Abstract
Described as a “don't eat me” signal, CD47 becomes a vital immune checkpoint in cancer. Its interaction with signal regulatory protein alpha (SIRPα) prevents macrophage phagocytosis. In recent years, a growing body of evidences have unveiled that CD47-based combination therapy exhibits a superior anti-cancer effect. Latest clinical trials about CD47 have adopted the regimen of collaborating with other therapies or developing CD47-directed bispecific antibodies, indicating the combination strategy as a general trend of the future. In this review, clinical and preclinical cases about the current combination strategies targeting CD47 are collected, their underlying mechanisms of action are discussed, and ideas from future perspectives are shared.
KEY WORDS: CD47, Combination strategies, Bispecific antibodies, Clinical data, Preclinical data, Cancer, Mechanisms, Future perspectives
Graphical abstract
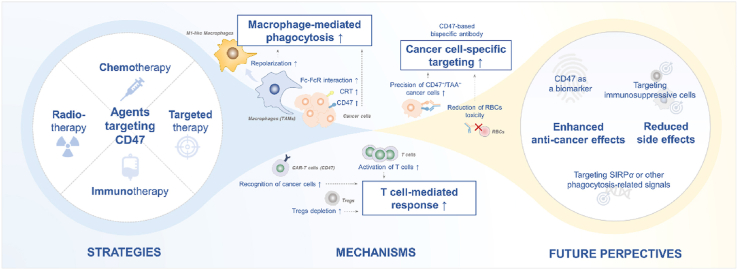
CD47-based combination strategy may represent the future trend of CD47-targeted therapy. Different combinational regimens, their underlying mechanisms, and some future perspectives are summarized in this review.
1. Introduction
CD47 is one of the most important anti-phagocytic signals in the immune system. It is a ligand for signal regulatory protein alpha (SIRPα) expressed on phagocytes such as macrophages, neutrophils, and dendritic cells (DCs)1, 2, 3. The interplay between CD47 and SIRPα leads to activation of immune receptor tyrosine-based inhibition motifs (ITIMs), subsequently causing recruitment of inhibitory molecules including Src homology 2 (SH2) domain-containing protein tyrosine phosphatase (SHP)-1 and SHP-24, 5, 6, and deactivation of proteins such as non-muscle myosin IIA7, thus limiting phagocytosis by phagocytes8. The blockade of CD47–SIRPα axis by targeting CD47 or SIRPα could be the first and foremost step in re-activating phagocytosis, and the focus of this review is targeting CD47 (CD47-based). The ubiquitous expression of CD47 in normal cells protects from elimination by phagocytes, while cancer cells also hijack this function to evade immunosurveillance9. Elevated expression of CD47 is found in solid tumor and hematological malignancies10,11, resulting in poor prognosis of patients with cancer but equally a potential therapeutic target for cancer treatment12.
Various types of CD47-based agents including antisense morpholino, anti-CD47 antibody, and SIRPα fusion protein have been developed in the past few years. Before antibodies become widely available, CD47 gene suppression with an antisense morpholino (CD47 morpholino) was a relatively common agent used in some preclinical studies13. Meanwhile, anti-CD47 antibodies and SIRPα fusion proteins are the mainstays of CD47-based agents with the fastest development and relatively abundant clinical evidence. However, encouraging clinical outcomes are rarely to find after an overview of the efficacy of these agents as monotherapy in cancer treatment. Monotherapy of magrolimab (NCT02216409), the first-in-class anti-CD47 antibody with IgG4 portion, presented that only 2 of 62 patients with advanced solid cancer had reduced tumor lesions after the treatment14. A clinical project (NCT02641002) of another anti-CD47 antibody named CC-90002 was terminated for its poor efficacy in patients with hematological malignancies15. Moreover, ALX148 (NCT03013218), a SIRPα–Fc fusion protein, also showed limited anti-cancer efficacy as monotherapy16. By contrast, no matter from clinical or preclinical evidence, CD47-based combination strategies obtained more intriguing therapeutic effects than monotherapy. Considering that many excellent prior reviews summarized CD47-based therapies mainly according to different types of single agents12,17, a systematic review collecting and analyzing these strategies purely from a combinatorial perspective is needed.
The combination regimens are divided into four sections, CD47-targeted therapy combined with chemotherapy, radiotherapy, targeted therapy, and immunotherapy. Since no drug targeting CD47 has been approved for clinical use, preclinical data about CD47-based combination strategies are of equal value as clinical data during the process of drug discovery. The preclinical data was first illustrated in accordance with different rationales, followed by the clinical information (if available) in each section. A notable detail is that bispecific antibodies or fusion proteins are also viewed as one type of combination regimen and included in this summary. After all data were collected, detailed working mechanisms underpinning the combinational effect were summarized in the discussion part. Lastly, future insights were provided from preclinical and clinical aspects.
2. Agents targeting CD47 with chemotherapy
Considering its remarkable anti-cancer effect, conventional chemotherapy maintains its irreplaceable role on the cancer treatment option list. With the development of immunotherapy in the past decade, the chemo-immunotherapy combination, especially the combination between chemotherapeutic agents and anti-PD-L1/PD-1 antibodies, has made great progress in certain types of cancer18, 19, 20. The distinct immune landscape built by chemotherapy is the key reason for its synergy with immunotherapy18. The same principle could be used in agents targeting CD47 with chemotherapy. Additionally, in a few cases, agents targeting CD47 are introduced to overcome the chemo-resistance.
Chemotherapeutic agents have been historically thought to kill cancer cells directly21. However, depth understanding of the immunological function of chemotherapy is more than simply killing cancer cells; it renders them to “antigenicity” (the property of being recognized by immune cells) and “adjuvanticity” (the property of delivering activating signals to immune cells) within the process of so-called immunogenic cell death (ICD)22,23. Calreticulin (CRT) is a marker of ICD and a pro-phagocytic signal in mediating macrophage phagocytosis24,25. Exposure of CRT on the surface of cancer cells caused by chemotherapeutic agents is viewed as one critical rationale for the combination with CD47-targeted therapy (Fig. 1A). Preclinical data observed that chemotherapeutic agents, including doxorubicin, temozolomide, and cisplatin, induced the CRT translocation to the cancer cell surface, all of which in combination with anti-CD47 antibody accounted for enhanced phagocytosis26,27. Notably, more detailed information about these data including experimental model, administration design, and key findings were mentioned in Table 1.
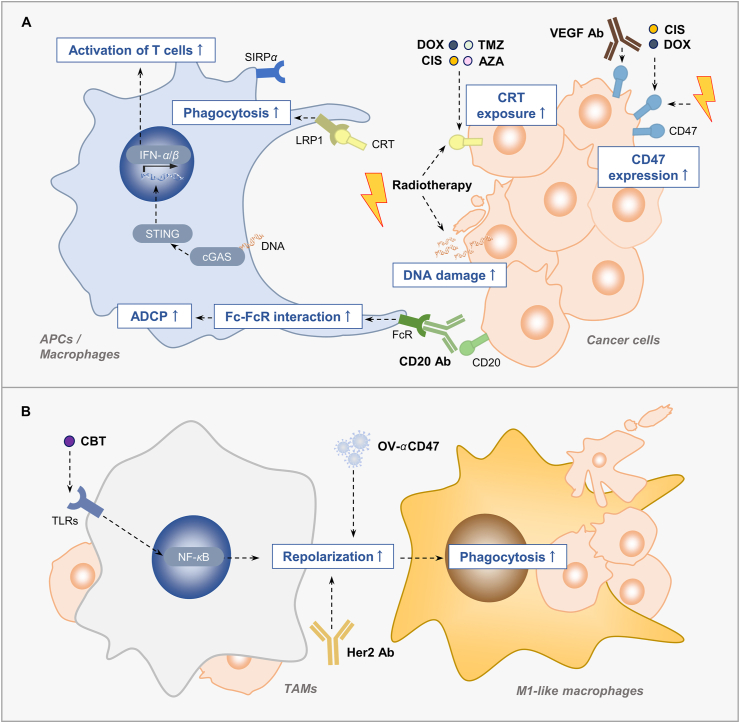
Figure 1.
Combination strategies enhance macrophage phagocytosis. (A) The combination treatment enhances phagocytosis by increasing the CRT on the cell surface, or enhancing Fc–FcR interaction. Chemotherapeutic drugs or radiotherapy enhance phagocytosis by increasing the surface expression of CRT. Some of them induces the ICD of cancer cells, cause more DNA damage, activate the cGAS–STING pathway of APC and finally leading to T cell activation. Anti-CD20 antibody augments ADCP through Fc–FcR interaction after combining with anti-CD47 antibody. Moreover, the up-regulation of CD47 on cancer cells by chemotherapy, radiotherapy or anti-VEGF antibody provides the opportunities to combine the agents targeting CD47. (B) Some combination strategies enhance macrophage phagocytosis by repolarizing the macrophage phenotype from TAMs to M1-like macrophage. CIS, cisplatin; TMZ, temozolomide; AZA, azacytidine; DOX, doxorubicin; CD20 Ab, anti-CD20 antibody; HER2 Ab, anti-HER2 antibody, CBT, cabazitaxel; OV-αCD47, the oncolytic virus expressing an anti-CD47 antibody.
Table 1.
Pre-clinical trials targeting CD47-based combination strategies in cancer.
| Agents targeting CD47 | Additional combinational regimen | Experiment model | Administration design | Key findings of combination regimen | Ref. |
|---|---|---|---|---|---|
| Chemotherapy | |||||
| MIAP301 | Cyclophosphamide or Paclitaxel | A20 B cell malignancy C57BL/6 mice |
i.p. Paclitaxel (40 mg/kg) or Cyclophosphamide (60 mg/kg) was administered 1 day before i.t. anti-CD47 antibody | Enhanced the anti-tumor effect; Preserved the memory immune response to tumor re-challenge |
33 |
| MIAP410 | Cisplatin | LLC lung cancer C57BL/6 mice |
Cisplatin (2.5 mg/kg) and anti-CD47 antibody (2.5 mg/kg) for 3 times | Enhanced anti-tumor effect; Prolonged survival time |
35 |
| B6H12 | Doxorubicin | MNNG/HOS osteosarcoma NSG mice |
i.v. Doxorubicin (1 mg/kg) on Days 0, 2, 4 i.p. anti-CD47 antibody (10 mg/kg) on Days 1, 3, 5 |
Reduced the tumor bioluminescence flux; Inhibited pulmonary metastases; Improved survival rate |
26 |
| B6H12 | Doxorubicin | Patient-derived xenograft liver cancer NOD/SCID mice |
i.p. anti-CD47 antibody (400 μg/mouse) i.v Doxorubicin (2 mg/kg) simultaneously |
Enhanced anti-tumor effect | 40 |
| CD47 morpholino | Doxorubicin | PLC/PRF/5, or patient-derived xenograft hepatocellular carcinoma Nude mice |
i.p. Doxorubicin (2 mg/kg) i.p. CD47 morpholino |
Triggered complete eradication of tumors in PLC/PRF/5 tumor models; Enhanced anti-tumor effect in patient-derived tumor xenograft models |
39 |
| MIAP410 | Temozolomide | GL261 glioblastoma or CT-2A astrocytoma C57BL/6 mice |
i.p. Temozolomide (20 mg/kg) on Days 7, 8, 9 i.p. Temozolomide (80 mg/kg) and MIAP410 (100 μg) on Days 11, 13, 15 |
Inhibited tumor growth; Prolonged survival |
27 |
| B6H12 | Gemcitabine | Patient-derived xenograft pancreatic cancer NU-Foxn1nu nude mice |
i.p. Gemcitabine (125 mg/kg) from Day 14 to 56, twice a week i.p. anti-CD47 (500 mg/mouse) from Day 14 to 35, every day |
Resulted in sustained tumor regression; Prevented disease relapse |
41 |
| B6H12 | Abraxane | Patient-derived xenograft pancreatic cancer NU-Foxn1nu nude mice |
i.v. Abraxane (50 mg/kg) from Day 14 to 28, every 4 days i.p. anti-CD47 (500 mg/mouse) from Day 14 to 35, every day |
Resulted in sustained tumor regression; Prevented disease relapse |
41 |
| B6H12 | Cabazitaxel | MDA-MB-231 breast cancer RAG2−/−, γc−/− BALB/c mice |
i.v. anti-CD47 antibody (4 mg/kg) once every week i.t. Cabazitaxel (40 μg/kg) once every week |
Inhibited tumor development | 30 |
| B6H12 | Paclitaxel | Daudi, or SUDHL-2 B cell malignancy RAG2−/−, γc−/− BALB/c mice; A20 B cell malignancy BALB/c mice |
i.v. or i.t. Nab-paclitaxel in combination with anti-CD47 antibody | Triggered tumor burden reduction; Prolonged survival times |
31 |
| Radiotherapy | |||||
| Hu5F9-G4 | Irradiation | Patient-derived orthotopic xenograft glioblastoma NSG mice |
Anti-CD47 antibody followed irradiation (10 Gy) | Inhibited tumor growth and increased median survival times (combo 137 days vs. anti-CD47 antibody 93.5 days vs. irradiation 116 days) | 53 |
| Pep-20-D12 | Irradiation | CT26 colon cancer C57BL/6 mice |
Locally irradiation (20 Gy) i.p. Pep-20-D12 (2 mg/kg) every day for 2 weeks |
Triggered tumor regression | 47 |
| CD47 morpholino | Irradiation | 15-12RM fibrosarcoma BALB/c mice |
i.p. CD47 morpholino (750 μL of 10 mmol/L), 48 h later, locally irradiation (10 Gy) | Reduced tumor growth (96% reduction in combo vs. saline alone) | 51 |
| Targeted therapy | |||||
| Anti-CD47 F (ab′) fragments |
Trastuzumab + Irradiation | 4T1 breast cancer BALB/c mice |
i.t. anti-CD47 F (ab′) fragments (100 μg/mouse) i.t. Trastuzumab (5 mg/kg) Locally irradiation (5 Gy per day for 2 days) |
Enhanced tumor inhibition; Increased necrotic area |
52 |
| MIAP410 | Trastuzumab | MM3MG, breast cancer BALB/c mice; KPL-4 breast cancer SCID-beige BALB/c mice |
i.p Trastuzumab (200 μg/mouse) i.p anti-CD47 antibody (300 μg/mouse) |
Prolonged survival rate; Delayed tumor growth |
67 |
| Hu5F9-G4 | Trastuzumab | BT474 breast cancer NSG mice |
i.p. Trastuzumab (100 μg/week) i.p. Hu5F9-G4 (250 μg) every other day |
Enhanced anti-tumor effect Prolonged survival rate in ADCC-tolerant cancer cells-xenograft model |
70 |
| B6H12 | Blinatumomab | Raji B cell malignancy NOD/SCID mice |
i.v. anti-CD47 antibody (10 mg/kg) every 3 days, 6 times in total i.v. Blinatumomab (3 mg/kg) daily, 16 times in total |
Enhanced anti-tumor effect | 96 |
| Immunotherapy | |||||
| A4 | Anti-PD-L1 antibody | B16F10 melanoma C57BL/6J mice | i.p. A4 (200 μg) for 14 days i.p. anti-PD-L1 (250 μg/dose) every other day for 14 days |
Delayed tumor growth; Prolonged the survival |
112 |
| A4 | Anti-PD-L1 antibody + anti-TRP-1 antibody | B16F10 melanoma C57BL/6J mice | i.p. A4 (200 μg/dose) for 14 days i.p. anti-PD-L1 antibody (250 μg/dose) every other day for 14 days i.p. anti-TRP-1 antibody every other day for 14 days |
Delayed tumor growth; Improved the disease-free survival; Presented a durable immune memory response |
112 |
| CD47 morpholino | Ipilimumab + Irradiation | B16F10 melanoma C57BL/6J mice | i.p. CD47 morpholino (10 μmol/L) 10 Gy local irradiation i.p. anti-CTLA4 antibody (100 μg) |
Increased tumor necrosis; Increased survival |
48 |
| A4 | GVAX + anti-CTLA-4 antibody | B16F10 melanoma C57BL/6J mice | s.c. irradiated GVAX cells (5 × 105) in 250 μL of HBSS i.p. A4 (200 μg/dose) for 14 days i.p. anti-CTLA-4 (200 μg/dose) every other day for 14 days |
Prolonged the survival; Enhanced the immune response after local delivery of A4; Caused considerable toxicity |
120 |
| MIAP301 | Poly(I:C) | MC38 colon cancer C57BL/6J mice | i.p. anti-CD47 antibody (200 mg/mouse) every 3 days, began on Day 8 and stopped on Day 23 i.p. Poly(I:C) (50 μg) on Days 8, 11, 14, 17, 20, 23 |
Suppressed tumor growth; Prolonged survival |
127 |
| MIAP301 | cGAMP | E0771 breast cancer C57BL/6J mice | i.t. anti-CD47 antibody (5 μg/mouse) i.t. cGAMP (0.5 μg/mouse) |
Suppressed tumor growth (5/9 mice were completely cured); Prolonged survival time; Induced immune memory response |
140 |
| αCD47-IgG1 | Oncolytic virus (OV-αCD47-G1) | A2780 ovarian cancer Athymic nude mice | i.v. 2 × 105 PFU OV-αCD47-G1 in 10 μL of saline | Slowed tumor progression | 142 |
| αmCD47-IgG2b | Oncolytic virus (OV-αmCD47-G2b) | ID8 ovarian cancer C57BL/6 mice | i.p 1 × 106 PFU OV-αmCD47-G2b in 100 μL of saline | Showed the strongest anti-tumor effect | 142 |
| αCD47-IgG1 | Oncolytic virus (OV-αCD47-G1) | GBM43 glioblastoma Athymic nude mice; CT2A-hCD47 glioblastoma C57BL/6 mice |
i.c. 2 × 105 PFU OV-αCD47-G1 in 3 μL of saline | Prolonged survival time in immunodeficient mice; Improved anti-tumor outcome in immunocompetent mice |
143 |
| A4-IgG2b | Oncolytic virus (OV-A4-IgG2b) | CT2A glioblastoma C57BL/6 mice | i.c. 2 × 105 PFU OV-A4-IgG2b in 3 μL of saline | Improved anti-tumor outcome | 143 |
| Bispecific antibody or fusion protein | |||||
| CD47/CD20 | Raji B cell malignancy NSG mice | i.p. (300, 500, or 1000 μg) | Prolonged survival significantly | 87 | |
| CD47/CD19 | Raji Non-Hodgkin lymphoma NOD/SCID mice | i.p. (400 μg) 3 times per week for 3 weeks | Induced tumor regression; Reduced “off-target” toxicity |
92 | |
| NI-1701 | Raji B cell malignancy NOD/SCID mice | i.v. (4 or 5 doses, 10 or 20 mg/kg) once a week for 4 or 5 weeks. | Controlled tumor growth | 91 | |
| NI-1701 | Patient-derived xenograft B cell malignancies Humanized NSG mice |
i.v. (20 mg/kg) on Days 7, 14, 21, 28, and 35 | Eradicated tumor burden | 91 | |
| CD47/Glypican3 | Hep3B hepatocellular carcinoma NOD/SCID mice | i.p. (10 mg/kg) twice a week for 3 weeks | Exerted superior antitumor effects by macrophages and neutrophils | 101 | |
| Bi-SP | A431 squamous cell carcinoma NOD/SCID mice | i.p. (10 or 40 mg/kg) on Days 0, 5, 10, 15 | Delayed tumor growth | 63 | |
| VEGFR1D2–SIRPαD1 fusion protein | U87 glioblastoma nude mice | i.p. SIRPα–VEGFR1 (10 mg/kg) twice a week | Reduced tumor volume | 77 | |
| PF-07257876 | B16F10 melanoma C57BL/6J mice | i.p. (20 mg/kg) three times a week for a total for 9 doses | Inhibited tumor growth | 111 | |
| PF-07257876 | MC38 colon cancer C57BL/6 mice | i.p. (10, 20, and 40 mg/kg) for a total of 6 doses |
Inhibited tumor growth | 111 | |
| PF-07257876 | CT26 colon cancer BALB/c mice | i.p. (5 mg/kg) every 3–4 days for a total for 3 doses | Inhibited tumor growth; Prolonged the survival times (75% combo vs. 12.5% monotherapy) |
111 | |
| Bispecific anti-PD-L1–SIRPα | MC38 colon cancer C57BL/6 mice | i.t. (50 μg/mouse) on Days 11 and 14 after tumor inoculation | Inhibited tumor growth; Extended mouse survival |
107 | |
| Bispecific anti-PD-L1–SIRPα | CT26 colon cancer BALB/c mice | i.t. (100 μg/mouse) on Days 10 and 14 after tumor inoculation | Extended mouse survival | 107 | |
| IBI322 | Raji B cell malignancy NOD-SCID mice | i.p. (0.17 mg/kg) dosage once every 2 days for 6 continuous doses | Presented rapid and nearly completed tumor regression | 110 | |
| IBI322 | A375 melanoma NOG mice | i.p. (1.1 mg/kg) dosage once every 2 days for 6 continuous doses | Inhibited tumor growth | 110 | |
| Anti-CTLA4–SIRPα fusion protein | Patient-derived xenograft lung cancer Humanized NSG mice | i.p. (30 μg) Day 12 i.p. after (200 μg) twice a week for a total of four times |
Inhibited tumor growth via tumor Treg depletion | 122 | |
| Anti-CTLA4–SIRPα fusion protein | MC38 colon cancer C57BL/6 mice | i.p. (20 μg) once on Day 13 | Inhibited tumor growth via tumor Treg depletion | 122 | |
| Anti-CTLA4–SIRPα fusion protein | CT26 colon cancer BALB/c mice | i.p. (50 μg) once on Day 6 | Inhibited tumor growth via tumor Treg depletion | 122 | |
| DSP107 | Diffuse large B cell lymphoma Humanized NSG mice | i.p. 250 μg or 150 μL DSP107 every other day for 6 consecutive treatments | Inhibited tumor growth (2 out of 6 mice being tumor-free at the end of the experiment) | 139 | |
| SIRPα–Fc–CD40L fusion protein | CT26 colon cancer; A20 B cell malignancy; WEHI-3 acute myelomonocytic leukemia BALB/c mice |
i.p. mSIRPα–Fc–CD40L fusion protein | Enhanced the anti-tumor effect; Enhanced a type I interferon response |
141 | |
i.c., intracranial injection; i.p., intraperitoneally injection; i.t., intratumoral injection; i.v., intravenously injection; s.c., subcutaneous injection.
In the tumor microenvironment (TME), the direct immunostimulatory effect of chemotherapy on the immune cells in the combination is also crucial (Fig. 1). Tumor-associated macrophages (TAMs) is canonically divided into M1 and M228. The M2 type of macrophage consists of a larger portion in TAMs and plays a tumor-promoting role, while the M1 type of macrophage plays a tumor-suppressive role29. By utilizing a screening system, Cao et al.30 identified that cabazitaxel repolarized macrophage to M1-like phenotype and enhanced its phagocytotic ability in breast cancer, possibly via NF-κB signaling pathway. Combination between cabazitaxel with anti-CD47 antibody exerted a stronger anti-cancer activity. The researchers recently used the same screening model and found that paclitaxel increased a certain population of TAMs and enhanced phagocytosis through Src family tyrosine kinase signaling, which potentiated the effect of anti-CD47 antibody in non-Hodgkin's lymphoma (NHL)31. Moreover, DNA damage caused by chemotherapeutic drugs potentially influences the innate sensing by propagating the stimulator of interferon genes (STING) pathway, which, in turn, enhances the type I IFN response, consequently arousing antigen presenting cell (APC)- and T cell-mediated immune response25,32. Based on this feature, treatment with cyclophosphamide, paclitaxel or temozolomide plus anti-CD47 antibody exerted potent tumor-fighting efficacy by augmenting APC infiltration and subsequently leading to more antigen presentation and T cell priming27,33.
Some chemotherapeutic agents induced the expression of CD47 on cancer cells and consequently caused these cancer cells to evade attacking from immune cells34. The countertherapeutic induction of CD47 by these agents provides opportunity to conduct the combination strategy (Fig. 1A). In both human cancer samples and murine model, cisplatin had been identified to up-regulate CD47 expression35,36. After cisplatin was combined with CD47 antibody, enhanced macrophage phagocytosis and tumor suppression were shown35. In addition, cancer stem cells (CSCs) in hepatocellular carcinoma (HCC) and pancreatic cancer are thought to be one of the main culprits of their chemo-resistance37,38. Elevated expression of CD47 was detected in these two types of CSCs after chemotherapy. Hence, targeting CSCs by CD47 morpholino, or anti-CD47 antibody exerted chemo-sensitizing effect in HCC and pancreatic cancer39, 40, 41.
Chemotherapeutic agents, including azacitidine (AZA), paclitaxel, doxorubicin, and docetaxel combined with different CD47 inhibitor, are under clinical trials (Table 2). Among all chemotherapy-related combination strategies, magrolimab plus AZA obtained preliminary phase Ib clinical trial results (NCT03248479). AZA is used to treat patients with high-risk myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) patients. It has been identified to synergize with magrolimab by up-regulating the expression of CRT42. The phase Ib results demonstrated that the objective response rate (ORR) of patients with MDS was 91% (30/33), and its complete remission (CR) rate reached 56% (14/33) after six-month combination treatment. In all patients with AML, the ORR and CR/CRi (complete remission/complete remission with incomplete count recovery) of the combination group were 64% (16/25) and 56%, respectively. Especially in TP53 mutant AML, the CR/CRi reached 75%. The combination therapy exhibited a better therapeutic efficacy than treatment with AZA alone and a similar tolerability43. Thus, it was approved by FDA as a breakthrough therapy for the treatment of MDS and AML (NCT04313881 and NCT04778397). As a special note, FDA has recently placed partial clinical hold on the aforementioned clinical trials (NCT04313881, NCT04778397, and NCT03248479) and other four trials (NCT05079230, NCT04778410, NCT04892446, and NCT02953509) due to an apparent imbalance in suspected unexpected serious adverse reactions between study arms, but soon lifted the hold on most of them after reviewing the comprehensive safety data from each clinical trial. The clinical trial results of the combination between magrolimab plus AZA are still meaningful. However, its safety should not be neglected in the future investigation. In addition, several other clinical studies about agents targeting CD47 plus chemotherapy are attempting to prove the feasibility of this kind of strategy. For example, high CR/CRi (94%) and CR (81%) rates were noted in newly diagnosed patients who are older/unfit or with high-risk AML patients after they received triplet combination regimen that included AZA, BCL-2 inhibitor venetoclax and magrolimab (NCT04435691). Another fully human anti-CD47 IgG4 antibody with lower red blood-cell binding affinity than magrolimab, namely, lemzoparlimab (TJC4), is in phase I clinical trials with the same triplet combination regimen in treating patients with AML or MDS (NCT04912063). Previously the initial result of a phase I/IIa study of lemzoparlimab showed that it was well-tolerated and caused moderate hematological adverse effects44.
Table 2.
Clinical trials targeting CD47-based combination strategies in cancer.
| Agent targeting CD47 | Additional combinational regimen | Statusa | Phase | Condition | Sponsor | CT number |
|---|---|---|---|---|---|---|
| Chemotherapy | ||||||
| Magrolimab | Azacitidine | Recruiting | III | Myelodysplastic syndromes | Gilead Sciences | NCT04313881 |
| Magrolimab | Azacitidine | Recruiting | III | Acute myeloid leukemia | Gilead Sciences | NCT04778397 |
| Magrolimab | Docetaxel | Recruiting | II | Solid tumor | Gilead Sciences | NCT04827576 |
| Magrolimab | Pembrolizumab + 5-Fluorouracil + Platinum/b Docetaxel | Recruiting | II | Head and neck squamous cell carcinoma | Gilead Sciences | NCT04854499 |
| Magrolimab | Venetoclax + Azacitidine/Mitoxantrone + Etoposide + Cytarabine/CC-486 | Recruiting | II | Myeloid malignancies | Gilead Sciences | NCT04778410 |
| AK117 | Nab paclitaxel/Paclitaxel | Recruiting | II | Metastatic triple-negative breast cancer locally advanced triple-negative breast cancer | Akeso | NCT05227664 |
| TTI-621 | Doxorubicin | Recruiting | II | Leiomyosarcoma | Pfizer | NCT04996004 |
| Magrolimab | Azacitidine + Venetoclax | Recruiting | I/II | Acute myeloid leukemia recurrent acute myeloid leukemia refractory acute myeloid leukemia |
M.D. Anderson Cancer Center | NCT04435691 |
| Evorpacept | Azacitidine | Recruiting | I/II | Higher risk myelodysplastic syndromes | ALX Oncology Inc. | NCT04417517 |
| AK117 | Azacitidine | Recruiting | I/II | Myelodysplastic syndrome | Akeso | NCT04900350 |
| AK117 | Azacitidine | Recruiting | I/II | Acute myeloid leukemia | Akeso | NCT04980885 |
| Evorpacept | Venetoclax + Azacitidine | Active, not recruiting | I/II | Acute myeloid leukemia | ALX Oncology Inc. | NCT04755244 |
| AO-176 | Paclitaxel | Active, not recruiting | I/II | Solid tumor | Arch Oncology | NCT03834948 |
| Magrolimab | Azacitidine | Active, not recruiting | I | Hematological malignancies | Gilead Sciences | NCT03248479 |
| IBI188 | Azacitidine | Recruiting | I | Myelodysplastic syndromes | Innovent Biologics (Suzhou) Co., Ltd. | NCT04485065 |
| Lemzoparlimab | Azacitidine + Venetoclax/Azacitidine | Recruiting | I | Acute myeloid leukemia myelodysplastic syndrome | AbbVie | NCT04912063 |
| TTI-622 | Azacitidine/Azacitidine + Venetoclax | Recruiting | I | Lymphoma multiple myeloma acute myeloid leukemia |
Pfizer | NCT03530683 |
| DSP107 | Venetoclax + Azacitidine | Recruiting | I | Acute myeloid leukemia myelodysplastic syndromes chronic myelomonocytic leukemia |
Kahr Medical | NCT04937166 |
| IBI188 | Azacitidine | Suspended | I | Myelodysplastic syndromes | Innovent Biologics (Suzhou) Co., Ltd. | NCT04511975 |
| Targeted therapy | ||||||
| Evorpacept | Trastuzumab + Ramucirumab + Paclitaxel | Recruiting | II/III | Gastric cancer gastroesophageal junction adenocarcinoma gastric adenocarcinoma |
ALX Oncology Inc. | NCT05002127 |
| Magrolimab | Daratumumab | Recruiting | II | Multiple myeloma | Gilead Sciences | NCT04892446 |
| Magrolimab | Cetuximab | Completed | I/II | Solid tumor colorectal cancer | Gilead Sciences | NCT02953782 |
| Magrolimab | Rituximab/Rituximab + Gemcitabine + Oxaliplatin | Active, not recruiting | I/II | Non-Hodgkin lymphoma | Gilead Sciences | NCT02953509 |
| CC-90002 | Rituximab | Completed | I | Hematologic neoplasms | Celgene | NCT02367196 |
| Hu5F9-G4 | Acalabrutinib + Rituximab | Completed | I | Non-Hodgkin lymphoma diffuse large B cell lymphoma | Acerta Pharma BV | NCT03527147 |
| Magrolimab | Obinutuzumab + Venetoclax | Recruiting | I | Follicular lymphoma marginal zone lymphoma mantle cell lymphoma etc. |
National Cancer Institute | NCT04599634 |
| TG-1801 | Ublituximab | Recruiting | I | B-cell lymphoma | TG Therapeutics, Inc. | NCT03804996 |
| TG-1801 | Ublituximab | Recruiting | I | Chronic lymphocytic lymphoma small lymphocytic lymphoma follicular lymphoma etc. |
TG Therapeutics, Inc. | NCT04806035 |
| Magrolimab | Dinutuximab | Recruiting | I | High risk neuroblastoma recurrent neuroblastoma recurrent osteosarcoma etc. |
National Cancer Institute | NCT04751383 |
| TTI-621 | Rituximab | Active, not recruiting | I | Hematologic malignancies solid tumor | Pfizer | NCT02663518 |
| Lemzoparlimab | Pomalidomide + Dexamethasone/Daratumumab + Dexamethasone | Terminated (Strategic considerations) | I | Multiple myeloma | AbbVie | NCT04895410 |
| Immunotherapy | ||||||
| Evorpacept | Pembrolizumab + Platinum + 5-Fluorouracil |
Recruiting | II | Head and neck cancer head and neck squamous cell carcinoma | ALX Oncology Inc. | NCT04675333 |
| Magrolimab | Pembrolizumab | Recruiting | II | Head and neck squamous cell carcinoma | Gilead Sciences | NCT04854499 |
| Magrolimab | Pembrolizumab | Recruiting | II | Hodgkin lymphoma classic Hodgkin lymphomarelapsed classical hodgkin lymphoma, etc. | Stanford University | NCT04788043 |
| Evorpacept | Pembrolizumab | Recruiting | II | Head and neck cancer head and neck squamous cell carcinoma | ALX Oncology Inc. | NCT04675294 |
| Anti-CD47 antibody | SHR2150 | Recruiting | I/II | Solid tumor | Chinese PLA General Hospital | NCT04588324 |
| DSP107 | Atezolizumab | Recruiting | I/II | Advanced solid tumor non-small cell lung cancer | Kahr Medical | NCT04440735 |
| AO-176 | Pembrolizumab | Active, not recruiting | I/II | Solid tumor | Arch Oncology | NCT03834948 |
| Magrolimab | Avelumab | Completed | I | Ovarian cancer | Gilead Sciences | NCT03558139 |
| IBI188 | Sintilimab/GM-CSF | Recruiting | I | Solid tumors lung adenocarcinoma osteosarcoma |
Innovent Biologics (Suzhou) Co., Ltd. | NCT04861948 |
| Evorpacept | Pembrolizumab/Pembrolizumab + 5-Fluorouracil + Cisplatin | Active, not recruiting | I | Metastatic cancer solid tumor advanced cancer etc. |
ALX Oncology Inc. | NCT03013218 |
| TTI-621 | Nivolumab | Active, not recruiting | I | Hematologic malignancies solid tumor | Pfizer | NCT02663518 |
| Magrolimab | Mogamulizumab | Suspended (Other - Amendment Request) | I/II | Recurrent mycosis fungoides recurrent mycosis fungoides and sezary syndrome recurrent sezary syndrome etc. |
National Cancer Institute | NCT04541017 |
| TTI-621 | PD-1 or PD-L1 inhibitor/pegylated interferon-α2a/T-Vec/Radiation | Terminated | I | Solid tumors mycosis fungoides melanoma Merkel-cell carcinoma etc. |
Trillium Therapeutics Inc. | NCT02890368 |
| Bispecific antibody or fusion protein | ||||||
| HX009 | Active, not recruiting | II | Advanced solid tumor | Waterstone Hanxbio Pty Ltd. | NCT04886271 | |
| DSP107 | Recruiting | I/II | Advanced solid tumor non-small cell lung cancer | Kahr Medical | NCT04440735 | |
| CPO107 | Recruiting | I/II | Non-Hodgkin lymphoma | Conjupro Biotherapeutics, Inc. | NCT04853329 | |
| IBI322 | Recruiting | I | Hematologic malignancy | Innovent Biologics (Suzhou) Co., Ltd. | NCT04795128 | |
| IBI322 | Recruiting | I | Advanced malignancies | Innovent Biologics (Suzhou) Co., Ltd. | NCT04338659 | |
| IBI322 | Recruiting | I | Advanced malignancies | Innovent Biologics (Suzhou) Co., Ltd. | NCT04328831 | |
| PF-07257876 | Recruiting | I | Non-small cell lung cancer head and neck squamous cell carcinoma Ovarian cancer |
Pfizer | NCT04881045 | |
| IMM0306 | Recruiting | I | Non-Hodgkin lymphoma | ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd. | NCT04746131 | |
| IMM2902 | Recruiting | I | Advanced solid tumor; advanced breast cancer; advanced gastric cancer | ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd. | NCT05076591 | |
| TG-1801 | Recruiting | I | B-cell lymphoma | TG Therapeutics, Inc. | NCT03804996 | |
| IBI322 | Not yet recruiting | I | Advanced solid tumor | Innovent Biologics (Suzhou) Co., Ltd. | NCT04912466 | |
| HX009 | Active, not recruiting | I | Advanced solid tumor | Waterstone Hanxbio Pty Ltd. | NCT04097769 | |
| SG2501 | Not yet recruiting | I | Hematological malignancy lymphoma | Hangzhou Sumgen Biotech Co., Ltd. | NCT05293912 | |
| SL-172154 | Terminated (Sponsor decision) | I | Cutaneous squamous cell Carcinoma squamous cell Carcinoma of head and neck | Shattuck Labs, Inc. | NCT04502888 | |
Data from https://clinicaltrials.gov/ (collected on August 22, 2022).
“/” is used to distinguish the different study arms of the clinical trial.
3. Agents targeting CD47 with radiotherapy
In line with chemotherapy, radiotherapy also induces ICD and remodels the TME to an inflammatory state45,46. Its potential to combine with agents targeting CD47 had been identified by many preclinical cases47, 48, 49, 50, 51, 52, 53. Moreover, the re-sensitization of radioresistant cancer cells by agents targeting CD47 is another important rationale for the combination.
In various cancer types, the enhanced anti-cancer effect of radiotherapy plus the CD47 blockade was possibly through increasing the “eat me” signals such as CRT on the irradiated cancer cells to mediate more phagocytosis47,50,53,54. Meanwhile, the modulation effect of the combination therapy on immune cells, particularly macrophages and T cells, was another main factor that contributed to the synergy (Fig. 1A). A peptide targeting CD47 to block the CD47–SIRPα interaction synergized with irradiation through the increased proportion of tumor-infiltrating macrophages and enhanced phagocytosis47. CD47 played a negative role in T cell response, possibly due to its interaction with thrombospondin-155,56. Regardless of macrophage-mediated immune response, one study using CD47 morpholino to suppress CD47 expression in CD8+ T cells in the context of radiotherapy was identified to activate T cells for combating fibrosarcoma and melanoma51. Enlightened by this finding and the T cell-targeting strategy, a triple combination consisting of anti-CTLA4 antibody, CD47 morpholino, and radiotherapy was conducted. The outcome suggested that the CD47 and CTLA4 blockade in the context of irradiation significantly shrank the tumor volume and prolonged the survival of tumor-bearing mice48.
In addition, up-regulation of CD47 expression after radiation was often accompanied by elevation of other factors such as PD-L1, HER2, or fatty acid oxidation enzymes, which is thought to cause radio-resistance and limit the efficacy of radiotherapy49,50,52. Therefore, anti-CD47 antibodies, in combination with inhibitors targeting these factors, respectively, are capable to re-sensitize the cancer cell resistance to radiotherapy49,50,52.
Clinical trial about radiotherapy-related combination strategies of CD47 is relatively insufficient. The efficacy and safety of TTI-621 (SIRPα-IgG1 Fc) plus radiation was accessed in a previous clinical trial (NCT02890368) but discontinued by the sponsor. The relevant clinical data were not disclosed.
4. Agents targeting CD47 with targeted therapy
The identification of specific mutation of oncogenic kinase in cancer has led to the advent of targeted therapy57. In recent years, its combination with immunotherapy has achieved encouraging clinical development58. The reshaping effect on the tumor immune microenvironment by targeted therapy make it an optimal assistant to couple with immunotherapy58. As for the combination with agents targeting CD47, reprogramming of macrophages or Fc receptor-dependent function of antibody-dependent cellular phagocytosis (ADCP) by some targeted therapy is one critical rationale for the enhanced efficacy (Fig. 1). Meanwhile, the generation of most bi-specific antibodies in the section is based on the co-expression of CD47 and other targets in certain types of cancer. Notably, CD47 blockade is used to overcome the drug resistance of anti-HER2 antibody in a few studies.
4.1. EGFR-targeted therapy
EGFR is usually mutated or overexpressed in various malignant tumors59. Meanwhile, higher expression of CD47 is ubiquitous in cancer9. Co-expression of CD47 and EGFR was found in some solid tumors, and it has been shown to be a poor prognostic factor in patients with cancer60. These features make it possible to design the two targets-based bi-specific antibody. Importantly, it is speculated that most CD47-based bispecific antibodies reduced the “off-target” rate, thereby eliciting a more potent ADCP effect than anti-CD47 antibody alone (Fig. 2). A CD47/EGFR (IgG1 with effector function) bispecific antibody was identified to selectively target different EGFR/CD47-double positive cancer cells. Compared with CD47/Mock-IgG1, the in vitro co-culture experiment identified that CD47/EGFR-IgG1 promoted macrophage-mediated ADCP and antigen cross-presentation, thus arousing adaptive immune response61. Another bispecific antibody fusion protein named Bi-SP (IgG1 with effector function) conjugated anti-EGFR IgG1 antibody and SIRPα variant-IgG1 (SIRPαV–Fc) together. An important detail is that the SIRPα variant without Fc portion previously showed enhanced phagocytosis to colon cancer cells when combined with anti-EGFR antibody cetuximab62. In the study of Bi-SP, potent macrophage phagocytosis and stronger anti-cancer effect were shown after the bispecific antibody treatment. Meanwhile, less hematotoxicity of Bi-SP than that of SIRPαV–Fc was found63.
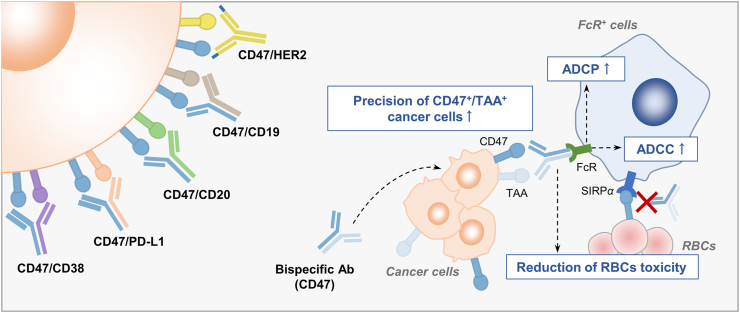
Figure 2.
CD47-based bispecific antibodies or fusion proteins enhance cancer cell-specific targeting. Several CD47-based bispecific antibody or fusion protein had already entered clinical investigation, including bispecific antibodies or fusion proteins targeting CD47/PD-L1, CD47/CD20, CD47/CD19, CD47/HER2 and CD47/CD38. These agents show great targeting precision to the dual antigens-expressing cells (CD47+/TAA+). They bind less to other type of cells such as RBCs and have less “off-target” toxicity. Meanwhile, the bi-specific antibodies or fusion protein induces strong ADCP or ADCC through Fc–FcR interaction. The FcR+ cells refer to macrophages (ADCP) or NK cells (ADCC).
A complete phase Ib/II clinical trial (NCT02953782) among subject with colorectal cancer demonstrated at most 45% (18/40) with stable disease (SD) after the combination treatment of magrolimab and cetuximab64. Currently, no CD47/EGFR bispecific antibody entered the clinical research.
4.2. HER2-targeted therapy
HER2, a driven gene usually overexpressed in breast cancers (∼20%), is one of the important indicators for metastasis and poor prognosis65,66. The close correlation between macrophage and antibody targeting HER2 provides opportunities to combine with agents targeting CD47. Macrophage-mediated ADCP via Fc–Fcγ receptor (FcγR) has been identified to be another anti-cancer mechanism of anti-HER2 antibody in addition to the antibody-dependent cell-mediated cytotoxicity (ADCC) effect by natural killer (NK) cells or neutrophils67,68. Monotherapy with the anti-HER2 antibody trastuzumab was observed to affect the infiltration and the phenotype of TAMs. Combination with anti-CD47 antibody further expanded TAMs infiltration and enhanced ADCP. Gene expression analysis of these TAMs showed that combination treatment polarized TAMs to a pro-inflammatory and anti-cancer phenotype, which is close related to M1 macrophage67.
Notably, the drug resistance of anti-HER2 therapy in some cases was caused by dysfunction of trastuzumab-mediated ADCC69. In a HER2 positive ADCC-tolerant breast cancer cells model, the addition of anti-CD47 antibody re-sensitized trastuzumab and prolonged the survival rate of mice via Fc–FcγR dependent ADCP70. Besides, as depicted in radiotherapy, correlation was established between CD47 and HER2 after irradiation, as both proteins were upregulated to abolish the efficacy of irradiation, and NF-κB was identified to be the common transcription factor of the two proteins. Inhibition of HER2 and CD47 simultaneously promoted the phagocytic effect of macrophage on radiation-resistant cells52.
In clinic, different agents targeting HER2 have remarkably improved the patient outcomes, among which, trastuzumab is a universally recommended agent by the current guidelines for stages I–III HER2-positive breast cancer66. However, the response rate of trastuzumab is still heterogeneous and resistance appears67. ALX148 (inactive Fc domain with no binding to Fcγ receptor) combined with trastuzumab enhanced the anti-cancer effect significantly in HER2-positive gastric cancer in a NOD-SCID mouse model71. On the basis of the results, the combination therapy was evaluated in phase I clinical trial (NCT03013218), and 21.1% (4/19) overall response rate and 26.3% (5/19) disease control rate were observed in patients with HER2-positive gastric or gastroesophageal junction cancer72. The treatment-related adverse effects were mostly in low grade, suggesting excellent safety and tolerability of this combination treatment72. Moreover, a CD47/HER2 bispecific antibody named IMM2902 (unknown subset of IgG with functional Fc portion) is ongoing a phase Ib study to test its safety, tolerability, and preliminary efficacy in patients with HER2-positive solid tumor (NCT05076591), and recently, it completed the first patient dosing.
4.3. VEGF-targeted therapy
VEGF, a signal protein that binds to VEGF receptor (VEGFR), maintains homeostasis in angiogenesis in normal tissues73. However, high levels of VEGF inhibit the functions of macrophages, DCs and T cells, leading to an immunosuppressive environment and promoting the tumor growth74. Tumor vascular normalization by agents targeting VEGF pathway ameliorated the immunosuppressive TME, which has been developed against several malignancies75. During anti-angiogenic therapy, upregulation of CD47 was found in a non-small cell lung cancer mouse model via the TNFα–NF-κB pathway. Compared with other VEGFR family members, VEGFR1 has a higher binding affinity to VEGF76. Researchers designed a VEGFR1–SIRPα fusion protein and found that co-targeting VEGFR1 and CD47 by the fusion protein induced more macrophage infiltration and showed synergetic effect in lung cancer and glioblastoma77,78.
4.4. CD20-targeted therapy
CD20 is a protein with a restricted expression starting from late pre-B cell but its expression could be lost in differentiated plasma cells, targeting CD20 by antibody provided potential therapeutic effect to B-cell malignancies79. In the combination context, FcγR activation by Fc portion of anti-CD20 antibody is thought to be the main rationale in exerting the additional anti-cancer effect in the presence of anti-CD47 antibody80,81. The anti-CD47 antibody could trigger phagocytosis without Fc portion, but once the rituximab had no Fc portion, the synergism was lost80. Chao et al.80 and Advani et al.81 found that the macrophage killing effect and the superior ADCP induced by two agents but not by the NK cells-mediated ADCC, were the predominant factors in combination therapy. However, several recent studies observed that the ADCC effect by anti-CD20 antibody could not be excluded in the synergism82, 83, 84.
In addition to integrating two separate agents together, several bispecific antibodies targeting CD47 and CD20 was also under preclinical investigation85, 86, 87. The CD20/CD47 bispecific antibodies had stronger binding affinity to the CD20/CD47 double-positive cancer cells and weaker binding to RBCs than rituximab or anti-CD47 antibody86,87. In vivo test also indicated that the bispecific antibody had a better tumor control and longer survival period than monotherapy86,87.
As the first generation of monoclonal antibody targeting CD20, rituximab combined with chemotherapy significant prolongs the patient's overall survival and the PFS in “mature” B-cell leukemias and diffuse large B-cell lymphoma (DLBCL)88. However, neither rituximab alone nor the already existing combo treatment could not fully control the tumor growth of many patients with NHL. Progress was made by rituximab combined with magrolimab (Hu5F9) in the treatment with DLCBL patients who received more than four prior therapies and are resistant to rituximab. Twenty-two patients (15 with DLBCL and 7 with follicular lymphoma) were enrolled in the test of Hu5F9 in combination with rituximab. After the combination treatment, 50% of patients had objective response with 8 complete responses. Additionally, at a median follow-up of 6.2 months in the DLBCL group and 8.1 months in the follicular lymphoma group, 91% of patients continued to respond81. The clinical evidence suggested the impressive efficacy of the combination strategy. Besides, the CD47/CD20 bispecific antibody, IMM0306 (IgG1 with effector function) utilized the dual-variable-domain immunoglobulin format. At present, it is ongoing phase I trial to study the safety and efficacy in patient with NHL (NCT04746131).
4.5. CD19-targeted therapy
CD19 has a more stable expression from pre-B cells till the differentiated plasma cells than CD20. Targeting CD19 was identified to be one of the alternative strategies when drug resistance occurred after anti-CD20 treatment, because some CD20-negative B-cell malignance expressed CD1989. Anti-CD19 antibody was more efficient in CD47−/− and the existence of CD47 limited the ADCP in the treatment of B-cell malignance in vitro90. The main combination pattern between CD47 and CD19 was the bispecific antibody. NI-1701, a CD47/CD19 antibody with a fully human IgG1 in its fully functional Fc portion91, was reported to target and enhance its ADCP to CD47/CD19 double-positive cells only, which accounted for weak antigen sink and RBCs clearances92. Compared with rituximab, NI-1701 had a better tumor control in Raji cells bearing NOD/SCID mouse. Different drug targets even made the combination between rituximab and NI-1701 have the best tumor regression. Safety evaluation in non-human primates indicated no drug related side effect such as hemagglutination and platelet aggregation occurred after treatment with NI-1701. Apart from enhanced the ADCP, NI-1701 demonstrated its potential to repolarize the M2 type of macrophages91. Another study also showed that co-engaging CD47 and CD19 by bi-specific antibody inhibited the B cell receptor (BCR)-mediated cell proliferation93. Moreover, MT103 (blinatumomab) is a clinical-used CD19/CD3-bispecific T cell engager antibody for treating patients with NHL and precursor B-ALL94,95. Co-treatment with MT103 and anti-CD47 caused persistent control of lymphoma96, possibly providing distinct ideas about the combination strategy in targeting CD47 and CD19, which build a closer connection between the innate and adaptive immune response.
In clinical, the CD47/CD19 bispecific antibody TG-1801 (its previous name is NI-1701) aimed at patients with aggressive lymphoma, DLBCL, CLL, SLL, and other types of B-cell lymphoma. It is ongoing phase I clinical trials and has not yielded results currently (NCT03804996 and NCT04806035).
4.6. Others
Researchers have never stopped discovering new combination schemes between CD47 and other targets. Those schemes shared some common features, one of which is that the selected targets to cooperate with CD47 are already identified TAAs and have even entered the clinic. A recent study demonstrated that anti-GD2 synergized with anti-CD47 antibody in GD2 positive neuroblastoma97. GD2 is a TAA for neuroblastoma and osteosarcoma, and anti-GD2 antibody dinutuximab has been implicated in treatment for patients with neuroblastoma98. One rationale for the regimen is that the tumor cell death and CRT exposure caused by the binding of GD2 to its antibody enhances the phagocytosis and anti-cancer activity of anti-CD47 antibody97. Currently, the regimen (magrolimab plus dinutuximab) is taking a phase I clinical trial in children and young adults with relapsed or refractory neuroblastoma or relapsed osteosarcoma (NCT04751383).
CD38 is a transmembrane glycoprotein highly expressed in many hematologic malignancies including T-ALL99. A newly published study proved that CD47 also overexpressed in most T-ALL patient samples100. The blockade of CD47–SIRPα axis had been identified to work with anti-CD38 antibody daratumumab to eliminate T-ALL cells in vitro and in patient derived xenograft mouse model, respectively. Elevated ADCP was presented after the combination, probably due to more Fc–FcγR interaction induced by daratumumab100. It is worth mentioning that two clinical trials (NCT04892446 and NCT05293912) were carried out to evaluate the safety and efficacy of this combination regimen, including a CD47/CD38 bispecific antibody named SG2501. Furthermore, some dual-targeted agents such as CD47/Glypican-3, CD47/mesothelin, and SIRPα–αCD123 fusion antibodies, also had compelling preclinical data to show their combinational anti-cancer efficacy92,101, 102, 103, 104.
5. Agents targeting CD47 with immunotherapy
Although CD47 is a vital signal to directly control macrophage phagocytosis, or indirectly increases antigen presentation by APC, and T cells activation, accumulating evidence indicates that solely blocking CD47 signal transduction is unable to trigger an intense immune response9. Therefore, further activation of the immune system, no matter further enhancing the innate immune response or the adaptive immune response, is the main rationale for agents targeting CD47 to combine with immunotherapy. Likewise, most of the bispecific agents in this section are acted as a bridge to connect macrophage phagocytosis and the further immune response, the related mechanisms were depicted in Fig. 3.
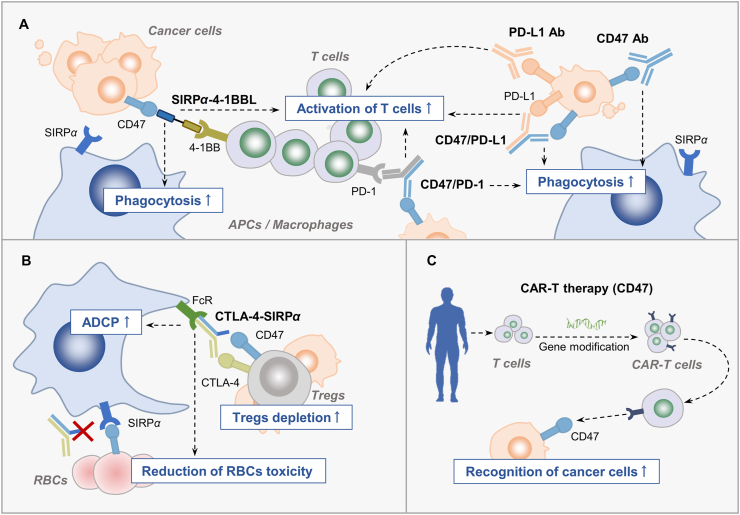
Figure 3.
Combination strategies regulate T cell-mediated response. (A) The SIRPα–4-1BBL fusion protein or CD47/PD-1 bispecific antibody could not only block CD47 to enhance phagocytosis but also activate T cell-mediated immune response. Besides, the combination effect of co-targeting CD47 and PD-L1 relies on T cell activation. (B) Unselective depletion of Tregs in periphery by anti-CTLA-4 antibody contributes to immune-related adverse effects. The anti-CTLA-4–SIRPα heterodimer is developed to reduce the binding of agents targeting CD47 to RBCs and precisely deplete tumor Tregs. (C) Selection of CD47 as the TAA for CAR-T therapy increases T cell recognition to cancer cells. CD47 Ab, anti-CD47 antibody; PD-L1 Ab, PD-L1 antibody; CD47/PD-L1, bispecific antibody targeting CD47 and PD-L1; CD47/PD-1, bispecific antibody targeting CD47 and PD-1; CTLA-4-SIRPα: anti-CTLA-4–SIRPα heterodimer.
5.1. Anti-PD-1 or anti-PD-L1 therapy
Antibodies targeting the PD-1–PD-L1 axis have been identified as the forefront immune checkpoint inhibitors in cancer treatment in clinic105. PD-L1 interacts with PD-1 to cause the dysfunction of T cells. Therefore, the blockade of PD-1–PD-L1 interaction induces a strong T cell-mediated adaptive immune response106. A positive correlation was speculated to exist between CD47 and PD-L1 for two reasons, namely that CD47 and PD-L1 have been found to be co-expressed in certain cancer types50,107, and some transcription factors and cytokines are shared by these two proteins106,108. All the aforementioned reasons enlarge the population of CD47+PD-L1+ cancer cells. Thus, the enhanced antitumor effect was largely depending on the specific elimination of the CD47+PD-L1+ cancer cells by the CD47/PD-L1 bispecific agents107,109, 110, 111. Meanwhile, less binding of red blood cells (RBCs) or normal cells of these bispecific antibodies reduced the hematological toxicity and “antigen sink” effect caused by CD47-based monotherapy107,110,111.
Combination strategies between agents targeting PD-L1 (or PD-1) and CD47 directly stimulate the innate and adaptive immune cells, thus enhancing the anti-cancer effect. Redoubled macrophage phagocytosis was shown after CD47 antagonist combined with anti-PD-L1 antibody112 or a CD47/PD-L1 bispecific antibody treatment111. This finding could be partially explained by the identification of the PD1–PD-L1 axis as another anti-phagocytosis checkpoint113. Except for macrophage, the cancer cell elimination by T cells plays the central role in the combination context. Robust CD8+ T cell response was dependent on the function of PD-1–PD-L1 and CD47–SIRPα blockade, the lack of each one abrogated the effect. An in-depth study further discovered that treatment with one CD47/PD-L1 bispecific antibody augmented the population of Tcf7+ stem-like progenitor CD8+ T cell in the TME and promoted its differentiation to an effector-like state111. In non-human primate experiment, the innate response in combination therapy was mostly relied on the blockade of the CD47–SIRPα axis, but T cells are still the main mediator in the anti-cancer process111.
In clinical stage, the combination schemes of targeting PD-L1 (or PD-1) and CD47 could be divided into the bispecific antibody and co-treatment with anti-PD-L1 (or anti-PD-1) antibody and agents targeting CD47. To date, bispecific antibodies such as IBI322 (CD47/PD-L1 bispecific antibody), HX009 (PD-1/CD47 bispecific antibody) and PF-07257875 (CD47/PD-L1 bispecific antibody) were in clinical trials. The phase I result of HX009, which was used for treatment with advanced solid tumor, indicated that dosing up to 7.5 mg/kg of HX009 was well-tolerated without any dose limiting toxicities114. Moreover, antitumor activity was observed in 1 and 5 mg/kg cohorts with objective responses in multiple tumor types (NCT04097769). Preliminary phase I results of IBI322 in patients with advanced solid tumors was released. IBI322 was well tolerated and showed a favorable safety profile. Within evaluable 20 patients, 4 achieved partial response (PR) and 7 achieved SD after treatment with IBI322 at active doses115. In addition, treatment with anti-PD-L1 antibody and anti-CD47 antibody also deserved exploration in clinic. A phase Ib clinical data about magrolimab combined with anti-PD-L1 antibody avelumab observed a 56% SD rate in enrolled platinum-resistant or refractory ovarian cancer patients116.
5.2. Anti-CTLA-4 therapy
As a negative regulator of T cell activation, CTLA-4 is an immune checkpoint expressed in conventional T cells and regulatory T cells (Tregs)117. Accordingly, an antibody targeting CTLA-4 was reported to expand and prime T effector lymphocytes118. Preclinical evidence illustrated that the dual targeting therapy unlocked two inhibitory signals on T cells (CD47-TSP-155 and CTLA-4-CD80/CD86 interaction inhibited T effectors cells activation), subsequently exerting anti-cancer effect by fully activated T effectors lymphocytes. A triple combination strategy among anti-CTLA-4 antibody, anti-sense morpholino silencing CD47, and irradiation was partly mentioned in the “combination with radiotherapy” section. In particular, experiment identified that CD47 morpholino plus anti-CTLA-4 antibody ipilimumab enhanced antigen-dependent killing of irradiated cancer cells48. CD47 and CTLA-4 targeted therapy in the context of irradiation prolonged the survival and increased tumor necrosis by recruiting CD8+ T cells, and intra-tumoral NK cells and expelling myeloid-derived suppressor cells (MDSCs).
The expression of CTLA-4 on Tregs is responsible for its immunosuppressive function. Utilization of anti-CTLA-4 antibody is able to deplete Tregs in the TME, partially via ADCP119. An enhanced phagocytosis of Tregs was presented by an inhibitor targeting CD47 plus anti-CTLA-4 antibody in a coculture system between Treg and macrophage120. However, sometimes, unselective depletion of Tregs in periphery by anti-CTLA-4 antibody contribute to immune-related adverse effects (irAEs)121. To reduce the binding of agents targeting CD47 to RBCs, anti-CTLA-4–SIRPα (hIgG1 with effector function) heterodimer was developed122. Anti-CTLA-4–SIRPα treatment demonstrated better tumor control than co-treatment with anti-CTLA-4 and SIRPα–Fc in different immunocompetent mouse models. Mechanism studies revealed that the precise ADCP of macrophage to tumor Tregs (ICOShigh immunosuppressive Tregs) was essential for the heterodimer to exert the synergized effect122.
5.3. TLR agonists-based therapy
Toll-like receptor (TLR) play a critical role in the immune system activation for their ability to recognize pathogen-associated molecular pattern signals expressed by pathogen and danger-associated molecular patterns signals released from stressed or dying cells123,124. TLRs are widely expressed in the immune cells, including macrophage, NK cells, DCs, neutrophils and T cells125. Activation of the TLR signaling pathway in different immune cell types leads to distinct immune response. TLR agonists were utilized by researchers to combine with other anti-cancer treatment for further therapeutic effect. Most preclinical studies about the combination between TLR agonists and agents targeting CD47 have focused on the function of TLR agonists in targeting macrophages. Previous researchers found that some TLR agonists enhanced macrophage phagocytosis when combined with CD47-targeted therapy126. Mechanism study revealed TLR signaling pathway activation in macrophage phosphorylated Bruton's tyrosine kinase, which contributed to CRT exposure of macrophage surface. The augment of “eat me” signal in macrophage and the blockade of CD47 explained for the higher phagocytosis rate126.
Besides, some recent studies addressed the importance of pro-inflammatory TME (including the release of pro-inflammatory cytokines and the existence of pro-inflammatory immune cells) in prompting efficacy of the CD47-targeted therapy. Zhong et al.127 discovered that the TLR3 activator Poly(I:C) was synergized with CD47 blockade via secretion of pro-inflammatory cytokines, mainly by IL-6. One recent study also presented that inflammatory stimulus, such as IFN-γ, GM-CSF treatment, and TLR agonists including the TLR4 agonist lipopolysaccharide (LPS), and Poly(I:C) stimulation bolstered macrophage phagocytotic function via pro-phagocytotic integrin CD11a/CD11c and the subsequent participation of CD18128. IFN-γ, LPS, and GM-CSF were canonical inducers that switched the macrophage phenotype to M129. In the combination context, M1 macrophage was a better assistant to CD47-targeted therapy. One study found that repolarization of macrophage by pretreatment of IFN-γ and LPS combined with SIRPα–Fc protein promoted phagocytosis of AML cells129. Besides, pyrimido [5,4-b] indole (PBI1), a potential TLR4 activator, was identified to polarize macrophage to M1 type. After cooperating with anti-CD47 antibody, PBI1 promoted macrophage engulfment to B-cell lymphoma cells130. Furthermore, re-programming the macrophage metabolism by TLR agonists also increased the cancer cell elimination. In vitro phagocytosis assay indicated that the TLR9 agonist CpG oligonucleotide showed an increased phagocytotic ability to PDAC cells after co-treatment with anti-CD47 antibody131.
TLRs signaling was related to APC activation132, a study arm in one phase I/II clinical trial selected a small molecule agonist of TLR7 (SHR2150) to combine with chemotherapy drugs plus anti-CD47 antibody to treat patients with unresectable/metastatic solid tumor (NCT04588324). The study was designed to evaluate the safety and clinical efficacy of this regimen, and patients are currently being recruited.
5.4. Others
Some other immunotherapy-based combination strategies of CD47 should also be mentioned. This section is divided on the basis of the dominant immune cells of action for these combined strategies. Research progress has been made in related combined strategies that are based on T cells. Chimeric antigen receptor (CAR) T-cell therapy has attracted tremendous attention these years133. CD47 in CAR-T therapy was firstly utilized as a tumor-associated antigen to ensure the precision of cancer cell-targeting134,135. Further development on CAR-T therapy identified that targeting multiple antigens, including CD47, and localizing the designed CAR-T injection had enhanced therapeutic effect136,137. Huang et al.138 recently designed a CAR-T therapy that simultaneously deliver SIRPα–Fc fusion protein to block CD47. The combination treatment increased the population of DCs and M1 type of macrophage in the tumor tissue, presenting a satisfied anti-cancer effect in solid tumor. Besides, a SIRPα–4-1BBL fusion protein (fusion between the extracellular domain of human SIRPα and human 4-1BBL, with no Fc portion) named DSP107 could not only block CD47 to enhance phagocytosis but also activate tumor-reactive T cell-mediated immune response. The co-stimulatory signal of T cells only occurred after the binding of 4-1BB to 4-1BBL expressed on the membrane, while soluble 4-1BBL has no activating function. Thus, the unique structure of DSP107 gained significant 4-1BB agonistic activity when bound to tumor-overexpressed CD47, aiding in driving T cell immune responses in the TME139. Critically, two phase I clinical trials about DSP107 are ongoing for treating patients with solid tumor and hematological malignances (NCT04440735 and NCT04937166).
Other immune cells also played a vital role in CD47-based combination therapies. The activation of APCs initiates the crosstalk between innate and adaptive response, which is facilitated by STING activation and the release of type I interferon. STING activation by its ligand cGAMP repolarized TAMs to M1-like macrophages, intra-tumoral treatment with cGAMP and anti-CD47 antibody was recently identified to have a systemic anti-cancer immune response140. Additionally, a SIRPa–Fc–CD40L fusion protein induced the production of type I interferon in the TME, potentiating the ADCP after combining another antibody such as anti-CD20 antibody, and had an outstanding tumor-fighting activity due to the activation of the whole immune response141. The SIRPa–Fc–CD40L fusion protein is undergoing phase I clinical trial (NCT04502888). Moreover, the role of NK cells in the combination therapy could not be neglected. Two recent studies about the combination between anti-CD47 antibody and oncolytic virus illustrated that the cancer-fighting synergy was mainly through NK cell mediated ADCC and macrophage repolarization142,143.
6. Discussion and future perspectives
More than 90% of CD47-related clinical trials are combination therapy. 30% and 70% of those combination therapy focuses on solid tumors and hematological malignancies, respectively. The current status of CD47-based combination therapy is that more exciting clinical trial results are obtained in hematological malignancies. AZA combined with CD47-targeted agents is the most common regimens in treating hematological malignancies including AML and MDS. In solid tumors, most clinical trials are centered around combination strategy between anti-PD-L1/PD-1 antibody plus CD47-targeted agents, and anti-HER2 antibody plus CD47-targeted agents (Table 2). Collectively, no matter in hematological or solid tumors, agent targeting CD47 plays an “indispensable” and “supportive” role in the combination strategy. The “indispensable” function was reflected in the clinical trial results of its combination strategy (NCT04435691) being superior to most other clinical combination regimens currently in the treatment for AML patients. Meanwhile, agent targeting CD47 in some combination schemes plays a strong supporting role, as its addition is able to enhance the efficacy of the already-existing treatment or overcome drug resistance. Various CD47-based dual-target agents are currently being tested in clinical trials (Table 2). Some dual-target agents such as IBI322 (CD47/PD-L1) and TG-1801 (CD47/CD19) hold various clinical trials in solid tumor and hematological malignancies. Compared with separated treatment, these bispecific agents maintain the combinational effect and simultaneously reduce side effects for their specificity to cancer cells. Hence, it is believed that they could have great therapeutic potential in the future.
Understanding the detailed mechanism sheds light on the subsequent development of combination strategies for CD47. Preclinical studies of combined strategies provide clues to answer the question (Table 1). First, combination therapies enhance macrophage phagocytosis. In-depth research on macrophage phagocytosis found that blocking the signal transduction of “don't eat me” alone sometimes could not trigger sufficient phagocytosis to eliminate cancer cells12, while adding more “eat me” signals greatly improved the phagocytic efficiency of macrophages144,145. In that case, the combination therapies further enhance the phagocytosis by up-regulating the “eat me” signals such as increasing the CRT exposure or inducing more Fc–FcR interaction144 (Fig. 1A). Another mechanism to increase phagocytosis is repolarizing TAMs to “M1-like” macrophage, which is a more pro-phagocytotic phenotype (Fig. 1B). Besides, the up-regulation of CD47 on cancer cells caused by other therapies provides an opportunity to combine agents targeting CD47 and consequently leads an increased phagocytosis (Fig. 1A).
Second, combination therapies enhance the tumor-targeting precision and reduced “off-target” toxicity. Of note, this advantage appears in most of CD47-based bispecific antibodies or fusion proteins146. The “on-target” rationales of these agents are the co-expression of CD47 and another TAA in cancer cell. Simultaneously blocking signal transduction of CD47 and another target stimulates two diverse functions. Compared with single target antibody, CD47-based bispecific agents could enhance macrophage ADCP or NK cells-mediated ADCC. Furthermore, the augmentation of cancer cells targeting by these agents circumvents the off-target toxicity, which is mainly the unnecessary “antigen sink” and anemia caused by the clearance of healthy RBCs (Fig. 2)147,148.
Third, combination therapies regulate T cell-mediated immune response. Single agent targeting CD47 is identified to stimulate the innate immune response. In that case, adding another agent to stimulate the adaptive immune response further eliminates cancer cells. Direct activation of CD8+ T cell by anti-PD-1/PD-L1 or 4-1BB ligand is helpful for further T cell activation and tumor regression (Fig. 3A). Furthermore, precise depletion to the tumor Tregs by macrophage and utilization of CD47-based CAR-T in the combination strategy perform a greater cancer fighting capacity, thus addressing the important role of T cells in the combination strategies (Fig. 3B, C).
In the future, the combination strategy of CD47 still needs to be deeply studied in terms of two aspects: enhancing the combined efficacy and reducing toxicity. These are also two major challenges that may halt the clinical translation of CD47-based therapy. Although some of them showed impressive outcomes, the efficacy of the current combination regimens could be further improved. Meanwhile, the design of some strategies focused more on enhancing the combinational effect but neglected to limit the toxicity. Therefore, some issues and insights need to be discussed after the data were integrated and the mechanism were summarized. 1) The idea of “precision medicine” is a reminder to explore the possibility of CD47 becoming a cancer biomarker. Retrospective analysis of several clinical studies speculated that the expression of CD47 in patients may predict the efficacy of CD47 antibodies and provide instruction into the need for further combination of CD47 antibodies in patients already receiving other treatments149, 150, 151, 152, 153. 2) Aside from targeting cancer cells, the utilization of macrophage phagocytosis to eliminate tumor resident immunosuppressive cells, such as MDSCs, Tregs, or CAFs could restore the immune activation of other immune cells. In recent years, the targeting of immunosuppressive cells in cancer has been approved to be a promising anti-cancer method154,155. The preliminary success of CD47-based dual target agents in preclinical study indicated that adding one more target largely extends their specificity to kill one certain type of cells146. Precise depletion of tumor Tregs by the anti-CTLA-4–SIRPα heterodimer has already set a good example122. 3) Beyond targeting CD47, targeting SIRPα is worthy of attention. As the receptor of CD47, SIRPα is expressed only in some myeloid cells. This feature allowed agents that target it to avoid binding to erythrocytes156,157. Therefore, SIRPα-based combination strategies may have less blood toxicity, but their anti-cancer potential still needs more preclinical and clinical data for support. 4) Moreover, with the continuous improvement of understanding of the mechanism of phagocytosis, other phagocytosis checkpoints were discovered, such as major histocompatibility complex class I–leukocyte immunoglobulin-like receptor 1 axis, CD24–sialic-acid-binding Ig-like lectin 10 axis, and so on144,158. Even if CD47 remains the mainstay of anti-phagocytic functions currently, future in-depth study might reveal that other phagocytosis checkpoints are functionally equivalent to, or more important than CD47 in certain types of cancer. Comprehensive identification and utilization of these phagocytosis checkpoints is of great significance for the pursuit of better anti-cancer effects. Meanwhile, if some checkpoints act more like co-effectors with CD47 in the anti-phagocytosis process, targeting them could combine with CD47-based therapy to synergistically enhance phagocytosis and further fight cancer.
Acknowledgments
This work was supported by The Science and Technology Development Fund, Macau SAR, China (File No.: 0129/2019/A3), Internal Research Grant of the State Key Laboratory of Quality Research in Chinese Medicine, University of Macau (File No.: QRCM-IRG2022-016, China), the 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong-Macau Joint Lab, File No.: 2020B1212030006, China) and the National Natural Science Foundation of China (File No.: 81973516).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Author contributions
Zi-Han Ye: Conceptualization, Data curation, Data collection, Writing - original draft, Writing - review & editing; Wei-Bang Yu: Data collection, Writing - original draft, Writing - review & editing; Mu-Yang Huang: Writing - review & editing; Jun Chen: Writing - review & editing; Jin-Jian Lu: Conceptualization, Data curation, Supervision, Project administration, Funding acquisition, Writing - review & editing.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPalpha axis. Eur J Cancer. 2017;76:100–109. doi: 10.1016/j.ejca.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Saito Y., Iwamura H., Kaneko T., Ohnishi H., Murata Y., Okazawa H., et al. Regulation by SIRPalpha of dendritic cell homeostasis in lymphoid tissues. Blood. 2010;116:3517–3525. doi: 10.1182/blood-2010-03-277244. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y., Buhring H.J., Zen K., Burst S.L., Schnell F.J., Williams I.R., et al. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem. 2002;277:10028–10036. doi: 10.1074/jbc.M109720200. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q., Qu J., Zhao M., Xu Q., Sun Y. Targeting SHP2 as a promising strategy for cancer immunotherapy. Pharmacol Res. 2020;152 doi: 10.1016/j.phrs.2019.104595. [DOI] [PubMed] [Google Scholar]
- 5.Zhao M., Guo W., Wu Y., Yang C., Zhong L., Deng G., et al. SHP2 inhibition triggers anti-tumor immunity and synergizes with PD-1 blockade. Acta Pharm Sin B. 2019;9:304–315. doi: 10.1016/j.apsb.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song Z., Wang M., Ge Y., Chen X.P., Xu Z., Sun Y., et al. Tyrosine phosphatase SHP2 inhibitors in tumor-targeted therapies. Acta Pharm Sin B. 2021;11:13–29. doi: 10.1016/j.apsb.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai R.K., Discher D.E. Inhibition of "self" engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180:989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veillette A., Chen J. SIRPalpha–CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol. 2018;39:173–184. doi: 10.1016/j.it.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Logtenberg M.E.W., Scheeren F.A., Schumacher T.N. The CD47–SIRPalpha immune checkpoint. Immunity. 2020;52:742–752. doi: 10.1016/j.immuni.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eladl E., Tremblay-LeMay R., Rastgoo N., Musani R., Chen W., Liu A., et al. Role of CD47 in hematological malignancies. J Hematol Oncol. 2020;13:96. doi: 10.1186/s13045-020-00930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willingham S.B., Volkmer J.P., Gentles A.J., Sahoo D., Dalerba P., Mitra S.S., et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Z., Sun H., Yu J., Tian W., Song Y. Targeting CD47 for cancer immunotherapy. J Hematol Oncol. 2021;14:180. doi: 10.1186/s13045-021-01197-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isenberg J.S., Romeo M.J., Abu-Asab M., Tsokos M., Oldenborg A., Pappan L., et al. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- 14.Sikic B.I., Lakhani N., Patnaik A., Shah S.A., Chandana S.R., Rasco D., et al. First-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol. 2019;37:946–953. doi: 10.1200/JCO.18.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeidan A.M., DeAngelo D.J., Palmer J.M., Seet C.S., Tallman M.S., Wei X., et al. A phase I study of CC-90002, a monoclonal antibody targeting CD47, in patients with relapsed and/or refractory (R/R) acute myeloid leukemia (AML) and high-risk myelodysplastic syndromes (MDS): final results. Blood. 2019;134:1320. [Google Scholar]
- 16.Lakhani N.J., LoRusso P., Hafez N., Krishnamurthy A., O'Rourke T.J., Kamdar M.K., et al. A phase 1 study of ALX148, a CD47 blocker, alone and in combination with established anticancer antibodies in patients with advanced malignancy and non-Hodgkin lymphoma. J Clin Oncol. 2018;36:3068. [Google Scholar]
- 17.Wang Y., Zhao C., Liu Y., Wang C., Jiang H., Hu Y., et al. Recent advances of tumor therapy based on the CD47–SIRPα axis. Mol Pharm. 2022;19:1273–1293. doi: 10.1021/acs.molpharmaceut.2c00073. [DOI] [PubMed] [Google Scholar]
- 18.Salas-Benito D., Perez-Gracia J.L., Ponz-Sarvise M., Rodriguez-Ruiz M.E., Martinez-Forero I., Castanon E., et al. Paradigms on immunotherapy combinations with chemotherapy. Cancer Discov. 2021;11:1353–1367. doi: 10.1158/2159-8290.CD-20-1312. [DOI] [PubMed] [Google Scholar]
- 19.Huang M.Y., Jiang X.M., Wang B.L., Sun Y., Lu J.J. Combination therapy with PD-1/PD-L1 blockade in non-small cell lung cancer: strategies and mechanisms. Pharmacol Ther. 2021;219 doi: 10.1016/j.pharmthera.2020.107694. [DOI] [PubMed] [Google Scholar]
- 20.Kiaie S.H., Sanaei M.J., Heshmati M., Asadzadeh Z., Azimi I., Hadidi S., et al. Immune checkpoints in targeted-immunotherapy of pancreatic cancer: new hope for clinical development. Acta Pharm Sin B. 2021;11:1083–1097. doi: 10.1016/j.apsb.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bracci L., Schiavoni G., Sistigu A., Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galluzzi L., Buque A., Kepp O., Zitvogel L., Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 23.Galluzzi L., Humeau J., Buque A., Zitvogel L., Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17:725–741. doi: 10.1038/s41571-020-0413-z. [DOI] [PubMed] [Google Scholar]
- 24.Chao M.P., Jaiswal S., Weissman-Tsukamoto R., Alizadeh A.A., Gentles A.J., Volkmer J., et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galluzzi L., Vitale I., Warren S., Adjemian S., Agostinis P., Martinez A.B., et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohanty S., Aghighi M., Yerneni K., Theruvath J.L., Daldrup-Link H.E. Improving the efficacy of osteosarcoma therapy: combining drugs that turn cancer cell ‘don't eat me' signals off and ‘eat me' signals on. Mol Oncol. 2019;13:2049–2061. doi: 10.1002/1878-0261.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Roemeling C.A., Wang Y., Qie Y., Yuan H., Zhao H., Liu X., et al. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat Commun. 2020;11:1508. doi: 10.1038/s41467-020-15129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Q., Guo N., Zhou Y., Chen J., Wei Q., Han M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B. 2020;10:2156–2170. doi: 10.1016/j.apsb.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan Z., Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther. 2021;6:127. doi: 10.1038/s41392-021-00506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao X., Li B., Chen J., Dang J., Chen S., Gunes E.G., et al. Effect of cabazitaxel on macrophages improves CD47-targeted immunotherapy for triple-negative breast cancer. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao X., Wang Y., Zhang W., Zhong X., Gunes E.G., Dang J., et al. Targeting macrophages for enhancing CD47 blockade-elicited lymphoma clearance and overcoming tumor-induced immunosuppression. Blood. 2022;139:3290–3302. doi: 10.1182/blood.2021013901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang M., Jia K., Wang L., Li W., Chen B., Liu Y., et al. Alterations of DNA damage response pathway: biomarker and therapeutic strategy for cancer immunotherapy. Acta Pharm Sin B. 2021;11:2983–2994. doi: 10.1016/j.apsb.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X., Pu Y., Cron K., Deng L., Kline J., Frazier W.A., et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samanta D., Park Y., Ni X., Li H., Zahnow C.A., Gabrielson E., et al. Chemotherapy induces enrichment of CD47+/CD73+/PDL1+ immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci U S A. 2018;115:E1239–E1248. doi: 10.1073/pnas.1718197115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui Z., Xu D., Zhang F., Sun J., Song L., Ye W., et al. CD47 blockade enhances therapeutic efficacy of cisplatin against lung carcinoma in a murine model. Exp Cell Res. 2021;405 doi: 10.1016/j.yexcr.2021.112677. [DOI] [PubMed] [Google Scholar]
- 36.Casey S.C., Tong L., Li Y., Do R., Walz S., Fitzgerald K.N., et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsui Y.M., Chan L.K., Ng I.O. Cancer stemness in hepatocellular carcinoma: mechanisms and translational potential. Br J Cancer. 2020;122:1428–1440. doi: 10.1038/s41416-020-0823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C., Heidt D.G., Dalerba P., Burant C.F., Zhang L., Adsay V., et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 39.Lee T.K., Cheung V.C., Lu P., Lau E.Y., Ma S., Tang K.H., et al. Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology. 2014;60:179–191. doi: 10.1002/hep.27070. [DOI] [PubMed] [Google Scholar]
- 40.Lo J., Lau E.Y., So F.T., Lu P., Chan V.S., Cheung V.C., et al. Anti-CD47 antibody suppresses tumour growth and augments the effect of chemotherapy treatment in hepatocellular carcinoma. Liver Int. 2016;36:737–745. doi: 10.1111/liv.12963. [DOI] [PubMed] [Google Scholar]
- 41.Cioffi M., Trabulo S., Hidalgo M., Costello E., Greenhalf W., Erkan M., et al. Inhibition of CD47 effectively targets pancreatic cancer stem cells via dual mechanisms. Clin Cancer Res. 2015;21:2325–2337. doi: 10.1158/1078-0432.CCR-14-1399. [DOI] [PubMed] [Google Scholar]
- 42.Chao M.P., Takimoto C.H., Feng D.D., McKenna K., Gip P., Liu J., et al. Therapeutic targeting of the macrophage immune checkpoint CD47 in myeloid malignancies. Front Oncol. 2020;9:1380. doi: 10.3389/fonc.2019.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sallman D.A., Malki M.A., Asch A.S., Lee D.J., Kambhampati S., Donnellan W.B., et al. Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in MDS and AML patients: phase Ib results. J Clin Oncol. 2020;38:7507. doi: 10.1200/JCO.22.02604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi J.Y., Li J., Jiang B., Jiang B., Liu H.Z., Cao X.X., et al. A phase I/IIa study of lemzoparlimab, a monoclonal antibody targeting CD47, in patients with relapsed and/or refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS): initial phase I results. Blood. 2020;136:30–31. [Google Scholar]
- 45.Deng L., Liang H., Xu M., Yang X., Burnette B., Arina A., et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLaughlin M., Patin E.C., Pedersen M., Wilkins A., Dillon M.T., Melcher A.A., et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20:203–217. doi: 10.1038/s41568-020-0246-1. [DOI] [PubMed] [Google Scholar]
- 47.Wang H., Sun Y., Zhou X., Chen C., Jiao L., Li W., et al. CD47/SIRPalpha blocking peptide identification and synergistic effect with irradiation for cancer immunotherapy. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz A.L., Nath P.R., Allgauer M., Lessey-Morillon E.C., Sipes J.M., Ridnour L.A., et al. Antisense targeting of CD47 enhances human cytotoxic T-cell activity and increases survival of mice bearing B16 melanoma when combined with anti-CTLA4 and tumor irradiation. Cancer Immunol Immunother. 2019;68:1805–1817. doi: 10.1007/s00262-019-02397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang N., Xie B., Xiao W., Fan M., Xu S., Duan Y., et al. Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat Commun. 2022;13:1511. doi: 10.1038/s41467-022-29137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsieh R.C., Krishnan S., Wu R.C., Boda A.R., Liu A., Winkler M., et al. ATR-mediated CD47 and PD-L1 up-regulation restricts radiotherapy-induced immune priming and abscopal responses in colorectal cancer. Sci Immunol. 2022;7 doi: 10.1126/sciimmunol.abl9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soto-Pantoja D.R., Terabe M., Ghosh A., Ridnour L.A., DeGraff W.G., Wink D.A., et al. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 2014;74:6771–6783. doi: 10.1158/0008-5472.CAN-14-0037-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Candas-Green D., Xie B., Huang J., Fan M., Wang A., Menaa C., et al. Dual blockade of CD47 and HER2 eliminates radioresistant breast cancer cells. Nat Commun. 2020;11:4591. doi: 10.1038/s41467-020-18245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gholamin S., Youssef O.A., Rafat M., Esparza R., Kahn S., Shahin M., et al. Irradiation or temozolomide chemotherapy enhances anti-CD47 treatment of glioblastoma. Innate Immun. 2020;26:130–137. doi: 10.1177/1753425919876690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gameiro S.R., Jammeh M.L., Wattenberg M.M., Tsang K.Y., Ferrone S., Hodge J.W. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–416. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller T.W., Kaur S., Ivins-O'Keefe K., Roberts D.D. Thrombospondin-1 is a CD47-dependent endogenous inhibitor of hydrogen sulfide signaling in T cell activation. Matrix Biol. 2013;32:316–324. doi: 10.1016/j.matbio.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouguermouh S., Van V.Q., Martel J., Gautier P., Rubio M., Sarfati M. CD47 expression on T cell is a self-control negative regulator of type 1 immune response. J Immunol. 2008;180:8073–8082. doi: 10.4049/jimmunol.180.12.8073. [DOI] [PubMed] [Google Scholar]
- 57.Bhullar K.S., Lagaron N.O., McGowan E.M., Parmar I., Jha A., Hubbard B.P., et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer. 2018;17:48. doi: 10.1186/s12943-018-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergholz J.S., Wang Q., Kabraji S., Zhao J.J. Integrating immunotherapy and targeted therapy in cancer treatment: mechanistic insights and clinical implications. Clin Cancer Res. 2020;26:5557–5566. doi: 10.1158/1078-0432.CCR-19-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Selvaggi G., Novello S., Torri V., Leonardo E., De Giuli P., Borasio P., et al. Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann Oncol. 2004;15:28–32. doi: 10.1093/annonc/mdh011. [DOI] [PubMed] [Google Scholar]
- 60.Arrieta O., Aviles-Salas A., Orozco-Morales M., Hernandez-Pedro N., Cardona A.F., Cabrera-Miranda L., et al. Association between CD47 expression, clinical characteristics and prognosis in patients with advanced non-small cell lung cancer. Cancer Med. 2020;9:2390–2402. doi: 10.1002/cam4.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hendriks M., Ploeg E.M., Koopmans I., Britsch I., Ke X., Samplonius D.F., et al. Bispecific antibody approach for EGFR-directed blockade of the CD47-SIRPalpha "don't eat me" immune checkpoint promotes neutrophil-mediated trogoptosis and enhances antigen cross-presentation. Oncoimmunology. 2020;9 doi: 10.1080/2162402X.2020.1824323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiskopf K., Ring A.M., Ho C.C., Volkmer J.P., Levin A.M., Volkmer A.K., et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y., Guo R., Chen Q., Liu Y., Zhang P., Zhang Z., et al. A novel bispecific antibody fusion protein co-targeting EGFR and CD47 with enhanced therapeutic index. Biotechnol Lett. 2018;40:789–795. doi: 10.1007/s10529-018-2535-2. [DOI] [PubMed] [Google Scholar]
- 64.Fisher G.A., Lakhani N.J., Eng C., Hecht J.R., Bendell J.C., Philip P.A., et al. A phase Ib/II study of the anti-CD47 antibody magrolimab with cetuximab in solid tumor and colorectal cancer patients. J Clin Oncol. 2020;38:114. [Google Scholar]
- 65.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 66.Tesch M.E., Gelmon K.A. Targeting HER2 in breast cancer: latest developments on treatment sequencing and the introduction of biosimilars. Drugs. 2020;80:1811–1830. doi: 10.1007/s40265-020-01411-y. [DOI] [PubMed] [Google Scholar]
- 67.Tsao L.C., Crosby E.J., Trotter T.N., Agarwal P., Hwang B.J., Acharya C., et al. CD47 blockade augmentation of trastuzumab antitumor efficacy dependent on antibody-dependent cellular phagocytosis. JCI Insight. 2019;4 doi: 10.1172/jci.insight.131882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi Y., Fan X., Deng H., Brezski R.J., Rycyzyn M., Jordan R.E., et al. Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcgamma receptors on macrophages. J Immunol. 2015;194:4379–4386. doi: 10.4049/jimmunol.1402891. [DOI] [PubMed] [Google Scholar]
- 69.Wilken J.A., Maihle N.J. Primary trastuzumab resistance: new tricks for an old drug. Ann N Y Acad Sci. 2010;1210:53–65. doi: 10.1111/j.1749-6632.2010.05782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Upton R., Banuelos A., Feng D., Biswas T., Kao K., McKenna K., et al. Combining CD47 blockade with trastuzumab eliminates HER2-positive breast cancer cells and overcomes trastuzumab tolerance. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2026849118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kauder S.E., Kuo T.C., Harrabi O., Chen A., Sangalang E., Doyle L., et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lakhani N.J., Chow L.Q.M., Gainor J.F., LoRusso P., Lee K.W., Chung H.C., et al. Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): a first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2021;22:1740–1751. doi: 10.1016/S1470-2045(21)00584-2. [DOI] [PubMed] [Google Scholar]
- 73.Simons M., Gordon E., Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 74.Yang J., Yan J., Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jayson G.C., Kerbel R., Ellis L.M., Harris A.L. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388:518–529. doi: 10.1016/S0140-6736(15)01088-0. [DOI] [PubMed] [Google Scholar]
- 76.Lal N., Puri K., Rodrigues B. Vascular endothelial growth factor B and its signaling. Front Cardiovasc Med. 2018;5:39. doi: 10.3389/fcvm.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang X., Wang Y., Fan J., Chen W., Luan J., Mei X., et al. Blocking CD47 efficiently potentiated therapeutic effects of anti-angiogenic therapy in non-small cell lung cancer. J Immunother Cancer. 2019;7:346. doi: 10.1186/s40425-019-0812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang X., Wang S., Nan Y., Fan J., Chen W., Luan J., et al. Inhibition of autophagy potentiated the anti-tumor effects of VEGF and CD47 bispecific therapy in glioblastoma. Appl Microbiol Biotechnol. 2018;102:6503–6513. doi: 10.1007/s00253-018-9069-3. [DOI] [PubMed] [Google Scholar]
- 79.Pavlasova G., Mraz M. The regulation and function of CD20: an "enigma" of B-cell biology and targeted therapy. Haematologica. 2020;105:1494–1506. doi: 10.3324/haematol.2019.243543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chao M.P., Alizadeh A.A., Tang C., Myklebust J.H., Varghese B., Gill S., et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Advani R., Flinn I., Popplewell L., Forero A., Bartlett N.L., Ghosh N., et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N Engl J Med. 2018;379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ni H., Cao L., Wu Z., Wang L., Zhou S., Guo X., et al. Combined strategies for effective cancer immunotherapy with a novel anti-CD47 monoclonal antibody. Cancer Immunol Immunother. 2022;71:353–363. doi: 10.1007/s00262-021-02989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evers M., Rosner T., Dunkel A., Jansen J.H.M., Baumann N., Ten Broeke T., et al. The selection of variable regions affects effector mechanisms of IgA antibodies against CD20. Blood Adv. 2021;5:3807–3820. doi: 10.1182/bloodadvances.2021004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Rees D.J., Brinkhaus M., Klein B., Verkuijlen P., Tool A.T.J., Schornagel K., et al. Sodium stibogluconate and CD47–SIRPalpha blockade overcome resistance of anti-CD20-opsonized B cells to neutrophil killing. Blood Adv. 2022;6:2156–2166. doi: 10.1182/bloodadvances.2021005367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Bommel P.E., He Y., Schepel I., Hendriks M.A.J.M., Wiersma V.R., van Ginkel R.J., et al. CD20-selective inhibition of CD47–SIRPα “don't eat me” signaling with a bispecific antibody-derivative enhances the anticancer activity of daratumumab, alemtuzumab and obinutuzumab. Oncoimmunology. 2018;7 doi: 10.1080/2162402X.2017.1386361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piccione E.C., Juarez S., Liu J., Tseng S., Ryan C.E., Narayanan C., et al. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs. 2015;7:946–956. doi: 10.1080/19420862.2015.1062192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma L., Zhu M., Gai J., Li G., Chang Q., Qiao P., et al. Preclinical development of a novel CD47 nanobody with less toxicity and enhanced anti-cancer therapeutic potential. J Nanobiotechnology. 2020;18:12. doi: 10.1186/s12951-020-0571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salles G., Barrett M., Foa R., Maurer J., O'Brien S., Valente N., et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34:2232–2273. doi: 10.1007/s12325-017-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katz B.Z., Herishanu Y. Therapeutic targeting of CD19 in hematological malignancies: past, present, future and beyond. Leuk Lymphoma. 2014;55:999–1006. doi: 10.3109/10428194.2013.828354. [DOI] [PubMed] [Google Scholar]
- 90.Gallagher S., Turman S., Lekstrom K., Wilson S., Herbst R., Wang Y. CD47 limits antibody dependent phagocytosis against non-malignant B cells. Mol Immunol. 2017;85:57–65. doi: 10.1016/j.molimm.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 91.Buatois V., Johnson Z., Salgado-Pires S., Papaioannou A., Hatterer E., Chauchet X., et al. Preclinical development of a bispecific antibody that safely and effectively Targets CD19 and CD47 for the treatment of B-cell lymphoma and leukemia. Mol Cancer Ther. 2018;17:1739–1751. doi: 10.1158/1535-7163.MCT-17-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dheilly E., Moine V., Broyer L., Salgado-Pires S., Johnson Z., Papaioannou A., et al. Selective blockade of the ubiquitous checkpoint receptor CD47 is enabled by dual-targeting bispecific antibodies. Mol Ther. 2017;25:523–533. doi: 10.1016/j.ymthe.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hatterer E., Barba L., Noraz N., Daubeuf B., Aubry-Lachainaye J.P., von der Weid B., et al. Co-engaging CD47 and CD19 with a bispecific antibody abrogates B-cell receptor/CD19 association leading to impaired B-cell proliferation. MAbs. 2019;11:322–334. doi: 10.1080/19420862.2018.1558698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Topp M.S., Stelljes M., Zugmaier G., Barnette P., Heffner L.T. Jr, Trippett T., et al. Blinatumomab retreatment after relapse in patients with relapsed/refractory B-precursor acute lymphoblastic leukemia. Leukemia. 2018;32:562–565. doi: 10.1038/leu.2017.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Topp M.S., Gokbuget N., Stein A.S., Zugmaier G., O'Brien S., Bargou R.C., et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 96.Xu L., Wang S., Li J., Li B. CD47/SIRPalpha blocking enhances CD19/CD3-bispecific T cell engager antibody-mediated lysis of B cell malignancies. Biochem Biophys Res Commun. 2019;509:739–745. doi: 10.1016/j.bbrc.2018.12.175. [DOI] [PubMed] [Google Scholar]
- 97.Theruvath J., Menard M., Smith B.A.H., Linde M.H., Coles G.L., Dalton G.N., et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat Med. 2022;28:333–344. doi: 10.1038/s41591-021-01625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nazha B., Inal C., Owonikoko T.K. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front Oncol. 2020;10:1000. doi: 10.3389/fonc.2020.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van de Donk N.W., Janmaat M.L., Mutis T., Lammerts van Bueren J.J., Ahmadi T., Sasser A.K., et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270:95–112. doi: 10.1111/imr.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Müller K., Vogiatzi F., Winterberg D., Rösner T., Lenk L., Bastian L., et al. Combining daratumumab with CD47 blockade prolongs survival in preclinical models of pediatric T-ALL. Blood. 2022;140:45–57. doi: 10.1182/blood.2021014485. [DOI] [PubMed] [Google Scholar]
- 101.Du K., Li Y., Liu J., Chen W., Wei Z., Luo Y., et al. A bispecific antibody targeting GPC3 and CD47 induced enhanced antitumor efficacy against dual antigen-expressing HCC. Mol Ther. 2021;29:1572–1584. doi: 10.1016/j.ymthe.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hatterer E., Chauchet X., Richard F., Barba L., Moine V., Chatel L., et al. Targeting a membrane-proximal epitope on mesothelin increases the tumoricidal activity of a bispecific antibody blocking CD47 on mesothelin-positive tumors. MAbs. 2020;12 doi: 10.1080/19420862.2020.1739408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tahk S., Vick B., Hiller B., Schmitt S., Marcinek A., Perini E.D., et al. SIRPα–αCD123 fusion antibodies targeting CD123 in conjunction with CD47 blockade enhance the clearance of AML-initiating cells. J Hematol Oncol. 2021;14:155. doi: 10.1186/s13045-021-01163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ferlin W.G., Buatois V., Masternak K., Shang L., Majocchi S., Hatterer E., et al. Remodeling tumor-associated macrophages with a CD47xMesothelin bispecific antibody for efficient elimination of solid tumor cells. J Clin Oncol. 2018;36 [Google Scholar]
- 105.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun C., Mezzadra R., Schumacher T.N. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu X., Liu L., Ren Z., Yang K., Xu H., Luan Y., et al. Dual targeting of innate and adaptive checkpoints on tumor cells limits immune evasion. Cell Rep. 2018;24:2101–2111. doi: 10.1016/j.celrep.2018.07.062. [DOI] [PubMed] [Google Scholar]
- 108.Huang C.Y., Ye Z.H., Huang M.Y., Lu J.J. Regulation of CD47 expression in cancer cells. Transl Oncol. 2020;13 doi: 10.1016/j.tranon.2020.100862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu B., Guo H., Xu J., Qin T., Guo Q., Gu N., et al. Elimination of tumor by CD47/PD-L1 dual-targeting fusion protein that engages innate and adaptive immune responses. MAbs. 2018;10:315–324. doi: 10.1080/19420862.2017.1409319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y., Ni H., Zhou S., He K., Gao Y., Wu W., et al. Tumor-selective blockade of CD47 signaling with a CD47/PD-L1 bispecific antibody for enhanced anti-tumor activity and limited toxicity. Cancer Immunol Immunother. 2021;70:365–376. doi: 10.1007/s00262-020-02679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen S.H., Dominik P.K., Stanfield J., Ding S., Yang W., Kurd N., et al. Dual checkpoint blockade of CD47 and PD-L1 using an affinity-tuned bispecific antibody maximizes antitumor immunity. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sockolosky J.T., Dougan M., Ingram J.R., Ho C.C., Kauke M.J., Almo S.C., et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A. 2016;113:E2646–E2654. doi: 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gordon S.R., Maute R.L., Dulken B.W., Hutter G., George B.M., McCracken M.N., et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roohullah A., Ganju V., Zhang F.M., Zhang L., Yu T., Wilkinson K., et al. First-in-human phase 1 dose escalation study of HX009, a novel recombinant humanized anti-PD-1 and CD47 bispecific antibody, in patients with advanced malignancies. J Clin Oncol. 2021;39:2517. [Google Scholar]
- 115.Wang J., Sun Y., Chu Q., Duan J., Wan R., Wang Z., et al. Abstract CT513: phase I study of IBI322 (anti-CD47/PD-L1 bispecific antibody) monotherapy therapy in patients with advanced solid tumors in China. Cancer Res. 2022;82:CT513. [Google Scholar]
- 116.Lakhani N.J., Patnaik A., Liao J.B., Moroney J.W., Miller D.S., Fleming G.F., et al. A phase Ib study of the anti-CD47 antibody magrolimab with the PD-L1 inhibitor avelumab (A) in solid tumor (ST) and ovarian cancer (OC) patients. J Clin Oncol. 2020;38:18. [Google Scholar]
- 117.Liu Y., Zheng P. How does an anti-CTLA-4 antibody promote cancer immunity?. Trends Immunol. 2018;39:953–956. doi: 10.1016/j.it.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Y., Zheng P. Preserving the CTLA-4 checkpoint for safer and more effective cancer immunotherapy. Trends Pharmacol Sci. 2020;41:4–12. doi: 10.1016/j.tips.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Silva P., Aiello M., Gu-Trantien C., Migliori E., Willard-Gallo K., Solinas C. Targeting CTLA-4 in cancer: is it the ideal companion for PD-1 blockade immunotherapy combinations?. Int J Cancer. 2021;149:31–41. doi: 10.1002/ijc.33415. [DOI] [PubMed] [Google Scholar]
- 120.Ingram J.R., Blomberg O.S., Sockolosky J.T., Ali L., Schmidt F.I., Pishesha N., et al. Localized CD47 blockade enhances immunotherapy for murine melanoma. Proc Natl Acad Sci U S A. 2017;114:10184–10189. doi: 10.1073/pnas.1710776114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sosa A., Lopez Cadena E., Simon Olive C., Karachaliou N., Rosell R. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol. 2018;10 doi: 10.1177/1758835918764628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang A., Ren Z., Tseng K.F., Liu X., Li H., Lu C., et al. Dual targeting of CTLA-4 and CD47 on Treg cells promotes immunity against solid tumors. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abg8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Urban-Wojciuk Z., Khan M.M., Oyler B.L., Fahraeus R., Marek-Trzonkowska N., Nita-Lazar A., et al. The role of TLRs in anti-cancer immunity and tumor rejection. Front Immunol. 2019;10:2388. doi: 10.3389/fimmu.2019.02388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaczanowska S., Joseph A.M., Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol. 2013;93:847–863. doi: 10.1189/jlb.1012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang L., Xu H., Peng G. TLR-mediated metabolic reprogramming in the tumor microenvironment: potential novel strategies for cancer immunotherapy. Cell Mol Immunol. 2018;15:428–437. doi: 10.1038/cmi.2018.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feng M., Chen J.Y., Weissman-Tsukamoto R., Volkmer J.P., Ho P.Y., McKenna K.M., et al. Macrophages eat cancer cells using their own calreticulin as a guide: roles of TLR and Btk. Proc Natl Acad Sci U S A. 2015;112:2145–2150. doi: 10.1073/pnas.1424907112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhong C., Wang L., Hu S., Huang C., Xia Z., Liao J., et al. Poly(I:C) enhances the efficacy of phagocytosis checkpoint blockade immunotherapy by inducing IL-6 production. J Leukoc Biol. 2021;110:1197–1208. doi: 10.1002/JLB.5MA0421-013R. [DOI] [PubMed] [Google Scholar]
- 128.Tang Z., Davidson D., Li R., Zhong M.C., Qian J., Chen J., et al. Inflammatory macrophages exploit unconventional pro-phagocytic integrins for phagocytosis and anti-tumor immunity. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.110111. [DOI] [PubMed] [Google Scholar]
- 129.Theocharides A.P., Jin L., Cheng P.Y., Prasolava T.K., Malko A.V., Ho J.M., et al. Disruption of SIRPalpha signaling in macrophages eliminates human acute myeloid leukemia stem cells in xenografts. J Exp Med. 2012;209:1883–1899. doi: 10.1084/jem.20120502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hardie J., Mas-Rosario J.A., Ha S., Rizzo E.M., Farkas M.E. Macrophage activation by a substituted pyrimido[5,4-b]indole increases anti-cancer activity. Pharmacol Res. 2019;148 doi: 10.1016/j.phrs.2019.104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu M., O'Connor R.S., Trefely S., Graham K., Snyder N.W., Beatty G.L. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don't-eat-me' signal. Nat Immunol. 2019;20:265–275. doi: 10.1038/s41590-018-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 133.Dana H., Chalbatani G.M., Jalali S.A., Mirzaei H.R., Grupp S.A., Suarez E.R., et al. CAR-T cells: early successes in blood cancer and challenges in solid tumors. Acta Pharm Sin B. 2021;11:1129–1147. doi: 10.1016/j.apsb.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Golubovskaya V., Berahovich R., Zhou H., Xu S., Harto H., Li L., et al. CD47–CAR-T cells effectively kill target cancer cells and block pancreatic tumor growth. Cancers (Basel) 2017;9:139. doi: 10.3390/cancers9100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.La H.T., Tran D.B.T., Tran H.M., Nguyen L.T. Third-generation anti-CD47-specific CAR-T cells effectively kill cancer cells and reduce the genes expression in lung cancer cell metastasis. J Immunol Res. 2021;2021 doi: 10.1155/2021/5575260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xie Y.J., Dougan M., Ingram J.R., Pishesha N., Fang T., Momin N., et al. Improved antitumor efficacy of chimeric antigen receptor T cells that secrete single-domain antibody fragments. Cancer Immunol Res. 2020;8:518–529. doi: 10.1158/2326-6066.CIR-19-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shu R., Evtimov V.J., Hammett M.V., Nguyen N.N., Zhuang J., Hudson P.J., et al. Engineered CAR-T cells targeting TAG-72 and CD47 in ovarian cancer. Mol Ther Oncolytics. 2021;20:325–341. doi: 10.1016/j.omto.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen H., Yang Y., Deng Y., Wei F., Zhao Q., Liu Y., et al. Delivery of CD47 blocker SIRPalpha-Fc by CAR-T cells enhances antitumor efficacy. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2021-003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cendrowicz E., Jacob L., Greenwald S., Tamir A., Pecker I., Tabakman R., et al. DSP107 combines inhibition of CD47/SIRPalpha axis with activation of 4-1BB to trigger anticancer immunity. J Exp Clin Cancer Res. 2022;41:97. doi: 10.1186/s13046-022-02256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kosaka A., Ishibashi K., Nagato T., Kitamura H., Fujiwara Y., Yasuda S., et al. CD47 blockade enhances the efficacy of intratumoral STING-targeting therapy by activating phagocytes. J Exp Med. 2021;218 doi: 10.1084/jem.20200792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Silva S., Fromm G., Shuptrine C.W., Johannes K., Patel A., Yoo K.J., et al. CD40 enhances type I interferon responses downstream of CD47 blockade, bridging innate and adaptive immunity. Cancer Immunol Res. 2020;8:230–245. doi: 10.1158/2326-6066.CIR-19-0493. [DOI] [PubMed] [Google Scholar]
- 142.Tian L., Xu B., Teng K.Y., Song M., Zhu Z., Chen Y., et al. Targeting Fc receptor-mediated effects and the "don't eat me" signal with an oncolytic virus expressing an anti-CD47 antibody to treat metastatic ovarian cancer. Clin Cancer Res. 2022;28:201–214. doi: 10.1158/1078-0432.CCR-21-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu B., Tian L., Chen J., Wang J., Ma R., Dong W., et al. An oncolytic virus expressing a full-length antibody enhances antitumor innate immune response to glioblastoma. Nat Commun. 2021;12:5908. doi: 10.1038/s41467-021-26003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feng M., Jiang W., Kim B.Y.S., Zhang C.C., Fu Y.X., Weissman I.L. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19:568–586. doi: 10.1038/s41568-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Suter E.C., Schmid E.M., Harris A.R., Voets E., Francica B., Fletcher D.A. Antibody:CD47 ratio regulates macrophage phagocytosis through competitive receptor phosphorylation. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang Y., Yang Z., Yang Y. Potential role of CD47-directed bispecific antibodies in cancer immunotherapy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.686031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Awwad S., Angkawinitwong U. Overview of antibody drug delivery. Pharmaceutics. 2018;10:83. doi: 10.3390/pharmaceutics10030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen Y.C., Shi W., Shi J.J., Lu J.J. Progress of CD47 immune checkpoint blockade agents in anticancer therapy: a hematotoxic perspective. J Cancer Res Clin Oncol. 2022;148:1–14. doi: 10.1007/s00432-021-03815-z. [DOI] [PubMed] [Google Scholar]
- 149.Sugimura-Nagata A., Koshino A., Inoue S., Matsuo-Nagano A., Komura M., Riku M., et al. Expression and prognostic significance of CD47–SIRPA macrophage checkpoint molecules in colorectal cancer. Int J Mol Sci. 2021;22:2690. doi: 10.3390/ijms22052690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu Y., Li J., Tong B., Chen M., Liu X., Zhong W., et al. Positive tumour CD47 expression is an independent prognostic factor for recurrence in resected non-small cell lung cancer. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Orozco-Morales M., Aviles-Salas A., Hernandez-Pedro N., Catalan R., Cruz-Rico G., Colin-Gonzalez A.L., et al. Clinicopathological and prognostic significance of CD47 expression in lung neuroendocrine tumors. J Immunol Res. 2021;2021 doi: 10.1155/2021/6632249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jiang W., Zeng H., Liu Z., Jin K., Hu B., Chang Y., et al. Immune inactivation by CD47 expression predicts clinical outcomes and therapeutic responses in clear cell renal cell carcinoma patients. Urol Oncol. 2022;40:166. doi: 10.1016/j.urolonc.2021.11.024. e15–e25. [DOI] [PubMed] [Google Scholar]
- 153.Sudo T., Takahashi Y., Sawada G., Uchi R., Mimori K., Akagi Y. Significance of CD47 expression in gastric cancer. Oncol Lett. 2017;14:801–809. doi: 10.3892/ol.2017.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Trac N.T., Chung E.J. Peptide-based targeting of immunosuppressive cells in cancer. Bioact Mater. 2020;5:92–101. doi: 10.1016/j.bioactmat.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li K., Shi H., Zhang B., Ou X., Ma Q., Chen Y., et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6:362. doi: 10.1038/s41392-021-00670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Adams S., van der Laan L.J., Vernon-Wilson E., Renardel de Lavalette C., Dopp E.A., Dijkstra C.D., et al. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol. 1998;161:1853–1859. [PubMed] [Google Scholar]
- 157.Wu Z.H., Li N., Mei X.F., Chen J., Wang X.Z., Guo T.T., et al. Preclinical characterization of the novel anti-SIRPα antibody BR105 that targets the myeloid immune checkpoint. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2021-004054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kamber R.A., Nishiga Y., Morton B., Banuelos A.M., Barkal A.A., Vences-Catalan F., et al. Inter-cellular CRISPR screens reveal regulators of cancer cell phagocytosis. Nature. 2021;597:549–554. doi: 10.1038/s41586-021-03879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]