Heart failure (HF) is a progressive and complex clinical syndrome resulting in 1 million hospitalizations in the United States. The American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HFSA) guidance on managing HF recommends that “evidence of clinical congestion” be assessed regularly to guide overall management and diuretic adjustments. Assessment of congestion, however, is not readily reproducible, because the signs and symptoms are generally nonspecific, and the clinical presentation of HF is nuanced. Thus, diagnosing and assessing HF status in pressured care environments may be challenging. As a result, the primary HF diagnosis, or detection of worsening HF, most often occurs at hospitalization. Furthermore, studies show that serial imaging and blood biomarkers have a low temporal resolution for congestion and are similarly poor markers to guide overall HF management.
This effect was quantified by the Remote Dielectric Sensing (ReDS) pilot study, where 43% of patients admitted owing to decompensation were still congested at discharge despite specialist input and repeated biomarker assessments.1 This study also confirmed that eliminating congestion could reduce the 30-day readmission dramatically (1.25% vs. 7.10%) and was sustained at 90 days (13.75% vs. 17.90%). Therefore, to meet the AHA/ACC/HFSA recommendations for HF management and guide diuretic adjustments appropriately, newer decision tools must overcome the limitations of bedside patient evaluation.
The gold standard assessment for congestion is a hemodynamic evaluation via right heart catheterization or semipermanent pulmonary artery catheter during hospital admission. Still, studies of these procedures have previously shown neutral or negative improvements in short-term outcomes. These results are, however, at odds with the positive outcome studies using remote invasive intracardiac pressure monitoring (ICPM) devices.2 To that end, it could be hypothesized that a reproducible, serial, noninvasive, and scalable assessment solution for congestion monitoring would feasibly lead to improved outcomes for patients with HF, where the gold standard test may have been impractical to yield a statistically significant outcomes improvement, but where surrogate solutions (invasive ICPM) have demonstrated clear benefit.
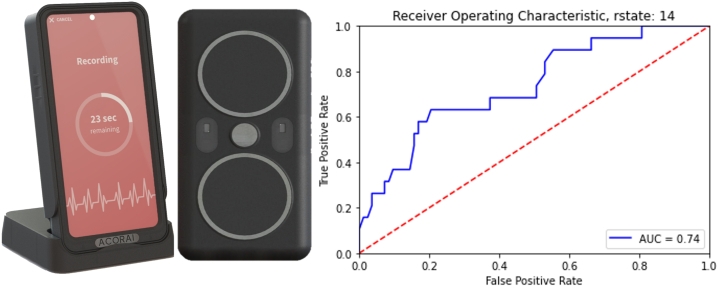
Acorai has developed a multisensor handheld medical device for noninvasive ICPM to improve HF management using the patented SAVE Sensor System (Figure 1). This ICPM is intended to produce accurate, absolute, and actionable information on appropriate hemodynamic parameters for discrete and serial measurements that could be incorporated into treatment algorithms for personalized care.
Figure 1.
Acorai Heart Monitor and SAVE Sensor System With ROC 0.74 AUC for >22 mm Hg Pulmonary Capillary Wedge Pressure
AUC = area under the curve.
The system is designed to present absolute intracardiac pressure measurements to assist practitioners with assessing congestion and the hemodynamic status of patients with HF or suspected decompensation. The hemodynamic variables intended to be quantified are the left atrial filling pressure estimated by pulmonary capillary wedge pressure, pulmonary congestion, or pulmonary hypertension by the pulmonary artery pressures (i.e., mean pulmonary artery pressure [mPAP]), systemic congestion by right atrial pressure, and cardiac output.
The device has duplicates of each sensor technology to cancel ambient noise, yet these may also improve overall spatial and temporal resolution compared with each sensor in isolation. The 4 sensor technologies have been chosen explicitly based on their standalone performance and published literature conferring their ability to detect and quantify physiological signals of interest. In combination, they could generate complimentary cardiac flow-dynamic insights.
The 4 sensor technologies that comprise the SAVE Sensor System are the following:
-
1)
Seismocardiography, a technique for measuring the mechanical vibrations generated by the heart, has the clinical utility to isolate vibration or wave patterns related to cardiovascular hemodynamics, left ventricular ejection patterns, left atrial filling, and pulmonary or intrathoracic pressure.3
-
2)
Phonocardiography collects audible signals from heart sounds using digital microphones targeted to specific frequencies to characterize physiological phenomena such as cardiac pressure gradients, muscle motion, blood flow, fluid dynamics, viscosity, pressure, and velocity.4
-
3)
Photoplethysmography is primarily used to derive the pulse wave transit time, which holds an inverse relationship with pressure, for the estimation of arterial blood pressure, blood viscosity, blood vessels stiffness, cardiac output, blood velocity, and isolated intracardiac or pulmonic pressure.5
-
4)
Electrocardiography sensors provide timing to other sensing modalities, and artificial intelligence enhancements can uncover underappreciated or previously unquantifiable information on the baseline, which may correlate with intracardiac pressure.
An observational study was initiated to demonstrate the feasibility of the device for estimating intracardiac pressure. A total of 281 subjects were included following local ethics procedures and EU MDR. The primary outcome was to determine the extent of correlation between the estimated measurements and those obtained from gold standard right heart catheterization in the same patient. Patients had the sensor data recorded immediately before a planned routine right heart catheterization. Then, a machine learning model was retrospectively trained on a subset of the data, following U.S. Food and Drug Administration good machine learning practice, to estimate intracardiac pressure from the senor information only. In this first feasibility analysis, we focused on the mPAP and the pulmonary capillary wedge pressure.
The results from the first-generation machined learning model have demonstrated a correlation between mPAP (P = 0.75, r2 = 0.55), a mean difference of measurements of 0.78 mm Hg. These results were higher than the expected correlation based on the literature for each sensor’s correlation with mPAP; the variability in gold standard data methods could account for some of the mean difference.
To understand the clinical usefulness of the device, the results were compared with the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) Trial bedside patient evaluation results, which defined the classification of severely congested HF as pulmonary capillary wedge pressure of >22 mm Hg. The area under the curve (AUC) of the Acorai Heart Monitor was 0.74, whereas cardiologist-led evaluation had an AUC of 0.63; BNP had an AUC of 0.55 for the same cutoff. The sensitivity and positive predictive values of the Acorai Heart Monitor were 95% and 96%, respectively. The specificity and negative predictive values were 31% and 24%, respectively. Thus, in the current version of the machine learning model, the performance is weighted toward a rule-in diagnostic.
Early preliminary data are sufficient to demonstrate a reasonable expectation that the device could estimate absolute intracardiac pressure parameters with enough accuracy to aid clinical decision-making. More data are required to confirm this performance and enhance the machine learning model. The Acorai study is being extended to U.S. sites, with approximately 1,000 additional patients enrolled to improve overall performance.
Footnotes
This work was presented at the 2023 CRF THT Shark Tank, March 20-23, 2023, Boston, Massachusetts. Editor’s Note: To view the authors’ full presentation at TCTMD Shark Tank, please visit https://www.jacc.org/journal/basic-translational/tht-2023-shark-tank
Mr Mace is a consultant to Acorai AB, receives a consultancy fee from Acorai AB and has an ownership interest. Mr Mace also has individual stock interest in Abbott Laboratories.
The author attests they are in compliance with human studies committees and animal welfare regulations of the author’s institution and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Bensimhon D., Alali S.A., Curran L., et al. The use of the reds noninvasive lung fluid monitoring system to assess readiness for discharge in patients hospitalized with acute heart failure: a pilot study. Heart Lung. 2021;50(1):59–64. doi: 10.1016/j.hrtlng.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld J., Zile M.R., Desai A.S., et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. 2021;398(10304):991–1001. doi: 10.1016/S0140-6736(21)01754-2. [DOI] [PubMed] [Google Scholar]
- 3.Azad Khurshidul M.D. UCF's Showcase of Text, Archives, Research & Scholarship (STARS) library; Orange County, FL: 2018. Seismocardiographic signal variability and pulmonary phase detection in adults. [Electronic thesis] [Google Scholar]
- 4.Xu J., Durand L.-G., Pibarot P. A new, simple, and accurate method for non-invasive estimation of pulmonary arterial pressure. Heart. 2002;88(1):76–80. doi: 10.1136/heart.88.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castaneda D.E., Esparza A., Ghamari M., et al. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int J Biosens Bioelectron. 2018;4(4):195–202. doi: 10.15406/ijbsbe.2018.04.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]