Abstract
Background
Sudden sensorineural hearing loss (SSNHL) can cause great panic in patients. Whether it is advantageous to add intravenous batroxobin in the treatment of SSNHL remains to be determined. This study aimed to compare the short-term efficacy of therapy combined with intravenous batroxobin and that without intravenous batroxobin in SSNHL patients.
Methods
This retrospective study harvested the data of SSNHL patients hospitalized in our department from January 2008 to April 2021. The hearing levels on the admitted day (before treatment) and the discharge day were considered pre-treatment hearing and post-treatment hearing, respectively. The hearing gain was the difference value of pre-treatment hearing and post-treatment hearing. We used Siegel's criteria and the Chinese Medical Association of Otolaryngology (CMAO) criteria to evaluate hearing recovery. The complete recovery rate, overall effective rate, and hearing gain at each frequency were considered outcomes. Propensity score matching (PSM) was conducted to balance the baseline characteristics between the batroxobin group and the non-batroxobin group. Sensitivity analysis was carried out in flat-type and total-deafness SSNHL patients.
Results
During the study period, 657 patients with SSNHL were admitted to our department. Among them, a total of 274 patients met the enrolled criteria of our study. After PSM, 162 patients (81 in each group) were included in the analysis. Once the hospitalized treatment was completed, the patients would be discharged the next day. Logistic regression analysis of the propensity score-matched cohort indicated that both the complete recovery rates [Siegel's criteria, OR: 0.734, 95% CI: 0.368–1.466, p = 0.381; CMAO criteria, OR: 0.879, 95% CI: 0.435–1.777, p = 0.720] and the overall effective rates [Siegel's criteria and CMAO criteria, OR: 0.741, 95% CI: 0.399–1.378, p = 0.344] were not significantly different between the two treatment groups. Sensitivity analysis has shown similar results. For flat-type and total-deafness SSNHL patients, no significant difference was found in post-treatment hearing gain at each frequency between the two groups after PSM.
Conclusion
There was no significant difference in short-term hearing outcomes between treatment with batroxobin and treatment without batroxobin in SSNHL patients by Siegel's and CMAO criteria after PSM. Future studies for better therapy regimens of SSNHL are still needed.
Keywords: intravenous batroxobin, sudden sensorineural hearing loss, short-term effects, propensity score matching, treatment
Introduction
Sudden sensorineural hearing loss (SSNHL) can cause great panic in patients. It is often accompanied by tinnitus and/or vertigo and can have severe influences on the living quality of patients. Approximately 5–27 per 100,000 people in the United States suffer from SSNHL each year (1). In Germany, SSNHL affects 160–400/100,000 people per year (2). However, the etiology of SSNHL is still unclear. Viral infections, circulatory disorders, autoimmune diseases, and inner ear or central nervous system abnormalities have been proposed to be possible causative factors (3). Among them, inner ear microcirculation obstruction resulting from circulatory factors is a frequently theorized cause (3–5).
Aimed at the possible inner ear microcirculation ischemia, one may assume the application of defibrinogenation agents in the treatment of SSNHL. However, defibrinogenation therapy for SSNHL is still controversial. Batroxobin is a kind of defibrinogenation drug from the venom of Bothrops atrox, which cleaves plasma fibrinogen into fibrin (6). It can augment local blood flow by reducing blood viscosity (7, 8), and thereby improve microcirculation. Batroxobin was originally used in the treatment of occlusive vascular diseases, such as stroke and myocardial infarction (9, 10). Some studies indicated that intravenous batroxobin may be effective in treating patients with SSNHL (8, 11). In the German Guideline “Sudden Idiopathic Sensorineural Hearing Loss” (2011) (2), the reduction of fibrinogen was presented as rheological therapy, one of the two main methods for the treatment of SSNHL. In the Chinese Guideline for diagnosis and treatment of sudden deafness (2015), intravenous batroxobin was recommended in the treatment of flat-type and total-deafness SSNHL (12). A study in Japan has shown that the recovery rate of profound hearing loss patients was better in batroxobin therapy compared to that in steroid treatment (7). Meanwhile, Jia et al. (13) also proposed that patients with SSNHL receiving combined treatment (steroid and intravenous batroxobin) could achieve superior recovery than steroid therapy alone. However, based on the Clinical Practice Guideline: Sudden Hearing Loss of the American Academy of Otolaryngology-Head and Neck Surgery Foundation (AAO-HNSF) (2019) (1), clinicians should not routinely apply defibrinogen therapy to SSNHL patients. There was not enough evidence to conclude the benefit of defibrinogenation therapy in the management of SSNHL. Whether it is advantageous to add intravenous batroxobin in the treatment of SSNHL remains to be determined.
This retrospective propensity score-matched study aimed to compare the short-term efficacy of therapy combined with intravenous batroxobin and that without intravenous batroxobin in SSNHL patients. We attempted to explore whether it is better to add intravenous batroxobin in the therapy of SSNHL and to provide a new direction and thinking for the treatment of SSNHL.
Materials and methods
SSNHL patients
The study harvested the data of SSNHL patients hospitalized in our department from January 2008 to April 2021. In this study, SSNHL referred to a rapid onset sensorineural hearing loss that occurs within a 72-h window with a decrease in the hearing of ≥30 decibels (dB) affecting at least three consecutive frequencies (1). Pure-tone audiometry was conducted on the admitted day (before treatment). Hearing thresholds at 250, 500, 1,000, 2,000, 4,000, and 8,000 Hz were recorded. Hearing loss was defined concerning the opposite ear's thresholds. The pre-treatment audiograms were categorized into four types (12): (1) low-frequency: the hearing loss was at frequencies below 1,000 Hz (1,000 Hz included), at least hearing loss at 250 and 500 Hz was ≥20 dB HL; (2) high-frequency: the hearing loss was at frequencies above 2,000 Hz (2,000 Hz included), at least hearing loss at 4,000 and 8,000 Hz was ≥20 dB; (3) flat: hearing decreases at all frequencies with the average hearing threshold at 250, 500, 1,000, 2,000, 4,000, and 8,000 Hz ≤ 80 dB; (4) total-deafness: hearing decreases at all frequencies with the average hearing threshold at 250, 500, 1,000, 2,000, 4,000, and 8,000 Hz ≥81 dB. All patients must meet the inclusion standards: (1) unilateral SSNHL; (2) age ≥18 years old; (3) time from the onset of hearing loss to the start of treatment ≤ 14 days; (4) first treatment in our department; (5) hospitalized for SSNHL treatment; (6) no acoustic neuroma or other retro-cochlear lesions from the magnetic resonance imaging of the internal acoustic canal; (7) no history of otitis media, Meniere's disease, autoimmune diseases, long-term exposure to noise, use of ototoxic drugs, previous ear surgery, and prior sudden deafness or trauma; (8) no history of familial deafness; (9) no contraindications of drugs here; and (10) with complete medical records. Pregnant women were excluded. The study was approved by the ethical committee of Xiangya Hospital, Central South University (No. 2019051092). All the patients were de-identified when collecting information. Given the retrospective and de-identified nature of this study, the committee waived the obligation to obtain informed consent. The study was performed in accordance with the STROBE guidelines.
Therapeutic regimens of intravenous batroxobin
The treatment regimen for each patient was formulated by sophisticated otolaryngologists in our department. Treatment including intravenous batroxobin was deemed as therapy combined with batroxobin (the batroxobin group). All other therapies were classified as therapy without batroxobin (the non-batroxobin group). No patient included in this study was treated with intravenous batroxobin alone. Batroxobin was administered intravenously once every other day for a total of three times. The initial dose was 10 BU and then decreased to a dose of 5 BU for the latter two times. The duration of each infusion was no <1 h. Serum fibrinogen was examined 24 h after each administration. If the level of serum fibrinogen was less than 100 mg/dl, it should be measured again after 24 h. The administration can carry on only when the fibrinogen level was higher than 100 mg/dl. In case complete recovery of hearing loss was confirmed by a pure-tone average (PTA), the treatment was interrupted.
Measurement of outcomes
The pure-tone audiometry was performed again on the discharge day. The hearing levels on the admitted day (before treatment) and the discharge day were considered the pre-treatment hearing and post-treatment hearing, respectively. The hearing gain was the difference value of pre-treatment hearing and post-treatment hearing. We used Siegel's criteria to evaluate hearing recovery (14): (1) no improvement: the hearing gain is <15 dB; (2) slight improvement: final hearing level was poorer than 45 dB with a hearing gain over 15 dB; (3) partial recovery: final hearing level was from 25 to 45 dB with a hearing gain more than 15 dB; and (4) complete recovery: final hearing level was better than 25 dB. All comparison was made using the average thresholds at 500, 1,000, and 2,000 Hz (14). We also used the Chinese Medical Association of Otolaryngology (CMAO) criteria (12) when grading hearing recovery: (1) complete recovery: the hearing thresholds at the influenced frequencies return to the normal level (within 20 dB), or the same hearing level of the contralateral ear; (2) excellent recovery: the average of hearing gains at the influenced frequencies is ≥30 dB; (3) partial recovery: the average of hearing gains at the influenced frequencies is ≥15 dB but <30 dB; and (4) no recovery: the average of hearing gains at the influenced frequencies is <15 dB. As for flat-type and total-deafness SSNHL patients, the calculation involved hearing thresholds at all frequencies. Overall, effective rates (including complete recovery, partial recovery, and a slight improvement for Siegel's criteria; including complete recovery, excellent recovery, and partial recovery for the CMAO criteria) were calculated. Hearing gain at each frequency for flat-type and total-deafness patients was recorded as well.
Potential confounders
The following data were collected as covariates: age, gender, course of the disease, type of the pre-treatment audiogram, history of hypertension and diabetes, pre-treatment platelet and fibrinogen level, use of steroids, and use of hyperbaric oxygen therapy (HBOT). The use of steroids was categorized as systemic corticosteroids alone, intratympanic corticosteroids alone, and together. Systemic corticosteroids were prednisone (≤ 60 mg/day, oral) or dexamethasone (10 mg/day, intravenous administration), full dose for 5 days and then halved, with a total duration of not more than 10 days. Intratympanic corticosteroids were dexamethasone (5 mg), which was injected every other day, three to five times in total. HBOT was conducted routinely twice a day, normally 10–14 times in total, with no more than 20 times.
Statistical analysis
Continuous variables were described as mean with standard deviation (SD) for normally distributed variables and median with an interquartile range (IQR) for non-normally distributed variables. Categorical variables were shown as numbers and percentages. Propensity score matching (PSM) was performed as an adjustment of possible confounders between patients treated combined with batroxobin and patients treated without batroxobin. The matching was performed in a 1:1 ratio without replacement and with a caliper of 0.1. The scores were calculated and matched using nearest neighbor matching. All covariates were included in the propensity score model, and a logistic regression analysis was performed to calculate the propensity score as a logistic model. The absolute value of the standardized difference (ASD) was used to evaluate the balance between the batroxobin group and the non-batroxobin group before and after PSM. This value was set at <0.1 to balance the two groups. We also use the distribution of propensity scores of the two groups to reflect whether the two groups are balanced. The more similar the patterns are between the treated group and the control group, the better these two groups are balanced. After confirming that the two groups were well-balanced, logistic regression analyses were performed for the complete recovery rate and overall effective rate in both the entire cohort and the propensity score-matched cohort. Subsequently, sensitivity analysis was conducted as follows: the abovementioned matched analysis using propensity scores, applying the same covariates and matching algorithm detailed earlier, was performed in flat-type and total-deafness SSNHL patients. We also conducted a paired t-test to compare hearing gain at each frequency in flat-type and total-deafness SSNHL patients between the batroxobin and non-batroxobin groups. A p-value of <0.05 was considered to be statistically significant. SPSS (version 23.0, IBM) and R (version 3.1.0, R Foundation for Statistical Computing) were utilized for statistical analysis. GraphPad Prism (version 8.0.2, GraphPad Software) was used for drawing bar charts.
Results
SSNHL patients
During the study period, 657 patients with SSNHL were admitted to our department. We excluded patients who did not meet our enrolled criteria. Finally, a total of 274 patients were included in the study: 148 for the batroxobin group and 126 for the non-batroxobin group. After PSM, a total of 162 patients (81 in each group) were included in the analysis (Figure 1). Baseline characteristics of the two groups before and after PSM are presented in Table 1. The two groups were well-balanced after PSM because all ASD values between the two groups were <0.1. The distributions of the standardized difference (SD) before and after PSM are presented in Supplementary Figure S3. After PSM, the distribution became more concentrated, being ~ 0. This indicated good balance, which was accorded with the results in Table 1. Moreover, the distribution of the propensity scores of the two groups became more similar after PSM (Supplementary Figure S1). This also exhibited that the two groups were balanced. The hospitalized treatment of SSNHL lasted for 7–10 days in our study. Once the hospitalized treatment was completed, the patients would be discharged the next day.
Figure 1.
Flowchart depicting patient selection.
Table 1.
Comparison of baseline characteristics between the batroxobin group and the non-batroxobin group in the entire cohort.
| Characteristics | Entire cohort (n = 274) | PS-matched group (n = 162) | ||||
|---|---|---|---|---|---|---|
| Batroxobin group (n = 148) | Non-batroxobin group (n = 126) | ASD | Batroxobin group (n = 81) | Non-batroxobin group (n = 81) | ASD | |
| Age, year | ||||||
| ≤ 40 | 68 (45.9%) | 70 (55.6%) | 43 (53.1%) | 44 (54.3%) | ||
| >40 | 80 (54.1%) | 56 (44.4%) | 0.192 | 38 (46.9%) | 37 (45.7%) | 0.025 |
| Gender | ||||||
| Male | 68 (45.9%) | 58 (46.0%) | 36 (44.4%) | 38 (46.9%) | ||
| Female | 80 (54.1%) | 68 (54.0%) | 0.002 | 45 (55.6%) | 43 (53.1%) | 0.049 |
| Course of disease | ||||||
| ≤ 7 days | 111 (75.0%) | 90 (71.4%) | 62 (76.5%) | 64 (79.0%) | ||
| >7 days | 37 (25.0%) | 36 (28.6%) | 0.082 | 19 (23.5%) | 17 (21.0%) | 0.057 |
| Type of pretreatment audiogram | ||||||
| Low-frequency | 13 (8.8%) | 24 (19.1%) | 10 (12.3%) | 12 (14.8%) | ||
| High-frequency | 11 (7.4%) | 8 (6.3%) | 0.043 | 3 (3.7%) | 5 (6.2%) | 0.097 |
| Flat | 62 (41.9%) | 56 (44.4%) | 0.104 | 39 (48.2%) | 36 (44.4%) | 0.074 |
| Total-deafness | 62 (41.9%) | 38 (30.2%) | 0.242 | 29 (35.8%) | 28 (34.6%) | 0.026 |
| History of hypertension | ||||||
| No | 130 (87.8%) | 112 (88.9%) | 74 (91.4%) | 72 (88.9%) | ||
| Yes | 18 (12.2%) | 14 (11.1%) | 0.032 | 7 (8.6%) | 9 (11.1%) | 0.075 |
| History of diabetes | ||||||
| No | 135 (91.2%) | 119 (94.4%) | 76 (93.8%) | 75 (92.6%) | ||
| Yes | 13 (8.8%) | 7 (5.6%) | 0.114 | 5 (6.2%) | 6 (7.4%) | 0.043 |
| Pretreatment plasma platelet count, 10 9 /L | ||||||
| ≤ 200 | 65 (43.9%) | 56 (44.4%) | 40 (49.4%) | 37 (45.7%) | ||
| >200 | 83 (56.1%) | 70 (55.6%) | 0.011 | 41 (50.6%) | 44 (54.3%) | 0.074 |
| Pretreatment fibrinogen level, g/L | ||||||
| ≤ 3 | 129 (87.2%) | 85 (67.5%) | 66 (81.5%) | 65 (80.2%) | ||
| >3 | 19 (12.8%) | 41 (32.5%) | 0.587 | 15 (18.5%) | 16 (19.8%) | 0.037 |
| Usage of steroids | ||||||
| Systemic alone | 82 (55.4%) | 112 (88.9%) | 67 (82.7%) | 67 (82.7%) | ||
| Intratympanic alone | 5 (3.4%) | 1 (0.8%) | 0.142 | 2 (2.5%) | 1 (1.2%) | 0.091 |
| Systemic and intratympanic | 61 (41.2%) | 13 (10.3%) | 0.471 | 12 (14.8%) | 13 (16.1%) | 0.034 |
| Use of HBOT | ||||||
| No | 22 (14.9%) | 22 (17.5%) | 10 (12.3%) | 11 (13.6%) | ||
| Yes | 126 (85.1%) | 104 (82.5%) | 0.073 | 71 (87.7%) | 70 (86.4%) | 0.035 |
PS, propensity score; ASD, the absolute value of the standardized mean difference; HBOT, hyperbaric oxygen therapy.
Hearing outcomes after propensity score matching
In the propensity score-matched cohort (Table 2), according to Siegel's criteria, the complete recovery rate was 30.9% (25 of 81) for the non-batroxobin group and 24.7% (20 of 81) for the batroxobin group. Based on the CMAO criteria, the complete recovery rate was 27.2% (22 of 81) for the non-batroxobin group and 24.7% (20 of 81) for the batroxobin group. The overall effective rate was the same for Siegel's criteria and the CMAO criteria. The overall effective rate of the non-batroxobin group was 58.0% (44 of 78), whereas that of the batroxobin group was 50.6% (41 of 81). The logistic regression analysis of the propensity score-matched cohort indicated that both the complete recovery rate [Siegel's criteria, OR: 0.734, 95% CI: 0.368–1.466, p = 0.381; CMAO criteria, OR: 0.879, 95% CI: 0.435–1.777, p = 0.720] and the overall effective rates [Siegel's criteria and CMAO criteria, OR: 0.741, 95% CI: 0.399–1.378, p = 0.344] were of no significant difference between the two treatment groups.
Table 2.
Complete recovery rate and overall effective rate before and after propensity score matching in the entire.
| Variables | Complete recovery rate | OR (95% CI) | p-value | Overall effective rate | OR (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Before PSM (entire cohort) | ||||||
| Siegel's criteria | ||||||
| Non-batroxobin group | 43 of 126 (34.1%) | 1 | 0.007 | 79 of 126 (62.7%) | 1 | 0.011 |
| Batroxobin group | 29 of 148 (19.6%) | 0.470 (0.272–0.814) | 70 of 148 (47.3%) | 0.534 (0.329–0.867) | ||
| CMAO criteria | ||||||
| Non-batroxobin group | 38 of 126 (30.2%) | 1 | 0.022 | 79 of 126 (62.7%) | 1 | 0.011 |
| Batroxobin group | 27 of 148 (18.2%) | 0.517 (0.294–0.909) | 70 of 148 (47.3%) | 0.534 (0.329–0.867) | ||
| After PSM (PS-matched cohort) | ||||||
| Siegel's criteria | ||||||
| Non-batroxobin group | 25 of 81 (30.9%) | 1 | 0.381 | 47 of 81 (58.0%) | 1 | 0.344 |
| Batroxobin group | 20 of 81 (24.7%) | 0.734 (0.368–1.466) | 41 of 81 (50.6%) | 0.741 (0.399–1.378) | ||
| CMAO criteria | ||||||
| Non-batroxobin group | 22 of 81 (27.2%) | 1 | 0.720 | 47 of 81 (58.0%) | 1 | 0.344 |
| Batroxobin group | 20 of 81 (24.7%) | 0.879 (0.435–1.777) | 41 of 81 (50.6%) | 0.741 (0.399–1.378) | ||
PSM, propensity score matching; PS, propensity score; OR, odds ratio; CI, confidence interval; CMAO, Chinese Medical Association of Otolaryngology.
Sensitivity analysis
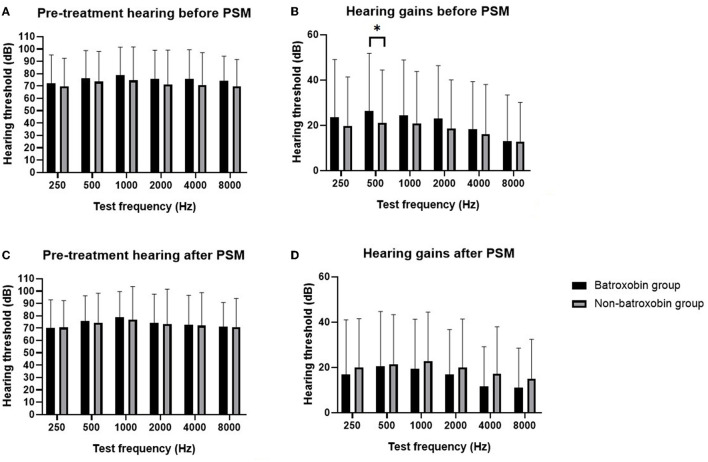
In the entire cohort, 218 patients were with flat-type or total-deafness pre-treatment audiograms. PSM was used to create two groups of 61 patients each. One group was for the batroxobin group, and the other one was for the non-batroxobin group, all with flat-type or total-deafness SSNHL. These groups were well-matched in the baseline characteristics (Table 3). The distribution of SD showed that after PSM, the SD value centered at ~ 0 (Supplementary Figure S4). This implied the balance between the two groups, which was consistent with the results in Table 3. The distribution of the propensity scores of the two groups also became similar (shown in Supplementary Figure S2). As presented in Table 4, after PSM, according to Siegel's criteria, the complete recovery rate was 16.4% (10 of 61) for the non-batroxobin group and 14.8% (9 of 61) for the batroxobin group. Based on the CMAO criteria, the complete recovery rate was 13.1% (eight of 61) for the non-batroxobin group and 14.8% (nine of 61) for the batroxobin group. The overall effective rate of the non-batroxobin group was 54.1% (33 of 66) while that of the batroxobin group was 42.6% (26 of 61) for both Siegel's criteria and the CMAO criteria. The logistic regression analysis of the propensity score-matched cohort indicated that both the complete recovery rate [Siegel's criteria, OR: 0.883, 95% CI: 0.331–2.352, p = 0.803; CMAO criteria, OR: 1.147, 95% CI: 0.411–3.200, p = 0.794] and the overall effective rate [Siegel's criteria and the CMAO criteria, OR: 0.630, 95% CI 0.308–1.288, p = 0.206] were not significantly different between the two groups. Figure 2 shows the pre-treatment hearing levels and post-treatment hearing gains at each frequency for the two groups (the data of Figure 2 are presented in Supplementary Table S1). In the propensity score-marched cohort, there was no significant difference in the pre-treatment hearing levels and the post-treatment hearing gains between the batroxobin group and the non-batroxobin group.
Table 3.
Comparison of baseline characteristics between the batroxobin group and the non-batroxobin group in the sensitivity analysis.
| Characteristics | Flat-type and total-deafness SSNHL patients (n = 218) | PS-matched group (n = 122) | ||||
|---|---|---|---|---|---|---|
| Batroxobin group (n = 124) | Non-batroxobin group (n = 94) | ASD | Batroxobin group (n = 61) | Non-batroxobin group (n = 61) | ASD | |
| Age, year | ||||||
| ≤ 40 | 52 (41.9%) | 46 (48.9%) | 33 (54.1%) | 32 (52.5%) | ||
| >40 | 72 (58.1%) | 48 (51.1%) | 0.141 | 28 (45.9%) | 29 (47.5%) | 0.033 |
| Gender | ||||||
| Male | 59 (47.6%) | 48 (51.1%) | 30 (49.2%) | 32 (52.5%) | ||
| Female | 65 (52.4%) | 46 (48.9%) | 0.069 | 31 (50.8%) | 29 (47.5%) | 0.065 |
| Course of disease | ||||||
| ≤ 7 days | 90 (72.6%) | 62 (66.0%) | 44 (72.1%) | 44 (72.1%) | ||
| >7 days | 34 (27.4%) | 32 (34.0%) | 0.148 | 17 (27.9%) | 17 (27.9%) | 0 |
| Type of pretreatment audiogram | ||||||
| Flat | 62 (50.0%) | 56 (59.6%) | 34 (55.7%) | 36 (59.0%) | ||
| Total-deafness | 62 (50.0%) | 38 (40.4%) | 0.191 | 27 (44.3%) | 25 (41.0%) | 0.065 |
| History of hypertension | ||||||
| No | 107 (86.3%) | 82 (87.2%) | 54 (88.5%) | 52 (85.2%) | ||
| Yes | 17 (13.7%) | 12 (12.8%) | 0.027 | 7 (11.5%) | 9 (14.8%) | 0.095 |
| History of diabetes | ||||||
| No | 112 (90.3%) | 88 (93.6%) | 57 (93.4%) | 57 (93.4%) | ||
| Yes | 12 (9.7%) | 6 (6.4%) | 0.111 | 4 (6.6%) | 4 (6.6%) | 0 |
| Pretreatment plasma platelet count, 10 9 /L | ||||||
| ≤ 200 | 60 (48.4%) | 46 (48.9%) | 30 (49.2%) | 31 (50.8%) | ||
| >200 | 64 (51.6%) | 48 (51.1%) | 0.011 | 31 (50.8%) | 30 (49.2%) | 0.033 |
| Pretreatment fibrinogen level, g/L | ||||||
| ≤ 3 | 108 (87.1%) | 64 (68.1%) | 52 (85.2%) | 51 (83.6%) | ||
| >3 | 16 (12.9%) | 30 (31.9%) | 0.533 | 9 (14.8%) | 10 (16.4%) | 0.049 |
| Usage of steroids | ||||||
| Systemic alone | 69 (55.7%) | 81 (86.1%) | 49 (80.3%) | 49 (80.3%) | ||
| Intratympanic alone | 4 (3.2%) | 1 (1.1%) | 0.149 | 2 (3.3%) | 1 (1.6%) | 0.096 |
| Systemic and intratympanic | 51 (41.1%) | 12 (12.8%) | 0.609 | 10 (16.4%) | 11 (18.1%) | 0.043 |
| Use of HBOT | ||||||
| No | 17 (13.7%) | 11 (11.7%) | 7 (11.5%) | 4 (6.6%) | ||
| Yes | 107 (86.3%) | 83 (88.3%) | 0.058 | 54 (88.5%) | 57 (93.4%) | 0.098 |
PS, propensity score; ASD, the absolute value of the standardized mean difference; HBOT, hyperbaric oxygen therapy.
Table 4.
Complete recovery rate and overall effective rate before and after propensity score matching in the sensitivity analysis.
| Variables | Complete recovery rate | OR (95% CI) | p-value | Overall effective rate | OR (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Before PSM (flat-type and total-deafness SSNHL patients' cohort) | ||||||
| Siegel's criteria | ||||||
| Non-batroxobin group | 15 of 94 (16.0%) | 1 | 0.317 | 50 of 94 (53.2%) | 1 | 0.127 |
| Batroxobin group | 14 of 124 (11.3%) | 0.670 (0.306–1.468) | 53 of 124 (42.7%) | 0.657 (0.383–1.126) | ||
| CMAO criteria | ||||||
| Non-batroxobin group | 10 of 94 (10.6%) | 1 | 0.879 | 50 of 94 (53.2%) | 1 | 0.127 |
| Batroxobin group | 13 of 124 (10.5%) | 1.069 (0.453–2.526) | 53 of 124 (42.7%) | 0.657 (0.383–1.126) | ||
| After PSM (PS-matched cohort) | ||||||
| Siegel's criteria | ||||||
| Non-batroxobin group | 10 of 61 (16.4%) | 1 | 0.803 | 33 of 61 (54.1%) | 1 | 0.206 |
| Batroxobin group | 9 of 61 (14.8%) | 0.883 (0.331–2.352) | 26 of 61 (42.6%) | 0.630 (0.308–1.288) | ||
| CMAO criteria | ||||||
| Non-batroxobin group | 8 of 61 (13.1%) | 1 | 0.794 | 33 of 61 (54.1%) | 1 | 0.206 |
| Batroxobin group | 9 of 61 (14.8%) | 1.147 (0.411–3.200) | 26 of 61 (42.6%) | 0.630 (0.308–1.288) | ||
PSM, propensity score matching; PS, propensity score; OR, odds ratio; CI, confidence interval; CMAO, Chinese Medical Association of Otolaryngology.
Figure 2.
Comparison of pre-treatment hearing and hearing gains between the batroxobin group and the non-batroxobin group in the sensitivity analysis. (A) Pre-treatment hearing level of batroxobin group and non-batroxobin group before PSM. (B) Hearing gains of batroxobin group and non-batroxobin group before PSM. (C) Pre-treatment hearing level of batroxobin group and non-batroxobin group after PSM. (D) Hearing gains of batroxobin group and non-batroxobin group after PSM. The black bar represents the batroxobin group and the gray bar represents the non-batroxobin group, * stands for a p-value of <0.05.
Discussion
Sudden sensorineural hearing loss is an urgent situation for the otolaryngology department. Patients are usually concerned about permanent hearing loss. Meanwhile, losing the sense of hearing can cause great inconvenience for patients. Impaired cochlea microcirculation was proposed to be a pathogenic factor (15–17). SSNHL is also associated with an increased risk of stroke and myocardial infarction (18–20). Batroxobin, which can increase cochlear blood flow (21), was suggested to be applied in the treatment of SSNHL (2), especially for flat-type and total-deafness SSNHL (12, 22). We used both Siegel's criteria and the CMAO criteria to grade hearing recovery. However, our results indicated that no matter the complete recovery rates or the overall effective rates were with no significant difference between the batroxobin group and the non-batroxobin group in the entire cohort after PSM by both criteria. For patients with flat-type and total-deafness SSNHL, the complete recovery rates and the overall effective rates by the two criteria were still without significant differences between the two treatment groups after PSM. Moreover, no meaningful difference was found between the two treatment groups in the post-treatment hearing gains at all frequencies after PSM.
Studies have demonstrated that no obvious difference was found in fibrinogen level (23, 24), activated partial thromboplastin time, and prothrombin time (25) between SSNHL patients and normal people. The hearing recovery of SSNHL patients was not related to the fibrinogen level (26). These findings may lead to negative results in our study. Older age, a longer course of disease (27, 28), total-deafness type of pre-treatment audiogram (29), and history of diabetes (30) have been considered adverse factors of hearing recovery for SSNHL patients. The use of steroids or HBOT (31) can also affect hearing outcomes. Our study has used PSM to balance the abovementioned potential confounders. Meanwhile, we also adjusted other underlining confounders such as gender, history of hypertension, and pre-treatment plasma platelet count. Before PSM, the complete recovery rates and the overall effective rates of the batroxobin group were significantly lower in the entire cohort (Table 2). According to Table 1, patients in the batroxobin group were older, and more patients suffering from diabetes and total deafness type of SSNHL. After using PSM to balance these possible confounders, the complete recovery rates and the overall effective rates became similar between the two groups (Table 2). Before PSM, hearing gain at 500 Hz of the batroxobin group was significantly higher in the flat-type and total-deafness SSNHL patients (Figure 2). However, patients in the batroxobin group were often treated together with intratympanic steroids or intratympanic and systematic steroids, while patients in the non-batroxobin group were often treated with systematic steroids alone (Table 3). It has been demonstrated that intratympanic steroids were better for PTA improvement than systematic steroids (32). Thus, the different applications of steroids between the two groups could be a confounding factor to the results. After PSM, the difference in hearing gains between the two groups became statistically unmeaningful (Figure 2). These indicated the importance and the advantage of using PSM. Kubo et al. once proposed that defibrinogenation therapy can achieve better hearing outcomes than steroid therapy (33). However, he did not rule out confounding factors such as age, and audiogram patterns. A randomized controlled trial in China (34) showed that batroxobin + Ginaton + corticosteroid had the highest efficiency in the treatment of total-deafness SSNHL patients, compared with batroxobin alone, batroxobin + Ginaton, and Ginaton + corticosteroid. However, among the four treatment groups, the two groups treated with corticosteroid were both with higher recovery rates, and the other two groups treated with batroxobin did not do so well. Therefore, better hearing outcomes in the trial may mainly result from the use of corticosteroids instead of batroxobin. In addition, a clinical trial has not evaluated hearing gains at each frequency for total-deafness SSNHL patients. The possible complications of batroxobin treatment should also be taken into account. Dizziness, nausea, and purpura on the skin have been reported in patients after treatment with batroxobin (13). There could be risks for hemorrhagic tendency as well.
To the best of our knowledge, our study was the first one that used PSM to analyze the effect of batroxobin in the treatment of SSNHL. PSM can partly eliminate the discrepancies between two cohorts of patients. After PSM, the baseline information becomes compatible. Using PSM can help researchers to avoid potential bias (35). The use of batroxobin in the treatment of SSNHL was controversial. We have added more evidence for medication options when treating patients with SSNHL.
There were still several limitations to this study. First of all, the number of cases was limited. The patients admitted were only in one single institution, which limited the generalization of our findings to other populations. Moreover, because of its retrospective design, there could be selection biases. Finally, we only assessed the hearing improvement right after therapy. As for the long-term hearing outcomes and complications after treatment, future studies are still needed.
Conclusion
There was no significant difference in short-term hearing outcomes between treatment combined with batroxobin and treatment without batroxobin in SSNHL patients by both Siegel's criteria and the CMAO criteria after PSM. Future studies for better therapy regimens of SSNHL are still needed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee of Xiangya Hospital, Central South University. The Ethics Committee waived the requirement of written informed consent for participation.
Author contributions
XWu, MJ, and HH: study design, data analysis and interpretation, and draft writing. XWu, MJ, HH, HW, and XWa: data collection. LM, CH, XC, LJ, and HW: technical and material support. All authors have read and approved the final manuscript.
Funding Statement
This study was supported by the Natural Science Foundation of Hunan Province (No. 2021JJ31108).
Abbreviations
SSNHL, sudden sensorineural hearing loss; CMAO, the Chinese Medical Association of Otolaryngology; PSM, propensity score matching; AAO-HNSF, the American Academy of Otolaryngology-Head and Neck Surgery Foundation; dB, decibels; PTA, pure-tone average; HBOT, hyperbaric oxygen therapy; SD, standard deviation; IQR, interquartile range; ASD, the absolute value of the standardized mean difference; PS, propensity score; OR, odds ratio; CI, confidence interval.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1102297/full#supplementary-material
Distribution of propensity scores before and after propensity score matching in the entire cohort. Treated as the batroxobin group, control as the non-batroxobin group.
Distribution of propensity scores before and after propensity score matching in flat-type and total-deafness SSNHL patients. Treated as the batroxobin group, control as the non-batroxobin group.
Distribution of standardized difference before and after propensity score matching in the entire cohort.
Distribution of standardized difference before and after propensity score matching in flat-type and total-deafness SSNHL patients.
Comparison of pre-treatment hearing and hearing gains between the batroxobin group and the non-batroxobin group in the sensitivity analysis.
References
- 1.Chandrasekhar SS, Tsai Do BS, Schwartz SR, Bontempo LJ, Faucett EA, Finestone SA, et al. Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. (2019) 161:S1–45. 10.1177/0194599819859885 [DOI] [PubMed] [Google Scholar]
- 2.Michel O, Deutsche Gesellschaft für Hals-Nasen-Ohren-Heilkunde, Kopf- und Hals-Chirurgie. Die aktuell gefasste Leitlinie “Hörsturz” (Akuter idiopathischer sensorineuraler Hörverlust) [The revised version of the german guidelines “sudden idiopathic sensorineural hearing loss”]. Laryngorhinootologie. (2011) 90:290–3. German. 10.1055/s-0031-1273721 [DOI] [PubMed] [Google Scholar]
- 3.Chau JK, Lin JR, Atashband S, Irvine RA, Westerberg BD. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope. (2010) 120:1011–21. 10.1002/lary.20873 [DOI] [PubMed] [Google Scholar]
- 4.Koçak HE, Filiz Acipayam AS, Acipayam H, Çakil Erdogan B, Alakhras WME, Kiral MN, et al. Microvascular dysfunction affects the development and prognosis of sudden idiopathic hearing loss. Clin Otolaryngol. (2017) 42:602–7. 10.1111/coa.12780 [DOI] [PubMed] [Google Scholar]
- 5.Jung SY, Shim HS, Hah YM, Kim SH, Yeo SG. Association of metabolic syndrome with sudden sensorineural hearing loss. JAMA Otolaryngol Head Neck Surg. (2018) 144:308–14. 10.1001/jamaoto.2017.3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waheed H, Moin SF, Choudhary MI. Snake venom: from deadly toxins to life-saving therapeutics. Curr Med Chem. (2017) 24:1874–91. 10.2174/0929867324666170605091546 [DOI] [PubMed] [Google Scholar]
- 7.Oya R, Horii A, Akazawa H, Osaki Y, Inohara H. Prognostic predictors of sudden sensorineural hearing loss in defibrinogenation therapy. Acta Otolaryngol. (2016) 136:271–6. 10.3109/00016489.2015.1104723 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Furukawa M, Kumagai M, Takahashi E, Matsuura K, Katori Y, et al. Defibrinogenation therapy for idiopathic sudden sensorineural hearing loss in comparison with high-dose steroid therapy. Acta Otolaryngol. (2003) 123:46–50. 10.1080/0036554021000028082 [DOI] [PubMed] [Google Scholar]
- 9.Stocker K, Fischer H, Meier J. Thrombin-like snake venom proteinases. Toxicon. (1982) 20:265–73. 10.1016/0041-0101(82)90225-2 [DOI] [PubMed] [Google Scholar]
- 10.Bell WR, Jr. Defibrinogenating enzymes. Drugs. (1997) 54:18–30. 10.2165/00003495-199700543-00005 [DOI] [PubMed] [Google Scholar]
- 11.Suoqiang Z, Ning Y, Guiliang Z, Yuhua Z, He Q. Effect of retreatment on the end-stage sudden deafness. Cell Biochem Biophys. (2012) 62:403–6. 10.1007/s12013-011-9314-1 [DOI] [PubMed] [Google Scholar]
- 12.Editorial Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Society of Otorhinolaryngology Head and Neck Surgery Chinese Medical Association . Guideline of diagnosis and treatment of sudden deafness (2015). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2015) 50:443–7. [PubMed] [Google Scholar]
- 13.Jia H, Yu Z, Li X, Wang J, Ge X, Chen ZT, et al. Efficacy of intratympanic corticosteroid, intravenous batroxobin and combined treatment for sudden sensorineural hearing loss with type-2 diabetes. Acta Otolaryngol. (2019) 139:522–8. 10.1080/00016489.2019.1592221 [DOI] [PubMed] [Google Scholar]
- 14.Siegel LG. The treatment of idiopathic sudden sensorineural hearing loss. Otolaryngol Clin North Am. (1975) 8:467–73. 10.1016/S0030-6665(20)32783-3 [DOI] [PubMed] [Google Scholar]
- 15.Capaccio P, Pignataro L, Gaini LM, Sigismund PE, Novembrino C, De Giuseppe R, et al. Unbalanced oxidative status in idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. (2012) 269:449–53. 10.1007/s00405-011-1671-2 [DOI] [PubMed] [Google Scholar]
- 16.Schweinfurth JM, Cacace AT. Cochlear ischemia induced by circulating iron particles under magnetic control: an animal model for sudden hearing loss. Am J Otol. (2000) 21:636–40. [PubMed] [Google Scholar]
- 17.Randolf HB, Haupt H, Scheibe F. Cochlear blood flow following temporary occlusion of the cerebellar arteries. Eur Arch Otorhinolaryngol. (1990) 247:226–8. 10.1007/BF00178990 [DOI] [PubMed] [Google Scholar]
- 18.Lammers MJW, Young E, Westerberg BD, Lea J. Risk of stroke and myocardial infarction after sudden sensorineural hearing loss: a meta-analysis. Laryngoscope. (2021) 131:1369–77. 10.1002/lary.29237 [DOI] [PubMed] [Google Scholar]
- 19.Lin SW, Lin C, Weng SF, Lin YS. Sudden sensorineural hearing loss is correlated with an increased risk of acute myocardial infarction: a population-based cohort study. Laryngoscope. (2013) 123:2254–8. 10.1002/lary.23837 [DOI] [PubMed] [Google Scholar]
- 20.Lin HC, Chao PZ, Lee HC. Sudden sensorineural hearing loss increases the risk of stroke: a 5-year follow-up study. Stroke. (2008) 39:2744–8. 10.1161/STROKEAHA.108.519090 [DOI] [PubMed] [Google Scholar]
- 21.Kawakami M, Makimoto K, Yamamoto H, Takahashi H. The effect of batroxobin on cochlear blood flow. Acta Otolaryngol. (1992) 112:991–7. 10.3109/00016489209137500 [DOI] [PubMed] [Google Scholar]
- 22.Shiraishi T, Kubo T, Okumura S, Naramura H, Nishimura M, Okusa M, et al. Hearing recovery in sudden deafness patients using a modified defibrinogenation therapy. Acta Otolaryngol Suppl. (1993) 501:46–50. 10.3109/00016489309126213 [DOI] [PubMed] [Google Scholar]
- 23.Quaranta N, Ramunni A, Brescia P, D'Elia A, Vacca A, Ria R. Soluble intercellular adhesion molecule 1 and soluble vascular cell adhesion molecule 1 in sudden hearing loss. Otol Neurotol. (2008) 29:470–4. 10.1097/MAO.0b013e318170b650 [DOI] [PubMed] [Google Scholar]
- 24.Haubner F, Martin L, Steffens T, Strutz J, Kleinjung T. The role of soluble adhesion molecules and cytokines in sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. (2011) 144:575–80. 10.1177/0194599810394324 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Gao G, Wang L, Ma X, Yu L, Ye F. Association between the number of intratympanic steroid injections and hearing recovery in sudden sensorineural hearing loss. Front Neurol. (2021) 12:798569. 10.3389/fneur.2021.798569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Zhou H, Feng Y, Zhao Y, Wang J, Chen Z, et al. Coagulation states in patients with sudden sensorineural hearing loss evaluated by thrombo elastography. Otolaryngol Head Neck Surg. (2021) 164:1280–6. 10.1177/0194599820965240 [DOI] [PubMed] [Google Scholar]
- 27.Kang WS, Yang CJ, Shim M, Song CI, Kim TS, Lim HW, et al. Prognostic factors for recovery from sudden sensorineural hearing loss: a retrospective study. J Audiol Otol. (2017) 21:9–15. 10.7874/jao.2017.21.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huy PT, Sauvaget E. Idiopathic sudden sensorineural hearing loss is not an otologic emergency. Otol Neurotol. (2005) 26:896–902. 10.1097/01.mao.0000185071.35328.6d [DOI] [PubMed] [Google Scholar]
- 29.Chang NC, Ho KY, Kuo WR. Audiometric patterns and prognosis in sudden sensorineural hearing loss in southern Taiwan. Otolaryngol Head Neck Surg. (2005) 133:916–22. 10.1016/j.otohns.2005.09.018 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Jiang Q, Wu X, Xie S, Feng Y, Sun H. The influence of metabolic syndrome on the prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol. (2019) 40:994–7. 10.1097/MAO.0000000000002352 [DOI] [PubMed] [Google Scholar]
- 31.Joshua TG, Ayub A, Wijesinghe P, Nunez DA. Hyperbaric oxygen therapy for patients with sudden sensorineural hearing loss: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. (2022) 148:5–11. 10.1001/jamaoto.2021.2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiang Q, Wu X, Yang T, Yang C, Sun H. A comparison between systemic and intratympanic steroid therapies as initial therapy for idiopathic sudden sensorineural hearing loss: a meta-analysis. Acta Otolaryngol. (2017) 137:598–605. 10.1080/00016489.2016.1260157 [DOI] [PubMed] [Google Scholar]
- 33.Kubo T, Matsunaga T, Asai H, Kawamoto K, Kusakari J, Nomura Y, et al. Efficacy of defibrinogenation and steroid therapies on sudden deafness. Arch Otolaryngol Head Neck Surg. (1988) 114:649–52. 10.1001/archotol.1988.01860180063031 [DOI] [PubMed] [Google Scholar]
- 34.Zheng H, Dai QQ, Zhou L, Feng NY, Qiu JH, Chen Y, et al. Multi-center study on the treatment of sudden total deafness. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2013) 48:379–84. Chinese. [PubMed] [Google Scholar]
- 35.Bi G, Liang J, Shan G, Zhan C. Propensity score matching for bias reduction in genomic profiling. J Clin Oncol. (2022) 40:1259–60. 10.1200/JCO.21.02449 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of propensity scores before and after propensity score matching in the entire cohort. Treated as the batroxobin group, control as the non-batroxobin group.
Distribution of propensity scores before and after propensity score matching in flat-type and total-deafness SSNHL patients. Treated as the batroxobin group, control as the non-batroxobin group.
Distribution of standardized difference before and after propensity score matching in the entire cohort.
Distribution of standardized difference before and after propensity score matching in flat-type and total-deafness SSNHL patients.
Comparison of pre-treatment hearing and hearing gains between the batroxobin group and the non-batroxobin group in the sensitivity analysis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.