Abstract
Significant maternal and fetal morbidity and mortality risk has been shown to be associated with cardiovascular disease in pregnancy. Several determinants, such as the increasing number of females with corrected congenital heart disease in reproductive age, a more advanced maternal age associated with cardiovascular risk factors, and a greater prevalence of preexisting comorbidities related to cardiac disorders such as cancer and COVID-19), lead to a higher incidence of cardiac complications in pregnancy in the last few decades. However, adopting a multidisciplinary strategy may influence maternal and neonatal outcomes. This review aims at assessing the role of the Pregnancy Heart Team, which should ensure careful pre-pregnancy counseling, pregnancy monitoring, and delivery planning for both congenital and other cardiac or metabolic disorders, addressing several emerging aspects in the multidisciplinary team-based approach.
Keywords: acquired heart disease, corrected congenital heart disease, pregnancy heart team, cardio obstetric team, pre-conception counseling, multidisciplinary team-Based approach, postpartum followup
Introduction
Maternal mortality (MM) has increased in the last twenty years (1). A substantial role of cardiovascular diseases (CVD) in this rising trend has been well-established (2), and more than 33% of pregnancy-related deaths have been attributed to CVD (3–6). Corrected congenital heart disease (cCHD), valvular heart diseases (VHD), and cardiomyopathies are the most frequent CVD in pregnancy (7). Moreover, because of the improvements in cCHD surgery, it has become more and more frequent that females survivors with cCHD embark on pregnancy (2, 8); in addition, a more advanced maternal age (9) and, consequently, a greater prevalence of cardiovascular (CV) risk factors have been shown to contribute to CV deaths and morbidity (2). Indeed it has been recognized that women over 30 years have a higher MM rate (9). Conversely, the development of complications such as intrauterine growth restriction (IUGR), preterm birth, preeclampsia, and other hypertensive disorder of pregnancy are more likely to be related to future CVD after delivery (10).
Remarkably, it has been estimated that more than 68% of CV-related MM could have been avoided (5). Therefore, in order to minimize MM, a multidisciplinary approach to pregnancy-associated conditions has been advocated (11, 12). In the latest decades, it has been proposed to create a cardio-obstetric or pregnancy heart team (PHT) involving cardiologists, gynecologists, obstetrics, anesthesiologists, nurses, and other specialists according to the specific clinical competencies required. Women with CVD or CV risk factors should be referred to this multidisciplinary team to improve care and outcomes for those at higher risk. The role of PHT is not only limited to the pregnancy period, but it is also crucial before pregnancy and in the post-delivery period (11–13). Indeed, women referred to PHT should receive appropriate counseling on maternal and fetal risk and the potential teratogenic effects of several drugs. Contraception should also be provided if required. An accurate clinical examination and close follow-up during pregnancy and, importantly, planning delivery should be provided. Finally, in the post-partum period, women should be carefully monitored for managing CV complications.
However, although the positive impact of team-based multidisciplinary strategies on pregnancy outcomes has been assessed, the role of the PHT has not been definitively recognized in clinical practice, and significant gaps exist in implementing a multiplanar approach for reducing pregnancy-associated comorbidity CVD burden. This paper aims to comprehensively discuss the efficacy and appropriateness of multidisciplinary evaluation, which enables an improvement in quality care.
Epidemiology
Maternal death is defined as a non-accidental, pregnancy-related fatal event occurring during pregnancy or within 42 days of its termination, irrespective of its duration and location (14). Moreover, all life-threatening events occurring during pregnancy and delivery are defined as severe maternal morbidities.
It has been estimated that the global maternal mortality ratio (MMR) is 216/100,000 live births (1), with a wide variability between developing and more developed countries (1). Indeed, social and geographic differences have also been considered to influence the pregnancy outcome, and a significant geographical heterogeneity has been shown. Black and Hispanic ethnicities belonging have been considered pregnancy-related mortality risk factors (15). Notably, a particularly high MMR (12/100.000) has been reported in the United States (1). Conversely, a lower mortality rate was recorded in European countries (1, 16), ranging from 2.7/100.000 to 10.9/100.000 live births in Norway and Slovakia, respectively (16). It has been reported that MM occurs mostly in preterm women or at delivery. However, a prevalence of MM of 20% until six weeks postpartum and later has been reported. CVD has been shown to cause more than ¼ of MM so that they are considered one of the major causes of MM (17).
Physiopathology
Blood volume expansion, higher cardiac output, lower systemic vascular resistance, obstruction of the vena cava, anemia, and systemic blood pressure (BP) fluctuations are hemodynamic changes that physiologically characterize each pregnancy and can result in the worsening of preexisting CVD (18). Therefore, underlying CV conditions (19) can be exacerbated by pregnancy.
Conversely, acquired CVD, can develop during pregnancy (19).
Pregnancy heart team
Although the awareness of MMR has increased in the last decades, the management of these patients still needs to be better organized.
In order to improve the quality of care for complex pregnant women avoiding discrepancies among different hospitals, the development of a PHT including cardiologists, gynecologists, anesthesiologists, and other specialized figures such as geneticists, neonatologists, cardiac surgeons, endocrinologists, and oncologists has been proposed (13, 17, 20, 21). Teams has been proposed. PHT should be finalized not only to guarantee accurate monitoring during the pregnancy and delivery but also should be organized to last from pre-conception counseling (22) to the postpartum follow-up, including labor and delivery time (14, 17–19).
Pre-conceptional counseling
Pre-conceptional counseling before pregnancy is crucial for identifying high-risk patients for maternal and fetal complications (22, 23). Contraception in young patients with CVD should also be encouraged by the PHT in order to avoid unplanned pregnancies (22). Moreover, women should be supported by the PHT and provided with the opportunity to choose the best timing for the pregnancy and undergo planned treatment. Remarkably, the inheritance of several conditions should also be faced. Heredity is expected to manifest in 50% of patients with genetic disorders associated with cCHD, such as DiGeorge (22q11 deletion) (24, 25), Marfan (26), Heart-hand syndromes (26, 27) Holt-Oram (28), Nooman (29), Alagille (30), CHARGE (31), Williams-Beuren (32), Cutis laxa (33), Vascular Ehlers–Danlos (vED) (34), and Silver-Russel syndromes (35). Several scores have been proposed to assess risk in pregnant women or those planning pregnancy. CARPREG II (Cardiac Disease in PregnancyStudy) (36), ZAHARA (Zwangerschap Bij Aangeboren Hartafwijking) (37), and modified WHO (World Health Organization) (38–40) have been validated to clinically evaluate the CVD burden, in order to favor not only preconception counseling, but also pregnancy and delivery management, and eventually termination of pregnancy in particularly high-risk conditions.
The modified World Health Organization (mWHO) risk score identifies five risk classes (WHO I, II, II-III, III, and IV) (38–40), investigating not only the risk assessment of CV events but also obstetric complications, such as miscarriage, postpartum hemorrhage, hypertensive disorders, prematurity, intrauterine growth restriction (IUGR), low birth weight (ELBW), and perinatal mortality (38).
Preconception counseling, frequency of controls during pregnancy, delivery time and modality, and postpartum care should be based on the risk assessment (8, 41–47).
Postpartum follow-up
Postpartum follow-up should be adequately monitored in women with known CVD, considering the fact that women with CVD remain at high risk for late CV complications (48). Females with an increased risk for adverse long-term CV outcomes can be identified using pregnancy risk prediction tools (48). The intrauterine device or progesterone-only subdermal implants can be used in the immediate postpartum period, taking into account the risk of thrombosis or bleeding.
Patients at great risk for developing CV complications should be monitored for the following 72 h (49). Several CV events, such as peripartum cardiomyopathy (PPCM), pulmonary embolism (PE), spontaneous coronary artery dissection (SCAD), and aortic dissection (AD), can occur postpartum. The so-called “red flag” symptoms are thought to be relevant in the early detection of CV complications. A self-monitoring of the patients is also beneficial. After discharge, the first visit should be performed for high-risk patients within three days (50). A postpartum evaluation within the first three weeks after delivery with interim follow-up has been recommended, including a comprehensive medical examination within 12 weeks (50). Nevertheless, heart failure (HF), arrhythmias, hypertensive disorders, and hemorrhagic and infective pregnancy-related events complications have been considered the most frequent causes of rehospitalizations in the first 42 days postpartum (51).
Discussion
An increment in MM has been reported in the last two decades. CVD is considered the leading cause of MM and morbidity (3–6). Nowadays, advanced maternal age is commonly observed, being related to a more prevalence of comorbidities and CV risk factors such as hypertension, diabetes, and obesity (15).
In addition, the widespread use of assisted reproductive technology has been correlated with greater CV risk. On the other hand, the improved management of cCHD resulted in a more prevalence of adult females with cCHD. Due to the difficult management of CVD in pregnant women, referring these patients to highly specialized centers would be advisable to ensure a high quality of care and a multidisciplinary approach during pregnancy and in the first few months of postpartum (Table 1).
Table 1.
Role of PHT evaluation before, during, and after pregnancy in the most common CVD.
| CVD | Before conception | During peripartum | Delivery | Long term follow-up |
|---|---|---|---|---|
| CAD (15, 18, 38, 52) |
|
|
|
|
| Cardiomyopathies (38, 40) |
|
|
|
|
| Hypertrophic cardiomyopathy (38) |
|
|
|
|
| Arrhythmias (38, 40) |
|
|
|
|
| VHD (18, 38) |
|
|
PHT for deciding mode and timing of delivery |
|
| Mitral stenosis (18, 38) |
|
|
|
|
| Aortic stenosis (18, 38) |
|
|
|
|
| Pulmonary artery hypertension (38) |
|
|
|
Counseling is necessary to discuss the need for ongoing therapies and to avoid future pregnancies |
| Atrial Septal Defect (38) |
|
|
|
|
| Coarctation of the aorta (19, 38) | Consider repair |
|
|
|
| Fontan circulation (19, 38) |
|
|
|
|
| Tetralogy of Fallot (19, 38) |
|
|
|
Close surveillance |
|
Aortic Disease (38)
Marfan syndrome Vascular Ehlers–Danlos syndrome Turner syndrome |
|
Monitoring by echocardiography every month in high risk patients and every three months in low-risk patients
|
Vaginal delivery if the ascending aorta diameter is <45 mm Cesarean delivery should be considered when the aortic diameter exceeds 45 mm, and is recommended in patients with vascular Ehlers–Danlos syndrome type IV |
FU of the dilated aorta |
| Hypertension (15, 38, 40, 52) |
|
|
|
|
CAD, coronary artery disease; ACS, acute coronary syndrome; ECG, electrocardiogram; CAG, coronary angiography; FU, follow-up; BNP, brain natriuretic peptide; NT-proBNP, N-terminal (NT)-pro hormone BNP; HF, heart failure; PPCM, peripartum cardiomyopathy; LVEF, left ventricular ejection fraction; LV, left ventricular; LVTO, left ventricular outflow tract obstruction; ECV, electrical cardioversion; VA, ventricular arrhythmias; CA, catheter ablation; sPAP, systolic pulmonary artery pressure; OMT, optical medical therapy; PAH, pulmonary arterial hypertension; MS, mitral stenosis; AS, aortic stenosis; ASD, atrial septal defect; BP, blood pressure; CoA, coarctation of the aorta.
Heart failure
Pregnancy-related HF is a dangerous condition that requires an appropriate multidisciplinary approach. PPCM (53, 54) and pre-existing CVD (36, 55) have been reported to be the leading causes of HF development during pregnancy. However, diastolic dysfunction may also evolve in overt HF (56, 57). Therefore, if clinical signs and/or symptoms occur, echocardiographic parameters and biomarkers should be strictly monitored in order to detect HF early (11).
Women may become symptomatic for HF in the second trimester or earlier due to increased plasma volume, especially if structural cardiac disorders coexist (58). Nevertheless, it has been reported that 60% of pregnancy-related HF occurs postpartum, particularly in the 30 days following delivery (59). However, the diagnosis is frequently delayed or under-recognized.
Beta-blockers (except atenolol), thiazides, and loop-diuretics should be recommended, whereas angiotensin-converting enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARBs), mineralocorticoid receptor antagonists, and angiotensin receptor-neprilysin inhibitor (ARNI) should not be used due to their fetotoxicity. Moreover, diuretics use should be limited to those cases in which pulmonary congestion (60). Hydralazine and nitrates may be safely used during pregnancy (61, 62). The delivery option should be evaluated if an acute refractory HF is detected. Sodium restriction should be recommended for all patients.
Peripartum cardiomyopathy (PPCM)
PPCM may occur during pregnancy or after delivery, generally in the earlier phases with idiopathic etiology. Its incidence ranges from 1 to 100 and 1–60,000 live births (63–67). African-American (AA) ancestry, a more advanced maternal age, multiple pregnancies, genetic predisposition, and hypertensive disorders have correlated with PPCM (63, 64, 68–70).
PPCM has been reported to be a leading cause of MM (71, 72). The diagnosis may be challenging because signs and symptoms may be masked by normal late pregnancy and postpartum features. A delay in detecting the diagnosis significatively increases MM so that an early diagnosis is crucial. Remarkably, PHT should provide the most appropriate medical strategy, carefully evaluating the potential teratogenic drugs effect and balancing advantages and drawbacks for the mother and fetus. Beta-blockers, loop diuretics, hydralazine/isosorbide dinitrate, and digoxin use may be encouraged, whereas ACE/ARB/aldosterone receptors antagonists must not be used. Moreover, to avoid thromboembolic events, anticoagulation should be considered during pregnancy in patients with LVEF <40%, prolonging to the first eight weeks after delivery (73).
Other pharmacological approaches, such as intravenous immune globulin use (74), pentoxifylline (an anti–tumor necrosis factor-alpha) (75), and bromocriptine prolactin inhibitor (76, 77) have also been proposed. After delivery, enalapril and spironolactone may be initiated, as well as beta-blockers and diuretics may be continued, preventing patients from fluid overload. Furthermore, if a severe LV dysfunction persists, PHT should consider wearable cardioverter/defibrillator options. Long-term follow-up also is recommended. Finally, contraception options should be guaranteed.
Coronary artery disease
Acute myocardial infarction associated with pregnancy (PAMI) has been shown to have a 3-fold increased prevalence in pregnancy compared to what has been expected in women of similar age and CV comorbidities (78), with a reported incidence of 1/16,000 deliveries (79). Pregnant women of all ages can be affected, particularly those aged more than 30 years (78). In addition to the traditional risk factors, other predisposing conditions, such as pre-eclampsia and eclampsia, have been described (79).
SCAD is the leading cause of PAMI, especially in the latest gestational period and in the early post-partum. The left anterior descending artery and left main segment are the most commonly involved vessels (80). Structural hormonally-mediated coronary alterations belonging to the hypercoagulable state of pregnancy have been proposed as PAMI-related mechanisms of coronary thrombosis in the absence of atherosclerosis. Transient spasms may also underline a SCAD if normal coronary artery anatomy is found (81).
ST-segment elevation myocardial infarction (STEMI) is the most common clinical manifestation of PAMI. LV function impairment and ventricular arrhythmias (VA) may occur (80). Due to very high mortality (ranging from 5% and 7%) in both mother and fetus (80, 82), PHT evaluation is crucial. A percutaneous coronary intervention should be recommended regardless of pregnancy. However, radiation risks must be carefully taken into account, lowering fetal exposure in order not to exceed the cutoff (<1 rad during pregnancy) (83). Moreover, the increased risk of SCAD should be considered.
Conversely, a conservative approach should be evaluated in non-ST-elevation myocardial infarction (NSTEMI) (80). Remarkably, the PHT approach in this context is mandatory.
Congenital heart disease (CHD)
Due to the improvement in cardiac surgery that has raised congenital patients' survival (84), the percentage of pregnant women with cCHD requiring a PHT evaluation has increased in the last decades. Although MM has been dramatically lowered up to 0.5% (47), cCHD causes a significant morbidity burden, often resulting in arrhythmias and HF (55, 85–88), so that strict clinical follow-up should be performed. Moreover, CHD must be classified into subcategories, accurately assessing the pregnancy-related risk according to the mWHO risk score (38–40). PHT plays a crucial role in managing these patients, who must be provided with appropriate counseling to raise awareness of pregnancy-related risks (89).
Remarkably, also according to the European Society of Cardiology (ESC) (18, 19, 40), Fontan circulation, systemic right ventricle (RV), and uncorrected cyanotic CHD are considered high-risk congenital disorders which mostly need a PHT evaluation.
Metabolic disorders
Metabolic disorders such as gestational diabetes mellitus should be detected and treated the earliest as possible (23), due to potential complications and adverse long-term consequences for both mother and fetus (90). Therefore, identifying undiagnosed prediabetes or diabetes at the beginning of the pregnancy is essential to improve pregnancy outcomes (91).
Pulmonary arterial hypertension (PAH)
Pulmonary Arterial Hypertension (PAH) is likely to be due to a multifactorial etiology. Idiopathic or heritable etiology, as well as connective tissue disease, CHD (Eisenmenger syndrome), left heart disorders, pulmonary diseases, and thromboembolic diseases, have been reported as mechanisms for the development of PAH (92). Patients with PAH must be carefully evaluated in order to be provided with the most appropriate treatment. Moreover, delivery must be planned early delivery. Remarkably, counseling is crucial for women with known PAH to decide strategies, including targeted therapies, physical exercise, oxygen support, and whether to interrupt pregnancy.
Valvular heart disease (VHD)
Congenital and acquired VHD are important causes of MM and morbidity, despite the fact that rheumatic etiology has diminished in the last decades, remaining a leading cause in developing countries (40, 93, 94). Remarkably, mechanical prosthetic valve management in pregnancy is particularly complex, requiring an appropriate anticoagulation strategy, requiring a PHT-based approach before and during pregnancy (40, 93, 94).
Cancer
Notably, due to the rising prevalence of cancer at young ages, the number of survivors who reach reproductive age and desire a pregnancy is significantly increased (95). In these cases, it is crucial that the patient is aware of the influence of cancer-related treatments on fertility, the outcome of the pregnancy, and potential CV complications (96).
It has been recognized that LV dysfunction may develop in women survivors who have undergone cancer therapies at reproductive age (37). Moreover, pregnancy-related hemodynamic stress is likely to result in LV impairment and HF (97).
The main risk factors of CV events during pregnancy include a reduced LV systolic function prior to the pregnancy, history of chemotherapy with anthracyclines (cumulative dose of doxorubicin ≥ 250 mg/m2) (98), history of radiotherapy (cumulative dose ≥ 35 Gy or direct radiation on the heart > 15 Gy), diagnosis and treatment of cancer at a young age (<10 years), a longer period of time from cancer treatment to first pregnancy (>15 years) (99, 100). The assessment of the basal BNP value during pregnancy allows early identification of systolic function impairment (101, 102). Moreover, women with a history of cardiomyopathy are at a higher risk of developing further LV failure during pregnancy (103).
Notably, late radiation-induced complications may occur after radiotherapy, manifesting as therapeutics-related cardiac dysfunction, premature CAD, valvular abnormalities, pericardial injury, HF, pericardial disease, and arrhythmias (104).
Therefore, it has been established that cancer survivors who are planning a pregnancy should undergo pre-conceptional counseling (100). Clinic surveillance, including echocardiographic evaluation, is advisable before pregnancy for patients previously treated with anthracyclines and chest radiation (100).
COVID-19
An increment of 33% in MMR during the COVID-19 pandemic has been reported (105).
It has been recognized that COVID-19 patients have associated injury of the heart and vessels involving microvascular and macrovascular damage. Arterial and venous thromboembolism, CAD, HF, and arrhythmias have been shown to increase in COVID-19. Pregnant women, compared to non-pregnant females affected by COVID-19 disease, are more likely to have adverse outcomes. Moreover, severe infections (10%), intensive care unit (ICU) admission (4%), mechanical ventilation (3%), and extracorporeal membrane oxygenation (ECMO) needing (0.2%) have been reported to be more frequent in COVID-pregnant patients (106). Furthermore, COVID-19 complications may lead to preterm delivery, and the management of the pregnancy is substantially modified (107).
Assessing pregnant women with COVID-19 requires PHT to recognize COVID-19-related CV complications and to distinguish them from other pregnancy-related CV risk conditions (107). Notably, a more advanced maternal age, obesity, hypertension, and diabetes not only result in increasing CV risk in pregnancy but also the risk of severe COVID-19 disease. Accordingly, a higher neonatal ICU rate has been recorded in children of mothers affected by COVID-19. The increased risk of CV complications has been associated with a low vaccination rate in pregnant women. A more adverse outcome has been reported in unvaccinated women compared to vaccinated ones. Remarkably, vaccination during pregnancy should be strongly encouraged and should be included in the PHT program (108).
Conclusions
Progress in cardiovascular care and cardiac surgery has determined significant improvement in the conditions of women who choose to become pregnant. A close assessment before the pregnancy and monitoring during and after by a multidisciplinary group is able to reduce adverse events and improve maternal-fetal outcomes.
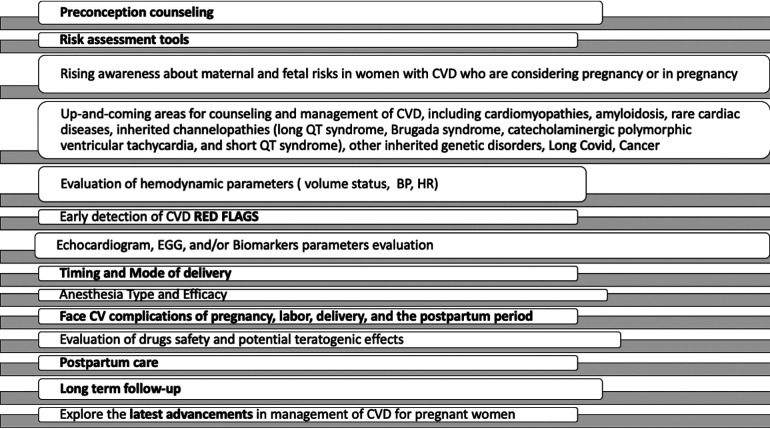
PHT management of comorbidities should be incorporated into pregnancy care in order to optimize appropriate and effective therapies (Figure 1).
Figure 1.
Pregnancy heart team: present and future directions. CVD, cardiovascular diseases; BP, blood pressure; HR, heart rate.
Implementing PHT care will require a multidisciplinary team to address therapeutic optimization, active comorbid disease management, and evidence-based interventions. Therefore, optimizing care pathways in cardio-obstetric patients is a promising area of care innovation that should substitute the traditional care approaches.
Author contributions
All authors have seen and approved the manuscript being submitted, have contributed significantly, attest to the validity and legitimacy of the data and its interpretation, and agree to its submission. All authors agree with the content, and all give explicit consent to submit. All authors whose names appear on the submission: 1. Made substantial contributions to the conception, and design of the work and to acquisition, analysis, or interpretation of data. 2. Drafted the work or revised it critically for important intellectual content; 3. approved the version to be published; 4. agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Group members of Management and Quality Working Group
Fabiana Lucà (MD, PhD, FESC) (Chairperson), Giorgio Caretta Ospedale S. Andrea - La Spezia, Stefano Cornara P.O. Levante Ospedale San Paolo - Savona, Irene Di Matteo ASST Ospedale Metropolitano Niguarda - Milano, Concetta Di Nora AOU Santa Maria della Misericordia - Udine, Silvia Favilli Azienda Ospedaliero Universitaria Meyer - Firenze, Simona Giubilato Azienda Ospedaliera Cannizzaro - Catania, Anna Pilleri ARNAS G. Brotzu - Cagliari, Andrea Pozzi ASST Papa Giovanni XXIII - Bergamo and Roberta Rossini Azienda Ospedaliera Santa Croce e Carle - Cuneo.
Group members of Pediatric Cardiology Group
Maria Giovanna Russo (MD) (Chairperson), Gabriele Egidy Assenza Ospedale Sant'Orsola Malpighi - Bologna, Annalisa Alaimo P.O. Giovanni di Cristina – Palermo, Roberta Ancona AORN Ospedale dei Colli P.O. Monaldi – Napoli, Domenico Sirico Università di Padova, Gaia Spaziani A.O.U. Meyer – Firenze, Stefano Domenicucci Agenzia Ligure Sanità Regione Liguria – Genova, Giovanni Di Salvo Azienda Ospedale-Università di Padova and Maria Giulia Gagliardi Ospedale Pediatrico Bambino Gesù – Roma.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN maternal mortality estimation inter-agency group. Lancet. (2016) 387:462–74. 10.1016/s0140-6736(15)00838-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirshberg A, Srinivas SK. Epidemiology of maternal morbidity and mortality. Semin Perinatol. (2017) 41:332–7. 10.1053/j.semperi.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 3.Von Dadelszen P, Bhutta ZA, Sharma S, Bone J, Singer J, Wong H, et al. The community-level interventions for pre-eclampsia (CLIP) cluster randomised trials in Mozambique, Pakistan, and India: an individual participant-level meta-analysis. Lancet. (2020) 396:553–63. 10.1016/S0140-6736(20)31128-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ukah UV, Dayan N, Potter BJ, Paradis G, Ayoub A, Auger N. Severe maternal morbidity and long-term risk of cardiovascular hospitalization. Circ Cardiovasc Qual Outcomes. (2022) 15:e008393. doi: 10.1161/CIRCOUTCOMES.121.008393 [DOI] [PubMed] [Google Scholar]
- 5.Keepanasseril A, Pfaller B, Metcalfe A, Siu SC, Davis MB, Silversides CK. Cardiovascular deaths in pregnancy: growing concerns and preventive strategies. Can J Cardiol. (2021) 37:1969–78. 10.1016/j.cjca.2021.09.022 [DOI] [PubMed] [Google Scholar]
- 6.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-Related mortality in the United States, 2011–2013. Obstet Gynecol. (2017) 130:366–73. 10.1097/aog.0000000000002114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah LM, Varma B, Nasir K, Walsh MN, Blumenthal RS, Mehta LS, et al. Reducing disparities in adverse pregnancy outcomes in the United States. Am Heart J. (2021) 242:92–102. 10.1016/j.ahj.2021.08.019 [DOI] [PubMed] [Google Scholar]
- 8.Siu SC, Sermer M, Colman JM, Alvarez AN, Mercier LA, Morton BC, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. (2001) 104:515–21. 10.1161/hc3001.093437 [DOI] [PubMed] [Google Scholar]
- 9.Nove A, Matthews Z, Neal S, Camacho AV. Maternal mortality in adolescents compared with women of other ages: evidence from 144 countries. Lancet Glob Health. (2014) 2:e155–164. 10.1016/s2214-109x(13)70179-7 [DOI] [PubMed] [Google Scholar]
- 10.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. (2005) 366:1797–803. 10.1016/s0140-6736(05)67726-4 [DOI] [PubMed] [Google Scholar]
- 11.Davis MB, Arendt K, Bello NA, Brown H, Briller J, Epps K, et al. Team-based care of women with cardiovascular disease from Pre-conception through pregnancy and postpartum: jACC focus seminar 1/5. J Am Coll Cardiol. (2021) 77:1763–77. 10.1016/j.jacc.2021.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parrini I, Lucà F, Favilli S, Domenicucci S, Russo MG, Sarubbi B, et al. Pregnancy and heart disease: the role of the pregnancy heart team. G Ital Cardiol. (2022) 23:631–44. 10.1714/3856.38394 [DOI] [PubMed] [Google Scholar]
- 13.Easter SR, Valente AM, Economy KE. Creating a multidisciplinary pregnancy heart team. Curr Treat Options Cardiovasc Med. (2020) 22:3. 10.1007/s11936-020-0800-x [DOI] [PubMed] [Google Scholar]
- 14.Briller JE. Severe maternal cardiovascular morbidity: below the tip of the iceberg. JACC Adv. (2022) 1:1–3. doi: 10.1016/j.jacadv.2022.100124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Mehran R, et al. Cardiovascular considerations in caring for pregnant patients: a scientific statement from the American heart association. Circulation. (2020) 141:e884–903. 10.1161/cir.0000000000000772 [DOI] [PubMed] [Google Scholar]
- 16.Diguisto C, Saucedo M, Kallianidis A, Bloemenkamp K, Bødker B, Buoncristiano M, et al. Maternal mortality in eight European countries with enhanced surveillance systems: descriptive population based study. Br Med J. (2022) 379:e070621. 10.1136/bmj-2022-070621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fry ETA, Wood MJ, Walsh MN. Maternal health: the heart of the matter. J Am Coll Cardiol. (2022) 80:1107–9. 10.1016/j.jacc.2022.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elkayam U, Goland S, Pieper PG, Silverside CK. High-risk cardiac disease in pregnancy: part I. J Am Coll Cardiol. (2016) 68:396–410. 10.1016/j.jacc.2016.05.048 [DOI] [PubMed] [Google Scholar]
- 19.Elkayam U, Goland S, Pieper PG, Silversides CK. High-risk cardiac disease in pregnancy: part II. J Am Coll Cardiol. (2016) 68:502–16. 10.1016/j.jacc.2016.05.050 [DOI] [PubMed] [Google Scholar]
- 20.Ouyang P, Sharma G. The potential for pregnancy heart teams to reduce maternal mortality in women with cardiovascular disease. J Am Coll Cardiol. (2020) 76:2114–6. 10.1016/j.jacc.2020.09.007 [DOI] [PubMed] [Google Scholar]
- 21.Wolfe DS, Yellin S. Maternal cardiology team: how to build and why it is necessary. Int J Cardiol Congenit Heart Dis. (2021) 5:100236. 10.1016/j.ijcchd.2021.100236 [DOI] [Google Scholar]
- 22.Lindley KJ, Bairey Merz CN, Davis MB, Madden T, Park K, Bello NA, et al. Contraception and reproductive planning for women with cardiovascular disease: JACC focus seminar 5/5. J Am Coll Cardiol. (2021) 77:1823–34. 10.1016/j.jacc.2021.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Hagen IM, Roos-Hesselink JW. Pregnancy in congenital heart disease: risk prediction and counselling. Heart. (2020) 106:1853–61. 10.1136/heartjnl-2019-314702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldmuntz E. 22q11.2 Deletion syndrome and congenital heart disease. Am J Med Genet C Semin Med Genet. (2020) 184:64–72. 10.1002/ajmg.c.31774 [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Rivera E, Liu YP, Verbitsky M, Anderson BR, Capone VP, Otto EA, et al. Genetic drivers of kidney defects in the DiGeorge syndrome. N Engl J Med. (2017) 376:742–54. 10.1056/NEJMoa1609009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuart AG, Williams A. Marfan's syndrome and the heart. Arch Dis Child. (2007) 92:351–6. 10.1136/adc.2006.097469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basson CT, Cowley GS, Solomon SD, Weissman B, Poznanski AK, Traill TA, et al. The clinical and genetic spectrum of the holt-oram syndrome (heart-hand syndrome). N Engl J Med. (1994) 330:885–91. 10.1056/nejm199403313301302 [DOI] [PubMed] [Google Scholar]
- 28.Spiridon MR, Petris AO, Gorduza EV, Petras AS, Popescu R, Caba L. Holt-oram syndrome with multiple cardiac abnormalities. Cardiol Res. (2018) 9:324–9. 10.14740/cr767w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linglart L, Gelb BD. Congenital heart defects in noonan syndrome: diagnosis, management, and treatment. Am J Med Genet C Semin Med Genet. (2020) 184:73–80. 10.1002/ajmg.c.31765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turnpenny PD, Ellard S. Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. (2012) 20:251–7. 10.1038/ejhg.2011.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blake KD, Prasad C. CHARGE syndrome. Orphanet J Rare Dis. (2006) 1:34. 10.1186/1750-1172-1-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins RT. 2nd. Cardiovascular disease in williams syndrome. Curr Opin Pediatr. (2018) 30:609–15. 10.1097/mop.0000000000000664 [DOI] [PubMed] [Google Scholar]
- 33.Roussin I, Sheppard MN, Rubens M, Kaddoura S, Pepper J, Mohiaddin RH. Cardiovascular complications of cutis laxa syndrome: successful diagnosis and surgical management. Circulation. (2011) 124:100–2. 10.1161/circulationaha.111.025056 [DOI] [PubMed] [Google Scholar]
- 34.Milewicz DM, Reid AJ, Cecchi AC. Vascular ehlers-danlos syndrome: exploring the role of inflammation in arterial disease. Circ Cardiovasc Genet. (2014) 7:5–7. 10.1161/circgenetics.114.000507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakeling EL, Brioude F, Lokulo-Sodipe O, O'Connell SM, Salem J, Bliek J, et al. Diagnosis and management of silver–russell syndrome: first international consensus statement. Nat Rev Endocrinol. (2017) 13:105–24. 10.1038/nrendo.2016.138 [DOI] [PubMed] [Google Scholar]
- 36.Silversides CK, Grewal J, Mason J, Sermer M, Kiess M, Rychel V, et al. Pregnancy outcomes in women with heart disease: the CARPREG II study. J Am Coll Cardiol. (2018) 71:2419–30. 10.1016/j.jacc.2018.02.076 [DOI] [PubMed] [Google Scholar]
- 37.Balci A, Sollie KM, Mulder BJ, de Laat MW, Roos-Hesselink JW, van Dijk AP, et al. Associations between cardiovascular parameters and uteroplacental doppler (blood) flow patterns during pregnancy in women with congenital heart disease: rationale and design of the zwangerschap bij aangeboren hartafwijking (ZAHARA) II study. Am Heart J. (2011) 161:269–275.e261. 10.1016/j.ahj.2010.10.024 [DOI] [PubMed] [Google Scholar]
- 38.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, et al. ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. (2018) 2018(39):3165–241. 10.1093/eurheartj/ehy340 [DOI] [PubMed] [Google Scholar]
- 39.Kim YY, Goldberg LA, Awh K, Bhamare T, Drajpuch D, Hirshberg A, et al. Accuracy of risk prediction scores in pregnant women with congenital heart disease. Congenit Heart Dis. (2019) 14:470–8. 10.1111/chd.12750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, et al. ESC guidelines on the management of cardiovascular diseases during pregnancy: the task force on the management of cardiovascular diseases during pregnancy of the European society of cardiology (ESC). Eur Heart J. (2011) 32:3147–97. 10.1093/eurheartj/ehr218 [DOI] [PubMed] [Google Scholar]
- 41.Siu SC, Sermer M, Harrison DA, Grigoriadis E, Liu G, Sorensen S, et al. Risk and predictors for pregnancy-related complications in women with heart disease. Circulation. (1997) 96:2789–94. 10.1161/01.cir.96.9.2789 [DOI] [PubMed] [Google Scholar]
- 42.Aranda-Gallardo M, Morales-Asencio JM, Canca-Sanchez JC, Barrero-Sojo S, Perez-Jimenez C, Morales-Fernandez A, et al. Instruments for assessing the risk of falls in acute hospitalized patients: a systematic review and meta-analysis. BMC Health Serv Res. (2013) 13:122. 10.1186/1472-6963-13-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hrvatin I, Rugelj D. Risk factors for accidental falls during pregnancy—a systematic literature review. J Matern Fetal Neonatal Med. (2022) 35:7015–24. 10.1080/14767058.2021.1935849 [DOI] [PubMed] [Google Scholar]
- 44.Nieva VF, Sorra J. Safety culture assessment: a tool for improving patient safety in healthcare organizations. Qual Saf Health Care. (2003) 12 Suppl 2:ii17–23. 10.1136/qhc.12.suppl_2.ii17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kampman MA, Balci A, van Veldhuisen DJ, van Dijk AP, Roos-Hesselink JW, Sollie-Szarynska KM, et al. N-terminal pro-B-type natriuretic peptide predicts cardiovascular complications in pregnant women with congenital heart disease. Eur Heart J. (2014) 35:708–15. 10.1093/eurheartj/eht526 [DOI] [PubMed] [Google Scholar]
- 46.Balci A, Sollie K, Mulder B, Roos-Hesselink J, Van Dijk A, Vliegen H, et al. Prospective assessment of pregnancy risk estimation models in women with congenital heart disease. Eur Heart J. (2010) 31:615–6. doi: 10.1136/heartjnl-2014-305597 [Google Scholar]
- 47.Roos-Hesselink JW, Ruys TP, Stein JI, Thilén U, Webb GD, Niwa K, et al. Outcome of pregnancy in patients with structural or ischaemic heart disease: results of a registry of the European society of cardiology. Eur Heart J. (2013) 34:657–65. 10.1093/eurheartj/ehs270 [DOI] [PubMed] [Google Scholar]
- 48.Siu SC, Lee DS, Rashid M, Fang J, Austin PC, Silversides CK. Long-term cardiovascular outcomes after pregnancy in women with heart disease. J Am Heart Assoc. (2021) 10:e020584. 10.1161/jaha.120.020584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melinda BD, Katherine A, Natalie AB, Brown H, Joan B, Kelly E. Team-based care of women with cardiovascular disease from pre-conception through pregnancy and postpartum. J Am Coll Cardiol. (2021) 77:1763–77. doi: 10.1016/j.jacc.2021.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKinney J, Keyser L, Clinton S, Pagliano C. ACOG committee opinion No. 736: optimizing postpartum care. Obstet Gynecol. (2018) 132:784–5. 10.1097/AOG.0000000000002849 [DOI] [PubMed] [Google Scholar]
- 51.Lima F, Nie L, Yang J, Owens A, Dianati-Maleki N, Avila C, et al. Postpartum cardiovascular outcomes among women with heart disease from a nationwide study. Am J Cardiol. (2019) 123:2006–14. 10.1016/j.amjcard.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 52.Park K, Bairey Merz CN, Bello NA, Davis M, Duvernoy C, Elgendy IY, et al. Management of women with acquired cardiovascular disease from Pre-conception through pregnancy and postpartum: jACC focus seminar 3/5. J Am Coll Cardiol. (2021) 77:1799–812. 10.1016/j.jacc.2021.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 75:207–21. 10.1016/j.jacc.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 54.Sliwa K, Petrie MC, van der Meer P, Mebazaa A, Hilfiker-Kleiner D, Jackson AM, et al. Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: an ESC EORP registry. Eur Heart J. (2020) 41:3787–97. 10.1093/eurheartj/ehaa455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruys TP, Roos-Hesselink JW, Hall R, Subirana-Domènech MT, Grando-Ting J, Estensen M, et al. Heart failure in pregnant women with cardiac disease: data from the ROPAC. Heart. (2014) 100:231–8. 10.1136/heartjnl-2013-304888 [DOI] [PubMed] [Google Scholar]
- 56.Vaught AJ, Kovell LC, Szymanski LM, Mayer SA, Seifert SM, Vaidya D, et al. Acute cardiac effects of severe pre-eclampsia. J Am Coll Cardiol. (2018) 72:1–11. 10.1016/j.jacc.2018.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kansal M, Hibbard JU, Briller J. Diastolic function in pregnant patients with cardiac symptoms. Hypertens Pregnancy. (2012) 31:367–74. 10.3109/10641955.2012.690056 [DOI] [PubMed] [Google Scholar]
- 58.Yoshida A, Kaji T, Yamada H, Yonetani N, Sogawa E, Yamao M, et al. Measurement of hemodynamics immediately after vaginal delivery in healthy pregnant women by electrical cardiometry. J Med Invest. (2019) 66:75–80. 10.2152/jmi.66.75 [DOI] [PubMed] [Google Scholar]
- 59.Mogos MF, Piano MR, McFarlin BL, Salemi JL, Liese KL, Briller JE. Heart failure in pregnant women: a concern across the pregnancy continuum. Circ Heart Fail. (2018) 11:e004005. 10.1161/circheartfailure.117.004005 [DOI] [PubMed] [Google Scholar]
- 60.Hilfiker-Kleiner D, Westhoff-Bleck M, Gunter HH, von Kaisenberg CS, Bohnhorst B, Hoeltje M, et al. A management algorithm for acute heart failure in pregnancy. The hannover experience. Eur Heart J. (2015) 36:769–70. 10.1093/eurheartj/ehv009 [DOI] [PubMed] [Google Scholar]
- 61.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC)Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 62.Sliwa K, Mebazaa A, Hilfiker-Kleiner D, Petrie MC, Maggioni AP, Laroche C, et al. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): eURObservational research programme in conjunction with the heart failure association of the European society of cardiology study group on PPCM. Eur J Heart Fail. (2017) 19:1131–41. 10.1002/ejhf.780 [DOI] [PubMed] [Google Scholar]
- 63.Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. (2011) 58:659–70. 10.1016/j.jacc.2011.03.047 [DOI] [PubMed] [Google Scholar]
- 64.Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. (2006) 368:687–93. 10.1016/s0140-6736(06)69253-2 [DOI] [PubMed] [Google Scholar]
- 65.Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. (2005) 80:1602–6. 10.4065/80.12.1602 [DOI] [PubMed] [Google Scholar]
- 66.Brar SS, Khan SS, Sandhu GK, Jorgensen MB, Parikh N, Hsu JW, et al. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol. (2007) 100:302–4. 10.1016/j.amjcard.2007.02.092 [DOI] [PubMed] [Google Scholar]
- 67.Kolte D, Khera S, Aronow WS, Palaniswamy C, Mujib M, Ahn C, et al. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: a nationwide population-based study. J Am Heart Assoc. (2014) 3:e001056. 10.1161/jaha.114.001056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, van der Werf R, Jongbloed JD, Paulus WJ, et al. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation. (2010) 121:2169–75. 10.1161/circulationaha.109.929646 [DOI] [PubMed] [Google Scholar]
- 69.Morales A, Painter T, Li R, Siegfried JD, Li D, Norton N, et al. Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation. (2010) 121:2176–82. 10.1161/circulationaha.109.931220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the heart failure association of the European society of cardiology working group on peripartum cardiomyopathy. Eur J Heart Fail. (2010) 12:767–78. 10.1093/eurjhf/hfq120 [DOI] [PubMed] [Google Scholar]
- 71.Hameed AB, Lawton ES, McCain CL, Morton CH, Mitchell C, Main EK, et al. Pregnancy-related cardiovascular deaths in California: beyond peripartum cardiomyopathy. Am J Obstet Gynecol. (2015) 213(379):e371–310. 10.1016/j.ajog.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 72.Briller J, Koch AR, Geller SE. Maternal cardiovascular mortality in Illinois, 2002–2011. Obstet Gynecol. (2017) 129:819–26. 10.1097/aog.0000000000001981 [DOI] [PubMed] [Google Scholar]
- 73.Elkayam U, Jalnapurkar S, Barakat M. Peripartum cardiomyopathy. Cardiol Clin. (2012) 30:435–40. 10.1016/j.ccl.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 74.Bozkurt B, Villaneuva FS, Holubkov R, Tokarczyk T, Alvarez RJ, Jr, MacGowan GA, et al. Intravenous immune globulin in the therapy of peripartum cardiomyopathy. J Am Coll Cardiol. (1999) 34:177–80. 10.1016/s0735-1097(99)00161-8 [DOI] [PubMed] [Google Scholar]
- 75.Sliwa K, Skudicky D, Candy G, Bergemann A, Hopley M, Sareli P. The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Fail. (2002) 4:305–9. 10.1016/s1388-9842(02)00008-9 [DOI] [PubMed] [Google Scholar]
- 76.Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, Becker A, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. (2010) 121:1465–73. 10.1161/circulationaha.109.901496 [DOI] [PubMed] [Google Scholar]
- 77.Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. (2013) 108:366. 10.1007/s00395-013-0366-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol. (2008) 52:171–80. 10.1016/j.jacc.2008.03.049 [DOI] [PubMed] [Google Scholar]
- 79.James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. (2006) 113:1564–71. 10.1161/circulationaha.105.576751 [DOI] [PubMed] [Google Scholar]
- 80.Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. (2014) 129:1695–702. 10.1161/circulationaha.113.002054 [DOI] [PubMed] [Google Scholar]
- 81.Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. (2012) 126:579–88. 10.1161/circulationaha.112.105718 [DOI] [PubMed] [Google Scholar]
- 82.Vaccarino V, Parsons L, Peterson ED, Rogers WJ, Kiefe CI, Canto J. Sex differences in mortality after acute myocardial infarction: changes from 1994 to 2006. Arch Intern Med. (2009) 169:1767–74. 10.1001/archinternmed.2009.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Colletti PM, Lee KH, Elkayam U. Cardiovascular imaging of the pregnant patient. AJR Am J Roentgenol. (2013) 200:515–21. 10.2214/ajr.12.9864 [DOI] [PubMed] [Google Scholar]
- 84.Mandalenakis Z, Giang KW, Eriksson P, Liden H, Synnergren M, Wåhlander H, et al. Survival in children with congenital heart disease: have we reached a peak at 97%? J Am Heart Assoc. (2020) 9:e017704. 10.1161/jaha.120.017704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drenthen W, Boersma E, Balci A, Moons P, Roos-Hesselink JW, Mulder BJ, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. (2010) 31:2124–32. 10.1093/eurheartj/ehq200 [DOI] [PubMed] [Google Scholar]
- 86.Drenthen W, Pieper PG, Roos-Hesselink JW, van Lottum WA, Voors AA, Mulder BJ, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol. (2007) 49:2303–11. 10.1016/j.jacc.2007.03.027 [DOI] [PubMed] [Google Scholar]
- 87.Fesslova VM, Villa L, Chessa M, Butera G, Salmona S, Acaia B. Prospective evaluation from single centre of pregnancy in women with congenital heart disease. Int J Cardiol. (2009) 131:257–64. 10.1016/j.ijcard.2007.10.030 [DOI] [PubMed] [Google Scholar]
- 88.Ford AA, Wylie BJ, Waksmonski CA, Simpson LL. Maternal congenital cardiac disease: outcomes of pregnancy in a single tertiary care center. Obstet Gynecol. (2008) 112:828–33. 10.1097/AOG.0b013e31818638c6 [DOI] [PubMed] [Google Scholar]
- 89.Kovacs AH, Harrison JL, Colman JM, Sermer M, Siu SC, Silversides CK. Pregnancy and contraception in congenital heart disease: what women are not told. J Am Coll Cardiol. (2008) 52:577–8. 10.1016/j.jacc.2008.05.013 [DOI] [PubMed] [Google Scholar]
- 90.McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. (2019) 5:47. 10.1038/s41572-019-0098-8 [DOI] [PubMed] [Google Scholar]
- 91.Green JB. Cardiovascular consequences of gestational diabetes. (2021) 143:988–90. doi: 10.1183/16000617.0079-2016 [DOI] [PubMed] [Google Scholar]
- 92.Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. (2016) 25:431–7. 10.1183/16000617.0079-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Canobbio MM, Warnes CA, Aboulhosn J, Connolly HM, Khanna A, Koos BJ, et al. Management of pregnancy in patients with Complex congenital heart disease: a scientific statement for healthcare professionals from the American heart association. Circulation. (2017) 135:e50–87. 10.1161/cir.0000000000000458 [DOI] [PubMed] [Google Scholar]
- 94.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP. 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. (2014) 129:2440–92. 10.1161/cir.0000000000000029 [DOI] [PubMed] [Google Scholar]
- 95.Hudson MM, Ehrhardt MJ. At the heart of safe and successful pregnancies in cancer survivors. JACC CardioOncol. (2020) 2:163–5. 10.1016/j.jaccao.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Poorvu PD, Frazier AL, Feraco AM, Manley PE, Ginsburg ES, Laufer MR, et al. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectr. (2019) 3:pkz008. 10.1093/jncics/pkz008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nolan MT, Marwick TH, Plana JC, Li Z, Ness KK, Joshi VM, et al. Effect of traditional heart failure risk factors on myocardial dysfunction in adult survivors of childhood cancer. JACC Cardiovasc Imaging. (2018) 11:1202–3. 10.1016/j.jcmg.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. (2017) 31:63–75. 10.1007/s10557-016-6711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chait-Rubinek L, Mariani JA, Goroncy N, Herschtal A, Wheeler GC, Dwyer MK, et al. A retrospective evaluation of risk of peripartum cardiac dysfunction in survivors of childhood, adolescent and young adult malignancies. Cancers. (2019) 11(8):1046. 10.3390/cancers11081046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nolan M, Oikonomou EK, Silversides CK, Hines MR, Thompson KA, Campbell BA, et al. Impact of cancer therapy-related cardiac dysfunction on risk of heart failure in pregnancy. JACC CardioOncol. (2020) 2(2):153–62. 10.1016/j.jaccao.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. AHA/ACC/HFSA guideline for the management of heart failure. J Am Coll Cardiol. (2022) 2022(79):e263–421. 10.1016/j.jacc.2021.12.012 [DOI] [PubMed] [Google Scholar]
- 102.Siegmund AS, Pieper PG, Bouma BJ, Rosenberg FM, Groen H, Bilardo CM, et al. Early N-terminal pro-B-type natriuretic peptide is associated with cardiac complications and function during pregnancy in congenital heart disease. Neth Heart J. (2021) 29:262–72. 10.1007/s12471-021-01540-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.DeFilippis EM, Haythe JH, Walsh MN, Kittleson MM. Intersection of heart failure and pregnancy: beyond peripartum cardiomyopathy. Circ Heart Fail. (2021) 14:e008223. 10.1161/circheartfailure.120.008223 [DOI] [PubMed] [Google Scholar]
- 104.Mitchell JD, Cehic DA, Morgia M, Bergom C, Toohey J, Guerrero PA, et al. Cardiovascular manifestations from therapeutic radiation. JACC Cardio Oncol. (2021) 3:360–80. 10.1016/j.jaccao.2021.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Slomski A. Maternal death rate increased during early COVID-19 pandemic. JAMA. (2022) 328:415–415. doi: 10.1093/cid/ciab344 [DOI] [PubMed] [Google Scholar]
- 106.Ko JY, DeSisto CL, Simeone RM, Ellington S, Galang RR, Oduyebo T, et al. Adverse pregnancy outcomes, maternal complications, and severe illness among US delivery hospitalizations with and without a coronavirus disease 2019 (COVID-19) diagnosis. Clin Infect Dis. (2021) 73:S24–31. 10.1093/cid/ciab344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Briller JE, Aggarwal NR, Davis MB, Hameed AB, Malhamé I, Mahmoud Z, et al. Cardiovascular complications of pregnancy-associated COVID-19 infections. JACC Adv. (2022) 1:100057. 10.1016/j.jacadv.2022.100057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. (2022) 28:583–90. 10.1038/s41591-022-01689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]