Abstract
Importance:
Evidence about the comparative effectiveness of chemoimmunotherapy versus immunotherapy alone in patients with advanced non-small cell lung cancer (aNSCLC) and high PD-L1 expression (≥50%) or very high PD-L1 expression (≥90%) is limited because of the lack of head-to-head clinical trials.
Objective:
To compare survival in aNSCLC patients receiving first-line chemoimmunotherapy vs. immunotherapy in both the PD-L1 expression ≥50% or ≥90% subgroups, accounting for potential confounders that may influence physician decision making.
Design, Setting, And Participants:
This cohort study used a nationwide electronic health record derived database to identify newly diagnosed cases of aNSCLC patients with PD-L1 of ≥50% who initiated first-line systemic therapy between from October 2016 to October 2021.
Exposures:
First-line therapy with chemoimmunotherapy or immunotherapy among patients with PD-L1 expression ≥50% or ≥90%.
Main Outcomes and Measures:
Survival was assessed using Kaplan-Meier curves and Cox regression. Propensity score-based inverse probability of weighting (IPW) was used to control for confounding. Because of non-proportionality of hazards, we estimated hazard ratios over the first 6 months and after 6 months for the overall cohort, and over the first 12 months and after 12 months for a subgroup of persons with a PD-L1 expression ≥90%.
Results:
We identified 3086 subjects who met inclusion criteria, of whom 32% received chemoimmunotherapy and 68% received immunotherapy alone. Chemoimmunotherapy was associated with no survival advantage versus immunotherapy alone during the entire follow-up period (IPW-adjusted Hazard Ratio [aHR] 0.98, 95% CI 0.86-1.12), but was associated with a survival benefit during the first 6 months (aHR 0.74, 95% CI 0.61-0.90). Similarly, in the subgroup of patients with a PD-L1 expression ≥90%, chemoimmunotherapy was associated with no overall survival advantage during the entire follow-up period (aHR 0.99, 95% CI 0.87-1.22), but was associated with a survival benefit during the first 12 months (aHR 0.74, 95% CI 0.57-0.97).
Conclusions and Relevance:
Chemoimmunotherapy was not associated with an overall benefit over immunotherapy alone, although was associated with an early survival advantage in both the overall cohort and the subgroup of patients with a PD-L1 expression ≥90%. Future studies should focus on identifying the characteristics of higher risk patients that may benefit from the addition of chemotherapy.
Micro Abstract
In this cohort study of 3086 patients with advanced non-small cell lung cancer (aNSCLC), chemoimmunotherapy was not associated with an overall benefit over immunotherapy alone in the first line, although was associated with an early survival advantage in both the overall cohort and the subgroup of patients with a PD-L1 expression ≥90%. The study results suggest that future studies should focus on identifying the characteristics of higher risk patients that may benefit from the addition of chemotherapy.
Introduction
Anti-programmed cell death ligand 1-(PD-L1) immunotherapy (including pembrolizumab), both as monotherapy and in combination with chemotherapy (i.e., chemoimmunotherapy), is a standard first-line anti-tumor strategy for advanced non-small cell lung cancer (aNSCLC), and confers favorable survival compared to conventional chemotherapy alone.1–4 PD-L1 is used as a biomarker to help predict which patients will benefit from immunotherapy, with high tumor expression of PD-(L)1 potentially responding more favorably. 1,5 While ≥50% is used as a cutoff for using immunotherapy alone, there is recent evidence that among aNSCLC patients treated with immunotherapy alone, having very high PD-L1 expression (≥90%) is associated with improved survival. This suggests that degree of PD-L1 positivity may also be associated with benefit from immunotherapy.6–8
In routine clinical practice, factors affecting choice of therapy include tumor histology, disease burden, symptoms, and tumor PD-L1 expression. 9 Many providers favor immunotherapy alone in patients with PD-L1 expression ≥50% because of a favorable side effect profile, better tolerability, and improved quality of life when compared to chemoimmunotherapy.9 However, clinicians often prefer chemoimmunotherapy for symptomatic patients with high disease burden and those with aggressively growing tumors,10 and use immunotherapy alone in patients with more indolent disease or less symptom burden. Given this potential bias resulting from confounding by indication, unadjusted comparisons of these treatments in the PD-L1 expression ≥50% group may be invalid and misleading.
Although chemoimmunotherapy and immunotherapy have both received Food and Drug Administration (FDA) approval based on superiority over conventional chemotherapy alone, the comparative effectiveness of these systemic therapies in patients with PD-L1 expression ≥50% and in the subgroup with PD-L1 expression ≥90% is unknown because of the lack of head-to-head trials. In the absence of head-to-head trials, and no evidence comparing the survival benefit of chemoimmunotherapy and immunotherapy, there is growing interest by clinicians, regulators and patients in leveraging real world data to inform best clinical practice.11 The FDA recently presented exploratory pooled clinical trial results that indirectly compared outcomes of anti-PD-(L)1 therapy users with or without chemotherapy for first-line treatment of aNSCLC among patients with PD-L1 expression ≥50%. They found no overall survival advantage for chemoimmunotherapy vs immunotherapy alone, although they reported improved progression-free survival and objective response rates that favored chemoimmunotherapy.12 However, this exploratory analysis was limited by its indirect cross-trial comparison, potential inter-trial heterogeneity, notable differences between clinical trial and real-world patients, and the inability to account for confounding and treatment effect heterogeneity among key subgroups, e.g. those with PD-L1 expression ≥90%. Therefore, the objective of the current study was to assess comparative overall survival following first line immunotherapy or chemoimmunotherapy in patients with aNSCLC and PD-L1 expression ≥50%, as well as in those with PD-L1 expression ≥90% after accounting for potential confounders that may influence physician decision making in selecting a therapeutic approach.
Methods
This cohort study was approved by the University of Pennsylvania’s Committee on Studies Involving Human Beings and is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.13
Data Source
We used data from the nationwide, deidentified longitudinal Flatiron Health database, which is derived from the electronic health records (EHRs) of approximately 280 US cancer clinics (approximately 880 sites of care), most of which are community oncology practices. Deidentified patient-level data included structured and unstructured data, such as clinician notes and pathology reports, all of which were curated via technology enabled abstraction, an approach which uses a software tool developed for the identification and targeted display of selected portions of the patient chart for abstraction which is then reviewed by clinically trained professionals, as described elsewhere.14–17 Data from unstructured EHR- derived digital documents were reviewed manually by trained medical record abstractors using abstraction protocols for each data element. 18,19 Quality control mechanisms involved in the abstraction process include duplicate chart abstraction, logic checks, and formal adjudication based on select variable complexity. 19–21
Study sample
We identified patients who 1) were 18 years of age or older, 2) had been diagnosed with advanced stage or metastatic stage IV or recurrent NSCLC (ICD- 9 codes 162.x or ICD- 10 codes C34.x or C39.9) between October 24, 2016 (based on FDA approval of first- line pembrolizumab in those with PD L1 expression of at least 50%1) and October 30th, 2021 (the date of data abstraction), 3) had at least two clinical visits on or after October 24, 2016, 4) initiated first line treatment with pembrolizumab alone (i.e., immunotherapy) or pembrolizumab plus platinum-based chemotherapy (i.e., chemoimmunotherapy) after diagnosis of advanced stage or metastasis, and 5) had a recorded value of percentage PD-L1 of at least 50% of tumor cells. Patients with incomplete treatment data, who received first-line therapy as a part of a randomized clinical trial, and those harboring sensitizing alterations in EGFR, ALK or ROS1 genes were excluded. We also excluded patients with a gap ≥90 days between diagnosis and first visit or medication order since this likely represents missing data.
Exposures and Variable Definitions
The exposure of interest was first-line systemic therapy, which was categorized as chemoimmunotherapy (e.g., carboplatin-based chemotherapy in combination with pembrolizumab) or immunotherapy alone (pembrolizumab).
Outcomes
The outcome of interest was overall survival, measured from the initiation of first-line chemoimmunotherapy or immunotherapy alone, which was considered the index date.22,23 Patients were followed until the first of the following dates: date of death, last structured EHR activity (including visit or medication administration), or October 29th, 2021.
Covariates
Ten covariates, determined a priori 8,9,24 in consultation with a medical oncologist, thought to potentially contribute to the use of chemoimmunotherapy and overall survival were used in the analysis: age at therapy initiation, self-reported race, sex, smoking history, PD-L1 expression ≥90%, tumor histology, presence of KRAS/BRAF mutation, practice type (academic vs community), and Eastern Cooperative Oncology Group performance status (ECOG PS). Information up to and including the index date was used to ascertain baseline covariates; ECOG PS value was ascertained within the 100 days prior to and including the index date. The baseline window for ECOG PS was parameterized by a lower and upper bound, each defined relative to the index date with the lower bound set to −100 days while the upper bound was set to +0 days. This window was designed to maximize completeness of available ECOG PS while ensuring its proximity to the index date. The ECOG PS with the date nearest to the index date was then selected. In the event, two or more eligible ECOG PS occurred on the same day, the highest ECOG PS (worse performance) was selected. PD-L1 expression was reported as a percentage of tumor cells with membranous staining and primarily assessed using the Dako PD-L1 IHC 22C3 or 28-8 pharmDx assay or Ventana PD-L1 SP142 or SP263 assay.
Statistical analysis
Baseline characteristics in the chemoimmunotherapy and immunotherapy groups were examined using descriptive statistics. Missing covariate data was imputed using multiple imputation via chained equations under the assumption that data were missing at random.25 Twenty imputed data sets were created, including all baseline covariates and outcomes listed above in the imputation model.26 Rubin rules 27 were used to generate pooled effect estimates across imputed data sets.28
To control for confounding, we used inverse probability weighting (IPW) by a function of the propensity score, which we defined as the probability of a patient having chemoimmunotherapy conditional on baseline variables. The propensity score model was estimated using logistic regression and included all baseline covariates listed above. Patients with chemoimmunotherapy were weighted by the inverse of the propensity score, and patients with immunotherapy were weighted by the inverse of the complement of the propensity score.29,30 Post weighting balance in covariates between treatment groups was evaluated using the standardized difference approach where absolute standardized differences were computed after weighting to assess whether any imbalances remained with imbalance defined as an absolute standardized difference of >0.1.31 Overlap of propensity score distributions between treatment was assessed groups using density plots.
Inverse probability weighted Kaplan-Meier curves comparing overall survival in the groups were plotted (median overall survival (OS), 6-month OS, 12-month OS and 36-month OS). Cox proportional hazards regression with IPW, using a robust variance estimator, was used to estimate weighted hazard ratios (HRs), and 95% confidence intervals (CIs) for chemoimmunotherapy compared with immunotherapy. The proportional hazard assumption was evaluated by testing the correlation of the scaled Schoenfeld residuals and time.32 After observing deviations from proportionality for the chemoimmunotherapy effect, we incorporated a time-varying coefficient for chemoimmunotherapy, allowing for a change point in the chemoimmunotherapy effect at 6 months. Similarly, because deviations from proportionality were observed for a PD-L1 expression ≥90% subgroup analysis, we incorporated a time-varying coefficient for chemoimmunotherapy, allowing for a change point in the chemoimmunotherapy effect at 12 months. These change-points (6-months and 12-months) were calculated using flexible parametric survival modelling and represented cut-points at which Kaplan-Meier curves were most divergent.33 Multivariable Cox proportional hazards regression was used to estimate HRs, and 95% CIs for the association between baseline risk factors and death within 12 months following therapy initiation. All statistical analyses were conducted using STATA V17.0/BE (College station, TX).
Results
Cohort characteristics
Of 3086 patients meeting inclusion criteria, 988 (32%) received chemoimmunotherapy and 2098 (68%) received immunotherapy alone. The median age was 71 years (interquartile range [IQR] 64-78), and half were male (52%), most were white (78%), had a history of smoking (93%), and received treatment at a community practice (95%). The prevalence of PD-L1 expression ≥90% was similar between groups: 43% in the chemoimmunotherapy group and 45% in immunotherapy alone group. Of patients with PD-L1 expression ≥90%, 97% had their staining assessed by Dako PD-L1 IHC 22C3 pharmDx assay. Similarly, among patients with PD-L1 expression 50-89%, 96% of patients had their staining assessed by Dako PD-L1 IHC 22C3 pharmDx assay. Unweighted baseline characteristics were generally similar between treatment groups, with two exceptions: the chemoimmunotherapy group had a lower proportion of patients categorized ECOGPS 2-4 (15% vs 23%) and were younger at therapy initiation (median age 68 vs 73 years) relative to the immunotherapy alone group. All baseline characteristics included in the propensity score model were well balanced between weighted treatment groups (absolute standardized differences <0.1) (Table 1). Subjects were followed for a median of 9.3 months (interquartile range (IQR) 2.9-21.6 months).
Table 1.
Baseline characteristics of study cohort
| Unweighted population | Weighted population (scaled) | |||||
|---|---|---|---|---|---|---|
| Chemoimmunotherapy | Immunotherapy alone | Absolute Standardized Difference Before IPW | Chemoimmunotherapy | Immunotherapy alone | Absolute Weighted Standardized Difference | |
| N | N=998 | N=2088 | N= 998 | N=2088 | ||
| Age at therapy initiation, median (IQRa) | 68 [61-75] | 73 [65-80] | 0.42 | 68 [61-75] | (73) [65-80] | 0.01 |
| Gender | 0.13 | <0.01 | ||||
| Female | 435 (44%) | 1041 (50%) | 48% | 48% | ||
| Male | 563 (56%) | 1047 (50%) | 52% | 52% | ||
| Race | 0.05 | <0.01 | ||||
| White | 682 (68%) | 1458 (70%) | 78% | 79% | ||
| Non-White | 185 (19%) | 406 (19%) | 22% | 21% | ||
| Missing | 131 (13%) | 224 (11%) | 0% | 0% | ||
| Smoking Status | <0.01 | <0.01 | ||||
| Current/Former | 927 (93%) | 1955 (94%) | 93% | 93% | ||
| Never | 71 (7%) | 133 (6%) | 7% | 7% | ||
| ECOG PS | 0.21 | 0.01 | ||||
| 0-1 | 622 (62%) | 1157(55%) | 74% | 74% | ||
| ≥2 | 150 (15%) | 482(23%) | 26% | 26% | ||
| Missing | 226 (23%) | 449 (22%) | 0% | 0% | ||
| PD-L1 Score | 0.06 | 0.01 | ||||
| ≥90% | 426(43%) | 946(45%) | 45% | 45% | ||
| 50-89% | 572(57%) | 1142(55%) | 55% | 55% | ||
| Practice type | 0.17 | 0.01 | ||||
| Academic | 22(2%) | 136(7%) | 5% | 5% | ||
| Community | 976(98%) | 1952(93%) | 95% | 95% | ||
| Tumor histology | 0.16 | 0.01 | ||||
| Non squamous cell carcinoma | 778 (78%) | 1476 (71%) | 73% | 73% | ||
| Squamous cell carcinoma | 169 (17%) | 513 (25%) | 21% | 23% | ||
| NSCLC NOS | 51 (5%) | 99(5%) | 6% | 4% | ||
| KRAS mutation | 0.20 | <0.01 | ||||
| Yes | 313 (31%) | 513 (25%) | 27% | 27% | ||
| No | 685 (69%) | 1575 (75%) | 73% | 73% | ||
| BRAF mutation | 0.04 | <0.01 | ||||
| Yes | 56 (6%) | 96 (5%) | 5% | 5% | ||
| No | 942 (94%) | 1992 (95%) | 95% | 95% | ||
IQR, interquartile range
Overall Survival
IPW-adjusted KM curves for the two treatment groups are displayed in Fig. 1. The IPW adjusted median overall survival was 16.6 months in the chemoimmunotherapy group and 15.2 months in the immunotherapy alone group (IPW-adjusted hazard ratio [aHR] 0.98, 95% CI 0.86-1.11). However, at 6 months, the IPW adjusted overall survival was moderately higher for chemoimmunotherapy than for immunotherapy alone (74% vs 68%; p=0.0001), and over the first 6 months, the hazard ratio for death for chemoimmunotherapy was reduced (aHR 0.74, 95% CI 0.61-0.90).
Figure 1.
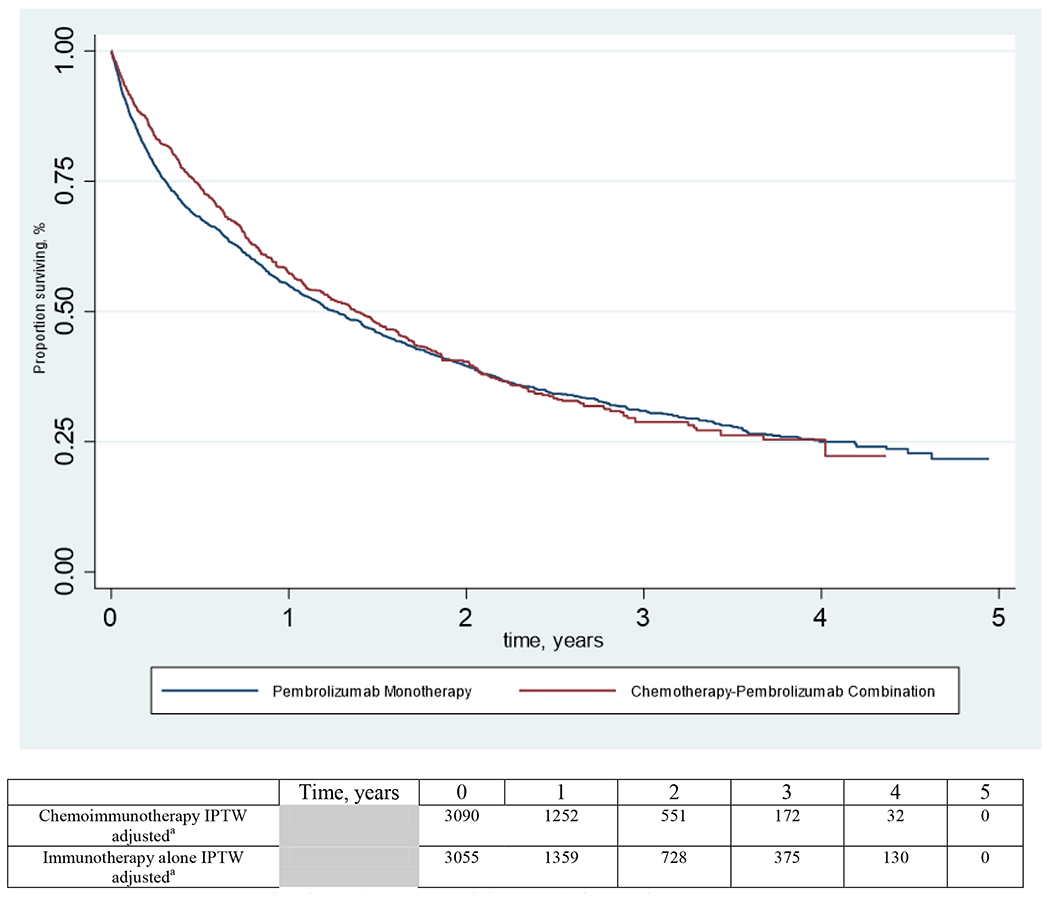
Inverse Probability of Weighting adjusted Kaplan-Meier Curves of Overall Survival by Pembrolizumab use
a Number of patients remaining in each group at risk at each time point
Baseline risk factors associated with mortality within 12 months following therapy initiation
In the multivariable analysis, male sex [HR 1.24 95% CI (1.11-1.39)], having received treatment in a community practice [HR 1.62 95% CI (1.19-2.23)] and poorer ECOG performance status HR 1.85 95% CI (1.65-2.11) were associated with higher mortality within the 12 months following therapy initiation, while having a very high PD-L1 expression (90-100%) [HR 0.85 95% CI (0.75-0.95)], a KRAS mutation [HR 0.87 95% CI (0.76-0.99)], and having received chemoimmunotherapy [HR 0.86 95% CI (0.76-0.97)] were associated with lower mortality.
Subgroup analyses based on PD-L1 expression
In the subgroup with PD-L1 expression ≥90%, chemoimmunotherapy was not associated with an overall survival benefit over immunotherapy alone (weighted mOS 19.8 vs 18.1 mo, aHR 0.99, 95% CI 0.81-1.22). However, within the first 12 months, chemoimmunotherapy was associated with a modest survival benefit in the PD-L1 expression ≥90% subgroup with a 12-month survival of 62% in the chemoimmunotherapy group and 57% in the immunotherapy group (p=0.03; aHR 0.74, 95% CI 0.57-0.97).
Discussion
We found no difference in overall survival for chemoimmunotherapy over immunotherapy alone in aNSCLC with PD-L1 expression ≥50%, after controlling for potential confounders affecting choice of therapy in routine clinical practice, and patient performance status. This finding is consistent with recent preliminary reports from the FDA pooled analysis and other systematic reviews. 34,35 However, when taking into account the violation of the proportional hazards assumption and evaluating time-dependent survival models, chemoimmunotherapy was associated with a relative reduction in mortality in the first 12 months among patients with PD-L1 expression ≥90% (adjusted OS at 12 months 62% vs 57%; aHR 0.74, 95% CI 0.57-0.97) and among patients with PD-L1 expression ≥50%, a relative reduction in mortality in the first 6 months (adjusted OS at 6 months 74% vs 69%; aHR 0.74, 95% CI 0.61-0.90). Providers and patients therefore need to balance the modest short-term survival benefit of chemoimmunotherapy with potential for increased toxicity. 36,37
Our observation of an early survival benefit of chemoimmunotherapy may be explained by at least two potential mechanisms: 1) immunotherapy enhancing the anti-tumor effect of chemotherapy; and/or 2) protection against hyperprogression or primary immunotherapy resistance.38–40 Despite great success with immunotherapy among patients with aNSCLC, only a limited portion of patients benefit from this treatment class, and identifying the non-responders remains a challenging clinical need. Although reported in a small subset of patients (e.g. ~14%)38, the phenomenon of hyper-progression has been demonstrated across several aNSCLC clinical trials, where immunotherapy alone strategies have identified a subset of patients experiencing early aggressive disease progression (i.e., tumor growth) likely due to immunotherapy resistance and subsequently reported as an increase in mortality in the first 3-6 months compared to the chemotherapy containing control arms.41–45 A recent network meta-analysis found, through indirect cross-trial comparisons, that the proportion of patients experiencing early disease progression is lower with chemoimmunotherapy than with immunotherapy alone.46 Interestingly, the FDA pooled analysis showed longer progression free survival in the chemoimmunotherapy group compared to immunotherapy alone.12 Our study did not examine progression free survival, but found an increase in mortality in those receiving immunotherapy alone in the first 6 months (31% patients died) compared to recipients of chemoimmunotherapy during the same period (24% patients died).
We found that male sex, receiving treatment in a community practice, and worse baseline ECOG PS ≥2 were associated with higher mortality after first-line therapy, while having very high PD-L1 expression, a KRAS mutation and receiving chemoimmunotherapy were associated with lower mortality during the first year. These prognostic indicators may aid providers in identifying patients who may be at risk of early mortality and therefore would derive maximum benefit from the addition of chemotherapy to their regimens.
In the absence of head-to-head trials, the choice of chemoimmunotherapy versus immunotherapy alone is risk/benefit discussion between providers and the patient revolving around the patient’s tumor histology, disease burden, symptoms, and tumor PD-L1 expression. Cross-trial comparisons between chemoimmunotherapy and immunotherapy alone versus chemotherapy alone suggest similar outcomes with different toxicity profiles, and therefore that immunotherapy alone may be a reasonable choice in patients with PD-L1 expression ≥50%.2,41,46,47 However, our results suggest that among aNSCLC patients with high and in those with very PD-L1 expression, there may be an early benefit (restricted to the first 6-12 months) of chemoimmunotherapy compared to immunotherapy alone, which may provide a rationale for initiating chemoimmunotherapy for the majority of patients for a short period of time as was done in Checkmate 9LA4 (2 cycles of upfront chemotherapy with immunotherapy).
This study has several strengths. The large sample size of over 3000 patients coupled with contemporary data allowed us to compare the effectiveness of chemoimmunotherapy and immunotherapy in clinically relevant PD-L1 expression subsets. Further, our analyses adjusted for numerous potential confounders.
Limitations
This study also has limitations. First, we did not study progression free survival and objective response rates and therefore were unable to compare findings with the FDA pooled analysis which found differences in these outcomes between chemoimmunotherapy and immunotherapy alone groups. Second, there is the potential of confounding because of unobserved factors that may be associated with the decision to use first line chemoimmunotherapy rather than immunotherapy alone. Such unobserved potential confounders may include visceral metastases, frailty, and body mass index that were not included in this real-world dataset. Third, ECOG PS was missing for nearly a quarter of the cohort although the strength and direction of association were similar when missing values were imputed. Fourth, as we observed deviations from proportionality for the chemoimmunotherapy effect, p-values for nonproportional hazards should be interpreted cautiously.
Conclusions
In this cohort study of those aNSCLC patients with PD-L1 expression ≥50%, chemoimmunotherapy was not associated with an overall survival advantage over immunotherapy alone, although was associated with a survival benefit in the first six months. Similarly, in the PD-L1 expression ≥90% subgroup, chemoimmunotherapy was not associated with an overall survival benefit, but associated with a survival benefit in the first 12 months. Future research should focus on identifying high risk features that may indicate a need for chemoimmunotherapy over immunotherapy in this patient population.
Supplementary Material
Figure 2.
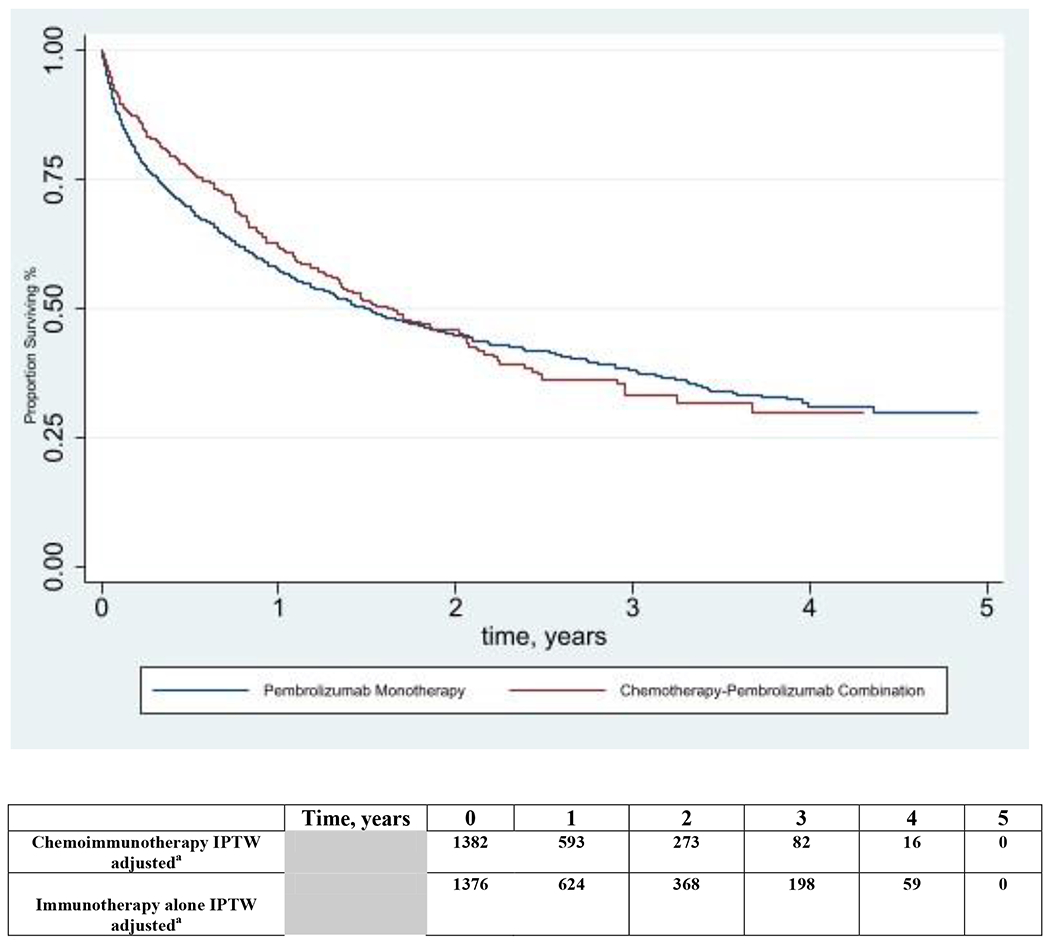
Inverse Probability of Treatment Weighting adjusted Kaplan-Meier Curves of Overall Survival by Immunotherapy use among Patients with PD-L1 expression ≥90%
a Number of patients remaining in each group at risk at each time point
Table 2:
Inverse probability of weighting-adjusted survival outcomes
| Patients with PD-L1 expression ≥50% | ||
|---|---|---|
| First-Line Chemoimmunotherapy | First-Line Immunotherapy alone | |
| Overall Survival (OS)a | ||
| Median OS (months) | 16.6 [IQR 5.6-48.2] | 15.2 [IQR 3.6-47.9] |
| 6-month OS | 74% | 68% |
| 12-month OS | 57% | 55% |
| 36-month OS | 29% | 31% |
| Overall, Hazard ratio | aHR 0.98, 95% CI 0.86-1.11 | 1 |
| Hazard ratio ≤ 6months | aHR 0.74, 95% CI 0.61-0.90 | 1 |
| Hazard ratio > 6 months | aHR 1.24, 95% CI 1.04-1.48 | 1 |
| No of deaths in 6 months n (%) | 240(24) | 653 (31) |
| No of deaths in 12 months b n (%) | 366(37) | 891(43) |
| Patients with PD-L1 expression ≥90% | ||
| Overall Survival (OS)a | ||
| Median OS (months) | 19.8 [IQR 6.8-NR] | 18.1 [IQR 3.6-NR] |
| 6-month OS | 77% | 70% |
| 12-month OS | 62% | 57% |
| 36-month OS | 33% | 38% |
| Overall, Hazard ratio | aHR 0.99, 95% CI 0.81-1.22 | 1 |
| Hazard ratio ≤ 12 months | aHR 0.74, 95% CI 0.57-0.97 | 1 |
| Hazard ratio > 12 months | aHR 1.51, 95% CI 1.04-2.18 | 1 |
| No of deaths in the first 6 months b n (%) | 93(22) | 277(29) |
| No of deaths in the first 12 months b n (%) | 137(32) | 378(40) |
Defined as time from first-line therapy initiation to date of death;
Following first-line therapy initiation
Interquartile range: IQR, NR; Not Reached, CI=confidence interval
Clinical Practice Points.
We identified 3086 subjects newly diagnosed cases of aNSCLC with PD-L1 expression of ≥50% who initiated first-line systemic therapy with pembrolizumab alone (n=2098) or its combination with platinum-based chemotherapy (n=988)
Chemoimmunotherapy was associated with no survival advantage versus immunotherapy alone during the entire follow-up period (IPW-adjusted Hazard Ratio [aHR] 0.98, 95% CI 0.86-1.12), but was associated with a survival benefit during the first 6 months (aHR 0.74, 95% CI 0.61-0.90).
Similarly, in the subgroup of patients with a PD-L1 expression ≥90%, chemoimmunotherapy was associated with no overall survival advantage during the entire follow-up period (aHR 0.99, 95% CI 0.87-1.22), but was associated with a survival benefit during the first 12 months (aHR 0.74, 95% CI 0.57-0.97).
We found that male sex, receiving treatment in a community practice, and numerically higher baseline ECOG PS ≥2 were associated with higher mortality after first-line therapy, while having very high PD-L1 expression, a KRAS mutation and receiving chemoimmunotherapy were associated with lower mortality during the first year. These prognostic indicators may aid providers in identifying patients who may be at risk of early mortality and therefore would derive maximum benefit from the addition of chemotherapy to their regimens.
Future research should focus on identifying high risk features that may indicate a need for chemoimmunotherapy over immunotherapy in this patient population.
Conflicts of Interest:
Mohsin Shah is a consultant epidemiologist at IQVIA Real World Solutions. Ronac Mamtani reports consulting fees from Bristol Myers Squibb, Seagen/Astellas, Flatiron Health and research funding from Merck outside the submitted work. Melina E. Marmarelis reports researching funding from Eli Lilly (Inst), AstraZeneca (Inst), Merck (Inst), AstraZeneca (Inst); consulting role with Astra Zeneca, Novocure, Boehringer Ingelheim, AstraZeneca, Janssen, Takeda, Blueprint Pharmaceuticals, Bristol Myers Squibb, Ikena; stock in Gilead Sciences, Portola Pharmaceuticals, Merck, Bluebird Bio, Johnson & Johnson, Pfizer. Sean Hennessy leads an educational program that has received support from Pfizer and Sanofi and has received consulting fees from Nektar Therapeutics, the Medullary Thyroid Consortium (Novo Nordisk, AstraZeneca, GlaxoSmithKline, Eli Lilly), Esteve Pharmaceuticals, Merck, Novo Nordisk, Biogen MA, and Intercept Pharmaceuticals, all unrelated to the submitted work.
Funding Support:
Shah was supported by National Institutes of General Medical Sciences (NIGMS) grant T32GM-075766.
Role of the Funder/Sponsor:
The funding body had no role in the design and conduct of this study; collection; management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; and the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N engl J med. 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Rodriguez-Abreu D, Robinson A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. 2019. [DOI] [PubMed]
- 3.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2018;379(21):2040–2051. [DOI] [PubMed] [Google Scholar]
- 4.Paz-Ares L, Ciuleanu T-E, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. The Lancet Oncology. 2021;22(2):198–211. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. New England journal of medicine. 2018;378(22):2078–2092. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar EJ, Ricciuti B, Gainor JF, et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Annals of Oncology. 2019;30(10):1653–1659. [DOI] [PubMed] [Google Scholar]
- 7.Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. The Lancet. 2021;397(10274):592–604. [DOI] [PubMed] [Google Scholar]
- 8.Shah M, Hubbard RA, Mamtani R, Marmarelis ME, Hennessy S. Very high PD-L1 expression as a prognostic indicator of overall survival among patients with advanced non-small cell lung cancer receiving anti-PD-(L)1 monotherapies in routine practice. Pharmacoepidemiol Drug Saf. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh RB, Min EJ, Wileyto EP, et al. Uptake and Survival Outcomes Following Immune Checkpoint Inhibitor Therapy Among Trial-Ineligible Patients With Advanced Solid Cancers. JAMA oncology. 2021;7(12):1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg SB, Herbst RS. Should chemotherapy plus immune checkpoint inhibition be the standard front-line therapy for patients with metastatic non–small cell lung cancer? Cancer. 2018;124(24):4592–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasello G, Pavan A, Attili I, et al. Real world data in the era of Immune Checkpoint Inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer treatment reviews. 2020;87:102031. [DOI] [PubMed] [Google Scholar]
- 12.Akinboro O, Vallejo JJ, Nakajima EC, et al. Outcomes of anti–PD-(L) 1 therapy with or without chemotherapy (chemo) for first-line (1L) treatment of advanced non–small cell lung cancer (NSCLC) with PD-L1 score≥ 50%: FDA pooled analysis. In: American Society of Clinical Oncology; 2022. [Google Scholar]
- 13.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. International journal of surgery. 2014;12(12):1495–1499. [DOI] [PubMed] [Google Scholar]
- 14.Ma X, Long L, Moon S, Adamson BJ, Baxi SS. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. Medrxiv. 2020. [Google Scholar]
- 15.Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv preprint arXiv:200109765. 2020. [Google Scholar]
- 16.Berger ML, Curtis MD, Smith G, Harnett J, Abernethy AP. Opportunities and challenges in leveraging electronic health record data in oncology. Future oncology. 2016;12(10):1261–1274. [DOI] [PubMed] [Google Scholar]
- 17.Liede A, Hernandez RK, Roth M, Calkins G, Larrabee K, Nicacio L. Validation of International Classification of Diseases coding for bone metastases in electronic health records using technologyenabled abstraction. Clin Epidemiol. 2015;7:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abernethy AP, Gippetti J, Parulkar R, Revol C. Use of electronic health record data for quality reporting. Journal of oncology practice. 2017;13(8):530–534. [DOI] [PubMed] [Google Scholar]
- 19.Singal G, Miller PG, Agarwala V, et al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non–small cell lung cancer using a clinicogenomic database. Jama. 2019;321(14):1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith SD, Tucker M, Bowser B, et al. Generating real-world tumor burden endpoints from electronic health record data: comparison of RECIST, radiology-anchored, and clinician-anchored approaches for abstracting real-world progression in non-small cell lung cancer. Advances in therapy. 2019;36(8):2122–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Presley CJ, Tang D, Soulos PR, et al. Association of broad-based genomic sequencing with survival among patients with advanced non–small cell lung cancer in the community oncology setting. Jama. 2018;320(5):469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Gossai A, Monroe S, Nussbaum NC, Parrinello CM. Validation analysis of a composite real-world mortality endpoint for US cancer patients. In: AACR; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Gossai A, Monroe S, Nussbaum NC, Parrinello CM. Validation analysis of a composite real-world mortality endpoint for patients with cancer in the United States. Health services research. 2021;56(6): 1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah M, Marmarelis ME, Mamtani R, Hennessy S. Association Between Survival and Very High Versus High PD-L1 Expression in Patients Receiving Pembrolizumab as First-line Treatment for Advanced Non-Small Cell Lung Cancer. Clin Lung Cancer. 2022. [DOI] [PubMed] [Google Scholar]
- 25.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Bmj. 2009;338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moons KG, Donders RA, Stijnen T, Harrell FE Jr. Using the outcome for imputation of missing predictor values was preferred. Journal of clinical epidemiology. 2006;59(10):1092–1101. [DOI] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple imputation for nonresponse in surveys. Vol 81: John Wiley & Sons; 2004. [Google Scholar]
- 28.Marshall A, Altman D, Holder R. Royston p.(2009). combining esimates of interest in prognosic modelling studies after muliple imputaion: current pracice and guidelines. D DĞĚŝĐĂů ZĞεĞĂЬĐŚ DĞĞŚŽĚŽůŽŐLǰ ϵ.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Statistics in medicine. 2013;32(16):2837–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statistics in medicine. 2015;34(28):3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z, Kim HJ, Lonjon G, Zhu Y. Balance diagnostics after propensity score matching. Annals of translational medicine. 2019;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoenfeld D Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. [Google Scholar]
- 33.Royston P, Parmar MKB. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Statistics in Medicine. 2002;21(15):2175–2197. [DOI] [PubMed] [Google Scholar]
- 34.He M, Zheng T, Zhang X, et al. First-line treatment options for advanced non-small cell lung cancer patients with PD-L1 ≥ 50%: a systematic review and network meta-analysis. Cancer Immunology, Immunotherapy. 2022;71(6):1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Han H, Zhang F, et al. Immune checkpoint inhibitors alone vs immune checkpoint inhibitors—combined chemotherapy for NSCLC patients with high PD-L1 expression: a network meta-analysis. British Journal of Cancer. 2022;127(5):948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho B, Wu Y, Lopes G, et al. FP13. 04 KEYNOTE-042 3-year survival update: 1L pembrolizumab vs platinum-based chemotherapy for PD-L1+ locally advanced/metastatic NSCLC. Journal of Thoracic Oncology. 2021;16(3):S225–S226. [Google Scholar]
- 37.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score≥ 50%. Journal of Clinical Oncology. 2021;39(21):2339–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non–small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA oncology. 2018;4(11):1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuentes-Antrás J, Provencio M, Díaz-Rubio E. Hyperprogression as a distinct outcome after immunotherapy. Cancer treatment reviews. 2018;70:16–21. [DOI] [PubMed] [Google Scholar]
- 40.Kurman JS, Murgu SD. Hyperprogressive disease in patients with non-small cell lung cancer on immunotherapy. Journal of thoracic disease. 2018;10(2):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non–small-cell lung cancer. New England Journal of Medicine. 2017;376(25):2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. [DOI] [PubMed] [Google Scholar]
- 43.Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. [DOI] [PubMed] [Google Scholar]
- 44.Reck M, Ciuleanu TE, Lee JS, et al. First-Line Nivolumab Plus Ipilimumab Versus Chemotherapy in Advanced NSCLC With 1% or Greater Tumor PD-L1 Expression: Patient-Reported Outcomes From CheckMate 227 Part 1. J Thorac Oncol. 2021;16(4):665–676. [DOI] [PubMed] [Google Scholar]
- 45.Jassem J, de Marinis F, Giaccone G, et al. Updated Overall Survival Analysis From IMpowerllO: Atezolizumab Versus Platinum-Based Chemotherapy in Treatment-Naive Programmed Death-Ligand 1-Selected NSCLC. J Thorac Oncol. 2021;16(11):1872–1882. [DOI] [PubMed] [Google Scholar]
- 46.Pathak R, De Lima Lopes G, Yu H, et al. Comparative efficacy of chemoimmunotherapy versus immunotherapy for advanced non–small cell lung cancer: A network meta-analysis of randomized trials. Cancer. 2021;127(5):709–719. [DOI] [PubMed] [Google Scholar]
- 47.Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. Progression after the next line of therapy (PFS2) and updated OS among patients (pts) with advanced NSCLC and PD-L1 tumor proportion score (TPS) ≥50% enrolled in KEYNOTE-024. Journal of Clinical Oncology. 2017;35(15_suppl):9000–9000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


