Abstract
The effectiveness of monophosphoryl lipid A (MPL) as a mucosal adjuvant was investigated following oral or intranasal (i.n.) administration of an aqueous adjuvant formulation of MPL (MPL-AF) added to soluble antigen or liposomal antigen or incorporated into liposomal antigen membranes. Groups of BALB/c female mice were immunized with 50 to 100 μg of free or liposomal Streptococcus mutans crude glucosyltransferase (C-GTF) with or without MPL-AF added to the vaccine or incorporated into the liposomal membrane. Plasma, saliva, vaginal wash, and fecal extract samples were collected biweekly following immunization and assessed for antigen-specific antibody activity by enzyme-linked immunosorbent assay (ELISA). Mice immunized by the i.n. route had higher levels of salivary, plasma, and vaginal immunoglobulin A (IgA) anti-C-GTF responses and higher levels of plasma IgG anti-C-GTF than the orally immunized groups. A second administration of the vaccine 14 weeks after the initial immunization resulted in an anamnestic response to C-GTF resulting in 10- and 100-fold increases in saliva and plasma IgA and plasma IgG, respectively (in the i.n. immunized groups). Mice receiving a second i.n. immunization with liposomal antigen and MPL-AF had higher salivary IgA anti-C-GTF responses than mice immunized with antigen plus MPL-AF or liposomal antigen (P < 0.05). Plasma IgG anti-C-GTF activity was highest in mice immunized by the i.n. route with antigen formulations containing MPL-AF (P < 0.05). These results demonstrate the effectiveness of MPL-AF as an adjuvant for potentiating mucosal and systemic immune responses to liposomal C-GTF following i.n. immunization.
Oral immunization with a variety of vaccines has been shown to induce disseminated secretory immune responses via the common mucosal immune system. However, often the responses are variable, transient, and low in magnitude. Recently, there has been much interest in determining the importance of Waldeyer's ring in humans as an induction site for mucosal responses, especially for responses in the upper respiratory tract and oral cavity (7). Experimental evidence has demonstrated that the nasal mucosa of mice contains nasal lymphoid tissue (NALT) (62), and it has been suggested that this tissue may be comparable to Waldeyer's ring in humans.
During the past several years, considerable effort has been devoted to the use of microbial antigens purified by in vitro culture or genetic recombination (i.e., subunit vaccines) for the development of new vaccines. These defined vaccines are considered safer than the whole microorganisms; however, they are often poorly immunogenic. Therefore, it has been necessary to utilize delivery vehicles and adjuvants to potentiate immune responses to these vaccine antigens. One of several approaches which are being investigated for effectiveness in augmenting immune responses to purified antigens is the use of liposomes (phospholipid artificial membrane vesicles) as a vehicle for antigen delivery (9, 33). It has been hypothesized that liposomes simulate biological membranes which can act as a vehicle for antigen delivery to immune processing cells for the induction of immune responses (37, 56). Numerous studies in various animal models have reported that intranasal (i.n.) immunization with liposomal vaccines results in increased antigen-specific antibody responses in pulmonary and oral secretions (1, 2, 4, 8, 13–15, 19).
Despite promising results in animals, human liposome immunization studies have not resulted in significant and persistent salivary responses. Therefore, recent attention has been given to the use of mucosal adjuvants such as nontoxic lipopolysaccharide (LPS). Monophosphoryl lipid A (MPL) has been used in humans as a systemic adjuvant and shown to potentiate responses to a coadministered antigen without causing toxic effects (17, 22, 51, 54). The mechanism(s) of MPL adjuvant effect appears to be the activation of macrophages and induction of cytokine synthesis (54), which result in increased immune responsiveness to relatively nonimmunogenic antigens, e.g., malarial sporozoite antigen (3, 43, 44, 57), gangliosides (42), polysaccharides (54), short synthetic peptides (16), and viral proteins (46, 47, 52). The studies with MPL (and other LPS preparations) have mostly used the systemic route; however, a study by Pierce and coworkers (39) reported that liposomal lipid A enhanced the mucosal response to enterically administered cholera toxin.
The purpose of this study was to determine the effectiveness of MPL in potentiating mucosal, especially salivary immune responses in mice to a Streptococcus mutans crude glucosyltransferase (C-GTF) antigen. In this study, we assessed differences in responses induced following nasal compared to oral immunization. Furthermore, differences in immune responses following i.n. immunization with free versus liposomal antigen with or without MPL were assessed.
MATERIALS AND METHODS
Bacteria, media, and reagents.
S. mutans serotype c strain GS-5 (F. Macrina, Virginia Commonwealth University, Richmond) was used to purify the GTF antigen. Stock cultures were maintained in glycerin/broth (50% [vol/vol]) at −80°C.
The components used for production of liposomes consist of d,l-α-dipalmitoyl phosphatidylcholine, cholesterol, and dicetylphosphate (obtained from Sigma Chemical Co., St. Louis, Mo.). Liposome-antigen preparations were suspended in phosphate-buffered saline (PBS). An aqueous, adjuvant formulation of MPL (MPL-AF) was provided by Corixa Corporation (Hamilton, Mont.). MPL was derived from the lipid A portion of LPS from Salmonella enterica serovar Minnesota R595 (20, 54). MPL-AF is an aqueous micellar suspension of MPL dispersed in dipalmitoyl phosphatidylcholine.
Heat-inactivated (56°C for 1 h) fetal calf serum (FCS; Flow Laboratories, Inc., Mclean, Va.) was used as the blocking reagent in the fecal extraction buffer and enzyme-linked immunosorbent assay (ELISA). Immunological reagents used for ELISA analysis consisted of standard calibrated pooled serum, unlabeled and biotin-conjugated rabbit anti-mouse immunoglobulin A (IgA) and IgG (Brookwood Biomedical, Birmingham, Ala.).
Antigen purification.
A C-GTF-enriched preparation was derived from S. mutans GS-5 grown in streptococcal defined medium (JRH Biosciences, Lenexa, Kans.) (55), as previously reported (12). Briefly, the culture supernatant containing C-GTF was concentrated by ultrafiltration (PLGC Pelicon Cassette System, 10,000-molecular-weight cutoff; Millipore Inc., Bedford, Mass.), followed by 60% ammonium sulfate precipitation and desalting of the resuspended pellet. The protein preparation was subjected to sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis (29), and the purity and enzymatic activity of the GTF were determined by Coomassie stain and periodic acid-Schiff stain following incubation of the gel with sucrose, as described by Mukasa et al. (34). Soluble C-GTF antigen for use as free antigen and for preparation of liposomal antigens for immunization was diluted in PBS to obtain a concentration of 500 μg/ml with or without 9 μg of MPL-AF per ml (C-GTF+MPL or C-GTF, respectively).
Liposomal and MPL preparations.
For liposome preparations, dipalmitoyl phosphatidylcholine, cholesterol, and dicetylphosphate were dissolved in chloroform at a molar ratio of 16:7:1, respectively. A lipid monolayer was formed in a round-bottom flask in an atmosphere of N2 by rotary evaporation and was dried by lyophilization. Liposomes were prepared by mixing C-GTF (500 μg/ml) with the lipid monolayer at 60°C, giving a total lipid concentration of 2 mg/ml. To incorporate MPL into the liposome membrane (MPL-L-C-GTF), MPL-AF was included in the aqueous antigen preparation at a concentration of 9 μg/ml. Following bath sonication (FS-14; Fisher Scientific, Norcross, Ga.), the resulting liposomes, which were heterogeneous in size, were made more homogeneous by filtration through a 5-μm-pore-size filter (Acrodisc; Gelman Scientific Co., Ann Arbor, Mich.; L-C-GTF), followed by microfiltration (Liposofast; Avestin Inc., Ottawa, Ontario, Canada) through a 100-nm-pore-size polycarbonate membrane (Poretics Corp., Livermore, Calif.). An immunization preparation consisting of MPL-AF and L-C-GTF was obtained by adding MPL-AF to a suspension of L-C-GTF to obtain a concentration of 9 μg/ml (MPL+L-C-GTF).
The liposome preparations were characterized by flow cytometry (FACStar; Becton Dickinson, Mountain View, Calif.), as previously described (10). Briefly, polystyrene 0.130-μm beads (Fluoresbrite beads; Polysciences Inc., Warrington, Pa.) were used as the standard to determine the relative size and homogeneity of the liposomes by flow cytometry. Forward scatter of the standard beads was recorded to establish the submicrometer gates. The percentage of particles (liposomes) in the established submicrometer region was determined by recording forward scatter of each liposome preparation. The purity, antigenicity, and biologic activity of C-GTF in liposome preparations were confirmed as described above for soluble C-GTF.
Immunization of mice.
Female BALB/c mice (6 to 8 weeks old) were used in this study. Mice were randomly allocated into groups of six and immunized by the oral or nasal route on days 0 and 1 (see Table 1 for experimental groups and doses). For oral immunization, mice were given 200 μl of the vaccine by gastric intubation, whereas for nasal immunization, mice were given multiple administrations of 10 to 15 μl of immunogen over a 2-h period with the aid of a micropipette (100-μl total volume). Animals were given boosters with the similar vaccine 14 weeks after the initial immunization. Blood, saliva, vaginal wash, and fecal samples were collected biweekly for 8 weeks and then prior to and following the booster. For blood collection, animals were anesthetized using ether vapor, and the retro-orbital plexus was punctured using heparinized capillary tubes. Plasma was collected after centrifugation and stored at −20°C until used for assessing antibody activity by ELISA. Saliva was collected with the aid of a Pasteur pipette after intraperitoneal injection of carbachol (Sigma; 5 μg in 0.05 ml) to stimulate flow. Vaginal wash was obtained by flushing the vagina twice with 50 μl of sterile saline. Saliva and vaginal wash were centrifuged, and the supernatants were frozen at −70°C. Fresh fecal pellets (3 to 4) were collected from each animal in separate tubes and processed as previously described (26). The extraction buffer for fecal samples contained 0.02% NaN3, 1 mM phenylmethylsulfonyl fluoride (Sigma), 0.005 M EDTA, and 2% FCS in borate-buffered saline (pH 8.2). Briefly, to each tube containing fecal pellets was added 0.6 ml of extraction buffer. The tubes were vortexed for 15 min and then centrifuged (13,000 × g, 10 min, 25°C), and the supernatants were collected and stored at −70°C until assayed for antibody activity.
TABLE 1.
Vaccine and immunization protocol used in this study
| Group | Dose (μg)a
|
Immunization route | |
|---|---|---|---|
| C-GTF | MPL-AF | ||
| L-C-GTF | 100 | Intragastric | |
| MPL+L-C-GTF | 100 | 1.8 | Intragastric |
| C-GTF | 50 | i.n. | |
| C-GTF+MPL | 50 | 0.9 | i.n. |
| L-C-GTF | 50 | i.n. | |
| MPL-L-C-GTF | 50 | 0.9 | i.n. |
| MPL+L-C-GTF | 50 | 0.9 | i.n. |
Mice were immunized with the dose indicated on days 0 and 1 and given boosters 14 weeks later (day 98).
All animal experiments performed in these studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Alabama at Birmingham.
ELISA.
An ELISA was used to determine relative concentrations of antibodies to C-GTF as previously described (11). Briefly, one-half of a polyvinyl chloride 96-well, flat-bottom microtiter plate (Dynatech Laboratories Inc., Chantilly, Va.) was coated overnight at room temperature with goat or rabbit anti-mouse IgA or IgG diluted in PBS, while the other half was coated with C-GTF (5 μg/ml in borate buffer [pH 9.6]). Following blocking with FCS (5% in PBS), 50 μl of five twofold dilutions of plasma, saliva, vaginal wash, or fecal extract (in duplicates) was added to wells. A calibrated mouse serum pool was used as the total immunoglobulin standard, and twofold serial dilutions were added in duplicate to individual wells which had been coated with anti-mouse IgG or IgA. Biotin-conjugated goat antiserum to mouse immunoglobulin (anti-IgA or -IgG; Southern Biotechnology, Inc., Birmingham, Ala.) was then added to appropriate wells. After incubation, wells were developed with streptavidin-peroxidase conjugate (0.4 μg/ml; Southern Biotechnology) followed by ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (Sigma) in citrate buffer. Color development (optical density [OD]) was recorded at 414 nm using an ELISA plate reader (Molecular Devices Corp., Menlo Park, Calif.). A four-parameter curve fitting program (Softmax; Molecular Devices) was used to construct reference curves for each ELISA plate from OD readings of the standard serum pool of known immunoglobulin concentration. Total immunoglobulin concentration and estimated concentrations of anti-C-GTF antibody in plasma, saliva, vaginal wash, and fecal extract samples were obtained from OD readings of the sample dilutions converted to nanograms per milliliter by multiplying values extrapolated from the standard curves (Softmax) by the dilution factor. In order to control for interplate variation, samples for each collection were analyzed on the same day. Plasma results are reported as nanograms of anti-C-GTF antibody activity per milliter, while saliva, vaginal wash, and fecal results were converted to ratios of IgA or IgG anti-C-GTF per total immunoglobulin levels to normalize for variation in sample collections.
Statistics.
The significance of differences in the means of antibody levels between groups was determined by analysis of variance (ANOVA) and Fisher probable least-squares difference (PLSD; P < 0.05) using JMP software (SAS Institute Inc., Cary, N.C.).
RESULTS
Salivary IgA anti-C-GTF response.
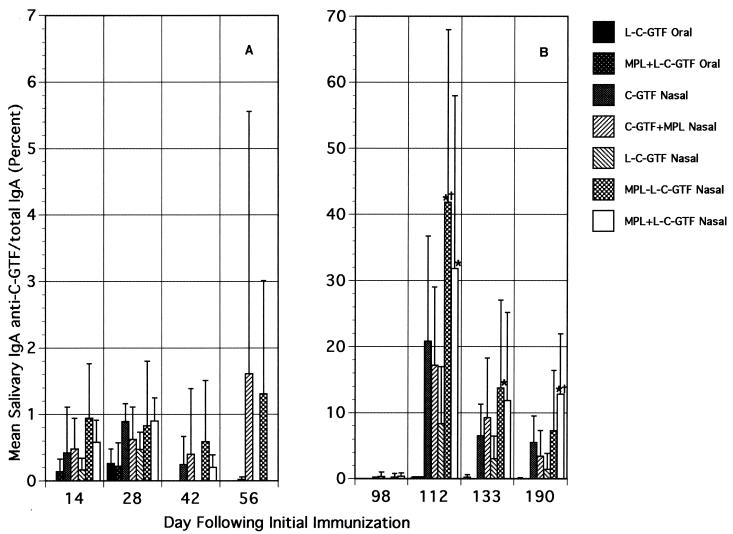
Differences in salivary IgA anti-C-GTF responses were observed on day 14 after immunization (Fig. 1A). However, no significant differences were detected between groups through day 56, except for the MPL-L-C-GTF i.n. immunized group compared to the L-C-GTF orally immunized group on day 14. The salivary IgA responses in mice immunized by the i.n. route with C-GTF+MPL and MPL-L-C-GTF persisted through 8 weeks (day 56) following initial immunization, whereas responses dropped in all other groups. By 14 weeks (day 98), only low or no salivary antibody activity was detected (Fig. 1B).
FIG. 1.
Salivary IgA antibody responses to C-GTF following initial (A) and booster (B) immunizations. BALB/c mice were immunized by the intragastric or i.n. route with C-GTF or L-C-GTF (with or without MPL-AF) on days 0 and 1 and given boosters on day 98. Values are the means + standard deviations of samples collected from animals on indicated days. ∗, significant difference in percent anti-C-GTF/total IgA compared to that seen in the i.n. immunized group given L-C-GTF. †, significant difference compared to C-GTF+MPL i.n. immunized group at a P value of <0.05 by ANOVA and Fisher PLSD.
A strong anamnestic response was observed by day 112 in all groups of i.n. immunized mice following a booster immunization on day 98, while no response was seen in the orally immunized animals (Fig. 1B). Two weeks after the booster immunization, salivary IgA anti-C-GTF activity in mice immunized by the nasal route was significantly higher than that seen in oral groups. Anti-C-GTF antibody activity diminished in the i.n. immunized groups; however, at all time points assessed, the mean antibody levels were higher in the i.n. immunized groups (compared to orally immunized groups). Furthermore, the MPL+L-C-GTF or MPL-L-C-GTF i.n. group or both had significantly (P < 0.05) higher mean levels of anti-C-GTF antibody activity than the other i.n. immunized groups at several time points following the booster (Fig. 1B). The salivary IgA responses following the nasal boost were more than 10-fold higher than those seen after the initial immunization. Although the responses decreased with time, they persisted for more than 3 months at a level higher than that seen after the first immunization.
Plasma anti-C-GTF response.
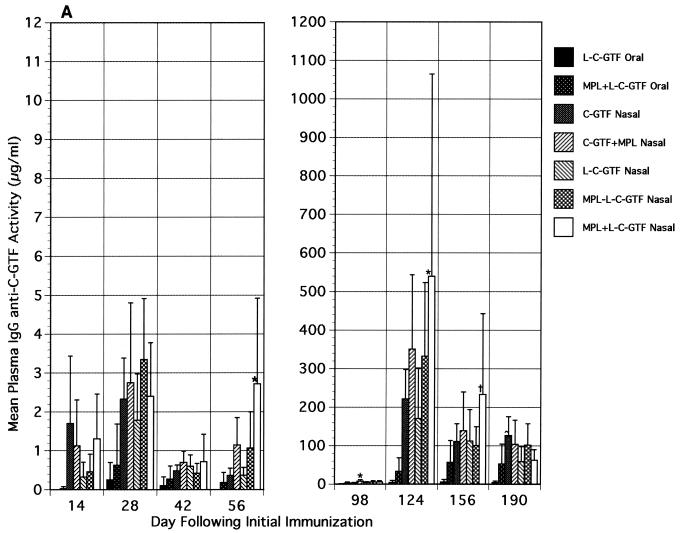
Plasma IgG (Fig. 2A) and, to a lesser extent, IgA (Fig. 2B) responses were induced in i.n. immunized mice following the primary and booster immunizations. The highest mean anti-C-GTF activity was observed in the i.n. immunized groups receiving antigen and MPL. Due to large variations within groups, many of the differences observed were not statistically significant. The IgG anti-C-GTF responses peaked on day 28 after the initial immunization in all groups and then decreased (day 42 [Fig. 2A]). However, the level of antibody activity on day 42 was maintained or increased from that time point until day 98 (prior to boost [Fig. 2A]). The mean specific antibody activity in plasma of i.n. immunized mice given liposomal antigen and MPL was significantly higher (P < 0.05) than that seen in mice orally immunized with L-C-GTF on day 28 (Fig. 2A). The response in the MPL+L-C-GTF i.n. immunized group was also significantly higher than that of the orally immunized groups on day 56. A significant but short-lived IgG response was also noted in the i.n. immunized group given antigen alone when compared to the orally immunized groups (day 14 [Fig. 2A]). An approximately 100-fold increase in plasma IgG anti-C-GTF antibody activity was observed 4 weeks (day 124) following the booster in the i.n. immunized animals (Fig. 2A). The boost also resulted in an anamnestic response in the group of mice orally immunized with MPL+L-C-GTF. The responses in i.n. immunized mice were significantly higher (P < 0.05) than those seen in L-C-GTF orally immunized mice when MPL was included in the i.n. immunogen (i.e., C-GTF+MPL, MPL-L-C-GTF, and MPL+L-C-GTF groups, day 124 [Fig. 2A]). The levels of IgG activity in the various groups decreased with time but remained high for 3 months (day 190) even for responses in mice orally immunized with MPL+L-C-GTF. Generally, responses in the i.n. immunized groups receiving MPL were higher than those seen in other i.n. immunized groups, except 3 months following the booster, when the anti-C-GTF activity was significantly higher in the groups immunized with antigen alone than in the i.n. group receiving MPL+L-C-GTF (Fig. 2A).
FIG. 2.
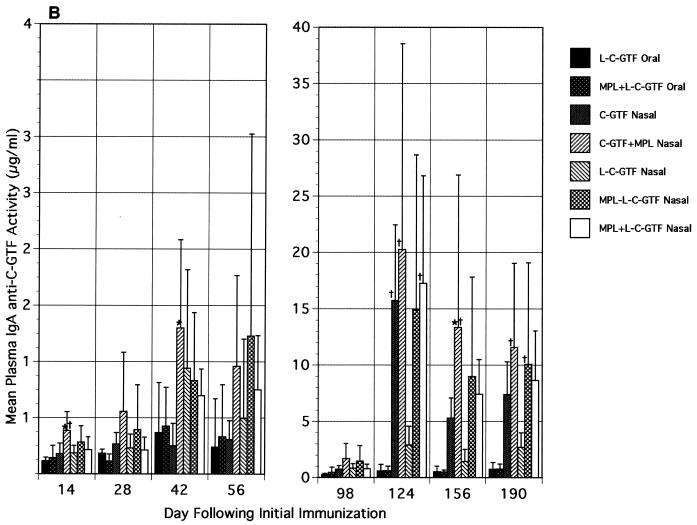
Plasma immunoglobulin antibody responses to C-GTF following initial and boost immunizations. (A) Plasma IgG antibody responses. BALB/c mice were immunized by the intragastric or i.n. route with C-GTF or L-C-GTF (with or without MPL-AF) on days 0 and 1 and given boosters on day 98. Values are the means + standard deviations of samples collected from animals on the indicated days. ∗, significant difference in anti-C-GTF activity compared to that seen in the i.n. immunized groups receiving C-GTF or L-C-GTF. †, difference compared to L-C-GTF and MPL-L-C-GTF i.n. immunized groups. ^, difference compared to L-C-GTF and MPL+L-C-GTF i.n. immunized groups at a P value of <0.05 by ANOVA and Fisher PLSD. (B) Plasma IgA antibody responses to C-GTF following initial and booster immunizations. BALB/c mice were immunized by the intragastric or i.n. route with C-GTF or L-C-GTF (with or without MPL-AF) on days 0 and 1 and given boosters on day 98. Values are the means + standard deviations of samples collected from animals on the indicated days. ∗, significant difference in anti-C-GTF activity compared to that seen in the i.n. immunized group receiving C-GTF. †, difference compared to L-C-GTF i.n. immunized group at a P value of <0.05 by ANOVA and Fisher PLSD.
The plasma IgA anti-C-GTF antibody responses peaked on day 42 (or day 56 for the MPL-L-C-GTF group) after the initial immunization (Fig. 2B). The C-GTF+MPL i.n. immunized group resulted in a significantly higher response than the orally immunized L-C-GTF group on days 14, 28, 56, and 98 (P < 0.05). As observed with plasma IgG responses, within the i.n. immunized groups, higher plasma IgA anti-C-GTF activity was observed in mice given antigen and MPL; however, differences between these groups were not significant. The responses for all groups were maintained above the day 14 level through day 98. Following the booster, a 10- to 20-fold increase was seen in the responses of all but one (L-C-GTF) of the i.n. immunized groups. The observed responses in the i.n. immunized groups decreased slightly during the next 3 months, while the orally immunized group responses remained low and constant. The responses in the i.n. immunized groups were significantly higher than the L-C-GTF orally immunized groups at several time points (Fig. 2B). These significantly higher responses were most consistently observed in the i.n. immunized groups receiving MPL. Furthermore, the i.n. immunized mice receiving a vaccine containing MPL had higher anti-C-GTF responses with some time points showing statistical significance than seen in mice receiving the vaccine without MPL by the i.n. route (Fig. 2B).
Vaginal and fecal IgA anti-C-GTF response.
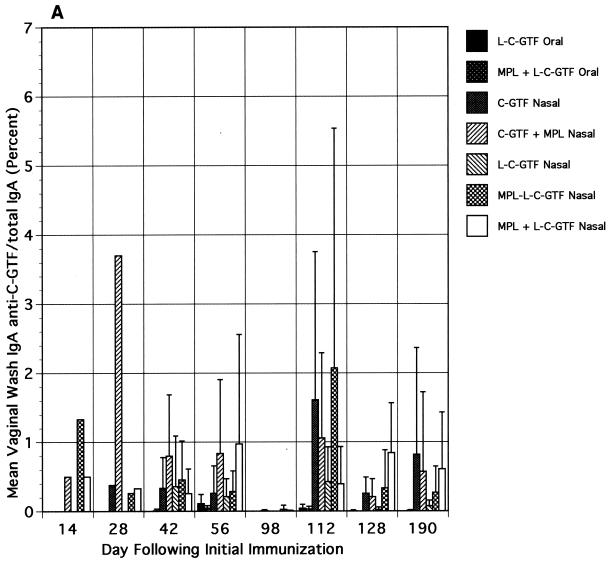
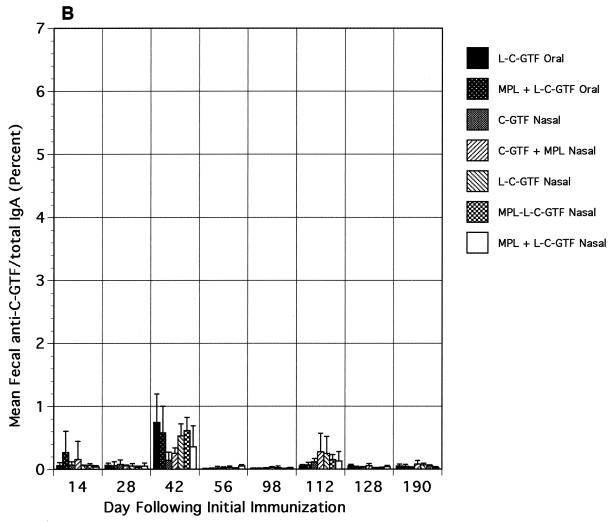
Relative to the salivary responses, low and variable levels of IgA anti-C-GTF activity were observed in vaginal wash and fecal samples collected after immunization (Fig. 3A and B, respectively). Although the differences in responses were not significant, the responses were higher in the i.n. immunized groups' vaginal washes than in those from orally immunized animals. Following the boost, a slight increase in the magnitude of the vaginal wash responses was seen, especially in the i.n. immunized groups; however, the differences between responses in the i.n. and orally immunized groups were not significant. Low fecal IgA responses were detected only on days 42 and 112 (after the booster) in groups immunized with liposomal antigen (Fig. 3B).
FIG. 3.
Vaginal wash (A) and fecal extract (B) IgA antibody responses to C-GTF. BALB/c mice were immunized by the intragastric or i.n. route with C-GTF or L-C-GTF (with or without MPL-AF) on days 0 and 1 and given boosters on day 98. Values are anti-C-GTF activity for pooled samples (vaginal, days 12 and 28) or the means + standard deviations of samples collected from animals on indicated days.
DISCUSSION
Lipid A and MPL have been used as systemic adjuvants for inducing cellular and humoral responses to various antigens given alone (35, 41, 42, 47, 52) or associated with liposomes (16, 32, 38, 40, 43, 59), while only limited studies have investigated their usefulness as mucosal adjuvants (5, 39, 46). The purpose of this study was to investigate the effects of free or liposome-associated MPL-AF on augmentation of immune responses to a vaccine antigen when administered by the oral versus nasal route. In the present study, we compared the effectiveness of the mucosal route of immunization for inducing salivary IgA responses. The i.n. route was selected to further evaluate compartmentalization within the common mucosal immune system. The vaccine antigen was found to be more immunogenic when given by the i.n. route than by the oral route. Furthermore, MPL was found to be a strong mucosal and systemic adjuvant when given by the i.n. route.
Little is known about memory responses in the mucosal immune system and about MPL as a mucosal adjuvant. In this study, a week 14 booster was chosen based on a previous study which used a recombinant Salmonella vector for oral delivery of a Porphyromonas gingivalis antigen (28) and showed the induction of a strong memory response when mice were given boosters at this time. In the present study, a booster at 14 weeks after the first immunization resulted in an excellent saliva and plasma memory response (10- to 100-fold increase). Liposomal-antigen i.n. administration with MPL (whether added to liposomal antigen or incorporated into liposome membrane) resulted in salivary IgA responses following a booster that were consistently higher than seen in mice immunized with liposomal or free antigen without MPL. MPL in most cases potentiated the plasma IgG and IgA antigen-specific memory responses following the booster.
Although primary and memory salivary responses were observed in groups following i.n. immunization, only slight primary and secondary responses were induced at other mucosal sites (intestinal and vaginal). Other studies have shown that i.n. immunization with various antigens and adjuvants can result in vaginal (18, 23, 27) and intestinal (61) responses in addition to salivary and systemic responses; however, these studies did not involve lipid A derivatives. It is possible that the low vaginal response observed following i.n. immunization was related to the timing of the immunization in female mice. It has been shown that the time of immunization in relation to the estrous cycle is important for the induction of mucosal responses (18, 36). Alternatively, the low vaginal and intestinal responses following the immunization protocol used in the study may suggest limited compartmentalization of the mucosal immune system (i.e., local mucosal response) (60).
Plasma IgG and IgA anti-C-GTF responses were induced in all of the i.n. immunization groups and the orally immunized MPL+L-C-GTF group. The induction of a systemic immune response following i.n. immunization was not surprising based on previous studies (6, 21, 24, 25, 30, 48–50, 53, 58, 61). Therefore, our findings as well as those of others demonstrate that the common mucosal immune system is not completely separate from the circulatory immune system. It has also been shown that parenteral immunization of humans results in mucosal as well as systemic responses (31).
Most murine studies published to date have involved the demonstration of adjuvant properties of MPL or other similar lipid A derivatives that were given systemically rather than mucosally (16, 35, 42, 43, 45, 47, 52, 54). Sasaki and coworkers (46), however, recently compared i.n. and intramuscular immunization using MPL as an adjuvant and observed the induction of similar systemic responses but higher intestinal responses in groups immunized by the i.n. route. Our findings further demonstrate that there is a systemic adjuvant effect of MPL when given via the i.n. route. While these findings are potentially beneficial for prevention of many invasive diseases, because of the localized nature of dental caries, a systemic response may not be important or desirable in interfering with the pathogenesis of this disease.
The findings of the studies reported herein will be helpful in developing mucosal vaccines, including an S. mutans antigen vaccine which is able to induce salivary responses in humans that are protective against the initiation of dental caries. MPL has been administered in human subjects by injection and has resulted in no observed side effects (other than minor irritation at the injection site) (22, 51, 54). Therefore, although further animal testing will help support the safe use of MPL for dental caries prevention and identification of the optimal immunization formulation, delivery system, and dosage regimen, clinical studies will benefit the understanding of the human response to mucosally administered MPL immunization protocols. These studies will hopefully provide insight into the usefulness of delivery and adjuvant systems for a myriad of diseases at mucosal surfaces, including S. mutans-induced dental caries.
ACKNOWLEDGMENTS
We thank Rosie Turner for secretarial help.
This work was supported in part by NIH grants DE09846, DE09081, DE11147, DE08182, DE08228, and DE06746 from the NIDCR.
REFERENCES
- 1.Abraham E. Intranasal immunization with bacterial polysaccharide containing liposomes enhances antigen-specific pulmonary secretory antibody response. Vaccine. 1992;10:461–468. doi: 10.1016/0264-410x(92)90395-z. [DOI] [PubMed] [Google Scholar]
- 2.Abraham E, Shah S. Intranasal immunization with liposomes containing IL-2 enhances bacterial polysaccharide antigen-specific pulmonary secretory antibody response. J Immunol. 1992;149:3719–3726. [PubMed] [Google Scholar]
- 3.Alving C R, Richards R L. Liposomes containing lipid A: a potent nontoxic adjuvant for a human malaria sporozoite vaccine. Immunol Lett. 1990;25:275–280. doi: 10.1016/0165-2478(90)90127-c. [DOI] [PubMed] [Google Scholar]
- 4.Aramaki Y, Fujii Y, Yachi K, Kikuchi H, Tsuchiya S. Activation of systemic and mucosal immune response following nasal administration of liposomes. Vaccine. 1994;12:1241–1245. doi: 10.1016/0264-410x(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 5.Baldridge J R, Yorgensen Y, Ward J R, Ulrich J T. Monophosphoryl lipid A enhances mucosal and systemic immunity to vaccine antigens following intranasal administration. Vaccine. 2000;18:2416–2425. doi: 10.1016/s0264-410x(99)00572-1. [DOI] [PubMed] [Google Scholar]
- 6.Balmelli C, Roden R, Potts A, Schiller J, DeGrandi P, Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J Virol. 1998;72:8220–8229. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandtzaeg P. The B-cell development in tonsillar lymphoid follicles. Acta Otolaryngol. 1996;523:55–59. [PubMed] [Google Scholar]
- 8.Brownlie R M, Brahmbhatt H N, White D C, Fountain M W, Rohde M, Wehland J, Timmis K N. Stimulation of secretory antibodies against Bordetella pertussis antigens in the lungs of mice after oral or intranasal administration of liposome-incorporated cell-surface antigens. Microb Pathog. 1993;14:149–160. doi: 10.1006/mpat.1993.1015. [DOI] [PubMed] [Google Scholar]
- 9.Childers N K, Michalek S M. Liposomes. In: O'Hagan D T, editor. Novel delivery systems for oral vaccines. Boca Raton, Fla: CRC Press; 1994. pp. 241–254. [Google Scholar]
- 10.Childers N K, Michalek S M, Eldridge J H, Denys F R, Berry A K, McGhee J R. Characterization of liposome suspensions by flow cytometry. J Immunol Methods. 1989;119:135–143. doi: 10.1016/0022-1759(89)90390-6. [DOI] [PubMed] [Google Scholar]
- 11.Childers N K, Zhang S S, Harokopakis E, Harmon C C, Michalek S M. Properties of practical oral liposome Streptococcus mutans glucosyltransferase vaccines for effective induction of caries protection. Oral Microbiol Immunol. 1996;11:172–180. doi: 10.1111/j.1399-302x.1996.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 12.Childers N K, Zhang S S, Michalek S M. Oral immunization of humans with dehydrated liposomes containing Streptococcus mutans glucosyltransferase induces salivary immunoglobulin A2 antibody responses. Oral Microbiol Immunol. 1994;9:146–153. doi: 10.1111/j.1399-302x.1994.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 13.de Haan A, Geerligs H J, Huchshorn J P, van Scharrenburg G J M, Palache A M, Wilschut J. Mucosal immunoadjuvant activity of liposomes: induction of systemic IgG and secretory IgA in mice by intranasal immunization with an influenza subunit vaccine and coadministered liposomes. Vaccine. 1995;13:155–162. doi: 10.1016/0264-410x(95)93129-w. [DOI] [PubMed] [Google Scholar]
- 14.de Haan A, Renegar K B, Small P A, Wilschut J. Induction of a secretory IgA response in the murine female urogenital tract by immunization of the lungs with liposome-supplemented viral subunit antigen. Vaccine. 1995;13:613–616. doi: 10.1016/0264-410x(94)00062-r. [DOI] [PubMed] [Google Scholar]
- 15.de Haan A, Tomee J F C, Huchshorn J P, Wilschut J. Liposomes as an immunoadjuvant system for stimulation of mucosal and systemic antibody responses against inactivated measles virus administered intranasally to mice. Vaccine. 1995;13:1320–1324. doi: 10.1016/0264-410x(95)00037-2. [DOI] [PubMed] [Google Scholar]
- 16.Friede M, Muller S, Briand J-P, Van Regenmortel M H V, Schuber F. Induction of immune response against a short synthetic peptide antigen coupled to small neutral liposomes containing monophosphoryl lipid A. Mol Immunol. 1993;30:539–547. doi: 10.1016/0161-5890(93)90028-a. [DOI] [PubMed] [Google Scholar]
- 17.Fries L, Gordon D, Richards R, Egan J, Hollingdale M, Gross M, Silverman C, Alving C. Liposomal malaria vaccine in humans: a safe and potent adjuvant strategy. Immunology. 1992;89:358–362. doi: 10.1073/pnas.89.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallichan W S, Rosenthal K L. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589–1595. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 19.Guink N E, Kris R M, Goodman-Snitkoff G, Small P A, Mannino R J. Intranasal immunization with proteoliposomes protects against influenza. Vaccine. 1989;7:147–151. doi: 10.1016/0264-410x(89)90055-8. [DOI] [PubMed] [Google Scholar]
- 20.Hagen S R, Thompson J D, Snyder D S, Myers K R. Analysis of a monophosphoryl lipid A immunostimulant preparation from Salmonella minnesota R595 by high-performance liquid chromatography. J Chromatogr. 1997;767:53–61. doi: 10.1016/s0021-9673(97)00041-1. [DOI] [PubMed] [Google Scholar]
- 21.Hirabayashi Y, Kurata H, Funato H, Nagamine T, Aizawa C, Tamura S, Shimada K, Kurata T. Comparison of intranasal inoculation of influenza HA vaccine combined with cholera toxin B subunit with oral or parenteral vaccination. Vaccine. 1990;8:243–248. doi: 10.1016/0264-410x(90)90053-o. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman S L, Edelman R, Bryan J P, Schneider I, Davis J, Sedegah M, Gordon D, Church P, Gross M, Silverman C, Hollingdale M, Clyde D, Sztein M, Losonsky G, Paparello S, Jones T R. Safety, immunogenicity, and efficacy of a malaria sporozoite vaccine administered with monophosphoryl lipid A, cell wall skeleton of mycobacteria, and squalane as adjuvant. Am J Trop Med Hyg. 1994;51:603–612. doi: 10.4269/ajtmh.1994.51.603. [DOI] [PubMed] [Google Scholar]
- 23.Hordnes K, Tynning T, Brown T A, Haneberg B, Jonsson R. Nasal immunization with group b streptococci can induce high levels of specific IgA antibodies in cervicovaginal secretions of mice. Vaccine. 1997;15:1244–1251. doi: 10.1016/s0264-410x(97)00021-2. [DOI] [PubMed] [Google Scholar]
- 24.Hotomi M, Saito T, Yamanaka N. Specific mucosal immunity and enhanced nasopharyngeal clearance of nontypeable Haemophilus influenzae after intranasal immunization with outer membrane protein P6 and cholera toxin. Vaccine. 1998;16:1950–1956. doi: 10.1016/s0264-410x(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 25.Isaka M, Yasuda Y, Kozuka S, Miura Y, Taniguchi T, Matano K, Goto N, Tochikubo K. Systemic and mucosal immune responses of mice to aluminium-adsorbed or aluminium-non-adsorbed tetanus toxoid administered intranasally with recombinant cholera toxin B subunit. Vaccine. 1998;16:1620–1626. doi: 10.1016/s0264-410x(98)00066-8. [DOI] [PubMed] [Google Scholar]
- 26.Jespersgaard C, Hajishengalis G, Greenway T E, Smith D J, Russell M W, Michalek S M. Functional and immunogenic characterization of two cloned regions of Streptococcus mutans glucosyltransferase I. Infect Immun. 1999;67:810–816. doi: 10.1128/iai.67.2.810-816.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansson E, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin b subunit of conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohler J J, Pathangey L B, Brown T A. Oral immunization with recombinant Salmonella typhimurium expressing a cloned Porphyromonas gingivalis hemagglutinin: effect of boosting on mucosal, systemic and immunoglobulin G subclass response. Oral Microbiol Immunol. 1998;13:81–88. doi: 10.1111/j.1399-302x.1998.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Lowell G H, Kaminski R W, Grate S, Hunt R E, Charney C, Zimmer S, Colleton C. Intranasal and intramuscular proteosome-staphylococcal enterotoxin b (SEB) toxoid vaccines: immunogenicity and efficacy against lethal SEB intoxication in mice. Infect Immun. 1996;64:1706–1713. doi: 10.1128/iai.64.5.1706-1713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lue C, Tarkowski A, Mestecky J. Systemic immunization with pneumococcal polysaccharide vaccine induces a predominant IgA2 response of peripheral blood lymphocytes and increases of both serum and secretory anti-pneumococcal antibodies. J Immunol. 1988;140:3793–3800. [PubMed] [Google Scholar]
- 32.Mbawuike I N, Acuna C, Caballero D, Pham-Nguyen K, Gilbert B, Petribon P, Harmon M. Reversal of age-related deficient influenza virus-specific CTL responses and IFN-γ production by monophosphoryl lipid A. Cell Immunol. 1996;173:64–78. doi: 10.1006/cimm.1996.0252. [DOI] [PubMed] [Google Scholar]
- 33.Michalek S M, Childers N K, Katz J, Dertzbaugh M T, Russell M W. Use of liposomes for the induction of mucosal immunity. Vaccine Res. 1992;1:241–247. [Google Scholar]
- 34.Mukasa H, Tsumori H, Shimamura A. Isolation and characterization of an extracellular glucosyltransferase synthesizing insoluble glucan from Streptococcus mutans serotype c. Infect Immun. 1985;49:790–796. doi: 10.1128/iai.49.3.790-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers K R, Beining P, Betts M, Snippe H, Inman J, Golding B. Monophosphoryl lipid A behaves as a T-cell-independent type 1 carrier for hapten-specific antibody responses in mice. Infect Immun. 1995;63:168–174. doi: 10.1128/iai.63.1.168-174.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nardelli-Haefliger D, Roden R, Balmelli C, Potts A, Schiller J, DeGrandi P. Mucosal but not parenteral immunization with purified human papillomavirus type 16 virus-like particles induces neutralizing titers of antibodies throughout the estrous cycle of mice. J Virol. 1999;73:9609–9613. doi: 10.1128/jvi.73.11.9609-9613.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostro M J. Liposomes. Sci Am. 1987;256:102–111. [PubMed] [Google Scholar]
- 38.Pierce N, Sacci J. Enhanced mucosal priming by cholera toxin and procholeragenoid with a lipoidal amine adjuvant (avridine) delivered in liposomes. Infect Immun. 1984;44:469–473. doi: 10.1128/iai.44.2.469-473.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierce N F, Sacci J B, Alving C R, Richardson E C. Enhancement by lipid A of mucosal immunogenicity of liposome-associated cholera toxin. Rev Infect Dis. 1984;6:563–566. doi: 10.1093/clinids/6.4.563. [DOI] [PubMed] [Google Scholar]
- 40.Rao M, Matyas G R, Grieder F, Anderson K, Jahrling P B, Alving C R. Cytotoxic T lymphocytes to Ebola Zaire virus are induced in mice by immunization with liposomes containing lipid A. Vaccine. 1999;17:2991–2998. doi: 10.1016/s0264-410x(99)00170-x. [DOI] [PubMed] [Google Scholar]
- 41.Ravindranath M H, Brazeau S M, Morton D L. Efficacy of tumor cell vaccine after incorporating monophosphoryl lipid A (MPL) in tumor cell membranes containing tumor-associated ganglioside. Experientia. 1994;50:648–653. doi: 10.1007/BF01952865. [DOI] [PubMed] [Google Scholar]
- 42.Ravindranath M H, Morton D L, Irie R F. Attachment of monophosphoryl lipid A (MPL) to cells and liposomes augments antibody response to membrane-bound gangliosides. J Autoimmun. 1994;7:803–816. doi: 10.1006/jaut.1994.1063. [DOI] [PubMed] [Google Scholar]
- 43.Richards R, Rao M, Wassef N, Glenn G, Rothwell S, Alving C. Liposomes containing lipid A serve as an adjuvant for induction of antibody and cytotoxic T-cell responses against RTS, S malaria antigen. Infect Immun. 1998;66:2859–2865. doi: 10.2307/1366431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richards R L, Swartz G M, Schultz C, Hayre M D, Ward G S, Ballou W R, Hockmeyer W T, Berman S L, Alving C R. Immunogenicity of liposomal malaria sporozoite antigen in monkeys: adjuvant effects of aluminum hydroxide and non-pyrogenic liposomal lipid A. Vaccine. 1989;7:506–512. doi: 10.1016/0264-410x(89)90274-0. [DOI] [PubMed] [Google Scholar]
- 45.Salkowski C A, Detore G R, Vogel S N. Lipopolysaccharide and monophosphoryl lipid A differentially regulate interleukin-12, gamma interferon, and interleukin-10 mRNA production in murine macrophages. Infect Immun. 1997;65:3239–3247. doi: 10.1128/iai.65.8.3239-3247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki S, Hamajima K, Fukushima J, Ihata A, Ishii N, Gorai I, Hirahara F, Mohri H, Okuda K. Comparison of intranasal and intramuscular immunization against human immunodeficiency virus type 1 with DNA-monophosphoryl lipid A adjuvant vaccine. Infect Immun. 1998;66:823–826. doi: 10.1128/iai.66.2.823-826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasaki S, Tsuji T, Hamajima K, Fukushimal J, Ishii N, Kaneko T, Xin K, Mohri H, Aoki I, Okubo T, Nishioka K, Okuda K. Monophosphoryl lipid A enhances both humoral and cell-mediated immune responses to DNA vaccination against human immunodeficiency virus type 1. Infect Immun. 1997;65:3520–3528. doi: 10.1128/iai.65.9.3520-3528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snider D P, Underdown B J, McDermott M R. Intranasal antigen targeting to MHC class II molecules primes local IgA and serum IgG antibody responses in mice. Immunology. 1997;90:323–329. doi: 10.1111/j.1365-2567.1997.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Staats H F, Nichols W G, Palker T J. Mucosal immunity to HIV-1—systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A) J Immunol. 1996;157:462–472. [PubMed] [Google Scholar]
- 50.Takahashi I, Okahashi N, Kanamoto T, Asakawa H, Koga T. Intranasal immunization of mice with recombinant protein antigen of serotype c Streptococcus mutans and cholera toxin B subunit. Arch Oral Biol. 1990;35:475–477. doi: 10.1016/0003-9969(90)90211-r. [DOI] [PubMed] [Google Scholar]
- 51.Thoelen S, Van Damme P, Mathei C, Leroux-Roels G, Desombere I, Safary A, Vandepapeliere P, Slaoui M, Mehues A. Safety and immunogenicity of a hepatitis B vaccine formulated with a novel adjuvant system. Vaccine. 1998;16:708–714. doi: 10.1016/s0264-410x(97)00254-5. [DOI] [PubMed] [Google Scholar]
- 52.Thompson H S G, Davies M L, Watts M J, Mann A E, Holding F P, O'Neill T, Beech J T, Thompson S J, Leesman G D, Ulrich J T. Enhanced immunogenicity of a recombinant genital warts vaccine adjuvanted with monophosphoryl lipid A. Vaccine. 1998;16:1993–1998. doi: 10.1016/s0264-410x(98)00088-7. [DOI] [PubMed] [Google Scholar]
- 53.Ugozzoli M, O'Hagan D T, Ott G S. Intranasal immunization of mice with herpes simplex virus type 2 recombinant gD2: the effect of adjuvants on mucosal and serum antibody responses. Immunology. 1998;93:563–571. doi: 10.1046/j.1365-2567.1998.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ulrich J T, Myers K R. Monophosphoryl lipid A as an adjuvant. In: Powell M F, Newman M J, editors. Vaccine design: the subunit and adjuvant approach. New York, N.Y: Plenum Press; 1995. pp. 495–524. [Google Scholar]
- 55.van der Rijn I, Kessler R E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980;27:444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Rooijen N, van Nieuwmegen R. Use of liposomes as biodegradable and harmless adjuvants. Methods Enzymol. 1983;93:83–95. doi: 10.1016/s0076-6879(83)93036-7. [DOI] [PubMed] [Google Scholar]
- 57.Verma J, Rao M, Amselem S, Krzych U, Alving C, Green S, Wassef N. Adjuvant effects of liposomes containing lipid A: enhancement of liposomal antigen presentation and recruitment of macrophages. Infect Immun. 1992;60:2438–2444. doi: 10.1128/iai.60.6.2438-2444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verweij W R, deHaan L, Holtrop M, Agsteribbe E, Brands R, vanScharrenburg G J M, Wilschut J. Musosal immunoadjuvant activity of recombinant Escherichia coli heat-labile enterotoxin and its B subunit: induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with influenza virus surface antigen. Vaccine. 1998;16:2069–2076. doi: 10.1016/s0264-410x(98)00076-0. [DOI] [PubMed] [Google Scholar]
- 59.White K, Krzych U, Gordon D M, Porter T G, Richards R L, Alving C R, Deal C D, Hollingdale M, Silverman C, Sylvester D R, Ballou W R, Gross M. Induction of cytolytic and antibody responses using Plasmodium falciparum repeatless circumsporozoite protein encapsulated in liposomes. Vaccine. 1993;11:1341–1346. doi: 10.1016/0264-410x(93)90105-7. [DOI] [PubMed] [Google Scholar]
- 60.Wu H-Y, Russell M W. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res. 1997;16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]
- 61.Wu H-Y, Russell M W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu H-Y, Nguyen H H, Russell M W. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46:506–513. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]