Abstract
Liver is the central hub regulating energy metabolism during feeding-fasting transition. Evidence suggests that fasting and refeeding induce dynamic changes in liver size, but the underlying mechanisms remain unclear. Yes-associated protein (YAP) is a key regulator of organ size. This study aims to explore the role of YAP in fasting- and refeeding-induced changes in liver size. Here, fasting significantly reduced liver size, which was recovered to the normal level after refeeding. Moreover, hepatocyte size was decreased and hepatocyte proliferation was inhibited after fasting. Conversely, refeeding promoted hepatocyte enlargement and proliferation compared to fasted state. Mechanistically, fasting or refeeding regulated the expression of YAP and its downstream targets, as well as the proliferation-related protein cyclin D1 (CCND1). Furthermore, fasting significantly reduced the liver size in AAV-control mice, which was mitigated in AAV Yap (5SA) mice. Yap overexpression also prevented the effect of fasting on hepatocyte size and proliferation. Besides, the recovery of liver size after refeeding was delayed in AAV Yap shRNA mice. Yap knockdown attenuated refeeding-induced hepatocyte enlargement and proliferation. In summary, this study demonstrated that YAP plays an important role in dynamic changes of liver size during fasting-refeeding transition, which provides new evidence for YAP in regulating liver size under energy stress.
KEY WORDS: Liver, Fasting, Refeeding, Yes-associated protein, Hepatocyte size, Hepatocyte proliferation, β-Catenin, Cyclin D1
Graphical abstract
YAP plays an important role in dynamic changes of liver size during fasting-refeeding transition, which provides new evidence for YAP in regulating liver size under energy stress.
1. Introduction
The liver is a critical metabolic organ in vertebrates, which plays a crucial role in maintaining whole body energy homeostasis via regulating metabolism of glucose, lipid, protein and xenobiotics. In contrast to other organs, liver size is precisely regulated by different signals to adjusting the body size and need, which has been called “hepatostat”1, 2, 3. A variety of stimuli influence liver size. For example, hormones, xenobiotics and virus infection induce liver enlargement4, 5, 6, whereas liver atrophy is associated with portacaval shunt, chemotherapy, hepatic fibrosis, cirrhosis and starvation7, 8, 9, 10.
Fasting, a form of dietary restriction, is defined as the absence of food and caloric beverages for periods, which induces a variety of metabolic, hormonal and immunological alterations11. It has been reported that fasting has a wide range of health benefits, which not only prolongs life- and health-span in animal models, but prevents and ameliorates various diseases such as obesity, diabetes, cancer and neurodegeneration, etc.11, 12, 13. In order to ensure energy supply of the whole body upon fasting, the metabolic mode is switched from glucose dependency to lipid metabolization (fatty acid oxidation, and ketogenesis). As the central hub for macronutrient metabolism, liver plays an important role during fasting-feeding switch. Fasting triggers the complex changes in hepatic gene expression regulating glycogenolysis, gluconeogenesis, fatty acid oxidation and ketogenesis. Several lines of evidence suggest that fasting reduces the liver weight, which returns to normal after refeeding10,14, 15, 16. However, the molecular mechanism remains unclear. Therefore, a better understanding of the molecular mechanisms that fasting and refeeding regulates liver size will contribute to the development of novel, preventive and therapeutic interventions for liver diseases.
Yes-associated protein (YAP), the key effector of Hippo signaling pathway, is an important regulator in organ size control17,18. YAP is phosphorylated and inactivated on multiple residues by the large tumor suppressor kinase 1/2 (LATS1/2), where phosphorylation of Ser127 results in 14-3-3 binding and cytoplasmic sequestration of YAP17. Phosphorylation on Ser397 ultimately leads to YAP degradation via the subsequent phosphorylation19. Dephosphorylated YAP can translocate into the nucleus from cytoplasm, and interacts with the transcriptional enhanced associate domain (TEAD) family of transcription factors, which initiates a range of genes transcription mainly involved in cell proliferation, survival and differentiation. Existing evidence suggests that YAP plays a critical role in liver homeostasis. Overexpression or activation of YAP in liver results in massive hepatomegaly17,18 and promotes liver regeneration after partial hepatectomy20,21. However, the enlarged liver rapidly returns to normal size after cessation of YAP overexpression, and the liver regeneration is also impaired by deletion of YAP. Recently, we reported that activation of pregnane X receptor (PXR), constitutive androstane receptor (CAR), peroxisome proliferator-activated receptor alpha (PPARα) significantly induces liver enlargement and promotes liver regeneration via interaction with YAP and inducing YAP translocation22, 23, 24, 25.
Therefore, the current study aims to elucidate the role of YAP in liver size during fasted and refed state using liver-specific Yap-knockdown mice and Yap-overexpression mice, which provides new evidence for the physiological role of YAP in regulating liver size under energy stress.
2. Materials and methods
2.1. Reagents
Anti-YAP (Cat# 14074S, RRID: AB_2650491), anti-Phospho-YAP (Ser127) (Cat# 13008S, RRID: AB_2650553), anti-Cyclin D1 (Cat# 2922S, RRID: AB_2228523), anti-Cyclin D1 (Cat# 55506S, RRID: AB_2827374), anti-Cyclin E1 (Cat# 20808, RRID: AB_2783554), anti-β-actin (Cat# 4970S, RRID, AB_2223172), anti-Lamin B1 (Cat# 13435S, RRID: AB_2737428), anti-GAPDH (Cat# 2118S, RRID: AB_561053), anti-rabbit, IgG HRP-linked antibody (Cat# 7074S, RRID: AB_2099233), anti-mouse IgG, HRP-linked antibody (Cat# 7076S, RRID: AB_330924), signalstain® boost IHC detection reagent (HRP, rabbit) (Cat# 8114S, RRID: AB_10544930), signalstain® boost IHC detection reagent (HRP, mouse) (Cat# 8125S, RRID: AB_10547893) were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-CCNA1 (Cat# D220507, RRID: AB_2819214), anti-CYR61 (Cat# D122190, RRID: AB_2819221), anti-ANKRD1 (Cat# D121628, RRID: AB_2819216) and anti-CTGF (Cat# D160212, RRID: AB_2819217) were purchased from Sangon Biotech (Shanghai, China). Anti-β-catenin (Cat# 610153, RRID: AB_397554) was purchased from BD Biosciences (Franklin Lakes, NJ, USA). Anti-caspase-3 (Cat# sc-56053, RRID: AB_781826) was purchased from Santa Cruz Biotechnology, Inc. (Carpinteria, CA, USA).
2.2. Animals and treatments
Male C57BL/6 mice (6–8 weeks old) were purchased from Guangdong Medical Laboratory Animal Center (Foshan, China). Mice were housed in a specific pathogen-free and temperature-controlled room (22–24 °C) with a standard 12-h light/dark cycle, and had free access to water and standard chow. For fasting experiments, 8-week-old C57BL/6 mice were fasted 24 or 48 h, respectively. For refeeding experiments, 8-week-old C57BL/6 mice were fasted 24 h and then fed normal chow for the times indicated. For liver-specific knockdown of YAP, 6-week-old C57BL/6 mice were intravenously injected with Adeno-associated virus (AAV) Control-ZsGreen or AAV Yap shRNA-ZsGreen (1.3 × 1011 genome copies per mouse, Hanbio, Shanghai, China) and were fasted 24 h after 4 weeks, then fed normal chow for the times indicated. For liver-specific overexpression of YAP (5SA), 6-week-old C57BL/6 mice were intravenously injected with AAV Control-ZsGreen or AAV Yap (5SA)-ZsGreen (1.5 × 1011 genome copies per mouse, Hanbio, Shanghai, China), and were fasted 24 h after 4 weeks. Serum and liver samples were collected and stored at −80 °C for further use. A portion of liver was immediately fixed in 4% paraformaldehyde for histological analysis. All animal experiments and protocols were approved by Institutional Animal Care and Use Committee of Sun Yat-sen University (Guangzhou, China).
2.3. Histological and biochemical analysis
Paraformaldehyde-fixed liver tissues were embedded in paraffin and sectioned. Paraffin-embedded sections were stained with hematoxylin and eosin (H&E; Biossci, Wuhan, China), β-catenin antibody or Cyclin D1 antibody. CX43 biological microscope (Olympus, Tokyo, Japan) was used to observe and obtain the images of stained liver sections.
The levels of serum alanine aminotransferase (ALT), aspartate transaminase (AST) and alkaline phosphatase (ALP) were measured using commercially available kits (URIT, Guilin, China) following the manufacturer's standard protocols and then analyzed by URIT-8021A automatic chemistry analyzer (URIT, Guilin, China).
2.4. Quantitative real-time polymerase chain reaction (RT-qPCR) analysis
Total RNA of liver samples was extracted by AG RNAex Pro reagent (Cat# AG21102, Accurate Biology, Hunan, China) according to instruction, and then cDNA was synthesized using Evo M-MLV RT Kit (Cat# AG11711, Accurate Biology). RT-qPCR was performed with Applied Biosystems 7500 (Thermo Fisher Scientific) using SYBR® Green Premix Pro Taq HS qPCR Kit (Cat# AG11701, Accurate Biology). The fold changes of mRNA levels were analyzed by ΔΔCt method. The primer sequences were listed in Supporting Information Table S1.
2.5. Western blot analysis
Total proteins, nuclear and cytoplasmic proteins were extracted from liver tissues as described previously22. In short, total protein and nuclear/cytoplasmic protein extracts of liver tissues were respectively homogenized with RIPA lysis buffer containing 1% 100 mmol/L PMSF (Cat# R0127, Biocolors, Shanghai, China) and NE-PER Nuclear and Cytoplasmic Extraction Reagents (Cat# 78835, Thermo Fisher Scientific). Protein concentration was determined using Pierce BCA Protein Assay Reagent Kit (Cat# 23225, Thermo Fisher Scientific). Proteins (30 μg/lane) were separated by 10% SDS-PAGE and transferred to NC membranes (Cat# 66485, Pall, New York, USA). The membranes were blocked using 5% skim milk (Cat# 1172GR500, BioFroxx, Einhausen, Germany) in TBST [Tris-buffered saline (TBS) containing 0.1% Tween-20] for 1 h at room temperature, and then incubated with different antibodies at 4 °C overnight. The blots were incubated by anti-rabbit IgG, HRP-linked antibody or anti-mouse IgG, HRP-linked antibody for 1 h at room temperature after washing with TBST three times the next day. Immunoreactive bands were visualized by chemiluminescence (Cat# WBKLS0500, Millipore, Burlington, VT, USA) following three washes with TBST.
2.6. Statistical analysis
All results are expressed as mean ± standard deviation (SD). Two-tailed Student's t tests was used for statistical analysis between two experimental groups and two-way ANOVA was used for statistical analysis among multiple unpaired experimental groups by GraphPad Prism 9.0 software (GraphPad Software, San Diego, CA, USA). P < 0.05 was considered as statistically significant.
3. Results
3.1. Fasting reduces liver size in mice
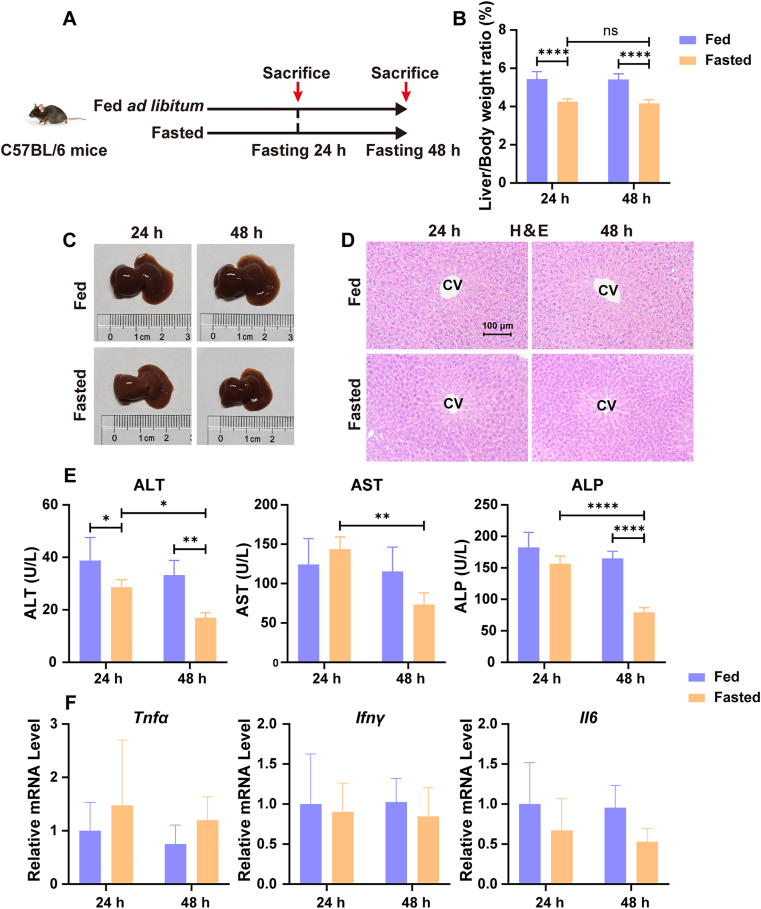
To evaluate the effect of fasting on liver size in mice, male C57BL/6 mice were fasted for 24 or 48 h (Fig. 1A). The results show that liver/body weight ratio was significantly decreased compared with the fed group after fasting for 24 and 48 h (−21.78%, P < 0.0001 after fasting for 24 h; −22.97%, P < 0.0001 after fasting for 48 h; Fig. 1B). The liver morphology also shows an obvious shrinkage after fasting for 24 and 48 h (Fig. 1C). H&E staining shows no significant liver necrosis among all groups (Fig. 1D). Serum levels of ALT, AST and ALP were examined. Overall, no obvious differences were observed between fed and 24 h-fasted groups; but the serum levels of ALT and ALP were significantly decreased after fasting for 48 h (Fig. 1E). In addition, the mRNA levels of pro-inflammation cytokines such as tumor necrosis factor alpha (Tnfα), interferon gamma (Ifnγ) and interleukin 6 (Il6) were not significantly altered after fasting for 24 and 48 h (Fig. 1F). These results reveal that 24-hour fasting reduced liver size but did not induce inflammatory liver injury.
Figure 1.
Fasting reduces liver size in mice. (A) C57BL/6 male mice were fed ad libitum (Fed) or fasted for 24 or 48 h (Fasted); (B) Liver/body weight ratio (n = 5); (C) Representative liver photographs of fed and fasted mice; (D) H&E staining of representative mice liver samples. Scale bar = 100 μm; (E) Serum ALT, AST and ALP levels (n = 5); (F) RT-qPCR analysis of pro-inflammatory cytokines Tnfα, Ifnγ, and Il6 (n = 5). Data are presented as mean ± SD. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗∗P < 0.0001 versus Fed group. Statistical significance was determined by two-way ANOVA. ns, not significant.
3.2. The liver size returns to normal level after refeeding
In order to explore change of liver size during fasting-feeding transition, male C57BL/6 mice were fasted for 24 h and then refed for 1, 3, 6, 12, and 24 h, respectively (Fig. 2A). The results show that fasting significantly decreased liver/body weight ratio, which was gradually recovered and basically returned to the normal level after refeeding for 6 h (Fig. 2B). Additionally, liver morphology also shows an obvious increase in liver size after refeeding, and liver size was almost back to normal level at 6 h post-refeeding (Fig. 2D). H&E staining shows no obvious liver necrosis during fasting and refeeding transition (Fig. 2C). Serum ALT, AST and ALP and the mRNA levels of pro-inflammation cytokines Tnfα, Ifnγ and Il6 were also examined, which show no significant increase during fasting and refeeding (Fig. 2E and F), indicating that there was no liver injury during fasting and refeeding transition.
Figure 2.
The liver size returns to normal level after refeeding. (A) C57BL/6 male mice were fed ad libitum (Fed) or fasted for 24 h (Fasted) and then refed for 1, 3, 6, 12 and 24 h (Refed); (B) Liver/body weight ratio (n = 5); (C) Representative liver photographs in different refed groups; (D) H&E staining of representative mice liver samples. Scale bar = 100 μm; (E) Serum ALT, AST and ALP levels (n = 5); (F) RT-qPCR analysis of pro-inflammatory cytokines Tnfα, Ifnγ, and Il6 (n = 5). Data are presented as mean ± SD. ∗P < 0.05, ∗∗∗P < 0.001 and ∗∗∗∗P < 0.0001 versus Fed group. Statistical significance was determined by two-tailed Student's t tests.
3.3. Effect of fasting and refeeding on hepatocyte size and proliferation
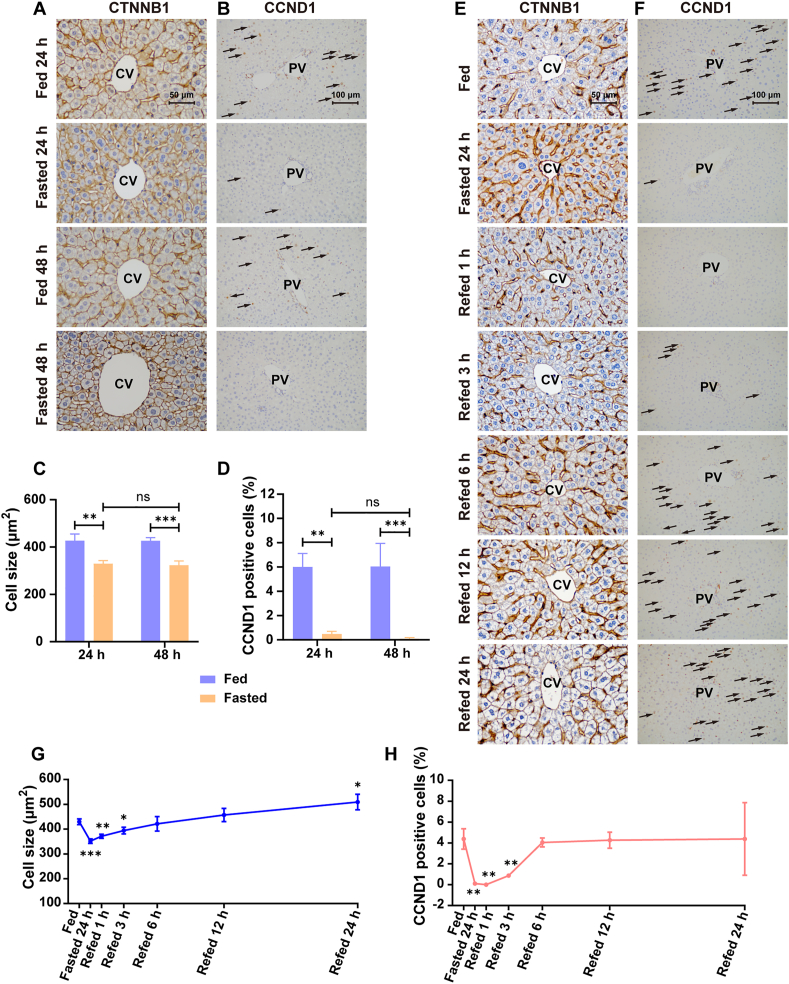
To explore the effect of fasting and refeeding on hepatocyte size, β-catenin (CTNNB1) staining was performed. A significant decrease of hepatocyte size around the central vein (CV) area was observed after fasting for 24 and 48 h (−22.73%, P < 0.01 after fasting for 24 h; −24.13%, P < 0.001; Fig. 3A and C). Likewise, the hepatocyte size around portal vein (PV) area was also dramatically reduced after fasting (−27.30%, P < 0.0001 after fasting for 24 h; −34.84%, P < 0.0001 after fasting for 48 h; Supporting Information Fig. S1A and S1C). Moreover, the reduced hepatocyte size around CV and PV by fasting were gradually increased after refeeding and basically returned to the normal level at 6 h post-refeeding (Fig. 3E and G, Supporting Information Fig. S1E and S1G).
Figure 3.
Effect of fasting and refeeding on hepatocyte size and proliferation. (A) Immunohistochemical staining of CTNNB1 around CV area for different fasted groups. Scale bar = 50 μm; (B) Immunohistochemical staining of CCND1 around PV area for different fasted groups. Scale bar = 100 μm; (C) Quantification of hepatocyte size in fasted mice (n = 3); (D) Quantification of CCND1 positive cells in fasted mice (n = 3); (E) Immunohistochemical staining of CTNNB1 around CV area for different refed groups. Scale bar = 50 μm; (F) Immunohistochemical staining of CCND1 around PV area for different refed groups. Scale bar = 100 μm; (G) Quantification of hepatocyte size for different refed groups (n = 3); (H) Quantification of CCND1 positive cells for different refed groups (n = 3). Data are presented as mean ± SD. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 versus Fed group. Statistical significance was determined by two-way ANOVA or two-tailed Student's t tests. ns, not significant.
The effect of fasting and refeeding on hepatocyte proliferation was further measured. Cyclin D1 (CCND1) staining was performed to evaluate hepatocyte proliferation. The percentage of CCND1 positive cell around the PV area was significantly decreased after fasting for 24 and 48 h (−92.00%, P < 0.01 after fasting for 24 h; −98.51%, P < 0.001 after fasting for 48 h; Fig. 3B and D), suggesting that fasting inhibited hepatocyte proliferation. In addition, the percentage of CCND1 positive cell around the CV area was also decreased after fasting for 24 and 48 h (−90.39%, P < 0.01 after fasting for 24 h; −99.23%, P < 0.01 after fasting for 48 h; Supporting Information Fig. S1B and S1D). The percentage of CCND1 positive cells around the CV and PV area was increased with the refeeding time and basically up to the normal level at 6 h post-refeeding (Fig. 3F and H, Supporting Information Fig. S1F and S1H), indicating that the suppression of hepatocyte proliferation by fasting was relieved after refeeding. Moreover, the proliferation-related proteins such as Cyclin A1 (CCNA1), CCND1 and Cyclin E1 (CCNE1) were measured in fasted and refed mice. The variation tendency of CCND1 was consistent with CCND1 staining, and CCNA1 and CCNE1 showed no significant change (Supporting Information Fig. S2A–S2D). These results indicate that fasting inhibited hepatocyte proliferation which could be reversed by refeeding in mice.
3.4. Fasting and refeeding regulate the hepatic YAP expression
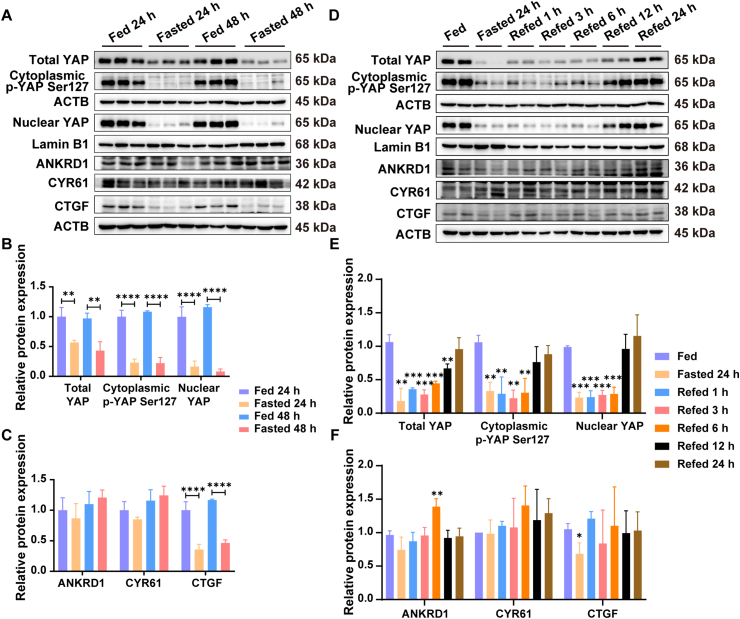
YAP plays a critical role in regulating organ size and tissue homeostasis. To investigate the effect of fasting and refeeding on YAP pathway, western blot analysis was performed to detect the expression of YAP and its downstream targets including ankyrin repeat domain 1 (ANKRD1), cysteine-rich angiogenic inducer 61 (CYR61) and connective tissue growth factor (CTGF). The results show that total YAP, cytoplasmic p-YAP (Ser127), nuclear YAP and CTGF were significantly decreased after fasting 24 and 48 h, whereas ANKRD1 and CYR61 were unchanged (Fig. 4A–C). During fasting to refeeding transition, the protein expressions of total YAP, cytoplasmic p-YAP (Ser127), nuclear YAP, CTGF and were gradually restored (Fig. 4D–F). These results were consistent with the alterations of hepatocyte size and proliferation during fasting and refeeding transition, suggesting that fasting and refeeding might regulate liver size via YAP.
Figure 4.
Effect of fasting and refeeding on YAP signaling pathway. (A) Western blot analysis of total YAP, cytoplasmic p-YAP (Ser127), nuclear YAP and its downstream targets ANKRD1, CYR61 and CTGF for different fasted groups; (B) Quantification of total YAP, cytoplasmic p-YAP (Ser127), nuclear YAP for different fasted groups (n = 3); (C) Quantification of ANKRD1, CYR61 and CTGF for different fasted groups (n = 3); (D) Western blot analysis of total YAP, cytoplasmic p-YAP (Ser127), nuclear YAP and its downstream targets ANKRD1, CYR61 and CTGF for different refed groups; (E) Quantification of total YAP, cytoplasmic p-YAP (Ser127), nuclear YAP for different refed groups (n = 3); (F) Quantification of ANKRD1, CYR61 and CTGF for different refed groups (n = 3). Data are presented as mean ± SD. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 and ∗∗∗∗P < 0.0001 versus Fed group. Statistical significance was determined by two-way ANOVA or two-tailed Student's t tests.
3.5. The overexpression of Yap resists the effect of fasting on liver size
AAV Yap (5SA) mice were used to investigate the effect of constitutively active YAP overexpression on liver size during fasting (Fig. 5A). ZsGreen fluorescence indicates that the AAV vectors had high transduction efficiency in mouse liver (Supporting Information Fig. S3A). Hepatic YAP was significantly up-regulated in AAV Yap (5SA) mice (Fig. 5B, Fig. S3B). The expression of YAP downstream targets including ANKRD1, CYR61 and CTGF were also remarkably increased (Supporting Information Fig. S4E and S4G), indicating that YAP signaling was activated after AAV Yap (5SA) treatment. Liver morphology shows an obvious enlargement in AAV Yap (5SA) mice, and compared to AAV Control mice, liver/body weight ratio of AAV Yap (5SA) mice was also significantly increased (+29.07%, P < 0.01; Fig. 5C and D), indicating that overexpression of Yap (5SA) induced liver enlargement. After fasting for 24 h, liver/body weight ratio both AAV Control and AAV Yap (5SA) mice were decreased compared with the counterpart, but the percentage reduction of liver/body weight ratio in fasting-treated AAV Yap (5SA) mice were lower than the counterpart in AAV Control mice (−15.93% versus −26.48%; Fig. 5C and D), indicating that overexpression of Yap prevented fasting-induced liver shrinkage. H&E staining showed hepatocyte necrosis and inflammatory cell infiltration in AAV Yap (5SA) mice (Fig. 5E). Serum biochemical indexes results showed no significant difference (Fig. S3C). The mRNA levels of pro-inflammation cytokines Tnfα, Ifnγ and Il6 was significantly increased in AAV Yap (5SA) mice, while there was a downward trend but no statistical significance in fasting-treated AAV Yap (5SA) mice, indicating that Yap (5SA) overexpression resulted in liver inflammatory injury and fasting might exert anti-inflammatory effects (Fig. S3D). CTNNB1 staining shows that the percentage reduction of hepatocyte size around CV and PV area after fasting in AAV Yap (5SA) mice was lower than the counterpart in AAV Control mice (CV area, −16.14% versus −26.39%, Fig. 5F and H; PV area, −11.06% versus −30.96%, Supporting Information Fig. S4A and S4C). Moreover, the percentage reduction of CCND1 positive cells around CV and PV area after fasting in AAV Yap (5SA) mice was also lower than the counterpart in AAV Control mice (CV area, −83.62% versus −90.00%, Fig. S4B and S4D; PV area, −68.65% versus −96.45%, Fig. 5G and I). In addition, the proliferation-related proteins such as CCNA1, CCND1 and CCNE1 were also measured, and CCND1 was up-regulated in AAV Yap (5SA) mice, while the protein expressions of CCNA1 and CCNE1 were no obvious changes (Fig. S4E and S4H). These data further demonstrate that YAP resists fasting-induced liver shrinkage to maintain the liver size after fasting.
Figure 5.
The overexpression of Yap resists the effect of fasting on liver size in AAV Yap (5SA) mice. (A) AAV Control and AAV Yap (5SA) mice were fed ad libitum (Fed) or fasted for 24 h (Fasted); (B) Western blot analysis of total YAP in AAV Control and AAV Yap (5SA) mice; (C) Representative liver photographs for fasted groups in AAV Control and AAV Yap (5SA) mice; (D) Liver/body weight ratio (n = 5); (E) H&E staining of representative mice liver samples. Scale bar = 100 μm; (F) Immunohistochemical staining of CTNNB1 around CV area. Scale bar = 50 μm; (G) Immunohistochemical staining of CCND1 around PV area. Scale bar = 100 μm; (H) Quantification of cell size (n = 3); (I) Quantification of CCND1 positive cells (n = 3). Data are presented as mean ± SD. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 versus Fed group. Statistical significance was determined by two-way ANOVA.
3.6. The knockdown of Yap delays the recovery of liver size following refeeding
To further confirm the role of YAP in fasting- or refeeding-induced liver size alteration, AAV Yap shRNA mice were used and treated with fasting and refeeding (Fig. 6A). ZsGreen fluorescence indicates that the AAV vectors had high transduction efficiency in mouse liver (Supporting Information Fig. S5A). The knockdown efficiency of AAV Yap shRNA was analyzed by Western blot, which showed that hepatic YAP was significantly down-regulated after AAV Yap shRNA treatment (Fig. 6B, Fig. S5B). However, the expression level of ANKRD1, CYR61 and CTGF showed no significant change (Supporting Information Fig. S6E and S6F). An obvious shrinkage of liver was observed after fasting in AAV Yap shRNA mice compared with the counterpart in AAV Control mice (Fig. 6C). Liver/body weight ratio were both significantly decreased after fasting in AAV Control mice and AAV Yap shRNA mice (Fig. 6D). Furthermore, shrunken liver by fasting was nearly returned to the normal level at 6 h post-refeeding in AAV Control mice, which was delayed in AAV Yap shRNA mice (Fig. 6C). Meanwhile, liver/body weight ratio in 6-hour refed group were also lower than that of the feeding group in AAV Yap shRNA mice (4.81% versus 4.38%, P < 0.05; Fig. 6D), compared to AAV Control mice, indicating that the knockdown of Yap delayed the recovery of liver size during refeeding.
Figure 6.
The knockdown of Yap delays the recovery of liver size following refeeding in AAV Yap shRNA mice. (A) AAV Control and AAV Yap shRNA mice were fed ad libitum (Fed) or fasted for 24 h (Fasted) and then refed for 6 and 24 h (Refed); (B) Western blot analysis of YAP in AAV Control and AAV Yap shRNA mice; (C) Representative liver photographs for refed groups in AAV Control and AAV Yap shRNA mice; (D) Liver/body weight ratio (n = 5); (E) H&E staining of representative mice liver samples. Scale bar = 100 μm; (F) Immunohistochemical staining of CTNNB1 around CV area. Scale bar = 50 μm; (G) Immunohistochemical staining of CCND1 around PV area. Scale bar = 100 μm; (H) Quantification of cell size (n = 3); (I) Quantification of CCND1 positive cells (n = 3). Data are presented as mean ± SD. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 and ∗∗∗∗P < 0.0001 versus Fed group. Statistical significance was determined by two-way ANOVA.
H&E staining shows no liver necrosis (Fig. 6E). Serum levels of ALT, AST were slightly increased after refeeding for 6 h in AAV Yap shRNA mice, but no significant changes of serum ALP and mRNA expression of pro-inflammation cytokines Tnfα, Ifnγ and Il6 were observed (Supporting Information Fig. S5C and S5D). Overall, these results suggested that fasting and refeeding did not induce obvious liver injury in AAV Yap shRNA mice. CTNNB1 staining shows that the hepatocyte size around CV and PV area were both significantly decreased after fasting in AAV Control mice and AAV Yap shRNA mice (Fig. 6F and H, Fig. S6A and S6C). No significant difference of hepatocyte size was observed between the fed group and 6-hour refed group in AAV Control mice, while hepatocyte size of the 6-hour refed group was significantly smaller than that of the feeding group in AAV Yap shRNA mice (Fig. 6F and H, Fig. S6A and S6C). In line with this, the decreased number of CCND1 positive cells by fasting were basically up to pre-fasting state after refed 6 h in AAV Control mice. However, the CCND1 positive cells were still barely seen after refeeding for 6 h in AAV Yap shRNA mice (Fig. 6G and I, Fig. S6B and S6D). Moreover, the expression of the proliferation-related proteins CCNA1, CCND1, and CCNE1 were measured and changes of CCND1 protein expression were consistent with CCND1 staining, while protein expressions of CCNA1 and CCNE1 were no significant changes (Fig. S6E and S6G). Taken together, these results suggest that YAP played an important role in the recovery of liver size during fasting to refeeding transition.
4. Discussion
Liver is a central hub for macronutrient metabolism, which size is strictly regulated. Several lines of evidence indicate that fasting reduced liver weight in rodents10,14, 15, 16, while the molecular mechanism remains unclear. As a key regulator of organ size control, YAP regulates liver size by promoting hepatocyte proliferation and hypertrophy17,22, 23, 24. The current study demonstrated that fasting significantly reduced liver size, which was recovered to the normal level after refeeding. Moreover, hepatocyte size was decreased and hepatocyte proliferation was inhibited after fasting. Conversely, refeeding promoted hepatocyte enlargement and proliferation compared to fasted state. Mechanistically, fasting or refeeding regulated the expression of YAP and its downstream targets, as well as the proliferation-related major cell cycle proliferative regulator, CCND1. Furthermore, fasting significantly reduced the liver size in AAV control mice, which was reversed in Yap overexpressed mice. Yap overexpression also prevented the effect of fasting on hepatocyte size and proliferation. Besides, the recovery of liver size after refeeding was delayed in AAV Yap shRNA mice. Yap knockdown attenuated refeeding-induced hepatocyte enlargement and proliferation. In summary, this study demonstrated that YAP plays an important role in dynamic changes of liver size during fasting-refeeding transition, which provides new evidence for YAP in regulating liver size under energy stress.
It has been reported that liver weight was decreased after fasting and was increased during refeeding periods14, and the liver function remained unaffected14,15,26. In agreement with these results, the current study confirmed that fasting reduced the liver size by reducing hepatocyte size and inhibiting hepatocyte proliferation, and then these phenotypes nearly recovered at 6 h post-refeeding, and there was no liver injury and necrosis during fasting and refeeding. Additionally, chronic inflammation is associated with metabolic diseases. Evidences have demonstrated that fasting was able to reduce inflammation, which can improve chronic inflammatory disease in human and mice27,28. The present studies show that the mRNA levels of pro-inflammatory factors Ifnγ and Il6 showed a downward trend after fasting, further confirming that long-term fasting has inhibitory effect on inflammation.
YAP is the key effector of Hippo signaling pathway, which regulates organ size, cell proliferation and apoptosis. The overexpression of YAP induces liver enlargement in mice17. Previous studies showed that YAP phosphorylation was higher in liver of fasted mice than in liver of refed mice. Compared with those of fasted mice, translocation of YAP into nucleus and transcription of its downstream target genes were significantly promoted in the livers of refed mice29. In agreement with these results, the current studies show that the protein expression of YAP was significantly downregulated after fasting, and then restored after refeeding. Therefore, we hypothesize that YAP might be involved in changes of liver size induced by fasting and refeeding. Therefore, to avoid degradation of exogenous YAP, constitutively active YAP (YAP (5SA)) was overexpressed in mice liver to investigate whether YAP resists the liver size reduction induced by fasting. The results showed that the percentage reduction of liver/body weight ratio in fasting-treated AAV Yap (5SA) mice were lower than the counterpart in AAV Control mice, indicating that constitutively active YAP resisted the shrinkage of liver induced by fasting. Furthermore, AAV Yap shRNA mice were used to reveal that the suppression of YAP delayed the recovery of liver size during refeeding, suggesting that YAP is essential for the recovery of liver size during fasting to refeeding transition. Interestingly, no difference was observed in mRNA expression of Yap between fed-state and fasted-state liver (data not shown), indicating that down-regulation of YAP protein was attributed to post-transcriptional mechanisms. YAP is usually phosphorylated by LATS1/2 and then interacts with 14-3-3, leading to its retention and degradation in the cytoplasm30. It was reported that activation of AMPK can lead to YAP phosphorylation and inhibition via multiple mechanisms29,31. Thus, we assumed that AMPK signaling pathway might also be involved in the regulation of YAP expression during fasting to refeeding transition. The above results indicated that YAP played an important role in regulating the changes of liver size during fasting and refeeding, suggesting the physiological relevance of energy homeostasis via the regulation of YAP activity.
Furthermore, that will be interesting to elucidate how YAP levels and function are regulated by feeding and fasting, and how this change is associated with other known factors in liver size/regeneration. Numerous signaling pathways have been reported to orchestrate liver size and liver regeneration, such as Hedgehog, Notch, and mTOR pathway32, 33, 34, 35. Hedgehog signaling pathways, a key regulator in liver development, is closely associated with liver size and regeneration32. Notch pathway is involved in liver regeneration by regulating hepatocyte proliferation and cholangiocyte differentiation33,34. Sengupta et al.35 demonstrated that activation of mTOR prevents the effects of fasting on liver shrinkage using Li-Tsc1KO mice expressing constitutively active mTOR. Notably, there also exist crosstalk between these signaling pathways and YAP. Hedgehog can function as an upstream regulator of YAP in regulating liver regeneration36. There also exists crosstalk between Notch and YAP: Notch ligands can drive the YAP-mediated transcriptional regulation; Notch and YAP signaling share the joined co-transcription regulation of the same downstream targets37. It was also reported that mTOR and Hippo–YAP signaling pathways collaborate to regulate organ size via controlling cell growth and proliferation38. Notably, there exist crosstalk between these signaling pathways and YAP36, 37, 38, and they might jointly participate in the regulation of liver size during the fasting-refeeding transition, which are needed further studies.
In normal adult liver, hepatocytes are usually maintained in a slow-turnover state39, and KI67 positive cells are almost undetectable by immunohistochemical staining. CCND1 is an important regulator of cell cycle, which is indispensable for G1-to-S phase transition40. Evidences demonstrate that fasting inhibits cell proliferation14,15,41, 42, 43. In agreement with these results, the present study shows that the number of CCND1 positive cells and CCND1 protein expression in liver were significantly decreased after fasting, and then recovered following refeeding. The recovery of CCND1 expression were impeded by Yap knockdown after fasting to feeding transition, and the down-regulation of CCND1 expression induced by fasting was ameliorated when hepatic YAP was overexpressed in mice. These results suggest that fasting could induce cell cycle arrest at the G1–S phase in hepatocytes and inhibit cell proliferation by down-regulating the protein level of YAP. Additionally, it was reported that fasting can induce cell apoptosis41,44. In line with these results, the current study shows that the protein level of cleaved caspase-3 was significantly increased after fasting for 24 h, then recovered after refeeding (Supporting Information Fig. S7), indicating that fasting might induce the cell apoptosis to decrease the hepatocyte numbers and liver size and reduce energy consumption, and then supply nutrient substance for liver cells to maintain the basic function of liver. However, the expression of cleaved caspase-3 was downregulated after fasting for 48 h. Regarding the difference, we hypothesized that the apoptotic pathway might be activated upon fasting conditions, which led to the decrease of hepatocyte numbers. However, with the prolonged fasting time, the hepatocyte apoptosis was suppressed by a negative feedback loop to maintain liver homeostasis.
Studies have demonstrated that fasting has profound effects on aging, health, and diseases obesity45. Fasting can drive a metabolic switch from glucose-based to ketone-based energy, with increased stress resilience, increased lifespan, and a decreased incidence of diseases, including cancer and obesity. Preclinical and clinical studies demonstrate that fasting has a great potential for cancer therapy11,46,47. Evidence indicates that fasting not only induces apoptosis of cancer cells but significantly sensitizes hepatocellular carcinoma (HCC) cell to antineoplastic agents48, 49, 50. YAP hyperactivation drives initiation and progression of multiple cancer types and resistance to various targeted and chemotherapies51. Although YAP is a potential drug target in various cancer, there is no direct inhibitors of YAP or FDA-approved clinical drug available51. In the present study, we demonstrated that fasting reduced liver size by down-regulating the protein expression of YAP. Given that YAP hyperactivation drives initiation and progression of multiple cancer types and resistance to various targeted and chemotherapies, our study might suggest fasting as a potential therapeutic strategy for cancer treatment by inhibiting YAP and inducing apoptosis of cancer cells.
5. Conclusions
In summary, for the first time, the current study demonstrates that fasting reduces the liver size by down-regulating YAP, while shrunken liver returns to normal level accompanied with an increase of YAP. Liver-specific knockdown of Yap delayed the recovery of liver size during refeeding while overexpression of Yap maintained the liver size after fasting. These findings explore the molecular mechanism of liver size alteration during fasting and refeeding transition, providing new evidence for physiological role of YAP in regulating liver size under energy stress.
Acknowledgments
The work was supported by the National Key R&D Program of China (2022YFA1104900), the Natural Science Foundation of China (Grant number: 82025034, 81973392), the Shenzhen Science and Technology Program (KQTD20190929174023858, China), the 111 project (Grant number: B16047, China), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (Grant number: 2017BT01Y093, China), and the National Engineering and Technology Research Center for New drug Druggability Evaluation (Seed Program of Guangdong Province, Grant number: 2017B090903004, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2022.12.011.
Contributor Information
Min Huang, Email: huangmin@mail.sysu.edu.cn.
Huichang Bi, Email: bihchang@smu.edu.cn.
Author contributions
Huichang Bi, Shicheng Fan and Min Huang conceived and designed the project. Xuan Li, Chenghui Cai, Yue Gao, Xinhui Wang, Yifei Zhang, Hangfei Liang, Huilin Li, Jie Yang performed the experiments. Huichang Bi, Xuan Li and Shicheng Fan wrote and revised the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Andersson E.R. In the zone for liver proliferation. Science. 2021;371:887–888. doi: 10.1126/science.abg4864. [DOI] [PubMed] [Google Scholar]
- 2.Michalopoulos G.K., Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18:40–55. doi: 10.1038/s41575-020-0342-4. [DOI] [PubMed] [Google Scholar]
- 3.Michalopoulos G.K., DeFrances M.C. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 4.Jiao T.Y., Yao X.P., Zhao Y.Y., Zhou Y.Y., Gao Y., Fan S.C., et al. Dexamethasone-induced liver enlargement is related to PXR/YAP activation and lipid accumulation but not hepatocyte proliferation. Drug Metab Dispos. 2020;48:830–839. doi: 10.1124/dmd.120.000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y.Y., Yao X.P., Jiao T.Y., Tian J.N., Gao Y., Fan S.C., et al. Schisandrol B promotes liver enlargement via activation of PXR and YAP pathways in mice. Phytomedicine. 2021;84 doi: 10.1016/j.phymed.2021.153520. [DOI] [PubMed] [Google Scholar]
- 6.Yao X.P., Jiao T.Y., Jiang Y.M., Fan S.C., Zhao Y.Y., Yang X., et al. PXR mediates mifepristone-induced hepatomegaly in mice. Acta Pharmacol Sin. 2022;43:146–156. doi: 10.1038/s41401-021-00633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S., Yoon H., Eom K. Retrospective quantitative assessment of liver size by measurement of radiographic liver area in small-breed dogs. Am J Vet Res. 2019;80:1122–1128. doi: 10.2460/ajvr.80.12.1122. [DOI] [PubMed] [Google Scholar]
- 8.Zaitoun A.A., Apelqvist G., Al-Mardini H., Gray T., Bengtsson F., Record C.O. Quantitative studies of liver atrophy after portacaval shunt in the rat. J Surg Res. 2006;131:225–232. doi: 10.1016/j.jss.2005.11.587. [DOI] [PubMed] [Google Scholar]
- 9.Shindoh J., Kobayashi Y., Kinowaki K., Mise Y., Gonoi W., Yoshida S., et al. Dynamic changes in normal liver parenchymal volume during chemotherapy for colorectal cancer: liver atrophy as an alternate marker of chemotherapy-associated liver injury. Ann Surg Oncol. 2019;26:4100–4107. doi: 10.1245/s10434-019-07740-x. [DOI] [PubMed] [Google Scholar]
- 10.Weindruch R., Sohal R.S. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longo V.D., Mattson M.P. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green C.L., Lamming D.W., Fontana L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol. 2022;23:56–73. doi: 10.1038/s41580-021-00411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofer S.J., Carmona-Gutierrez D., Mueller M.I., Madeo F. The ups and downs of caloric restriction and fasting: from molecular effects to clinical application. EMBO Mol Med. 2022;14 doi: 10.15252/emmm.202114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kouda K., Nakamura H., Kohno H., Ha-Kawa S.K., Tokunaga R., Sawada S. Dietary restriction: effects of short-term fasting on protein uptake and cell death/proliferation in the rat liver. Mech Ageing Dev. 2004;125:375–380. doi: 10.1016/j.mad.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Sokolović M., Sokolović A., Wehkamp D., Ver Loren van Themaat E., de Waart D.R., Gilhuijs-Pederson L.A., et al. The transcriptomic signature of fasting murine liver. BMC Genomics. 2008;9:528. doi: 10.1186/1471-2164-9-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tessitore L., Tomasi C., Greco M. Fasting-induced apoptosis in rat liver is blocked by cycloheximide. Eur J Cell Biol. 1999;78:573–579. doi: 10.1016/S0171-9335(99)80023-5. [DOI] [PubMed] [Google Scholar]
- 17.Dong J.X., Feldmann G., Huang J.B., Wu S., Zhang N.L., Comerford S.A., et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camargo F.D., Gokhale S., Johnnidis J.B., Fu D., Bell G.W., Jaenisch R., et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 19.Zhao B., Li L., Tumaneng K., Wang C.Y., Guan K.L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grijalva J.L., Huizenga M., Mueller K., Rodriguez S., Brazzo J., Camargo F., et al. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014;307:G196–G204. doi: 10.1152/ajpgi.00077.2014. [DOI] [PubMed] [Google Scholar]
- 21.Lu L., Finegold M.J., Johnson R.L. Hippo pathway coactivators Yap and Taz are required to coordinate mammalian liver regeneration. Exp Mol Med. 2018;50:e423. doi: 10.1038/emm.2017.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y.M., Feng D.C., Ma X.C., Fan S.C., Gao Y., Fu K.L., et al. Pregnane X receptor regulates liver size and liver cell fate by Yes-associated protein activation in mice. Hepatology. 2019;69:343–358. doi: 10.1002/hep.30131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan S.C., Gao Y., Qu A.J., Jiang Y.M., Li H., Xie G.M., et al. YAP-TEAD mediates PPARα-induced hepatomegaly and liver regeneration in mice. Hepatology. 2022;75:74–88. doi: 10.1002/hep.32105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Y., Fan S.C., Li H., Jiang Y.M., Yao X.P., Zhu S.G., et al. Constitutive androstane receptor induced-hepatomegaly and liver regeneration is partially yes-associated protein activation. Acta Pharm Sin B. 2021;11:727–737. doi: 10.1016/j.apsb.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y.H., Meng Q., Yang M.B., Liu D.Y., Hou X.Y., Tang L., et al. Current trends in drug metabolism and pharmacokinetics. Acta Pharm Sin B. 2019;9:1113–1144. doi: 10.1016/j.apsb.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J.B., Cheng Y., Su Q., Ai W., Gong L., Wang Y.Y., et al. Effects of intermittent fasting on liver physiology and metabolism in mice. Exp Ther Med. 2021;22:950. doi: 10.3892/etm.2021.10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan S., Tung N., Casanova-Acebes M., Chang C., Cantoni C., Zhang D.C., et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell. 2019;178:1102–1114. doi: 10.1016/j.cell.2019.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller H., de Toledo F.W., Resch K.L. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Scand J Rheumatol. 2001;30:1–10. doi: 10.1080/030097401750065256. [DOI] [PubMed] [Google Scholar]
- 29.Wang W., Xiao Z.D., Li X., Aziz K.E., Gan B., Johnson R.L., et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma S.H., Meng Z.P., Chen R., Guan K.L. The Hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88:577–604. doi: 10.1146/annurev-biochem-013118-111829. [DOI] [PubMed] [Google Scholar]
- 31.Mo J.S., Meng Z., Kim Y.C., Park H.W., Hansen C.G., Kim S., et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochoa B., Syn W.-K., Delgado I., Karaca G.F., Jung Y., Wang J., et al. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology. 2010;51:1712–1723. doi: 10.1002/hep.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams J.M., Jafar-Nejad H. The roles of Notch signaling in liver development and disease. Biomolecules. 2019;9:608. doi: 10.3390/biom9100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geisler F., Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology. 2015;61:382–392. doi: 10.1002/hep.27268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sengupta S., Peterson T.R., Laplante M., Oh S., Sabatini D.M. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- 36.Swiderska-Syn M., Xie G.H., Michelotti G.A., Jewell M.L., Premont R.T., Syn W.K., et al. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64:232–244. doi: 10.1002/hep.28542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Totaro A., Castellan M., Di Biagio D., Piccolo S. Crosstalk between YAP/TAZ and Notch signaling. Trends Cell Biol. 2018;28:560–573. doi: 10.1016/j.tcb.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Csibi A., Blenis J. Hippo–YAP and mTOR pathways collaborate to regulate organ size. Nat Cell Biol. 2012;14:1244–1245. doi: 10.1038/ncb2634. [DOI] [PubMed] [Google Scholar]
- 39.Fausto N., Campbell J.S. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 40.Tchakarska G., Sola B. The double dealing of cyclin D1. Cell Cycle. 2020;19:163–178. doi: 10.1080/15384101.2019.1706903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tessitore L. Apoptosis and cell proliferation are involved in the initiation of liver carcinogenesis by a subnecrogenic dose of diethylnitrosamine in refed rats. J Nutr. 2000;130:104–110. doi: 10.1093/jn/130.1.104. [DOI] [PubMed] [Google Scholar]
- 42.Okada T., Fukuda S., Hase K., Nishiumi S., Izumi Y., Yoshida M., et al. Microbiota-derived lactate accelerates colon epithelial cell turnover in starvation-refed mice. Nat Commun. 2013;4:1654. doi: 10.1038/ncomms2668. [DOI] [PubMed] [Google Scholar]
- 43.Weng M.L., Chen W.K., Chen X.Y., Lu H., Sun Z.R., Yu Q., et al. Fasting inhibits aerobic glycolysis and proliferation in colorectal cancer via the Fdft1-mediated AKT/mTOR/HIF1α pathway suppression. Nat Commun. 2020;11:1869. doi: 10.1038/s41467-020-15795-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Nagai M., Noguchi R., Takahashi D., Morikawa T., Koshida K., Komiyama S., et al. Fasting-refeeding impacts immune cell dynamics and mucosal immune responses. Cell. 2019;178:1072–1087. doi: 10.1016/j.cell.2019.07.047. [DOI] [PubMed] [Google Scholar]
- 45.de Cabo R., Mattson M.P. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381:2541–2551. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 46.Clifton K.K., Ma C.X., Fontana L., Peterson L.L. Intermittent fasting in the prevention and treatment of cancer. CA Cancer J Clin. 2021;71:527–546. doi: 10.3322/caac.21694. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J., Deng Y.L., Khoo B.L. Fasting to enhance cancer treatment in models: the next steps. J Biomed Sci. 2020;27:58. doi: 10.1186/s12929-020-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi J., Chen X.Y., Wu Q.C., Wang J., Zhang H., Mao A.R., et al. Fasting induces hepatocellular carcinoma cell apoptosis by inhibiting SET8 expression. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/3985089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W.Z., Wang Y.F., Zhou X.B., Pan X.H., Lü J.H., Sun H.L., et al. The anti-tumor efficacy of 20(S)-protopanaxadiol, an active metabolite of ginseng, according to fasting on hepatocellular carcinoma. J Ginseng Res. 2022;46:167–174. doi: 10.1016/j.jgr.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krstic J., Reinisch I., Schindlmaier K., Galhuber M., Riahi Z., Berger N., et al. Fasting improves therapeutic response in hepatocellular carcinoma through p53-dependent metabolic synergism. Sci Adv. 2022;8:eabh2635. doi: 10.1126/sciadv.abh2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen C.D.K., Yi C.L. YAP/TAZ signaling and resistance to cancer therapy. Trends Cancer. 2019;5:283–296. doi: 10.1016/j.trecan.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.