Abstract
Gas therapy has been proven to be a promising and advantageous treatment option for cancers. Studies have shown that nitric oxide (NO) is one of the smallest structurally significant gas molecules with great potential to suppress cancer. However, there is controversy and concern about its use as it exhibits the opposite physiological effects based on its levels in the tumor. Therefore, the anti-cancer mechanism of NO is the key to cancer treatment, and rationally designed NO delivery systems are crucial to the success of NO biomedical applications. This review summarizes the endogenous production of NO, its physiological mechanisms of action, the application of NO in cancer treatment, and nano-delivery systems for delivering NO donors. Moreover, it briefly reviews challenges in delivering NO from different nanoparticles and the issues associated with its combination treatment strategies. The advantages and challenges of various NO delivery platforms are recapitulated for possible transformation into clinical applications.
Key words: Nitric oxide, Nitric oxide donor, Nitric oxide-based delivery system, Anti-cancer, Binding mechanisms, Synergistic treatment strategy, Inorganic platforms, Organic platforms
Graphical abstract
Roles of NO in cancer treatment, nanoplatforms for NO donors’ delivery alone as well as in combination with other modalities, and the challenges.

1. Introduction
The diagnosis and treatment of tumor has always been a global problem. At present, cancer treatment mainly includes surgery, radiotherapy, chemotherapy and molecular targeted therapy. Although these methods can inhibit tumor growth to a certain extent, there are still some disadvantages. For example, surgery can be traumatic to the patient with the chances of recurrence. Radiotherapy and chemotherapy have high systemic toxicity and side effects. Molecular targeted therapies have a limited range of action. Therefore, there is an urgent need to develop safe and effective new methods for cancer treatment. Relevant studies have shown that a class of special gas signal molecules in human body, including nitric oxide (NO), carbon monoxide, and hydrogen sulfide, possess unique biological activities and chemical properties1. Among the many gaseous molecular transmitters currently studied, NO has shown extremely superior physiological and pathological effects on various cells2. NO is mainly produced in vivo by the oxidation reaction of NO synthase (NOS)3. NOS widely exists in mammalian endothelial cells, macrophages, neurophagocytic cells, and nerve cells4. The research over the years has revealed that NO signals are important in mucus production, pain perception, vasodilation, sleep control and regulation, sphincter contraction and relaxation, erection regulation, and the normal functioning of the immune system5.
In addition to regulating some normal physiological activities, a large number of studies have confirmed that NO is closely related to the occurrence and development of many diseases, especially tumors6. The NO affects tumor apoptosis, metastasis, drug resistance and so on. Existing data show that there is a dual relationship between NO and tumor: appropriate concentration of NO can promote tumor growth, while high concentration of NO has anti-tumor effect7. Therefore, the use of NO for the treatment of cancer has attracted extensive attention of scientists. However, NO is a radical gaseous species with a short half-life and significant limitations for direct transport. To solve this problem, researchers have developed a variety of stimuli-responsive NO delivery donors and have tried to use different nano-delivery systems to transport various NO donors. This review summarizes the endogenous production of NO, its physiological mechanisms of action, the application of NO in cancer treatment, and nano-delivery systems for delivering NO donors (Fig. 1). Moreover, it briefly reviews challenges in delivering NO from different nanoparticles (NPs) and its combination synergistic treatment strategies. The advantages and challenges of various NO delivery platforms are recapitulated for possible transformation into clinical applications.
Figure 1.
The general illustration showing anticancer mechanisms of NO, NO donors and diverse platforms for NO delivery.
1.1. Endogenous production of NO
NO is synthesized in mammals NOS-dependent pathway (l-arginine-NO pathway) and independent (nitrate-nitrite-NO pathway) pathways (Fig. 2). NOS is an isoenzyme that can generate endogenous NO and is present in endothelial cells, macrophages, neurophagocytes, and nerve cells. In the presence of nicotinamide adenine dinucleotide phosphate (NADPH) and molecular oxygen, it catalyzes the release of NO from the nitrogen residue of l-arginine8. There are three subtypes of NOS: neural type (nNOS/NOS1), inducible type (iNOS/NOS2), and endothelial type (eNOS/NOS3)9. NOS1 is mainly expressed in the brain and peripheral nervous system, where the produced NO functions as a neurotransmitter and participates in the regulation and control of stroke and neurodegenerative diseases10. NOS3 is mainly expressed in vascular endothelium. NO, catalyzed by NOS3, plays a key role in regulating cell proliferation, vascular tension, platelet aggregation, and leukocyte adhesion11. The enzymatic activities of NOS1 and NOS3 are also calcium dependent. In contrast, NOS2, whose expression is most easily observed in monocytes or macrophages of human patients with infectious diseases, can produce NO for a longer time and in a relatively high amount as it is not calcium-dependent12,13. The NO produced by NOS2 can fight against inflammation, foreign bacteria, and mutated tumor cells. The NOS is essentially a dimer, and each monomer has two different catalytic domains: the N-terminal oxygenase domain and the C-terminal reductase domain. Although these three forms of NOS are regulated and translated from different genes, their structures and catalytic conditions are similar; NADPH, L-arginine, and oxygen serve as substrates, whereas flavin adenine dinucleotide oxidized form, calmodulin, flavin mononucleotide, and tetrahydrobiopterin are required as cofactors14. As a result, these enzymes malfunction during hypoxia. Furthermore, the absence of certain substrates or cofactors in the catalytic process can lead to NOS uncoupling, where these enzymes convert NO production to superoxide production.
Figure 2.
Nitric oxide synthase (NOS)-dependent (l-Arginine-NO pathway) and independent (Nitrate-nitrite-NO pathway) pathways.
In order to explore the above problems, researchers have revealed an alternative way of NO production when NOS is absent in the human body15. The nitrate-nitrite-NO pathway is an alternative system for NO production, supporting and supplementing the normative NOS-dependent NO production, especially if the later fails16. Nitrate and nitrite are not only the oxidation products produced by NOS-dependent NO, but are also components of our diet (such as green vegetables)17. Nitrate is reduced to nitrite by facultative anaerobic bacteria on the surface of the tongue. Nitrite can be further reduced to NO and other bioactive nitrogen oxides by non-enzymatic mechanisms (such as acidic and hypoxic conditions) and several proteins (such as hemoglobin, myoglobin, neuroglobin, xanthine oxidoreductase and complexes)18. Above all, these two pathways maintain the delicate steady state of this highly diffusive and reactive gas at the same time and regulate human physiological balance.
1.2. Physiological effects of NO
The characteristic of NO with radical electrons (lone pair electrons) endows it with reactivity to inorganic molecules (oxygen, superoxide or transition metal), prosthetic groups and DNA structure, thus depicting its wide range of biological activities19. The effect of NO is mainly mediated through two methods: cyclic guanosine phosphate (cGMP)-dependent and cGMP-independent. NO, catalyzed by NOS1 and NOS3, acts mainly through a cGMP-dependent pathway. At physiological concentrations, NO catalyzes the conversion of its substrate guanosine triphosphate into the secondary signal molecule, cGMP, by binding to the heme group of soluble guanylate cyclase (sGC), thereby it regulates the protein kinase G signaling pathway20. Protein kinase G also regulates many other proteins in this signal channel, such as NOS, sGC, etc21. cGMP can also activate cGMP-dependent protein kinase (cGK), which mediates local and general signaling. Activation of the NO/cGMP/cGKI pathway can also induce smooth muscle relaxation by reducing cytosolic calcium levels and/or calcium desensitization of contractile elements22. Therefore, NO regulates a variety of physiological functions through cGMP behavior, such as proliferation, intestinal peristalsis, platelet aggregation, apoptosis, vascular tone, and neurotransmission.
The cGMP-independent pathway mainly occurs through the reaction of NO with superoxide (O2-·), molecular O2, mercaptans, and transition metals (such as zinc). According to research, the cGMP independent effect of NO is mainly implemented in three ways. Firstly, the interaction of NO with O2 and O2- results into various oxidative homologues at a very fast reaction rate, making it possible for NO to trigger various forms of modification of targeted nodes in vivo. These oxidative homologues include increased reactivity (nitrous oxide, N2O3), enhanced toxicity (such as nitrogen dioxide, NO2), greater oxidation activity (peroxynitrite, ONOO−), and higher stability nitrite (NO2-) and nitrate (NO3-) species23. According to research, low-concentration reactive nitrogen substances (RNS) have a beneficial effect in immune response and regulate a series of physiological responses such as maintaining the normal metabolism and redox homeostasis of cells24,25. On the contrary, abnormal levels of RNS can also irreversibly destroy biological functions of macromolecules (lipids, nucleic acids, and proteins) to cause an imbalance of oxidation and antioxidant effects in the body26. The production of these RNS has an important influence on the cellular stress response induced by NO. The main reason is that rather than the direct cytotoxic interaction of NO with the biological targets, it can cause irreversible modification of genetic material and biological macromolecules27,28. Secondly, it can be considered that the reaction of NO with the metals (usually iron, copper, and manganese) bound to the protein is biologically significant. NO binding to the metals in the active part of the protein could prevent cells from performing normal functions. For example, NO can inactivate, catalase, cytochrome oxidase, protease, nitrile hydratase, and cytochrome P45029. Thirdly, post-translational modification regulates cell signaling, mainly through the formation of S-nitrosothiol. NO can react with thiols in proteins or peptides to form S-nitrosothiols (SNOs), which are thiol derivatives generated by substituting nitroso cations for the protons on the ‒SH functional group. SNOs are not only the main storage pathway of NO in the body, but also the source of sulfur-based free radicals that regulate some physiological processes30, 31, 32. Another more common post-translational modification mediated by NO is S-glutathionylation, such as formation of mixed disulfides between glutathione (GSH) and target protein cysteine residues33. This modification also changes the structure and function of redox-sensitive proteins containing sulfhydryl groups and is related to many pathological mechanisms in mammalian cells34,35. At the same time, cysteine residues are also essential for maintaining NOS3 function36. Chen et al.37 proved that excessive production of highly reactive molecules such as reactive oxygen species (ROS) and RNS in vivo by peroxidation can alter NOS3 activity through the S-GSH pathway.
1.3. Mechanism of NO in cancer treatment
It has been observed that NO can affect the development, growth, and metastasis of tumor cells. However, many studies have reported contrasting results. The proper concentration of NO in the body can not only kill cancer cells directly, but also enhance the efficacy of other treatment methods. However, NO at a certain concentration can also promote the canceration of cells. Therefore, the scientific community has been debating over the years whether NO is tumor-killing or carcinogenic. Fortunately, in 2008, Ridnour et al.38 put much of the controversy to rest by reporting a specific NO concentration threshold. Increased cGMP at NO concentrations of 1–30 nmol/L promotes angiogenesis and endothelial cell proliferation, whereas, at NO concentration of 30‒100 nmol/L, a significant increase in protein kinase B and extracellular regulated protein kinase signals the proliferation and anti-apoptotic response of tumor cells. At 100‒300 nmol/L, hypoxia-inducible factor (HIF)-1α stabilizes and increases vascular endothelial growth factor. Hence, these levels of NO promote tumor growth39. However, above 300 nmol/L, NO has anticancer properties. It leads to increased phosphorylation of p53, MKP-1 expression, and inhibition of cellular respiration40,41.
The mechanisms by which NO can be used in cancer treatment are mainly to produce high cytotoxicity, promote cancer cell apoptosis, inhibit cancer cell metastasis, and improve the efficacy of other drugs42. The tumoricidal or tumor-promoting effects of NO also depend on time and location43. NO can regulate the transition between apoptosis and autophagy by disrupting beclin 1/Vps34 association and increasing BCL-2/beclin 1 interactions44. The apoptosis effect of NO is also related to the cell type45,46. Studies have found that NO promotes apoptosis in HCC cells47, while NO inhibits apoptosis in melanoma cells48. Due to the dichotomous role of NO in cancer, the role of NO on metastasis cannot be easily divided into “pro-metastatic” or “anti-metastatic” because it may depend on other factors, such as cell type49, dose50,51, organ52, and even metastasis stage. For the metastasis of tumor cells, NO mainly regulates the expression and inhibition of galectin-3. Galectin-3 overexpression promotes tumor transformation and maintains the transformed phenotype, and enhances the adhesion of tumor cells to the extracellular matrix and increases metastatic spread. Scientists have demonstrated that in human breast cancer (BT549) cells, galectin-3 enhances the metastatic potential through the NOS2 cytotoxic pathway to allow cancer cells to escape53. On the other hand, NO has also been shown to reduce cancer aggressiveness, which ultimately hinders the metastatic properties of cells54.
Understanding the antitumor properties of NO is of great significance for exploiting its role in tumor biology. This review comprehensively explains the antitumor mechanisms of NO from the following aspects (Table 1)55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73.
Table 1.
The main mechanism of NO for cancer treatment.
| Role of NO | Mechanism of NO activity | Ref. |
|---|---|---|
| Causes genotoxicity | Depolarization through mitochondria, caspase-3 activation and DNA fragmentation | 55, 56, 57, 58, 59, 60 |
| Suppression of cellular respiration and shifts in iron metabolism | ||
| Generation of oxygen free radicals Inactivation certain key enzymes in cell metabolism | ||
| Promotes cancer cell apoptosis | Decrease in BCL-2 expression | 61, 62, 63, 64, 65 |
| Promotion of the production of ceramide | ||
| Macrophage activation P53 accumulation | ||
| Inhibits cancer cell metastasis | Decrease in galectin-3 expression Inhibition of platelet aggregation and tumor metastasis |
66,68,69 |
| Improves the efficacy of other drugs | Upregulation of MMP-2 and 9 to loose tumor tissue structure | 67,72 |
| Destruction of the cell membrane to inhibit multidrug resistance | ||
| Inhibition of the ATPase activity of P-gp to inhibit multidrug resistance Normalization of blood vessels at tumor sites to increase the penetration of chemotherapy drugs | ||
| Reverses MDR | Decreased expression of P-gp and MRP | 70,71,73 |
| Depletion of GSH | ||
| Regulation of NF-κB and HIF |
GSH, glutathione; HIF, hypoxia-induced factors; MDR, multidrug resistance; MRP, multidrug resistance related protein; NO, nitric oxide; P-gp, P-glycoprotein.
1.3.1. Regulation of the oxygen concentration
The killing of malignant tumors by NO can be carried out by regulating the oxygen concentration at the tumor site. Hypoxia is a universally recognized biological feature of tumors74. Insufficient hemoperfusion caused by local space limitations and delayed angiogenesis can easily lead to the formation of a hypoxic microenvironment in the tumor75. HIF-1 is a regulatory factor produced by cells in order to adapt to the hypoxic environment76. HIF-1 plays a crucial role in tumor growth and metastasis, because it can bind to the corresponding target genes to regulate cell growth and proliferation, energy metabolism, and angiogenesis. HIF has been shown to directly regulate the activity of hypoxia-induced NOS in a variety of cell types, including liver cancer cells and glioblastoma77,78. NO generated from various NO donors has been shown to inhibit the expression of HIF-1α via activation of the prolyl hydroxylasedomain proteins. Therefore, inhibiting NO synthesis can lead to hyponitroxia which is exacerbated by the lack of NO-regulated blood flow79. Although the hypoxic microenvironment can inhibit the growth of tumors to a certain extent by inhibiting the energy metabolism of tumors via inhibition of the cytochrome oxidase, however, some malignant solid tumors can adapt to the hypoxic microenvironment by changing their own metabolism and malignant biological behavior. A tumor-friendly environment is formed to transform the microenvironment by causing tumor cells to release biomolecules and biosignals. Therefore, the mutually reinforcing cycle of hypoxia/hyponitroxia will promote the progression of malignant tumors to a certain extent, and both O2 and NO can be used as targets for killing tumors. O2 restoration is a complex and difficult operation, whereas NO has become a relatively operable target. The continuous low NO concentration caused by hypoxia in cancer is the best “environment” to promote the development of cancer. If the NO concentration moves up and down slightly, the “safe area” will become the “kill area” or “inhibition area” for cancer80. This provides a treatment plan to increase or reduce NO concentration by increasing exogenous NO donors or regulating NO-related factors such as NOS to induce tumor death.
Different NO donors (sodium nitroprusside, nitroglycerin, and isosorbide dinitrate) have been studied to reduce HIF-1α protein levels to alleviate the hypoxic environment under hypoxic conditions in Hep3B cells81. Yasuda et al.82 demonstrated that the rates of immunoreactive cells for HIF-1α in tumor tissues treated with nitroglycerin were lower than those without nitroglycerin.
1.3.2. Genotoxicity
Reports in recent years have shown that the NO provided by either exogenous donors or some cells (macrophages or endothelial cells) have inhibitory or even kill effects on various tumors83. These studies indicate that NO can be used as a cytotoxic molecule. NO-mediated cytotoxicity involves inhibition of mitochondrial respiration and DNA strand synthesis in cancer cells55. One of the mechanisms by which NO kills tumor cells is to damage DNA. NO can mediate DNA damage through any of the following three possible mechanisms: (i) the involvement of NO in a series of reactions that damage DNA56, 57, 58. (ii) Modification of DNA strands directly84,85; and (iii) nitrosation of NO directly inhibits specific DNA repair systems. For example, NO-mediated cysteine nitrosation at the active site inhibits several key DNA repair enzymes, such as alkyltransferases and zinc finger proteins59. Furthermore, studies have shown that NO can cause DNA double-strand to break in mammalian cells. In addition, nitrosation and oxidative stress seem to produce different types of mutations in DNA. For example, the highly nitrosative NO product, N2O3, can deaminate DNA bases and cause DNA mutations60. NO-mediated oxidative stress also has a damaging effect on DNA86.
The physiological regulation of mitochondrial respiration by NO is also associated with tumor cytotoxicity. In an earlier study, NO produced by l-arginine was shown to inhibit mitochondrial respiration in tumor target cells87. At both low and high concentrations, NO had inhibitory effects on mitochondria, resulting in a decrease in mitochondrial membrane potential and disruption of normal mitochondrial function88,89. NO at low concentrations can reversibly inhibit cytochrome oxidase, while NO at high concentrations alternately inhibit complexes in the respiratory chain, such as reversible inhibition of complex IV and irreversible inhibition of complex I. The effects of low concentration NO are reversible, but these last longer at higher concentrations.
1.3.3. Cancer cell apoptosis
Apoptosis is a gene-regulated form of programmed cell death, which is an important regulatory process for the normal growth and development of organisms90,91. The process of apoptosis is briefly summarized as the following steps: (1) the cell receives apoptotic signals to initiate the apoptotic process; (2) the “death receptor” on the cell surface binds to its corresponding ligand (such as Fas, TNFR1 or TRAIL); (3) proteolytic enzymes (caspase) closely related to apoptosis are activated, and 4) entering a continuous reaction process92. Signaling events in cell apoptosis occur very quickly and can be understood by identifying the key roles of apoptosis-inhibiting molecules, caspases, BCL-2 family members, and mitochondria.
Several reports indicated that NO and some RNS can directly cause cell necrosis or apoptosis or programmed cell death61. For example, transfection of NOS2 gene into murine melanoma cells can eliminate tumorigenicity and metastatic ability, which indicates endogenous NO-mediated apoptosis62. Although the decisive elements for NO-induced apoptosis have not been clearly identified, NO-induced apoptosis may trigger the accumulation of cell cycle regulator P5363. Studies have demonstrated that NO at high concentrations can lead to phosphorylation and acetylation of P5364. The phosphorylation of P53 prevents it from binding to mouse double minute 2 homolog and prevents P53 from being ubiquitinated and degraded. NO can stabilize P53 through the phosphorylation of the serine/threonine part, leading to a high level of wild-type P53; ERK phosphatase MKP-1 is also activated by NO, preventing cell proliferation and causing cell apoptosis93. The induction of apoptosis often requires high NO levels provided by exogenous NO donors. When the concentration of NO approaches or exceeds 1 μmol/L, cell death is caused by protein nitrosation, nitrification, and alkylation94. NOS2 usually produces NO at a concentration of 1 mol/L for several days95, and non-physiological high concentrations of NO/S-nitrosothiol can promote cell death96.
NO can also regulate other signaling factors related to cell apoptosis through various mechanisms, including activation of c-Jun N-terminal kinase and ERK in the MAPK signaling pathway97. The MAPK signaling pathway is related to the activation of the caspase cascade, and the P38 MAPK signaling pathway is closely involved in the activation of caspase-8 and -965. Overall, it is a subject of considerable significance and potential importance to understand the factors that control the effects of exposure to high concentrations of NO on cell viability and apoptosis in tumors.
1.3.4. Inhibition of cancer cell metastasis
One of the main reasons for the failure of surgery to cure cancer is the metastasis of tumor that leads to its recurrence from time to time98. When tumor expands to a certain volume, tumor cells enter the blood vessels, and then invade other normal tissues and organs via bloodstream. Cancer cells invade normal tissues in the form of epithelial sheets or single cells. In most cases, metastasis to the regional lymph nodes or distant organs occurs before the primary tumor is diagnosed99. Furthermore, metastases are often so widespread or inaccessible that delivery of therapeutic agents with tolerable toxic effects is compromised100.
Compared with benign tumors, malignant tumors are more likely to produce secondary metastasis. Pre-metastatic malignant tumors include lung cancer, digestive system cancer (liver cancer, esophageal cancer, colorectal cancer, and pancreatic cancer), leukemia, breast cancer, lymphoma, and brain cancer. Metastatic secondary tumors are tumors that receive signals to take root in otherwise healthy places through a series of movements. In this process, tumor cells complete multiple sequential, complex, and highly selective steps52,101,102. Tumors generally metastasize through the following main steps: (a) tumor cells receive and respond to growth signals and begin to proliferate excessively. (b) Tumor masses (diameter of <2 mm) first lose the ability to adhere to adjacent tumor cells and receive oxygen and nutrients through diffusion. (c) Tumor cells invade into the extracellular matrix and then migrate into the blood and lymphatic vessels. Integrins may contribute to locomotion such as synchronized forward migration by the operation of actin cytoskeletal filaments. Uncontrollable invasion of cancer cells into the blood circulation and normal tissues is an indispensable step in metastasis. In addition, loose blood vessels common in fast-growing tumors allow tumor cells to easily enter the bloodstream without the need for complex invasion operations. (d) After entering the venous bloodstream, the cancer cells are either single cells or homotypic aggregates or heterotypic aggregates with white blood cells and/or platelets. (e) Some larger tumor thrombi are blocked and captured by tiny blood vessels where they adhere, divide, and proliferate around the blood vessels and the cells of the parenchymal organs, and a small tumor cell clone is formed around the blood vessels, which is called micrometastasis. Tumor cells lost their tight tissue structure by reducing cell adhesion to facilitate cell clusters or single cell invasion. Additionally, tumor cells and stromal cells can also degrade the extracellular matrix by secreting proteases (such as matrix metalloproteinases). Studies have shown that tumor metastasis depends on the growth of blood vessels. Tumor-induced angiogenesis regulates many angiogenic factors, such as: platelet-derived growth factor, vascular endothelial growth factor, pancreatin-like growth factor and transforming factor-α. These angiogenic factors degrade the vascular endothelial matrix, promote the division, migration and proliferation of endothelial cells to induce the growth of host capillaries that promote the formation and enlargement of metastases103.
NO· has been shown to affect certain key factors in the metastatic process of tumor cells, including invasiveness, motility and adhesion66. However, whether high concentrations of NO promote or inhibit metastasis is still a controversial issue. For example, NO regulates MMPs which are involved in the breakdown of tumor matrix. NO produced from donor spermine-NONOate or ONOO− of SIN-1 has been shown to inhibit MMP-9 through a mechanism involving caveolin-1 at the interface of human non-small cell lung cancer cells and endothelial cells in co-culture expression and activation67. However, some indirect evidences suggest that ONOO− produced by NO· and superoxide (O2-·) under pathological conditions may activate proMMPs to MMPs in tumors68. Another possible mode of action of NO in controlling the cancer metastasis is via inhibition of galectin-369,104. For example, paclitaxel has been shown to inhibit the metastasis-enhancing effect of galectin-3 by stimulating the production of NO in human liver cancer cells, HepG2105. Contrarily, many studies have also revealed the pro-angiogenic effect of NO that promotes metastasis106.
1.3.5. Multidrug resistance (MDR) reversal
In the history of the fight against cancer, many treatment options, including chemotherapy, radiation therapy, and surgical resection have made huge contributions. However, the MDR of cancer cells is still an important obstacle to overcome. Resistance to anticancer drugs is caused by a decrease in the effective drug concentration in tumor cells, which is usually attributed from the enhanced metabolism or inactivation of drugs in the body, poor penetration across tissues, and insufficient blood supply to tumors107. One of the most critical factors associated with drug resistance is the overexpression of the membrane transporters (ABC transporters) in cancer cells which can expel a variety of cytotoxic drugs from cancer cells, resulting in a significant reduction in their efficacy. ABCB1, called P-glycoprotein (P-gp), ABCC1-6, called MDR-related protein (MRP) 1–6 and ABGC2, called breast cancer resistance protein, are the main efflux transporters that cause MDR108. Research on anti-tumor drugs and drug delivery systems that can evade MDR has aroused great interest in the scientific community.
The properties of NO have been used in anti-cancer treatments to overcome drug resistance70. For example, NO can reduce the expression of MRP, P-gp, and tumor cell MDR markers. Therefore, developing a controllable and stable NO donor to reverse MDR seems a decent therapeutic approach. According to Riganti's discovery, NO reversed the drug resistance of colon cancer cells through ABC efflux transporter nitrification, inhibiting its activity and increasing the accumulation of anticancer drugs in MDR cells71. It has also been experimentally proven that the NO donor JS-K can inhibit the ATPase activity of P-gp in NCI/ADR-RES cells to overcome MDR109. In addition, NO causes the consumption of GSH, which usually inactivates platinum-based drugs72. Due to the high affinity of platinum for sulfur groups, thiol-containing molecules can cause the inactivation of platinum-based drugs110. Therefore, the effectiveness of platinum-based drugs can be improved by inducing endogenous NOS activity and providing exogenous NO donors. NO also inhibits the activation of HIF, which regulates gene transcription and induces MDR under anaerobic conditions. The reversal of the MDR effect could be triggered by the instability or inactivation of HIF-1α as the dimerization of HIF1α/β induces the expression of MRP73. It has also been shown that NO increases the chemosensitivity of certain cancer cells through NF-κB related pathways. It is known that NF-κB not only regulates survival and metastasis pathways, but also regulates drug resistance111.
2. NO donors
Insufficient NO production in the body is often closely related to the formation of various diseases112, so exogenous NO is of great significance for the prevention and treatment of these diseases113. Since NO is difficult to control as a gas, compounds that release NO in situ (NO donors) have been developed and used in biological research, and have become an important focus in NO research. NO donor is a kind of compound that releases NO after simple enzymatic hydrolysis or non-enzymatic action in the body. Small molecule NO donors mainly include metal-NO-complexes, SNOs, BNN6, NO-drug hybrids, and N-diazenium diolates (NONOates)114 (Fig. 3). NO donors, which were originally used as a research tool, were inefficient in controlling the release of NO due to spontaneous decomposition. Considering that the formation of NO in vivo is precisely regulated by enzymes and signal transduction mechanisms, a controllable NO donor is pivotal in the development of potential therapeutic agents. Therefore, NO donors that can be released in response to external factors have attracted widespread attention. Herein, this article will introduce NO donors from two aspects: endogenous stimuli sensitive donors and exogenous stimuli sensitive donors.
Figure 3.
Common small molecule NO donors.
2.1. Endogenous stimuli sensitive donors
The endogenous stimuli include pH, GSH, NOS2, and glucose. NONOates are well-known and widely used compounds that are triggered by acidic environments, such as tumor microenvironment, to release NO115. They can be synthesized from amines and NO. The rate of decomposition depends not only on the pH of the solution, but also on the substituents of the secondary amine groups. Once NONOate is dissolved in an aqueous solution within the physiological pH range, it can be hydrolyzed to generate two molecules of NO without unique metabolism or redox reaction116. Phenylsulfonyl furoxan (PSF) and nitrate polymers can be triggered by the high concentration of GSH in cancer cells to release NO117. Arginine is the precursor of NO, which produces NO under the action of NOS2 in the cytoplasm of phagocytes. In addition, l-arginine can also be used as a glucose-sensitive NO donor, because glucose oxidase (GOx) can convert glucose into gluconic acid and H2O2, and H2O2 can promote the release of NO from l-arginine118.
2.2. Exogenous stimuli sensitive donors
The commonly used external stimuli are light and X-ray. The trigger lights are generally ultraviolet light (UV) and near infrared light (NIR). BNN6 is a UV-sensitive NO donor, which is degraded into NO and N,Nʹ-2, 5-cyclohexadiene-1,4-di-sec-butylamine (BHA) under UV stimulation119. The release of NO in response to UV may cause certain phototoxicity to the body, thus limiting its practical application. Therefore, the use of a more penetrating NIR light-sensitive NO donor has broader application prospects120. SNOs is a commonly used multiple response NO donor. Liu et al.121 designed and synthesized hyaluronic acid (HA)-modified Ag@S-nitrosothiol core-shell NPs by combining NO-based chemotherapy and photothermal therapy (PTT). In response to NIR and the generated heat, the polymer shell with SNOs released high concentration of free NO. SNOs not only respond to the NIR and the generated heat, but also respond to X-ray. Fan and colleagues122 constructed a smart X-ray controlled NO release up-conversion nanotherapy system by designing upconversion NPs (UCNPs) with S-nitrosothiol-grafted mesoporous silica (Si). The NPs were designed to decompose the S-N bond of S-NO in response to x-ray radiation to release NO, resulting in X-ray-induced NO release for on-demand hypoxia by radiation sensitization.
3. The binding mechanisms of NO-donor and carrier
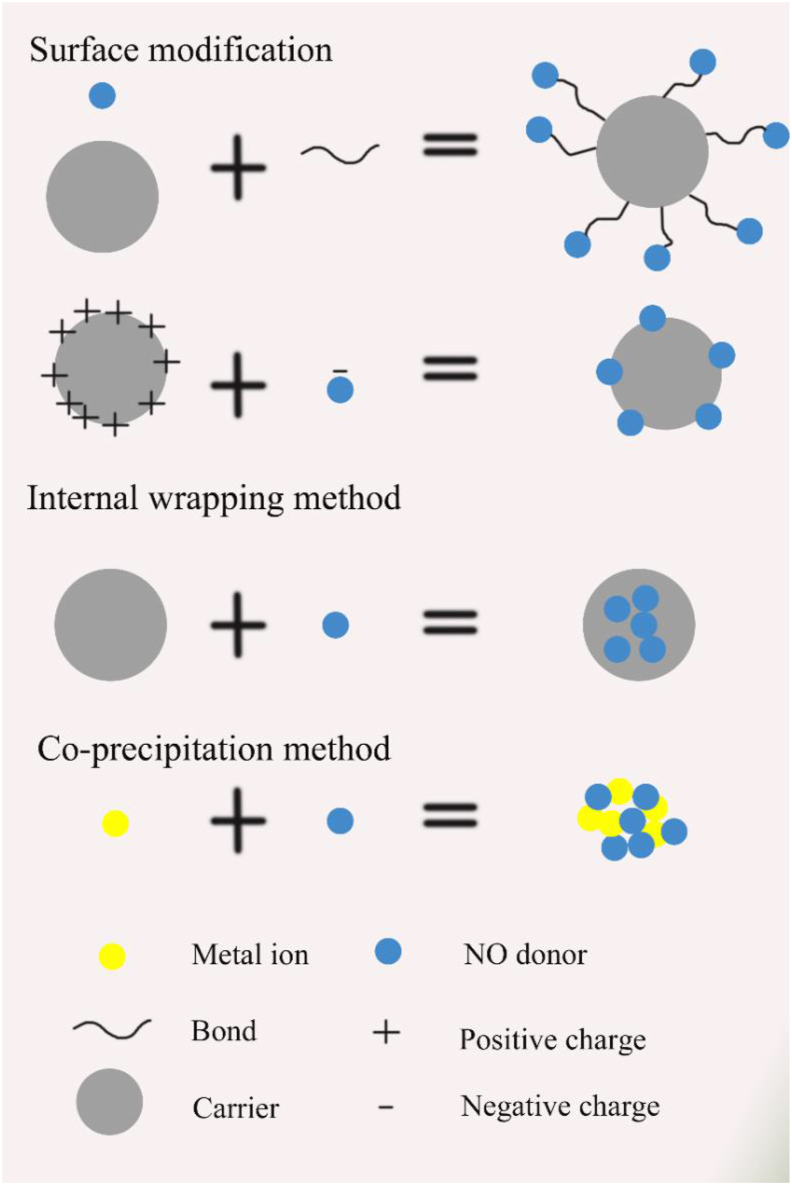
NO donor can be loaded on different carriers by mainly three approaches, surface modification, internal wrapping, and co-precipitation (Fig. 4). Here, some representative NO donors are mentioned to illustrate these three methods.
Figure 4.
The binding mechanism of NO-donor and carrier.
SNOs decompose into disulfides in the solution and release NO stably without vascular tolerance. Therefore, these compounds are currently one of the most widely used NO donors. The most commonly used way of binding NO donor to the carrier is through some linkages such as sulfhydryl modification on the surface of the carrier. Dong et al.123 modified 3-mercaptopropyltrimethoxysilane (MPTMS) to introduce thiol groups onto the surface of mesoporous silica NPs (MSN), and then reacted it with NaNO2 to convert the thiols into SNOs and generate NPs loaded with SNOs (Fig. 5). Katayama et al.124 added terminal sulfhydryl groups to HSA by incubating 0.15 mmol/L rHSA with 3 mmol/L Traut's reagent (2-imminothiolane) in 100 mmol/L potassium phosphate buffer containing 0.5 mmol/L DTPA (pH 7.8) for 1 h at room temperature. The terminal sulfhydryl groups were then incubated with 15 mmol/L isoamyl nitrite for 3 h at room temperature for S-nitrosylation. In addition, Taladriz-Blanco et al.125 demonstrated the surface modification of citrate stabilized gold NPs with DL-penicillamine (PEN) and N-acetyl-d,l-penicillamine (NAP) through the thiol functionality. The sulfhydryl groups on the surface of the gold NPs were nitrosated into the corresponding nitrosothiol. In addition to the SNOs, there are many other NO donors that can also be connected to the surface of the carrier through surface modification, such as the N-diazoniumdiolates. In addition to bonding, the NO donor can also be functionalized on the surface of the carrier through electrostatic interactions. For example, a large number of amino groups on the chitosan can bind with Roussin's black salt (RBS) through electrostatic interaction of RBS [NH4] [Fe4S3(NO)7]126.
Figure 5.
(A) Schematic representation of DN@MSN preparation and (B) the proposed mechanism for NO donor-mediated collagen depletion and improved nanoparticle penetration in tumors. Reprinted with the permission from Ref. 123. Copyright © 2020 Taylor & Francis Group.
The internal packaging methods may include oil-in-water emulsification and electrostatic adsorption. The dinitrosyl iron complex (DNIC) is a water-insoluble NO donor. Zhang et al.127 encapsulated formaldehyde and nitric oxide obtained from nitrated or nitrosated urotropine in liposomes by thin lipid film hydration and sequential extrusion. Since the concentration of H2O2 in tumors is much higher than that in normal organs, l-arginine, which can spontaneously react with hydrogen peroxide to produce NO, is an excellent NO donor. It is mostly combined with the carrier by internal loading. Zhang et al.128 loaded l-arginine into the cavity and mesopores of MSN. The combination of NO donor and carrier depends on the nature of the carrier.
Whether it is external modification or internal packaging, low donor loading efficiency and the complexity in the structure limit potential utilization of NO donors. The co-precipitation method has been developed to address this issue. Hu et al.129 created a GSH-sensitive NO donor (1,5-bis[(l-proline-1-yl) diazen-1-ium-1,2-diol-O2-yl]-2,4-dinitrobenzene, BPDB) (Fig. 6). Two carboxyl groups in the chemical structure of BPDB endow active sites for the coordination with metal ions. Therefore, the GSH sensitive NO donor, BPDB, coordinated with iron ions through simple precipitation followed by partial ion exchange process to form nano-scale coordination polymer (NCP). This loading method was simple and had excellent NO release ability. This approach not only provides higher loading of NO donor high, but also offers basis for NCP degradation by the high concentration of GSH (∼10 mmol/L) in tumor cells. Four NO molecules were released from each BPDB molecule triggered by two GSH molecules. This approach can also be used for the synthesis of new NO donors by engineering complexes of NO with different metals.
Figure 6.
(A) Synthetic route of BPDB (Compound 4); (B) Schematic illustration of Fe(III)-BNCP and Fe(II)-BNCP preparation; and (C) The mechanism of NO release in response to high concentrations of GSH. Reprinted with the permission from Ref. 129. Copyright © 2020 Taylor & Francis Group.
4. Platforms for NO delivery
This review discusses recent developments in NO donor delivery strategies categorized from inorganic and organic delivery systems. It will provide ideas for future research trends in NO transport (Table 2)123,128,130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152.
Table 2.
Characteristics of NO platforms.
| Carrier platform | NO donor | Stimuli | Mechanism of NO | NO donor loading site | Size (nm) | Tumor type | Ref. |
|---|---|---|---|---|---|---|---|
| Si NPs | SNOs | Spontaneous | Regulating the content and activity of MMP-1 and -2 | External modification | ∼100 | Breast cancer | 123 |
| l-Arginine | Ultrasound | apoptosis | Inner parcel | ∼500 | Panc-1 cells | 128 | |
| NONOates | Spontaneous | Cytotoxicity | External modification | 90–161 | Ovarian cells | 130 | |
| SNOs | Spontaneous | Cytotoxicity | External modification | 30 | Human alveolar epithelial cells | 131 | |
| DEA/NO | Endogenous esterase | Promoting the penetration | External modification | ∼210 | MCF-7/ADR cells | 132 | |
| AuNPs | l-Arginine | NIR | Overcoming multidrug resistance | Inner parcel | 128.6 | MCF-7/ADR cells | 133 |
| Nitro benzimidazole derivatives | UV | Cytotoxicity | External modification | 7 | HeLa cells | 134 | |
| QDs | PSF | GSH, | Cytotoxicity | Inner parcel | ∼7.7 | HeLa cells | 135 |
| PSF | GSH | Overcoming multidrug resistance | Inner parcel | ∼7.9 | SGC7901/ADR cells | 136 | |
| The nitroaniline derivative NO photodonor | UV | Cytotoxicity | External modification | N/A | HeLa cells, human xenograft BxPC-3 pancreatic | 137 | |
| UCNPs | RBS | NIR | Cytotoxicity, overcoming multidrug resistance | External modification | ∼70 | HeLa cells, MCF-7/ADR cells | 138 |
| BNN6 | UV | Overcoming multidrug resistance, Apoptosis |
Inner parcel | N/A | MCF-7/ADR cells | 139 | |
| Protein nanoparticles | SNOs | Spontaneous | Apoptosis | External modification | N/A | C26 cells | 140 |
| Polymer nanomicelles | SNOs | UV | Promote apoptosis | Inner parcel | 50 | A neuroblastoma BE(2)-C cell line | 141 |
| TNO3 | GSH | Cell cytotoxicity, apoptosis, Sensitization effect | Inner parcel | 133.9 | Hepatocarcinoma HepG2 cancer cells | 142 | |
| TNO3 | reduction oxidation | Tumor vascular normalization, overcoming multidrug resistance | Inner parcel | 10.0 | MCF-7/ADR cells | 143 | |
| l-Arginine | H2O2 | Overcoming multidrug resistance | External modification | 55 | Human oral Squamous cell Carcinoma Cell line | 144 | |
| Endogenous NO donor | Cu2+ | Dilating blood vessels | N/A | 92.9 | Vascular endothelial cells, 4T1 tumor cells | 145 | |
| Lipid NPs | DNIC | Acid | Tumor vascular normalization, improving immune function | Inner parcel | 119 | Hepatocellular carcinoma cells | 146 |
| JS-K | GSTs, S1P | Promoting osmosis | Inner parcel | 189 | Glioblastoma multiforme cells | 147 | |
| FZ-ss-FZ | FZ-ss-FZ | GSH | Cytotoxicity | co-precipitation | 160 | 4T1 cells | 148 |
| DSPE-mPEG5k | NRh-Bn-NO, NRh-Et-NO | NIR | Cytotoxicity | Inner parcel | 30 | U87MG cells | 149 |
| A nitrogluconic acid-containing copolymer | A nitrogluconic acid-containing copolymer | GSH | Overcoming multidrug resistance | Inner parcel | 150 | Human umbilical vein endothelial cells and human breast adenocarcinoma MDA-MB-231 triple-negative cancer cells | 150 |
| HA | HN | NIR | Improving penetration | Inner parcel | 264 | Human umbilical vein endothelial cells, 4T1 cells | 151 |
| AuNC@CBSA-PTX-ICG@HA-NO3 | SNOs | Spontaneous | Anti-metastasis effect | External modification | 300, 200, 100 | 4T1 cells | 152 |
DEA/NO, 1,1-diethyl-2-hydroxy-2-nitrosohydrazine; DNIC, dinitrosyl iron complex; GSH, glutathione; GSTs, glutathione S-transferases; HA, hyaluronic acid; NIR, near infrared light; NO, nitric oxide; NONOates, N-diazenium diolates; NPs, nanoparticles; PSF, phenylsulfonyl furoxan; RBS, roussin's black salt; SNOs, S-nitrosothiols; TNO3, nitrate functionalized d-α-tocopheryl polyethylene glycol succinate; UV, ultraviolet light.
4.1. Inorganic platforms for NO delivery
4.1.1. Silica NPs (Si NPs)
Si NPs are widely used as drug-loading systems due to their good drug loading capacity and biocompatibility153. Nanoporous Si, as a drug carrier, can realize immediate, sustained, and pH or temperature-sensitive release of drugs by virtue of its nanopore structure, morphology or control of the pore channels and surface functionalization154,155. The inorganic-organic hybrid Si can be prepared by the co-condensation of tetraethoxy or tetramethoxysilane and aminoalkoxysilane under appropriate conditions of ethanol (or methanol), ammonia and water156. Shin et al.157 converted the amine functional group in inorganic-organic hybrid Si to N-diazadiene diol salt by supplying NO (5 atm) under high pressure and alkaline conditions. This Si-derived NO storage/transport system has advantages over previously reported macromolecular NO donors, including: (1) loading of a large amount of NO (50‒1780 nmol/mg); (2) modulation of the release kinetics of NO (half-lives and NO release durations); and (3) ease of tuning the particle size (20‒500 nm). Stevens et al.130 designed NO-releasing Si NPs to improve the delivery of NO to human ovarian cancer cells while reducing the cytotoxicity to normal cells. They compared the cytotoxicity of NPs with the previously reported small molecule NO donor PYRRO/NO. Nanoparticle-derived NO showed greater inhibitory effect on the growth of ovarian tumor cells as a toxic agent. However, just by loading NO donors on the surface of Si NPs without the targeting of cancerous tissues could lead to lower circulation time and carrier deposition in normal tissues leading to serious side effects.
In order to increase the targeting of NO donors loaded Si NPs to the desired sites, serious efforts have been reported in the literature. Zhang et al.131 reported the synthesis of Fe3O4/Si core/shell NPs and their functionalization with SNOs. The NPs were spherical with a narrow particle size distribution, a core diameter of 10 nm and a shell thickness of 20 nm Fe3O4/Si surface-modified functionalized SNOs could store large amounts of NO, and the integrated superparamagnetic core conferred by Fe3O4 showed extremely strong magnetic targeting potential. An increase in the viability of the human alveolar epithelial cells treated with SNOs modified on Fe3O4/Si surface noted, suggesting the improved biocompatibility of the designed NPs. In addition to adding ligands for targeting tumor tissues on the loaded Si system, it is also important to integrate the knowledge of different physiological characteristics of tumor tissues. For instance, the fibrinogen content of the pancreatic cancer cell matrix is more abundant than other tumors, the mesoporous Si carrier system constructed by Zhang and colleagues123, 128, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152 were modified with fibrinogen cyclic decapeptide CGLIIQKNEC (CLT1-G-propargy1). l-Arginine as H2O2 responsive NO donor in this platform was found to possess seven-fold higher retention rate in a subcutaneous xenograft mouse model of Panc-1. As a result, inhibition was more pronounced and survival was increased (80 ± 5% improvement in 60-day survival).
NO has been frequently used to enhance cell sensitivity and enhance anti-cancer activity of chemotherapeutics. The NO donor-modified Si NPs have been loaded with chemotherapeutic drugs to achieve synergistic anti-cancer effect. Mesoporous Si NPs (MSNs) with an advantage of adjustable dielectric porosity have gradually replaced non-porous inorganic-organic hybrid Si as drug carriers158. Munaweera et al.159 developed a strategy to use lower dose cisplatin for anticancer therapy by exploiting the capability of NO to increase the chemosensitivity of cells. Amine-functionalized hybrid corrugated MSN (AMS) was first synthesized by the condensation of tetraethylorthosilicate and 3-triethoxysilylpropyldiethylenetriamine (Si-DETA). Cisplatin-AMS was then formed by adsorption of cisplatin and AMS. The experimental results showed that the average Pt uptake in NSCLC cells treated with NO-Si-DETA-cisplatin-AMS was 46.6% (H596) and 32.4% (A549) higher than in cells treated with Si-DETA-cisplatin-AMS, while for the normal cell lines, the increase was lower (WI-38) or non-existent (BEAS-2B). NO can also improve the tumoral permeation of nanomedicines. Studies have found that although nano-drugs can accumulate at the tumor site through the enhanced permeation and retention (EPR) effect, most of them remain only around the tumor blood vessels due to the obstruction of the tumor matrix (collagen, HA, etc.)160. Therefore, the targeted efficacy of nanomedicine in tumors is minimal, which has also become a major bottleneck restricting the clinical transformation of nanomedicines. Recently, Dong et al.123 prepared MSN loaded with doxorubicin (DOX), externally modified with NO donor nitrosothiol and PEG chains to treat breast cancer (DN@MSN). After intravenous administration, DN@MSN accumulated in tumor tissue through EPR effect and released NO through the decomposition of SNOs. NO-releasing NPs (N@MSN and DN@MSN) resulted in an approximately two-fold increase in the amount of 3-nitrotyrosine, a biomarker of ONOO−. Moreover, a 3.5-fold increase in levels of MMP-1 and -2 in tumors resulted in a decrease in collagen 1. The results suggested that the sustained NO released by S-nitrosothiol in this study facilitated NPs’ penetration in tumor tissues by virtue of higher MMP that caused lowering of collagen content (Fig. 5). The use of MSNs to load powerful anti-cancer drugs such as DOX and paclitaxel can not only enhance the anti-cancer effects, but also reduce the dose of anti-cancer drugs, thus, greatly enhancing the safety of the therapy.
In summary, MSNs are currently the most studied Si NPs for NO donor delivery due to following advantages: (1) in contrast to the organic carriers such as micelles and liposomes, MSNs have better stability and greater loading capacity. (2) The amine groups on the nanoporous Si surface can be easily modified into N-diazadiene glycolates for efficient NO transport. (3) The surface of MSNs is rich in a large number of hydroxyl groups, which makes it convenient to modify certain targets or functional materials, equipping them with targeting or stimuli-responsive properties (such as redox response, pH response, enzyme response, light response, or magnetic response). (4) The mesoporous structure can be loaded with a large number of chemotherapeutic drugs to achieve NO/chemotherapy synergistic therapy. Although MSNs have many advantages as a NO carrier, there are also some disadvantages that need to be further explored. For example, co-condensation is a commonly used MSNs’ preparation method that involves acidic or alkaline conditions, leading to the decomposition or denaturation of some coupling agents and NO donors. Moreover, the introduction of large number of functional groups could also destroy the mesoporous structure.
4.1.2. Gold NPs (AuNPs)
Gold (Au), one of the earliest metals discovered in history, due to its good chemical inertness, have been extensively studied in the field of inorganic nanomaterials161,162. In the fields of biochemistry and pharmacy, AuNPs are favored by researchers due to their dense gold core, small size, higher drug adsorption, biocompatibility, and stability under various conditions163,164. AuNPs have been explored as inorganic nano-delivery carriers for NO donors with the following advantages: (1) the surface modifiable AuNPs can improve the targeting of nanoformulations. (2) The excellent imaging function can monitor the changes in levels of NO. AuNPs have special electromagnetic properties, which enable them to exhibit peculiar optical phenomena, such as surface plasmon resonance and fluorescence, when excited by resonance. (3) AuNPs can be used for both covalent and non-covalent drug delivery. Non-covalent drug loading involves use of the structural features, such as hollow pores for drug loading. Covalent drug loading involves coupling of NO donors to the surface of AuNPs, such as SNOs or N-diazeniumdiolate NO donor molecules. (4) Excellent drug releasing capability of AuNPs could also be an advantage. On the one hand, the high concentration of GSH in tumor cells has stronger affinity for AuNPs, which can replace the drugs loaded by AuNPs through ligand displacement reaction. On the other hand, certain shapes of AuNPs tend to generate heat when irradiated by a specific wavelength of laser light. The generated heat promotes the breaking of chemical bonds on the surface, releasing anti-tumor drugs. (5) Compared to the spherical AuNPs (solid structure), Au nanorods with rod-like structure and hollow gold nanoshells with hollow spherical structure have better photothermal conversion efficiency. It can not only perform PTT/NO therapy with NO donors, but also release heat-sensitive NO donors. Levy and colleagues165 modified the Au nanoshells with the NO donor ammonium N-nitroso(4-mercaptomethylphenyl) hydroxylamine (TCF) through thiol bonds for photothermal conversion. NO of TCF was released due to the heat generated by hollow Au nanoshells in response to NIR exposure. AuNPs can also release NO from the NO donor by means of resonance energy transfer (RET). RET, as an early development of optical word technology, has now become a commonly used method in analytical detection and biological imaging. In RET, the coupling between the donor chromophore and the acceptor chromophore can be carried out through dipole-dipole interaction, and the energy is transferred from the donor to the acceptor in a non-radiative manner166. Wang et al.133 applied this principle to overcome MDR in early breast cancer by releasing higher concentrations of NO. Using l-arginine as an NO donor and DOX as a model drug, they prepared a drug-carrying liposome (ADLAu@CuS YSNPs) that could hydrolyze in response to NO. Under the irradiation of 808 nm laser, the NPs with Au NPs as the core effectively transformed l-arginine into NO due to the intense RET process and ROS generation in a confined space. NO further destroyed the phospholipid bilayer structure of the NO-responsive liposome layer, resulting in NO and DOX release. They detected the expression of P-gp in MCF-7/ADR cells and the fluorescence intensity of DOX in cells. These results demonstrated that NO could increase DOX accumulation in MCF-7/ADR cells by inhibiting the expression of P-gp. In MCF-7/ADR-bearing mice, the excellent tumor inhibition effect of ADLAu@CuS YSNPs further verified the potential of NO to inhibit MDR. In order to enhance the stability and controllability of NO release from NO donor groups on AuNPs, Sudhesh et al.134 synthesized 2-mercapto-5-nitrobenzo imidazole (MNBI) terminated AuNPs under aqueous conditions (MNBI-GNPs). This condition was intended to release NO in a controlled manner under visible light radiation. MNBI-GNPs showed improved NO release due to the chromophore interactions and chromophore plasmon-electron interactions of the dense photoresponsive capping agent (Fig. 7). The cytotoxic effects of 10 μL (10 μg/mL) cisplatin and 4 μL (4 μg/mL) MNBI-GNPs were almost the same, suggesting 60% lower dose of GNPs as effective as the 10 μg/mL cisplatin. In addition, Wang and his colleagues167 demonstrated that AuNPs can catalyze the generation of NO from endogenous or exogenous NO donors via S-nitrosothiol. Besides serving as an inorganic carrier to deliver NO donors, AuNPs are also commonly used as NO detectors in vivo. A small concentration of NO (∼10−8 M) can be detected by surface-enhanced Raman scattering spectroscopy of the complexes with AuNPs as cores as demonstrated by Peng's group168.
Figure 7.
Schematic representation showing HeLa cell apoptosis in the presence of MNBI-Stabilized GNPs. Reprinted with the permission from Ref. 147. Copyright © 2020 Taylor & Francis Group.
AuNPs’ excellent light-to-heat conversion effect could control NO release more accurately than other carriers. Despite a promising future, AuNPs have many challenges and shortcomings as delivery vehicles. Firstly, it is not easy to accurately control the size and shape of AuNPs. In fact, many synthesis processes produce variable dispersion of the particles. Secondly, the inhomogeneity and aggregation of AuNPs not only affect drug loading, but also cause drug loss in the blood circulation. Moreover, the small specific surface area of AuNPs limits the loading capacity for NO donors. Therefore, the design of efficient AuNPs still needs to be optimized.
4.1.3. Quantum dots (QDs)
QDs refer to semiconductor nanocrystals with dimensions smaller than or close to the exciton Bohr radius (1–10 nm) in all three dimensions169. By applying a certain electric field or light stimuli to the nano-semiconductor materials, they emit light that changes in frequency as the size of the semiconductor changes. These nano-semiconductors have the property of confining electrons and electron holes, which is similar to atoms or molecules in nature, therefore they are called quantum dots. The novel electronic and optical properties of QDs can be used in many important fields, such as electronics, optoelectronics, photovoltaics, and biomedicine170,171. The advantages of QDs as anti-tumor drug delivery carriers are: (1) the size of quantum dots can be precisely regulated, and the average size distribution is about 5%–10%. Appropriate size of QDs not only allows passage through the gaps between tumor vessels, but also avoids being cleared by the mononuclear phagocyte system (MPS). (2) The composition of QDs is easy to control. Different metal ions have different properties, safety and biocompatibility. (3) The emission wavelength of QDs can be tuned by changing the size and chemical composition. It avoids the problem of some weak penetrating and harmful lights such as UV light to living beings. A suitable emission wavelength is beneficial to the detection of living organisms, and at the same time, the interference of the fluorescent background on the detection signal is greatly reduced. (4) Wide excitation wavelength range and narrow emission wavelength range. This optical property of QDs permits selection of a more suitable excitation wavelength in its continuous excitation spectrum, so as to minimize the autofluorescence of biological samples and to improve the resolution and sensitivity. (5) Surface modification of QDs can improve fluorescence quantum yield, reduce toxicity, and improve stability and dispersion in the medium, as well as provide deeper therapy for targeted tumor cells. The traditional QDs that have been developed for drug delivery are CdSe QDs and ZnSe QDs172. But these may also incur heavy metal toxicity.
Recently, ZnO QDs have been studied as an effective drug carrier173. It is less toxic than conventional QDs such as cadmium selenide/zinc selenide QDs due to the absence of heavy metals. It is very suitable as a carrier for tumor therapy as it degrades under the acidic conditions of tumors, and the released Zn2+ ions can also inhibit the growth of cancer cells by inhibiting the calcium ion signal of tumor cell hyperactivation174. Therefore, combining ZnO QDs with other types of treatments such as NO therapy and chemotherapy can achieve synergistic anticancer effects. Moreover, the surface-capped amino groups of ZnO QDs can easily react with other groups such as carboxyl and epoxy groups, which means that ZnO QDs can be easily modified for certain targets. For example, Ding et al.135 prepared PSF loaded RGD-conjugated ZnO QDs (PSF@ZnO-RGD). GSH-responsive NO-donor PSF were loaded inside ZnO QDs because of the formation of coordination bonds with PSF through Zn2+, whereas the surface amino-rich ZnO QDs were bonded with the RGD. RGD with tumor-targeting function released PSF and Zn2+ due to decomposition by acidic environment of tumor cells. NO generated by PSF due to the abundant GSH in the cells had an inhibitory effect on cancer cells in combination with Zn2+. Regrettably, PSF@ZnO-RGD were tested for its synergistic anti-cancer effect at the cellular levels only. 2, 3-Dimethylmaleic anhydride (DMMA)-modified ZnO NPs were designed by solvothermal synthesis136 in which DOX and PSF were loaded via a step-by-step strategy. DOX was loaded by forming a coordinate bond with Zn2+, while PSF was loaded by amide reaction with amine groups. DMMA, a molecule with pH-responsive charge-reversal capability, was also modified on NPs through amine groups. The obtained (DOX, PSF) @ZnO-DMMA nanospheres were designed to synergistically treat drug-resistant gastric cancer by increasing the sensitivity of cancer cells to DOX by NO, and inhibiting cancer cells by combining DOX with Zn2+. The average diameter of complex multifunctional nanosphere was only 7.9 nm that demonstrated excellent anticancer effects against sGC7901/ADR cells and tumor-bearing model animals.
Carbon quantum dots (CDs) usually refer to monodispersed spherical carbon nanomaterials with size less than 10 nm, composed of sp2/sp3 carbon core and outer oxygen/nitrogen functional groups175. CDs are an ideal substitute material for traditional semiconductor QDs because they have properties similar to traditional semiconductor QDs but with lower toxicity and higher biocompatibility. The main reason for the limited application of some photosensitive NO donors is their lower absorption window than the phototherapy window (650–1350 nm)176, where the penetration of light through human tissues is only a few millimeters. A material with two-photon excitation (TPE) offers two major advantages in this problem: (1) it allows to be excited in a therapeutic window where soft tissue penetration depths greater than one centimeter can be achieved; (2) it allows for achieving high spatial resolution in three dimensions. The TPE cross-section adjustment function of CDs can shift the spectrum of the NO donor from 400 nm to 800 nm through energy resonance transfer, so that the regulating effect of NO can be combined with photodynamic therapy (PDT). This feature of CDs can be used to design light-sensitive NO donor delivery systems. Fowley et al.137 reported a conjugate between CDs and the nitroaniline derivative NO photodonor capable of generating anticancer NO radicals through an energy transfer mechanism. To determine the ability of these nanohybrids to produce cytotoxic effects against cancer cells in the treatment window, cell viability of HeLa cells was measured using MTT assay. This nanohybrid significantly reduced tumor volume in mice bearing human xenograft BxPC-3 pancreatic tumors under highly biocompatible 800 nm light (Fig. 8).
Figure 8.
The carboxylic-terminated CQDs 1, the NO photodonor 2 and the NO photoreleasing CQDs 3. Reprinted with the permission from Ref. 160. Copyright © 2020 Taylor & Francis Group.
Another advantage of CDs as drug carriers is that they can easily load drugs by following two ways. Firstly, drug loading on CDs via π‒π stacking can improve the solubility of poorly soluble NO donors. Secondly, the surface of CDs contains a large number of ‒COOH, which provides a basis to conjugate drugs. Carboxylic acid-terminal CDs are not only easily grafted with NO donors (such as nitroaniline NO donors), but also with other functional components. For example, Deng et al.177 reported a NO-delivery nanoplatform (Ru-NO@FA@CDs), which firstly combined ethylenediamine with abundant ‒COOH on the surface of CDs, and then facilitated covalent binding of Ru-NO, nitrosoruthenium and folic acid (FA) molecules through covalent amide bonds. When Ru-NO was covalently grafted, the electron absorption of Ru-NO@FA@CDs in the visible region was greatly enhanced compared to the bare CDs. This was beneficial because it allowed the use of longer wavelengths of light, rather than the UV light typically used for ruthenium nitroso groups, to trigger NO release. Many regulatory tasks undertaken by NO in cells are related to mitochondria. For example, NO at high concentrations inhibits many components of the respiratory pathway, including the oxygen-binding site of cytochrome oxidase. Targeted NO release systems can produce high cytotoxicity to cancer cells by specifically damaging the mitochondria of cancer cells. Xu and colleagues178 modified a triphenylphosphonium moiety onto CDs to obtain a mitochondrial targeting and photoresponsive NO releasing nanosystem. By monitoring the shift of JC-1 dye fluorescence emission, the change of mitochondrial membrane potential of HeLa cells after NO treatment was observed. The results showed that NO could lead to the loss of mitochondrial membrane potential and cause apoptosis through mitochondrial damage.
QDs are potential carrier systems for NO. However, the inertness of QDS in vivo, that is, the long-term toxicity to the organism, remains to be verified. At this stage, most of the application of QDs in human is still at the experimental stage. Therefore, QDs as the NO transporters need further and more extensive research.
4.1.4. Upconversion NPs (UCNPs)
UCNPs, a new type of fluorescent material, are a class of nanomaterials doped with lanthanides179. The up-conversion luminescence process refers to the process in which a single higher-energy photon is released after two or more low-energy photons are obtained, that is, NIR light can be converted into higher-energy UV light and visible light. Due to its significant advantages such as photostability, high chemical stability, large light penetration depth, no background light interference, and almost no damage to biological tissues, it has a wide range of application prospects in biomedicine180,181.
Using light as a trigger can solve the problems of difficult localization and insufficient sensitivity of drugs on the one hand, and control the site and dose by adjusting the irradiation power and duration on the other hand. By exploiting the specificity of photostimulation, it is possible not only to track drugs through imaging, but also to fine-tune the cytotoxicity of agents through light-controlled NO release systems. Photosensitive NO precursors, generally, only receive UV light or visible light to release NO, but their penetration is not as deep and safe as NIR penetration182. In search of a solution, scientists discovered that UCNPs can act as sensors to activate photosensitive NO precursors. The application of UCNPs to the photosensitive NO donor delivery has the following advantages183,184. First, the low-energy NIR can be upconverted into higher-energy light to achieve the spectrum required by the photosensitive NO donor. Second, NIR can be used as the excitation light source. Compared with the use of UV and visible light as the excitation light source, NIR is safer and has a deeper light transmission depth, which allows NO to be used for the treatment of deeper tumors. Third, UCNPs can be magnetically coated to localize and target cancer cells. Zhang et al.138 synthesized and developed the mesoporous-Si-coated white-light emitting UCNPs (NaYbF4:Tm@NaYF4:Yb/Er) as the NIR photosensitizing platform for dose-controllable NO release by using photolytic [NH][Fe4S3(NO)7] (RBS) as the NO donor. The NPs had a particle size of 70 nm with a shell-core structure. Spherical core of NaYbF4:Tm UCNPs had a size of 40 nm covered with the mesoporous shell of NaYF4:Yb/Er. The encapsulated RBS efficiently released NO by absorbing 980 nm NIR photons, converting them to higher-energy photons, and then transferring the energy to the NO donor. By controlling the output power of 980 nm NIR light, they were able to fine-tune the NO concentration-dependent biological effects of cancer therapy. In vitro cytotoxicity of HeLa and MCF-7 cells was evaluated by CCK-8 method. The results showed that high concentration of NO had strong cytotoxic effect. At the same time, DOX intake and related protein expression in MCF-7/ADR cells suggested that low concentration of NO can be used as P-gp modulator in combination with chemotherapy to overcome MDR. Li et al.139 also designed a core-shell UCNPs@MgSiO3 with upconversion effect. The cavity structure of UCNPs@MgSiO3 not only increased the loading of NO donors BNN6 and DOX, but also the branched flower-like structure on its surface increased the uptake of NPs by tumor cells. UCNPs converted NIR to UV to trigger the release of NO by BNN6. Further molecular and proteomic studies showed that NO down-regulated the ubiquitin-proteasome system and NF-κB signaling in cancer cells, reversing MDR and inducing apoptosis. They demonstrated the anticancer activity of UCNPs@MgSiO3-BNN6/ADR using MCF-7/ADR cells and tumor-bearing mice. With the rapid developments in nanotechnology and functionalization of NPs’ surfaces, targeted UCNPs have also emerged185,186.
The energy transfer properties of UCNPs are favorable, but there are also some defects in the application process. For example, there may be low energy transfer efficiency and normal tissue overheating may also occur. Generally, UCNPs require control of size and surface modification. In addition, the effects of surface modification and size on the in vivo behavior of UCNPs need to be further explored. UCNPs are mostly taken up by MPS, such as the liver and spleen. More optimized approaches need to be developed to improve pharmacokinetic dynamics and tumor targeting.
4.2. Organic platforms for NO delivery
4.2.1. Protein NPs
Entry of many exogenous vectors into the body stimulates the body's immune and inflammatory reactions, which could cause serious adverse reactions. Albumin NPs have been selected as a carrier for many drugs because of the endogenous nature, stable structure, and unique spatial construction of albumin that can increase the solubility of poorly soluble drugs in plasma187,188. The most interesting application of albumin NPs is to use them as carriers of anti-tumor drugs, because there are many binding sites such as tumor cell surface receptor gp60 and caveolin-1 in tumor cells for albumin, which can increase targeting and reduce drugs' side effects189. In addition, there are multiple functional groups on the surface of albumin, which can be modified with corresponding ligands to achieve further specific targeting190. Human serum albumin (HSA), one of the abundant plasma proteins, is a single-chain protein with a relative molecular mass of 67,000, consisting of amino acid residue (Cys-34) that can be used by S-nitrosylated free thiols to generate NO191. Regrettably, one molecule of HSA only has one Cys-34 available for conversion to NO donor, which is not enough. To address this issue, Katayama et al.192 increased the number of free sulfhydryl groups on HSA by sulfhydrylation via an iminothio ring. Then, NO-HSA was prepared by mixing HSA with isoamyl nitrite (6.64 mol NO/mol HSA). They investigated the inhibitory effect of NO-HSA on murine colon 26 carcinoma (C26). The prominent the anti-tumor effect was noted in in vitro experiments on C26 cells and in vivo C26 tumor-bearing mice. Experiments showed that NO-HSA generated ROS at tumor sites, activated caspase-3, and fragmented the DNA. Immunohistochemical analysis of tumor masses indicated that inhibition of tumor growth by NO-HSA was mediated through induction of apoptosis. Later on, they also evaluated the antitumor activity of nitrosylated HSA against the tumor cell line LY-80, a variant of Yoshida's sarcoma124. Mitochondrial depletion polarization, caspase-3 activation, and DNA fragmentation in two tumor cells in a dose-dependent manner were identified as the causes for nitrosylated HSA activity.
Unfortunately, HSA-based nanodelivery systems usually do not have circulation stability, that is, the NPs dissociate and release the drug prematurely before reaching the tumor targets193. Therefore, efforts have been continuously made to optimize the structure of nitrosylated HSA to obtain a more stable and longer-acting delivery systems. Studies have shown that poly-S-nitrosothiol-HSA releases NO very quickly at the cellular level, resulting in rapid cell apoptosis140. Experiments in tumor-bearing mice also showed that the half-life of the S-nitroso moiety in Poly-S-nitrosothiol-HSA was estimated to be only 18.9 min in vivo. It indicates that Poly-S-nitrosothiol-HSA is unstable and releases NO rapidly in cell or animal experiments. Therefore, Ishima and colleagues194 optimized the structure of Poly-S-nitrosothiol-HSA and converted it into a PEG-Poly-S-nitrosothiol-HSA dimer. They experimentally demonstrated that PEGylation can enhance the circulation time of Poly-S-nitrosothiol-HSA in vivo, and this dimerization enhanced the tumor-suppressive effect by 10-folds in C26 tumor-bearing mice due to the efficient delivery of NO. These data suggest that PEGylation and dimerization of poly- S-nitrosothiol-HSA are a very powerful strategy for boosting NO stability in tumors. It is well known that NO is an important regulator and promoter of EPR195. Seki et al.196 reported that NO donor nitroglycerin increased drug accumulation in solid tumors via EPR effect. In addition, Islam et al.197 investigated the role of three NO donors (nitroglycerin, l-arginine, and hydroxyurea) in ameliorating the EPR effect in various solid tumor models. The results showed that the different NO donors significantly increased (1.5-2-fold) the delivery of nano-drugs to different solid tumor models, while significantly improving (2-3-fold) the antitumor effects of these drugs. S-Nitrified HSA dimer as a novel nano-EPR enhancer had also been applied to the self-assembled systems such as micelles and liposomes198. The combination of micelles and liposomes composed of S-nitrified HSA dimer with Dox not only enhanced the antitumor effect, but also reduced the side effects of DOX199.
Although, albumin as NO donor has temperature and pH stability, its application has also some challenges. For example, availability and cost of HSA limit its extensive use, whereas, bovine serum albumin (BSA) is known to induce a mild immune response upon injection. Moreover, even if the NO donor is brought into the tumor tissues by a carrier, the anti-cancer effect is poor because it cannot be fully released. Studies have shown that pH- and GSH-sensitive keratin itself can promote NO production in cancer cells200, thereby sensitizing the tumor cells to drugs. Therefore, the nanosystem formed by keratin-coated DOX responded to pH, enzymes, and GSH at the tumor site, and keratin induced NO production by promoting NO release from S-nitrosothiol201. Some scientists also regard keratin as a new type of NO donor. Lu and his group202 designed a MB@keratin/PBA/d-α-tocopherol PEG 1000 succinate (TPGS) NPs (MB@KPTNPs) and demonstrated that keratin can produce a large amount of NO in the body and can deplete GSH in tumor tissue to increase the effect of PDT.
4.2.2. Polymer nanomicelles
Polymer nanomicelles are functional systems with hydrophobic core and hydrophilic shell formed by self-assembly of amphiphilic block polymers in aqueous solution by non-covalent bonds (hydrogen bonds, coordination bonds, hydrophilic and hydrophobic interactions). Hydrophobic segments of polymer micelles can be loaded with insoluble drugs, thereby improving their solubility and bioavailability. Moreover, the particle size of polymer micelles is tens of nanometers, which is conducive to avoiding MPS system, and the particles could passively target cancer sites through EPR effect203,204. NO donors equipped micelles could offer many advantages: (1) synthesis of micelles wrapped with NO donors is very easy, simple, and time and effort saving. (2) Micelles show a high drug loading efficiency, which means that the NO donors can effectively exert the therapeutic effect. (3) More importantly, the amphiphilic micelle system can encapsulate both water-soluble and insoluble NO donors.
The types of micelles are diverse because the commonly used hydrophilic end materials include PEG, HA, hydroxypropyl cellulose, etc., while the commonly used hydrophobic segment materials include cholesterol, poly (lactic-co-glycolic acid) (PLGA), polyamino acids, etc. Different NO donors can be chemically modified to the micellar surface or physically encapsulated internally. For example, Duong et al.141 bonded nitrosoglutathione to POEGMA-b-PVDM micelles by coupling the amino group of nitrosoglutathione to the azlactone, so that the water stability of nitrosoglutathione was increased. TPGS is regarded as a natural micelle due to its lipophilic tail and hydrophilic polar head. Song et al.142 functionalized nitrate-TPGS into nitrate functionalized d-α-tocopheryl polyethylene glycol succinate (TNO3) so that it can produce NO. They used DOX as a model drug to synthesize DOX&TNO3 with a particle size of 133.9 nm. They comprehensively investigated the ability of DOX&TNO3 to release NO in vitro, cytotoxicity and uptake ability of hepatoma cells (HepG2), as well as the kinetic behavior of DOX&TNO3 in vivo in rats. The cytotoxicity of DOX&TNO3 on HepG2 cells was compared with that of free DOX to evaluate the combined effect of TNO3 and DOX. The cell viabilities were 60 and 20% after exposure to free DOX of 10 μg/mL for 24 and 72 h, respectively. However, DOX&TNO3 exhibited a significantly low cell viability of 10% after incubation for 24 h. These results suggest that NO can induce cytotoxicity and sensitize tumor cells to chemotherapeutic agents. The synergistic effect of DOX & TNO3 was further verified against H22 and S180 tumor bearing mice. Later, they also constructed DOX-ADD@TPGS-NO micelles as pH-sensitive prodrugs by linking adjudin (ADD) to DOX143. They focused on examining low concentrations of NO to overcome MDR of DOX by MCF-7/ADR cells by normalizing tumor vasculature and increasing oxygenation at the tumor site. ADD acted synergistically with NO by disrupting mitochondrial function and inhibiting P-gp activity. Jin et al.144 prepared a redox micelle called micelle-DOX-Arg-GOx, which was formed by linking the hydrophilic fragment NO donor l-arginine and the hydrophobic fragment DOX with PEGylated disulfide bonds. The NPs were very stable, and the particle size only increased from the initial 45–55 nm after 7 days. Arginine is commonly used as a highly biocompatible NO donor in response to H2O2, however H2O2 is less abundant in the tumor environment. Therefore, they included GOx to catalyze the production of gluconic acid and H2O2 from glucose, within the micelles. These micelles released DOX and NO for synergistic treatment after redoxed by a large amount of GSH in the tumor. Cell and animal experiments on WSUHN6 (a human oral Squamous cell carcinoma cell line) and the tumor-bearing mice demonstrated the therapeutic effect of micellar DOX-Arg-GOx against tumor.
In addition to loading NO donors onto micelles, some scientists prepare micelles that can stimulate endogenous NO donors to release large amounts of NO. For example, Cu2+ is a good choice due to its ability to catalyze the decomposition of endogenous NO donors. Wei et al.145 prepared a micelle with Cu2+ modified on the surface. The micelles’ hydrophobic segments were polylactic acid and poly(ε-caprolactone) segments, and hydrophilic segment was PEG, whereas DOX was loaded inside. Cu2+ was modified on PEG to stimulate tumor cells to produce NO. This multifunctional hybrid polymer micelle was designed to selectively dilate tumor blood vessels using NO to enhance the accumulation of polymer micelles for effective cancer therapy. Costa et al.205 also prepared a micelle based on 3-methyl-5-phenylpyrazoline-1-(S-benzyldithiocarbamate) to induce NO production in macrophages.
Compared to other nanodelivery systems, the biggest challenge for micelles is their stability when intravenously injected into the body. The stability of micelles as NO transport vehicles may be affected by hemodilution effects, changes in blood pH, salt concentration, and blood flow rates. The main reason is that the micelles do not possess sufficient thermodynamically stability in an aqueous environment, which leads to the rapid release of NO donors after being diluted. Premature NO donors release not only increases the toxic and side effects, but also causes the low efficacy of NO in vivo.
4.2.3. Lipid NPs
Liposomes are biomimetic NPs that consist of phospholipid bilayer membranes and hydrophilic cavity206. They are used as nanocarriers for drug/gene delivery due to good biodegradation, biocompatibility, and absence of immune response207,208. Conventional liposomes can be modified to extend half-life, target the surface of cancer cells, and imaging via charge reversal, PEG modification, and ligand functionalization209. Liposomes can also encapsulate both fat-soluble and water-soluble drugs210. Therefore, liposomes have gradually become very famous in drug carrier research.
There are two types of drug loading methods in liposomes: active drug loading and passive drug loading. In the passive drug loading method, the formation of liposomes and drug loading is simultaneous, while the active drug loading method is to form blank liposomes followed by loading the drug. Sung et al.146 prepared a nano-delivery system (NanoNO) by oil-in-water single emulsion with biodegradable PLGA polymer as the main component. They dissolved PLGA, DSPE-PEG, TPGS, cholesterol, 1, 2-dioleoyl-sn-glycero-3-phosphocholine, and NO donor DNIC in the organic phase and then dropped the organic phase into the deionized water phase. Finally, NanoNO was obtained by sonication and centrifugation, with a particle size of about 120 nm. They used the concept that low doses of NO promote concentration-dependent changes in TME that lead to normalization of tumor blood vessels and enhance delivery of small anticancer drugs and large therapeutic molecules. They demonstrated NanoNO's ability to stimulate anti-tumor immunity by levels of immune factors. Their findings demonstrated the ability of nanoscale NO delivery to efficiently reprogram tumor vasculature and immune microenvironments to overcome resistance to cancer therapy. Liu et al.147 prepared a liposome delivery system (S1P/JS-K/Lipo), which included NO donor JS-K responsive to GSH S-transferases (GSTs) and sphingosine 1-phosphate (S1P) signaling molecule to treat glioma. This liposome was prepared by extrusion using membrane hydration and membrane filtration. The experimental results demonstrated that S1P/ks-k/Lipo effectively penetrated the brain–blood–tumor barrier under the action of microvesicle protein 1. It was observed that NO was catalytically released by GSTs to play a role in glioma mouse imaging. Masetto et al.211 encapsulated a novel NO-releasing gemcitabine prodrug (NO-GEM) in liposomes against GEM-resistant pancreatic ductal adenocarcinoma and observed encouraging anti-tumor results.
In the field of drug delivery, lipid NPs can be regarded as the universal carrier, but these do have some disadvantages. Lipid NPs delivering NO may prematurely leak loaded NO donors. Oxidative hydrolysis of phospholipids and particle aggregation are also the major stability issues. Additionally, the ordinary liposomes are mainly concentrated in the MPS organs such as liver, spleen and kidney. These problems limit the application of liposomes and need further efforts.
5. A synergistic treatment strategy of NO
With the great progress and continuous scientific exploration in the field of materials science, the understanding of the application and transport of NO has become more mature and comprehensive. By summarizing a large body of recent literature, we found that NO alone can be used as a drug to suppress tumors because of its genotoxic effect at high concentrations. For example, Gu et al.148 combined a NO-releasing prodrug dimer precursor (FZ-SS-FZ), that is, two NO donor molecules (FZ) connected by disulfide and ester bonds. FZ-SS-FZ@FA NPs were made by FZ-SS-FZ self-assembly in water, which was further functionalized with folic acid for targeting. Experiments on 4T1 cells and tumor-bearing mice demonstrated that the NPs exerted their genotoxic and tumor-inhibiting effects in response to NO released by GSH. Tiemuer and coworkers149 also designed two NIR light-triggered NO-releasing donors (NRh-Bn-NO and NRh-Et-NO) containing rhodamine for precise controllable release and in vivo detection of NO. Interestingly, the excitation wavelength they chose to control the release of NO was inconsistent with the excitation wavelength for monitoring NO in vivo. In this way, the accumulation of nanoformulations in tissues and organs can be detected without affecting the release of NO, thereby increasing the biological safety. To further increase the safety, they encapsulated the NO donor with DSPE-mPEG5k to obtain a PEG-Bn-NO nanosystem with significant antitumor activity.
Compared to the NO treatment alone, NO therapy when used in combination with other therapies yields more pronounced effects. Chemotherapy is a systemic treatment that uses chemicals to inhibit tumor growth, relieve tumor metastasis, and ultimately cure cancer. Although it is still the mainstream treatment method in clinical practice, MDR, low tumor accumulation, and low safety are unavoidable pitfalls of chemotherapy. However, NO has been shown in multiple ways to enhance the uptake and sensitivity of MDR cells to chemotherapeutic drugs by inhibiting the expression of P-gp and MRP. Zhang et al.132 integrated endogenous esterase-responsive PSF and DOX into GSH-responsive MSNs (MPNDs). They aminated and recarboxylated MSNs so that 4-(bromomethyl) phenol (P1) could be linked via an ester bond to form GSH-responsive MSNs (MSN@P1). They expressed good responsive release properties of NO and DOX via esterase and GSH in vitro. Moreover, they used MCF-7 tumor spheroids for in vivo experiments, and proved that MPNDs use not only achieved therapeutic effects, but also promoted drug penetration. Besides, Li et al.150 loaded a DOX prodrug (borate-DOX) into a nitrogluconic acid-containing copolymer. They found that after the self-assembled NPs entered the tumor site, where NO produced by the reduction of nitrogluconic acid by GSH could alleviate the hypoxic conditions at the tumor site and overcome the MDR. This seems to be a suitable strategy for the treatment of hypoxic solid tumors. Xu et al.212 encapsulated paclitaxel in S-nitrosated HSA to form NPs, which disrupted blood vessels and allowed T cells to infiltrate tumor sites.
In addition to combining with chemotherapeutics, the combined treatment strategy of NO and PDT has also been explored by many scientists. In PDT, under specific light irradiation, photosensitizers can generate high levels of ROS to kill tumor cells. However, there are still many shortcomings in PDT treatment of tumors, including: (1) lack of oxygen in the tumor microenvironment; (2) excessive GSH content at tumor sites; (3) insufficient penetration depth of light sources and photosensitizers; and (4) tumor immune escape. NO can make up for some deficiencies of PDT in various aspects to enhance anti-tumor efficacy. For example, Liu et al.213 combined the photosensitizer Ce6 and NO in NPs. They nitrified mannan to generate NO-mannan responsive to GSH, and then self-assembled to encapsulate Ce6 to form NPs. On the one hand, NO acted as a vasodilator to relieve tumor hypoxia, and on the other hand, GSH was consumed due to continuous generation of NO from NO-mannan. Based on the experimental results of 4T1 tumor-bearing mice, NO greatly enhanced the tumor suppressive effect of PDT. There are various mechanisms by which NO can improve the efficacy of PDT. For example, Xiang et al.214 combined the NO donor l-arginine and Ce6 to form NPs. They demonstrate that NO can increase the therapeutic effect of PDT through two pathways. First, NO can alleviate hypoxia by inhibiting mitochondrial respiration in cancer cells by changes in mitochondrial membrane potential (MMP, ΔΨm); second, NO hinders oxidative phosphorylation (OXPHOS), which leads to intracellular adenosine triphosphate (ATP) consumption. A low ATP environment makes tumor cells more sensitive to PDT by inhibiting DNA proliferation.
PTT involves irradiation of a photothermal agent by a specific wavelength light, causing the photothermal agent to heat up and kill tumor cells. In contrast to PDT, PTT does not need oxygen for its action. Recently, the combination of NO therapy and PTT has attracted much attention. Wu et al.215 encapsulated the thermosensitive NO donor BNN6 in a mesoporous structured photothermal agent polydopamine (M-PDA) to form M/B@R NPs. Experiments based on 4T1 cells and 4T1 tumor-bearing mice proved that the PTT/NO therapy had synergistic therapeutic effect. Xu et al.216 nitrified the surface of photothermal agent aza-BODIPY to generate S-NO. DSPE-mPEG5000 was used to encapsulate S-NO and to improve biocompatibility. In vitro experiments revealed the shedding of NO in S-NO to S-H after NIR irradiation as aza-BODIPY with increased electrons could enhance the heat generating ability. Lee et al.217 loaded NO donor DETA/NO and PTT agent indocyanine green (ICG) onto polylactic acid monolith for the treatment of osteosarcoma and bone regeneration.
Harnessing the immune system to battle cancer has been investigated for many years whereas combination of NO therapy with immunotherapy is a promising strategy. Chen et al.218 found that NO itself has the function of activating the immune system and promoting drug penetration. They designed a nanomotor loaded with immune checkpoint inhibitor anti-PD-1 driven by the NO domain called heparin-folate-cy5.5/l-arginine. The NO released from arginine facilitate penetration by disintegrating the cellular matrix and increased the immune effect by normalizing blood vessels. Analysis of tumor tissue in experimental MCF-7 tumor-bearing mice revealed that the efficiency of T cell infiltration increased from 2.1 to 28.2%. In addition, Wu et al.219 found that hollow iron oxide (Fe3O4) NPs could convert M2 TAMs with immune evasion functions into M1 TAMs. They loaded NO donor L-amino acids into Fe3O4 NPs to achieve immunotherapy/NO synergistic therapy.
In addition to the above, NO can also synergize with radiotherapy, ultrasound therapy, and Pt-based drugs220. In addition to combining a single mode of tumor treatment with NO gas therapy, some researchers have also tried to combine NO gas therapy with multiple modes in order to achieve better therapeutic effects. For example, Wang et al.221 combined photothermal agents Cu2‒xSe, NO donors N-diazeniumdiolate and DOX to form multifunctional NPs to exert PTT/NO therapy/chemotherapy effect against MDR breast cancer. Hu et al.151 constructed multifunctional smart NPs (IDDHN) by using NIR-sensitive NO donor, small-sized dendrimeric prodrug of DOX, and ICG along with a hyaluronidase-triggered shrink sized hyaluronic acid shell. As a result, IDDHN combined gas therapy, PTT and chemotherapy coupled with biodegradable HA for deeper infiltration of tumor. Liu et al.152 also constructed a combination nanoplatform (AuNC@CBSA-PTX-ICG@HA-NO3) employing multiple treatment options, such as BSA capped gold nanoclusters, paclitaxel, and NO donor for enhanced antitumor therapy.
6. Conclusions and future perspectives
As a hot point of gas therapy, NO provides a unique solution for the treatment of cancer. Therefore, during the past decade, scientists have been very interested in it and conducted a lot of research on NO production, mechanisms and applications in tumors, and safe delivery. In this paper, we summarized the endogenous production of NO via two pathways; enzyme-dependent and enzyme-independent. However, the in vivo generation of NO is complex, and more detailed studies are needed in this regard. The physiological role of NO in vivo is also mediated through two pathways; cGMP-dependent and cGMP-independent. In particular, the role of cGMP-independent pathway is considered responsible for the cytotoxicity of NO against tumors. It is generally known that NO has both tumor-promoting and tumor-inhibiting functions. Due to the inherent complexity, NO may play different functions depending upon the concentration of NO, tumor site, metabolic stage, etc. Herein, we summarized the anti-tumor aspects of NO in NO-based nano-delivery systems. It is mainly involved in hypoxia regulation, genotoxicity, pro-apoptosis, anti-metastatic, and MDR reversal. The dual role of NO in the body has attracted researchers to develop exogenous NO donors that can release significant quantities of NO at specific locations and times. These NO donors are generally loaded onto nanocarriers through external modifications, internal packaging, and/or co-precipitation with itself as a carrier to form NO-based NPs. The release of NO donors is influenced by both endogenous and exogenous factors.
Compared to pure NO donors, NO delivery systems have shown encouraging results in the past few years and opened new avenues for NO-mediated therapy. This paper summarizes the development of NO nanosystems in recent years from the aspects of inorganic and organic carriers. Inorganic carrier systems such as QDs, CDs, AuNPs, and MSNs have been designed and synthesized for tumor diagnosis and treatment, while each having advantages and disadvantages. For example, surface-modified MSNs can be loaded with NO donors to improve the drug loading capacity. UCNPs with a core-shell structure can shift the spectra of NO donors responsive to UV and visible light. AuNPs activate the release of NO donors through their own photothermal effect to enhance the anticancer effect of PTT. Although, a large number of inorganic nanomaterials are currently studied as drug carriers, some toxic organic solvents are used in the preparation process. Therefore, it is necessary to establish a comprehensive evaluation system for such drugs delivery. In addition, the design and preparation of some inorganic delivery systems are relatively complicated, leading to inability to rapidly scale up to industrial production or clinical trials. Compared with inorganic nanocarriers, organic delivery systems based on liposomes, micelles, and proteins NPs have special attributes for drug loading and controlled drug release. For example, micellar nano-delivery systems can be loaded with a large number of hydrophilic or hydrophobic NO donors and drugs. However, organic nanomaterials also face some obstacles in their real-time applications, such as variability during purification, antigenicity, and uncertain thermal and pH stability. Further research will be beneficial to the expand clinical applications of NO treatment modalities.
Although NO therapy is considered to have strong potential in cancer treatment, the common NO donor drugs (organic nitrates and metal-NO complexes) in clinical practice are mainly used to relieve diseases such as angina pectoris or chronic pharyngitis. Based on the medical applications of NO, many NO donors have been developed. However, there are still many problems in the clinical application of nano-delivery systems for delivering NO donors in cancer treatment.
-
1)
There is a need for the tools to monitor the release behavior of NO-based nanoformulations in vivo. A sophisticated system for monitoring the degree of NO release at the location of the release would play a significant role in establishing the correlation between the overall effects of NO and the NO biodistribution. It is known that the generation of NO is often accompanied by the production of toxic ROS and toxic ONOO−. Therefore, detecting the release behavior of NO is beneficial for establishing biosafety. To achieve this, loading fluorescein in nanosystems is a viable option. The N2O3 generated by the action of NO and oxygen reacts with fluorescein to generate fluorescence. The optical fiber sensor detects the light signal converted by fluorescence so that the behavior and concentration of NO in the body can be detected. This assay has good sensitivity, imaging capability, and detection of NO concentration in vivo without interference with NO signaling pathways. However, the fluorescein is sensitive to the product N2O3 rather than NO itself, so the presence of N2O3 in vivo may interfere with the detection of NO. In addition, some NO may be wasted due to the consumption of N2O3, thereby weakening the anti-cancer effect of NO.
-
2)
Encapsulation of NO donors in nanocarrier systems is below par. Some inorganic carriers, such as MSNs and MOFs, exhibit a mesoporous structure, which may lead to the leakage of NO donors during their systemic distribution. To solve this problem, use of biological membranes, such as erythrocyte membrane around the nanocarriers to seal the pores would be beneficial. In addition, NO donors can be conjugated to certain moieties of the nanocarriers to avoid drug loss.
-
3)
The targeting of NO-based nano-drug delivery systems also require considerable attention for the success of this therapeutic module. The accumulation of nano-formulations at tumor sites is one of the determinants for the efficacy of NO. Insufficient concentrations of nanoformulations at the target site may affect PTT therapy and the release of heat-sensitive NO donors. Use of multiple modalities, such as external/internal stimuli and targeting tools along with the control of physicochemical attributes of the nanocarrier would augment the NO targetability.
The adjuvant role of NO donors in increasing the penetration of antitumor drugs and sensitization of the resistant tumors against conventional anticancer agents, other than the ability to be use along with non-conventional therapeutic approaches (PDT or PTT) seem to be the more plausible aspect of the NO donors encapsulated nanocarriers. The clinical applications and real-time evaluation of these nanocarriers will decide the role of NO donors in future against cancers.
Acknowledgments
This work was supported by the Foundation of Anhui Province Key Laboratory of Pharmaceutical Preparation Technology and Application (No. 2021KFKT04, China), the National Natural Science Foundation of China (No. 81973488, China), and College Students Innovation Project for the R&D of Novel Drugs (No. J1310032, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Jia Liu, Email: jyszyyliujia@163.com.
Zhipeng Chen, Email: czpcpu2000@hotmail.com.
Yanyu Xiao, Email: cpuyanyuxiao@163.com.
Author contributions
Dan Gao, Sajid Asghar, Su Chen and Ruixin Niu collected references and drafted the manuscript. Rongfeng hu, Jia Liu, Zhipeng Chen and Yanyu Xiao proposed the concept and revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Verbeure W., van Goor H., Mori H., van Beek A.P., Tack J., van Dijk P.R. The role of gasotransmitters in gut peptide actions. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.720703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul S., Pan S., Mukherjee A., De P. Nitric oxide releasing delivery platforms: design, detection, biomedical applications, and future possibilities. Mol Pharm. 2021;18:3181–3205. doi: 10.1021/acs.molpharmaceut.1c00486. [DOI] [PubMed] [Google Scholar]
- 3.Shah A.M. Inducible nitric oxide synthase and cardiovascular disease. Cardiovasc Res. 2000;45:148–155. doi: 10.1016/s0008-6363(99)00316-8. [DOI] [PubMed] [Google Scholar]
- 4.Murayama T., Nomura Y. The actions of NO in the central nervous system and in thymocytes. Jpn J Pharmacol. 1998;76:129–139. doi: 10.1254/jjp.76.129. [DOI] [PubMed] [Google Scholar]
- 5.Wang P.G., Xian M., Tang X., Wu X., Wen Z., Cai T., et al. Nitric oxide donors: chemical activities and biological applications. Chem Rev. 2002;102:1091–1134. doi: 10.1021/cr000040l. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter A.W., Schoenfisch M.H. Nitric oxide release: part II. therapeutic applications. Chem Soc Rev. 2012;41:3742–3752. doi: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkeby O.J., Kutzsche S., Risöe C., Rise I.R. Cerebral nitric oxide concentration and microcirculation during hypercapnia, hypoxia, and high intracranial pressure in pigs. J Clin Neurosci. 2000;7:531–538. doi: 10.1054/jocn.2000.0788. [DOI] [PubMed] [Google Scholar]
- 8.Corpas F.J., Gonzalez-Gordo S., Palma J.M. Nitric oxide and hydrogen sulfide modulate the NADPH-generating enzymatic system in higher plants. J Exp Bot. 2021;72:830–847. doi: 10.1093/jxb/eraa440. [DOI] [PubMed] [Google Scholar]
- 9.Moncada S., Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 10.Carlstrom M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat Rev Nephrol. 2021;17:575–590. doi: 10.1038/s41581-021-00429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bredt D.S., Hwang P.M., Snyder S.H., Lowenstein C., Reed R.R., Snyder S.H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991;351:714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- 12.Xie Q.W., Cho H.J., Calaycay J., Mumford R.A., Swiderek K.M., Lee T.D., et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 13.Xu M.W., Liu L.Z. Nitric oxide: from a mysterious labile factor to the molecule of the Nobel Prize. Recent progress in nitric oxide research. Cell Res. 1998;8:251–258. doi: 10.1038/cr.1998.25. [DOI] [PubMed] [Google Scholar]
- 14.Vannini F., Kashfi K., Nath N. The dual role of iNOS in cancer. Redox Biol. 2015;6:334–343. doi: 10.1016/j.redox.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hezel M.P., Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015;21:7–16. doi: 10.1111/odi.12157. [DOI] [PubMed] [Google Scholar]
- 16.Schiffer T.A., Lundberg J.O., Weitzberg E., Carlström M. Modulation of mitochondria and NADPH oxidase function by the nitrate-nitrite-NO pathway in metabolic disease with focus on type 2 diabetes. Biochim Biophys Acta Mol Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165811. [DOI] [PubMed] [Google Scholar]
- 17.Li M., Fan Y., Zhang X., Hou W. Fruit and vegetable intake and risk of type 2 diabetes mellitus: meta-analysis of prospective cohort studies. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zweier J.L., Wang P., Samouilov A., Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 19.Yeo C.T., Stancill J.S., Oleson B.J., Schnuck J.K., Stafford J.D., Naatz A., et al. Regulation of ATR-dependent DNA damage response by nitric oxide. J Biol Chem. 2021;296 doi: 10.1016/j.jbc.2021.100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bredt D.S. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 21.Soderling S.H., Beavo J.A. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 2000;12:165–183. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 22.Fukumura D., Kashiwagi S., Jain R.K. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 23.Toledo J.C., Jr., Augusto O. Connecting the chemical and biological properties of nitric oxide. Chem Res Toxicol. 2012;25:975–989. doi: 10.1021/tx300042g. [DOI] [PubMed] [Google Scholar]
- 24.del Río L.A., Sandalio L.M., Palma J.M., Bueno P., Corpas F.J. Metabolism of oxygen radicals in peroxisomes and cellular implications. Free Radical Biol Med. 1992;13:557–580. doi: 10.1016/0891-5849(92)90150-f. [DOI] [PubMed] [Google Scholar]
- 25.Emerit J., Beaumont C., Trivin F. Iron metabolism, free radicals, and oxidative injury. Pharmacother. 2001;55:333–339. doi: 10.1016/s0753-3322(01)00068-3. [DOI] [PubMed] [Google Scholar]
- 26.Dean R.T., Gieseg S., Davies M.J. Reactive species and their accumulation on radical-damaged proteins. Trends Biochem Sci. 1993;18:437–441. doi: 10.1016/0968-0004(93)90145-d. [DOI] [PubMed] [Google Scholar]
- 27.Dizdaroglu M. Chemical determination of free radical-induced damage to DNA. Free Radical Biol Med. 1991;10:225–242. doi: 10.1016/0891-5849(91)90080-m. [DOI] [PubMed] [Google Scholar]
- 28.Fang F.C. Antimicrobial reactive oxygen and nitrogen species: concepts, and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 29.Wink D.A., Mitchell J.B. Chemical biology of nitric oxide: insights into regulatory cytotoxic and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 30.Tabuchi A., Oh E., Taoka A., Sakurai H., Tsuchiya T., Tsuda M. Rapid attenuation of AP-1 transcriptional factors associated with nitric oxide (NO)-mediated neuronal cell death. J Biol Chem. 1996;271:31061–31067. doi: 10.1074/jbc.271.49.31061. [DOI] [PubMed] [Google Scholar]
- 31.Stamler J.S. S-nitrosothiols and the bioregulatory actions of nitrogen oxides through reactions with thiol groups. Curr Top Microbiol Immunol. 1995;196:1919–1936. doi: 10.1007/978-3-642-79130-7_4. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z. Protein S-nitrosylation and cancer. Cancer Lett. 2012;320:123–129. doi: 10.1016/j.canlet.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Grek C.L., Zhang J., Manevich Y., Townsend D.M., Tew K.D. Causes and consequences of cysteine S-glutathionylation. J Biol Chem. 2013;288:26497–26504. doi: 10.1074/jbc.R113.461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying J., Clavreul N., Sethuraman M., Adachi T., Cohen R.A. Thiol oxidation in signaling and response to stress: detection and quantification of physiological and pathophysiological thiolmodifications. Free Radic Biol Med. 2007;43:1099–1108. doi: 10.1016/j.freeradbiomed.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill B.G., Bhatnagar A. Role of glutathiolation in preservation, restoration and regulation of protein function. IUBMB Life. 2007;59:21–26. doi: 10.1080/15216540701196944. [DOI] [PubMed] [Google Scholar]
- 36.Hofmann H., Schmidt H.H. Thiol dependence of nitric oxide synthase. Biochemistry. 1995;34:13443–13452. doi: 10.1021/bi00041a023. [DOI] [PubMed] [Google Scholar]
- 37.Chen C.A., Wang T.Y., Varadharaj S., Reyes L.A., Hemann C., Talukder M.A., et al. S-Glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridnour L.A., Thomas D.D., Switzer C., Flores-Santana W., Isenberg J.S., et al. Molecular mechanisms for discrete nitric oxide levels in cancer. Nitric Oxide. 2008;19:73–76. doi: 10.1016/j.niox.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soni Y., Softness K., Arora H., Ramasamy R. The yin yang role of nitric oxide in prostate cancer. Am J Men's Health. 2020;14:1–8. doi: 10.1177/1557988320903191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu S.C., Lu C.Y., Chen Y.L., Lo F.C., Wang T.Y., Chen Y.J., et al. Water-soluble dinitrosyl iron complex (DNIC): a nitric oxide vehicle triggering cancer cell death via apoptosis. Inorg Chem. 2016;55:9383–9392. doi: 10.1021/acs.inorgchem.6b01562. [DOI] [PubMed] [Google Scholar]
- 41.Yan Y., Wang C.H., Cui S.H., Zhai L., Sun J., Liu X.Y., et al. Enhanced cancer therapeutic efficiency of NO combined with siRNA by caspase-3 responsive polymers. J Control Release. 2021;339:506–520. doi: 10.1016/j.jconrel.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Qin L., Gao H. The application of nitric oxide delivery in nanoparticle-based tumor targeting drug delivery and treatment. Asian J Pharm Sci. 2019;14:380–390. doi: 10.1016/j.ajps.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kashfi K. Nitric oxide in cancer and beyond. Biochem Pharmacol. 2020;176:1–7. doi: 10.1016/j.bcp.2020.114006. [DOI] [PubMed] [Google Scholar]
- 44.Pattingre S., Tassa A., Qu X., Garuti R., Liang X.H., Mizushima N. Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Ji Y.Y., Ma Y.J., Wang J.W. Cytoprotective role of nitric oxide in HepG2 cell apoptosis induced by hypocrellin B photodynamic treatment. J Photochem Photobiol B. 2016;163:366–373. doi: 10.1016/j.jphotobiol.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Lim S.H., Hung A.C., Porter A.G. Focused PCR screen reveals p53 dependence of nitric oxide-induced apoptosis and up-regulation of maspin and plasminogen activator inhibitor-1 in tumor cells. Mol Cancer Res. 2009;7:55–66. doi: 10.1158/1541-7786.MCR-08-0331. [DOI] [PubMed] [Google Scholar]
- 47.Liu Z.Y., Li G.M., Gou Y., Xiao D.Y., Luo G., Saavedra J.E., et al. JS-K, a nitric oxide prodrug, induces DNA damage and apoptosis in HBV-positive hepatocellular carcinoma HepG2.2.15 cell. Biomed Pharmacother. 2017;92:989–997. doi: 10.1016/j.biopha.2017.05.141. [DOI] [PubMed] [Google Scholar]
- 48.He H., Feng Y.S., Zang L.H., Liu W.W., Ding L.Q., Chen L.X., et al. Nitric oxide induces apoptosis and autophagy; autophagy down-regulates NO synthesis in physalin A-treated A375-S2 human melanoma cells. Food Chem Toxicol. 2014;71:128–135. doi: 10.1016/j.fct.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Fu Q.Z., Liu X.F., Liu Y., Yang J.X., Lv G.Y., Dong S.F. MicroRNA-335 and -543 suppress bone metastasis in prostate cancer via targeting endothelial nitric oxide synthase. Int J Mol Med. 2015;36:1417–1425. doi: 10.3892/ijmm.2015.2355. [DOI] [PubMed] [Google Scholar]
- 50.Deng Y., Jia F., Chen X.H., Jin Q., Ji J. ATP suppression by pH-activated mitochondria-targeted delivery of nitric oxide nanoplatform for drug resistance reversal and metastasis inhibition. Small. 2020;16:2001747–2001760. doi: 10.1002/smll.202001747. [DOI] [PubMed] [Google Scholar]
- 51.Flaherty R.L., Intabli H., Falcinelli M., Bucca G., Hesketh A., Patel B.A., et al. Stress hormone-mediated acceleration of breast cancer metastasis is halted by inhibition of nitric oxide synthase. Cancer Lett. 2019;459:59–71. doi: 10.1016/j.canlet.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 52.Cheng H., Wang L., Mollica M., Re A.T., Wu S., Zuo L. Nitric oxide in cancer metastasis. Cancer Lett. 2014;353:1–7. doi: 10.1016/j.canlet.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moon B.K., Lee Y.J., Battle P., Jessup J.M., Raz A., Kim H.R. Galectin-3 protects human breast carcinoma cells against nitric oxide-induced apoptosis. Am J Pathol. 2001;159:1055–1060. doi: 10.1016/S0002-9440(10)61780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lahiri M., Martin J.H.J. Nitric oxide decreases motility and increases adhesion in human breast cancer cells. Oncol Rep. 2009;21:275–281. [PubMed] [Google Scholar]
- 55.Isaksson R., Casselbrant A., Elebring E., Hallberg M., Larhed M., Fändriks L., et al. Direct stimulation of angiotensin II type 2 receptor reduces nitric oxide production in lipopolysaccharide treated mouse macrophages. Eur J Pharmacol. 2020;868 doi: 10.1016/j.ejphar.2019.172855. [DOI] [PubMed] [Google Scholar]
- 56.Lintas C.L., Clark A., Fox J., Tannenbaum S.R., Newberne P.M. In vivo stability of nitrite and nitrosamine formation in the dogstomach: effect of nitrite and amine concentration and of ascorbic acid. Carcinogenesis. 1982;3:161–165. doi: 10.1093/carcin/3.2.161. [DOI] [PubMed] [Google Scholar]
- 57.Correa P., Haenszel W., Cuello C., Tannenbaum S., Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 58.Taylor H.W., Lijinsky W. Tumor induction in rats by feeding heptamethyleneimine and nitrite in water. Cancer Res. 1975;35:812–815. [PubMed] [Google Scholar]
- 59.Graziewicz M., Wink D.A., Laval M. Nitric oxide inhibits DNA ligase activity. Potential mechanisms for NO mediated DNA damage. Carcinogenesis. 1996;17:2501–2505. doi: 10.1093/carcin/17.11.2501. [DOI] [PubMed] [Google Scholar]
- 60.Wink D.A., Mitchell J.B. The chemical biology of NO: insights into regulation, protective and toxic mechanisms of nitric oxide. Curr Top Cell Regul. 1996;34:159–187. doi: 10.1016/s0070-2137(96)80006-9. [DOI] [PubMed] [Google Scholar]
- 61.Xie K., Huang S. Contribution of nitric oxide-mediated apoptosis to cancer metastasis inefficiency. Free Radic Biol Med. 2003;34:969–986. doi: 10.1016/s0891-5849(02)01364-3. [DOI] [PubMed] [Google Scholar]
- 62.Xie K., Huang S., Dong Z., Juang S.H., Gutman M., Xie Q.W., et al. Transfection with the inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis by K-1735 murine melanoma cells. J Exp Med. 1995;181:1333–1343. doi: 10.1084/jem.181.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Udo K.M., Maria A., Pierluigi N., Bernhard B. p53 expression in nitric oxide-induced apoptosis. FEBS Lett. 1994;355:23–26. doi: 10.1016/0014-5793(94)01161-3. [DOI] [PubMed] [Google Scholar]
- 64.Forrester K., Ambs S., Lupold S.E., Kapust R.B., Spillare E.A., Weinberg W.C., et al. Nitric oxide induced p53 accumulation and regulation of inducible nitric oxide synthase (NOS2) expression by wild type p53. Proc Natl Acad Sci U S A. 1996;93:2442–2447. doi: 10.1073/pnas.93.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin J.D., Seano G., Jain R.K. Normalizing function of tumor vessels: progress, opportunities, and challenges. Ann Rev Physiol Rev. 2019;81:505–534. doi: 10.1146/annurev-physiol-020518-114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pereira B.P., do Valle G.T., Salles B.C.C., Costa K.C.M., Ângelo M.L., Torres L.H.L., et al. Pyrrolidine dithiocarbamate reduces alloxan-induced kidney damage by decreasing nox4, inducible nitric oxide synthase, and metalloproteinase-2. Naunyn-Schmiedeberg’s Arch Pharmacol. 2020;393:1899–1910. doi: 10.1007/s00210-020-01906-1. [DOI] [PubMed] [Google Scholar]
- 67.Inoue S., Kawanishi S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995;371:86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- 68.Azouz A.A., Saleh E., Abo-Saif A.A. Aliskiren, tadalafil, and cinnamaldehyde alleviate joint destruction biomarkers; MMP-3 and RANKL; in complete Freund's adjuvant arthritis model: downregulation of IL-6/JAK2/STAT3 signaling pathway. Saudi Pharm J. 2020;28:1101–1111. doi: 10.1016/j.jsps.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim S.J., Kang H.G., Kim K., Kim H.Y., Zetterberg F., Park Y.S., et al. Crosstalk between WNT and STAT3 is mediated by galectin-3 in tumor progression. Gastric Cancer. 2021;24:1050–1062. doi: 10.1007/s10120-021-01186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding Y., Ma Y., Du C., Wang C.W., Chen T.T., Wang Y., et al. NO-releasing polypeptide nanocomposites reverse cancer multidrug resistance via triple therapies. Acta Biomater. 2021;123:335–345. doi: 10.1016/j.actbio.2021.01.015. [DOI] [PubMed] [Google Scholar]
- 71.Riganti C., Miraglia E., Viarisio D., Costamagna C., Pescarmona G., Ghigo D., et al. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005;65:516–525. [PubMed] [Google Scholar]
- 72.Kim J., Pramanick S., Lee D., Park H., Kim W.J. Polymeric biomaterials for the delivery of platinum-based anticancer drugs. Biomater Sci. 2015;3:1002–1017. doi: 10.1039/c5bm00039d. [DOI] [PubMed] [Google Scholar]
- 73.Callapina M., Zhou J., Schmid T., Köhl R., Brüne B. NO restores HIF-1a hydroxylation during hypoxia: role of reactive oxygen species. Free Radic Biol Med. 2005;39:925–936. doi: 10.1016/j.freeradbiomed.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y., Li M., Yao Q., Chen C. Recent advances in tumor hypoxia: tumor progression, molecular mechanisms, and therapeutic implications. Med Sci Monit. 2007;13:175–180. [PubMed] [Google Scholar]
- 75.North S., Moenner M., Bikfalvi A. Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Lett. 2005;218:1–14. doi: 10.1016/j.canlet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Jensen B.L. Get use to the -dustats: roxadustat and molidustat, members of the hypoxia-inducible factor (HIF) prolyl hydroxylase (PHD) inhibitor drug class promote kidney function, perfusion and oxygenation in rats through nitric oxide. Acta Physiol (Oxf) 2021;233 doi: 10.1111/apha.13706. [DOI] [PubMed] [Google Scholar]
- 77.Bonfili L., Gong C., Lombardi F., Cifone M.G., Eleuteri A.M., et al. Strategic modification of gut microbiota through oral bacteriotherapy influences hypoxia inducible factor-1alpha: therapeutic implication in Alzheimer's disease. Int J Mol Sci. 2021;23:357. doi: 10.3390/ijms23010357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gomes M.T.R., Guimarães E.S., Marinho F.V., Macedo I., Aguiar E.R.G.R., Barber G.N., et al. STING regulates metabolic reprogramming in macrophages via HIF-1 alpha during Brucella infection. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y., Huang K., Wang M., Wang Q.S., Chang H.S., Liang Y.K., et al. Ubiquitination flow repressors: enhancing wound healing of infectious diabetic ulcers through stabilization of polyubiquitinated hypoxia-inducible factor-1alpha by theranostic nitric oxide nanogenerators. Adv Mater. 2021;33 doi: 10.1002/adma.202103593. [DOI] [PubMed] [Google Scholar]
- 80.Wink D.A., Vodovotz Y., Laval J., Laval F., Dewhirst M.W., Mitchell J.B. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 81.Takabuchi S., Hirota K., Nishi K., et al. The inhibitory effect of sodium nitroprusside on HIF-1 activation is not dependent on nitric oxide-soluble guanylyl cyclase pathway. Biochem Biophys Res Commun. 2004;324:417–423. doi: 10.1016/j.bbrc.2004.09.064. [DOI] [PubMed] [Google Scholar]
- 82.Yasuda H., Nakayama K., Watanabe M., Suzuki S., Fuji H., Okinaga S., et al. Nitroglycerin treatment may enhance chemosensitivity to docetaxel and carboplatin in patients with lung adenocarcinoma. Clin Cancer Res. 2006;12:6748–6757. doi: 10.1158/1078-0432.CCR-06-1124. [DOI] [PubMed] [Google Scholar]
- 83.Liu X.R., Zhang Y.P., Wang Y.J., Yang M.W., Hong F.F., Yang S.L. Protein phosphorylation in cancer: role of nitric oxide signaling pathway. Biomolecules. 2021;11:1009. doi: 10.3390/biom11071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wink D.A., Cook J.A., Kim S.Y., Vodovotz Y., Pacelli R., Krishna M.C., et al. Superoxide modulates the oxidation and nitrosation of thiols by nitric oxide derived reactive intermediates. J Biol Chem. 1997;272:11147–11151. doi: 10.1074/jbc.272.17.11147. [DOI] [PubMed] [Google Scholar]
- 85.Miles A.M., Gibson M.F., Kirshina M., Cook J.C., Pacelli R., Wink D., et al. Effects of superoxide on nitric oxide-dependent N-nitrosation reactions. Free Radic Res. 1995;23:379–390. doi: 10.3109/10715769509065259. [DOI] [PubMed] [Google Scholar]
- 86.Roy A., Saqib U., Wary K., Baig M.S., et al. Macrophage neuronal nitric oxide synthase (NOS1) controls the inflammatory response and foam cell formation in atherosclerosis. Int Immunopharm. 2020;83 doi: 10.1016/j.intimp.2020.106382. [DOI] [PubMed] [Google Scholar]
- 87.Hibbs J.B., Vavrin Z., Taintor R.R. l-Arginine is required for the expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987;138:550–565. [PubMed] [Google Scholar]
- 88.Richter C., Gogvadze V., Schlapbach R., et al. Nitric oxide kills hepatocytes by mobilizing mitochondrial calcium. Biochem Biophys Res Commun. 1994;205:1143–1150. doi: 10.1006/bbrc.1994.2785. [DOI] [PubMed] [Google Scholar]
- 89.Brown G.C. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995;369:136–139. doi: 10.1016/0014-5793(95)00763-y. [DOI] [PubMed] [Google Scholar]
- 90.Bal-Price A., Brown G.C. Nitric-oxide-induced necrosis and apoptosis in PC12 cells mediated by mitochondria. J Neurochem. 2000;75:1455–1464. doi: 10.1046/j.1471-4159.2000.0751455.x. [DOI] [PubMed] [Google Scholar]
- 91.Rudin C.M., Thompson C.B. Thompson, Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu Rev Med. 1997;48:267–281. doi: 10.1146/annurev.med.48.1.267. [DOI] [PubMed] [Google Scholar]
- 92.Ashkenazi A., Dixit V.M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 93.Ambs S., Hussain S.P., Harris C.C. Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. Faseb J. 1997;11:443–448. doi: 10.1096/fasebj.11.6.9194524. [DOI] [PubMed] [Google Scholar]
- 94.Borutaite V., Brown G.C. S-nitrosothiol inhibition of mitochondrial complex I causes a reversible increase in mitochondrial hydrogen peroxide production. Biochim Biophys Acta. 2006;1757:562–566. doi: 10.1016/j.bbabio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 95.Singh S., Gupta A. Nitric oxide: role in tumour biology and iNOS/NO-based anticancer therapies. Cancer Chemother Pharmacol Res. 2011;67:1211–1224. doi: 10.1007/s00280-011-1654-4. [DOI] [PubMed] [Google Scholar]
- 96.Monteiro H.P., Costa P.E., Reis A.K., Stern A. Nitric oxide: protein tyrosine phosphorylation and protein S-nitrosylation in cancer. Biomed J. 2015;38:380–388. doi: 10.4103/2319-4170.158624. [DOI] [PubMed] [Google Scholar]
- 97.Dodson M., Darley-Usmar V., Zhang J. Nitric oxide induces coupling of mitochondrial signalling with the endoplasmic reticulum stress response. Nat Cell Biol. 2004;6:1129–1134. doi: 10.1038/ncb1188. [DOI] [PubMed] [Google Scholar]
- 98.Fidler I.J. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. clowes memorial award lecture. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- 99.Fidler I.J., Balch C.M. The biology of cancer metastasis and implications for therapy. Curr Probl Surg. 1987;24:137–209. doi: 10.1016/0011-3840(87)90002-5. [DOI] [PubMed] [Google Scholar]
- 100.Jain R.K. Barriers to drug delivery in solid tumors. Sci Am. 1994;271:58–65. doi: 10.1038/scientificamerican0794-58. [DOI] [PubMed] [Google Scholar]
- 101.Ehrenfeld P., Cordova F., Duran W.N., Sanchez F.A. S-nitrosylation and its role in breast cancer angiogenesis and metastasis. Nitric Oxide. 2019;87:52–59. doi: 10.1016/j.niox.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 102.Yilmaz M., Christofori G., Lehembre F. Distinct mechanisms of tumor invasion and metastasis. Trends Mol Med. 2007;13:535–541. doi: 10.1016/j.molmed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 103.O'Reilly M.S., Holmgren L., Shing Y., Chen C., Rosenthal R.A., Moses M., et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 104.Wang J.S., Xiao W.W., Zhong Y.S., Li X.D., Du S.X., Xie P., et al. Galectin-3 deficiency protects lipopolysaccharide-induced chondrocytes injury via regulation of TLR4 and PPAR-gamma-mediated NF-kappaB signaling pathway. J Cell Biochem. 2019;120:10195–10204. doi: 10.1002/jcb.28304. [DOI] [PubMed] [Google Scholar]
- 105.Sayed-Ahmad M.M., Mohamad M.A. Contribution of nitric oxide and epidermal growth factor receptor in anti-metastatic potential of paclitaxel in human liver cancer cell (HebG2) J Egypt Natl Canc Inst. 2005;17:35–41. [PubMed] [Google Scholar]
- 106.Gallo O., Masini E., Morbidelli L., Franchi A., Fini-Storchi I., Vergari W.A., et al. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J Natl Cancer Inst. 1998;90:587–596. doi: 10.1093/jnci/90.8.587. [DOI] [PubMed] [Google Scholar]
- 107.Huang Y., Zhang J., Zhang Y., Shi L., Qin X., Lu B., et al. P-glycoprotein suppression by photothermal-responsive nitric oxide releasing nanoplatform for triple-combination therapy of multidrug resistant cancer. Mater Des. 2021;211 [Google Scholar]
- 108.Kim M., Park S.C., Lee D.Y. Glycyrrhizin as a nitric oxide regulator in cancer chemotherapy. Cancers (Basel) 2021;13:5762. doi: 10.3390/cancers13225762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sinha B.K., Perera L., Cannon R.E. Reversal of drug resistance by JS-K and nitric oxide in ABCB1- and ABCG2-expressing multi-drug resistant human tumor cells. Biomed Pharmacother. 2019;120:109468–109478. doi: 10.1016/j.biopha.2019.109468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Turchi J.J. Nitric oxide and cisplatin resistance: NO easy answers. Proc Natl Acad Sci USA. 2006;103:4337–4338. doi: 10.1073/pnas.0601001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reynaert N.L., Ckless K., Korn S.H., Vos N., Guala A.S., Wouters E.F.M., et al. Nitric oxide represses inhibitory kB kinase through S-nitrosylation. Proc Natl Acad Sci USA. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Viaro F., Nobre F., Evora P.R. Expression of nitric oxide synthases in the pathophysiology of cardiovascular diseases. Arq Bras Cardiol. 2000;74:380–393. doi: 10.1590/s0066-782x2000000400009. [DOI] [PubMed] [Google Scholar]
- 113.Megson I.L., Webb D.J. Nitric oxide donor drugs: current status and future trends. Expet Opin Invest Drugs. 2002;11:587–601. doi: 10.1517/13543784.11.5.587. [DOI] [PubMed] [Google Scholar]
- 114.Janczuk A.J., Jia Q., Xian M., Wen Z., Wang P.G., Cai T., et al. NO donors with anticancer activity. Expert Opin Ther Pat. 2002;12:819–826. [Google Scholar]
- 115.Hrabie J.A., Keefer L.K. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem Rev. 2002;102:1135–1154. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 116.Makings L.R., Tsien R.Y. Caged nitric oxide, stable organic molecules from which nitric oxide can be photoreleased. J Biol Chem. 1994;269:6282–6285. [PubMed] [Google Scholar]
- 117.Duan W., Li J., Inks E.S., Chou C.J., Jia Y., Chu X., et al. Design, synthesis, and antitumor evaluation of novel histone deacetylase inhibitors equipped with a phenylsulfonylfuroxan module as a nitric oxide donor. J Med Chem. 2015;58:4325–4338. doi: 10.1021/acs.jmedchem.5b00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fan W.P., Lu N., Huang P., Liu Y., Yang Z., Wang S., et al. Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving-like/gas therapy. Angew Chem Int Ed Engl. 2017;129:1249–1253. doi: 10.1002/anie.201610682. [DOI] [PubMed] [Google Scholar]
- 119.Fan J., He N., He Q., Liu Y., Ma Y., Fu X., et al. A novel self-assembled sandwich nanomedicine for NIR-responsive release of NO. Nanoscale. 2015;7:20055–20062. doi: 10.1039/c5nr06630a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fan W., Yung B.C., Chen X. Stimuli-responsive NO release for on-demand gas-sensitized synergistic cancer therapy. Angew Chem Int Ed Engl. 2018;57:8383–8394. doi: 10.1002/anie.201800594. [DOI] [PubMed] [Google Scholar]
- 121.Liu T., Li X., Wang J., Zhang P., Huang X., Zhang Z., et al. Ag@S-nitrosothiol core-shell nanoparticles for chemo and photothermal synergistic tumor targeted therapy. J Mater Chem B. 2020;8:5483–5490. doi: 10.1039/d0tb00734j. [DOI] [PubMed] [Google Scholar]
- 122.Fan W., Bu W., Zhang Z., Shen B., Zhang H., He Q., et al. X-ray radiation-controlled NO-release for on-demand depth-Independent hypoxic radiosensitization. Angew Chem Int Ed Engl. 2015;54:14026–14030. doi: 10.1002/anie.201504536. [DOI] [PubMed] [Google Scholar]
- 123.Dong X., Liu H.J., Feng H.Y., Yang S.C., Liu X.L., Lai X., et al. Enhanced drug delivery by nanoscale integration of a nitric oxide donor to induce tumor collagen depletion. Nano Lett. 2019;19:997–1008. doi: 10.1021/acs.nanolett.8b04236. [DOI] [PubMed] [Google Scholar]
- 124.Katayama N., Nakajou K., Ishima Y., Ikuta S., Yokoe J., Yoshida F., et al. Nitrosylated human serum albumin (SNO-HSA) induces apoptosis in tumor cells. Nitric Oxide. 2010;22:259–265. doi: 10.1016/j.niox.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 125.Taladriz-Blanco P., Pastoriza-Santos V., Perez-Juste J., Hervés P. Controllable nitric oxide release in the presence of gold nanoparticles. Langmuir. 2013;29:8061–8069. doi: 10.1021/la4014762. [DOI] [PubMed] [Google Scholar]
- 126.Tan L.J., Huang R., Li X.Q., Liu S.P., Shen Y.M. Controllable release of nitric oxide and doxorubicin from engineered nanospheres for synergistic tumor therapy. Acta Biomater. 2017;57:498–510. doi: 10.1016/j.actbio.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Y., Feng S.J., Meng X., Luo J., Xu Y.R., Ning X.H. Identifying urotropine derivatives as co-donors of formaldehyde and nitric oxide for improving antitumor therapy. Chem Commun (Camb) 2021;57:7581–7584. doi: 10.1039/d1cc02177j. [DOI] [PubMed] [Google Scholar]
- 128.Zhang K., Xu H.X., Jia X.Q., Chen Y., Ma M., Sun L.P., et al. Ultrasound-triggered nitric oxide release platform based on energy transformation for targeted inhibition of pancreatic tumor. Acs Nano. 2016;10:10816–10828. doi: 10.1021/acsnano.6b04921. [DOI] [PubMed] [Google Scholar]
- 129.Hu Y., Lv T., Ma Y., Xu J., Zhang Y., Hou Y., et al. Nanoscale coordination polymers for synergistic NO and chemodynamic therapy of liver cancer. Nano Lett. 2019;19:2731–2738. doi: 10.1021/acs.nanolett.9b01093. [DOI] [PubMed] [Google Scholar]
- 130.Stevens E.V., Carpenter A.W., Shin J.H., Liu J.S., Der C.J., Schoenfisch M.H. Nitric oxide-releasing silica nanoparticle inhibition of ovarian cancer cell growth. Mol Pharm. 2010;7:775–785. doi: 10.1021/mp9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang X.F., Mansouri S., Mbeh D.A., Yahia L.H., Sacher E., Veres T. Nitric oxide delivery by core/shell superparamagnetic nanoparticle vehicles with enhanced biocompatibility. Langmuir. 2012;28:12879–12885. doi: 10.1021/la302357h. [DOI] [PubMed] [Google Scholar]
- 132.Zhang C., Meng X.D., Gong C.C., Zhao J.M., Zhang K., Yang Z. Glutathione-responsive biodegradable nanoplatform with endogenous esterase-triggered nitric oxide release for gas therapy and enhanced chemotherapy. ACS Appl Bio Mater. 2021;4:5212–5221. doi: 10.1021/acsabm.1c00384. [DOI] [PubMed] [Google Scholar]
- 133.Wang L., Chang Y., Feng Y.L., Li X., Cheng Y., Jian H., et al. Nitric oxide stimulated programmable drug release of nanosystem for multidrug resistance cancer therapy. Nano Lett. 2019;19:6800–6811. doi: 10.1021/acs.nanolett.9b01869. [DOI] [PubMed] [Google Scholar]
- 134.Sudhesh P., Tamilarasan K., Arumugam P., Berchmans S. Nitric oxide releasing photoresponsive nanohybrids as excellent therapeutic agent for cervical cancer cell lines. ACS Appl Mater Inter. 2013;5:8263–8266. doi: 10.1021/am402086m. [DOI] [PubMed] [Google Scholar]
- 135.Ding H., Wang L., Zhang L., Zhu B., Hou L., Huang G., et al. RGD-modified ZnO nanoparticles loaded with nitric oxide precursor for targeted cancer therapy. Chem Phys Lett. 2020;749:137445–137450. [Google Scholar]
- 136.Tan L.J., He C.Y., Chu X.J., Chu Y.Q., Ding Y.M. Charge-reversal ZnO-based nanospheres for stimuli-responsive release of multiple agents towards synergistic cancer therapy. Chem Eng J. 2020;395:125177–125187. [Google Scholar]
- 137.Fowley C., McHale A.P., McCaughan B., Fraix A., Sortino S., Callan J.F. Carbon quantum dot-NO photoreleaser nanohybrids for two-photon phototherapy of hypoxic tumors. Chem Commun (Camb) 2015;51:81–84. doi: 10.1039/c4cc07827f. [DOI] [PubMed] [Google Scholar]
- 138.Zhang X., Tian G., Yin W.Y., Wang L.M., Zheng X.P., Yan L., et al. Controllable generation of nitric oxide by near-infrared-sensitized upconversion nanoparticles for tumor therapy. Adv Funct Mater. 2015;25:3049–3056. [Google Scholar]
- 139.Li S.H., Song X.R., Zhu W., Chen Y.L., Zhu R., Wang L.P., et al. Light-switchable yolk-mesoporous shell UCNPs@MgSiO3 for nitric oxide-evoked multidrug resistance reversal in cancer therapy. ACS Appl Mater Interfaces. 2020;12:30066–30076. doi: 10.1021/acsami.0c06102. [DOI] [PubMed] [Google Scholar]
- 140.Ishima Y., Yoshida F., Kragh-Hansen U., Watanabe K., Katayama N., Nakajou K., et al. Cellular uptake mechanisms and responses to NO transferred from mono- and poly-S-nitrosated human serum albumin. Free Radic Res. 2011;45:1196–1206. doi: 10.3109/10715762.2011.606814. [DOI] [PubMed] [Google Scholar]
- 141.Duong H.T., Kamarudin Z.M., Erlich R.B., Li Y., Jones M.W., Kavallaris M., et al. Intracellular nitric oxide delivery from stable NO-polymeric nanoparticle carriers. Chem Commun (Camb) 2013;49:4190–4192. doi: 10.1039/c2cc37181b. [DOI] [PubMed] [Google Scholar]
- 142.Song Q., Tan S., Zhuang X., Guo Y., Zhao Y., Wu T., et al. Nitric oxide releasing d-alpha-tocopheryl polyethylene glycol succinate for enhancing antitumor activity of doxorubicin. Mol Pharm. 2014;11:4118–4129. doi: 10.1021/mp5003009. [DOI] [PubMed] [Google Scholar]
- 143.Qi Y., Qin X.Y., Yang C.L., Wu T.T., Qiao Q., Song Q.L., et al. Micelle system based on molecular economy principle for overcoming multidrug resistance and inhibiting metastasis. Mol Pharm. 2018;15:1005–1016. doi: 10.1021/acs.molpharmaceut.7b00922. [DOI] [PubMed] [Google Scholar]
- 144.Jin R.H., Liu Z.N., Liu T., Yuan P., Bai Y., Chen X., et al. Redox-responsive micelles integrating catalytic nanomedicine and selective chemotherapy for effective tumor treatment. Chin Chem Lett. 2021;32:3076–3082. [Google Scholar]
- 145.Wei G.Q., Wang Y., Huang X.H., Yang G., Zhao J., Zhou S., et al. Enhancing the accumulation of polymer micelles by selectively dilating tumor blood vessels with NO for highly effective cancer treatment. Adv Healthc Mater. 2018;7:1801094–1801109. doi: 10.1002/adhm.201801094. [DOI] [PubMed] [Google Scholar]
- 146.Sung Y.C., Jin P.R., Chu L.A., Hsu F.F., Wang M.R., Chang C.C., et al. Delivery of nitric oxide with a nanocarrier promotes tumour vessel normalization and potentiates anti-cancer therapies. Nat Nanotechnol. 2019;14:1160–1171. doi: 10.1038/s41565-019-0570-3. [DOI] [PubMed] [Google Scholar]
- 147.Liu Y., Wang X., Li J., Tang J., Li B., Zhang Y., et al. Sphingosine 1-phosphate liposomes for targeted nitric oxide delivery to mediate anticancer effects against brain glioma tumors. Adv Mater. 2021;33 doi: 10.1002/adma.202101701. [DOI] [PubMed] [Google Scholar]
- 148.Gu G.L., Chen C., Zhang S.C., Yin B., Wang J., et al. Self-assembly dual-responsive NO donor nanoparticles for effective cancer therapy. ACS Appl Mater Interfaces. 2021;13:50682–50694. doi: 10.1021/acsami.1c12646. [DOI] [PubMed] [Google Scholar]
- 149.Tiemuer A., Yu H., Zhao C., Sun W., Zhang Y., Jiang Y., et al. Nitroso-caged upconversion luminescent prodrug: near infrared light-activatable NO nano-donor for gas therapy. Chem Eng J. 2022;430 [Google Scholar]
- 150.Li Q.Y., Zhang J.N., Li J.N., Ye H., Li M., Hou W., et al. Glutathione-activated NO-/ROS-generation nanoparticles to modulate the tumor hypoxic microenvironment for enhancing the effect of HIFU-combined chemotherapy. ACS Appl Mater Interfaces. 2021;13:26808–26823. doi: 10.1021/acsami.1c07494. [DOI] [PubMed] [Google Scholar]
- 151.Hu C., Cun X.L., Ruan S.B., Liu R., Xiao W., et al. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy. Biomaterials. 2018;168:64–75. doi: 10.1016/j.biomaterials.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 152.Liu R., Xiao W., Hu C., Xie R., Gao H.L. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J Control Release. 2018;278:127–139. doi: 10.1016/j.jconrel.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 153.Sun D.D., Wang Z.K., Zhang P., Yin C.Y., Wang J.Y., Sun Y., et al. Ruthenium-loaded mesoporous silica as tumor microenvironment-response nano-fenton reactors for precise cancer therapy. J Nanobiotechnol. 2021;19:1–16. doi: 10.1186/s12951-021-00848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ke F.Y., Yi J.H., Zhang S., Zhou S.Q., Ravikovitch P.I., Kruk M. Structures and dimensions of micelle-templated nanoporous silicas derived from swollen spherical micelles of temperature-dependent size. J Colloid Interface Sci. 2019;544:312–320. doi: 10.1016/j.jcis.2019.02.057. [DOI] [PubMed] [Google Scholar]
- 155.Juere E., Kleitz F. On the nanopore confinement of therapeutic drugs into mesoporous silica materials and its implications. Micropor Mesopor Mat. 2018;270:109–119. [Google Scholar]
- 156.Lim M.H., Stein A. Comparative studies of grafting and direct syntheses of inorganic-organic hybrid mesoporous materials. Chem Mater. 1999;11:3285–3295. [Google Scholar]
- 157.Shin J.H., Metzger S.K., Schoenfisch M.H. Synthesis of nitric oxide-releasing silica nanoparticles. J Am Chem Soc. 2007;129:4612–4619. doi: 10.1021/ja0674338. [DOI] [PubMed] [Google Scholar]
- 158.Xiong L., Du X., Shi B.Y., Bi J.X., Kleitz F., Qiao S.Z. Tunable stellate mesoporous silica nanoparticles for intracellular drug delivery. J Mater Chem B. 2015;3:1712–1721. doi: 10.1039/c4tb01601g. [DOI] [PubMed] [Google Scholar]
- 159.Munaweera I., Shi Y., Koneru B., Patel A., Dang M.H., Pasqua A.J.D., et al. Nitric oxide- and cisplatin-releasing silica nanoparticles for use against non-small cell lung cancer. J Inorg Biochem. 2015;153:23–31. doi: 10.1016/j.jinorgbio.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 160.Thakkar S., Sharma D., Kalia K., Tekade R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: a review. Acta Biomater. 2020;101:43–68. doi: 10.1016/j.actbio.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 161.Paidari S., Ibrahim S.A. Potential application of gold nanoparticles in food packaging: a mini review. Gold Bull. 2021;54:31–36. [Google Scholar]
- 162.Wang Y., Zeng Z., Qiao J.Y., et al. Ultrasensitive determination of nitrite based on electrochemical platform of AuNPs deposited on PDDA-modified MXene nanosheets. Talanta. 2021;221 doi: 10.1016/j.talanta.2020.121605. [DOI] [PubMed] [Google Scholar]
- 163.Mehmet L.Y. Sensitive sandwich-type voltammetric immunosensor for breast cancer biomarker HER2 detection based on gold nanoparticles decorated Cu-MOF and Cu2ZnSnS4 NPs/Pt/g-C3N4 composite. Mikrochim Acta. 2021;188:78. doi: 10.1007/s00604-021-04735-y. [DOI] [PubMed] [Google Scholar]
- 164.Botteon C.E.A., Silva L.B., Ccana-Ccapatinta G.V., Silva T.S., Ambrosio S.R., Veneziani R.C.S., et al. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci Rep. 2021;11:1974. doi: 10.1038/s41598-021-81281-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Levy E.S., Morales D.P., Garcia J.V., Reich N.O., Ford P.C. Near-IR mediated intracellular uncaging of NO from cell targeted hollow gold nanoparticles. Chem Commun (Camb) 2015;51:17692–17695. doi: 10.1039/c5cc07989f. [DOI] [PubMed] [Google Scholar]
- 166.Frost M.C., Meyerhoff M.E. Controlled photoinitiated release of nitric oxide from polymer films containing S-Nitroso-N-acetyl-DL-penicillamine derivatized fumed silica filler. J Am Chem Soc. 2004;126:1348–1349. doi: 10.1021/ja039466i. [DOI] [PubMed] [Google Scholar]
- 167.Wang L., Xin X., Li P., Dou J., Han X., She J., et al. Stepwise immobilization of keratin-dopamine conjugates and gold nanoparticles on PET sheets for potential vascular graft with the catalytic generation of nitric oxide. Colloids Surf B. 2021;205 doi: 10.1016/j.colsurfb.2021.111855. [DOI] [PubMed] [Google Scholar]
- 168.Peng Y., Qin L., Kang S.Z., Li G.D., Li X.Q. A novel AuNPs-based nanosensors for smart detection of NO with low concentration. Talanta. 2019;191:457–460. doi: 10.1016/j.talanta.2018.08.091. [DOI] [PubMed] [Google Scholar]
- 169.Efros A.L., Brus L.E. Brus, Nanocrystal quantum dots: from discovery to modern development. ACS Nano. 2021;15:6192–6210. doi: 10.1021/acsnano.1c01399. [DOI] [PubMed] [Google Scholar]
- 170.Chen H., Liu Z., Wei B., Huang J., You X.R., Zhang J.Y., et al. Redox responsive nanoparticle encapsulating black phosphorus quantum dots for cancer theranostics. Bioact Mater. 2021;6:655–665. doi: 10.1016/j.bioactmat.2020.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chung S., Revia R.A., Zhang M. Graphene quantum dots and their applications in bioimaging, biosensing, and therapy. Adv Mater. 2021;33 doi: 10.1002/adma.201904362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu S., Gu T., Fu J., Li X., Chronakis I.S., Ge M., et al. Quantum dots-hyperbranched polyether hybrid nanospheres towards delivery and real-time detection of nitric oxide. Mater Sci Eng C Mater Biol Appl. 2014;45:37–44. doi: 10.1016/j.msec.2014.08.070. [DOI] [PubMed] [Google Scholar]
- 173.Fu B., Tao C., Chen N., Lin J.R., Zhao P. ZnO QD covalently coated, GSH/pH dual-responsive drug delivery system for chemotherapeutic/ionic synergistic therapy. J Drug Deliv Sci Tec. 2021;66 [Google Scholar]
- 174.Choi S., Cui C., Luo Y., Kim S.H., Ko J.K., Huo X., et al. Selective inhibitory effects of zinc on cell proliferation in esophageal squamous cell carcinoma through Orai1. FASEB J. 2018;32:404–416. doi: 10.1096/fj.201700227RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li S., Li L., Tu H.Y., Zhang H., Silvester D.S., Banks C.E., et al. The development of carbon dots: from the perspective of materials chemistry. Mater Today. 2021;51:188–207. [Google Scholar]
- 176.Smith A.M., Mancini M.C., Nie S.M. Bioimaging: second window for in vivo imaging. Nat Nanotechnol. 2009;4:710–711. doi: 10.1038/nnano.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Deng Q., Xiang H.J., Tang W.W., et al. Ruthenium nitrosyl grafted carbon dots as a fluorescence-trackable nanoplatform for visible light-controlled nitric oxide release and targeted intracellular delivery. J Inorg Biochem. 2016;165:152–158. doi: 10.1016/j.jinorgbio.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 178.Xu J.S., Zeng F., Wu H., Hu C.P., Yu C.M., Wu S.Z. Preparation of a mitochondria-targeted and NO-releasing nanoplatform and its enhanced pro-apoptotic effect on cancer cells. Small. 2014;10:3750–3760. doi: 10.1002/smll.201400437. [DOI] [PubMed] [Google Scholar]
- 179.Zhang Y., Zhu X., Zhang Y. Exploring heterostructured upconversion nanoparticles: from rational engineering to diverse applications. ACS Nano. 2021;15:3709–3735. doi: 10.1021/acsnano.0c09231. [DOI] [PubMed] [Google Scholar]
- 180.Zhang L., Chen C., Tay S.S., Wen S., Cao C., Biro M., et al. Optimizing the polymer cloak for upconverting nanoparticles: an evaluation of bioactivity and optical performance. ACS Appl Mater Interfaces. 2021;13:16142–16154. doi: 10.1021/acsami.1c01922. [DOI] [PubMed] [Google Scholar]
- 181.Ouyang Q., Wang L., Ahmad W., Rong Y.W., Li H.H., Hu Y.Q., et al. A highly sensitive detection of carbendazim pesticide in food based on the upconversion-MnO2 luminescent resonance energy transfer biosensor. Food Chem. 2021;349 doi: 10.1016/j.foodchem.2021.129157. [DOI] [PubMed] [Google Scholar]
- 182.Lan Y., Zhu X.H., Tang M., Wu Y.H., Zhang J., Liu J.L., et al. Construction of a near-infrared responsive upconversion nanoplatform against hypoxic tumors via NO-enhanced photodynamic therapy. Nanoscale. 2020;12:7875–7887. doi: 10.1039/c9nr10453d. [DOI] [PubMed] [Google Scholar]
- 183.Yang N.L., Gong F., Yang N. Recent advances in upconversion nanoparticle-based nanocomposites for gas therapy. Chem Sci. 2022;13:1883–1898. doi: 10.1039/d1sc04413c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chu H.Q., Cao T.M., Dai G.M., Liu B., Duan H.J., Kong C.C., et al. Recent advances in functionalized upconversion nanoparticles for light-activated tumor therapy. RSC Adv. 2021;11:35472–35488. doi: 10.1039/d1ra05638g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang H., Liu Y., Wang Z.H., Yang M., Gu Y.Q. 808 nm-light-excited upconversion nanoprobe based on LRET for the ratiometric detection of nitric oxide in living cancer cells. Nanoscale. 2018;10:10641–10649. doi: 10.1039/c8nr03078b. [DOI] [PubMed] [Google Scholar]
- 186.Dai Y., Yang D., Yu D., Xie S., Wang B., Bu J., et al. Engineering of monodisperse core-shell up-conversion dendritic mesoporous silica nanocomposites with a tunable pore size. Nanoscale. 2020;12:5075–5083. doi: 10.1039/c9nr10813k. [DOI] [PubMed] [Google Scholar]
- 187.Hornok V. Serum albumin nanoparticles: problems and prospects. Polymers (Basel) 2021;13:3759. doi: 10.3390/polym13213759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xin X., Huang H.L., Lu W.X., Li Y., Zeng F., Wang S., et al. Albumin concentration is a candidate indicator for predicting the risk of calf venous thromboembolism in patients undergoing gynecologic surgery. Int J Gynaecol Obstet. 2022;156:139–144. doi: 10.1002/ijgo.13675. [DOI] [PubMed] [Google Scholar]
- 189.Xu Y., Liu Y.L., Liu Q., Lu S., Chen X., Xu W., et al. Co-delivery of bufalin and nintedanib via albumin sub-microspheres for synergistic cancer therapy. J Control Release. 2021;338:705–718. doi: 10.1016/j.jconrel.2021.08.049. [DOI] [PubMed] [Google Scholar]
- 190.Minanni C.A., Machado-Lima A., Iborra R.T., Okuda L., Pinto R., Santana M., et al. Persistent effect of advanced glycated albumin driving inflammation and disturbances in cholesterol efflux in macrophages. Nutrients. 2021;13:3633. doi: 10.3390/nu13103633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Peters T.J. Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- 192.Katayama N., Nakajou K., Komori H., Uchida K., Yokoe J.I., Yasui N., et al. Design and evaluation of S-nitrosylated human serum albumin as a novel anticancer drug. J Pharmacol Exp. 2008;325:69–76. doi: 10.1124/jpet.107.132100. [DOI] [PubMed] [Google Scholar]
- 193.Kianfar E. Protein nanoparticles in drug delivery: animal protein, plant proteins and protein cages, albumin nanoparticles. J Nanobiotechnol. 2021;19:159. doi: 10.1186/s12951-021-00896-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ishima Y., Yoshida F., Kragh-Hansen U., Yin H., Liao L., Katayama N., et al. Tuning of poly-S-nitrosated human serum albumin as superior antitumor nanomedicine. J Pharm Sci. 2014;103:2184–2188. doi: 10.1002/jps.24020. [DOI] [PubMed] [Google Scholar]
- 195.Yoshikawa T., Mori Y., Feng H.T., Phan Q.P., Kishimura A., Kang J.H., et al. Rapid and continuous accumulation of nitric oxide-releasing liposomes in tumors to augment the enhanced permeability and retention (EPR) effect. Int J Pharm. 2019;565:481–487. doi: 10.1016/j.ijpharm.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 196.Seki T., Fang J., Maeda H. Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application. Cancer Sci. 2009;100:2426–2430. doi: 10.1111/j.1349-7006.2009.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Islam W., Fang J., Imamura T., Etrych T., Subr V., Ulbrich K., et al. Augmentation of the enhanced permeability and retention effect with nitric oxide-generating agents improves the therapeutic effects of nanomedicines. Mol Cancer Ther. 2018;17:2643–2653. doi: 10.1158/1535-7163.MCT-18-0696. [DOI] [PubMed] [Google Scholar]
- 198.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 199.Kinoshita R., Ishima Y., Ikeda M., Ulrich K.H., Fang J., Nakamura H., et al. S-Nitrosated human serum albumin dimer as novel nano-EPR enhancer applied to macromolecular anti-tumor drugs such as micelles and liposomes. J Control Release. 2015;217:1–9. doi: 10.1016/j.jconrel.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 200.Li Y.M., Zhi X.L., Lin J.T., You X., Yuan J. Preparation and characterization of DOX loaded keratin nanoparticles for pH/GSH dual responsive release. Mater Sci Eng C Mater Biol Appl. 2017;73:189–197. doi: 10.1016/j.msec.2016.12.067. [DOI] [PubMed] [Google Scholar]
- 201.Sun Z., Yi Z., Cui X.X., Chen X., Su W., Ren X., et al. Tumor-targeted and nitric oxide-generated nanogels of keratin and hyaluronan for enhanced cancer therapy. Nanoscale. 2018;10:12109–12122. doi: 10.1039/c8nr03265c. [DOI] [PubMed] [Google Scholar]
- 202.Lu T.Y., Lu W.F., Wang Y.H., Liao M.Y., Wei Y., Fan T.J., et al. Keratin-based nanoparticles with tumor-targeting and cascade catalytic capabilities for the combinational oxidation phototherapy of breast cancer. ACS Appl Mater Inter. 2021;13:38074–38089. doi: 10.1021/acsami.1c10160. [DOI] [PubMed] [Google Scholar]
- 203.Weinstain R., Slanina T., Kand D., Klán P., et al. Visible-to-NIR-light activated release: from small molecules to nanomaterials. Chem Rev. 2020;120:13135–13272. doi: 10.1021/acs.chemrev.0c00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xie L., Liu R., Chen X., He M., Zhang Y., Chen S. Micelles based on lysine, histidine, or arginine: designing structures for enhanced drug delivery. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.744657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Costa A.R., Franca R.N., Silva-Jardim I., Silva R.J.S., Santos J.L., Salay L.C., et al. Self-assembling micellar system based on Pluronic and pyrazole-dithiocarbazate-conjugate stimulates production of nitric oxide from macrophages. Colloids Interface Sci Commun. 2021;41 [Google Scholar]
- 206.Zhang G., Sun J.M. Lipid in chips: a brief review of liposomes formation by microfluidics. Int J Nanomed. 2021;16:7391–7416. doi: 10.2147/IJN.S331639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.de Lima L.S., Mortari M.R. Therapeutic nanoparticles in the brain: a review of types, physicochemical properties and challenges. Int J Pharm. 2022;612 doi: 10.1016/j.ijpharm.2021.121367. [DOI] [PubMed] [Google Scholar]
- 208.Antoniou A.I., Giofrè S., Seneci P., Passarella D., Pellegrino S. Stimulus-responsive liposomes for biomedical applications. Drug Discov Today. 2021;26:1794–1824. doi: 10.1016/j.drudis.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 209.Costa C., Liu Z.H., Simoes S.I., Correia A., Rahikkala A., Seitsonen J., et al. One-step microfluidics production of enzyme-loaded liposomes for the treatment of inflammatory diseases. Colloids Surf B Biointerfaces. 2021;199 doi: 10.1016/j.colsurfb.2020.111556. [DOI] [PubMed] [Google Scholar]
- 210.Yu H., Liang Z.H., Zhu L.J., Zhao C., He X., Chen K., et al. Nucleic acid-modified liposome: construction methods and biological applications. Adv Mater Interfac. 2021;9 [Google Scholar]
- 211.Masetto F., Chegaev K., Gazzano E., Mullappilly N., Rolando B., Arpicco S., et al. MRP5 nitration by NO-releasing gemcitabine encapsulated in liposomes confers sensitivity in chemoresistant pancreatic adenocarcinoma cells. Biochim Biophys Acta Mol Cell Res. 2020;1867 doi: 10.1016/j.bbamcr.2020.118824. [DOI] [PubMed] [Google Scholar]
- 212.Xu Y., Liu J.W., Liu Z.Y., Chen G., Li X., Ren H. Damaging tumor vessels with an ultrasound-triggered NO release nanosystem to enhance drug accumulation and T cells infiltration. Int J Nanomed. 2021;16:2597–2613. doi: 10.2147/IJN.S295445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu F.R., Gong S.L., Shen M.L., He T., Liang X., Shu Y., et al. A glutathione-activatable nanoplatform for enhanced photodynamic therapy with simultaneous hypoxia relief and glutathione depletion. Chem Eng J. 2021;403 [Google Scholar]
- 214.Xiang Q.Q., Qiao B., Luo Y.L., Cao J., Fan K., Hu X., et al. Increased photodynamic therapy sensitization in tumors using a nitric oxide-based nanoplatform with ATP-production blocking capability. Theranostics. 2021;11:1953–1969. doi: 10.7150/thno.52997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wu C.G., Liang J.L., Wang X., Zhou X., Cai X., Xu J., et al. Light-activated nitric-oxide overproduction theranostic nanoplatform based on long-circulating biomimetic nanoerythrocyte for enhanced cancer gas therapy. Sci China Chem. 2021;64:1796–1810. [Google Scholar]
- 216.Xu Y.J., Wang S.Q., Chen Z.J., Hu R., Zhao Y., Wang K., et al. Nitric oxide release activated near-Infrared photothermal agent for synergistic tumor treatment. Biomaterials. 2021;276 doi: 10.1016/j.biomaterials.2021.121017. [DOI] [PubMed] [Google Scholar]
- 217.Lee J.H., Uyama H., Kwon O.K., Kim Y.J. Nitric oxide and reactive oxygen species-releasing polylactic acid monolith for enhanced photothermal therapy of osteosarcoma. J Ind Eng Chem. 2021;94:498–506. [Google Scholar]
- 218.Chen H., Shi T., Wang Y., Liu Z.Y., Liu F.C., Zhang H.Y., et al. Deep penetration of nanolevel drugs and micrometer-level T cells promoted by nanomotors for cancer immunochemotherapy. J Am Chem Soc. 2021;143:12025–12037. doi: 10.1021/jacs.1c03071. [DOI] [PubMed] [Google Scholar]
- 219.Wu X., Cheng Y., Zheng R., Xu K., Yan J., Song P., et al. Immunomodulation of tumor microenvironment by arginine-loaded iron oxide nanoparticles for gaseous immunotherapy. ACS Appl Mater Interfaces. 2021;13:19825–19835. doi: 10.1021/acsami.1c04638. [DOI] [PubMed] [Google Scholar]
- 220.Thakar S.B., Ghorpade P.N., Shaker B., Lee J., Na D. Gas-mediated cancer therapy combined with starvation therapy, ultrasound therapy, chemotherapy, radiotherapy, and photodynamic therapy: a review. Environ Chem Lett. 2021;19:2981–2993. [Google Scholar]
- 221.Wang J., Wu C.C., Qin X.R., Huang Y.Y., Zhang J.N., Chen T.T., et al. NIR-II light triggered nitric oxide release nanoplatform combined chemo-photothermal therapy for overcoming multidrug resistant cancer. J Mater Chem B. 2021;9:1698–1706. doi: 10.1039/d0tb02626c. [DOI] [PubMed] [Google Scholar]