Abstract
Skeletal muscle atrophy is represented by a dramatic decrease in muscle mass, and it is related to a lower life expectancy. Among the different causes, chronic inflammation and cancer promote protein loss through the effect of inflammatory cytokines, leading to muscle shrinkage. Thus, the availability of safe methods to counteract inflammation-derived atrophy is of high interest. Betaine is a methyl derivate of glycine and it is an important methyl group donor in transmethylation. Recently, some studies found that betaine could promote muscle growth, and it is also involved in anti-inflammatory mechanisms. Our hypothesis was that betaine would be able to prevent tumor necrosis factor-α (TNF-α)-mediated muscle atrophy in vitro. We treated differentiated C2C12 myotubes for 72 hr with either TNF-α, betaine, or a combination of them. After the treatment, we analyzed total protein synthesis, gene expression, and myotube morphology. Betaine treatment blunted the decrease in muscle protein synthesis rate exerted by TNF-α, and upregulated Mhy1 gene expression in both control and myotube treated with TNF-α. In addition, morphological analysis revealed that myotubes treated with both betaine and TNF-α did not show morphological features of TNF-α-mediated atrophy. We demonstrated that in vitro betaine supplementation counteracts the muscle atrophy led by inflammatory cytokines.
Keywords: betaine, cytokines, hypertrophy, inflammation-mediated atrophy, morphometry, muscle wasting, myotubes, skeletal muscle, supplementation
Introduction
Skeletal muscle encompasses about 40% of total human body mass in a healthy-weight population, and it has a pivotal role not only in motor tasks like locomotion and breathing, but it is also fundamental in the regulation of energy expenditure and metabolic processes.1,2 Muscle mass is regulated by a solid balance between protein synthesis and degradation. When skeletal muscle protein synthesis exceeds protein degradation, hypertrophy occurs. Stimuli leading to muscle hypertrophy and health include physical exercise, proper nutrition, or pharmacological treatments.3–8 Conversely, if protein degradation occurs more rapidly than protein synthesis (i.e., net negative protein balance), skeletal muscle undergoes atrophy. 9 Skeletal muscle atrophy is a debilitating condition that occurs with aging, physical inactivity, poor nutritional habits, and importantly, it is a comorbidity in a plethora of diseases. 10 Among the different causes, inflammatory disease and cancer dramatically increase skeletal muscle catabolism, ultimately leading to muscle atrophy, and this condition contributes to negative patients’ prognosis, and both low expectancy and quality of life. 11 Muscle atrophy related to cancer and inflammatory diseases is mainly due to the increase of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α).12,13 TNF-α mainly plays its role by activating NF-κB signaling pathway, increasing the transcription of gene involved in inflammation. 14 In addition, TNF-α is able to increase the transcription of E3 ubiquitin ligases such as Atrogin and MuRF-1 in an NF-κB-dependent fashion. It is well documented that an increased synthesis of these ubiquitin ligases promotes the ubiquitination and the degradation of sarcomeric proteins by the ubiquitin-proteasome system, leading to muscle atrophy.15,16 As skeletal muscle mass and quality are important predictors of mortality, the need to find new molecules able to counteract inflammation-mediated atrophy is of primary importance.
Betaine is a substance naturally found in high concentration in different foods (e.g., beets, spinach, wheat bran). 17 It is a methyl group donor in the process of transmethylation of homocysteine, and it also works as an essential osmoprotectant mainly in the liver and kidneys. 18 Moreover, being a methyl donor, betaine is also known to affect DNA methylation, which is involved in the regulation of gene expression. 19 Betaine accomplishes its anti-inflammatory functions by regulating diverse pathways, including NF-κB and different downstream genes in various tissues.20–22
In addition, betaine supplementation can enhance muscle hypertrophy both in vitro and in vivo models. It has been shown that betaine promotes muscle protein synthesis via mammalian target of rapamycin complex 1 protein kinase, and this enhanced muscle hypertrophy, strength, and motor function in mice. 23 In another mice study, betaine supplementation increased muscle mass, promoted muscle formation, and regulated the ratio of fiber types in skeletal muscle. 24 Betaine promotes skeletal muscle fiber differentiation and myotube hypertrophy through the activation of the insulin growth factor-1 in vitro. 25 Nevertheless, human data on the effect of betaine on skeletal muscle hypertrophy are contrasting, but the number of studies including human subjects is still very low, and the selected populations are different.26,27
However, although in literature it is clear that betaine ameliorates inflammation and regulates skeletal muscle metabolism, the effect of betaine supplementation in muscle atrophy driven by inflammatory status has never been studied so far. Our hypothesis was that betaine would be able to prevent the atrophic effect mediated by the inflammatory stimulus provided by TNF-α. Thus, in this study, the effect of betaine on TNF-α-mediated muscle atrophy in differentiated myotubes was evaluated using a molecular and morphological approach, checking for total protein synthesis, gene expression, and muscle morphology.
Materials and Methods
Cell Culture and Experimental Design
C2C12 myoblasts were purchased from ATCC (Mannassan, VA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher Scientific, Waltham, MA) supplemented with 10% FBS (fetal bovine serum), 1% penicillin/streptomycin, and 2 mM l-glutamine. When cells reached ~85% of confluence, they were cultured in DMEM 2% FBS to start the differentiation phase.
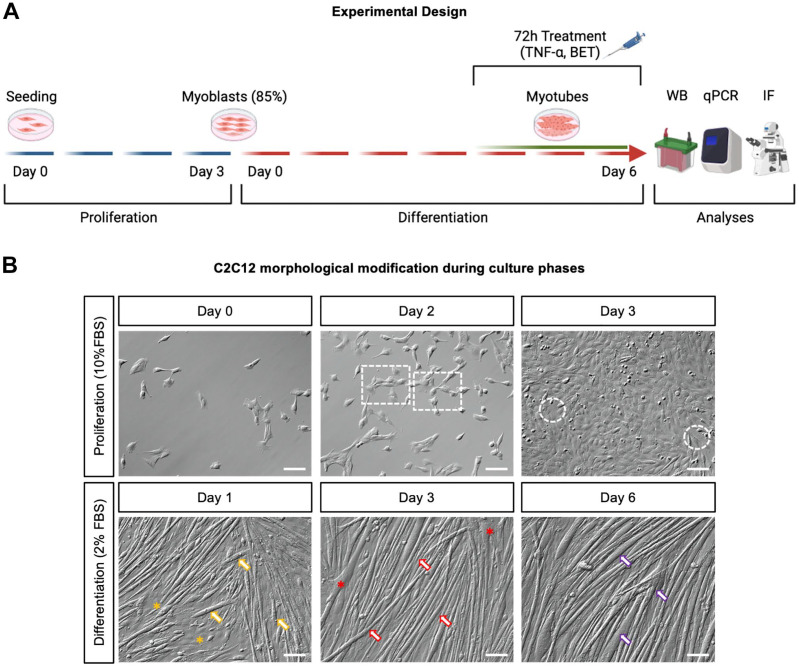
Treatments (TNF-α 25 ng/ml, betaine 10 mM, alone and in combination) were administered at the fourth day of differentiation (for 72 hr), until myotubes were completely mature in the cell culture. After 72 hr of treatment, Western blot, quantitative real-time polymerase chain reaction (qPCR), and immunohistochemistry were performed to evaluate total protein synthesis, mRNA transcription, and myotube morphology (Fig. 1A).
Figure 1.
(A) Experimental design. From day 0 (seeding day) to day 3 of the proliferation phase (highlighted by blue lines), myoblasts were cultured with DMEM 10% FBS. At day 3 of the proliferation phase, myoblasts reached ~85% of confluence, and they underwent differentiation phase in DMEM 2% FBS for 7 days (from day 0 to day 6, highlighted by red lines). From day 4 to day 6 of differentiation, myotubes were treated with 10 mM BET and TNF-α, alone or in combination. After 72 hr of treatment, WB, qPCR, and immunofluorescence were performed to evaluate total protein synthesis rate, mRNA content, myotube morphology, and architecture, respectively. (B) Proliferation and differentiation time course of C2C12. At day 0 of the proliferation phase (upper left panel), C2C12 myoblasts were present in culture as single cells or in small clusters formed by two to four cells. At day 2 of proliferation (upper central panel), myoblasts started to form bigger clusters (highlighted by white dashed rectangles), and at day 3 of the proliferation (upper right panel), cells reached 85–90% of confluency; indeed only small empty areas were visible (white dashed circles). Thus, the differentiation started when cells reached ~85% confluence. At day 1 of differentiation (lower left panel), short, immature myotubes were visible in culture (orange arrows), and some clusters of non-fused myoblasts were present as well (orange asterisks). At day 3 of differentiation (lower central panel), myotubes were longer (red arrows) but the diameter was not maximal yet, and several points of fusion were evident (red asterisks). At day 6 of the differentiation phase (i.e., the last day of treatment), the whole culture was represented by mature myotubes (lower right panel), where most of them reached the highest diameter (purple arrows). Scale bars, 100 µm. The experimental design picture (panel A) was created using BioRender.com. Abbreviations: DMEM, Dulbecco’s Modified Eagle Medium; FBS, fetal bovine serum; BET, betaine; TNF-α, tumor necrosis factor-α; WB, Western blot; qPCR: quantitative real-time polymerase chain reaction; IF, immunofluorescence.
SUnSET Method for Total Protein Synthesis Rate Determination
The Synthesis Measurement by Surface Sensing of Translation (SUnSET) method was used to measure total protein synthesis rate of myotubes following 72 hr of treatments. This method relies on the capacity of puromycin (that is a structural analogue of aminoacyl tRNA) to be incorporated in newly synthesized proteins and be measured by Western blot. Each well was incubated with 1 µM of puromycin (540411; Sigma Aldrich, St. Louis, MO) 1 hr before the end of the treatment.28,29 Myotubes were washed twice with 1 ml of cold PBS, and they were lysed in ice-cold RIPA Lysis and extraction buffer (89900; Thermo Fisher Scientific) containing 10 µl/ml Halt Protease and Phosphatase Inhibitor Single-Use Cocktail (78442; Thermo Fisher Scientific) as recommended by the manufacturer. The samples were left in ice for 5 min, and the plates were swirled occasionally to ensure uniform spreading of the lysis buffer. Protein lysate was collected and stored at −20C. Protein concentrations were determined with Pierce 660 nm Protein Assay Kit (22662; Thermo Fisher Scientific). In all, 20 µg of proteins were dissolved in 4× Bolt LDS Sample Buffer (B0007; Thermo Fisher Scientific), 10× Bolt Sample Reducing Agent (B0009; Thermo Fisher Scientific), and ddH2O. Then the samples were subjected to electrophoretic separation on NuPAGE 4–12%, Bis-Tris, 1.0 mm polyacrylamide gels using the Mini Gel Tank.
Proteins were transferred to a nitrocellulose membrane using the Power Blotter (Thermo Fisher Scientific). This step was verified by Ponceau Red staining. The membrane was blocked in 5% bovine serum albumin (BSA) in Tris-buffered saline-0.05% Tween 20 (TBST) for 15 min and then underwent an overnight incubation in primary antibody at 4C in TBST and a second incubation in fluorescent secondary antibody at room temperature for 1 hr.
The secondary antibody used was Alexa Fluor 488 anti-mouse (1:1000). The signal intensity was measured using iBright CL1500 Imaging System (Thermo Fisher Scientific). Protein quantifications were performed using iBright Analysis Software (Thermo Fisher Scientific). Results are expressed as puromycin intensity normalized by Ponceau Red staining.
RNA Extraction, Reverse Transcription, and qPCR
Cells were lysed with QIAzol lysis reagent (QIAGEN; Hilden, Germany). The total RNA was extracted using the miRNeasy Mini Kit (QIAGEN) according to the manufacturer’s procedure. For reverse transcription, 1 μg of RNA was retrotranscribed by the high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific). The primers used for the experiments are summarized in Table 1.
Table 1.
Primers Used for Quantitative Real-time Polymerase Chain Reaction.
| Gene | FWD—Sequence (5′-3′) | REV—Sequence (5′-3′) |
|---|---|---|
| 18S | CATGGCCGTTCTTAGTTGGT | CGCTGAGCCAGTCAGTGTAG |
| Myh7 | TGCCCCATATATACAGCCCCT | TGCTGAGGCTTCCTTTCTCG |
| Myh2 | TCCAAGTTCCGCAAGATCCA | GGGACAGCCTTACTCTTCGC |
| Myh1 | CGGTCGAAGTTGCATCCCTA | TAGTTCCGCCTTCGGTCTTG |
| ACTA1 | TCTTGTGTGTGACAACGGCT | GGGTCAGGATACCTCGCTTG |
For all the examined mRNAs, qPCR analysis was performed using SYBR green (PowerUp SYBR Green Master mix; Thermo Fisher Scientific) in Quant Studio 3 (Thermo Fisher Scientific) as previously reported. 30 Briefly, the samples were analyzed in triplicate in MicroAmp optical 96-well reaction plates (Thermo Fisher Scientific) and 18S was used as a housekeeping gene. The samples were run as follows: step 1, 95C for 10 min; step 2, 95C for 15 sec; and step 3, 60C for 1 min. Steps 2 and 3 were repeated for 40 cycles. The authenticity of the PCR products was verified by the melt-curve analysis. For data analysis, the Ct value of all genes analyzed was normalized to the Ct of the housekeeping gene 18S, resulting in the ΔCt value. Next, the ΔΔCt value was obtained subtracting the ΔCt value of each experimental condition from ΔCt value of the control condition. Finally, the fold change was generated using the formula 2−ΔΔCt. The 18s primer sequence was obtained from a previous study, 31 while Myh7, Myh2, Myh1, and ACTA1 were designed using PrimerBlast.
Immunofluorescence and Morphometric Analysis
Immunofluorescence analysis was performed as previously described. 32 Briefly, cells were fixed with 4% paraformaldehyde for 10 min. After fixation, cells were washed three times with PBS, permeabilized with 0.5% Triton X-100 for 15 min, and incubated in 5% BSA for 20 min at room temperature. Cells were subsequently incubated overnight with antisarcomeric α-actinin (1:100; ab9465; Abcam, Cambridge, MA), and antimyosin (1:100; PA5-31466; Invitrogen, Carlsbad, CA) primary antibodies. Cells were washed three times with PBS and incubated 1 hr at room temperature with the appropriate secondary antibody conjugated with Alexa Fluor 488 and Alexa Fluor 546 (1:100; Invitrogen). Nuclei were counterstained with 4’,6-diamidino-2-phenyilndole (DAPI, Thermo Fisher Scientific).
Brightfield images were acquired using a Zeiss Axio vert.A1 equipped with Zeiss Axiocam 503 mono (Carl Zeiss; Jena, Germany) while fluorescent images were acquired using Evos M7000 (Thermo Fisher Scientific). The image analyses for immunofluorescence pictures were performed by Celleste Image Analysis Software (Thermo Fisher Scientific). Random fields were chosen to measure myotube diameter, and all the myotubes in the pictures were measured. The average diameter per myotube was calculated as the mean of 10 measurements taken along the length of the myotube. 33 Nuclei per fiber were calculated as total number of nuclei into the myotubes divided by the number of myotubes in the field. 34 Myotubes area was obtained tracing myotubes perimeter and the Celleste Image Analysis Software provided the results in µm2. Finally, the line profile function was used to evaluate the alternation of sarcomeric protein (α-actinin and myosin). A 10-µm line profile was used to visually evaluate sarcomere aspect and a Pearson’s correlation coefficient (r) between α-actinin and myosin fluorescence was also computed to numerically evaluate the protein alternation (i.e., a positive correlation indicates protein colocalization, while a 0 to negative correlation indicates that fluorescence peaks—that is, protein of interest—do not colocalize and thus their alternation pattern). For all the analysis, the researcher was blinded for the condition, and random areas were analyzed for sarcomeres architecture evaluation.
Statistical Analysis
The one-way analysis of variance (ANOVA) was used to check for difference between the experimental conditions for total protein synthesis, gene expression, area, diameter, and nuclei per fiber. When one-way ANOVA showed statistical significance, Tukey’s post hoc test was used for multiple comparisons. Pearson’s correlation coefficient (r) was used to evaluate the correlation between myosin and α-actinin pixels to evaluate their mutual relationship and thus sarcomere structure. All data are reported as mean ± standard deviation of n=3 replicates. Results were considered statistically significant when p<.05. All statistical analyses were performed with GraphPad PRISM Version 9.4.1 (GraphPad Software, San Diego, CA).
Results
Betaine Prevents the TNF-α-Mediated Decrease in Protein Synthesis
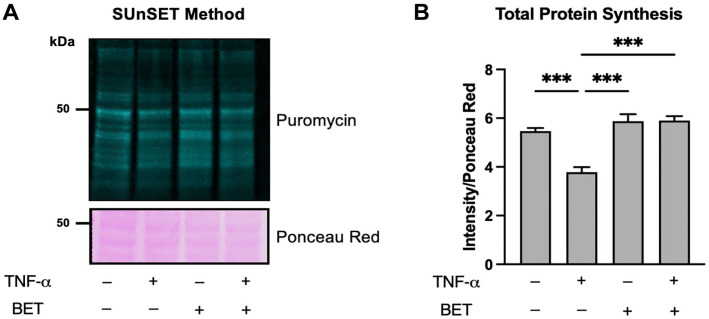
To quantify total protein synthesis rate, puromycin intensity was normalized for Ponceau Red staining (Fig. 2A). Statistical analysis showed that TNF-α treatment significantly decreased total protein synthesis rate compared with control, betaine, and TNF-α + betaine condition (Fig. 2B). Nevertheless, betaine and TNF-α + betaine conditions did not exhibit significant differences compared with control although they showed slightly higher values (Fig. 2B). Overall, betaine blunted the decrease in total protein synthesis rate exerted by TNF-α treatment.
Figure 2.
(A) Representative SUnSET image showing puromycin signal (green, fluorescent lanes) indicating total protein synthesis rate in the different conditions and Ponceau Red (pink bands) used for data normalization. (B) Histograms representing statistical analysis performed to determine differences in total protein synthesis rate. Data are reported as mean ± standard deviation. Differences were considered significant when p<0.05. Abbreviations: SUnSET, Synthesis Measurement by Surface Sensing of Translation; TNF-α, tumor necrosis factor-α; BET, betaine.*p<0.05, **p<0.01, ***p<0.001.
TNF-α and Betaine Selectively Regulate the mRNA Content of Myosin Heavy Chains and Sarcomeric Actin
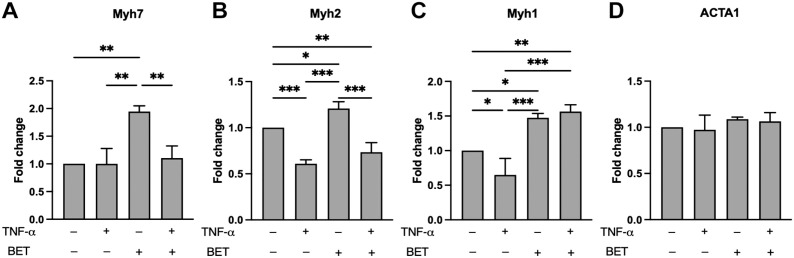
The gene expression profiles of Myh7, Myh2, Myh1, and ACTA1 are presented in Fig. 3. The mRNA content of Myh7 (encoding for myosin heavy chain type I) was significantly increased by betaine, compared with control, TNF-α, and TNF-α + betaine as well (Fig. 3A).
Figure 3.
Gene expression profiles of Myh7 (A), Myh2 (B), Myh1 (C), and ACTA1 (D). Data are reported as mean ± standard deviation. Differences were considered significant when p<0.05. Abbreviations: TNF-α, tumor necrosis factor-α; BET, betaine. *p<0.05, **p<0.01, ***p<0.001.
Betaine treatment increased Myh2 (encoding for myosin heavy chain type IIa) expression compared with control, TNF-α, and TNF-α + betaine (Fig. 3B). In addition, Myh2 was significantly less expressed in TNF-α and TNF-α + betaine compared with control (Fig. 3B). Similarly, TNF-α treatment decreased Myh1 expression compared with control (Fig. 3C). In addition, the mRNA content of Myh1 was significantly higher in both, betaine and TNF-α + betaine, compared with control and TNF-α alone (Fig. 3C). On the contrary, ACTA1 expression was not affected by any treatment (Fig. 3D).
Betaine Supplementation Reverts the Decrease in Myotubes Area and Diameter, and Sarcomere Structure Modifications Mediated by TNF-α
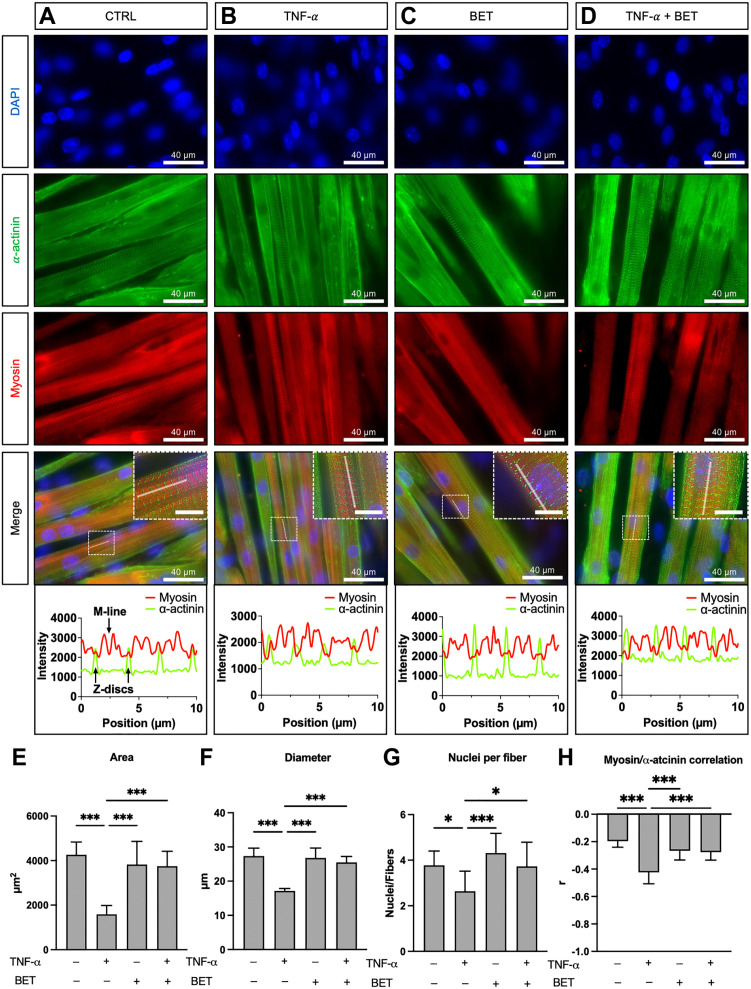
Myotubes immunofluorescence images and statistical analysis for area, diameter, nuclei per fiber, and myosin-α-actinin correlation are showed in Fig. 4. TNF-α treatment significantly decreased myotubes area, diameter, and nuclei per fiber compared with control condition and betaine treatment (Fig. 4E to G, respectively). In addition, myotubes treated with TNF-α showed a different sarcomere architecture as depicted from a difference in the alternation between α-actinin and myosin arrangement (Fig. 4H). On the contrary, when betaine was added along with TNF-α, an increase in area, diameter, and nuclei per fiber occurred, and all these variables were not different compared with control and betaine conditions. Moreover, the sarcomere architecture returned similar to control levels in TNF-α + betaine condition (Fig. 4H) as demonstrated by the line profile graph. Indeed, the α-actinin fluorescence peaks (representing Z discs) were located at the lowest myosin fluorescent peaks (Fig. 4D, bottom right panel showing the line profile results on the XY graph) as in the control and betaine conditions.
Figure 4.
Immunofluorescence and morphology analysis. Control (A), TNF-α (B), betaine (C), and TNF-α + betaine (D). In the line profile panels showed on the XY graphs, the green fluorescence peaks (α-actinin) represent Z discs, that are localized in between two red fluorescence peaks (myosin), that represent the thick filament portion of two adjacent sarcomeres. The M-line is denoted by a sharp decrease of myosin fluorescence intensity as myomesin (and not myosin) is abundant in that sarcomere region. Pearson’s r denotes the correlation coefficient between red (myosin) and green (α-actinin) pixels, and it was used to assess possible effect of the treatment on the physiological alternance between these two proteins. Scale bars: 40 µm and 10 µm (inserts). Ten µm of line profile is showed to highlight the sarcomeric protein alternation. Histograms report area (E), diameter (F), nuclei per fiber (G), and myosin/α-actinin correlation (H). Data are reported as mean ± standard deviation. Differences were considered significant when p<0.05. Abbreviations: TNF-α, tumor necrosis factor-α; BET, betaine. *p<0.05, ***p<0.001.
Discussion
Main Findings
In this study, the effect of sustained in vitro betaine treatment (72 hr) on TNF-α-mediated muscle atrophy was investigated on C2C12 differentiated myotubes. The most important findings were that (1) betaine was able to prevent the decreased total muscle protein synthesis mediated by TNF-α; (2) betaine increased the expression of all the analyzed gene encoding myosin heavy chains compared with control, but only avoided the decrease in Myh1 led by TNF-α; and (3) betaine provided hypertrophy on myotubes treated with TNF-α, showing a protective effect on muscle atrophy mediated by inflammatory stimuli.
Betaine Is Able to Restore Normal Muscle Protein Synthesis Rate in TNF-α-Treated Myotubes
Different studies showed that betaine supplementation can enhance skeletal muscle protein synthesis, differentiation, and hypertrophy in vitro.23–25 However, the mechanisms by which betaine regulates muscle mass are still not fully elucidated. Betaine acts as a methyl donor and may increase muscle creatine availability 35 and can activate the canonical pathway of protein kinase B-mechanistic target of rapamycin. 36 Importantly, betaine affects DNA methylation, which is highly involved in the regulation of gene expression. 37 After 12 weeks intervention, mice treated with betaine displayed a 1.3- to 1.5-fold higher protein synthesis rate (measured by SUnSET method) than mice in age-matched control group. 23 Moreover, the same study showed that betaine increased muscle protein synthesis and hypertrophy in a time-dependent manner. 23 To treat myotubes, a concentration of 10 mM betaine has been chosen based on previous works performing dose-response on cell viability, proliferation, and differentiation of C2C12. In addition, 10 mM betaine showed the highest hypertrophic potential in vitro as well. 25
We detected an increase in muscle protein synthesis compared with myotubes treated with TNF-α but not compared with control condition. This could suggest that, in vitro, after 72 hr, the hypertrophic stimulus lead by betaine is sufficient to prevent the catabolic effect of TNF-α and lead protein synthesis at normal levels but is not able to provide a clear increase in protein synthesis compared with control. These results can reflect several in vivo data; indeed, betaine supplementation in moderately trained healthy subjects did not show further improvement in strength and muscle hypertrophy after 6 weeks of training. 38 However, the number of studies investigating the effects of betaine supplementation on hypertrophy in humans is limited and controversial.
Numerous evidence has shown that betaine exerts anti-inflammatory functions in different diseases. 22 To provide an atrophic stimulus, we treated C2C12 myotubes with the pro-inflammatory cytokine TNF-α, which is known to activate NF-κB signaling, increasing the transcription of inflammatory genes and enhancing catabolism in skeletal muscle. 39 When we added betaine to TNF-α-treated myotubes, the total protein synthesis rate increased, returning similar to control levels, thus inhibiting the catabolic action of TNF-α. This effect is probably explained by the fact that betaine performs its anti-inflammatory effect mainly by inhibiting NF-κB activity. 20 In addition, it has been shown that betaine inhibits upstream molecules that induce NF-κB activation. Indeed, betaine prevented the activation of NF-κB induced by lipopolysaccharide in murine macrophage cells. 40 Thus, in skeletal muscle, the well-known inhibition of NF-κB provided by betaine could prevent the catabolic events linked to NF-κB pathway activation.
TNF-α and Betaine Regulate Gene Expression in a Selective Manner
Our data showed that TNF-α and betaine regulated differently the gene expression of Myh7, Myh2, and Myh1, whereas ACTA1 (encoding for sarcomeric actin) was not affected by either treatment. It is well known that atrophy caused by chronic inflammation includes a diminished gene and protein expression of myosin heavy chains, and this affects muscle mass and function. 41 O’Brien and colleagues 42 showed that the expression of the early and late differentiation markers MyoG and myosin heavy chain was significantly reduced with TNF-α at day 5 of differentiation in primary human myoblasts. In C2C12 differentiated myotubes, treatments with both TNF-α + IFN-γ led to a significant decrease in the expression of the myofibrillar protein myosin heavy chain, and the inhibition of NF-κB blocked cytokine-mediated decreases in myosin heavy chain. 43 In this study, TNF-α decreased Myh2 and Myh1 (both genes encoding fast-type myosin heavy chain proteins) gene expression compared with control, while Myh7 (encoding slow-type myosin heavy chain protein) was not affected by the inflammatory cytokine. On the contrary, different studies showed that betaine is able to increase gene expression of myosin heavy chains. Betaine treatment increased the MyHC expression about 1.5-fold and 2.3-fold of 15- and 18-month-old mice, respectively. 23 Similarly, in a previous study in which C2C12 myoblasts were treated with 10 mM betaine, the expression of genes encoding for diverse myosin heavy chains (Myh7 and Myh1) isoforms was differently regulated. 24 Moreover, despite the changes in myosin transcripts, we did not detect modification in ACTA1 gene: This is unsurprising as even if both Myh and ACTA1 encode for sarcomeric proteins, betaine can regulate in a different way (up, down, or not modified) the gene expression of highly related proteins. 24
Here, we report that 10 mM betaine treatment increased gene expression of Myh7, Myh2, and Myh1 compared with control condition. Moreover, when added along with TNF-α, betaine was able to prevent the decrease in Myh1 led by TNF-α. Thus, in accordance with the existing literature, we have seen that TNF-α regulates differently the various myosin heavy chain isoforms and that 72 hr betaine treatment is able to prevent the decrease in fast-type myosin transcript.
Betaine Exerts a Hypertrophic Action and Restores Morphology on TNF-α-Treated Myotubes
In this study, the results obtained for total protein synthesis rate were reflected in the morphological analysis as well. Indeed, TNF-α exerted its atrophic effect on myotubes, decreasing both area and diameter. However, when betaine was added along with TNF-α, the atrophic effect was blunted, thus myotubes area and diameter increased, being similar to control and betaine conditions. The same trend was seen for the number of nuclei per fiber, indicating that betaine restores the normal myonuclear fusion activity. Consistently, fusion index, myotube length, and myotube area were higher in muscle cells treated with betaine than in the controls. 24 Also, Senesi and colleagues 25 showed that betaine supplementation enhanced C2C12 myoblast differentiation in vitro; in addition, they observed that 10 mM betaine supplementation induced a higher number of newly formed myotubes, which were longer than control. Moreover, betaine treatment increased the cross-sectional area of the quadricep muscle about 1.4-fold and 1.3-fold in 15- and 18-months mice, respectively. 23 Taken together, these data suggested that betaine supplementation promotes skeletal muscle differentiation and hypertrophy both in vitro and in vivo. However, in our setting, betaine had hypertrophic effect in myotubes treated with TNF-α only, showing a protective effect on inflammation-derived atrophy but not a net effect in control myotubes. These findings are not in line with previous studies showing a hypertrophic effect of betaine in physiological muscle condition 23 ; however, these discrepancies could be due to the highly different experimental models employed (cells exposed for 72 hr vs. animals supplemented with a long-term diet).
Other than being thinner and containing a lower number of nuclei, we have seen that myotubes treated with TNF-α had disorganized sarcomeres as shown by a modification on the alternation of myosin and α-actinin proteins. The components of the sarcomeric contractile apparatus are in a dynamic homeostasis by means of highly regulated protein turnover and assembly. Fine-tuned mechanisms (i.e., gene expression and post-translational modifications) ensure the physiological arrangement and function of the contractile apparatus. 44 It has been shown that during inflammation-derived atrophy, pro-inflammatory cytokine (i.e., TNF-α and IFN-γ) signaling pathway leads to SENP3 protein degradation. Depleted SENP3 protein level ultimately leads to reduced myosin heavy chain gene expression. In turn, low levels of myosin protein disrupt the sarcomere assembly and affect muscle function. 45
Accordingly, in our study, Myh2 and Myh1 mRNA decreased when myotubes were treated with TNF-α, and simultaneously microscopy analysis highlighted the disarrangement of the contractile apparatus, modifying the normal alternation between myosin and actinin probably due to the shrinkage of myosin protein. On the contrary, betaine treatment reverted the effect of TNF-α, restoring the normal sarcomere arrangement. Interestingly, it has been highlighted that in a different cellular model, betaine is able to inhibit NF-κB pathway 21 : As NF-κB represents the main effector of the TNF-α signaling, mechanistic studies should be performed on skeletal muscle cells to determine whether betaine is able to antagonize the TNF-α-induced muscle atrophy by contrasting NF-κB pathway activation.
In conclusion, betaine treatment has shown to prevent the atrophic effect of TNF-α, mainly regulating protein synthesis rate, myosin gene expression, hypertrophy, myonuclear fusion, and sarcomere architecture. Nonetheless, more research is needed to figure out if betaine should be included in the management of inflammation-induced atrophy and in normal population as well.
Practical Applications
The inclusion of betaine supplementation could be useful in contrasting inflammation-induced muscle atrophy. However, the number of existing studies on the effect of betaine on skeletal muscle is considerably low and more research (both in vivo and in vitro) is needed to clarify the effect and usefulness of betaine supplementation. Although the effect of betaine on in vivo models is controversial, with our morphological and molecular approach, we demonstrate that betaine prevents the atrophic effect of TNF-α on skeletal muscle cells.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Conceptualization: ADC, GG, and ADB; Methodology: ADC, GG, and PI; Data analysis: ADC, PI, and DV; Investigation: ADC and GG; Resources: ADB and BG; Writing and original draft preparation: ADC and GG; writing, review, and editing: PB, ADB, and BG; Supervision: ADB and BG; funding acquisition, ADB. All authors have read and agreed to the published version of the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was granted by MUR ex 60% funds.
ORCID iDs: Andrea Di Credico  https://orcid.org/0000-0002-8388-9305
https://orcid.org/0000-0002-8388-9305
Pascal Izzicupo  https://orcid.org/0000-0001-6944-8995
https://orcid.org/0000-0001-6944-8995
Pasqualina Buono  https://orcid.org/0000-0003-0755-6143
https://orcid.org/0000-0003-0755-6143
Angela Di Baldassarre  https://orcid.org/0000-0002-4473-4909
https://orcid.org/0000-0002-4473-4909
Barbara Ghinassi  https://orcid.org/0000-0002-3529-2790
https://orcid.org/0000-0002-3529-2790
Contributor Information
Andrea Di Credico, Department of Medicine and Aging Sciences, “G. D’Annunzio” University of Chieti-Pescara, Chieti, Italy; Reprogramming and Cell Differentiation Lab, Center for Advanced Studies and Technology, Chieti, Italy.
Giulia Gaggi, Department of Medicine and Aging Sciences, “G. D’Annunzio” University of Chieti-Pescara, Chieti, Italy; Reprogramming and Cell Differentiation Lab, Center for Advanced Studies and Technology, Chieti, Italy.
Pascal Izzicupo, Department of Medicine and Aging Sciences, “G. D’Annunzio” University of Chieti-Pescara, Chieti, Italy.
Daniela Vitucci, Department of Movement Sciences and Wellness, University Parthenope, Napoli, Italy; CEINGE-Biotecnologie Avanzate Franco Salvatore, Napoli, Italy.
Pasqualina Buono, Department of Movement Sciences and Wellness, University Parthenope, Napoli, Italy; CEINGE-Biotecnologie Avanzate Franco Salvatore, Napoli, Italy.
Angela Di Baldassarre, Department of Medicine and Aging Sciences, “G. D’Annunzio” University of Chieti-Pescara, Chieti, Italy; Reprogramming and Cell Differentiation Lab, Center for Advanced Studies and Technology, Chieti, Italy.
Barbara Ghinassi, Department of Medicine and Aging Sciences, “G. D’Annunzio” University of Chieti-Pescara, Chieti, Italy; Reprogramming and Cell Differentiation Lab, Center for Advanced Studies and Technology, Chieti, Italy.
Literature Cited
- 1.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997Jul;77(3):731–58. [DOI] [PubMed] [Google Scholar]
- 2.Baskin KK, Winders BR, Olson EN. Muscle as a “mediator” of systemic metabolism. Cell Metab. 2015Feb3;21(2):237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res. 2010Oct;24(10):2857–72. [DOI] [PubMed] [Google Scholar]
- 4.Jäger R, Kerksick CM, Campbell BI, Cribb PJ, Wells SD, Skwiat TM, Purpura M, Ziegenfuss TN, Ferrando AA, Arent SM, Smith-Ryan AE, Stout JR, Arciero PJ, Ormsbee MJ, Taylor LW, Wilborn CD, Kalman DS, Kreider RB, Willoughby DS, Hoffman JR, Krzykowski JL, Antonio J.International Society of Sports Nutrition Position Stand: protein and exercise. J Int Soc Sports Nutr. 2017Jan3;14(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen S, Reitelseder S, Kalsen A, Kreiberg M, Onslev J, Gad A, Ørtenblad N, Backer V, Holm L, Bangsbo J, Hostrup M. β 2-adrenergic agonist salbutamol augments hypertrophy in MHCIIa fibers and sprint mean power output but not muscle force during 11 weeks of resistance training in young men. J Appl Physiol. 2021Mar1;130(3):617–26. [DOI] [PubMed] [Google Scholar]
- 6.Di Credico A, Gaggi G, Ghinassi B, Mascherini G, Petri C, Di Giminiani R, Di Baldassarre A, Izzicupo P. The influence of maturity status on anthropometric profile and body composition of youth goalkeepers. IJERPH. 2020Nov8;17(21):8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condello G, Capranica L, Stager J, Forte R, Falbo S, Di Baldassarre A, Segura-Garcia C, Pesce C.Physical activity and health perception in aging: do body mass and satisfaction matter? A three-path mediated link. López Lluch G, editor. PLoS ONE. 2016Sep9;11(9):e0160805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falone S, Mirabilio A, Passerini A, Izzicupo P, Cacchio M, Gallina S, Baldassarre AD, Amicarelli F.Aerobic performance and antioxidant protection in runners. Int J Sports Med. 2009Nov;30(11):782–8. [DOI] [PubMed] [Google Scholar]
- 9.Sartori R, Romanello V, Sandri M.Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun. 2021Jan12;12(1):330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanzani A, Conraads VM, Penna F, Martinet W.Molecular and cellular mechanisms of skeletal muscle atrophy: an update. J Cachexia Sarcopenia Muscle. 2012Sep;3(3):163–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE.Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011May;12(5):489–95. [DOI] [PubMed] [Google Scholar]
- 12.Llovera M, García-Martínez C, López-Soriano J, Agell N, López-Soriano FJ, Garcia I, Argilés JM. Protein turnover in skeletal muscle of tumour-bearing transgenic mice overexpressing the soluble TNF receptor-1. Cancer Lett. 1998Aug14;130(1–2):19–27. [DOI] [PubMed] [Google Scholar]
- 13.Reid MB, Li YP.Tumor necrosis factor-alpha and muscle wasting: a cellular perspective. Respir Res. 2001;2(5):269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003Sep;3(9):745–56. [DOI] [PubMed] [Google Scholar]
- 15.Pijet B, Pijet M, Litwiniuk A, Gajewska M, Pająk B, Orzechowski A.TNF-α and IFN-s-dependent muscle decay is linked to NF-κ B- and STAT-1 α-stimulated Atrogin1 and MuRF1 genes in C2C12 myotubes. Mediators Inflamm. 2013;2013:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Martínez C, Agell N, Llovera M, López-Soriano FJ, Argilés JM. Tumour necrosis factor-alpha increases the ubiquitinization of rat skeletal muscle proteins. FEBS Lett. 1993Jun1;323(3):211–4. [DOI] [PubMed] [Google Scholar]
- 17.Zeisel SH, Mar MH, Howe JC, Holden JM.Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003May;133(5):1302–7. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann L, Brauers G, Gehrmann T, Häussinger D, Mayatepek E, Schliess F, Schwahn BC.Osmotic regulation of hepatic betaine metabolism. Am J Physiol Gastrointest Liver Physiol. 2013May1;304(9):G835–46. [DOI] [PubMed] [Google Scholar]
- 19.Lever M, Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem. 2010Jun;43(9):732–44. [DOI] [PubMed] [Google Scholar]
- 20.Go EK, Jung KJ, Kim JM, Lim H, Lim HK, Yu BP, Chung HY.Betaine modulates age-related NF-kappaB by thiol-enhancing action. Biol Pharm Bull. 2007;30(12):2244–9. [DOI] [PubMed] [Google Scholar]
- 21.Yi EY, Kim YJ. Betaine inhibits in vitro and in vivo angiogenesis through suppression of the NF-κB and Akt signaling pathways. Int J Oncol. 2012Nov;41(5):1879–85. [DOI] [PubMed] [Google Scholar]
- 22.Zhao G, He F, Wu C, Li P, Li N, Deng J, Zhu G, Ren W, Peng Y.Betaine in inflammation: mechanistic aspects and applications. Front Immunol. 2018May24;9:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Lu X, He T, Yishake D, Tan X, Hou M, Luo Y, Long J, Tang Z, Zhong R, Fang A, Zhu H. Betaine delayed muscle loss by attenuating Samtor complex inhibition for mTORC1 signaling via increasing SAM level. Mol Nutr Food Res. 2021Aug;65(15):2100157. [DOI] [PubMed] [Google Scholar]
- 24.Du J, Shen L, Zhang P, Tan Z, Cheng X, Luo J, Zhao X, Yang Q, Gu H, Jiang A, Ma J, Tang Q, Jin L, Shuai S, Li M, Jiang Y, Tang G, Bai L, Li X, Wang J, Zhang S, Zhu L. The regulation of skeletal muscle fiber-type composition by betaine is associated with NFATc1/MyoD. J Mol Med. 2018Jul;96(7):685–700. [DOI] [PubMed] [Google Scholar]
- 25.Senesi P, Luzi L, Montesano A, Mazzocchi N, Terruzzi I.Betaine supplement enhances skeletal muscle differentiation in murine myoblasts via IGF-1 signaling activation. J Transl Med. 2013Dec;11(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cholewa JM, Wyszczelska-Rokiel M, Glowacki R, Jakubowski H, Matthews T, Wood R, Craig SA, Paolone V. Effects of betaine on body composition, performance, and homocysteine thiolactone. J Int Soc Sports Nutr. 2013Jan3;10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwab U, Törrönen A, Toppinen L, Alfthan G, Saarinen M, Aro A, Uusitupa M.Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am J Clin Nutr. 2002Nov;76(5):961–7. [DOI] [PubMed] [Google Scholar]
- 28.Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, Hornberger TA. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 2011Mar;25(3):1028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halle JL, Counts-Franch BR, Prince RM, Carson JA. The effect of mechanical stretch on myotube growth suppression by colon-26 tumor-derived factors. Front Cell Dev Biol. 2021Jul30;9:690452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaggi G, Di Credico A, Guarnieri S, Mariggiò MA, Ballerini P, Di Baldassarre A, Ghinassi B.Human fetal membrane-mesenchymal stromal cells generate functional spinal motor neurons in vitro. iScience. 2022Oct;25(10):105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaggi G, Di Credico A, Izzicupo P, Alviano F, Di Mauro M, Di Baldassarre A, Ghinassi B. Human mesenchymal stromal cells unveil an unexpected differentiation potential toward the dopaminergic neuronal lineage. Int J Mol Sci. 2020Sep9;21(18):6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaggi G, Di Credico A, Guarnieri S, Mariggiò MA, Di Baldassarre A, Ghinassi B.Human mesenchymal amniotic fluid stem cells reveal an unexpected neuronal potential differentiating into functional spinal motor neurons. Front Cell Dev Biol. 2022Jul22;10:936990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001Nov;3(11):1009–13. [DOI] [PubMed] [Google Scholar]
- 34.Jones JM, Player DJ, Martin NRW, Capel AJ, Lewis MP, Mudera V.An assessment of myotube morphology, matrix deformation, and myogenic mRNA expression in custom-built and commercially available engineered muscle chamber configurations. Front Physiol. 2018May8;9:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman JR, Ratamess NA, Kang J, Rashti SL, Faigenbaum AD. Effect of betaine supplementation on power performance and fatigue. J Int Soc Sports Nutr. 2009Jan6;6(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apicella JM, Lee EC, Bailey BL, Saenz C, Anderson JM, Craig SAS, Kraemer WJ, Volek JS, Maresh CM. Betaine supplementation enhances anabolic endocrine and Akt signaling in response to acute bouts of exercise. Eur J Appl Physiol. 2013Mar;113(3):793–802. [DOI] [PubMed] [Google Scholar]
- 37.Anderson OS, Sant KE, Dolinoy DC.Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem. 2012Aug;23(8):853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moro T, Badiali F, Fabbri I, Paoli A.Betaine supplementation does not improve muscle hypertrophy or strength following 6 weeks of cross-fit training. Nutrients. 2020Jun5;12(6):1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatnagar S, Panguluri SK, Gupta SK, Dahiya S, Lundy RF, Kumar A.Tumor necrosis factor-α regulates distinct molecular pathways and gene networks in cultured skeletal muscle cells. Vij N, editor. PLoS ONE. 2010Oct12;5(10):e13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li JM, Ge CX, Xu MX, Wang W, Yu R, Fan CY, Kong LD.Betaine recovers hypothalamic neural injury by inhibiting astrogliosis and inflammation in fructose-fed rats. Mol Nutr Food Res. 2015Feb;59(2):189–202. [DOI] [PubMed] [Google Scholar]
- 41.Combaret L, Dardevet D, Béchet D, Taillandier D, Mosoni L, Attaix D.Skeletal muscle proteolysis in aging. Curr Opin Clin Nutr Metab Care. 2009Jan;12(1):37–41. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien ME, Londino J, McGinnis M, Weathington N, Adair J, Suber T, Kagan V, Chen K, Zou C, Chen B, Bon J, Mallampalli RK.Tumor necrosis factor alpha regulates skeletal myogenesis by inhibiting SP1 interaction with cis-acting regulatory elements within the Fbxl2 gene promoter. Mol Cell Biol. 2020May28;40(12):e00040-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-κB is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003Jan;278(4):2294–303. [DOI] [PubMed] [Google Scholar]
- 44.Nayak A, Amrute-Nayak M.SUMO system—a key regulator in sarcomere organization. FEBS J. 2020Jun;287(11):2176–90. [DOI] [PubMed] [Google Scholar]
- 45.Nayak A, Lopez-Davila AJ, Kefalakes E, Holler T, Kraft T, Amrute-Nayak M.Regulation of SETD7 methyltransferase by SENP3 is crucial for sarcomere organization and cachexia. Cell Rep. 2019May;27(9):2725–36.e4. [DOI] [PubMed] [Google Scholar]