Abstract
Human heart mast cells (HHMC) have been identified in heart tissue, perivascularly, and in the intima of coronary arteries. In vitro activation of isolated HHMC induces the release of vasoactive and proinflammatory mediators (histamine, tryptase, and cysteinyl leukotriene C4 [LTC4]). We investigated the effects of several bacterial proteins on HHMC activation in vitro. HHMC released histamine, tryptase, and LTC4 in response to Staphylococcus aureus Cowan 1 and the immunoglobulin (Ig)-binding protein A, but not to S. aureus Wood 46, which does not synthesize protein A. The effect of protein A was inhibited by preincubation with monoclonal IgM VH3+. Some strains of Peptostreptococcus magnus express an Ig light chain-binding surface protein called protein L. Such bacteria and soluble protein L stimulated the release of preformed and newly synthesized mediators from HHMC. Preincubation of HHMC with either protein A or protein L resulted in complete cross-desensitization to a subsequent challenge with the heterologous stimulus or anti-IgE. Monoclonal IgE (κ chains) blocked protein L-induced release, whereas IgE (λ chains) had no effect. Streptococcal protein G, formyl-containing tripeptide, and pepstatin A did not activate HHMC. Bacterial products protein A and protein L and intact bacteria (S. aureus and P. magnus) activate HHMC by acting as Ig superantigens.
Mast cells and basophils, the only cells expressing high-affinity receptors (FcɛRI) for immunoglobulin E (IgE) and synthesizing histamine, are widely recognized as effector cells in several inflammatory disorders (18, 40, 41). Mast cells play a fundamental role in the pathophysiology of inflammatory diseases through the elaboration and release of a myriad of proinflammatory (13, 41, 45) and immunoregulatory molecules (5, 17, 64), and they express a wide spectrum of surface receptors for cytokines and chemokines (8, 53). Mast cells are present in the human heart (2, 46), around coronary arteries (15, 29), and in atherosclerotic plaques (27, 28). Human heart mast cells (HHMC) have been implicated in the pathophysiology of coronary artery diseases (9, 29), eosinophil myocarditis (14, 47), and dilated cardiomyopathy (48).
Infections and cardiovascular diseases are the most common causes of mortality and morbidity in humans (35). Mast cells are widely distributed in all vascularized tissues, including the heart (2, 18, 46). The strategic location of these cells within and around blood vessels and their ability to release inflammatory mediators (18, 40, 41), cytokines (5, 17, 19), and chemokines (64) suggest that they might be implicated in bacterial infections. In addition, mast cells have been preserved through evolution (7) and are probably essential for natural and acquired immunity (32, 36, 51). Although an association has been reported between bacterial infections and the development of cardiovascular diseases (1, 20, 24, 34, 43), the mechanisms by which infectious agents can contribute to these diseases are still largely unknown (11, 21).
Mast cells tend to accumulate at sites of chronic bacterial infection and can phagocytize bacteria (37). Intact bacteria and their products can activate human FcɛRI+ cells to release proinflammatory mediators and cytokines through diverse mechanisms (32, 36, 38, 39, 44, 51). We have established a technique for the efficient dispersion of mast cells from human heart tissue and identified some of the immunological and nonimmunological stimuli that induce HHMC to release vasoactive and proinflammatory mediators in vitro (46, 47). The experiments described here were designed to investigate the mechanism by which the bacterial Ig-binding proteins A and L activate HHMC to release preformed and de novo-synthesized mediators.
MATERIALS AND METHODS
Reagents and buffers.
We purchased 60% HClO4 from Baker Chemical. Bovine serum albumin, human recombinant C5a, piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), hyaluronidase, collagenase type II, chymopapain, pepstatin A, and synthetic leukotriene C4 (LTC4) were from Sigma Chemical. Hanks' balanced salt solution and fetal calf serum were from GIBCO. DNase, formyl-containing tripeptide (formylmethionylleucylphenylalanine [FMLP]), and pronase were from Calbiochem. RPMI 1640 with 25 mM HEPES buffer and Eagle's minimum essential medium were from Flow Laboratories. Percoll was from Pharmacia Fine Chemicals. [3H]LTC4 (168 Ci/mmol) was from New England Nuclear. The rabbit anti-IgE was a kind gift from Teruko Ishizaka and Kimishige Ishizaka (La Jolla Institute for Allergy and Immunology, La Jolla, Calif.). Rabbit anti-LTC4 was generously donated by Edward Kusner (Zeneca Pharmaceuticals, Philadelphia, Pa.). The tryptase radioimmunoassay (RIA) kit (Pharmacia Tryptase RIACT 50; Pharmacia Diagnostics AB) was kindly supplied by Kabi Pharmacia S.p.A. (Milan, Italy). The PIPES buffer used in these experiments was a mixture of 25 mM PIPES, 110 mM NaCl, and 5 mM KCl, pH 7.4 (referred to as P). PCG contains 2 mM CaCl2 and 1 g of dextrose per liter in addition to P (48). pH was titrated to 7.4 with sodium bicarbonate. P-EDTA is P buffer containing 4 mM EDTA. PGMD is 0.25 g of MgCl2 · 6H2O, 10 mg of DNase, and 1 g of gelatin per liter in addition to P, pH 7.4. Phosphate-buffered saline contained 0.15 M NaCl and 0.06 M phosphate, pH 7.2.
Bacteria, protein A, protein L, and protein G.
Staphylococcus aureus Cowan 1 and Wood 46 were obtained from the National Type Culture Collection (London, United Kingdom). The bacteria were killed by incubation with 0.5% formaldehyde (3 h, 22°C), heat treated (3 min, 80°C), washed, and stored in small aliquots at −80°C. The bacteria were counted in a Neubauer chamber (39). Heat-killed protein L-expressing and nonexpressing strains (312 and 644, respectively) of the anaerobic bacterial species Peptostreptococcus magnus were obtained as described elsewhere (42, 44). The binding properties of protein L have been described previously (42). Proteins A and G were from Pharmacia Fine Chemicals. Protein A (1 mg/ml) was iodinated with KI in the presence of chloramine T (1.6 mg/ml), and the reaction was stopped by the addition of sodium metabisulfite (4.8 mg/ml) as described elsewhere (39).
Purification of human monoclonal IgE and IgM.
IgE myeloma proteins were purified from the sera of three myeloma patients by repeated gel filtration on Sephadex G-200, followed by elution through a Sepharose CL-4B column (39, 54). RIA showed no IgG, IgM, or IgA contamination. Monoclonal IgM antibodies were purified from the sera of patients with Waldenström's macroglobulinemia by gel permeation as described elsewhere (49). Variable regions of these monoclonal IgM antibodies were determined using a well-characterized panel of primary sequence-dependent VH and VK family-specific reagents that identify framework regions (49).
Purification of HIgG and RIgG.
Human polyclonal IgG (HIgG) and rabbit polyclonal IgG (RIgG) were purified by precipitation of normal human or rabbit serum with 50% saturated ammonium sulfate followed by chromatography as described elsewhere (39).
Isolation and partial purification of HHMC.
The heart tissue used in this study was obtained from patients (29 to 65 years old) undergoing heart transplantation at the Deutsches Herzzentrum (Berlin, Germany), mostly for cardiomyopathy, and from donors without cardiovascular disease who had died in car accidents. The explanted heart was immediately immersed in cold (4°C) cardioplegic solution and processed within 5 to 18 h of removal. The heart tissue (100 to 600 g) was dissected to separate the left and right ventricles and the septum. Fat tissue, large vessels, and pericardium were removed. The tissue was finely minced (2- to 5-mm fragments), suspended in P buffer (10 ml/g of wet tissue), and washed three times by centrifugation (once at 150 × g at 4°C for 8 min; then twice at 150 × g at 22°C for 8 min). After each centrifugation, the heart fragments were filtered through 150-μm-pore-size Nytex cloth (Tetko, Elmsford, N.Y.). Fragments were incubated (15 min, 37°C) under constant stirring in P buffer containing 10 mg of collagenase/g of wet tissue. At the end of the first incubation, the cell suspension was filtered through 150-μm-pore-size Nytex cloth. The residual tissue was weighed, and three further cycles of enzymatic digestion were performed, using a new preparation of collagenase each time. After the last enzymatic digestion, the cell suspension was centrifuged (150 × g, 22°C, 8 min) and filtered first through 150-μm-pore-size Nytex cloth and then through 60-μm-pore-size Nytex cloth to remove large particles and large cells (mostly myocytes). Finally, cells were washed twice in PGMD (25 mM P, 110 mM NaCl, 1 mM Mg, 1 g of gelatin per liter, 20 mg of DNase per ml [pH 7.37]) by centrifugation (150 × g, 22°C, 8 min). At this stage of the procedure, Alcian blue-positive cells (mast cells) accounted for <0.1% of total cells (46, 47). Cell pellets were resuspended in 250 ml of P buffer containing 2% bovine serum albumin and centrifuged (25 × g, 22°C, 2 min) to remove sedimented myocytes. Myocytes (>100 μm long) were pelleted and discarded; supernatants containing endothelial cells, fibroblasts, and mast cells were then collected and centrifuged (150 × g, 22°C, 8 min). HHMC were partially purified by flotation through a discontinuous Percoll gradient as detailed elsewhere (46). The enzymatic dispersion of tissue yields ≈5 × 104 mast cells/g of heart tissue. Short-term (≈16-h) cultures of HHMC were prepared by resuspending 2 × 106 to 5 × 106 cells/ml in a solution of RPMI 1640 containing 25 mM HEPES, 1% penicillin-streptomycin solution, 2 mM l-glutamine, and 10% fetal calf serum at 37°C in humidified 95% air–5% CO2. The viability of mast cells was routinely evaluated by trypan blue exclusion and was always >95% (46, 47).
Solid-phase protein binding assay.
The ability of protein A and hyperiodinated protein A to react with RIgG, HIgG, and human monoclonal IgM was evaluated by a solid-phase binding assay as described elsewhere (39).
Histamine release assay.
Cells (≈3 × 104 HHMC/tube) were resuspended in PCG, and 0.3 ml of the cell suspension was placed in 12- by 75-mm polyethylene tubes and warmed to 37°C; 0.2 ml of each prewarmed releasing stimulus was added, and incubation was continued at 37°C for 30 min (46). At the end of this step, the reaction was stopped by centrifugation (1,000 × g, 22°C, 2 min), and the cell-free supernatants were stored at −80°C for subsequent assay of histamine, tryptase, and LTC4 content. Histamine was assayed with an automated fluorometric technique (57). To calculate histamine release as a percentage of total cellular histamine, the spontaneous release from mast cells (3 to 10% of the total cellular histamine) was subtracted from both numerator and denominator (53). The total histamine content in mast cells was obtained by cell lysis with 8% HC1O4 (49). All values are based on the means of duplicate or triplicate determinations. Replicates differed from each other by less than 10% in histamine content.
RIA of tryptase and LTC4.
Total tryptase was assessed by lysis induced by incubating cells with 100 μl of Triton X-100 (0.1%). Tryptase was assayed by a solid-phase RIA (Pharmacia Tryptase RIACT 50) (48). LTC4 was analyzed on 100-μl fractions taken from the supernatant fluids. The samples were stored at −80°C. LTC4 was measured by RIA within 24 h of the experiment to minimize degradation of the compound (48).
LDH assay.
To test whether protein A, protein L, protein G, and pepstatin A have cytotoxic effects on HHMC, lactate dehydrogenase (LDH) activity was determined in the supernatants of HHMC. The cell-free supernatants were collected, and the concentrations of LDH were measured as previously described (47). None of stimuli used in these experiments caused LDH release in the supernatants of HHMC after incubation for 30 min at 37°C.
Statistical analysis.
The results are the means ± the standard errors of the means (SEM). Student's t test with Bonferroni's correction was used for multiple comparisons between groups. The data subjected to linear regression were calculated by the least-squares method (y = a + b x) in which a was the xy axis intercept and b was the slope of the line. The level of statistical significance was P < 0.05 (59).
RESULTS
Effect of S. aureus Cowan 1 and Wood 46 and of protein A on histamine release from HHMC.
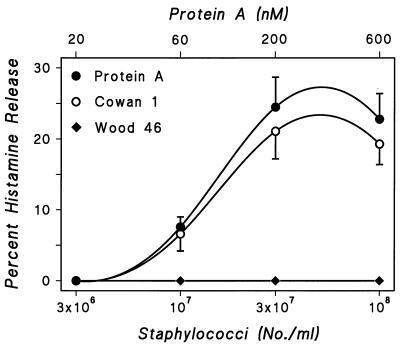
S. aureus is one of the most common pathogens to cause endocarditis and toxic shock syndrome (34, 35). The majority of clinical isolates of S. aureus synthesize protein A, a 45-kDa bacterial cell wall protein which has unique Ig-binding properties. Protein A has a classical site that binds to Fcγ, a constant region of IgG (16), and an alternative site that binds the Fab portion of 15 to 50% of human polyclonal IgM, IgA, IgG, and IgE (26). Increasing numbers of S. aureus Cowan 1 (3 × 106 to 108 staphylococci per tube), which synthesize protein A, induced gradual increases in histamine release from HHMC (Fig. 1). S. aureus Wood 46 (3 × 106 to 108 bacteria per tube), which does not contain protein A, did not induce histamine release in any of the six HHMC preparations. Soluble protein A (20 to 600 nM) induced concentration-dependent histamine release from HHMC. These results suggest protein A mediates the Staphylococcus-induced activation of HHMC.
FIG. 1.
Effect of increasing concentrations of S. aureus Cowan 1, S. aureus Wood 46, and protein A on histamine secretion from HHMC from six donors. Each point is the mean ± SEM. Error bars are not shown when graphically too small.
Cross-desensitization between S. aureus Cowan 1 and protein A.
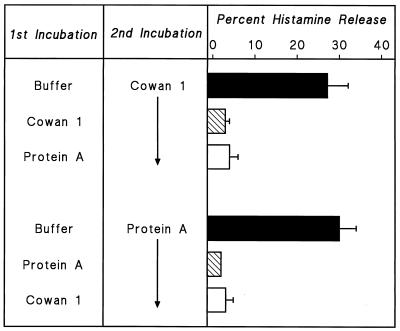
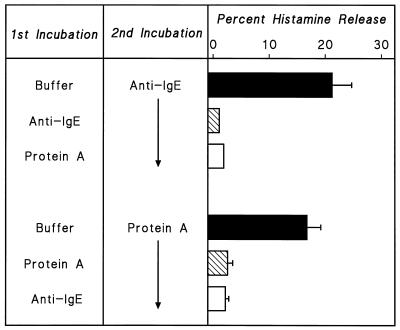
HHMC were treated with S. aureus Cowan 1 or protein A in P-EDTA for 30 min at 37°C, washed, and suspended in PCG. HHMC pretreated with either S. aureus Cowan 1 or protein A released virtually no histamine when challenged with optimal concentrations of either S. aureus Cowan 1 or protein A (Fig. 2). Similar results were obtained in two other experiments. These results show that there is cross-desensitization between soluble protein A and intact S. aureus Cowan 1 and support the idea that the bacterial cell wall protein A may be responsible for the activation of HHMC by S. aureus.
FIG. 2.
Effect of desensitization to one stimulus on the response to a second stimulus. Cells were desensitized to S. aureus Cowan 1 (108 bacteria per ml) or to protein A (200 nM) by preincubation with the stimuli in Ca2+-free P-EDTA for 30 min at 37°C. Cells were then washed twice at 4°C, resuspended in PCG, and challenged with S. aureus Cowan 1 (3 × 107 bacteria per ml), or protein A (200 nM) for 30 min at 37°C. Each bar shows the mean ± SEM of triplicate determinations. Error bars are not shown when graphically too small.
Effect of hyperiodination of protein A.
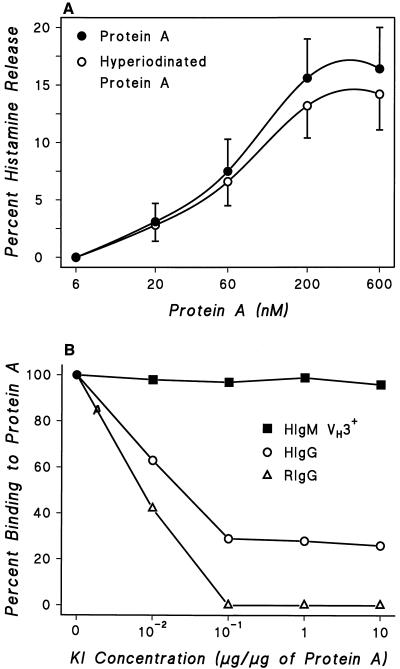
Protein A has two binding sites for Igs. The classical site binds the Fcγ of IgG1, IgG2, and IgG4 (16), and the alternative site(s) bind the Fab portion of a percentage of IgG, IgE, IgA, and IgM (26). Hyperiodination selectively alters the Fcγ-binding region of protein A (39, 58). The histamine-releasing activity of protein A was only slightly reduced by hyperiodination (10 μg of KI/10 μg of protein A) (Fig. 3A). This treatment abolished the ability of protein A to react with RIgG, which shows only Fcγ-protein A reactivity, and strongly reduced its reactivity with HIgG, which possesses both Fcγ and F(ab′)2 reactivity (23, 52) (Fig. 3B). Binding of human monoclonal IgM VH3+ was not affected by this treatment. These findings suggest that activation of HHMC induced by protein A is not mediated by interaction through the classical site of the protein.
FIG. 3.
(A) Effect of hyperiodination of protein A on its ability to induce histamine secretion from HHMC from three donors. Protein A was iodinated with KI (10 μg/10 μg of protein A). Each point is the mean ± SEM. Error bars are not shown when graphically too small. (B) Effect of inactivation of tyrosyl residues of protein A on its ability to react with human monoclonal IgM VH3+, HIgG, and RIgG. The binding of 125I-labeled IgM, 125I-labeled HIgG, and 125I-labeled RIgG to protein A coupled to polyvinyl plate wells was evaluated before and after inactivation of tyrosyl residues by treatment of protein A with various concentrations of KI. Each point shows the mean of duplicate determinations of a typical experiment. Similar results were obtained in two other experiments.
The alternative F(ab′)2-binding site on protein A is responsible for S. aureus Cowan 1-induced activation of HHMC.
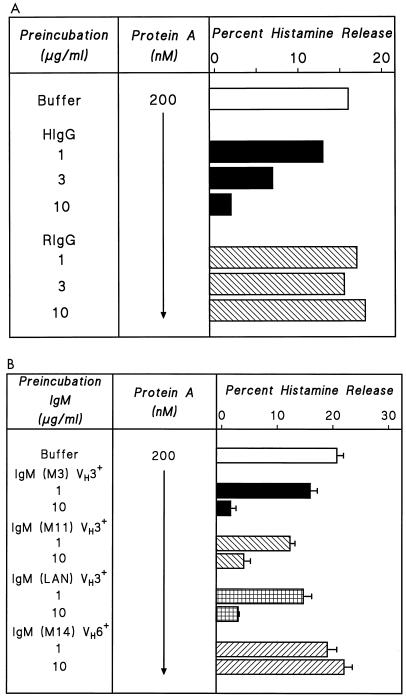
We investigated how the F(ab′)2-binding regions of protein A affect the activation of HHMC induced by protein A-containing S. aureus. We first studied the effect on protein A-induced HHMC activation of molecules that show only Fcγ-protein A reactivity, such as RIgG, and those with both Fcγ and F(ab′)2 reactivity, such as HIgG (23, 52). HIgG dose dependently inhibited protein A-induced histamine release, whereas RIgG, which does not bind the alternative site of protein A (25), had no such effect (Fig. 4A). In a parallel series of experiments, we tried to inhibit selectively the Fcγ and F(ab′)2 reactivity of intact S. aureus Cowan 1 by preincubation with the same Igs. The results were similar to those obtained with protein A (data not shown). These findings indicate that both protein A and protein A-expressing S. aureus induce histamine release by binding Igs bound to FcɛRI on HHMC through the alternative site.
FIG. 4.
(A) Effect of preincubation of protein A with HIgG or RIgG on the activation of HHMC. Protein A (200 nM) was preincubated for 15 min at 37°C with increasing concentrations of HIgG (1 to 10 μg/ml) or RIgG (1 to 10 μg/ml). HHMC were then added, and incubation continued for another 30 min at 37°C. Each bar shows the mean of duplicate determinations of a typical experiment. Similar results were obtained in three other experiments. (B) Effect of preincubation of protein A with monoclonal IgM VH3+ or IgM VH6+ on histamine release from HHMC. Protein A (200 nM) was preincubated 10 min at 37°C with increasing concentrations (1 to 10 μg/ml) of human monoclonal IgM VH3+ (M3, M11, and LAN) or IgM VH6+ (M14). HHMC were then added, and incubation was continued for another 30 min at 37°C. Each bar shows the mean ± SEM of triplicate determinations.
Protein A induces mediator release from HHMC by interaction with the VH3 region of Igs.
We further examined the structural basis for the interaction between human Igs and protein A. The specificity of alternative binding site(s) of protein with human Igs is encoded by the germ line sequences of many of the commonly expressed VH3 genes (23, 52). To assess the mechanism by which protein A activates HHMC, the protein was preincubated with monoclonal IgM of different VH families (49). In three experiments, preincubation of HHMC with three preparations of monoclonal IgM (M3, M11, and LAN), which possess a VH3 domain, concentration dependently inhibited the histamine-releasing activity of protein A (Fig. 4B). In contrast, a monoclonal IgM (M14) which has a VH6 domain had no such effect. These results suggest that binding to the VH3 domain inhibits the binding of protein A to IgE bound to FcɛRI on HHMC.
Cross-desensitization between protein A and anti-IgE.
We examined the relationship between anti-IgE and protein A by cross-desensitization between anti-IgE and protein A. Anti-IgE activates mast cells to release histamine by binding to the Fcɛ portion of the IgE molecule on the cell membrane (13, 45, 46). HHMC were treated with anti-IgE (3 μg/ml) or protein A (200 nM) in P-EDTA for 30 min at 37°C. At the end of incubation, cells were washed and suspended in PCG. Cells preincubated with P buffer and then challenged with anti-IgE released histamine, whereas cells preincubated with anti-IgE released less than 5% histamine. Cells desensitized to protein A released ≈90% less histamine than control cells. In reverse experiment, cells were preincubated with protein A or anti-IgE in P-EDTA before challenge with protein A. As expected, when protein A-pretreated cells were challenged with protein A, they had lost their ability to release with the homologous stimulus (Fig. 5). Similar results were obtained in two other experiments. Thus, it appears that the releasing activity of protein A is mediated mainly by interaction with IgE present on the mast cell membrane (18, 40, 41).
FIG. 5.
Effect of desensitization to one stimulus on response to a second stimulus. Cells were desensitized to anti-IgE (3 μg/ml) or to protein A (200 nM) by preincubation with the stimuli in Ca2+-free P-EDTA for 30 min at 37°C. Cells were then washed twice at 4°C, resuspended in PCG, and challenged with anti-IgE (3 μg/ml) or protein A (200 nM) for 30 min at 37°C. Each bar shows the mean ± SEM. Error bars are not shown when graphically too small.
Effect of IgE stripping on protein A-induced histamine release from HHMC.
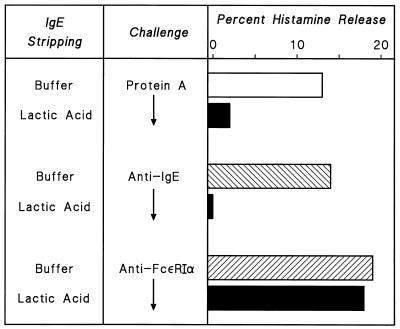
A second line of evidence that protein A induces histamine release by binding to IgE comes from the finding that protein A does not induce histamine release from HHMC stripped of IgE from FcɛRI by brief exposure to low pH (44). Figure 6 illustrates the representative results of one of three experiments showing that lactic acid-induced dissociation of IgE from mast cells completely eliminated anti-IgE secretion and markedly reduced protein A-induced release from HHMC. In contrast, this treatment did not affect the response to a monoclonal antibody cross-linking the α chain of FcɛRI (anti-FcɛRIα) (46).
FIG. 6.
Effect of lactic acid treatment on histamine release from HHMC induced by protein A, anti-IgE, and anti-FcɛRIα. HHMC were either untreated (buffer) or treated with lactic acid (0.01 M, pH 3.9, 5 min, 22°C) and washed twice. Cells were then challenged with protein A (200 nM), anti-IgE (3 μg/ml), or anti-FcɛRIα (1 μg/ml) for 30 min at 37°C. Each bar shows the mean of duplicate determinations of a typical experiment. Similar results were obtained in two other experiments.
Effect of P. magnus on histamine release from HHMC.
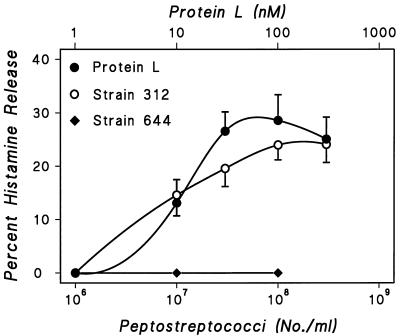
P. magnus is an anaerobic bacterium expressing a cell wall protein L that binds human Ig molecules, regardless of the heavy-chain class, through high-affinity interaction with Ig light chains and is thus an Ig superantigen (42, 56). The Ig-binding activity is mediated through five highly homologous domains, which interact with framework regions in the variable domain of Ig light chains (3, 63). P. magnus is part of the indigenous flora of the skin, the oral cavity, and the gastrointestinal and genitourinary tracts. However, these bacteria are also the causative agents in a variety of infections, including endocarditis and cardiac abscesses (50). Given the correlation between protein L expression and P. magnus virulence (30), we investigated the effects on histamine release from HHMC of increasing numbers of two strains of P. magnus, one that synthesizes protein L (strain 312) and one that does not (strain 644). Protein L binds with high affinity predominantly to κ L chains (4, 42), and this interaction involves exclusively the VL portion of Igs (42). With strain 312 peptostreptococci in a range from 106 to 3 × 108 bacteria/tube, histamine secretion gradually increased with the numbers of bacteria. Strain 644 peptostreptococci (105 to 108 bacteria/tube), which do not synthesize protein L (42, 44), did not induce histamine release in any of the five preparations of HHMC studied (Fig. 7). HHMC were also treated with protein L (1 to 300 nM), which induced concentration-dependent histamine release. A significant correlation was found between the maximal percent histamine release induced by protein L and that induced by peptostreptococcal strain 312. These findings suggest that protein L is responsible for the activation of basophils by P. magnus strain 312.
FIG. 7.
Effect of increasing concentrations of P. magnus strains 312 and 644 and of protein L on histamine secretion from HHMC from five donors. Each point is the mean ± SEM. Error bars are not shown when graphically too small.
Comparison of effects of protein L, protein A, and protein G with anti-IgE-induced histamine release from HHMC.
In eight experiments, HHMC were challenged with a wide range of concentrations of protein A (60 to 600 nM), protein L (10 to 100 nM), protein G (10 to 600 nM), and anti-IgE (3 × 10−1 to 3 μg/ml). Protein G is the IgG-binding protein of group C and G streptococci (4, 42). Protein G binds to the CH2-CH3 interface region of human IgG Fc (4, 42). Proteins A and L and anti-IgE concentration dependently induced histamine release from HHMC, protein L and anti-IgE being significantly more potent than protein A. In fact, the maximal protein A-induced histamine secretion was significantly lower (12.3% ± 1.8%) than that caused by protein L (18.6% ± 3.1%; P < 0.05) and anti-IgE (17.0% ± 2.3%; P < 0.05). HHMC were essentially unresponsive to protein G, which binds exclusively to IgG (4, 16).
Cross-desensitization between protein L and anti-IgE.
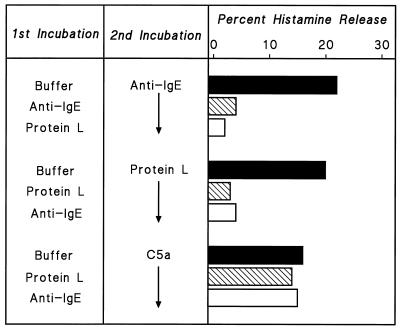
We tested anti-IgE and protein L for cross-desensitization. The results of one of three experiments are illustrated in Fig. 8. HHMC were preincubated with anti-IgE (3 μg/ml) or protein L (100 nM) in P-EDTA for 30 min at 37°C; then the cells were washed, resuspended in PCG, and challenged with anti-IgE, protein L, or C5a. Cells preincubated with protein L released ≈20% of their histamine content when challenged with anti-IgE (1 μg/ml) or protein L (30 nM). Similarly, HHMC preincubated with anti-IgE released less than 5% of their histamine content in response to either challenge. In contrast, cells preincubated with anti-IgE or protein L were not desensitized in response to challenge with C5a, which activates a receptor independent of the IgE receptor on HHMC (46, 47). These results are consistent with the hypothesis that the releasing property of protein L is mediated by interaction with IgE on the mast cell surface.
FIG. 8.
Effect of desensitization to one stimulus on response to a second stimulus. Cells were desensitized to protein L (100 nM) or to anti-IgE (3 μg/ml) by incubation with the stimuli in Ca2+-free P-EDTA for 30 min at 37°C. Cells were then washed twice at 4°C, resuspended in PCG, and challenged with protein L (30 nM), anti-IgE (1 μg/ml), or C5a (10−6 M) for 30 min at 37°C. Each bar shows the mean of duplicate determinations of a typical experiment. Similar results were obtained in two other experiments.
Interactions between protein L or anti-IgE and different IgE myeloma proteins.
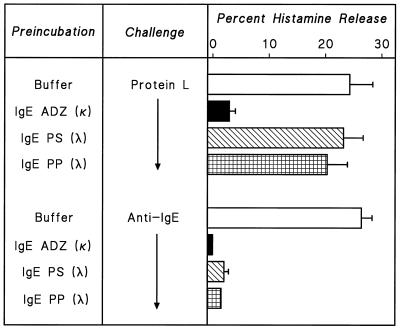
The binding specificity of protein L is directed to the L chains of Ig, and the affinity constant for IgG, IgA, and IgM is around 1010 M−1 (3, 4). Protein L contains multiple κ-binding domains (31), making this bacterial product similar to the divalent anti-IgE antibodies (13, 45). In contrast to κ chains, protein L binds λ light chains poorly or not at all (42). To evaluate the mechanism of activation of HHMC by protein L, we preincubated protein L or anti-IgE with three different IgE myelomas, designated PS, ADZ, and PP. IgE myelomas PS and PP have λ L chains, whereas IgE myeloma ADZ has κ chains (39, 54). IgE purified from all three sources (3 μg/ml) blocked the histamine-releasing activating of anti-IgE. IgE purified from myelomas PS and PP (λ chains) did not modify the histamine-releasing activity of protein L, whereas IgE from myeloma ADZ (κ chains) completely blocked the releasing activity of protein L (Fig. 9).
FIG. 9.
Effect of preincubation of protein L and anti-IgE with monoclonal IgE on histamine release from HHMC. Protein L (10 nM) or anti-IgE (1 μg/ml) was preincubated for 15 min at 37°C with human monoclonal IgE (3 μg/ml), PS (3 μg/ml), or PP (3 μg/ml). HHMC were then added, and incubation continued for another 30 min at 37°C. Each bar shows the mean ± SEM. Error bars are not shown when graphically too small.
Effect of protein A and protein L on LTC4 synthesis from HHMC.
HHMC challenged with anti-IgE synthesize de novo LTC4 (46, 47), a proinflammatory mediator with vasoactive and biological properties (22, 61). Protein L and protein A acted as complete secretagogues because they too caused the de novo synthesis of LTC4 by HHMC. Table 1 summarizes the results of five experiments in which HHMC were challenged with different concentrations of protein L, protein A, or anti-IgE. As previously shown (46), anti-IgE induced concentration-dependent de novo synthesis of LTC4, and of histamine. Protein L and protein A also induced the synthesis of LTC4. There was a significant correlation between the percent histamine secretion and the release of LTC4 by anti-IgE (r = 0.84; P < 0.01), protein L (r = 0.78; P < 0.01), and protein A (r = 0.91; P < 0.01) from HHMC.
TABLE 1.
Effect of increasing concentrations of protein L, protein A, and anti-IgE on histamine release and de novo synthesis of LTC4 from HHMC
| Protein or antibody | Mean ±
SEM
|
|
|---|---|---|
| % Histamine release | LTC4 (ng/106 mast cells) | |
| Protein L (nM) | ||
| 10 | 2.2 ± 1.0 | 6.0 ± 1.7 |
| 30 | 13.2 ± 1.9 | 10.6 ± 3.4 |
| 100 | 19.2 ± 2.7 | 23.0 ± 3.1 |
| Protein A (nM) | ||
| 60 | 1.0 ± 0.4 | 3.0 ± 1.3 |
| 200 | 7.2 ± 1.2 | 13.6 ± 2.1 |
| 600 | 12.4 ± 1.4 | 21.2 ± 3.3 |
| Anti-IgE (μg/ml) | ||
| 0.3 | 2.2 ± 1.0 | 3.6 ± 1.0 |
| 1 | 11.6 ± 3.3 | 18.6 ± 3.9 |
| 3 | 15.0 ± 2.5 | 15.8 ± 3.2 |
Correlation between histamine and tryptase release from HHMC induced by protein A and protein L.
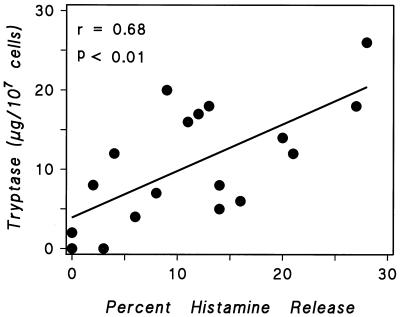
HHMC secretory granules contain tryptase, a neutral protease that can be immunologically released (46–48). We investigated whether histamine release was correlated to the secretion of tryptase induced by protein A and protein L (Fig. 10) and found a significant correlation between the maximum histamine and tryptase release (r = 0.68; P < 0.01). These findings indicate that protein A and protein L release tryptase in parallel with histamine from HHMC.
FIG. 10.
Correlation between the maximum percentage of histamine secretion and tryptase release induced by protein A and protein L from HHMC. Each point is the mean of duplicate determinations from separate experiments.
Effect of FMLP and pepstatin A on HHMC.
Previous studies have reported a remarkable degree of selectivity of bacterial products in their capacity to induce the release of mediators from human basophils or mast cells. For example, pepstatin A, a pentapeptide isolated from cultures of actinomycetes (38), and the bacterial formylated tripeptide FMLP activate a specific seven-transmembrane receptor independent of the FcɛRI on human basophils (12). FMLP and pepstatin A selectively induce the release of chemical mediators from human basophils (12, 38) but not from human lung mast cells (6). In six experiments, we investigated the effects of FMLP (10−8 to 10−5 M) and pepstatin A (10−8 to 10−5 M) on histamine and LTC4 release from HHMC. FMLP and pepstatin A did not induce histamine release or the de novo synthesis of LTC4 from HHMC in any experiment (data not shown). These results underline the immunological heterogeneity of HHMC in response to different bacterial products.
DISCUSSION
This study demonstrates that two bacterial products, protein A of S. aureus and protein L of P. magnus, induce the release of preformed and de novo-synthesized vasoactive and proinflammatory mediators from mast cells isolated from human heart tissue. We also demonstrate that the protein A-containing bacterial strain S. aureus Cowan 1 and the protein L-containing P. magnus induce mediator release from HHMC. Protein A's releasing activity appears to be mediated by interaction with the VH3 region of IgE on HHMC, whereas the activity of protein L is mediated by interaction with the κ light chain of human IgE. This is the first demonstration that bacterial products and intact bacteria can activate HHMC in vitro to release preformed and de novo-synthesized proinflammatory mediators.
The releasing activity of protein A and S. aureus Cowan 1 appears to be mediated by interaction of the alternative F(ab′)2-binding site with IgE present on HHMC. This is borne out by the lack of effect of hyperiodination of protein A, which selectively alters the Fcγ-binding region of the protein, and by the correlation between the maximum histamine release induced by anti-IgE and that induced by protein A. In addition, there was complete cross-desensitization between protein A and anti-IgE. Finally, HHMC from which IgE had been dissociated by brief exposure to lactic acid no longer released histamine in response to protein A and anti-IgE. In contrast, this treatment did not affect the response to a monoclonal antibody cross-linking the α chain of FcɛRI (anti-FcɛRI). These findings are in agreement with the notion that the reactivity of protein A for human Ig is at least divalently expressed in the molecule structure (23, 25, 26, 52, 56). Therefore, protein A can function as a natural cross-linking agent which reproduces the releasing activity of rabbit IgG anti-human IgE on HHMC (46, 48).
This study provides insight into the mechanisms of interaction between protein L or protein A and IgE bound on HHMC. Three different monoclonal IgM antibodies with a VH3 domain inhibited the release of mediators induced by protein A from HHMC, whereas IgM VH6+ had no effect. This suggests that protein A's releasing activity depends on binding to an Ig structure located in the VH3 domain, a fragment common to all Ig classes and subclasses (23, 25, 52, 56). The releasing activity of protein L-containing bacteria, such as P. magnus strain 312, and soluble protein L appears to be mediated by interaction with IgE present on HHMC. This is borne out by the observation that protein L binds with high affinity (≈1010 M−1) to all human Ig isotypes (3, 42, 63). In addition, there is a highly significant correlation between the maximal percent histamine release induced by anti-IgE and by protein L and complete cross-desensitization between protein L and anti-IgE. Finally, two IgE myeloma proteins (PS and PP), which both possess a λ chain, did not prevent the release of histamine induced by protein L from HHMC, whereas IgE myeloma ADZ (which has κ chains) blocked this release. These results indicate that protein L interacts with the κ light chain of IgE on the cardiac mast cell to induce the release of vasoactive and proinflammatory mediators. In conclusion, the data show that protein A and protein L act as Ig superantigens by activating HHMC in vitro.
The various proteins expressed by different bacteria vary widely in the ability to promote the release of proinflammatory mediators from human FcɛRI+ cells. Protein G, synthesized by streptococci, binds with high affinity to all isotypes of human IgG (4) but did not activate either basophils (39) or HHMC. Moreover, the bacterial products FMLP and pepstatin A, which activate a specific seven-transmembrane receptor independent of the FcɛRI on human basophils (12, 38), did not activate HHMC. These findings indicate that different bacterial products selectively activate human basophils and mast cells through specific mechanisms.
Given the biological importance of mast cell-derived mediators such as histamine (33, 62), tryptase (55), and cysteinyl leukotrienes (22, 61) in heart pathophysiology, our findings might explain how some bacterial products cause tissue damage in the heart and coronary vessels of patients with infections. Proteins A and L are complete secretagogues, capable not only of releasing preformed mediators (histamine and tryptase) but also of inducing the de novo synthesis of LTC4 from HHMC. In vivo administration of cysteinyl leukotrienes can increase coronary vascular resistance in humans (58). Given the biological importance of leukotrienes in inflammation and cardiovascular pathophysiology (22, 58), this finding could have biological and clinical relevance. Tryptase, a neutral protease present in the cytoplasmic granules of HHMC (46–48), can activate complement, leading to the formation of anaphylatoxins (C3a and C5a) (55). C5a receptors are present on HHMC, and their engagement by C5a leads to HHMC activation and the release of proinflammatory mediators (46). Mast cells are found in human heart tissue (2, 46), perivascularly (29), and in the intima of coronary arteries (9, 15, 27, 29). Therefore, the release of preformed and de novo-synthesized mediators caused by certain bacterial products from HHMC might act as an amplification factor, contributing to the pathogenesis of myocardial damage in patients with bacterial infections.
Our data provide the first indication that intact bacteria and soluble bacterial products specifically activate HHMC, thus acting as Ig superantigens (56). Human mast cells synthesize a still-growing list of proinflammatory mediators (41, 45), cytokines, and chemokines (5, 19, 64), thus playing a much more complex proinflammatory and immunoregulatory role than previously believed. Recent studies have yielded circumstantial evidence linking cardiovascular diseases with bacterial infections (1, 20, 24, 43). However, the mechanisms by which bacteria might cause cardiovascular diseases in humans are largely unknown (11, 21).
Interestingly, protein L and A both activate HHMC through an interaction with membrane-bound IgE on these cells, although with different types of Fab specificity. It is intriguing that patients with coronary heart disease may have high serum IgE levels (10, 60). The mechanism of HHMC activation by Ig-binding bacterial proteins could serve as a new model for the pathogenetic link between bacterial infections, IgE-mediated activation of HHMC, and cardiovascular diseases. In conclusion, we demonstrate that S. aureus Cowan 1 and soluble protein A can activate HHMC to release mediators, by interacting with the VH3 region of IgE. Protein L and P. magnus activate HHMC through a specific interaction with κ light chains of IgE bound on HHMC. Thus, certain bacterial products can act as Ig superantigens activating HHMC.
ACKNOWLEDGMENTS
This study was supported by grants from the Consiglio Nazionale delle Ricerche (Targeted Project Biotechnology no. 99.000216.PF31 and 99.00401.PF49), Ministero dell'Università e Ricerca Tecnologica (MURST) of Italy (Rome, Italy), and the Swedish Medical Research Council (Project 7480).
We thank Lina Tagliaferri for excellent secretarial assistance.
REFERENCES
- 1.Bachmaier K, Neu N, de la Maza L M, Pal S, Hessel A, Penninger J M. Chlamydiainfections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–1339. doi: 10.1126/science.283.5406.1335. [DOI] [PubMed] [Google Scholar]
- 2.Bankl H C, Radaszkiewicz T, Klappacher G W, Glogar D, Sperr W R, Großschmidt K, Bankl H, Lechner K, Valent P. Increase and redistribution of cardiac mast cells in auricular thrombosis. Possible role of kit ligand. Circulation. 1995;91:275–283. doi: 10.1161/01.cir.91.2.275. [DOI] [PubMed] [Google Scholar]
- 3.Björck L. Protein L: a novel bacterial cell wall protein with affinity for Ig L chains. J Immunol. 1988;140:1194–1197. [PubMed] [Google Scholar]
- 4.Björck L, Kronvall G. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J Immunol. 1984;133:969–974. [PubMed] [Google Scholar]
- 5.Bradding P, Feather I H, Howarth P H, Mueller R, Roberts J A, Britten K, Bews J P A, Hunt T C, Okayama Y, Heusser C H, Bullock G R, Church M K, Holgate S T. Interleukin 4 is localized to and released by human mast cells. J Exp Med. 1992;176:1381–1386. doi: 10.1084/jem.176.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casolaro V, Galeone D, Giacummo A, Sanduzzi A, Melillo G, Marone G. Human basophil/mast cell releasability. V. Functional comparisons of cells obtained from peripheral blood, lung parenchyma, and bronchoalveolar lavage in asthmatics. Am Rev Respir Dis. 1989;139:1375–1382. doi: 10.1164/ajrccm/139.6.1375. [DOI] [PubMed] [Google Scholar]
- 7.Chieffi Baccari G, de Paulis A, Di Matteo L, Gentile M, Marone G, Minucci S. In situ characterization of mast cells in the frog Rana esculenta. Cell Tissue Res. 1998;292:151–162. doi: 10.1007/s004410051045. [DOI] [PubMed] [Google Scholar]
- 8.Columbo M, Horowitz E M, Botana L M, MacGlashan D W, Jr, Bochner B S, Gillis S, Zsebo K M, Galli S J, Lichtenstein L M. The human recombinant c-kitreceptor ligand, rhSCF, induces mediator release from human cutaneous mast cells and enhances IgE-dependent mediator release from both skin mast cells and peripheral blood basophils. J Immunol. 1992;149:599–608. [PubMed] [Google Scholar]
- 9.Constantinides P. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92:1083. doi: 10.1161/01.cir.92.5.1083. [DOI] [PubMed] [Google Scholar]
- 10.Criqui M H, Lee E R, Hamburger R N, Klauber M R, Coughlin S S. IgE and cardiovascular disease. Results from a population-based study. Am J Med. 1987;82:964–968. doi: 10.1016/0002-9343(87)90159-8. [DOI] [PubMed] [Google Scholar]
- 11.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 12.de Paulis A, Ciccarelli A, de Crescenzo G, Cirillo R, Patella V, Marone G. Cyclosporin H is a potent and selective competitive antagonist of human basophil activation by N-formyl-methionyl-leucyl-phenylalanine. J Allergy Clin Immunol. 1996;98:152–164. doi: 10.1016/s0091-6749(96)70237-3. [DOI] [PubMed] [Google Scholar]
- 13.de Paulis A, Ciccarelli A, Marinò I, de Crescenzo G, Marinò D, Marone G. Human synovial mast cells. II. Heterogeneity of the pharmacologic effects of antiinflammatory and immunosuppressive drugs. Arthritis Rheum. 1997;40:469–478. doi: 10.1002/art.1780400313. [DOI] [PubMed] [Google Scholar]
- 14.Estensen R D. Eosinophilic myocarditis: a role for mast cells? Arch Pathol Lab Med. 1984;108:358–359. [PubMed] [Google Scholar]
- 15.Forman M B, Oates J A, Robertson D, Robertson R M, Roberts II L J, Virmani R. Increased adventitial mast cells in a patient with coronary spasm. N Engl J Med. 1985;313:1138–1141. doi: 10.1056/NEJM198510313131807. [DOI] [PubMed] [Google Scholar]
- 16.Forsgren A, Sjöquist J. “Protein A” from S. aureus. I. Pseudoimmune reaction with human γ-globulin. J Immunol. 1966;99:822–827. [PubMed] [Google Scholar]
- 17.Frangogiannis N G, Lindsey M L, Michael L H, Youker K A, Bressler R B, Mendoza L H, Spengler R N, Smith C W, Entman M L. Resident cardiac mast cells degranulate and release preformed TNF-α, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 18.Galli S J. New concepts about the mast cell. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 19.Gauchat J F, Henchoz S, Mazzel G, Aubry J P, Brunner T, Blasey H, Life P, Talabot D, Flores-Romo L, Thompson J, Kishi K, Butterfield J, Dahinden C, Bonnefoy J Y. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 20.Grayston J T, Kuo C, Coulson A S, Campbell L A, Lawrence R D, Lee M J, Strandness E D, Wang S. Chlamydia pneumoniae(TWAR) in atherosclerosis of the carotid artery. Circulation. 1995;92:3397–3400. doi: 10.1161/01.cir.92.12.3397. [DOI] [PubMed] [Google Scholar]
- 21.Gura T. Chlamydiaprotein linked to heart disease. Science. 1999;283:1238–1239. doi: 10.1126/science.283.5406.1238. [DOI] [PubMed] [Google Scholar]
- 22.Henderson W R., Jr The role of leukotrienes in inflammation. Ann Intern Med. 1994;121:684–697. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- 23.Hillson J L, Karr N S, Oppliger I R, Mannik M, Sasso E H. The structural basis of germline-encoded VH3 immunoglobulin binding to staphylococcal protein A. J Exp Med. 1993;178:331–336. doi: 10.1084/jem.178.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu H, Pierce G N, Zhong G. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J Clin Investig. 1999;103:747–753. doi: 10.1172/JCI4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inganäs M, Johansson S G O, Bennich H H. Interaction of human polyclonal IgE and IgG from different species with protein A from Staphylococcus aureus: demonstration of protein-A-reactive sites located in the Fab′2fragments of human IgG. Scand J Immunol. 1980;12:23–31. doi: 10.1111/j.1365-3083.1980.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 26.Inganäs M. Comparison of mechanisms of interaction between protein A from Staphylococcus aureus and human monoclonal IgG, IgA and IgM in relation to the classical Fcγ and the alternative F(ab′)2protein interactions. Scand J Immunol. 1981;13:343–352. doi: 10.1111/j.1365-3083.1981.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaartinen M, Penttilä A, Kovanen P T. Accumulation of activated mast cells in the shoulder region of human coronary atheroma, the predilection site of atheromatous rupture. Circulation. 1994;90:1669–1678. doi: 10.1161/01.cir.90.4.1669. [DOI] [PubMed] [Google Scholar]
- 28.Kaartinen M, Penttilä A, Kovanen P T. Mast cells in rupture-prone areas of human coronary atheromas produce and store TNF-α. Circulation. 1996;94:2787–2792. doi: 10.1161/01.cir.94.11.2787. [DOI] [PubMed] [Google Scholar]
- 29.Kalsner S, Richards R. Coronary arteries of cardiac patients are hyperreactive and contain stores of amines: a mechanism for coronary spasm. Science. 1984;223:1435–1437. doi: 10.1126/science.6701530. [DOI] [PubMed] [Google Scholar]
- 30.Kastern W, Holst E, Nielsen E, Sjöbring U, Björck L. Protein L, a bacterial immunoglobulin-binding protein and possible virulence determinant. Infect Immun. 1990;58:1217–1222. [Google Scholar]
- 31.Kastern W, Sjöbring U, Björck L. Structure of peptostreptococcal protein L and identification of a repeated immunoglobulin light chain-binding domain. J Biol Chem. 1992;267:12820–12825. [PubMed] [Google Scholar]
- 32.Lantz C S, Boesiger J, Song C H, Mach N, Kobayashi T, Mulligan R C, Nawa Y, Dranoff G, Galli S J. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 33.Levi R, Malm J R, Bowman F O, Rosen M R. The arrhythmogenic actions of histamine on human atrial fibers. Circ Res. 1981;49:545–550. doi: 10.1161/01.res.49.2.545. [DOI] [PubMed] [Google Scholar]
- 34.Libman H, Arbeit R D. Complications associated with Staphylococcus aureusbacteremia. Arch Intern Med. 1984;144:541–545. [PubMed] [Google Scholar]
- 35.Lowy F D. Staphylococcus aureusinfections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 36.Malaviya R, Ikeda T, Ross E, Abraham S N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 37.Malaviya R, Twesten N J, Ross E A, Abraham S N, Pfeifer J D. Mast cells process bacterial Ags through a phagocytic route for class I MHC presentation to T cells. J Immunol. 1996;156:1490–1496. [PubMed] [Google Scholar]
- 38.Marone G, Columbo M, Soppelsa L, Condorelli M. The mechanism of basophil histamine release induced by pepstatin A. J Immunol. 1984;133:1542–1546. [PubMed] [Google Scholar]
- 39.Marone G, Tamburini M, Giudizi M G, Biagiotti R, Almerigogna F, Romagnani S. Mechanism of activation of human basophils by Staphylococcus aureusCowan 1. Infect Immun. 1987;55:803–809. doi: 10.1128/iai.55.3.803-809.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marone G, Lichtenstein L M, Galli S J. Mast cells and basophils. London, England: Academic Press; 2000. [Google Scholar]
- 41.Metcalfe D D, Baram D, Mekori Y A. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 42.Nilson B H K, Solomon A, Björck L, Akerström B. Protein L from Peptostreptococcus magnusbinds to the κ light chain variable domain. J Biol Chem. 1992;267:2234–2239. [PubMed] [Google Scholar]
- 43.Pasceri V, Cammarota G, Patti G, Cuoco L, Gasbarrini A, Grillo R L, Fedeli G, Gasbarrini G, Maseri A. Association of virulent Helicobacter pyloristrains with ischemic heart disease. Circulation. 1998;97:1675–1679. doi: 10.1161/01.cir.97.17.1675. [DOI] [PubMed] [Google Scholar]
- 44.Patella V, Casolaro V, Björck L, Marone G. Protein L. A bacterial Ig-binding protein that activates human basophils and mast cells. J Immunol. 1990;145:3054–3061. [PubMed] [Google Scholar]
- 45.Patella V, Bouvet J-P, Marone G. Protein Fv produced during viral hepatitis is a novel activator of human basophils and mast cells. J Immunol. 1993;151:5685–5698. [PubMed] [Google Scholar]
- 46.Patella V, Marinò I, Lampärter B, Arbustini E, Adt M, Marone G. Human heart mast cells. Isolation, purification, ultrastructure, and immunologic characterization. J Immunol. 1995;154:2855–2865. [PubMed] [Google Scholar]
- 47.Patella V, de Crescenzo G, Marinò I, Genovese A, Adt M, Gleich G J, Marone G. Eosinophil granule proteins activate human heart mast cells. J Immunol. 1996;157:1219–1225. [PubMed] [Google Scholar]
- 48.Patella V, Marinò I, Arbustini E, Lamparter-Schummert B, Verga L, Adt M, Marone G. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97:971–978. doi: 10.1161/01.cir.97.10.971. [DOI] [PubMed] [Google Scholar]
- 49.Patella V, Giuliano A, Bouvet J-P, Marone G. Endogenous superallergen protein Fv induces IL-4 secretion from human FcɛRI+ cells through interaction with the VH3 region of IgE. J Immunol. 1998;161:5647–5655. [PubMed] [Google Scholar]
- 50.Pouedras P, Donnio P Y, Sire J M, Avril J L. Prosthetic valve endocarditis and paravalvular abscess caused by Peptostreptococcus magnus. Clin Infect Dis. 1992;15:185. doi: 10.1093/clinids/15.1.185. [DOI] [PubMed] [Google Scholar]
- 51.Prodeus A P, Zhou X, Maurer M, Galli S J, Carroll M C. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 52.Roben P W, Salem A N, Silverman G J. VH3 family antibodies bind domain D of staphylococcal protein A. J Immunol. 1995;154:6437–6445. [PubMed] [Google Scholar]
- 53.Romagnani P, de Paulis A, Beltrame C, Annunziato F, Dente V, Maggi E, Romagnani S, Marone G. Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. Am J Pathol. 1999;155:1195–1204. doi: 10.1016/S0002-9440(10)65222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sala P, Tonutti E, Ruscio M, Colle R, Antonutto G, Falconieri G. IgE myeloma. Report of a new case and review of the literature. Haematologica. 1981;66:788–795. [PubMed] [Google Scholar]
- 55.Schwartz L B, Kawahara M S, Hugli T E, Vik D, Fearon D T, Austen K F. Generation of C3a anaphylatoxin from human C3 by human mast cell tryptase. J Immunol. 1983;130:1891–1895. [PubMed] [Google Scholar]
- 56.Silverman G J. B-cell superantigens. Immunol Today. 1997;18:379–386. doi: 10.1016/s0167-5699(97)01101-8. [DOI] [PubMed] [Google Scholar]
- 57.Siraganian R P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974;57:383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 58.Sjöholm I, Bierken A, Sjöquist J. Protein A from Staphylococcus aureus. XVI. The effect of nitration of protein A with tetranitromethane and subsequent reduction. J Immunol. 1973;110:1562–1569. [PubMed] [Google Scholar]
- 59.Snedecor G W, Cochran W G. Statistical methods. Ames, Iowa: The Iowa State University Press; 1980. [Google Scholar]
- 60.Szczeklik A, Sladek K, Szczerba A, Dropinski J. Serum immunoglobulin E response to myocardial infarction. Circulation. 1988;77:1245–1249. doi: 10.1161/01.cir.77.6.1245. [DOI] [PubMed] [Google Scholar]
- 61.Vigorito C, Giordano A, Cirillo R, Genovese A, Rengo F, Marone G. Metabolic and hemodynamic effects of peptide leukotriene C4 and D4in man. Int J Clin Lab Res. 1997;27:178–184. doi: 10.1007/BF02912454. [DOI] [PubMed] [Google Scholar]
- 62.Vigorito C, Poto S, Picotti G B, Triggiani M, Marone G. Effect of activation of the H1receptor on coronary hemodynamics in man. Circulation. 1986;73:1175–1182. doi: 10.1161/01.cir.73.6.1175. [DOI] [PubMed] [Google Scholar]
- 63.Wilkström M, Sjöbring U, Drakenberg T, Forsén S, Björck L. Mapping of the immunoglobulin light chain-binding site of protein L. J Mol Biol. 1995;250:128–133. doi: 10.1006/jmbi.1995.0364. [DOI] [PubMed] [Google Scholar]
- 64.Yano K, Yamaguchi M, de Mora F, Lantz C S, Butterfield J H, Costa J J, Galli S J. Production of macrophage inflammatory protein-1α by human mast cells: increased anti-IgE-dependent secretion after IgE-dependent enhancement of mast cell IgE-binding ability. Lab Investig. 1997;77:185–193. [PubMed] [Google Scholar]