Abstract
The invasive enteropathogenic bacterium Shigella flexneri activates apoptosis in macrophages. Shigella-induced apoptosis requires caspase-1. We demonstrate here that tripeptidyl peptidase II (TPPII), a cytoplasmic, high-molecular-weight protease, participates in the apoptotic pathway triggered by Shigella. The TPPII inhibitor Ala-Ala-Phe-chloromethylketone (AAF-cmk) and clasto-lactacystin β-lactone (lactacystin), an inhibitor of both TPPII and the proteasome, protected macrophages from Shigella-induced apoptosis. AAF-cmk was more potent than lactacystin and irreversibly blocked Shigella-induced apoptosis by 95% at a concentration of 1 μM. Conversely, peptide aldehyde and peptide vinylsulfone proteasome inhibitors had little effect on Shigella-mediated cytotoxicity. Both AAF-cmk and lactacystin prevented the maturation of pro-caspase-1 and its substrate pro-interleukin 1β in Shigella-infected macrophages, indicating that TPPII is upstream of caspase-1. Neither of these compounds directly inhibited caspase-1. AAF-cmk and lactacystin did not impair macrophage phagocytosis or the ability of Shigella to escape the macrophage phagosome. TPPII was also found to be involved in apoptosis induced by ATP and the protein kinase inhibitor staurosporine. We propose that TPPII participates in apoptotic pathways.
Gram-negative bacteria of the genus Shigella cause bacillary dysentery, a potentially fatal diarrheal disease. In the lower gastrointestinal tract, Shigella provokes an acute inflammation that leads to tissue destruction and promotes bacterial invasion (15). Early during infection, Shigella is phagocytosed by resident tissue macrophages. Shigella escapes the phagolysosome and kills macrophages in vitro (18, 43) and in vivo (45) by inducing apoptosis. Apoptotic macrophages release mature interleukin-1β (IL-1β) and IL-18, cytokines which trigger the acute inflammation characteristic of dysentery (36, 44). Bacterial virulence factors are encoded on a 220-kb plasmid and include a type III secretion apparatus and the invasion plasmid antigens (Ipa) A to D (17, 25).
The invasin IpaB is secreted by Shigella into the macrophage cytoplasm (37). IpaB is necessary and sufficient to induce apoptosis (6, 42) and binds specifically to caspase-1 (IL-1β converting enzyme; ICE) (19). Caspases are cysteine proteases that cleave after aspartic acid residues. These enzymes are synthesized as zymogens and are activated by proapoptotic stimuli (39). In macrophages infected with Shigella, pro-caspase-1 is proteolytically processed to its mature form which cleaves pro-IL-1β and activates apoptosis (19).
In addition to caspases, other endogenous proteases have been implicated in apoptosis. The eukaryotic 26S proteasome is an N-terminal threonine protease complex with multiple endoproteolytic activities (trypsin-, chymotrypsin-, and caspase-like). The proteasome is required for the cell's general ATP- and ubiquitin-dependent protein turnover, cell cycle progression, and immunosurveillance (3, 31). Moreover, the proteasome participates in apoptotic pathways triggered by growth factor deprivation, radiation, and chemicals (9, 13, 27).
Another high-molecular-weight protease is the cytoplasmic serine protease tripeptidyl peptidase II (TPPII) (40). TPPII is an amino-endopeptidase and exists as a homo-oligomer in the cytoplasm (1, 2, 11) or as a membrane-bound complex (33). Tripeptidyl peptidases are involved in general protein degradation (40), but TPPII also cleaves specific substrates such as neuropeptides (33). In lymphoma cells adapted to proteasome inhibitors, a proteolytic system with TPPII-like activity compensates for the loss of proteasome activity (12), and TPPII substitutes some proteasomal functions (11). Both the proteasome and TPPII share a similar overall structure, as determined by electron microscopy (3, 11, 24).
TPPII and the proteasome differ in their substrate spectra and inhibition patterns. The Streptomyces metabolite lactacystin and its active, more-cell-permeable form, clasto-lactacystin β-lactone (lactacystin), inhibit the proteasome (8, 10) and, less efficiently, TPPII (11). Ala-Ala-Phe-chloromethylketone (AAF-cmk) irreversibly blocks purified TPPII but not the proteasome. Conversely, the proteasome inhibitor N-acetyl-Leu-Leu-norleucinal (LLnL) does not inhibit TPPII (11). LLnL blocks the proteasome more potently than N-acetyl-Leu-Leu-methioninal (LLM) (32). Finally, 4-hydroxy-5-iodo-3-nitrophenylacetyl-Leu-Leu-Leu-vinylsulfone (LLL-vs) inhibits the trypsin-, chymotrypsin-, and caspase-like proteasomal activities irreversibly (5). Using these inhibitors, we demonstrate here that TPPII participates in Shigella-induced apoptosis upstream of caspase-1 and in the apoptotic pathways triggered by staurosporine and ATP.
MATERIALS AND METHODS
Cells, bacteria, and reagents.
Mouse J774 macrophage-like cells, mouse peritoneal macrophages (from B57BL/6 or Swiss Webster mice), and human THP.1 monocytic leukemia cells (American Type Culture Collection [ATCC]) were used in this study. Macrophages were routinely cultured in a humidified atmosphere of 5% CO2 at 37°C in RPMI 1640 glutamine medium (Mediatech; Fisher Scientific, Pittsburgh, Pa.) supplemented with 10% fetal calf serum (Gibco-BRL, Gaithersburg, Md.), l-glutamine (2 mM), and penicillin and streptomycin (50 μg/ml each). For infections, J774 cells and peritoneal macrophages were cultured overnight. THP.1 cells were differentiated for 3 days in the presence of 160 nM phorbol 12-myristate 13-acetate (PMA). To induce pro-IL-1β, the macrophages were treated overnight with 1 μg of lipopolysaccharide (LPS; Shigella serotype 1A; Sigma, St. Louis, Mo.) per ml.
The wild-type Shigella flexneri strain M90T, a serotype 5A clinical isolate (35), and the virulence plasmid-cured, nonvirulent derivative BS176 were grown aerobically in tryptic soy broth medium (Gibco-BRL) at 37°C. The bacteria were centrifuged and resuspended in serum-free RPMI 1640 medium (SFM) prior to infecting the macrophages.
All chemicals were obtained from Sigma unless otherwise stated. The caspase inhibitors and substrates acetyl-Tyr-Val-Ala-Asp-aldehyde (YVAD), acetyl- Tyr-Val-Ala-Asp-chloromethylketone (YVAD-cmk), acetyl-Tyr-Val-Ala-Asp-7-amido-4-methyl-coumarin (YVAD-amc), and carboxybenzyl-Val-Ala-Asp-fluoromethylketone (zVAD-fmk) were purchased from Bachem (King of Prussia, Pa.). Lactacystin was from Boston Biochem, Inc. (Cambridge, Mass.), LLL-vs was from Calbiochem (La Jolla, Calif.), and carboxybenzyl-Phe-Ala-fluoromethylketone (zFA-fmk) from Enzyme Systems Products (Livermore, Calif.). The inhibitors and substrates were prepared as 5 or 10 mM stock solutions in dimethyl sulfoxide (DMSO) and stored at −20°C. Purified caspase-1 was kindly provided by N. Thornberry (Merck, Rahway, N.J.).
Cytotoxicity assays.
Cytotoxicity induced by Shigella (multiplicity of infection [MOI] of 10 to 50; 2 to 3 h) (7), neutralized ATP (5 mM, 30 min) (21), or staurosporine (1 μM, 12 h) was routinely analyzed with the CytoTox96 Kit (Promega, Madison, Wis.) as described earlier (46). The percentage of cytotoxicity was calculated by quantifying the amount of lactate dehydrogenase (LDH) released from dying cells according to the following formula: [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100 (19). LDH release is not specific for apoptosis; however, Shigella (43), ATP (21), and staurosporine (30) represent well-established apoptotic systems. Shigella-induced apoptosis of differentiated THP.1 cells was assayed by using the Cell Death Detection ELISAPLUS Kit (Boehringer Mannheim, Indianapolis, Ind.) according to the manufacturer's instructions. Prior to the addition of the apoptotic agent, the macrophages were incubated with proteasome inhibitors (2 to 3 h) or caspase inhibitors (1 h) when indicated. The viability of macrophages treated with the inhibitors alone was assayed by LDH release, enzyme-linked immunosorbent assay (ELISA), and oxidation of the tetrazolium salt MTT [3,(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide] (16). Within the time frame of the Shigella infections and ATP-induced apoptosis, the inhibitors alone were not cytotoxic for the macrophages, and the toxicity of 5% DMSO was less than 4% (data not shown).
Maturation of caspase-1 and IL-1β in macrophages infected with Shigella.
Peritoneal macrophages from B57BL/6 mice were infected with Shigella (MOI of 5) and lysed in situ at the indicated time points, and the maturation of caspase-1 was analyzed by Western blotting using the rabbit anti-mouse caspase-1 antibody R10311 (kindly provided by D. Miller, Merck) as described previously (18, 19). When indicated, the macrophages were preincubated with AAF-cmk (10 μM, 2 h), lactacystin (30 μM, 4 h), or YVAD-cmk (100 μM, 1 h).
THP.1 cells were infected with Shigella at an MOI of 50. When indicated, the cells were treated with AAF-cmk (10 μM, 2 h), lactacystin (135 μM, 3 h), or YVAD-cmk (0.5 mM, 1 h) prior to the infection. Maturation of IL-1β was analyzed by Western blotting as described above, using a goat anti-human IL-1β antibody (R&D Systems, Minneapolis, Minn.).
Gentamicin protection assay.
To discriminate between phagocytosed Shigella and extracellular Shigella, a modification of the gentamicin protection assay was used (26). At 20 min postinfection, J774 macrophages infected with Shigella (MOI of 50) were washed twice with 0.2 ml of phosphate-buffered saline (PBS)-gentamicin (0.1 mg/ml), incubated for another hour in SFM-gentamicin (0.1 mg/ml), and lysed with PBS–Triton X-100 (0.1%). The lysate was diluted and plated on nutrient broth agar (Difco Laboratories, Detroit, Mich.) supplemented with 0.5% sodium chloride, and the CFU were counted. When indicated, the macrophages were treated with either AAF-cmk (1 μM, 1 h), lactacystin (5 μM, 2 h), YVAD-cmk (100 μM, 1 h), zVAD-fmk (100 μM, 1 h), or cytochalasin B (2.5 μg/ml, 1 h) prior to infection.
Detection of actin tails in Shigella-infected macrophages.
Shigella-infected J774 macrophages were labeled with fluorescein isothiocyanate (FITC)-conjugated phalloidin and propidium iodide as described for epithelial cells (4). Briefly, at 30 min postinfection (MOI of 20), the macrophages were fixed, treated with RNase (1 mg/ml, 1 h), and stained with phalloidin-FITC (50 μg/ml) and propidium iodide (5 μg/ml) for 45 min. Prior to the infection, some macrophages were incubated with either AAF-cmk (1 μM) or lactacystin (5 μM) for 2 h. The samples were analyzed with a Leica DMRBE confocal microscope equipped with an argon-krypton laser.
Fluorometric assays of TPPII and caspase-1.
The hydrolytic activity of TPPII during Shigella infection was measured with the fluorogenic peptide substrate Ala-Ala-Phe-7-amido-4-methyl-coumarin (AAF-amc). J774 cells were cultured overnight and infected with Shigella (MOI of 10) in PBS as described above. At different time points, the cells were lysed with digitonin (final concentration, 20 μg/ml), AAF-amc (100 μM) was added, and the reaction was stopped after 30 min with trifluoroacetic acid (final concentration, 0.2%). Prior to analyzing the hydrolysis of AAF-amc, some macrophages were treated for 2 h with different concentrations of AAF-cmk or lactacystin in PBS. The fluorescence of the leaving group 7-amino-4-methyl-coumarin (amc) was measured at 380-nm excitation and 460-nm emission wavelengths with a MicroMax-2 spectrofluorometer (Instruments SA, Edison, N.J.).
The activity of purified caspase-1 was assayed as described above, using YVAD-amc as a fluorogenic peptide substrate (38). When indicated, aliquots of purified caspase-1 were treated with different concentrations of either AAF-cmk, lactacystin, or YVAD-cmk (2 h, 4°C) prior to assaying the hydrolysis of YVAD-amc.
RESULTS
Effect of TPPII and proteasome inhibitors on Shigella-induced macrophage apoptosis.
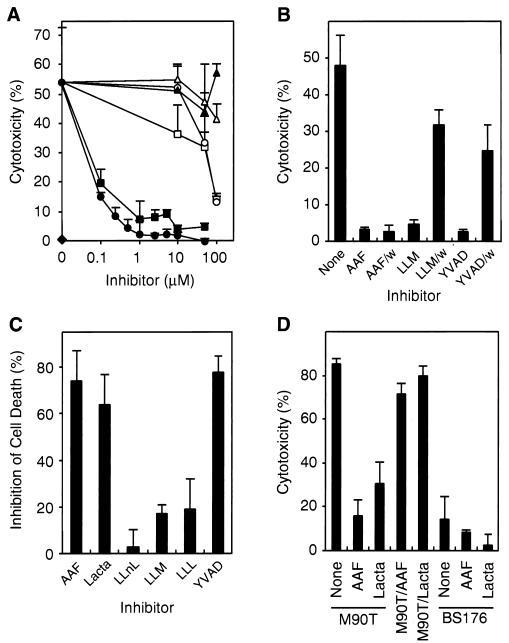
To identify novel components of the apoptotic pathway triggered by S. flexneri, we treated J774 macrophages with various inhibitors of TPPII and the proteasome. The TPPII inhibitor AAF-cmk protected macrophages from the Shigella wild-type strain M90T in a dose-dependent manner by more than 95% at concentrations of as low as 1 μM (Fig. 1A). clasto-Lactacystin β-lactone (referred to as “lactacystin”) blocked Shigella-induced macrophage cell death by approximately 90% at concentrations of between 1 and 10 μM. In contrast different proteasome inhibitors at concentrations as high as 100 μM had little (LLM and LLL-vs) or no (LLnL) effect on Shigella-induced apoptosis. The peptide inhibitor zFA-fmk, used as a negative control, did not affect Shigella-induced cell death at 100 μM. As expected, the nonvirulent S. flexneri strain BS176 was not cytotoxic. These data indicate that TPPII rather than the proteasome is necessary for Shigella-induced macrophage apoptosis.
FIG. 1.
TPPII is involved in Shigella-induced macrophage apoptosis. (A) Mouse J774 macrophages were treated with the TPPII inhibitor AAF-cmk (●), the proteasome-TPPII inhibitor clasto-Lactacystin β-lactone (lactacystin) (■), or the proteasome inhibitors LLnL (▴), LLM (□), LLL-vs (○), or with zFA-fmk (▵) and then infected with S. flexneri wild-type strain M90T or the nonvirulent strain BS176 (⧫) at an MOI of 10. The cytotoxicity was assayed by measuring the LDH release after 2 h. (B) J774 macrophages were treated with AAF-cmk (5 μM), LLM (100 μM), or the caspase-1 inhibitor YVAD (50 μM) and washed (AAF/w, LLM/w, and YVAD/w) prior to M90T infection. (C) Peritoneal macrophages were preincubated with a 10 μM concentration of the proteasome-TPPII inhibitors indicated or with YVAD-cmk (100 μM) and infected with M90T. (D) J774 macrophages were infected with M90T grown in the presence of 1 μM AAF-cmk (M90T/AAF) or 5 μM lactacystin (M90T/Lacta). In parallel, untreated macrophages (none) or macrophages treated with 1 μM AAF-cmk (AAF) or 5 μM lactacystin (Lacta) were infected with mock-treated M90T or BS176. Means and standard deviations of experiments done in triplicate are shown. Similar results were obtained in at least three (A and D) or two (B and C) independent experiments.
AAF-cmk, a peptide chloromethylketone, irreversibly blocks the serine protease TPPII (11, 12) and reversibly inhibits TPPI (41). To distinguish between TPPII and TPPI, we washed J774 macrophages treated with AAF-cmk prior to infection with M90T. AAF-cmk (5 μM) irreversibly protected the macrophages from Shigella-induced apoptosis (Fig. 1B), ruling out a role for TPPI. As expected, the peptide aldehydes LLM (100 μM) and YVAD (50 μM), a competitive inhibitor of caspase-1 (6), reversibly protected the macrophages from Shigella-mediated apoptosis.
Primary macrophages treated with inhibitors of high-molecular-weight proteases prior to an infection with M90T showed a protection profile very similar to J774 cells. The TPPII inhibitor AAF-cmk decreased Shigella-mediated apoptosis in mouse peritoneal macrophages by 75% at a concentration of 10 μM (Fig. 1C). At the same concentration, lactacystin decreased Shigella cytotoxicity by 64%, LLnL had almost no effect, and LLM or LLL-vs decreased cytotoxicity by approximately 20%. The caspase-1 inhibitor YVAD-cmk (100 μM) decreased Shigella-induced apoptosis by about 80%.
Human monocytic THP.1 cells differentiated with PMA were also protected from Shigella-mediated cytotoxicity by AAF-cmk and lactacystin. However, whereas 10 μM AAF-cmk completely blocked Shigella-induced apoptosis, lactacystin and YVAD-cmk were effective only at 10-fold-higher concentrations (data not shown).
To analyze whether the TPPII inhibitors have an effect on Shigella rather than on macrophages, we cultured M90T for 2 h in presence of 1 μM AAF-cmk or 5 μM lactacystin. Within this time period, treated bacteria reached the same optical density as untreated cultures, demonstrating that the TPPII inhibitors did not affect the ability of Shigella to grow (data not shown). M90T, thus treated and washed with serum-free medium, caused only slightly less cell death in infected macrophages compared to mock-treated M90T (Fig. 1D). In contrast, macrophages preincubated with AAF-cmk (1 μM) or lactacystin (5 μM) were protected from Shigella-induced apoptosis by 82 or 64%, respectively. These data demonstrate that the TPPII inhibitors block Shigella-mediated cytotoxicity by affecting only macrophages and not Shigella. As expected, preincubation of macrophages with the TPPII inhibitors had no effect on an infection with the nonvirulent strain BS176.
Maturation of caspase-1 and IL-1β in Shigella-infected macrophages is inhibited by AAF-cmk and lactacystin.
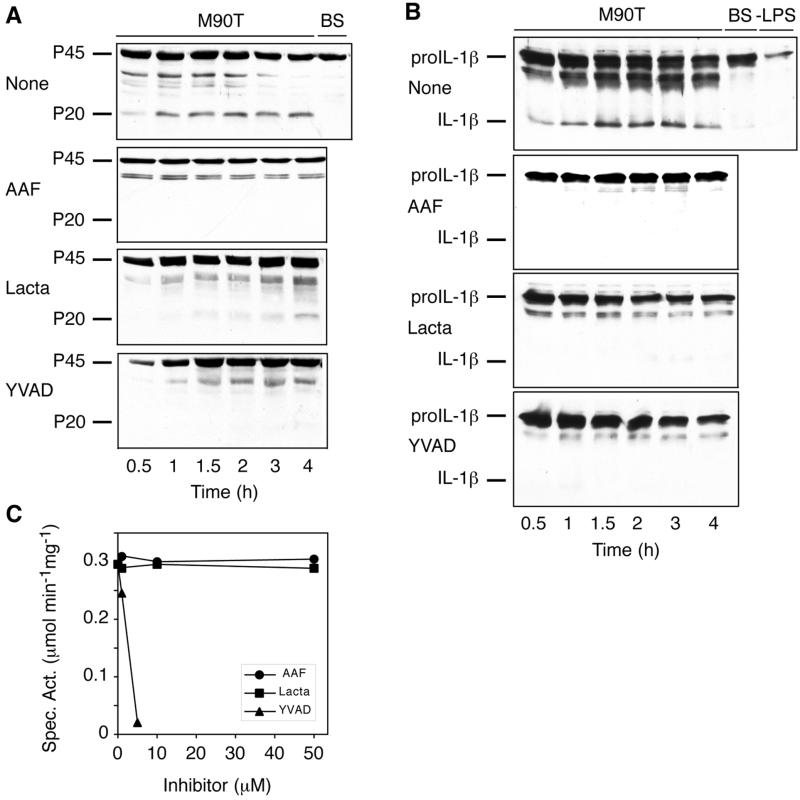
During a Shigella infection, caspase-1 matures by limited proteolysis from a 45-kDa precursor to the active enzyme composed of 20- and 10-kDa polypeptides (6, 19). To test the effect of the TPPII inhibitors AAF-cmk and lactacystin on the maturation of pro-caspase-1, we preincubated mouse peritoneal macrophages with either compound and infected the cells with Shigella. Lysates of the infected macrophages were prepared at the indicated time points and analyzed by Western blotting with an antibody recognizing the 45-kDa precursor and the 20-kDa processed form of caspase-1. In untreated macrophages, mature caspase-1 was detected as soon as 30 min after infection with the wild-type Shigella strain M90T (Fig. 2A). Macrophages infected with the avirulent strain BS176 did not process pro-caspase-1 even by 4 h postinfection. AAF-cmk (10 μM) and, less potently, lactacystin (30 μM) blocked the processing of pro-caspase-1, indicating that TPPII participates upstream of caspase-1 in the apoptotic pathway. Whereas AAF-cmk completely abolished the maturation of caspase-1 throughout the 4-h infection, lactacystin was less efficient, and at 2 h postinfection the 20-kDa caspase-1 fragment began to appear. As previously shown (19), the caspase-1 inhibitor YVAD-cmk (100 μM) abrogated the processing of pro-caspase-1 during the Shigella infection.
FIG. 2.
TPPII is required for the maturation of caspase-1 in Shigella-infected macrophages. (A) Peritoneal macrophages (None) and macrophages treated with AAF-cmk (AAF, 10 μM), lactacystin (Lacta, 30 μM), or YVAD-cmk (YVAD, 100 μM) were infected with virulent (M90T, 0.5 to 4 h) or nonvirulent (BS, 4 h) Shigella strains. Maturation of caspase-1 was analyzed by Western blotting in lysates of infected macrophages. (B) Differentiated, LPS-stimulated THP.1 cells (None) and cells treated with AAF-cmk (AAF, 10 μM), lactacystin (Lacta, 135 μM), or YVAD-cmk (YVAD, 0.5 mM) were infected with virulent (M90T, 0.5 to 4 h) or nonvirulent (BS, 4 h) Shigella strains. Maturation of IL-1β was analyzed by Western blotting in lysates of infected and naive (−LPS) macrophages. (C) The specific activity of purified caspase-1 against the fluorogenic peptide substrate YVAD-amc was determined in the absence or in presence of AAF-cmk, lactacystin, or YVAD-cmk at the concentrations indicated. The specific activity is defined as the number of micromoles of amc released per minute per milligram of protein. The experiments were done at least twice, and results similar to those shown were obtained each time.
M90T but not BS176 triggered the maturation of IL-1β in differentiated THP.1 cells treated with LPS, indicating that caspase-1 was activated during Shigella-induced apoptosis (Fig. 2B). AAF-cmk (10 μM) and lactacystin (135 μM) prevented the maturation of IL-1β in M90T-infected THP.1 cells. As expected, the caspase-1 inhibitor YVAD-cmk (0.5 mM) also abolished the processing of IL-1β. THP.1 cells not treated with LPS (−LPS) expressed only little pro-IL-1β.
To test whether AAF-cmk or lactacystin directly inhibit caspase-1, we incubated purified caspase-1 with these compounds and assayed the enzymatic activity with the fluorogenic peptide substrate YVAD-amc. Neither AAF-cmk nor lactacystin at concentrations up to 50 μM had any effect on caspase-1 activity (Fig. 2C). Therefore, AAF-cmk and, as shown previously (34), lactacystin do not inhibit caspase-1 directly. In contrast, the caspase-1 inhibitor YVAD-cmk (5 μM) almost completely abolished the activity of purified caspase-1.
Shigella is protected within macrophages from gentamicin by AAF-cmk and lactacystin.
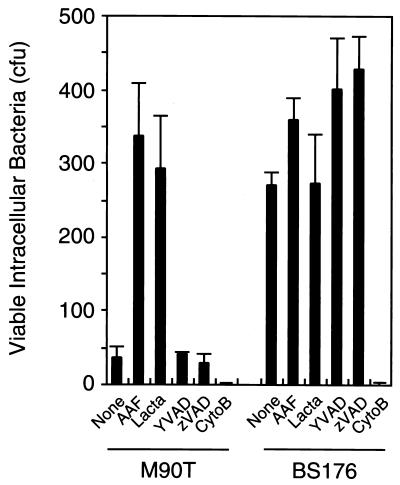
Intracellular bacteria are protected from the antibiotic gentamicin, which is used to discriminate between intra- and extracellular bacteria (26). As expected, the avirulent S. flexneri strain BS176 was protected within macrophages from gentamicin (Fig. 3). In contrast, gentamicin killed the wild-type strain M90T and, therefore, apoptotic macrophages appear to become permeable to the antibiotic. To test whether blocking apoptosis would result in protection of M90T from gentamicin, we incubated macrophages with TPPII and caspase inhibitors. Interestingly, treatment of macrophages with either AAF-cmk or lactacystin completely protected intracellular wild-type Shigella from gentamicin. The caspase-1 inhibitor YVAD-cmk or the broad-spectrum caspase inhibitor zVAD-fmk, on the other hand, did not protect M90T from gentamicin. However, both caspase inhibitors abolished Shigella-mediated cytotoxicity as determined by LDH release (data not shown).
FIG. 3.
Shigella is protected within macrophages from gentamicin by AAF-cmk or lactacystin. J774 macrophages were treated with the TPPII inhibitors AAF-cmk (AAF, 1 μM) or lactacystin (Lacta, 5 μM), with the caspase inhibitors YVAD-cmk or zVAD-fmk (YVAD or zVAD, 100 μM), or with cytochalasin B (CytoB, 2.5 μg/ml), an inhibitor of actin polymerization. After infection with either wild-type (M90T) or avirulent (BS176) S. flexneri and gentamicin treatment, the CFU were determined. Means and standard deviations of experiments done in quadruplicate are shown. Similar results were obtained in three independent experiments.
To analyze whether TPPII or caspase inhibitors block bacterial uptake by macrophages, we treated the macrophages with the inhibitors prior to an infection with strain BS176. The inhibitors had no effect on the number of viable avirulent Shigella within macrophages, indicating that these inhibitors did not impair the phagocytic activity of the macrophages (Fig. 3). As another control, we preincubated macrophages with cytochalasin B, a potent inhibitor of actin polymerization. No bacteria were recovered from cytochalasin B-treated macrophages, demonstrating that phagocytosis was completely abrogated and that gentamicin quantitatively eradicated extracellular Shigella.
Bacterial escape from the phagosome is not impaired by AAF-cmk or lactacystin.
Since the TPPII inhibitors AAF-cmk and lactacystin did not affect the phagocytic activity of macrophages, we investigated whether these inhibitors impair the escape of Shigella from the macrophage phagosome. Shortly after entry into a host cell, wild-type Shigella escapes the phagosome and moves intracellularly by polymerizing actin, resulting in “actin tails” at a single pole of the bacterium (4, 29). To assay the formation of cytoplasmic actin tails, we labeled infected macrophages with phalloidin-FITC and stained both macrophage nuclei and bacteria with propidium iodide. The wild-type Shigella strain M90T formed bacterium-associated actin tails in untreated macrophages, as well as in macrophages preincubated for 2 h with 1 μM AAF-cmk or 5 μM lactacystin (data not shown). Therefore, AAF-cmk and lactacystin did not prevent the escape of Shigella from the phagosome.
TPPII activity is not altered during Shigella infection of macrophages.
To test whether the activity of TPPII is regulated during an infection of macrophages with Shigella, we assayed the hydrolysis of the fluorogenic peptide substrate AAF-amc. TPPII hydrolyzes only peptides with a free amino terminus, and AAF-amc is hydrolyzed by TPPII several orders of magnitude more efficiently than by the proteasome (11). To confirm that the AAF-amc hydrolysis observed was actually due to TPPII activity, we preincubated macrophages with the TPPII inhibitors AAF-cmk and lactacystin and determined the extent of AAF-amc hydrolysis in lysates of macrophages permeabilized with digitonin. AAF-cmk and lactacystin (10 μM each) blocked the hydrolysis of AAF-amc in macrophage lysates to 94 and 57%, respectively (data not shown), suggesting that the activity measured was due to TPPII.
Up to 1 h postinfection, the specific activity of AAF-amc hydrolysis was similar in lysates of mock-infected macrophages and macrophages infected with either the wild-type strain M90T or nonvirulent BS176 (approximately 40 pmol of amc released min−1 mg of protein−1; data not shown). Since the hydrolytic activity against a prototypic TPPII peptide substrate was not altered in apoptotic macrophages infected with Shigella, TPPII activity is presumably not regulated during the bacterial infection.
TPPII is involved in apoptosis induced by ATP and staurosporine.
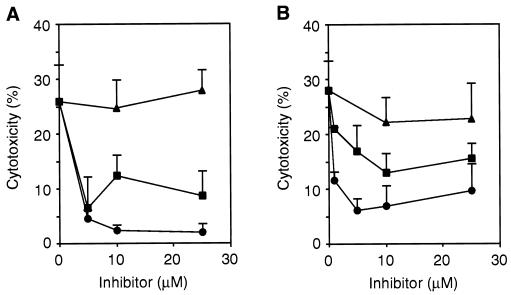
To test if TPPII is specifically involved in Shigella-induced apoptosis or whether it also plays a role in other apoptotic pathways, we analyzed cell death induced by the protein kinase inhibitor staurosporine (30) and ATP (21). At a concentration of 5 μM, the TPPII inhibitors AAF-cmk and lactacystin decreased staurosporine-induced apoptosis in J774 macrophages by 83 and 75%, respectively (Fig. 4A). Similarly, AAF-cmk and lactacystin (10 μM each) inhibited ATP-induced apoptosis in mouse peritoneal macrophages by 75 and 54%, respectively (Fig. 4B). In contrast, the proteasome inhibitors LLnL and LLM (data not shown) did not affect apoptosis induced by either staurosporine or ATP. The induction of apoptosis by staurosporine requires a 12-h incubation period. Within this period, AAF-cmk and LLnL were not cytotoxic to the macrophages. However, lactacystin alone caused 40% cell death (data not shown). In summary, the inhibition pattern obtained for staurosporine- and ATP-induced apoptosis indicates that TPPII is also involved in these apoptotic pathways.
FIG. 4.
TPPII is involved in apoptosis induced by staurosporine and ATP. J774 macrophages were incubated with staurosporine (1 μM, 12 h) (A) or LPS-activated mouse peritoneal macrophages were treated with ATP (5 mM, 30 min) (B) in the absence of in the presence of AAF-cmk (●), lactacystin (■), or LLnL (▴) at the concentrations indicated. The cytotoxicity was determined by measuring the LDH release and is corrected for cell death caused by lactacystin alone. Means and standard deviations of experiments done in triplicate are shown. Similar results were obtained in three (A) or two (B) independent experiments.
DISCUSSION
TPPII participates in Shigella-induced macrophage apoptosis.
In this report we describe the high-molecular-weight protease TPPII as a previously unidentified component of the apoptotic pathway triggered by S. flexneri in macrophages (Fig. 1). The TPPII inhibitors AAF-cmk and lactacystin, but not the proteasome inhibitor LLnL, potently blocked Shigella-induced apoptosis. This inhibition profile is characteristic for purified TPPII and not the proteasome (11). The proteasome inhibitor LLM but not LLnL protected macrophages to some extent from Shigella-induced apoptosis, which is inversely correlated to the potential of these compounds to block the proteasome (32). In contrast, LLnL inhibited apoptosis more potently than LLM in apoptotic pathways involving the proteasome (14, 34).
AAF-cmk blocks TPPII irreversibly and the lysosomal protease TPPI reversibly (28, 41). AAF-cmk irreversibly blocked Shigella-induced apoptosis (Fig. 1), suggesting that cytoplasmic TPPII is involved. Consistent with the idea that AAF-cmk and lactacystin act on a cytoplasmic macrophage protease, we found that the inhibitors (i) block Shigella-induced apoptosis by acting on macrophages but not on Shigella (Fig. 1), (ii) do not impair phagocytosis of bacteria (Fig. 3), and (iii) do not affect bacterial escape from the phagosome (data not shown). Shigella needs to escape the macrophage phagosome in order to induce apoptosis (42) and, therefore, resides in the same subcellular compartment (i.e., the cytoplasm) as TPPII. At present we cannot exclude the possibility that other proteases with an inhibition profile similar to TPPII are also or alternatively involved in Shigella-induced apoptosis.
Role of TPPII in Shigella-induced apoptosis.
TPPII appeared to be upstream of caspase-1 in Shigella-induced apoptosis. The TPPII inhibitors AAF-cmk and lactacystin prevented the maturation of caspase-1 and IL-1β in macrophages infected with Shigella, without inhibiting caspase-1 directly (Fig. 2). By activating caspase-1 and consequently IL-1β, TPPII participates in a proinflammatory apoptotic pathway and contributes to an innate immune response. Similarly, lactacystin has been shown to block the activation of caspase-1 and IL-1β in macrophages treated with ATP (34). In this and other studies, however, the authors concluded that the proteasome participates upstream of caspases and mitochondrial events in thymocyte apoptosis (14, 20) and neuronal programmed cell death (34).
Macrophages undergoing Shigella-triggered apoptosis did not protect the intracellular bacteria from gentamicin (Fig. 3). Wild-type Shigella was protected from the antibiotic in macrophages treated with the TPPII inhibitors AAF-cmk and lactacystin, suggesting that TPPII is involved in the process. TPPII might be involved in the exclusion of the antibiotic from the cytoplasm or, alternatively, by preventing its action within the cytoplasm. The caspase inhibitors YVAD-cmk and zVAD-fmk, on the other hand, abolished Shigella-induced apoptosis (Fig. 1) (6, 18) but did not protect intracellular Shigella from gentamicin. Therefore, caspase-independent processes seem to occur during Shigella-induced apoptosis that are controlled by TPPII.
It is unclear how TPPII participates in Shigella-induced apoptosis. The activity of TPPII seems not to be regulated during Shigella-mediated apoptosis (data not shown), and TPPII probably operates upstream of caspase-1 (Fig. 2). The Shigella invasin IpaB is sufficient to induce macrophage apoptosis and directly binds to and presumably activates caspase-1 (6, 19). Possible roles for TPPII might be to (i) form a complex with IpaB and caspase-1, directly promoting the activation of the caspase, (ii) degrade an inhibitor allowing cell death to be initiated by the IpaB–caspase-1 complex, or (iii) cleave caspase-1 substrates that are part of the apoptotic cascade. A more precise definition of the role of TPPII in apoptosis awaits the identification of its proapoptotic substrate(s).
TPPII is involved in apoptosis triggered by staurosporine and ATP.
TPPII might also participate in the apoptotic pathways triggered by staurosporine and ATP. AAF-cmk and lactacystin, but not proteasome peptide inhibitors, protected macrophages from apoptosis triggered by staurosporine and ATP (Fig. 4). The apoptotic pathways triggered by both ATP (23) and staurosporine (22) do not require caspase-1, suggesting that TPPII might also promote the activation of caspases other than caspase-1.
The participation of TPPII in apoptotic pathways is a previously unidentified function for this protease. In addition to its putative role in general protein turnover (40), TPPII also operates as a neuro-endopeptidase (33). Moreover, in EL-4 lymphoma cells adapted to proteasome inhibitors, TPPII or an as-yet-unidentified AAF-amc hydrolyzing activity seems to compensate for the functional loss of the proteasome (11, 12). Cell cycle progression, turnover of ubiquitinated proteins, and generation of some major histocompatibility complex class I-presented peptides was not impaired in these cells. However, it is presently unclear to what extent TPPII has the potential to substitute proteasomal functions. Like TPPII, the proteasome not only functions in immunoregulation but also in apoptosis (9, 13, 27). It currently appears that, depending on the proapoptotic stimulus, either TPPII, the proteasome, or both might participate in a particular apoptotic pathway.
ACKNOWLEDGMENTS
We thank D. Miller for kindly providing the antibody against caspase-1 and N. Thornberry for purified caspase-1. We are also indebted to D. Hersh for late-night technical assistance and A. Aliprantis, L. Devi, J. Moss, and Y. Weinrauch for critical reading of the manuscript.
H.H. was supported by a fellowship from the Swiss National Science Foundation. This work was supported by NIH grant AI42780 to A.Z.
REFERENCES
- 1.Bålöw R M, Ragnarsson U, Zetterqvist O. Tripeptidyl aminopeptidase in the extralysosomal fraction of rat liver. J Biol Chem. 1983;258:11622–11628. [PubMed] [Google Scholar]
- 2.Bålöw R M, Tomkinson B, Ragnarsson U, Zetterqvist O. Purification, substrate specificity, and classification of tripeptidyl peptidase II. J Biol Chem. 1986;261:2409–2417. [PubMed] [Google Scholar]
- 3.Baumeister W, Walz J, Zuhl F, Seemüller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 4.Bernardini M L, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogyo M, McMaster J S, Gaczynska M, Tortorella D, Goldberg A L, Ploegh H. Covalent modification of the active site threonine of proteasomal beta subunits and the Escherichia coli homolog HslV by a new class of inhibitors. Proc Natl Acad Sci USA. 1997;94:6629–6634. doi: 10.1073/pnas.94.13.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Smith M R, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by directly binding ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 7.Clerc P L, Ryter A, Mounier J, Sansonetti P J. Plasmid-mediated early killing of eukaryotic cells by Shigella flexneri as studied by infection of J774 macrophages. Infect Immun. 1987;55:521–527. doi: 10.1128/iai.55.3.521-527.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craiu A, Gaczynska M, Akopian T, Gramm C F, Fenteany G, Goldberg A L, Rock K L. Lactacystin and clasto-lactacystin beta-lactone modify multiple proteasome beta-subunits and inhibit intracellular protein degradation and major histocompatibility complex class I antigen presentation. J Biol Chem. 1997;272:13437–13445. doi: 10.1074/jbc.272.20.13437. [DOI] [PubMed] [Google Scholar]
- 9.Fenteany G, Schreiber S L. Lactacystin, proteasome function, and cell fate. J Biol Chem. 1998;273:8545–8548. doi: 10.1074/jbc.273.15.8545. [DOI] [PubMed] [Google Scholar]
- 10.Fenteany G, Standaert R F, Lane W S, Choi S, Corey E J, Schreiber S L. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 11.Geier E, Pfeifer G, Wilm M, Lucchiari-Hartz M, Baumeister W, Eichmann K, Niedermann G. A giant protease with potential to substitute for some functions of the proteasome. Science. 1999;283:978–981. doi: 10.1126/science.283.5404.978. [DOI] [PubMed] [Google Scholar]
- 12.Glas R, Bogyo M, McMaster J S, Gaczynska M, Ploegh H L. A proteolytic system that compensates for loss of proteasome function. Nature. 1998;392:618–622. doi: 10.1038/33443. [DOI] [PubMed] [Google Scholar]
- 13.Grimm L M, Osborne B A. Apoptosis and the proteasome. Results Probl Cell Differ. 1999;23:209–228. doi: 10.1007/978-3-540-69184-6_10. [DOI] [PubMed] [Google Scholar]
- 14.Grimm L G, Goldberg A L, Poirier G G, Schwartz L M, Osborne B A. Proteasomes play an essential role in thymocyte apoptosis. EMBO J. 1996;15:3835–3844. [PMC free article] [PubMed] [Google Scholar]
- 15.Hale T L. Genetic basis of virulence in Shigella species. Microbiol Rev. 1991;55:206–224. doi: 10.1128/mr.55.2.206-224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen M B, Nielsen S E, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 17.Hilbi H. Host responses to secreted Shigella virulence factors. Curr Opin Infect Dis. 1999;12:221–228. doi: 10.1097/00001432-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1β-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun. 1997;65:5165–5170. doi: 10.1128/iai.65.12.5165-5170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilbi H, Moss J E, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell R A, Yuan J, Sansonetti P J, Zychlinsky A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem. 1998;273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch T, Dallaporta B, Zamzami N, Susin S A, Ravagnan L, Marzo I, Brenner C, Kroemer G. Proteasome activation occurs at an early, premitochondrial step of thymocyte apoptosis. J Immunol. 1998;161:35–40. [PubMed] [Google Scholar]
- 21.Hogquist K A, Nett M A, Unanue E R, Chaplin D D. Interleukin-1 is processed and released during apoptosis. Proc Natl Acad Sci USA. 1991;88:8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobsen M D, Weil M, Raff M C. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol. 1996;133:1041–1051. doi: 10.1083/jcb.133.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, Towne E, Tracey D, Wardwell S, Wei F-Y, Wong W, Kamen R, Seshadri T. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 24.Macpherson E, Tomkinson B, Bålöw R M, Hoglund S, Zetterqvist O. Supramolecular structure of tripeptidyl peptidase II from human erythrocytes as studied by electron microscopy, and its correlation to enzyme activity. Biochem J. 1987;248:259–263. doi: 10.1042/bj2480259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ménard R, Dehio C, Sansonetti P J. Bacterial entry into epithelial cells: the paradigm of Shigella. Trends Microbiol. 1996;4:220–226. doi: 10.1016/0966-842X(96)10039-1. [DOI] [PubMed] [Google Scholar]
- 26.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti P J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992;60:237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orlowski R Z. The role of the ubiquitin-proteasome pathway in apoptosis. Cell Death Differ. 1999;6:303–313. doi: 10.1038/sj.cdd.4400505. [DOI] [PubMed] [Google Scholar]
- 28.Page A E, Fuller K, Chambers T J, Warburton M J. Purification and characterization of a tripeptidyl peptidase I from human osteoclastomas: evidence for its role in bone resorption. Arch Biochem Biophys. 1993;306:354–359. doi: 10.1006/abbi.1993.1523. [DOI] [PubMed] [Google Scholar]
- 29.Prévost M C, Lesourd M, Arpin M, Vernel F, Mounier J, Hellio R, Sansonetti P J. Unipolar reorganization of F-actin layer at bacterial division and bundling of actin filaments by plastin correlate with movement of Shigella flexneri within HeLa cells. Infect Immun. 1992;60:4088–4099. doi: 10.1128/iai.60.10.4088-4099.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raff M C, Barres B A, Burne J F, Coles H S, Ishizaki Y, Jacobson M D. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- 31.Rock K L, Goldberg A L. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu Rev Immunol. 1999;17:739–779. doi: 10.1146/annurev.immunol.17.1.739. [DOI] [PubMed] [Google Scholar]
- 32.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 33.Rose C, Vargas F, Facchinetti P, Bourgeat P, Bambal R B, Bishop P B, Chan S M, Moore A N, Ganellin C R, Schwartz J C. Characterization and inhibition of a cholecystokinin-inactivating serine peptidase. Nature. 1996;380:403–409. doi: 10.1038/380403a0. [DOI] [PubMed] [Google Scholar]
- 34.Sadoul R, Fernandez P-A, Quiquerez A-L, Martinou I, Maki M, Schröter M, Becherer J D, Irmler M, Tschopp J, Martinou J C. Involvement of the proteasome in the programmed cell death of NGF-deprived sympathetic neurons. EMBO J. 1996;15:3845–3852. [PMC free article] [PubMed] [Google Scholar]
- 35.Sansonetti P J, Kopecko D J, Formal S B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982;35:852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sansonetti P J, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, Takeda K, Zychlinsky A. Caspase-1 activation of IL-1β and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–590. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 37.Thirumalai K, Kim K, Zychlinsky A. IpaB, a Shigella flexneri invasin, colocalizes with interleukin-1β converting enzyme (ICE) in the cytoplasm of macrophages. Infect Immun. 1997;65:787–793. doi: 10.1128/iai.65.2.787-793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thornberry N A. Interleukin-1β converting enzyme. Methods Enzymol. 1994;244:615–631. doi: 10.1016/0076-6879(94)44045-x. [DOI] [PubMed] [Google Scholar]
- 39.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 40.Tomkinson B. Tripeptidyl peptidases: enzymes that count. Trends Biochem Sci. 1999;24:355–359. doi: 10.1016/s0968-0004(99)01435-8. [DOI] [PubMed] [Google Scholar]
- 41.Vines D, Warburton M J. Purification and characterization of a tripeptidyl aminopeptidase I from rat spleen. Biochim Biophys Acta. 1998;1384:233–242. doi: 10.1016/s0167-4838(98)00012-0. [DOI] [PubMed] [Google Scholar]
- 42.Zychlinsky A, Kenny B, Ménard R, Prévost M C, Holland I B, Sansonetti P J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 43.Zychlinsky A, Prévost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–168. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 44.Zychlinsky A, Sansonetti P J. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]
- 45.Zychlinsky A, Thirumalai K, Arondel J, Cantey J R, Aliprantis A, Sansonetti P J. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64:5357–5365. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zychlinsky A, Fitting C, Cavaillon J M, Sansonetti P J. Interleukin-1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J Clin Investig. 1994;94:1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]