Abstract
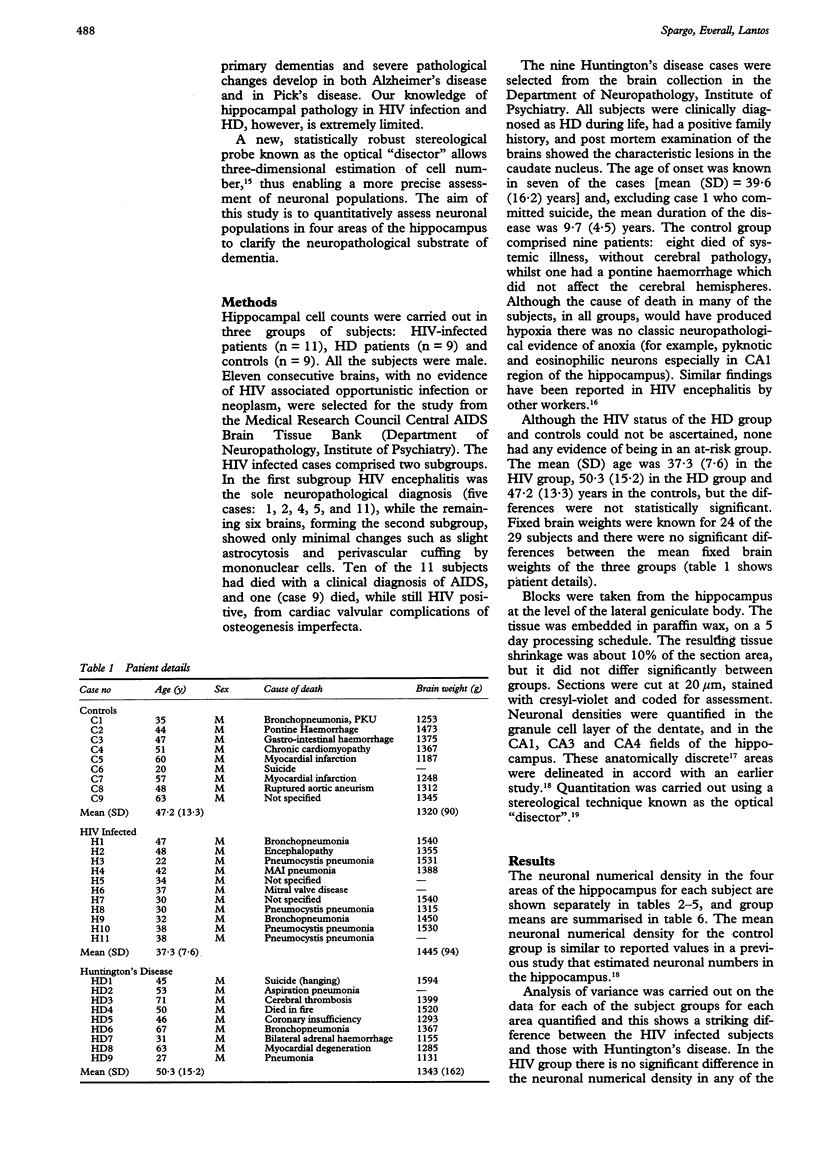
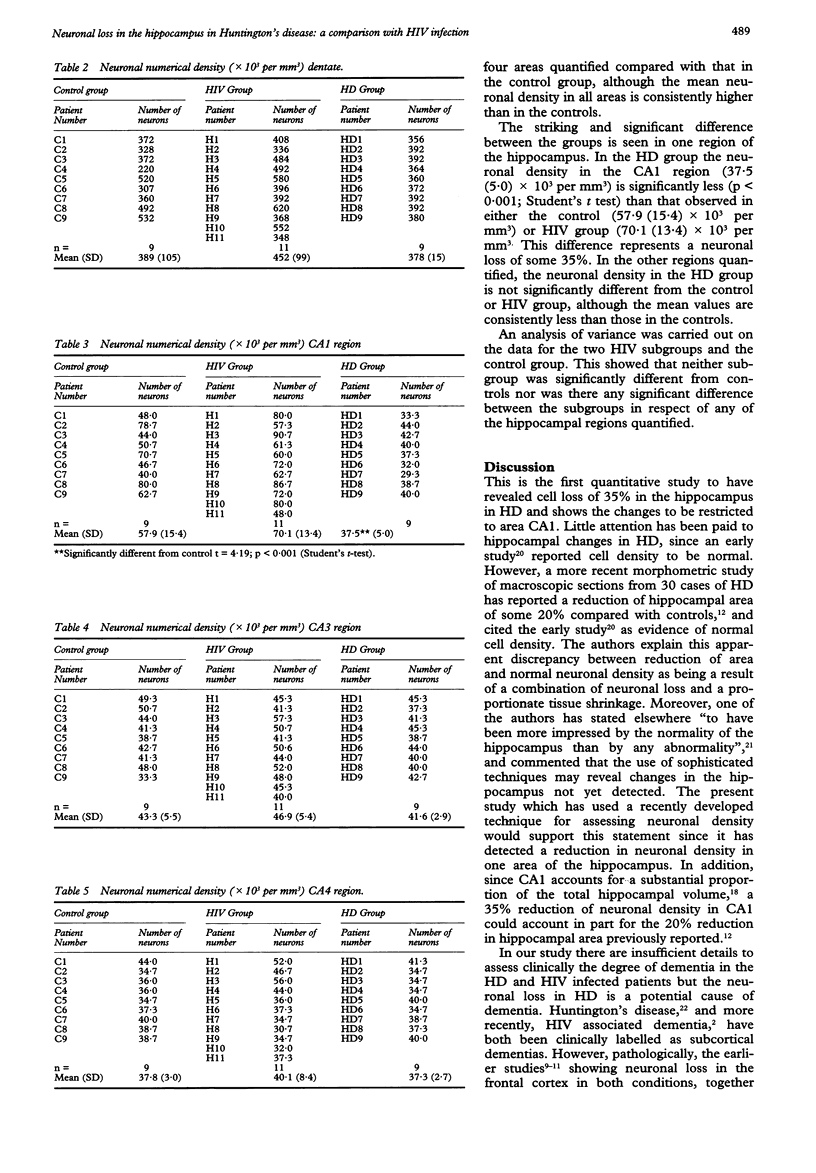
The hippocampus is usually affected in primary dementias and the pathological changes may be severe. Knowledge of hippocampal pathology in HIV infection and Huntington's disease (HD), however, is extremely limited. A stereological technique (the optical "disector") has been used to assess neuronal populations in four areas of the hippocampus in 11 patients with HIV infection and in nine patients with HD. The HIV patients died without opportunistic infections or neoplasms affecting the brain; they had HIV encephalitis or minimal changes. The HD cases were all clinically diagnosed, had a positive family history and showed the characteristic lesions in the caudate nucleus. The neuronal counts were compared with those in nine controls. In the granule cell layer of the dentate, CA3 and CA4, there was no significant difference in the neuronal numerical density between the three groups. A striking difference between the HIV and HD groups was seen in the CA1 region. The neuronal numerical density in the CA1 area was significantly lower in the HD patients than in either the HIV patients or the controls (mean (SD) 37.5 (5.0); 70.1 (13.4); 57.9 (15.4) x 10(3) per mm3, p < 0.001 (Students' t test)). This difference represents a neuronal loss of 35%. In all four hippocampal areas the neuronal density was higher in the HIV group than in the controls but the differences were not significant and can be explained by the higher average age of the control group. These findings contribute to the understanding of the mechanism of dementia in both AIDS and in Huntington's disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball M. J. Topographic distribution of neurofibrillary tangles and granulovacuolar degeneration in hippocampal cortex of aging and demented patients. A quantitative study. Acta Neuropathol. 1978 May 24;42(2):73–80. doi: 10.1007/BF00690970. [DOI] [PubMed] [Google Scholar]
- Ball M. J. Topography of Pick inclusion bodies in hippocampi of demented patients. A quantitative study. J Neuropathol Exp Neurol. 1979 Nov;38(6):614–620. doi: 10.1097/00005072-197911000-00006. [DOI] [PubMed] [Google Scholar]
- Budka H., Costanzi G., Cristina S., Lechi A., Parravicini C., Trabattoni R., Vago L. Brain pathology induced by infection with the human immunodeficiency virus (HIV). A histological, immunocytochemical, and electron microscopical study of 100 autopsy cases. Acta Neuropathol. 1987;75(2):185–198. doi: 10.1007/BF00687080. [DOI] [PubMed] [Google Scholar]
- Budka H. Neuropathology of human immunodeficiency virus infection. Brain Pathol. 1991 Apr;1(3):163–175. doi: 10.1111/j.1750-3639.1991.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Cudkowicz M., Kowall N. W. Degeneration of pyramidal projection neurons in Huntington's disease cortex. Ann Neurol. 1990 Feb;27(2):200–204. doi: 10.1002/ana.410270217. [DOI] [PubMed] [Google Scholar]
- Everall I. P., Luthert P. J., Lantos P. L. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991 May 11;337(8750):1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- Gupta S. R., Naheedy M. H., Young J. C., Ghobrial M., Rubino F. A., Hindo W. Periventricular white matter changes and dementia. Clinical, neuropsychological, radiological, and pathological correlation. Arch Neurol. 1988 Jun;45(6):637–641. doi: 10.1001/archneur.1988.00520300057019. [DOI] [PubMed] [Google Scholar]
- Lang W., Miklossy J., Deruaz J. P., Pizzolato G. P., Probst A., Schaffner T., Gessaga E., Kleihues P. Neuropathology of the acquired immune deficiency syndrome (AIDS): a report of 135 consecutive autopsy cases from Switzerland. Acta Neuropathol. 1989;77(4):379–390. doi: 10.1007/BF00687372. [DOI] [PubMed] [Google Scholar]
- London E. D., Yamamura H. I., Bird E. D., Coyle J. T. Decreased receptor-binding sites for kainic acid in brains of patients with Huntington's disease. Biol Psychiatry. 1981 Feb;16(2):155–162. [PubMed] [Google Scholar]
- Maj M. Organic mental disorders in HIV-1 infection. AIDS. 1990 Sep;4(9):831–840. doi: 10.1097/00002030-199009000-00001. [DOI] [PubMed] [Google Scholar]
- Masliah E., Ge N., Morey M., DeTeresa R., Terry R. D., Wiley C. A. Cortical dendritic pathology in human immunodeficiency virus encephalitis. Lab Invest. 1992 Mar;66(3):285–291. [PubMed] [Google Scholar]
- Mattson M. P., Guthrie P. B., Kater S. B. Intrinsic factors in the selective vulnerability of hippocampal pyramidal neurons. Prog Clin Biol Res. 1989;317:333–351. [PubMed] [Google Scholar]
- Miller A. K., Alston R. L., Mountjoy C. Q., Corsellis J. A. Automated differential cell counting on a sector of the normal human hippocampus: the influence of age. Neuropathol Appl Neurobiol. 1984 Mar-Apr;10(2):123–141. doi: 10.1111/j.1365-2990.1984.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Navia B. A., Cho E. S., Petito C. K., Price R. W. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986 Jun;19(6):525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Price R. W., Sidtis J. J., Brew B. J. AIDS dementia complex and HIV-1 infection: a view from the clinic. Brain Pathol. 1991 Apr;1(3):155–162. doi: 10.1111/j.1750-3639.1991.tb00655.x. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Foster A. C., French E. D., Whetsell W. O., Jr, Köhler C. Excitotoxic models for neurodegenerative disorders. Life Sci. 1984 Jul 2;35(1):19–32. doi: 10.1016/0024-3205(84)90148-6. [DOI] [PubMed] [Google Scholar]
- Snider W. D., Simpson D. M., Nielsen S., Gold J. W., Metroka C. E., Posner J. B. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983 Oct;14(4):403–418. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- Sterio D. C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984 May;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- West M. J., Gundersen H. J. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990 Jun 1;296(1):1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- de la Monte S. M., Vonsattel J. P., Richardson E. P., Jr Morphometric demonstration of atrophic changes in the cerebral cortex, white matter, and neostriatum in Huntington's disease. J Neuropathol Exp Neurol. 1988 Sep;47(5):516–525. doi: 10.1097/00005072-198809000-00003. [DOI] [PubMed] [Google Scholar]


