Abstract
Background
Tumour-treating fields therapy is a locoregional, anti-cancer treatment. Efficacy and safety of tumour-treating fields therapy in adults with newly diagnosed glioblastoma were demonstrated in the pivotal phase 3 EF-14 study (NCT00916409). Here, we report post-approval data of tumour-treating fields therapy in Japanese patients with newly diagnosed glioblastoma.
Methods
Unsolicited post-marketing surveillance data from Japanese patients with newly diagnosed glioblastoma treated with tumour-treating fields therapy (December 2016–June 2020) were retrospectively analysed. The primary endpoints were skin, neurological and psychiatric adverse events. The secondary endpoints were 1- and 2-year overall survival rates, and the 6-month progression-free survival. adverse events were analysed using MedDRA v24.0. The overall survival and progression-free survival were assessed using the Kaplan–Meier survival analysis (log-rank testing). The Cox proportional hazard regression analyses were also performed.
Results
Forty patients with newly diagnosed glioblastoma were enrolled (62.5% male; median age 59 years; median baseline Karnofsky Performance Scale score 90). The most common tumour-treating-fields-therapy-related adverse event was beneath-array local skin reaction (60% of patients). The adverse events were mostly mild to moderate in severity. Neurological disorders were observed in 2.5% patients (one patient reported dysesthesia). No psychiatric disorders were reported. The 1- and 2-year overall survival rates were 77.9% (95% CI 60.6–88.3) and 53.6% (35.5–68.7%), respectively. The 6-month progression-free survival was 77.5% (61.2–87.6%). These survival rates compare favourably with those in the EF-14 trial (1- and 2-year overall survival rates: 73% [69–77%] and 43% [39–48%], respectively; 6-month progression-free survival rate: 56% (51–61%).
Conclusion
This post-approval, real-world evidence study revealed no new safety signals and suggests the safety and efficacy of tumour-treating fields therapy in Japanese patients with newly diagnosed glioblastoma.
Keywords: skin, new technology/instruments, interventional therapy, CNS
This post-approval, real-world study revealed no new safety signals and is suggestive of the safety and efficacy of tumour-treating fields therapy in Japanese patients with newly diagnosed glioblastoma.
Introduction
Glioblastoma (GBM) is the most common malignant primary brain tumour, accounting for 12% of all the primary brain tumours in Japan (1), and with an estimated global incidence rate of 3.2 per 100 000 individuals (2,3). GBM is associated with a very poor prognosis, with an estimated 5-year survival rate of 15.5% in neurosurgical cases (1). Regardless of the treatment, GBM invariably recurs within a median time interval of <7 months and treatment options for the management of recurrences are lacking (2).
The Society for Neuro-Oncology (SNO), the European Association of Neuro-Oncology (EANO), the Japan Society of Clinical Oncology (JSCO), the Japan Neurosurgical Society (JNS) and the Japan Society for Neuro-Oncology (JSNO) recommend that patients with newly diagnosed (nd) GBM undergo maximal safe tumour resection, followed by radiation therapy with concomitant and then adjuvant temozolomide (TMZ) chemotherapy (2,4). However, the addition of Tumor Treating Fields therapy (TTFields; Optune®, Novocure® GmbH, Device Manufacturer) to maintenance TMZ for ndGBM has now been incorporated into this standard of care (SOC) following approval in China, EU, Japan and USA, (5,6).
TTFields are low intensity, intermediate frequency (150–200 kHz), alternating electric fields that cause mitotic arrest and apoptosis leading to the suppression of tumour growth and spread (7–13). TTFields have also been shown to exert biophysical forces on a variety of charged and polarizable molecules, impacting DNA repair, autophagy, cancer cell migration, membrane permeability and immunological response processes, in addition to their antimitotic effects (7,8,14–17). TTFields therapy is locoregional, rather than systemic, since the electric fields are continuously generated by a wearable device, and delivered via arrays placed directly on the surface of the scalp at the site of the tumour.
TTFields therapy is approved for the treatment of adult patients with malignant pleural mesothelioma, ndGBM and recurrent GBM (5,6,18), based on efficacy and safety data from the STELLAR, EF-14 and EF-11 clinical studies, respectively (19–23).
The pivotal phase 3 EF-14 clinical study (NCT00916409), which included patients with ndGBM whose tumour was resected or biopsied and who had completed concomitant radiochemotherapy, compared subsequent TTFields therapy with TMZ versus maintenance TMZ alone (22). Compared with the patients receiving TMZ only, the patients receiving TTFields therapy had significant improvements in progression-free survival (PFS) (6.7 vs. 4.0 months [hazard ratio (HR), 0.63; 95% confidence interval (CI) 0.52–0.76; P < 0.001]) and overall survival (OS) (20.9 vs. 16.0 months [HR, 0.63; 95% CI 0.53–0.76; P < 0.001]). Furthermore, a post hoc analysis of the EF-14 data reported that continuation of TTFields therapy after the first progression significantly improved OS (24). Survival benefits were also evident among a subgroup of vulnerable elderly patients (25).
The addition of TTFields therapy to TMZ did not significantly increase the rates of systemic adverse events (AEs), and the safety profile of the two treatment groups were comparable, with the exception of a higher incidence of localized skin reactions among the patients receiving concomitant TTFields therapy (22). An absence of systemic AEs was also observed in the pivotal EF-11 study (NCT00379470) in patients with recurrent GBM (23). In this study, TTFields monotherapy was comparable in efficacy with the best available treatment according to the physician’s choice, but displayed a more favourable safety profile. The patients experienced fewer severe AEs, and the AEs were generally localized to the scalp rather than the systemic events typically associated with chemotherapy. Advice on the prevention and management of dermatologic scalp AEs, which are known to be associated with TTFields therapy, is available (26,27).
Real-world evidence from a global surveillance study of >10 000 patients with GBM (28) confirmed the favourable safety profile of TTFields therapy in GBM reported in the pivotal EF-11 and EF-14 studies. Overall, the AEs were mainly localized to the treatment area and the paediatric patients experienced similar rates of skin irritation as the adult and elderly patients. There were also no increases in systemic effects or the new safety concerns (28).
This paper reports additional real-world safety and efficacy data for TTFields therapy in Japanese patients with ndGBM (EF-29).
Methods
Study design and patient population
Unsolicited post-marketing surveillance data from Japanese patients with ndGBM who received TTFields therapy (December 2016–June 2020) were retrospectively analysed. Patients with histologically confirmed supratentorial ndGBM were eligible for inclusion. Exclusion criteria were as follows: patients treated outside of the contract or enrollment periods, patients with a history of treatment with the relevant NovoTTF device before enrollment, patients who did not give or withdrew consent and those who were lost to follow-up.
Endpoints
The primary endpoints were the rate of the following AEs: skin irritations, neurological and psychiatric disorders.
The secondary endpoints included assessment of 1- and 2-year OS and 6-month PFS rates. The exploratory endpoints included the analysis of the following factors potentially influencing survival: patient age, surgical resection method and baseline Karnofsky Performance Scale (KPS) score.
Safety analysis
The rates of AEs, patient information and device usage data were collected and analysed. All the events entered in the ‘AE report’ field in the paper case report form were subjected to safety assessments, in accordance with an internal standard operating procedure. In the case of queries surrounding the entry of events in areas other than the AE report field, follow-up investigations were conducted with the physician who made the entry and data clarified accordingly. The AEs that occurred after the discontinuation of the device, and which had been assessed as not causally related by the physician, were excluded from the analysis. AEs were named according to the Medical Dictionary for Regulatory Activities (MedDRA) version 24.0 preferred terms. AEs were classed as mild, moderate, severe, life-threatening and fatal, and defined as related, not related or unknown by the treating physician. Serious AEs were based on the ICH International Pharmaceutical Glossary (MedDRA/J) version 24.0 Classification.
Efficacy analysis
Disease progression was assessed every 2 months. Where a magnetic resonance imaging scan was available, disease progression was determined according to the Response Assessment in Neuro-Oncology criteria.
Statistical analysis
A minimum of 40 patients was calculated as necessary to detect one case of anxiety (a rate of incidence 33/437) with a power of 95%, and this was therefore considered to be the required minimum sample size for analysis.
Survival rates (OS and PFS) were estimated using the Kaplan–Meier method. OS of the following subgroups was assessed (potential differences were investigated using a log-rank test): sex; age; treatment history of GBM; surgical resection method (biopsy, partial resection and gross total resection); KPS score ≥70 or <70 at baseline; average daily treatment time ≥18 h or <18 h. Descriptive statistics of the AEs (occurrence rate) was conducted as part of the primary analysis endpoint.
The Cox proportional hazard regression model was used to analyse the impact of the following covariates on prognosis: age degree of surgical resection (biopsy, partial resection and gross total resection) KPS. Statistical significance at P < 0.05 was determined using the chi-squared test. In addition, the survival rates were analysed by the Cox proportional hazard regression analyses using the Wald chi-square test. The analyses aims to examine whether the baseline characteristics: age, degree of surgical resection (biopsy, partial resection and gross total resection) and baseline KPS are prognostic factors.
Results
Study population
In total, retrospective data from 40 patients, across nine Japanese sites, were collected and evaluated (Fig. S1). Baseline characteristics are summarized in Table 1. The majority (62.5%) of patients were male; median age was 59 years, with 60.0% of patients <65 years of age, and median KPS score was 90.
Table 1.
Baseline characteristics
| Total (N = 40) | |
|---|---|
| Age, (years), median (range) | 59 (19–75) |
| Age, (years), n (%) | |
| <65 | 24 (60) |
| ≥65 | 11 (27.5) |
| Unknown | 5 (12.5) |
| Male, n (%) | 25 (62.5) |
| KPS score, median (range) | 90 (60–100) |
| 60, n (%) | 1 (2.5) |
| 70, n (%) | 4 (10.0) |
| 80, n (%) | 8 (20.0) |
| 90, n (%) | 17 (42.5) |
| 100, n (%) | 9 (22.5) |
| Unknown, n (%) | 1 (2.5) |
| Concomitant steroids, n (%) | |
| Yes | 32 (80.0) |
| No | 7 (17.5) |
| Unknown | 1 (2.5) |
| History of GBM resection, n (%) | |
| Biopsy | 3 (7.5) |
| Partial | 12 (30.0) |
| Gross total | 23 (57.5) |
| Unknown/data not provided | 2 (5.0) |
GBM, glioblastoma; KPS, Karnofsky Performance Score.
Safety analysis
Overall, 24 (60%) patients reported ≥1 AE. All these patients experienced skin irritation reactions that were generally mild-to-moderate in severity. Most were localized skin irritations with dermatitis being the most frequently reported (25%) (Table 2). In 21 patients (53%), skin-related AEs occurred within 6 months of starting treatment. Treatment was permanently stopped in one patient and temporarily stopped in three patients as a result of skin AEs; all four patients experienced resolution. One patient experienced dysesthesia within 12 months of starting treatment; this was not considered to be a serious neurological event. No psychiatric AEs were reported. Other AEs reported were malignant neoplasm progression, discomfort at site of medical device and skin injury, all of which were reported by one patient each. TTFields-therapy-related AEs comprised of skin irritation symptoms, skin-related injury and discomfort at the treatment site (Table 2). In total, 10 patients experienced serious AEs (15 events in total); none were related to TTFields therapy (Table 3).
Table 2.
Adverse events and association with TTFields therapy
| TTFields-therapy-related (Y/N) | Total (N = 40) | |
|---|---|---|
| Number of reported AEs, events | – | 31 |
| Patients with ≥1 AE, n (%) | – | 24 (60.0) |
| Skin-related AEs, n (%) | ||
| Dermatitis | Y | 10 (25.0) |
| Itching | Y | 5 (12.5) |
| Erythema | Y | 3 (7.5) |
| Eczema | Y | 2 (5.0) |
| Skin erosion | Y | 2 (5.0) |
| Blisters | Y | 1 (2.5) |
| Rash | Y | 1 (2.5) |
| Skin disorder | Y | 1 (2.5) |
| Skin pain | Y | 1 (2.5) |
| Skin ulcer | Y | 1 (2.5) |
| Neurological AEs, n (%) | ||
| Dysesthesia | N | 1 (2.5) |
| Other, n (%) | ||
| Malignant neoplasm progression | N | 1 (2.5) |
| Discomfort at site of medical device use | Y | 1 (2.5) |
| Skin injury | Y | 1 (2.5) |
AEs, adverse events; N, no; TTFields, Tumor Treating Fields; Y, yes.
Table 3.
Incidence of serious adverse events
| Serious adverse events, n (%) | Total (N = 40) |
|---|---|
| Malignant neoplasm progression | 6 (15.0) |
| Vertebral compression fracture | 2 (5.0) |
| Epilepsy | 1 (2.5) |
| Monoplegia | 1 (2.5) |
| Seizure | 1 (2.5) |
| Cholecystitis | 1 (2.5) |
| Gait disturbance | 1 (2.5) |
| Lymphocyte decrease | 1 (2.5) |
| Wrist fracture | 1 (2.5) |
Survival analysis
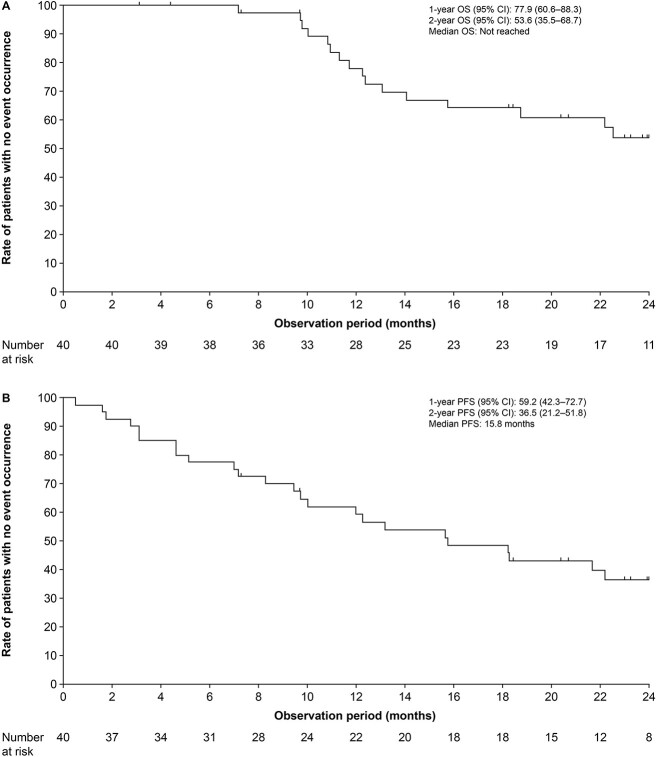
One and 2-year OS rates were 77.9% (95% CI 60.6–88.3) and 53.6% (95% CI 35.5–68.7), respectively (Fig. 1A). Six-month PFS rate was 77.5% (95% CI 61.2–87.6) (Fig. 1B).
Figure 1.
Kaplan–Meier curves of (A) overall survival and (B) progression-free survival.
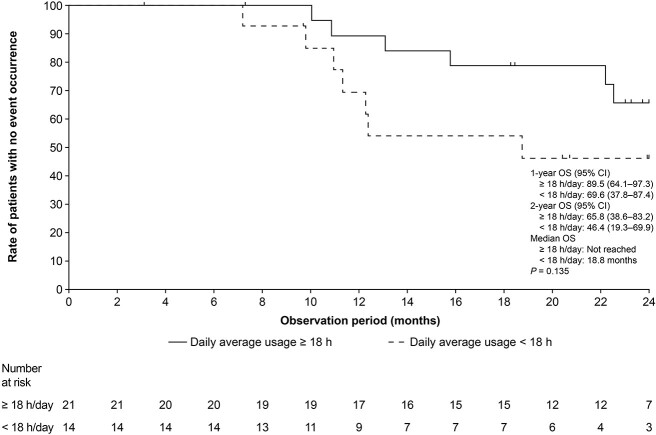
The 2-year OS rate in patients who used TTFields therapy for an average of ≥18 h/day during the duration of the contract was 65.8% (95% CI 38.6–83.2), demonstrating a trend towards increased survival relative to patients who used TTFields therapy for an average of <18 h/day (46.4% [95% CI 19.3–69.9]), P = 0.14 (Fig. 2).
Figure 2.
Kaplan–Meier curve of overall survival, according to time on TTFields therapy. TTFields, Tumor Treating Fields.
As age was unknown for five patients, the Cox proportional hazard regression analysis was performed using the survival data from 35 patients. Analyses of OS using the Cox proportional hazard modelling found survival to be significantly improved relative to each year decrease in age (univariate HR: 1.050 [95% CI 1.002–1.100], P = 0.040; multivariate HR: 1.053, [95% CI 1.003–1.105] P = 0.037; Table S1). Degree of surgical resection and baseline KPS score were not significant prognostic factors for OS (Table S1). Likewise, age, degree of surgical resection and baseline KPS score were not significantly associated with PFS (all P > 0.05) (Table S2).
Discussion
This post-marketing surveillance study in Japanese patients adds to the growing body of real-world safety data for TTFields therapy in patients with ndGBM. The study revealed no new safety signals; most events were mild-to-moderate, localized skin irritations associated with TTFields therapy.
Results are in line with previous studies which have also shown skin reactions to be the most common TTFields-therapy-related AEs. In the pivotal phase 3 EF-14 study, 52% of patients experienced mild-to-moderate skin irritation (22), while in the large-scale global post-marketing surveillance data analysis, 38% of patients with ndGBM experienced skin reactions with TTFields therapy (28). Similar high rates of occurrence of skin AEs have been reported for the TTFields-therapy-treated patients with recurrent GBM (28,29). Skin-related AEs can be managed by early prophylactic interventions, such as optimal shaving and periodic shifting of the array position, and good patient management strategies; for example, use of topical corticosteroids or antibiotics (5,27). In EF-14, the AEs associated with concomitant TMZ (e.g. leukopenia or lymphopenia) were reported and were within the expected levels (22). However, the AEs associated with any concurrent anti-cancer treatments were not captured in this study, likely due to the retrospective design and potential reporting bias towards the TTFields-therapy-related AEs of the treating physicians.
Of note, while the proportion of GBM patients ≥65 years of age has been reported to be 45% in the Japanese brain tumour registry (1), only 28% (11/40) of patients were ≥65 years of age in this study. The slightly younger population in this study may potentially reflect differences in patient and/or healthcare professional drivers and barriers to initiating TTFields therapy for younger versus older patients. The male:female ratio of patients receiving TTFields therapy in this study (62.5% male) was slightly higher than the GBM patient population as a whole in Japan (58% male) (1), but in line with that previously reported in the global TTFields therapy post-marketing study (66.3% male) (28).
Although the median KPS was consistent across this study and EF-14, the median age of patients in this study was slightly higher than in EF-14 (59.0 [19–75] years vs. 56.0 [19–83] years, respectively). Despite this, survival data in this study compared favourably with rates observed in the EF-14 study population. In EF-14, 73% and 43% of the TTFields-therapy-treated patients were alive after 1 and 2 years, respectively, with 56% experiencing PFS for 6 months (22).
In this study, a trend of an increased 2-year OS was seen in patients who used TTFields therapy for ≥18 h/day compared with those who used it for <18 h/day. This is consistent with the previous observations that TTFields therapy with a maximal monthly compliance rate ≥75% (≥ 18 h/day) corresponds to greater survival benefit in patients with GBM (2,3,29–31).
The retrospective study design and small sample number represent limitations of the study, as the analyses could not be statistically powered. The lack of a control arm means that there was no way of comparing TTFields therapy with other treatment strategies. Additionally, the study did not include reporting on concurrent therapies, such as steroids, or other treatments such as bevacizumab. As such, the impact of these treatments on safety outcomes cannot be adjusted for, and this should therefore be taken into account when evaluating the data, as concurrent therapies may have potentially affected the AE incidences, for example the use of topical steroids may have affected skin fragility, impacting the placement of arrays. Furthermore, as data were collected retrospectively, reporting of AEs may not have been carried out consistently.
This analysis provides evidence that TTFields therapy is well tolerated in Japanese patients, with survival rates comparable with those observed in the pivotal phase 3 EF-14 study. Although these data are suggestive of efficacy in a Japanese population, results should be interpreted with caution, due to the limitations described above.
Conclusion
TTFields therapy was generally well tolerated, with no new safety signals in Japanese patients with ndGBM, despite the population having a high burden of disease. The AE profile was comparable with published clinical trial data and real-world evidence, with localized skin reactions being the most frequently reported TTFields-therapy-associated AEs.
In this post-marketing study, the younger patients with better performance scores tended to be included, in contrast to a real-world population in which patients with ndGBM would typically be older with a less favourable KPS score. Even so, data reported here are suggestive of the safety and efficacy of TTFields therapy in Japanese patients with ndGBM, and support use in this patient population.
Supplementary Material
Acknowledgements
The authors would like to thank the patients, their families and all the investigators involved in this study. Medical writing support under the direction of the authors was provided by Robert Merker, PhD, Novocure Global Publications, New York, USA and Prime, Knutsford, UK. Writing and editorial support provided by Prime was funded by Novocure Inc. and conducted according to the Good Publication Practice guidelines (Link). The Sponsor was involved in the study design, collection, analysis and interpretation of data, as well as in the data checking of the information provided in the manuscript. However, the ultimate responsibility for the opinions, conclusions and data interpretation lies with the authors.
Contributor Information
Ryo Nishikawa, Department of Neuro-Oncology/Neurosurgery, Saitama Medical University International Medical Center, Saitama, Japan.
Fumiyuki Yamasaki, Department of Neurosurgery, Hiroshima University Hospital, Hiroshima, Japan.
Yoshiki Arakawa, Department of Neurosurgery, Kyoto University Graduate School of Medicine, Kyoto, Japan.
Yoshihiro Muragaki, Department of Neurosurgery, Tokyo Women’s Medical University Hospital, Tokyo, Japan.
Yoshitaka Narita, Department of Neurosurgery and Neuro-Oncology, National Cancer Center Hospital, Tokyo, Japan.
Shota Tanaka, Department of Neurosurgery, The University of Tokyo Hospital, Tokyo, Japan.
Shigeru Yamaguchi, Department of Neurosurgery, Hokkaido University Hospital, Sapporo, Japan.
Akitake Mukasa, Department of Neurosurgery, Kumamoto University Hospital, Kumamoto, Japan.
Masayuki Kanamori, Department of Neurosurgery, Tohoku University Hospital, Sendai, Japan.
Funding
This study was funded by Novocure Inc.
Disclosures
R.N.: Grants from Chugai Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd., Advisory board for Novocure Co., Ltd., Honoraria from Varian Medical Systems, Inc., Ono Pharmaceutical Co., Ltd., Eisai Co., Ltd.
F.Y.: Nothing to disclose.
Y.A.: Nothing to disclose.
Y.M.: Nothing to disclose.
Y.N.: Nothing to disclose.
S.T.: Nothing to disclose.
S.Y.: Nothing to disclose.
A.M.: Nothing to disclose.
M.K.: Nothing to disclose.
Data sharing
The datasets generated during and/or analysed during this study are available for 3 years after the date of publication. Please contact Piet Hinoul, (phinoul@novocure.com) for inquiries about access.
References
- 1. Brain Tumor Registry of Japan . Brain tumor registry of Japan (2005-2008). Neurol Med Chir (Tokyo) 2017;57:9–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol 2020;22:1073–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostrom QT, Patil N, Cioffi G, et al. CBTRUS statistical report: primary brain and other central nervous system Tumors diagnosed in the United States in 2013-2017. Neuro Oncol 2020;22:iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Japan Society for Neuro-Oncology . Adult glioblastoma (GBM) guidelines - CQ6 how to treat adult recurrent glioblastoma? Kanehara-shuppan, Tokyo, Japan. 2016. https://www.jsn-o.com/guideline3/CQ/006.html. [Google Scholar]
- 5. Novocure . Optune®: instructions for use. 2019. https://www.optune.com/Content/pdfs/Optune_IFU_8.5x11.pdf.
- 6. Novocure . Optune®: instructions for use (EU). 2020. https://www.optune.de/wp-content/uploads/2020/11/Optune_User_Manual_ver2.0.pdf.
- 7. Voloshin T, Kaynan N, Davidi S, et al. Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol Immunother 2020;69:1191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voloshin T, Schneiderman RS, Volodin A, et al. Tumor treating fields (TTFields) hinder cancer cell motility through regulation of microtubule and acting dynamics. Cancers (Basel) 2020;12:3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirson ED, Dbalý V, Tovaryš F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A 2007;104:10152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res 2004;64:3288–95. [DOI] [PubMed] [Google Scholar]
- 11. Kirson ED, Giladi M, Gurvich Z, et al. Alternating electric fields (TTFields) inhibit metastatic spread of solid tumors to the lungs. Clin Exp Metastasis 2009;26:633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giladi M, Schneiderman RS, Voloshin T, et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep 2015;5:18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giladi M, Schneiderman RS, Porat Y, et al. Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology 2014;14:54–63. [DOI] [PubMed] [Google Scholar]
- 14. Rominiyi O, Vanderlinden A, Clenton SJ, et al. Tumor Treating Fields therapy for glioblastoma: current advances and future directions. Br J Cancer 2021;124:697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang E, Patel CB, Pohling C, et al. Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov 2018;4:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shteingauz A, Porat Y, Voloshin T, et al. AMPK-dependent autophagy upregulation serves as a survival mechanism in response to tumor treating fields (TTFields). Cell Death Dis 2018;9:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giladi M, Munster M, Schneiderman RS, et al. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol 2017;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Novocure . Optune®: instructions for use for unrescetable malignant pleural mesothelioma. 2021. https://www.optunelua.com/pdfs/Optune-Lua-MPM-IFU.pdf?uh=18f20e383178129b5d6cd118075549592bae498860854e0293f947072990624c&administrationurl=https%3A%2F%2Foptunelua-admin.novocure.intouch-cit.com%2F.
- 19. Ceresoli GL, Aerts J, Madrzak J, et al. Final results of phase II STELLAR trial: TTFields with chemotherapy in unresectable malignant pleural mesothelioma. Cancer Res 2019;79(13 Suppl):CT201. [Google Scholar]
- 20. Ceresoli GL, Aerts JG, Dziadziuszko R, et al. Tumor Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): a multicentre, single-arm phase 2 trial. Lancet Oncol 2019;20:1702–9. [DOI] [PubMed] [Google Scholar]
- 21. Grosso F, Ceresoli GL. Radiological response patterns in the phase 2 STELLAR trial of TTFields with chemotherapy for first-line treatment of malignant pleural mesothelioma (MPM). J Clin Oncol 2019;37 (15 Suppl):8551. [Google Scholar]
- 22. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017;318:2306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 2012;48:2192–202. [DOI] [PubMed] [Google Scholar]
- 24. Kesari S, Ram Z. Tumor-treating fields plus chemotherapy versus chemotherapy alone for glioblastoma at first recurrence: a post hoc analysis of the EF-14 trial. CNS Oncol 2017;6:185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ram Z, Kim CY, Hottinger AF, et al. Efficacy and safety of tumor treating fields (TTFields) in elderly patients with newly diagnosed glioblastoma: subgroup analysis of the phase 3 EF-14 clinical trial. Front Oncol 2021;11:671972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lacouture M, Davis ME, Elzinga G, et al. Characterization and management of dermatologic adverse events with the NovoTTF-100A system, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin Oncol 2014;41(Suppl 4):S1–14. [DOI] [PubMed] [Google Scholar]
- 27. Lacouture M, Anadkat MJ, Ballo MT, et al. Prevention and management of dermatologic adverse events associated with tumor treating fields in patients with glioblastoma. Front Oncol 2020;10:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi W, Blumenthal DT, Oberheim Bush NA, et al. Global post-marketing safety surveillance of Tumor Treating Fields (TTFields) in patients with high-grade glioma in clinical practice. J Neurooncol 2020;148:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mrugala MM, Engelhard HH, Dinh Tran D, et al. Clinical practice experience with NovoTTF-100A™ system for glioblastoma: the patient registry dataset (PRiDe). Semin Oncol 2014;41(Suppl 6):S4–S13. [DOI] [PubMed] [Google Scholar]
- 30. Kanner AA, Wong ET, Villano JL, Ram Z. Post hoc analyses of intention-to-treat population in phase III comparison of NovoTTF-100A™ system versus best physician’s choice chemotherapy. Semin Oncol 2014;41(Suppl 6):S25–34. [DOI] [PubMed] [Google Scholar]
- 31. Toms SA, Kim CY, Nicholas G, Ram Z. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial. J Neurooncol 2019;141:467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.