptom research primarily utilizes interviewer-rated assessments, which do not adequately assess the impact of dynamic environmental contexts. Although “second-generation” interviewer-based scales such as the Clinical Assessment Interview for Negative Symptoms (CAINS; (Kring et al., 2013) and Brief Negative Symptom Scale (BNSS; (Kirkpatrick et al., 2011) provide a more nuanced assessment grounded in recent conceptualizations, the limitations inherent to interviewer-rated scales diminish our ability to capture context-dependent symptom changes. For example, these scales require interviewers to derive a global negative symptom rating across time (e.g., past week) and changing contexts (e.g., activities, locations) (Cohen et al., 2019b). These low-resolution ratings represent a broad temporal range that cannot be used to infer how symptoms vary as a function of environmental context (Cohen et al., 2020b, 2021), making it challenging to precisely quantify which contexts exacerbate or reduce symptoms. Further, interviewer-rated scales have high participant recall demands, requiring retrospective reports of higher-order states like motivation and pleasure over extended timeframes (e.g., past week). This, along with work showing that the working memory impairments commonly seen in SZ may impact participants’ ability to effectively recall symptoms during clinical interviews (Moran et al., 2017), suggest that interviewer-rated rates scales do not allow for a precise assessment of how negative symptoms vary across contexts.
One method that allows for a higher resolution negative symptom assessment, especially experiential negative symptoms (i.e., anhedonia, avolition, and asociality), across contexts is Ecological Momentary Assessment (EMA). EMA involves the repeated administration of surveys while participants are engaged in daily life outside of the laboratory, providing greater ecological validity and temporal specificity. Studies have found that assessing negative symptoms in SZ via EMA is feasible, valid, and could overcome retrospective recall demands of interviewer-rated scales (Badal et al., 2021; Moran et al., 2017; Parrish et al., 2022). In addition, researchers have begun to use EMA to better isolate the effects of dynamic environmental contexts on emotional experience and symptoms in SZ. Research suggests that SZ report greater positive affect when they are with others compared to being alone (Depp et al., 2016; Edwards et al., 2018; Mote et al., 2019; Oorschot et al., 2013). Initial work deconstructing this finding based on social partner (e.g., friend, family) suggested that when SZ and controls were with family and friends, they reported greater positive affect and social motivation (Badal et al., 2021). When with “others” (e.g., strangers), SZ instead reported greater anxiety, while controls reported greater social motivation. However, these findings offer limited insight into the role that different social partners have on other negative symptom domains (e.g., anhedonia) as well as how interaction modality (i.e., in person, phone call) influences negative symptoms. Similarly, few studies have examined the effect activity or location may have on negative symptoms in SZ. Extant studies have demonstrated that being out of the home (versus home) is associated with greater negative affect (e.g., anxiety, sadness) (Badal et al., 2021; Parrish et al., 2020). Although these findings indicate that EMA can help elucidate the impact of context on emotional experience in daily life in SZ, they also point to a gap in our understanding about the role of context (e.g., method of interacting, location, activity) on state fluctuations in negative symptoms.
The present study used EMA to address this gap and more comprehensively assess experiential negative symptom levels across contexts. Specifically, we examined the role that activity (e.g., resting, recreation), location (e.g., home, public), social partner (e.g., alone, with strangers), and social modality (e.g., electronically, in person) had on concurrent negative symptoms of avolition, anhedonia, and asociality measured via EMA. SZ and healthy controls (CN) completed six days of EMA where eight daily surveys assessing negative symptoms and context were delivered. We hypothesized that avolition, anhedonia, and asociality would significantly vary across contexts in both SZ and CN. We anticipated that asociality would show the greatest fluctuations between social and non-social contexts, while the remaining analyses were exploratory given that little work has been done in this area.
Method
Participants
Participants included 52 SZ outpatients (1444 EMA samples) and 55 CN (1786 EMA samples). Groups did not differ on age, sex, parental education, or race; however, SZ had lower personal education than CN (see Table 1). There was a trend toward SZ completing fewer EMA surveys than CN; however, completion rates were in line with prior SZ EMA studies (Mote and Fulford, 2020).
Table 1.
Group Demographic and Clinical Characteristics
| SZ (n = 52) | CN (n = 55) | Test Statistic | p-value | |
|---|---|---|---|---|
| Age, mean (SD) | 38.98 (11.97) | 39.07 (10.62) | F = .002 | .97 |
| Parental Education, mean (SD) | 13.83 (2.90) | 13.63 (2.85) | F = .12 | .73 |
| Participant Education, mean (SD) | 13.21 (2.28) | 15.40 (2.82) | F = 19.32 | <.001 |
| Male; n (%) | 18 (35%) | 17 (31%) | χ2 = .17 | .68 |
| Race (n, %) | χ2 = 8.62 | .13 | ||
| African American | 17, 32.7% | 16, 29.1% | - | - |
| Caucasian | 30, 57.7% | 24, 43.6% | - | - |
| Asian American | 0, 0% | 4, 7.3% | - | - |
| Hispanic/Latino | 2, 3.8% | 6, 10.9% | - | - |
| Biracial | 3, 5.8% | 3, 5.5% | - | - |
| Other | 0, 0% | 2, 3.6% | - | - |
| EMA Survey Adherence, M (SD) | .58 (.26) | .67 (.24) | F = 3.52 | .06 |
| BNSS Domain scores, M (SD) | ||||
| BNSS – Anhedonia | 4.49 (4.81) | - | - | - |
| BNSS – Avolition | 3.88 (3.25) | - | - | - |
| BNSS – Asociality | 2.73 (2.69) | - | - | - |
| BNSS – Blunted Affect | 2.18 (3.42) | - | - | - |
| BNSS – Alogia | 0.69 (1.86) | - | - | - |
Note. BNSS = Brief Negative Symptom Scale; CN = control group; EMA = ecological momentary assessment; SZ = schizophrenia group.
Participants were recruited from the local community via online or printed advertisements; SZ participants were also recruited from outpatient mental health centers. SZ diagnoses were made using the Structured Clinical Interview for DSM5 (SCID-5; First et al., 2015). CN had no current major psychiatric diagnoses based on the SCID-5, no current schizophrenia-spectrum personality disorders (via the SCID-PD; First et al., 2016), no lifetime psychotic or bipolar disorders, no psychosis family history, and no current psychotropic medication prescription. All participants denied lifetime neurological disorders and past six month substance abuse. Following prior methods (Granholm et al., 2020; Strassnig et al., 2021), participants were compensated $20 per hour of assessments and $1 per EMA survey completed. An $80 bonus was provided for returning the study phone. All participants provided written informed consent for a protocol approved by the University of Georgia Institutional Review Board.
Procedures
Study procedures occurred in three phases over a one-week period (see Narkhede et al., 2022; Raugh et al., 2021) for additional details).
Phase 1: Initial Laboratory Visit.
Participants provided informed consent, completed diagnostic/symptom interviews, and received training for the mEMA app (www.ilumivu.com) and smartphone used for EMA. Participants completed a practice survey to ensure they understood the response formats. SZ completed the SCID-5 and BNSS (BNSS; Kirkpatrick et al., 2011). CN completed the SCID-5 and SCID-PD.
Phase 2: EMA.
Data were collected over six days; 8 daily surveys were delivered randomly within 90-minute epochs from 9AM to 9PM. Both SZ and CN completed the EMA procedures to allow for group comparisons and since negative symptoms have been found at subclinical levels in healthy controls and the general population (Kaiser et al., 2011; Stefanis et al., 2002; Strauss et al., 2016; Werbeloff et al., 2015).
EMA surveys included items assessing context and negative symptoms1 of anhedonia, avolition, and asociality (see supplement for items). Following BNSS procedures, negative symptom items were created to assess internal experience components of anhedonia, avolition, and asociality via questions about enjoyment (current/consummatory and anticipatory) and interest; participants completed these items for their concurrently endorsed contexts (e.g., “How interested are you in the activity?”). Context items assessed current activity (“What are you doing?”), location (“Where are you?”), social partner (“Who are you interacting with?”), and social modality (“How are you interacting with them?”). Contexts were not mutually exclusive; participants could select as many as applied up to 15 minutes before the survey.
Phase 3: Final Laboratory Visit.
Following EMA, participants returned the study phone and received monetary compensation.
Data Analysis
To account for nesting and repeated measures in the data, all models presented used multilevel modeling with random intercepts within person and day. As all effects evaluated were among categorical variables, no random slopes were used. Models were conducted separately for each negative symptom domain (anhedonia (anticipatory and consummatory combined), avolition, asociality) and context domain (activity, location, social partner, social modality). Only contexts observed 200 times or more (out of 3212 surveys) were included to ensure adequate observations for stable estimates and statistical inference. Seven activity contexts were evaluated: resting, working, watching TV, eating, computer use, errands, and recreation/hobbies. Four location contexts were evaluated: home, work, public, and friend/family residence. Six social partner contexts were evaluated: no one/alone, family, significant other, coworkers, friends, and strangers. Four social modality contexts were evaluated: no one/alone, in-person, electronic, and phone. Resting, being at home, and being alone were selected as “baseline” or reference contexts against which others were compared.
Primary models for each negative symptom domain evaluated the effect of Group (CN, SZ), Context (see above), and Group X Context interaction. A main effect of Group would indicate a difference between CN and SZ regardless of context (i.e., between-group), while a main effect of Context would indicate differences between contexts in both groups (i.e., within-group). An interaction can be interpreted as reflecting both how the difference in negative symptoms by groups varies by context (between-group) and how the differences between contexts vary for each group (within-group). Only instances where contexts were endorsed were included in models to isolate the relative effects of each context (i.e., does context B reduce symptoms compared to context A). Context was treated as a repeated-measures variable within each survey instance. Model omnibus effects were evaluated using Wald χ2 tests. Significant omnibus effects were evaluated for within-group and between-group differences, and significant Group X Context interactions were plotted to help interpret effects. The false discovery correction was applied to correct for multiple comparisons.
To contextualize results, several exploratory models were planned and are presented in the supplemental materials. Group differences in Context endorsement (i.e., the probability of each group endorsing a Context) were evaluated. Second, we evaluated the context effects outlined above on overall negative symptoms (mean of anhedonia, avolition, and asociality). Additional models evaluated differences in anticipatory and consummatory anhedonia based on Group, Context, and their interaction. Given that mood symptoms are often linked to negative symptoms (Krynicki et al., 2018), we also conducted analyses to examine the utility of controlling for these symptoms.
Results
Table 2 presents omnibus effects for models evaluating the context effects on anhedonia, avolition, and asociality. Two-way interactions are displayed in Figures 1–3. Means for each symptom domain, including by context and group are in the supplement (See Supplemental Table 1 and 2). As correlations of mood with contexts and negative symptoms were largely minimal (see Supplemental Table 3), mood was not used as a covariate in any analyses.
Table 2.
Primary model omnibus effects
| Context | Total R2 | Fixed-effect R2 | Group | Context | Group × Context |
|---|---|---|---|---|---|
| Anhedonia | |||||
| Activity | 0.57 | 0.06 | 8.37** | 283.37*** | 49.89*** |
| Location | 0.47 | 0.02 | 4.66* | 65.44*** | 18.48*** |
| Social partner | 0.5 | 0.03 | 1.74 | 97.34*** | 16.11 ** |
| Social modality | 0.48 | 0.02 | 1.6 | 21.87*** | 6.07 |
| Avolition | |||||
| Activity | 0.53 | 0.04 | 6.17* | 173.44*** | 21.7 ** |
| Location | 0.42 | 0.02 | 3.71 | 25.79*** | 6.86 |
| Social partner | 0.44 | 0.02 | 2.42 | 35.59*** | 6.82 |
| Social modality | 0.43 | 0.01 | 2.23 | 11.53 ** | 4.74 |
| Asociality | |||||
| Activity | 0.49 | 0.03 | 1.32 | 92.96*** | 37.96*** |
| Location | 0.42 | 0.02 | 0.01 | 43.41*** | 9.45 * |
| Social partner | 0.63 | 0.29 | 5.29* | 1396.14*** | 75.55*** |
| Social modality | 0.61 | 0.28 | 6.22* | 1188.54*** | 43.79*** |
Note. All effects in bold remained after false discovery rate correction.
= p < .05,
= p < .01,
= p < .001
Figure 1.
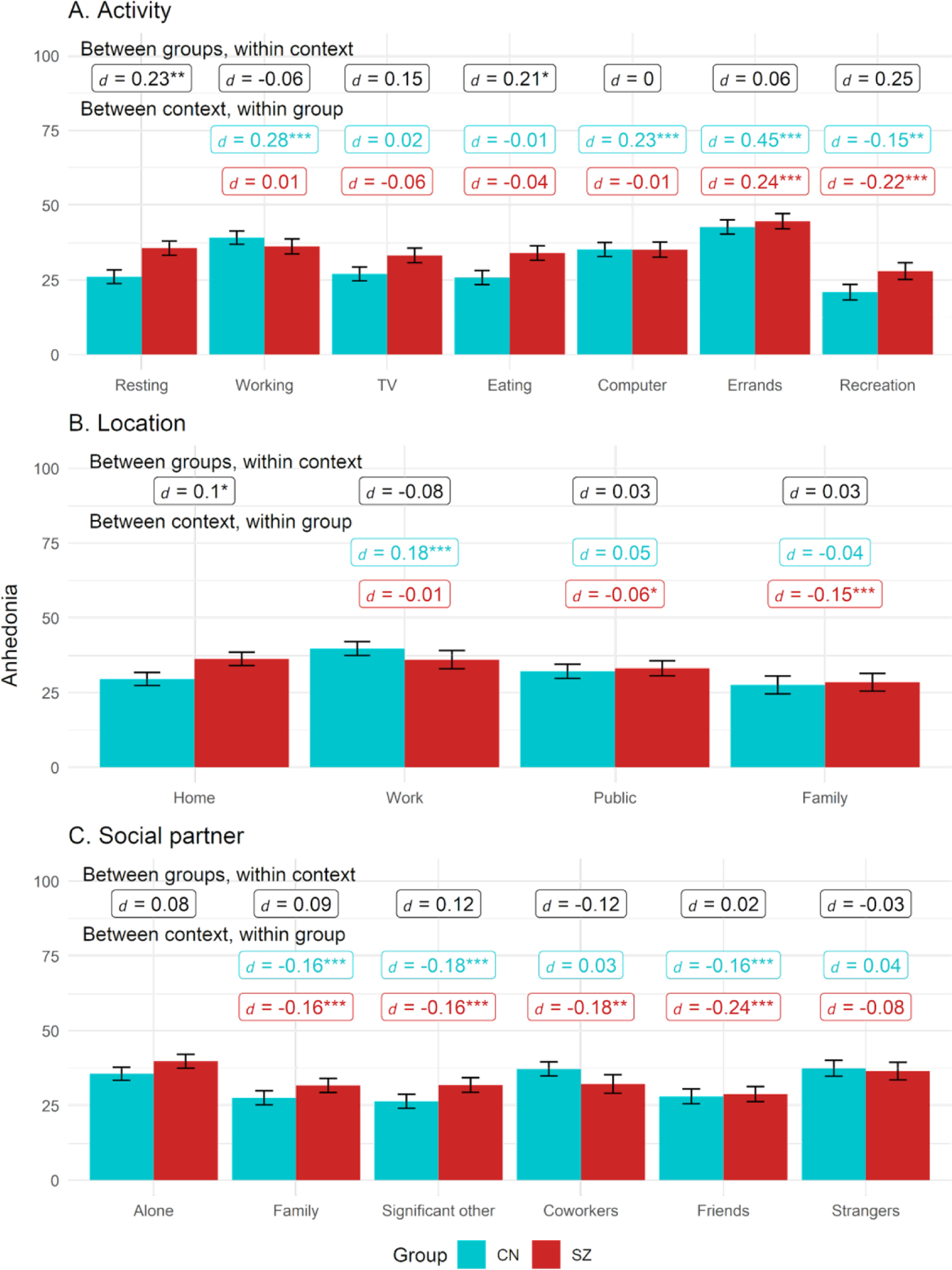
Anhedonia based on group and activity, location, or social partner context
Note. Black labels reflect contrast between groups within each context while colored labels reflect contrast within group relative to reference context (resting, being at home, or being alone). Figures use estimated marginal means and error bars reflect standard error.
* = p < .05, ** = p < .01, ** = p < .001
Figure 3.
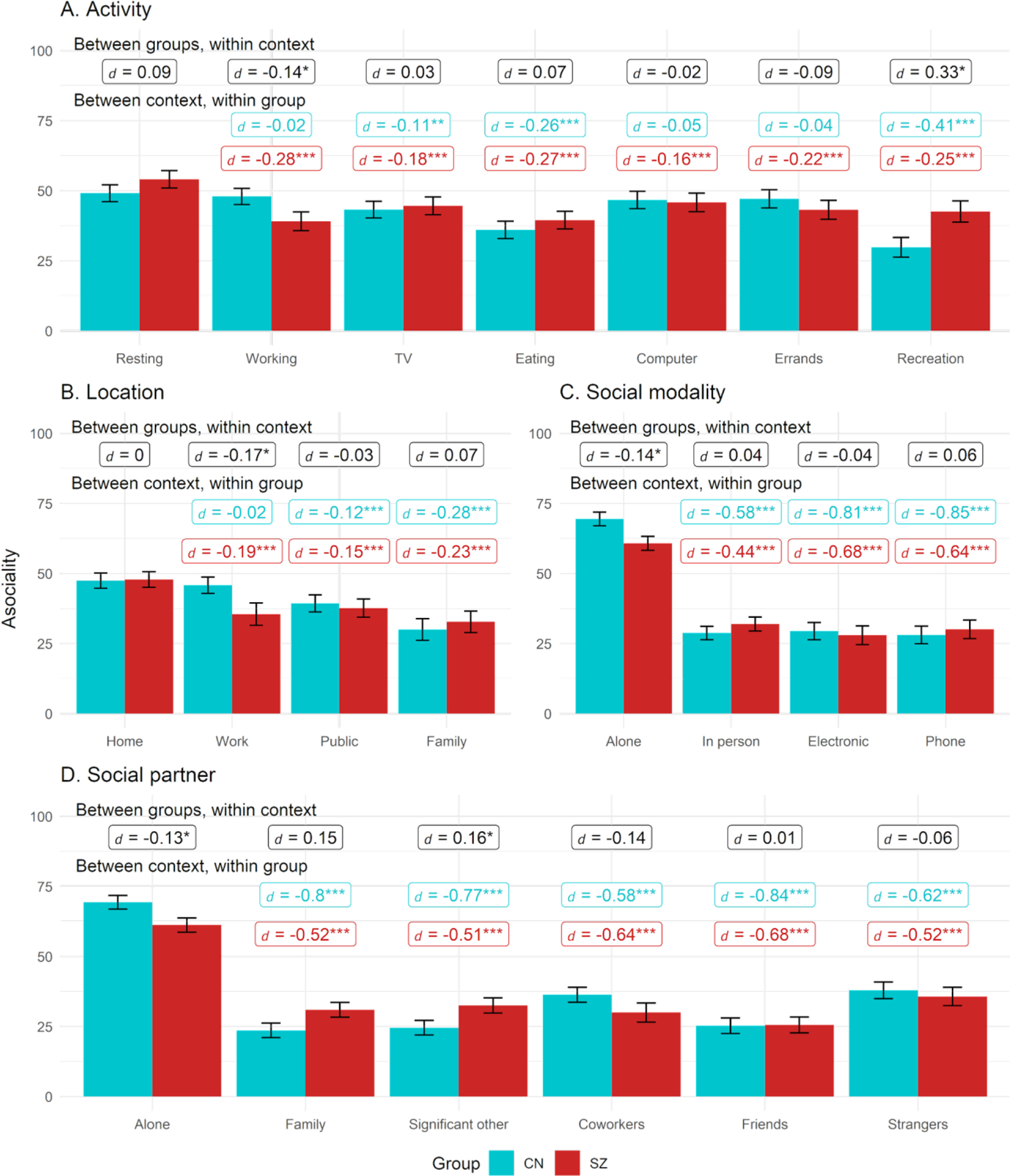
Asociality activity, location, partner, modality
Note. Black labels reflect contrast between groups within each context while colored labels reflect contrast within group relative to reference context (resting, being at home, or being alone). Figures use estimated marginal means and error bars reflect standard error.
* = p < .05, ** = p < .01, ** = p < .001
Anhedonia
Activity
For activity, the Group, Context, and Group X Context effects were all significant (see Table 2); thus, the effect of activity context on anhedonia levels varied by group. Follow-up comparisons found that SZ showed higher anhedonia than CN when resting and eating, but groups did not differ in anhedonia levels while completing work, TV, computer, errands, or recreational activities (see Figure 1). In SZ, compared to when resting, anhedonia was elevated when running errands but reduced when in recreational activities. In CN, compared to resting, anhedonia was higher when working, using the computer, and running errands, while it was lower during recreation. In both groups, anhedonia was lower during recreation relative to every other activity.
Location
For location, the Group, Context, and Group X Context effects were all significant, indicating the effect of location context on anhedonia levels also varied by group. SZ had higher anhedonia when at home compared to CN, while anhedonia did not differ across groups in any other locations. Within SZ, compared to being at home, anhedonia was lower when in public and with family. For CN, compared to being at home, anhedonia was higher when at work.
Social partner
For social partner, Context and Group x Context effects were significant, while Group was nonsignificant. However, no group differences in anhedonia reached statistical significance for any of the social partners. Compared to being alone, SZ reported lower anhedonia for all social partners except strangers. In CN, compared to being alone, CN showed lower anhedonia with family, significant others, and friends but similar levels when with coworkers and strangers.
Social modality
For social modality, the effect of Context was significant, while the Group and Group X Context effects were nonsignificant. In the combined sample, compared to being alone, anhedonia was lower for all interaction modalities: in person, electronic, and phone interactions (ps < .003). Anhedonia also did not differ during in-person versus electronic and phone interactions (ps > .29).
Avolition
Activity
For activity, the Group, Context, and Group X Context effects were all significant (Table 2). SZ showed higher avolition than CN when resting and eating but similar levels in other activities (Figure 2). In SZ, compared to resting, avolition was elevated when running errands but reduced during recreational activities and watching TV. In CN, compared to resting, avolition was higher when working, using the computer, and running errands, while it was lower during recreation. In both groups, avolition was lower during recreation compared to all other contexts (ps < .03, ds > 0.13).
Figure 2.
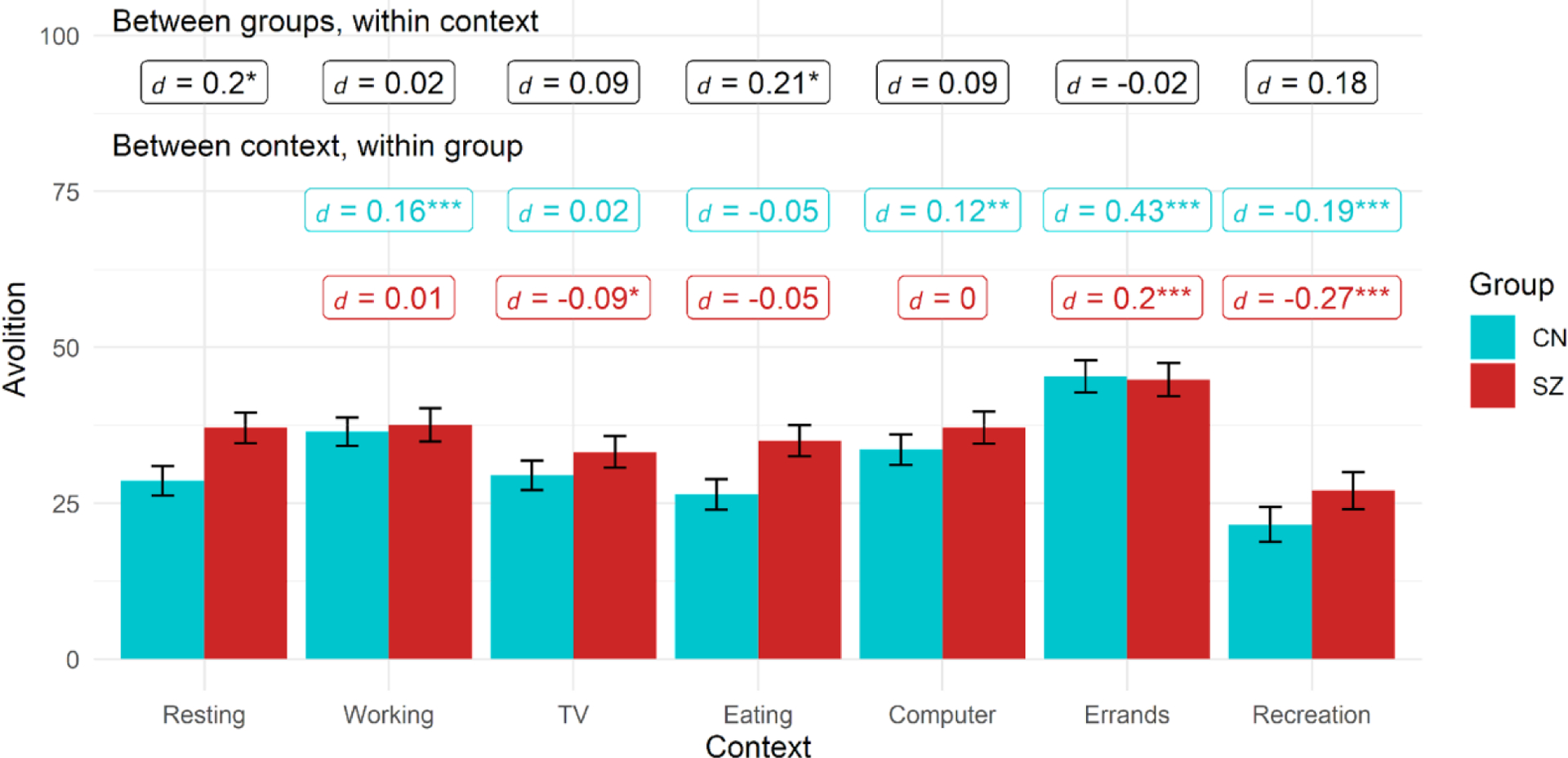
Avolition by group based on activity context
Note. Black labels reflect contrast between groups within each context while colored labels reflect contrast within group relative to reference context (resting). Figures use estimated marginal means and error bars reflect standard error.
* = p < .05, ** = p < .01, ** = p < .001
Location
For location, the effect of Context was significant, while the Group and Group X Context effects were nonsignificant. Thus, across both groups, there was a significant effect of location context on avolition. In the full combined sample, relative to being at home or work, avolition was lower when in public (ps < .01, ds > .05) and with family (p < 0.001, d > 0.10).
Social partner
For social partner, the effect of Context was significant, while the Group and Group X Context effects were nonsignificant. Across groups, relative to being alone, avolition was lower when with family (p < .001, d = 0.1), significant other (p < .001, d = 0.12), friends (p < .001, d = 0.17), and strangers (p = .017, d = 0.07).
Social modality
For social modality, the effect of Context was significant, while the Group and Group X Context effects were nonsignificant. Relative to being alone, avolition was lower during in person (p < .001= .00, d = 0.08), electronic (p = .009, d = 0.08) and phone (p = 0.03547, d = 0.0665) interactions across groups. Avolition also did not differ during in-person versus electronic and phone interactions (ps > .36).
Asociality
Activity
For activity, Context and Group X Context effects were significant, while Group was nonsignificant (Table 2). Asociality was greater in SZ than CN during recreational activities but was greater in CN than SZ when working (see Figure 3). In SZ, compared to resting, asociality was lower in all other contexts. Within CN, in relation to resting, asociality was lower while watching TV, eating, and during recreation.
Location
For location, Context and Group X Context effects were significant, while Group was nonsignificant. CN reported greater asociality than SZ while at work. Compared to being at home, asociality was lower in both groups when in public or at a family/friends home. Compared to being at home, SZ also reported lower asociality when at work.
Social partner
For social partner, the Group, Context, and Group X Context effects were all significant. Compared to SZ, CN had higher asociality while alone but lower asociality when interacting with a significant other. Within both groups, asociality was higher while alone compared to all other contexts. Within CN, asociality was greater with coworkers or strangers compared to other partners, while this was only observed with strangers in SZ.
Social modality
For social modality, the Group, Context, and Group X Context effects were all significant. CN had higher asociality while alone compared to SZ, but groups did not differ on asociality for in-person, electronic, or phone interactions. Within both groups, asociality was higher while alone compared to all other contexts, and asociality levels did not differ during in-person versus electronic or phone interactions.
Discussion
This study leveraged EMA to evaluate the impact of dynamic contextual factors on the negative symptoms of anhedonia, avolition, and asociality in schizophrenia (SZ) and controls (CN). In contrast to the long-standing notion that negative symptoms are largely stable across time and settings (Stahl and Buckley, 2007), results indicated that in both SZ and CN, levels of anhedonia, avolition, and asociality largely varied across activity, location, social partner, and social modality contexts. Further, in contrast to past work with interviewer-rated scales indicating consistent elevations in negative symptoms in SZ compared to CN (Strauss et al., 2016; Xie et al., 2018), SZ and CN did not report significantly different levels of negative symptoms across the majority of individual contexts (e.g., activity or social partner types). In fact, SZ and CN had more similarities than differences in symptoms across individual contexts. Further, our results also identified several contexts (e.g., recreation, close social interactions) where negative symptoms were similarly reduced in each group.
A key finding is contexts had differential associations with negative symptom domains. Notably, anhedonia levels did not significantly differ between groups across social partners (e.g., family, significant others, co-workers, friends, strangers), suggesting in the moment social pleasure may largely be intact in SZ. Although this differs from studies showing social anhedonia deficits in SZ using interviewer-rated scales (Strauss et al., 2016; Xie et al., 2018), our results align with laboratory and EMA studies demonstrating that SZ and CN generally report similar levels of in-the-moment positive affect when exposed to pleasant stimuli (Cohen and Minor, 2010) as well as similar increases in positive affect when interacting with others (compared to alone) in daily life (Depp et al., 2016; Edwards et al., 2018; Gard et al., 2007; Mote et al., 2019; Oorschot et al., 2013). Indeed, we found that groups did not differ in consummatory anhedonia when engaging with any social partner. However, anticipatory anhedonia across social partners also appeared intact in SZ, as no group differences emerged for any social partner. This contrasts some (Chan et al., 2010; Gard et al., 2007; Mote et al., 2014) but not all (Frost and Strauss, 2016; Strauss et al., 2011) prior work showing that self-reported anticipatory (but not consummatory) pleasure is impaired in SZ. However, whereas this past work largely used self-report measures assessing anticipatory anhedonia for a range of imagined activities (e.g., eating, going to an amusement park), our assessment of anticipatory anhedonia had greater temporal precision for real-world activities. Thus, our method reduced participant recall demands, potentially creating a more intact and easily accessible value representation of the activity which could then be used to generate a more specific report of future expected pleasure during social contexts.
However, anhedonia differed between group in some individual contexts. Resting, eating, and being at home were associated with greater anhedonia in SZ than CN. Although somewhat surprising since these contexts are generally thought to elicit pleasure, our findings could indicate that these contexts may fundamentally differ (i.e., involve different activities or food) across groups. This may be due to differences in resources (e.g., finances), frequency of engagement (which may result in habituation and decreased pleasure), or associated cognitions (e.g., defeatist beliefs) not assessed in the current study. Finally, the activities of running errands, using the computer, watching TV, and working as well as being alone resulted in similarly higher levels of anhedonia in both groups (i.e., no group differences). This suggests that similar levels of lower pleasure were reported across groups for these activities. Taken together, these findings provide further support for no longer viewing anhedonia in SZ as a diminished hedonic capacity (Strauss and Gold, 2012); the experience of pleasure was largely normative, with anhedonia group differences present in only a minority of contexts.
Additionally, we identified key contexts where avolition varied in SZ. Similar to anhedonia, recreational activities led to similar reductions in avolition for both SZ and CN. Although it is not surprising that groups reported the greatest interest and enjoyment when engaged in recreation, it is striking that avolition and anhedonia largely appeared to be “normalized” in SZ during recreational activities. This aligns with prior work indicating recreation and greater community participation are associated with enhanced self-efficacy and recovery (Fenton et al., 2017; Pegg and Patterson, 2002; Snethen et al., 2012) and provides support for recreation being a critical intervention target for avolition and anhedonia. Further, the activities of running errands, working, or using the computer resulted in similarly high reports of avolition in both groups, largely in line with what we observed in anhedonia. These largely “productive” activities could lead to increases in these symptom domains. This is particularly surprising as many strategies to promote functional recovery involve going to work or engaging in more goal-directed activities such as using the computer. Future work is needed to identify moderators of this relationship (e.g., job type, congruence between job and interests, computer activity type) and how best to align SZ with meaningful jobs and “productive” activities that truly facilitate symptomatic improvement. Alternatively, greater focus on activities enhancing hedonic and eudaimonic well-being over productivity may provide a useful avenue for intervention.
In line with our hypotheses, the largest fluctuations in asociality were between social versus non-social contexts in both groups. However, there was also variability in asociality among social, activity, and location contexts. Replicating prior EMA work showing that closer social relationships were associated with higher social motivation in SZ (Badal et al., 2021), location contexts (i.e., friend/family member’s home) and social partners involving relatively close relationships (family, friends), also generally led to similarly lower levels of asociality in both groups. However, there were some notable exceptions, with SZ reporting greater asociality or less interest in interactions when with significant others or engaging in recreational activities compared to CN. This may reflect differences in conversation type (i.e., more conflict, less interactive, or more likely to activate negative cognitions in SZ) or recreational activity (i.e., more pleasure-based, solo activities). Indeed, our finding aligns with a prior study showing that more intimate relationships were linked with greater happiness in CN but not SZ (Mote et al., 2019). On the other hand, we observed that being at work—a place where fewer intimate conversations are typically held—was associated with greater social interest in SZ than CN, suggesting that workplace interactions provide a meaningful opportunity for socialization in SZ. Along these lines, SZ reported greater social interest when alone than CN. This may be due to CN having greater social resources (e.g., larger social networks (Gayer-Anderson and Morgan, 2013)) and more social skills, enabling them to meet their social needs. Finally, social interest levels were similarly high (compared to being alone) in both groups across different social modality interaction methods of in-person, electronic, or phone; this pattern was also observed for avolition and anhedonia. This affirms that electronic communication may be an equally meaningful and impactful way for SZ to connect with others, supporting the utility of mobile health and socially interactive online interventions (e.g., Alvarez-Jimenez et al., 2021; Luther et al., 2020a). Future work is needed to identify the engagement level and frequency needed to sustain asociality reductions.
Together these findings align with the increasing focus on the role that context and environment have on negative symptoms. This study supports a key hypothesis of the Bioecosystem Theory of Negative Symptoms (Strauss, 2021) by showing that immediate contexts (i.e., the microsystem) influence experiential negative symptom levels in SZ. Further, these findings provide additional support against historical descriptions suggesting that SZ show continuous negative symptom declines (Bleuler, 1950; Kraepelin et al., 1919) as well as more recent findings that these symptoms are invariant across time (Kirkpatrick et al., 2011; Kring et al., 2013). Our findings instead suggest that negative symptoms can significantly vary across different contexts even in the chronic phase of SZ.
Although the number of contexts examined is a strength of this study, additional contexts (e.g., spiritual activities), possible moderating factors (e.g., context quality) as well as their interactions could be assessed in future, larger and longer EMA studies. This study also focused on experiential negative symptoms and did not examine contextual influences on alogia or blunted affect; however ambulatory videos (Cohen et al., 2020a; Cowan et al., 2022) and passive vocal recording (Cohen et al., 2019a) could assess how contexts influence these symptoms. Similarly, although anhedonia, avolition, and asociality are inherently experiential, making self-reports a critical facet of these symptom assessments, future work is needed to evaluate how context impacts behavioral components of these domains (e.g., using passive digital phenotyping measures). Supplemental analyses examining the associations between negative affect and context and negative symptoms suggested generally minimal associations; however, additional studies are needed that are designed to expressly disentangle the complex role of mood on context and negative symptoms. Finally, we examined SZ in the chronic illness phase, and future work is needed to examine the contextual effects on negative symptoms in earlier illness stages.
Despite these limitations, findings suggest that experiential negative symptoms vary notably across the contexts of activities, locations, social partner, and social modality. Results may guide behavioral activation or other approaches aimed at improving negative symptoms by encouraging SZ to reduce time in certain contexts (e.g., resting, being at home, eating, errands, general computer activities) or increase time in others (e.g., recreation, most social interactions). These results may also inform just-in-time adaptative (Nahum-Shani et al., 2018) mobile interventions where intervention content could be delivered to a person’s phone when they enter a context where their negative symptoms are prone to increase. Finally, our results bolster support for prior negative symptom interventions that involve adapting environments (Velligan et al., 2008) and suggest that modifying contexts associated with symptom exacerbations could also facilitate negative symptom improvement.
Supplementary Material
Funding:
This work was supported by R21-MH112925 to G.P.S. from the National Institute of Mental Health. Study sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interests:
Dr. Gregory Strauss is one of the original developers of the Brief Negative Symptom Scale (BNSS) and receives royalties and consultation fees from Medavante-ProPhase LLC in connection with commercial use of the BNSS and other professional activities; these fees are donated to the Brain and Behavior Research Foundation. Dr. Strauss has received honoraria and travel support from Medavante-ProPhase LLC for training pharmaceutical company raters on the BNSS. In the past 2 years, Dr. Strauss has consulted for and/or been on the speaker bureau for Minerva Neurosciences, Acadia, Lundbeck, Sunovion, Boehringer Ingelheim, and Otsuka pharmaceutical companies. All other authors have no relevant disclosures to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We use the term symptoms throughout the manuscript given that negative symptom-like experiences at subclinical levels have been found in non-clinical groups, including the general population and healthy controls (Kaiser et al., 2011; Stefanis et al., 2002; Strauss et al., 2016; Werbeloff et al., 2015). However, in both groups, these items are assessing reductions in pleasure, motivation, and social motivation and interaction.
References
- Alvarez-Jimenez M, Koval P, Schmaal L, Bendall S, O’Sullivan S, Cagliarini D, D’Alfonso S, Rice S, Valentine L, Penn DL, Miles C, Russon P, Phillips J, McEnery C, Lederman R, Killackey E, Mihalopoulos C, Gonzalez-Blanch C, Gilbertson T, Lal S, Gleeson JFM, 2021. The Horyzons project: a randomized controlled trial of a novel online social therapy to maintain treatment effects from specialist first-episode psychosis services. World Psychiatry 20, 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badal VD, Parrish EM, Holden JL, Depp CA, Granholm E, 2021. Dynamic contextual influences on social motivation and behavior in schizophrenia: a case-control network analysis. NPJ Schizophr. 7, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E, 1950. Dementia praecox or the group of schizophrenias. International Universities Press, New York. [Google Scholar]
- Chan RCK, Wang Ya, Huang J, Shi Y, Wang Yuna, Hong X, Ma Z, Li Z, Lai MK, Kring AM, 2010. Anticipatory and consummatory components of the experience of pleasure in schizophrenia: cross-cultural validation and extension. Psychiatry Res. 175, 181–183. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Cowan T, Le TP, Schwartz EK, Kirkpatrick B, Raugh IM, Chapman HC, Strauss GP, 2020a. Ambulatory digital phenotyping of blunted affect and alogia using objective facial and vocal analysis: Proof of concept. Schizophr. Res 220, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Fedechko TL, Schwartz EK, Le TP, Foltz PW, Bernstein J, Cheng J, Holmlund TB, Elvevåg B, 2019a. Ambulatory vocal acoustics, temporal dynamics, and serious mental illness. J. Abnorm. Psychol 128, 97–105. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS, 2010. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr. Bull 36, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Schwartz E, Le TP, Cowan T, Cox C, Tucker R, Foltz P, Holmlund TB, Elvevåg B, 2020b. Validating digital phenotyping technologies for clinical use: the critical importance of “resolution”. World Psychiatry 19, 114–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Schwartz E, Le TP, Cowan T, Kirkpatrick B, Raugh IM, Strauss GP, 2021. Digital phenotyping of negative symptoms: the relationship to clinician ratings. Schizophr. Bull 47, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Schwartz E, Le TP, Fedechko T, Kirkpatrick B, Strauss GP, 2019b. Using biobehavioral technologies to effectively advance research on negative symptoms. World Psychiatry 18, 103–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan T, Cohen AS, Raugh IM, Strauss GP, 2022. Ambulatory audio and video recording for digital phenotyping in schizophrenia: Adherence & data usability. Psychiatry Res. 311, 114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depp CA, Moore RC, Perivoliotis D, Holden JL, Swendsen J, Granholm EL, 2016. Social behavior, interaction appraisals, and suicidal ideation in schizophrenia: The dangers of being alone. Schizophr. Res 172, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CJ, Cella M, Emsley R, Tarrier N, Wykes THM, 2018. Exploring the relationship between the anticipation and experience of pleasure in people with schizophrenia: An experience sampling study. Schizophr. Res 202, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton L, White C, Gallant KA, Gilbert R, Hutchinson S, Hamilton-Hinch B, Lauckner H, 2017. The Benefits of Recreation for the Recovery and Social Inclusion of Individuals with Mental Illness: An Integrative Review. Leis. Sci 39, 1–19. [Google Scholar]
- First MB, Williams JBW, Benjamin LS, Spitzer RL, 2016. Structured Clinical Interview for DSM-5® Personality Disorders (SCID-5-PD). American Psychiatric Publishing, Washington, D.C. [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL, 2015. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association, Arlington, VA. [Google Scholar]
- Frost KH, Strauss GP, 2016. A review of anticipatory pleasure in schizophrenia. Curr. Behav. Neurosci. Rep 3, 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P, 2015. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr. Bull 41, 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF, 2007. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr. Res 93, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayer-Anderson C, Morgan C, 2013. Social networks, support and early psychosis: a systematic review. Epidemiol. Psychiatr. Sci 22, 131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Holden JL, Mikhael T, Link PC, Swendsen J, Depp C, Moore RC, Harvey PD, 2020. What Do People With Schizophrenia Do All Day? Ecological Momentary Assessment of Real-World Functioning in Schizophrenia. Schizophr. Bull 46, 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PM, Beck AT, 2009. Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophr. Bull 35, 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Docherty NM, Serper MR, Rasmussen M, 1990. Cognitive deficits and thought disorder: II. An 8-month followup study. Schizophr. Bull 16, 147–156. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Heekeren K, & Simon JJ (2011). The negative symptoms of schizophrenia: category or continuum?. Psychopathology 44(6), 345–353. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, Marder SR, 2011. The brief negative symptom scale: psychometric properties. Schizophr. Bull 37, 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E, Robertson GM, Barclay RM, 1919. Dementia praecox and paraphrenia. Chicago Medical Book Co., New York. [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP, 2013. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am. J. Psychiatry 170, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynicki CR, Upthegrove R, Deakin JFW, & Barnes TR, 2018. The relationship between negative symptoms and depression in schizophrenia: a systematic review. Acta Psychiatr. Scand 137(5), 380–390. [DOI] [PubMed] [Google Scholar]
- Luther L, Fischer MW, Johnson-Kwochka AV, Minor KS, Holden R, Lapish CL, McCormick B, Salyers MP, 2020a. Mobile enhancement of motivation in schizophrenia: A pilot randomized controlled trial of a personalized text message intervention for motivation deficits. J. Consult. Clin. Psychol 88, 923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther L, Suor JH, Rosen C, Jobe TH, Faull RN, Harrow M, 2020b. Clarifying the direction of impact of negative symptoms and neurocognition on prospective work functioning in psychosis: A 20-year longitudinal study. Schizophr. Res 220, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EK, Culbreth AJ, & Barch DM (2017). Ecological momentary assessment of negative symptoms in schizophrenia: Relationships to effort-based decision making and reinforcement learning. J Abnorm. Psychology 126(1), 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote J, Fulford D, 2020. Ecological momentary assessment of everyday social experiences of people with schizophrenia: A systematic review. Schizophr. Res 216, 56–68. [DOI] [PubMed] [Google Scholar]
- Mote J, Gard DE, Gonzalez R, Fulford D, 2019. How did that interaction make you feel? The relationship between quality of everyday social experiences and emotion in people with and without schizophrenia. PLoS ONE 14, e0223003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote J, Minzenberg MJ, Carter CS, Kring AM, 2014. Deficits in anticipatory but not consummatory pleasure in people with recent-onset schizophrenia spectrum disorders. Schizophr. Res 159, 76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, Murphy SA, 2018. Just-in-Time Adaptive Interventions (JITAIs) in Mobile Health: Key Components and Design Principles for Ongoing Health Behavior Support. Ann. Behav. Med 52, 446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkhede SM, Luther L, Raugh IM, Knippenberg AR, Esfahlani FZ, Sayama H, Cohen AS, Kirkpatrick B, Strauss GP, 2022. Machine learning identifies digital phenotyping measures most relevant to negative symptoms in psychotic disorders: implications for clinical trials. Schizophr. Bull 48, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oorschot M, Lataster T, Thewissen V, Lardinois M, Wichers M, van Os J, Delespaul P, Myin-Germeys I, 2013. Emotional experience in negative symptoms of schizophrenia--no evidence for a generalized hedonic deficit. Schizophr. Bull 39, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish EM, Chalker S, Cano M, Harvey PD, Taylor CT, Pinkham A, Moore RC, Ackerman RA, Depp CA, 2022.Ecological Momentary Assessment of Social Approach and Avoidance Motivations in Serious Mental Illness: Connections to Suicidal Ideation and Symptoms. Arch. Suicide Res 14:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish EM, Depp CA, Moore RC, Harvey PD, Mikhael T, Holden J, Swendsen J, Granholm E, 2020. Emotional determinants of life-space through GPS and ecological momentary assessment in schizophrenia: What gets people out of the house? Schizophr. Res 224, 67–73. [DOI] [PubMed] [Google Scholar]
- Pegg S, Patterson I, 2002. The Impact of a Therapeutic Recreation Program on Community-Based Consumers of a Regional Mental Health Service. Journal of Park & Recreation Administration. [Google Scholar]
- Pinkham AE, Mueser KT, Penn DL, Glynn SM, McGurk SR, Addington J, 2012. Social and functional impairments . In: Lieberman JA, Stroup TS, Perkins DO (Eds.), Essentials of Schizophrenia . American Psychiatric Publishing, Inc., Arlington, VA, pp. 93–130. [Google Scholar]
- Pogue-Geile MF, Harrow M, 1985. Negative symptoms in schizophrenia: their longitudinal course and prognostic importance. Schizophr. Bull 11, 427–439. [DOI] [PubMed] [Google Scholar]
- Raugh IM, James SH, Gonzalez CM, Chapman HC, Cohen AS, Kirkpatrick B, Strauss GP, 2021. Digital phenotyping adherence, feasibility, and tolerability in outpatients with schizophrenia. J. Psychiatr. Res 138, 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snethen G, McCormick BP, Van Puymbroeck M, 2012. Community involvement, planning and coping skills: pilot outcomes of a recreational-therapy intervention for adults with schizophrenia. Disabil. Rehabil 34, 1575–1584. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Buckley PF, 2007. Negative symptoms of schizophrenia: a problem that will not go away. Acta Psychiatr. Scand 115, 4–11. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, Hanssen M, Smirnis NK, Avramopoulos DA, Evdokimidis IK, Stefanis CN, … & Van Os J, 2002. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol. Medicine 32(2), 347–358. [DOI] [PubMed] [Google Scholar]
- Strassnig MT, Harvey PD, Miller ML, Depp CA, Granholm E, 2021. Real world sedentary behavior and activity levels in patients with schizophrenia and controls: An ecological momentary assessment study. Ment. Health Phys. Act 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Bartolomeo LA, Harvey PD, 2021. Avolition as the core negative symptom in schizophrenia: relevance to pharmacological treatment development. NPJ Schizophr. 7, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Gold JM, 2012. A new perspective on anhedonia in schizophrenia. Am. J. Psychiatry 169, 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Vertinski M, Vogel SJ, Ringdahl EN, Allen DN, 2016. Negative symptoms in bipolar disorder and schizophrenia: A psychometric evaluation of the brief negative symptom scale across diagnostic categories. Schizophr. Res 170, 285–289. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Wilbur RC, Warren KR, August SM, Gold JM, 2011. Anticipatory vs. consummatory pleasure: what is the nature of hedonic deficits in schizophrenia? Psychiatry Res. 187, 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, 2021. A bioecosystem theory of negative symptoms in schizophrenia. Front. Psychiatry 12, 655471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Diamond PM, Mintz J, Maples N, Li X, Zeber J, Ereshefsky L, Lam Y-WF, Castillo D, Miller AL, 2008. The use of individually tailored environmental supports to improve medication adherence and outcomes in schizophrenia. Schizophr. Bull 34, 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werbeloff N, Dohrenwend BP, Yoffe R, van Os J, Davidson M, & Weiser M, 2015. The association between negative symptoms, psychotic experiences and later schizophrenia: a population-based longitudinal study. PloS one, 10(3), e0119852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D-J, Shi H-S, Lui SSY, Shi C, Li Y, Ho KKY, Hung KSY, Li W-X, Yi Z-H, Cheung EFC, Kring AM, Chan RCK, 2018. Cross Cultural Validation and Extension of the Clinical Assessment Interview for Negative Symptoms (CAINS) in the Chinese Context: Evidence from a Spectrum Perspective. Schizophr. Bull 44, S547–S555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


