Abstract
Flagella from diverse gram-negative bacteria induce tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) synthesis by human monocytes (F. Ciacci-Woolwine, P. F. McDermott, and S. B. Mizel, Infect. Immun. 67:5176–5185, 1999). In this study, we establish that purified flagellin (FliC or FljB), the major filament protein from Salmonella enterica serovar Enteritidis, S. enterica serovar Typhimurium, and Pseudomonas aeruginosa, is an extremely potent inducer of TNF-α production by human monocytes and THP-1 myelomonocytic cells. Fifty percent of maximal TNF-α production (EC50) was obtained with 1.5 × 10−11 M flagellin (0.75 ng/ml). Mutagenesis studies revealed that the central hypervariable region of flagellin is essential for the TNF-α-inducing activity of the protein. Although less active than the wild-type protein, a Salmonella flagellin mutant composed of only the central hypervariable region retained substantial TNF-α-inducing activity at nanomolar concentrations. In contrast, the conserved amino- and carboxy-terminal regions are inactive. Mutational analysis of the hypervariable region revealed that it contains two equally active TNF-α-inducing domains. The ability of THP-1 cells to respond to purified flagellins is dramatically reduced by mild trypsin treatment of the cells. Taken together, our results demonstrate that the cytokine-inducing activity of flagellins from gram-negative bacteria results from the interaction of these proteins with high-affinity cell surface polypeptide receptors on monocytes.
A large number of studies have demonstrated that multiple components of gram-negative and gram-positive bacteria possess the ability to stimulate the release of proinflammatory cytokines from monocytes and macrophages (4). These cytokine inducers have collectively been termed bacterial modulins. Lipopolysaccharide (LPS), the best-studied bacterial modulin from gram-negative organisms, has been shown to induce the synthesis of tumor necrosis factor alpha (TNF-α) and other cytokines in vivo and in vitro. Although TNF-α plays an essential role in resistance to bacterial infections, it is also a major contributor to the deleterious effects leading to septic shock. More recently, other bacterial modulins have been identified that elicit the production of proinflammatory cytokines (4). These include lipoteichoic acid and peptidoglycan from gram-positive organisms, lipoarabinomannan from mycobacteria, lipoproteins from mycoplasmas, and porins from gram-negative organisms. We (1, 3) and others (14) have shown that flagella or small fragments of flagella from several species of gram-negative bacteria stimulate TNF-α and interleukin-1β synthesis in human peripheral blood mononuclear cells (PBMC). Using genetic and biochemical approaches, we demonstrated that the expression of the major flagellar filament subunit, flagellin, is required for cytokine induction by gram-negative organisms (1).
Gram-negative bacteria such as Escherichia coli, Salmonella enterica serovar Enteritidis, and Pseudomonas aeruginosa produce flagellins with molecular masses of approximately 40 to 60 kDa (6). For example, the salmonella flagellins have a molecular mass of approximately 50 kDa. Alignment of amino acid sequences from different gram-negative species shows a high degree of sequence similarity in the amino- and carboxy-terminal regions, comprising approximately the N-terminal 150 and C-terminal 85 residues of the protein. In contrast, the central hypervariable regions of these proteins are quite divergent. Differences in length within the hypervariable domains account for most of the variation in molecular mass among different species.
Although flagellin expression is essential for the TNF-α-inducing activity of flagella (1), it was thought possible that the role of flagellin is simply to stabilize the actual inducer and not to induce cytokine synthesis. For example, the flagellin may be required to present FliD, the flagellum cap protein. To address this question, we prepared purified recombinant flagellins from S. enterica serovar Enteritidis and S. enterica serovar Typhimurium as well as P. aeruginosa and tested each protein for TNF-α-inducing activity in cultures of PBMC and THP-1 cells, a human myelomonocytic cell line. Using deletion mutants of flagellin, we defined the region(s) of the flagellin protein required for TNF-α-inducing activity. In addition, we evaluated the possibility that flagellin induces cytokine production in monocytes via interaction with a cell surface polypeptide.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. enterica serovar Enteritidis CD5, S. enterica serovar Typhimurium SL1344, and P. aeruginosa PAO1 strains, used in this study, have been described elsewhere (3). E. coli BL21(DE3) cells were purchased from Novagen. All strains were grown in Luria-Bertani medium (Sigma Chemical Co.) at 37°C with aeration. To maintain plasmid pET29a (Novagen), kanamycin (Sigma) was added at a final concentration of 25 μg/ml. Bacterial strains were stored at −70°C in 25% glycerol.
FliC cloning and mutagenesis.
PCR oligonucleotide primers were designed to amplify wild-type fliC from serovars Enteritidis and Typhimurium and P. aeruginosa PAO and wild-type fliB from serovar Typhimurium chromosomal DNA, based on National Center for Biotechnology Information database sequences. Primers were synthesized and DNA sequences were determined at the Wake Forest University Comprehensive Cancer Center Core Facility by using ABI technology. Bacterial chromosomal DNA was prepared using cetyltrimethylammonium bromide (CTAB) as previously described (13), and 20 ng of DNA was added to each PCR mixture. The cloning primers contained terminal restriction sites to allow directional cloning between the NdeI and XhoI sites of the pET29a expression vector (Novagen) in frame with the T7 promoter and C-terminal His tag.
FliC deletion mutants were constructed using overlap extension PCR (5). In first-round PCR, DNA was amplified under standard buffer conditions (1.5 mM MgCl2, 0.1 mM (each) deoxynucleoside triphosphates, 0.5 μM oligonucleotide primers, 20 ng of template DNA, 2.5 U of Taq polymerase [Promega]) using a cloning primer containing a terminal NdeI or XhoI restriction site and one of several mutagenic primers flanking the sequence to be deleted. The PCR products were extracted with phenol-chloroform, precipitated in ethanol, and treated with mung bean nuclease for 10 min at 37°C (Promega) to remove template-independent 3′ adenine overhangs. The two PCR products (with overlapping ends) were mixed in equimolar amounts and used as the template in a second amplification procedure with the cloning primers as above. The resulting amplification products were digested with NdeI and BamHI and ligated with pET29a linearized with the same enzymes.
Plasmids were introduced into E. coli BL21(DE3) (Novagen) by electroporation using a Gene Pulser apparatus (Bio-Rad, Richmond, Calif.) and selected in the presence of kanamycin (25 μg/ml). All mutants were confirmed by sequence analysis. FliC production was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG).
Purification and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of FliC.
E. coli BL21(DE3) containing either wild-type or mutant pet29a::fliC was grown at 37°C in Luria-Bertani medium containing kanamycin (25 μg/ml) to an optical density at 595 nm of approximately 0.8. IPTG was then added to a final concentration of 1 mM, and incubation was continued for an additional 5 h at 37°C. The cells were chilled on ice and harvested by centrifugation at 5,000 × g for 15 min. Cell-free lysates were prepared in 8 M urea and purified on Ni-nitrilotriacetic acid agarose (Qiagen) as specified by the manufacturer. The purified proteins were extensively dialyzed against phosphate-buffered saline (pH 7.2). Protein concentrations were determined using the bicinchoninic protein assay (Pierce). The amount of LPS in each preparation of purified protein was determined using the E-toxate Limulus amoebocyte lysate assay (Sigma). The samples were filter sterilized and stored in aliquots at −70°C until needed.
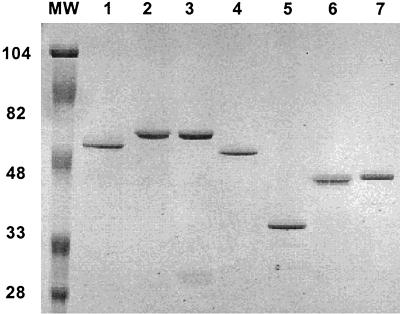
To assess protein purity, equivalent amounts of total protein were resolved by PAGE on SDS–12.5% polyacrylamide gels under reducing conditions. Protein bands were visualized by Coomassie blue staining. The purified proteins exhibited a single stained band (Fig. 1).
FIG. 1.
SDS-PAGE of purified flagellin proteins. Proteins were synthesized as fusions to a C-terminal His6 tag as described in Materials and Methods. An aliquot of each protein was loaded onto 12.5% polyacrylamide gel and subjected to SDS-PAGE. Molecular weight markers (Bio-Rad) are indicated in thousands. Lanes: 1, FliCSE; 2, FliCST; 3, FljBST; 4, FliCPAO; 5, FliC 103; 6, FliC 108; 7, FliC 109.
Cell cultures and TNF-α stimulation.
The human myelomonocytic cell line THP-1 (American Type Culture Collection) was grown at 37°C under 5% CO2 in RPMI 1640 culture medium containing 20% fetal bovine serum, 2.5 μM 2-mercaptoethanol and 50 μg of gentamicin per ml. For FliC stimulation, cells were seeded at 5 × 106/well in 24-well tissue culture plates (Costar).
PBMC were prepared from healthy donors by Isolymph density centrifugation (Gallard-Schlesinger Industries, Carle Place, N.Y.) as described previously (3). PBMC (5 × 106) were allowed to adhere in serum-free RPMI for 2 h (at 37°C under 5% CO2) in 24-well tissue culture plates. Nonadherent cells were removed by washing, and the cultures were incubated overnight in fresh culture medium containing 10% fetal bovine serum and gentamicin. Adherent cells were washed three times with prewarmed culture medium just prior to incubation with flagellins.
Dialyzed preparations of concentrated recombinant flagellin (generally greater than 200 μg/ml) contained 20 to 40 μg of endotoxin per ml. Serial flagellin dilutions were prepared in RPMI 1640 medium containing 100 μg of polymyxin B per ml and incubated at room temperature for 10 min before being added to the cells. This amount of polymyxin B was sufficient to neutralize up to 1.0 μg of endotoxin per ml (data not shown), a concentration of endotoxin that was several orders of magnitude greater than the actual amount of endotoxin present in the diluted flagellin samples that were added to the monocyte cultures (where the LPS concentration was usually lower than 0.5 ng/ml). To ensure that the polymyxin B was neutralizing the LPS activity in each assay, a control set of cells were incubated in the presence or absence of polymyxin B and a concentration of LPS that was equivalent to the largest amount present in the least dilute flagellin sample.
PBMC and THP-1 cells were incubated in serum-free RPMI medium with the various concentrations of the flagellin preparations for 4 h at 37°C under 5% CO2. Triton X-100 was then added to a final concentration of 0.5% to lyse the cells. The lysates were cleared by centrifugation (16,000 × g at 4°C) and stored at −20°C until tested for TNF-α content.
TNF-α enzyme-linked immunosorbent assay.
The amount TNF-α in total-cell lysates (cells plus medium) was determined using a commercial enzyme-linked immunosorbent assay kit (Abraxis, Hatboro, Pa.).
RESULTS
Purified recombinant flagellins induce TNF-α production in cultures of PBMC and THP-1 cells.
In previous work, we showed that partially purified flagellum preparations from S. enterica serovar Enteritidis, S. enterica serovar Typhimurium, P. aeruginosa, Yersinia enterocolitica, and E. coli stimulate TNF-α synthesis in cultures of PBMC (3). To determine if purified recombinant flagellins (FliC and FljB) were active as TNF-α inducers, we prepared and tested purified recombinant FliC and FljB from S. enterica serovar Enteritidis (FliCSE and FljBSE), S. enterica serovar Typhimurium (FliCST), and P. aeruginosa (FliCPAO) for their capacity to induce TNF-α synthesis in PBMC and THP-1 cells.
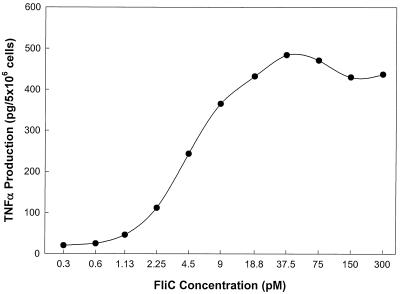
Titration experiments demonstrated that purified FliCSE induced TNF-α synthesis in a concentration-dependent manner in cultures of PBMC (Fig. 2 and Table 1) as well as THP-1 cells (Table 1). Half-maximal stimulation of TNF-α production in cultures of PBMC was achieved with a concentration (EC50) of 1.5 × 10−11 M FliCSE, with maximal stimulation occurring at 4.0 × 10−11 M. In THP-1 cells, the EC50 was similar, averaging 2.9 × 10−11 M (Table 1). These results clearly establish that purified FliC is an extraordinarily potent monocyte-activating factor. As noted in Materials and Methods, polymyxin B added to samples before testing was used to eliminate any contribution from the small amount of LPS present in the purified FliC preparation. LPS and LPS-plus-polymyxin B controls were included in every experiment to ensure that the observed TNF-α induction was solely due to the action of flagellin.
FIG. 2.
TNF-α induction in adherent human PBMC by FliCSE. Serial dilutions of FliCSE were made in complete RPMI containing polymyxin B (100 μg/ml) and were incubated with cells for 4 h. TNF-α production was measured by ELISA. Results from a representative experiment are shown. Cells receiving 0.55 ng of LPS per ml (the concentration of LPS in the most concentrated flagellin sample tested) produced 850 pg of TNF-α/5 × 106 cells. This level was reduced to 45 pg of TNFα/5 × 106 cells in the presence of polymyxin B.
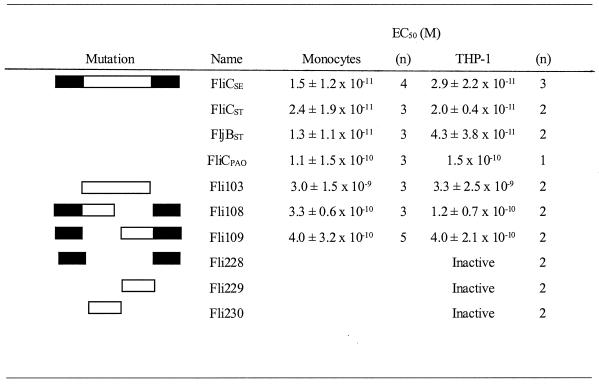
TABLE 1.
Effect of wild-type and mutant flagellins on TNF-α production by human monocytes and THP-1 cellsa
Each titration experiment involved testing TNF-α-inducing activity at a minimum of 10 concentrations. The n value refers to the number of times each sample was independently tested in a 10-point titration. Fli228, Fli229, and Fli230 were inactive up to the highest concentration tested (3.4 × 10−8 M).
In contrast to S. enterica serovar Entereditis, serovar Typhimurium is capable of synthesizing two different types of flagellin, FliC and FljB. This process is controlled at the genetic level by a regulatory recombinase, Hin, which catalyzes reversible site-specific recombination in a 1-kb segment of the Salmonella chromosome (10). The impact of this phase variation on virulence is not known. One hypothesis is that flagellar antigen switching provides a means of immune evasion, expediting bacterial colonization of luminal epithelial cells. In view of this possibility, we determined whether recombinant FliCST and FljBST from serovar Typhimurium (Fig. 1) differed in their relative capacity to stimulate TNF-α production. As shown in Table 1, FljBST and FliCST exhibited EC50s that were similar to each other as well as to that of FliCSE. Thus it is unlikely that phase variation in flagellin synthesis in serovar Typhimurium serves as a virulence mechanism to reduce macrophage-derived proinflammatory cytokine synthesis.
In a previous study, we found that P. aeruginosa strain PAO1 flagella are approximately 50% as active as Salmonella flagella in the induction of TNF-α synthesis by PBMC (3). To determine whether the observed lower potency of Pseudomonas flagella is due to an intrinsic difference between FliC proteins from Salmonella and Pseudomonas, purified recombinant FliCPAO was prepared (Fig. 1) and assayed for TNF-α-inducing activity. In cultures of PBMC and THP-1 cells, FliCPAO exhibited approximately 10% of the TNF-α-inducing activity of FliCSE (Table 1). Since the amino- and carboxy-terminal domain sequences are highly conserved between Salmonella and Pseudomonas, the observed difference in potency is likely to be due to differences in the structure or sequence of the hypervariable domain in the two proteins.
The hypervariable region of FliC may contain two independent TNF-α-inducing domains.
During flagellar biosynthesis in vivo, incorporation of subunits into the growing filament involves interaction between the conserved terminal domains of monomeric FliC (8), which is directed by hook-associated protein HAP2 (7). In the mature flagellum, the central hypervariable domain is positioned on the filament surface, with the conserved terminal domains forming the walls of the hollow core (9). Since intact flagella are active in the induction of TNF-α synthesis, we reasoned that the exposed hypervariable region may provide the site(s) that are involved in stimulating cytokine synthesis. To test this hypothesis, we analyzed the cytokine-inducing activity of a mutant FliC protein containing only the hypervariable domain (amino acids 146 to 465).
As shown in Table 1, the hypervariable peptide was also quite active as an inducer of TNF-α production in monocytes and THP-1 cells, exhibiting an EC50 of approximately 3 × 10−9 M. Although the hypervariable peptide is quite potent, it is 1 to 5% as active as the wild-type protein. The results with the hypervariable peptide are consistent with the conclusion that the hypervariable region contains the site(s) required for TNF-α-inducing activity but that optimal activity is dependent on the presence of the conserved amino- and carboxy-terminal domains. To test this hypothesis, we made a FliC construct containing only the conserved amino- and carboxy termini (Δ152–421) (Fli228). The resultant peptide was inactive up to the highest concentration tested (3.4 × 10−8 M) (Table 1). Thus, the hypervariable domain appears to be responsible for the cytokine-inducing activity of flagellin. However, the conserved termini appear to play an important role in generating an optimal conformation of the hypervariable domain.
Given the observed biologic activity of the hypervariable region, we next determined if the entire hypervariable region or only a restricted portion was required for TNF-α-inducing activity. Two deletion mutants were generated that contained the amino- and carboxy-terminal conserved domains but lacked different portions of the hypervariable region. These deletion mutants, Fli108 (Δ151–289) and Fli109 (Δ296–421) (Fig. 1), lacking either half of the hypervariable region, were constructed based on the domain designations of Wei and Joys (12).
Titration experiments revealed that Fli108 and Fli109 were equally active in cultures of PBMC and THP-1 cells (Table 1). However, these mutants reproducibly exhibited EC50s approximately 5% of that of the wild-type protein (EC50s of approximately 3 × 10−10 M). The reduced activity of these proteins was not due to nonspecific aggregation, since gel filtration profiles showed the same elution profile as wild-type protein did (data not shown). The folding of these domains appears to have a marked influence on the biologic activity of the proteins, as evidenced by the lack of activity of hypervariable domains lacking the amino and carboxy termini (Table 1). In view of these results, it is possible that the hypervariable region of FliC contains two independent TNF-α-inducing domains and that the engagement of both of these properly folded domains with receptors on the monocyte provides for a very high-avidity interaction that ultimately translates into TNF-α induction at very low concentrations of the wild-type protein.
Trypsin sensitivity of the receptor(s) for flagellins.
As a first step in the identification of the flagellin receptor on monocytes, we used the following experimental design to determine if the flagellin responsiveness of monocytes involves a cell surface polypeptide. THP-1 cells (106) were incubated with or without 10 μg of trypsin per ml for 20 min at 37°C, washed, and then incubated with 10−9 M FliC for 60 min at 4°C. The control and trypsin-treated cells were incubated at 4°C with FliC to prevent any significant synthesis of new FliC receptors during this incubation. After the FliC incubation, the cells were washed, shifted to 37°C for 4 h, and assayed for TNF-α. Trypsin treatment resulted in a 65 to 73% loss of FliC responsiveness compared to control cells (Table 2). These results are consistent with the notion that the flagellin receptor(s) is, at least in part, a polypeptide.
TABLE 2.
THP-1 sensitivity to FliC is trypsin sensitive
| Trypsin treatmenta | TNF-α production (pg/106 cells) | % Inhibitionb |
|---|---|---|
| Expt 1 | ||
| No | 309 | |
| Yes | 103 | 67 |
| Expt 2 | ||
| No | 155 | |
| Yes | 53 | 66 |
| Expt 3 | ||
| No | 360 | |
| Yes | 97 | 73 |
THP-1 cells (106) were incubated in the presence or absence of 10 μg of trypsin per ml for 20 min at 37°C, washed, and then incubated with 10−9 M FliC for 1 h at 4°C. The cells were then washed and shifted to 37°C for 4 h. The TNF-α production in each culture was measured by ELISA.
Inhibition due to trypsin treatment.
DISCUSSION
Bacteria possess a number of cell-associated and secreted molecules, termed bacterial modulins, that stimulate the release of proinflammatory mediators in the host (4). In previous work, we (1, 3) and others (14) have demonstrated that isolated flagella or fragments of isolated flagella from gram-negative bacteria elicit the production of TNF-α in cultures of adherent human PBMC and monocyte-like cell lines. Genetic complementation in a fliC deletion mutant identified flagellin as the key component of the flagella that was essential for the induction of cytokine synthesis (1). Although flagella from other gram-negative organisms, such as E. coli, P. aeruginosa, and Y. enterocolitica, also stimulated TNF-α synthesis by human monocytes, flagella from Salmonella strains were generally the most potent inducers (1).
In the present study, we demonstrate that purified Salmonella FliC and FljB are exceptionally potent inducers of TNF-α synthesis, with detectable amounts of TNF-α being induced in cells exposed to less than 1.5 × 10−12 M flagellin (Fig. 2). Although less potent than its Salmonella counterpart, FliC from P. aeruginosa was also extremely active as a TNF-α inducer (EC50 = 1 × 10−10 M). Based on our observations that (i) the LPS-nonresponsive U38 cell line and LPS-tolerant human monocytes respond to flagella or purified flagellin (1–3); (ii) trypsin treatment of flagellin preparations destroys their ability to induce cytokine production (reference 2 and unpublished observations), (iii) polymyxin B-treated flagellin retains full biologic activity (see above), and (iv) passage of flagellin preparations through an endotoxin removal column does not result in a loss of monocyte activating activity (unpublished observations), it is highly unlikely that the observed biologic activity of purified flagellin is due to contaminating LPS. Furthermore, all of the flagellin proteins were produced in the same strain of E. coli, were purified in an identical manner, and contained the same level of endotoxin (as measured by the E-toxate Limulus amoebocyte lysate assay). Thus, the differential activity of the individual flagellin forms is completely inconsistent with the notion that the observed biologic activity is due to contaminating LPS.
Sequence alignment of predicted fliC proteins from diverse gram-negative bacterial species reveals a high degree of sequence conservation in the amino- and carboxy-terminal domains, with most of the variability occurring in the central hypervariable domain (12). The hypervariable domain varies substantially in size and sequence among different bacterial species. The conserved terminal regions interact to form the internal walls of the hollow filament, and the hypervariable region forms the exterior surface. Although the hypervariable domain peptide (Fli103) retained high-level activity at nanomolar concentrations, it was approximately 1% as active as the wild-type protein (Table 1). This result indicates that full agonist activity is not conferred solely by linear sequences of amino acids in the hypervariable region but requires an appropriate secondary structure that is dependent on the conserved amino- and carboxy-terminal domains. This conclusion is supported by the observations that peptides containing only the amino and carboxy termini or only the hypervariable half sites of FliC were inactive (Table 1). Although it is formally possible that the conserved termini directly participate in the binding of flagellin to specific receptors on monocytes, we consider this possibility unlikely since intact flagella are active and the conserved termini are buried within the flagellum.
In an attempt to identify regions of FliC that might be involved in interactions with receptors on monocytes, we analyzed deletion mutants lacking either half of the hypervariable domain (Fli108 and Fli109). Not only were both mutants active, but also they exhibited similar EC50 values (Table 1). These findings are consistent with the hypothesis that the hypervariable region may contain two independent sites that are capable of eliciting cytokine production. These deletions correspond approximately to structural domains D2 and D3 of flagellin, which form the outer surface of the filament. Taken together with the mutant Fli103 findings, it is possible that two surface-exposed domains within the hypervariable region possess independent TNF-α-inducing activity but can act cooperatively to produce a very high-avidity interaction with receptors on the monocyte. It remains to be determined if the two hypervariable region binding domains of a single flagellin molecule interact with a single receptor or with two receptors. The latter possibility would provide a mechanism for enhanced monocyte responsiveness due to enhanced receptor cross-linking, a well-described mechanism for augmenting receptor signaling. Given the reduced potency of the P. aeruginosa FliC protein and its shorter hypervariable region, it is possible that this protein might possess only a single TNF-α-inducing domain.
The data presented in Table 1 and the observation that trypsin treatment of THP-1 cells markedly reduces flagellin responsiveness (Table 2) are consistent with the hypothesis that monocytes express high-affinity cell surface polypeptide receptors for flagellin. In future studies, we plan to evaluate this hypothesis using a radiolabeled form of flagellin in a receptor binding assay.
LPS-triggered cytokine production is a key event in septic shock due to gram-negative bacteria. Following this early phase of elevated cytokine synthesis and excessive proinflammatory activity, a second phase occurs that is characterized by a state of acquired immunodeficiency in which proinflammatory cytokine synthesis is dramatically reduced (11). During this latter phase, monocytes and neutrophils are LPS tolerant; i.e., they no longer respond to levels of LPS that initially triggered substantial cytokine synthesis. The extraordinary sensitivity of monocytes to flagellin may provide a mechanism for the continued response of the innate immune system to gram-negative pathogens following the induction of LPS tolerance. This conclusion is supported by our observation that LPS-tolerant human PBMC retain responsiveness to flagella (3). Thus, studies on the mechanism of action of flagellin may ultimately contribute to a more complete understanding of the host-pathogen interaction as well as the pathogenesis of sepsis due to gram-negative bacteria.
ACKNOWLEDGMENT
This study was supported by NIH grant AI38670.
REFERENCES
- 1.Ciacci-Woolwine F, Blomfield I C, Richardson S H, Mizel S B. Salmonella flagellin induces tumor necrosis factor alpha in a human promonocytic cell line. Infect Immun. 1998;66:1127–1134. doi: 10.1128/iai.66.3.1127-1134.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciacci-Woolwine F, Kucera L S, Richardson S H, Iyer N P, Mizel S B. Salmonellae activate tumor necrosis factor alpha production in a human promonocytic cell line via a released polypeptide. Infect Immun. 1997;65:4624–4633. doi: 10.1128/iai.65.11.4624-4633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciacci-Woolwine F, McDermott P F, Mizel S B. Induction of cytokine synthesis by flagella from gram-negative bacteria may be dependent on the activation or differentiation state of human monocytes. Infect Immun. 1999;67:5176–5185. doi: 10.1128/iai.67.10.5176-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 6.Joys T M. The flagellar filament protein. Can J Microbiol. 1988;34:452–458. doi: 10.1139/m88-078. [DOI] [PubMed] [Google Scholar]
- 7.Maki S, Vonderviszt F, Furukawa Y, Imada K, Namba K. Plugging interactions of HAP2 pentamer into the distal end of flagellar filament revealed by electron microscopy. J Mol Biol. 1998;277:771–777. doi: 10.1006/jmbi.1998.1663. [DOI] [PubMed] [Google Scholar]
- 8.Mimori-Kiyosue Y, Vonderviszt F, Namba K. Locations of terminal segments of flagellin in the filament structure and their roles in polymerization and polymorphism. J Mol Biol. 1997;270:222–237. doi: 10.1006/jmbi.1997.1111. [DOI] [PubMed] [Google Scholar]
- 9.Namba K, Yamashita I, Vonderviszt F. Structure of the core and central channel of bacterial flagella. Nature. 1989;342:648–654. doi: 10.1038/342648a0. [DOI] [PubMed] [Google Scholar]
- 10.Szekely E, Simon M. DNA sequence adjacent to flagellar genes and evolution of flagellar-phase variation. J Bacteriol. 1983;155:74–81. doi: 10.1128/jb.155.1.74-81.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volk H D, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller J M, Docke W D, Kox W J. Monocyte deactivation—rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22:S474–S481. doi: 10.1007/BF01743727. [DOI] [PubMed] [Google Scholar]
- 12.Wei L N, Joys T M. Covalent structure of three phase-1 flagellar filament proteins of Salmonella. J Mol Biol. 1985;186:791–803. doi: 10.1016/0022-2836(85)90397-3. [DOI] [PubMed] [Google Scholar]
- 13.Wilson K. Preparation and analysis of DNA. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 2.4.1–2.4.5. [Google Scholar]
- 14.Wyant T L, Tanner M K, Sztein M B. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect Immun. 1999;67:3619–3624. doi: 10.1128/iai.67.7.3619-3624.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]