Abstract
Messenger RNA (mRNA) has drawn much attention in the medical field. Through various treatment approaches including protein replacement therapies, gene editing, and cell engineering, mRNA is becoming a potential therapeutic strategy for cancers. However, delivery of mRNA into targeted organs and cells can be challenging due to the unstable nature of its naked form and the low cellular uptake. Therefore, in addition to mRNA modification, efforts have been devoted to developing nanoparticles for mRNA delivery. In this review, we introduce four categories of nanoparticle platform systems: lipid, polymer, lipid-polymer hybrid, and protein/peptide-mediated nanoparticles, together with their roles in facilitating mRNA-based cancer immunotherapies. We also highlight promising treatment regimens and their clinical translation.
KEY WORDS: Cancer immunotherapy, Lipid nanoparticles, Lipid–polymer hybrid nanoparticles, Messenger RNA, mRNA delivery, Polymeric nanoparticles, Protein/peptide-mediated nanoparticles
Graphical abstract
mRNA has becoming a potential treatment strategy for cancer therapy. In this review, we highlight representative delivery systems of mRNA and promising treatment regimens for their clinical translation.
1. Introduction
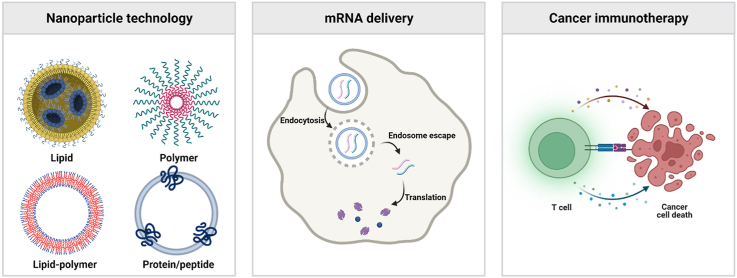
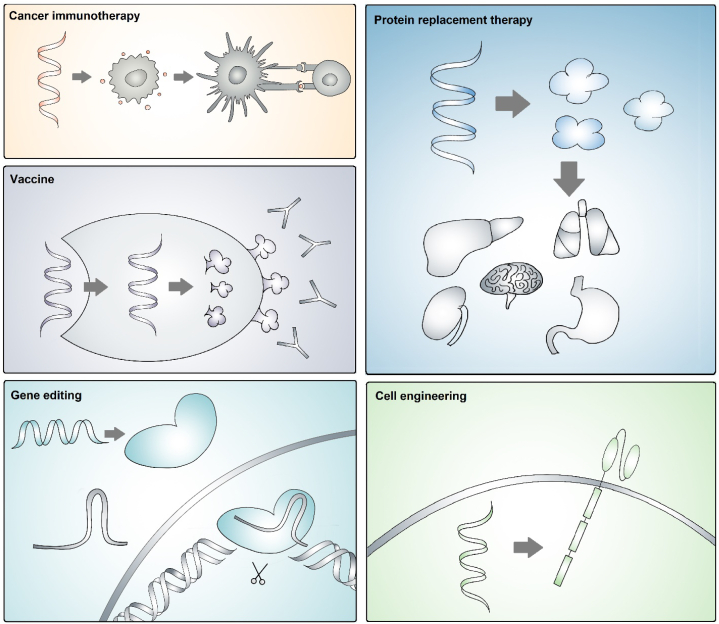
Two U.S. Food and Drug Administration (FDA)-approved coronavirus disease 2019 (COVID-19) vaccines, BNT162b and mRNA-1273 produced by Pfizer/BioNTech and Moderna, have drawn public attention to the messenger RNA (mRNA)-based therapy1,2. Over the last few decades, mRNA has emerged as an innovative and potent therapeutical strategy for cancer treatment3, 4, 5, 6. Effective mRNA engineering and delivery can be exploited in various biomedical applications through protein replacement, cell engineering7, gene editing8,9, and anti-cancer immune activation (Fig. 1)10, 11, 12, 13, 14, 15. Besides, mRNA therapeutics have shown great advantages in cellular reprogramming by integrating antigen receptors, exhibiting biocompatibility with minimal immunogenicity, and facilitating tissue and organ targeting for local or systemic administration16, 17, 18.
Figure 1.
Illustration of representative mRNA-based biomedical applications: cancer immunotherapy by activation and recruitment of immune cells; protein replacement therapy by supplementing defective or absent protein; vaccines by antigen production and presentation; utilization of clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9 (CRISPR‒Cas9) technologies for gene editing; cell engineering to modify functional cells.
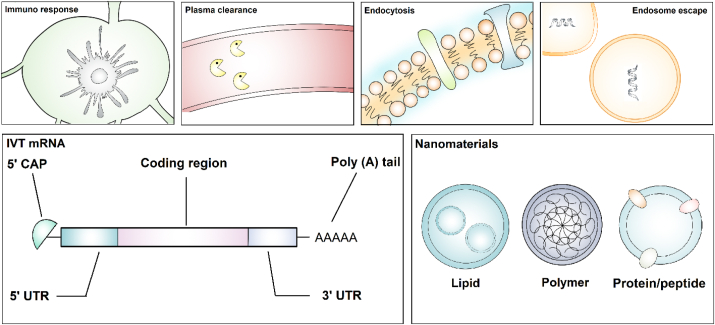
Despite the expeditious development of mRNA therapy, the delivery of the mRNA molecules is facing challenges such as undesired immune responses, exposure to systemic circulations, and inefficient cellular uptake and endosomal escape (Fig. 2)3. To encounter these barriers, mRNA is modified to prevent rapid degradation, thereby improving the systemic stability and therapeutic efficiency19, 20, 21, 22, 23, 24. General modifications include capping 5ʹ guanine and prolonging 3ʹ with repetitive adenosine tails to achieve ribosome attachment for protein translation and transportation to the cytoplasm20,25. Transcript stability along with reduced immunogenicity can be achieved by chemically editing ribonucleotides using N1/6-methyladenosine, 5-methylcytosine, 5-hydroxymethylcytosine, N1-methylpseudouridine (m1Ψ), or ribose methylation26,27.
Figure 2.
Challenges for mRNA delivery and methods to overcome these barriers, including mRNA modifications and nanomaterial-based delivery platforms.
Albeit the accomplishment in optimizing mRNA moieties, targeted cellular uptake is an urgent need for safe, precise, and efficient delivery materials. In recent decades, multiple drug delivery nanomaterials have been proposed and developed, including lipids28, 29, 30, 31, 32, polymers33, lipid‒polymer hybrid34, and protein/peptide-mediated carriers (Fig. 2)35,36. Altogether these materials are advantageous in eluding non-specific binding-induced plasma clearance, penetrating cellular membranes, releasing mRNA drugs through readily endosomal escape, and eliciting adjuvant mRNA therapeutic immune responses5,7,10,37, 38, 39, 40. In this context, a wide range of mRNA therapeutics are conducted in clinical trials for cancer immunotherapy (Table 1)20,38,41, 42, 43, 44, 45. This review describes recent advances in four main nanoparticle platforms to deliver mRNAs, including lipid, polymer, lipid-polymer hybrid, protein/peptide-mediated nanoparticles, together with their applications in delivering various mRNA modalities for cancer immunotherapies. We also present future perspectives and research directions for nanoparticle delivery systems and mRNA therapeutics.
Table 1.
Recent clinical trials of mRNA-nanoparticle therapeutics against cancer.
| Organization | Cancer type | mRNA | Nanoparticle carrier | Administration route | Phase | NCT number |
|---|---|---|---|---|---|---|
| AstraZeneca | Solid tumors | MEDI 1191 (IL-12) | LNP | Intratumoral | I | NCT03946800 |
| BioNTech SE | Prostate cancer | W_pro1/BNT 112 (targeting 5 antigens) | Liposome | IV | I/II | NCT04382898 |
| Melanoma | BNT 122 (RO7198457) | Lipoplex | IV | II | NCT03815058 | |
| BNT 111 | Lipo-MERIT | IV | N/A | NCT02410733 | ||
| Colorectal cancer | BNT 122 (RO7198457) | Lipoplex | IV | II | NCT04486378 | |
| Solid tumor | CLDN6 | Lipoplex | IV | I/II | NCT04503278 | |
| Head and neck | BNT 113 | Lipoplex | IV | II | NCT04534205 | |
| Squamous cell | BNT 113 | Lipoplex | ID | I/II | NCT03418480 | |
| Carcinoma | ||||||
| Non-small cell lung cancer (NSCLC) | BNT 116 | Lipoplex | IV | I | NCT05142189 | |
| CureVac AG | NSCLC | mRNA CV9201 | RNActive® Protamine | ID | I/II | NCT00923312 |
| mRNA CV9202 | RNActive® Protamine | ID | I/II | NCT03164772 | ||
| Hormonal refractory prostate cancer | mRNA CV9301 | RNActive® Protamine | ID | I/II | NCT00831467 | |
| Merck Sharp & Dohme LLC | Carcinoma, NSCLC, neoplasms | mRNA-5671/V941 | LNP | IM | I | NCT03948763 |
| Moderna Therapeutics Inc. | Relapsed/refractory solid tumor malignancies or lymphoma | mRNA-2752 (OX40L, IL-23, IL-36γ) | LNP (lipid5, DSPC, cholesterol, PEG) | Intratumoral | I | NCT03739931 |
| mRNA-2416 (OX40L) | LNP | Intratumoral | I/II | NCT03323398 | ||
| Solid tumors | mRNA-4157 (personalized TAAs) | LNP | IM | I | NCT03313778 | |
| Melanoma | mRNA-4157 (personalized TAAs) | LNP | IV | II | NCT03897881 | |
| Pancreatic, colorectal neoplasms, NSCLC | mRNA-5671/Merck V941 | LNP | IM | I | NCT03948763 | |
| University Medical Center Groningen | Ovarian cancer | W_ova1 vaccine | Liposome | IV | I | NCT04163094 |
| University of Florida | Melanoma | Autologous total tumor mRNA | DOTAP liposome | IV | I | NCT05264974 |
| Adult glioblastoma | Autologous total tumor and full-length LAMP mRNA | DOTAP liposome | IV | I | NCT04573140 | |
| Changhai Hospital, Stemirna Therapeutics (layout) | Advanced esophageal | Personalized mRNA tumor vaccine | Lipopolyplex | SQ | N/A | NCT03468244 |
| Squamous carcinoma | ||||||
| Gastric adenocarcinoma | ||||||
| Pancreatic adenocarcinoma | ||||||
| Colorectal adenocarcinoma |
2. Nanoparticle-mediated mRNA delivery for cancer treatments
2.1. Lipid nanoparticles (LNPs)
Lipid nanoparticles (LNPs) have emerged as promising and widely used platforms to deliver mRNA therapeutics45. Typically, LNPs consist of cationic/ionizable lipids to encapsulate mRNA molecules through electronic interaction, helper lipids such as cholesterol, phospholipid, and polyethylene glycol (PEG)–lipids to maintain particle stability and compatibility. However, certain cationic lipids were reported to induce organ injuries including liver and spleen toxicity45, 46, 47. In addition, PEG-lipids may induce expression of anti-PEG which might lead to rapid clearance of LNPs43,45,48, 49, 50, 51. Therefore, efforts have been made to developing lipid materials with high delivery efficiency, organ/cell targeting, and low toxicity. Currently, many mRNA drugs utilizing LNPs as delivery systems are under clinical investigations for the treatment of various solid tumors20,45,52, 53, 54.
In 2018, the FDA approved ionizable lipid, (6Z,9Z,28Z,31Z)-heptatriaconta-6,9,28,31-tetraen-19-yl 4-(dimethylamino) butanoate (MC3), for siRNA delivery55. More recently, MC3-based LNPs were used as delivery systems in mRNA-based cancer immunotherapy. For example, Wang et al.56 utilized MC3-based LNPs to deliver mRNAs encoding chemokine (C‒C motif) ligand 2 (CCL2) and chemokine (C‒C motif) ligand 5 (CCL5) linked by a single domain antibody (BisCCL2/5i). Intravenous administration of the BisCCL2/5i mRNA-LNP blocked both chemokine ligand signaling pathways and polarized the macrophages towards cancer inhibitory phenotype (M1), thereby achieving a 50% survival rate in pancreatic cancer liver metastasis mouse models. Co-administration of BisCCL2/5i mRNA-LNP with PD-1 ligand inhibitor (PD-Li) resulted in a 57% complete response, establishing a potential therapeutic strategy targeting liver malignancy56.
Also, the need for higher mRNA delivery efficiency drives the optimization of lipid structure57,58. For example, Sabnis et al.59 reported a MC3-derived material lipid 5, heptadecan-9-yl 8-((2-hydroxyethyl) (8-(nonyloxy)-8-oxooctyl) amino) octanoate, by substituting MC3 linoleic tail with a new one containing ester lipids. This optimization exploits a similar cellular uptake mechanism through an apolipoprotein E-mediated low-density lipoprotein-dependent manner with less organelle aggregation driven by spatial configuration59. Hewitt et al.60 leveraged this lipid 5 delivery platform to deliver interleukin 12 (IL12)-encoding mRNA for cancer immunotherapy. The expression of IL12 stimulated the upregulation of T-helper 1 type (TH1) immune response genes, CD8+ T cell production, and interferon-γ (IFN-γ) expression, resulting in tumor shrinkage and prolonged survival time. In MC38 mouse models with colon adenocarcinoma, a single intratumorally injection of (murine)IL-12 mRNA-LNP resulted in around 86% complete tumor clearance rate, and nearly all mice were resistant to tumor rechallenge. Furthermore, an (human)IL-12 mRNA-LNP therapy (MEDI1191) is currently active in phase I clinical trial with 87 patients enrolled for solid tumor treatment (NCT03946800)60. Hewitt et al.61 also used lipid 5-based LNP to deliver a tri-mRNA encoding IL-23, IL-36γ, and tumor necrosis factor receptor superfamily member 4 ligand (OX 40L). The IL-23/IL-36γ/OX40L triplet mRNA-LNP is under phase I clinical trial for multiple solid tumor treatment (NCT03739931). In murine colon and hepatocellular carcinoma models, the tri-mRNA-LNP triggered cytokine and chemokine expression and increased complete response rates. Furthermore, four weekly injections of tri-mRNA-LNP resulted in an over 70% complete recovery rate, demonstrating the low toxicity and reproducibility of the lipid 5 delivery system during metabolism and elimination61. Likewise, Li et al.58 delivered mRNA encoding OX40 for expressing costimulatory receptors in combination with an anti-OX40 antibody using a phospholipid-derived LNP system (PL1-LNPs). The combination of PL1-OX40 mRNA and anti-OX40 antibody showed a 60% complete response rate in A20 mouse models. The study also revealed that this combinational therapy significantly enhanced the immune response to anti-programmed cell death protein 1 antibodies (αPD-1) and anti-cytotoxic T-lymphocyte-associated antigen 4 antibodies (αCTLA-4) in the B16F10 tumor model58.
Besides, LNPs are applicable in activating adaptive immune responses by delivering tumor antigens mRNAs to induce long-term anti-tumor effects62, 63, 64. Recently, Moderna reported the phase I clinical trial results of LNP-delivered mRNA-4157 encoding tumor-associated neoantigens for solid tumor treatment (NCT03897881). Patients were intramuscularly injected once every three weeks for up to 9 cycles. 11 out of 13 patients remained disease free during the study (3 with melanoma, 8 with non-small cell lung cancer (NSCLC), and 2 with colorectal cancer). The trial is currently moving forward to phase II stage for efficacy study with 157 patients enrolled65. In a pre-clinical study, Zhang et al.62 reported that an ovalbumin OVA (257-264)-encoded mRNA-lipid nanoparticle system exhibited therapeutic efficacy in treating OVA-specific mouse colon cancer models. Here, the formulation consists of synthetic lipidoid material C1 (PAMAM dendrimer G0 mixed with 1,2-epoxydodecane) and 1,2-distearoyl-sn-glycerol-3-Phosphoethanolamine-N-[amino(polyethylene glycol)-2000 (DSPE-PEG2000). The mRNA-lipid nanoparticles enabled the expression of OVA (257-264) in dendritic cells, thereby activating the immune system via Toll-like receptor 4 signaling pathway. After three subcutaneous injections in the B16-OVA mouse model, the tumor size in the OVA-mRNA/LNP treated group was 3-fold smaller than that in the PBS control62. In another study, Oberli et al.63 screened an ionizable lipid library and identified a lead formulation using (3,6-bis[4-[bis(2-hydroxydodecyl)amino]butyl]-2,5-piperazinedione (cKK-E12) to delivery mRNAs encoding OVA, glycoprotein 100 (gp100), and tyrosinase-related protein 2 (TRP2). Intravenous injection of OVA mRNA LNP promoted CD8 T cell activation, leading to a two-fold longer survival time and a 2.5-fold reduction in tumor size in the xenografted B16F10 mouse models. The gp100 mRNA treatment dramatically reduced tumor size by more than twofold, and co-administration of gp100 and TRP2 mRNAs prolonged the survival time by 20 days63. Using the same formulation, Rybakova et al.64 delivered mRNA encoding anti-human epidermal growth factor receptor 2 (HER2) antibody to xenografted mice with human breast cancer. Compared with HER2-negative tumor growth, HER2-positive tumors were four times smaller after the treatment. Notably, pharmacokinetic results such as the area under the curve and half-life in mRNA therapy outperformed those of single antibody-based administration.
Leveraging LNPs to deliver gene-editing systems is another commonly used strategy for cancer immunotherapy16,66. For example, Rosenblum et al.67 used lipid 8-based LNPs to knockdown polo-like kinase 1 (PLK1) gene in xenograft mouse models by co-delivering Cas9 and single-guide RNA encoding polo-like kinase (sgPLK1). Intracerebral injection of Lipid 8-Cas9/sgPLK1 mRNA into glioblastoma (GBM)-bearing mice resulted in up to 70% gene being edited, and intraperitoneal injection into OV8 xenograft mice achieved 80% gene editing efficiency67,68. Liu et al.69 used the disulfide-integrated LNP BAMEA-O16B to deliver Cas9/sgGFP to human embryonic kidney cells with 90% knockout efficiency. Intravenous injection of BAMEA-O16B-Cas9/sg-proprotein convertase subtilisin/kexin type 9 (PCSK9) demonstrated high accumulation in mice hepatocytes, broadening liver-targeted therapeutic strategies of CRISPR-Cas9 mRNA delivery. Zhang et al.70 developed a biodegradable lipid-like N-methyl-1,3-propanediamine (MPA-Ab) for Cas9/sgGFP delivery and the result showed a 41% reduction in fluorescence signal via intratumoral injection into 293T xenografted mouse models. Miller et al.71 reported that ZA3-Ep10, an LNP composed of a zwitterionic amino phospholipid, cholesterol, and PEG, delivered Cas9/sgLoxP to induce tdTomato expression in livers, kidneys, and lungs of engineered mouse models.
2.2. Polymeric nanoparticles
Polymeric nanoparticles have been developed for decades to deliver nucleic acids. The advantages of polymeric nanoparticles lie in their tunability and high encapsulation capability. On one hand, the morphological structures of the polymeric nanoparticles can be tuned by combining different monomer ratios72,73. On the other, if positively charged, polymers can bind with mRNAs through electrostatic interactions. Accordingly, polymeric nanoparticles have been formulated for mRNA delivery and applied in cancer immunotherapy. However, particle aggregation issues and low delivery efficiency greatly limit the application of polymeric nanoparticles43,74. Therefore, a number of co-polymers have been developed to address these limitations. For example, Zhang et al.75 developed a polymeric nanoparticle composed of poly (beta-amino ester) (PbAE), poly-glutamic acid (PGA), and Di-mannose moieties to deliver mRNAs encoding transcriptional regulatory factor 5 (IRF5) and inhibitor of nuclear factor kappa-B kinase subunit beta (IKKβ) to treat ovarian cancer, melanoma, and glioblastoma. Expression and presentation of IRF5/IKKβ in tumor-associated macrophages induced immune responses through boosting secretions of IL-12, IFN-γ, and tumor necrosis factor alpha (TNF-α), resulting in an increase of M1-like macrophage proportion by twentyfolds75. To explore a therapeutic efficiency for prostate cancer, the group modified the polymeric nanoparticles to target circulating T cells by replacing the mannose moieties with an anti-CD8 antibody76. They transplanted LNCaP C42 prostate cancer cells into NSG mice and treated these mice intravenously with anti-receptor tyrosine kinase-like orphan receptor 1 (antiROR1)-mRNA nanoparticles once per week for six weeks. The survival rate of mice treated with anti-ROR1-mRNA-NP was twofold higher compared with the control group76.
Polymeric nanoparticles carrying mRNAs encoding specific tumor antigens can also induce long-term effects of antitumor immunity by activating antigen-specific immune cells. Haabeth et al.77 developed a polymer named charge-altering releasable transporters (CARTs) to deliver an OVA-encoded mRNA in a B-cell lymphoma-bearing mouse model. As synthetic oligomers, CARTs are advantageous in their degradation mechanism from polycationic backbone into neutral molecules, therefore facilitating endosomal escape and mRNA payload release in the cytoplasm. Composed of 13 lipid blocks and 11 cationic blocks, CART D13:A11 encapsulated with OVA-mRNA was injected into A20-OVA mice. CART D13:A11/OVA-mRNA induced activation of antigen-specific CD4+ and CD8+ T cells, thereby fulfilling the therapeutic efficiency presented by tumor volume reduction and resistance to re-challenge77,78. CART delivery system was also used to deliver enhanced green fluorescent protein (EGFP) mRNA to natural killer (NK) cells. Compared to commercially available lipofectamine, CART O5:N6:A9, showed more efficient delivery in NK cells with minimal phenotypic changes79,80.
Abbasi et al.81 delivered both Cas mRNA and Ai9 sgRNA to knock out tdTomato STOP cassette using a PEGylated polyplex (polyethylene glycol-b-poly{N-[N-(2-aminoethyl)-2aminoethyl] aspartamide}) (PAsp(DET)). Intraparenchymal injection of PM-Cas/Ai9 sgRNA into transgenic mice models revealed tdTomato expression. Yan et al.82 formulated a polymeric nanoparticle that contained polyester coupled with A17 (Cysteamine hydrochloride) and C12 (1-Dodecanethiol) modifications. An outer layer of Pluronic F127 was assembled in a ratio of 5% for in vivo stabilization by utilizing poly (propylene oxide) segments to leave hydrophobic PEG regions in the outer layer to protect the particle from aggregation and protein absorption. They delivered luciferase-encoded mRNAs to non-obese diabetic/severe combined immunodeficiency mouse models to observe optimal expression via fluorescent visualization. The result showed increased signal intensity in the lungs by twofold, indicating the nanoparticle PE4K-A17-0.33C12 a potential platform to deliver mRNAs in lung-targeting cancer treatments82.
2.3. Lipid-polymer hybrid nanoparticles
Lipid-polymer hybrid nanoparticles generally refer to nanoparticles comprising of polymers and lipid components83. Since these hybrid nanoparticles leverage complementary characteristics of lipid and polymer materials, they are considered promising platforms for mRNA delivery. For example, Kong et al.84 used synthetic lipopolymer hybrid nanoparticles to deliver p53-mRNA for the restoration of tumor suppressors. This formulation consists of an ionizable lipid GO-C14 for mRNA condensation and cytoplasmic transport, a poly-(disulfide amide) that rapidly releases payloads by disrupting disulfide bonds when exposed to a glutathione (GSH)-rich tumor environment, DSPE-PEG2000, and 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)] (DMPE-PEG) to prevent degradation. The intravenous delivery of p53-mRNA-NP delayed cancer growth by triggering cell cycle arrest and apoptosis. Furthermore, co-administration of p53-mRNA-NP with the immunosuppressant everolimus achieved almost complete tumor clearance in p53-null liver cancers and metastasis muse models84. In addition to p53 restoration for potential cancer therapy, Islam et al.85 reported the mRNA delivery of PTEN, another tumor suppressor gene, for prostate cancer therapy. Modifications to the previous formulation include replacing poly (disulfide amide) (PDSA) with poly (lactic-co-glycolic acid) (PLGA) for core stabilization with higher biocompatibility and biodegradability, as well as substituting SPE-PEG2000/DMPE-PEG mixture with ceramide-PEG for higher drug retention. Facilitated with micropinocytosis and released by the proton-sponge effect, PTEN-mRNA-PGCP NP reduced PTEN-null PCa LNCaP and LN3 subclone cell viability via inhibiting PI3K-AKT signaling pathway. Six intravenous injections of PTEN-mRNA-NP in PCa xenografts and advanced PCa mouse models showed tumor sizes five and fourfold smaller than those treated with PBS and EGFP-mRNA-NP controls, respectively85. In both studies, restoration of tumor suppressor genes in null animal models provides a potential approach to reactivate cancer immune responses to kill tumors.
Lipid-polymer hybrid nanoparticles can also deliver tumor-specific antigens to activate adaptive immune responses to fight against cancers. For example, Persano et al.86 developed a lipopolyplex (LPP) to deliver OVA-encoded mRNA to treat lung metastases of murine B16 tumors. This hybrid nanoparticle contains a poly(beta-aminoester) core wrapped in a lipid shell made of 49% 1,2-dioleoyl-sn-glycero-3-ethylphosphorylcholine (EDOPC), 49% DOPE, and 2% DSPE-PEG-2000. Following cellular entry via micropinocytosis, OVA-mRNA-LPP promoted dendritic cell maturation indicated by upregulated expression of INF-α, INF-β, and IL-12 in C57BL/6 mice compared with PBS control. In B16-OVA lung metastasis mouse models, the reduction in the number of lung nodules, overexpression of IFN-γ, and activation of TRP2-positive CD8+ T cells demonstrated anti-tumor efficiency after three OVA-mRNA-LPP tail vein administrations86.
The introduction of anti-angiogenic factors by hybrid nanoparticles is another approach to inhibit tumor growth by minimizing the tumor vascular network and preventing newly formed blood vessels. Uchida et al.87 designed a lipopolymer-based nanoparticle to deliver anti-angiogenic protein sFlt-1-encoded mRNA to BxPC3 xenograft mouse models. The nanoparticles were prepared by introducing a cholesterol moiety to the block polymer made of poly[N-{Nʹʹ-[Nʹʹʹ-(2-aminoethyl)-2-aminoethyl]-2-aminoethyl}2-aminoethyl) aspartamide] (PEG-PASp(TEP)-Chol). Compared with HEPES-treated controls, intravenous administration of the sFlt-1 mRNA-loaded lipopolymer NPs substantially decreased the relative tumor volume by fourfold, along with reduced blood vessel density around the tumor area87.
Zhao et al.88 reported luciferase mRNA delivery via a lipid polymer hybrid nanoparticle (LPN) composed of a PLGA4 core and a lipid shell prepared from N1,N3,N5-tris(3-(didodecylamino)propyl)benzo-1), 3,5-tricarboxamide (TT3), DOPE, cholesterol, and DMG-PEG2000. Luci-mRNA/PLGA4-LPN increased luciferase intensity by twofold compared with TT3 lipid nanoparticles. Xiong et al.89 formulated a theranostic dendrimer-based lipid nanoparticle (DLNP) from dendrimer 4A3-SC8, DOPE, Cholesterol, and PBD-lipid which was prepared by a PEG-DMG-2000 connected to 4,4-difluoro-4-bora-3a, 4a-diaza-s-indacene (BODIPY) core with an aryl linker. High bioluminescence intensity around tumor areas was observed in SUM159 breast cancer xenografted mouse models 6 h post intratumoral injections of Luci-mRNA-DLNP.
2.4. Protein/peptide-mediated nanoparticles
Proteins or peptides-mediated nanoparticles are another important mRNA carrier. Due to the nature of macro-biomolecules, the sizes of protein/peptide-mediated nanoparticles are large enough to avoid rapid clearance in the blood circulation before reaching target sites. Moreover, protein/peptide employs a unique mechanism to penetrate targeted cell membranes through direct translocation via ligand-receptor interactions35,36,90.
Clinical studies have been conducted for cancer immunotherapy by delivering mRNAs encoding tumor-associated antigens through a self-adjuvant RNActive® platform, a mixture of protamine-mRNA and free mRNA at a weight ratio of 1:2. For example, CV9103 (NCT00831467) is a clinical trial that investigates the use of RNActive®/mRNAs encoding PSA, PSCA, PSMA, and STEAP1 for prostate cancer treatment91. CV9201 (NCT00923312) delivers MAGE-C1, MAGE-C2, NY-ESO-1, survivin, and 5T4-encoded mRNAs to treat stage IIIB/IV disease of NSCLC92,93. Patients in both studies were administered intradermally in weeks 1, 3, 7, 15, and 23. In the CV9301 study, 76 percent of patients elicited detectable cellular immune responses. Among these responders, the median overall survival was 29.3 months for non-metastatic disease patients and 31.4 months for metastatic disease patients91. In CV9201 phase II trial, the survival rates for 1, 2, and 3 years were 44.4%, 26.7%, and 20.7%, respectively93.
Besides, pre-clinical studies involved delivering OVA, a commonly used tumor antigen, in the form of mRNA by Udhayakumar et al.94 using cell-penetrating peptide nanocomplexes. The nanoparticle contained a RALA peptide made of 30 amino acids with rich arginine and lysine to condensate mRNA by charge and an alanine- and leucine-rich hydrophobic layer shield. The OVA-mRNA/RALA complex increased inductions of INF-β and IL-6 transcript levels by three-hundred and seven fold, respectively. Remarkably, the RALA complex, if incorporated with pseudouridine and 5-methylcytidine modified mRNA, instigated cytotoxic T-cell immunity to achieve a 70% antigen-specific killing rate94.
Mai et al.95 delivered mRNA encoding cytokeratin 19 (CK19), a protein widely expressed in epithelial cell membranes, to elicit anti-tumor responses through a protamine-liposome nanoparticle system containing protamine, dioleoyl-3-trimethylammonium propane (DOTAP), cholesterol, and DSPE-PEG. The cell-penetrating properties of these arginine-rich peptides allowed CK19-mRNA-NP to target aggressive Lewis lung cancer cells in a mouse model. Through intranasal administration, CK19-mRNA-NP accelerated dendritic cell activation by overexpressing MHC II molecules and CD86, resulting in substantial inhibitory effect by at least four-fold reduction of tumor volume95. A similar protamine-liposome system was applied to deliver interleukin-encoded mRNAs for the regulation of cell growth, differentiation, and activation, thereby modulating inflammation and immune responses. Lei et al.96 delivered IL15-mRNA to fight against colon cancer via a protamine/liposome system (CLPP) incorporating 50% protamine, 25% DOTAP, and 25% cholesterol. The overexpression of IL-15 proteins by IL15-mRNA-CLPP subsequently increased lymphocyte viability, activated T cells and NK cells, and initiated TNF-α signaling pathway. The safety and efficiency of CLPP were also demonstrated in C26 xenograft and C26 lung metastasis mouse models through intraperitoneal administration, which was evidenced by a four-fold reduction in tumor weight without significant pathological changes96.
The CLPP carrier system was also utilized to deliver an mRNA encoding Survivin-T34A to treat C26 colon cancer97. Through a lipid raft-mediated pathway, Survivin-T34A-mRNA-CLPP penetrated cell membranes and exposed mRNAs for protein expression. The result showed a 56% cellular proliferation inhibition supported by elevated expression levels of TNF-α, IFN-γ, and IL-6 cytokines, along with the infiltration of CD4+, CD8+, NK, and macrophage cells, shifting the cellular microenvironment to the tumor-suppressive phase. In C26 abdominal cavity and pulmonary metastatic mouse models, therapeutic efficiency achieved a thirty fold reduction in nodule weight and 78% inhibition of metastatic growth97. Wang et al.98 delivered herpes simplex virus 1-thymidine kinase (HSV1-tk)-encoded mRNA to nude mice bearing NSCLC H460 xenografts. The lipid/protamine/mRNA (LPR) formulation contains a protamine-mRNA core encapsulated by a self-assembling DOTAP/cholesterol liposome, surrounded by DSPE-PEG and DSPE-PEG-AA. The coadministration of ganciclovir (GCV) as a prodrug system inhibited tumor growth and reduced tumor volume by more than twenty fold compared with the untreated group98.
Gao et al.99 designed a fusion of two cell-penetrating peptides, cRGD-R9, to facilitate cellular uptake by a cyclic Arg-Gly-Asp for delivering mRNAs encoding Bim, another suicide gene, into C26 colon xenografted mice. By fusing these peptides to DMP nano skeletons containing DOTAP and methoxy poly (ethylene glycol)-poly(ε-caprolactone) (mPEG-PCL), they described multiple cell-penetrating mechanisms of the formulation, including pinocytosis, caveolin- and clathrin-mediated endocytosis. Therapeutic data showed a more than 97% inhibition of C26 cancer cell growth and tumor shrinkage in pulmonary metastasis mouse models through local administration99.
3. Conclusions
The nanoparticle advancements have led to an increasing number of mRNA-related clinical trials over the past decade, particularly after the emergence of COVID-19 mRNA vaccines. Despite the significant progress, mRNA-based cancer immunotherapy is still in its early stage and thus more investigations are needed to elaborate pharmacokinetic profiles, improve the efficacy, and minimize toxicity43,90,100. Although utilizations of highly biodegradable materials greatly improve the delivery of mRNA-based drugs, how to maintain the balance between efficacy and safety remains a concern for clinical translation of mRNA-based cancer treatment. Collectively, with the development of functional nanoparticle-based delivery systems, mRNA may provide revolutionary and promising therapeutic effects for cancer immunotherapy.
Acknowledgments
Yizhou Dong acknowledges the support from the Maximizing Investigators' Research Awards (R35GM119679, USA) and (R35GM144117, USA) from the National Institute of General Medical Sciences.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Author contributions
Yichen Zhong, Shi Du, and Yizhou Dong: writing and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
Yizhou Dong is a scientific advisory board member of Oncorus Inc, Arbor Biotechnologies, and FL85. Other authors declare no conflicts of interest.
References
- 1.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu A.M., Choi Y.H., Tu M.J. RNA drugs and RNA targets for small molecules: principles, progress, and challenges. Pharmacol Rev. 2020;72:862–898. doi: 10.1124/pr.120.019554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics — developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 5.Kaczmarek J.C., Kowalski P.S., Anderson D.G. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9:60. doi: 10.1186/s13073-017-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao W., Hou X., Vick O.G., Dong Y. RNA delivery biomaterials for the treatment of genetic and rare diseases. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue Y., Che J., Ji X., Li Y., Xie J., Chen X. Recent advances in biomaterial-boosted adoptive cell therapy. Chem Soc Rev. 2022;51:1766–1794. doi: 10.1039/d1cs00786f. [DOI] [PubMed] [Google Scholar]
- 8.Yan J., Kang D.D., Turnbull G., Dong Y. Delivery of CRISPR-Cas9 system for screening and editing RNA binding proteins in cancer. Adv Drug Deliv Rev. 2022;180 doi: 10.1016/j.addr.2021.114042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X., Zhao W., Nguyen G.N., Zhang C., Zeng C., Yan J., et al. Functionalized lipid-like nanoparticles for in vivo mRNA delivery and base editing. Sci Adv. 2020;6 doi: 10.1126/sciadv.abc2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong Q., Lee G.Y., Ding J., Li W., Shi J. Biomedical applications of mRNA nanomedicine. Nano Res. 2018;11:5281–5309. doi: 10.1007/s12274-018-2146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel S., Athirasala A., Menezes P.P., Ashwanikumar N., Zou T., Sahay G., et al. Messenger RNA delivery for tissue engineering and regenerative medicine applications. Tissue Eng Part A. 2019;25:91–112. doi: 10.1089/ten.tea.2017.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo D., Saltzman W.M. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 14.Chandler R.J. Messenger RNA therapy as an option for treating metabolic disorders. Proc Natl Acad Sci USA. 2019;116:20804–20806. doi: 10.1073/pnas.1914673116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao Y., Tang Z., Huang X., Chen W., Zhou J., Liu H., et al. Emerging mRNA technologies: delivery strategies and biomedical applications. Chem Soc Rev. 2022;51:3828–3845. doi: 10.1039/d1cs00617g. [DOI] [PubMed] [Google Scholar]
- 16.Yan J., Kang D.D., Dong Y. Harnessing lipid nanoparticles for efficient CRISPR delivery. Biomater Sci. 2021;9:6001–6011. doi: 10.1039/d1bm00537e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damase T.R., Sukhovershin R., Boada C., Taraballi F., Pettigrew R.I., Cooke J.P. The limitless future of RNA therapeutics. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.628137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di C., Syafrizayanti, Zhang Q., Chen Y., Wang Y., Zhang X., et al. Function, clinical application, and strategies of pre-mRNA splicing in cancer. Cell Death Differ. 2019;26:1181–1194. doi: 10.1038/s41418-018-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gómez-Aguado I., Rodríguez-Castejón J., Vicente-Pascual M., Rodríguez-Gascón A., Solinís M.Á., del Pozo-Rodríguez A. Nanomedicines to deliver mRNA: state of the art and future perspectives. Nanomaterials. 2020;10:364. doi: 10.3390/nano10020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo S., Vieweger M., Zhang K., Yin H., Wang H., Li X., et al. Ultra-thermostable RNA nanoparticles for solubilizing and high-yield loading of paclitaxel for breast cancer therapy. Nat Commun. 2020;11:972. doi: 10.1038/s41467-020-14780-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roundtree I.A., Evans M.E., Pan T., He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauger D.M., Cabral B.J., Presnyak V., Su S.V., Reid D.W., Goodman B., et al. mRNA structure regulates protein expression through changes in functional half-life. Proc Natl Acad Sci U S A. 2019;116:24075–24083. doi: 10.1073/pnas.1908052116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boo S.H., Kim Y.K. The emerging role of RNA modifications in the regulation of mRNA stability. Exp Mol Med. 2020;52:400–408. doi: 10.1038/s12276-020-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramanathan A., Robb G.B., Chan S.-H. mRNA capping: biological functions and applications. Nucleic Acids Res. 2016;44:7511–7526. doi: 10.1093/nar/gkw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi H., Chai P., Jia R., Fan X. Novel insight into the regulatory roles of diverse RNA modifications: re-defining the bridge between transcription and translation. Mol Cancer. 2020;19:78. doi: 10.1186/s12943-020-01194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H., Yao J., Bao R., Dong Y., Zhang T., Du Y., et al. Cross-talk of four types of RNA modification writers defines tumor microenvironment and pharmacogenomic landscape in colorectal cancer. Mol Cancer. 2021;20:29. doi: 10.1186/s12943-021-01322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z., Kuo J.C.T., Zhang C., Huang Y., Zhou Z., Lee R.J. A squalene-based nanoemulsion for therapeutic delivery of resiquimod. Pharmaceutics. 2021;13:2060. doi: 10.3390/pharmaceutics13122060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z., Kuo J.C.T., Zhang C., Huang Y., Lee R.J. Ivermectin enhanced antitumor activity of resiquimod in a co-loaded squalene emulsion. J Pharmaceut Sci. 2022;111:3038–3046. doi: 10.1016/j.xphs.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Li B., Luo X., Deng B., Wang J., McComb D.W., Shi Y., et al. An orthogonal array optimization of lipid-like nanoparticles for mRNA delivery in vivo. Nano Lett. 2015;15:8099–8107. doi: 10.1021/acs.nanolett.5b03528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao L., Lin J., Huang Y., Li L., Delcassian D., Ge Y., et al. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat Commun. 2020;11:2424. doi: 10.1038/s41467-020-16248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Sun C., Wang C., Jankovic K.E., Dong Y. Lipids and lipid derivatives for RNA delivery. Chem Rev. 2021;121:12181–12277. doi: 10.1021/acs.chemrev.1c00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie J., Gonzalez-Carter D., Tockary T.A., Nakamura N., Xue Y., Nakakido M., et al. Dual-sensitive nanomicelles enhancing systemic delivery of therapeutically active antibodies specifically into the brain. ACS Nano. 2020;14:6729–6742. doi: 10.1021/acsnano.9b09991. [DOI] [PubMed] [Google Scholar]
- 34.Xue Y., Feng J., Liu Y., Che J., Bai G., Dong X., et al. A synthetic carrier of nucleic acids structured as a neutral phospholipid envelope tightly assembled on polyplex surface. Adv Healthc Mater. 2020;9 doi: 10.1002/adhm.201901705. [DOI] [PubMed] [Google Scholar]
- 35.Miao Y., Yang T., Yang S., Yang M., Mao C. Protein nanoparticles directed cancer imaging and therapy. Nano Converg. 2022;9:2. doi: 10.1186/s40580-021-00293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandra F., Khaliq N.U., Sunna A., Care A. Developing protein-based nanoparticles as versatile delivery systems for cancer therapy and imaging. Nanomaterials. 2019;9:1329. doi: 10.3390/nano9091329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchida S., Perche F., Pichon C., Cabral H. Nanomedicine-based approaches for mRNA delivery. Mol Pharm. 2020;17:3654–3684. doi: 10.1021/acs.molpharmaceut.0c00618. [DOI] [PubMed] [Google Scholar]
- 38.Miao L., Zhang Y., Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho W., Gao M., Li F., Li Z., Zhang X., Xu X. Next-generation vaccines: nanoparticle-mediated DNA and mRNA delivery. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li B., Zhang X., Dong Y. Nanoscale platforms for messenger RNA delivery. WIREs Nanomed Nanobiotechnol. 2019;11 doi: 10.1002/wnan.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alfarouk K.O., Stock C.-M., Taylor S., Walsh M., Muddathir A.K., Verduzco D., et al. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. doi: 10.1186/s12935-015-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorentzen C.L., Haanen J.B., Met Ö., Svane I.M. Clinical advances and ongoing trials of mRNA vaccines for cancer treatment. Lancet Oncol. 2022;23:e450–e458. doi: 10.1016/S1470-2045(22)00372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang X., Kong N., Zhang X., Cao Y., Langer R., Tao W. The landscape of mRNA nanomedicine. Nat Med. 2022;28:2273–2287. doi: 10.1038/s41591-022-02061-1. [DOI] [PubMed] [Google Scholar]
- 45.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kedmi R., Ben-Arie N., Peer D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials. 2010;31:6867–6875. doi: 10.1016/j.biomaterials.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 47.Sedic M., Senn J.J., Lynn A., Laska M., Smith M., Platz S.J., et al. Safety evaluation of lipid nanoparticle–formulated modified mRNA in the Sprague-Dawley rat and Cynomolgus monkey. Vet Pathol. 2018;55:341–354. doi: 10.1177/0300985817738095. [DOI] [PubMed] [Google Scholar]
- 48.Knop K., Hoogenboom R., Fischer D., Schubert U.S. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 49.Wang X., Ishida T., Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release. 2007;119:236–244. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 50.McSweeney M.D., Wessler T., Price L.S.L., Ciociola E.C., Herity L.B., Piscitelli J.A., et al. A minimal physiologically based pharmacokinetic model that predicts anti-PEG IgG-mediated clearance of PEGylated drugs in human and mouse. J Control Release. 2018;284:171–178. doi: 10.1016/j.jconrel.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abu Lila A.S., Kiwada H., Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release. 2013;172:38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 52.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4 doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim S.A., Cox A., Tung M., Chung E.J. Clinical progress of nanomedicine-based RNA therapies. Bioact Mater. 2022;12:203–213. doi: 10.1016/j.bioactmat.2021.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kong N., Zhang R., Wu G., Sui X., Wang J., Kim N.Y., et al. Intravesical delivery of KDM6A-mRNA via mucoadhesive nanoparticles inhibits the metastasis of bladder cancer. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2112696119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoy S.M. Patisiran: first global approval. Drugs. 2018;78:1625–1631. doi: 10.1007/s40265-018-0983-6. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Tiruthani K., Li S., Hu M., Zhong G., Tang Y., et al. mRNA delivery of a bispecific single-domain antibody to polarize tumor-associated macrophages and synergize immunotherapy against liver malignancies. Adv Mater. 2021;33 doi: 10.1002/adma.202007603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J.-Q., Zhang C., Zhang X., Yan J., Zeng C., Talebian F., et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J Control Release. 2022;345:306–313. doi: 10.1016/j.jconrel.2022.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li W., Zhang X., Zhang C., Yan J., Hou X., Du S., et al. Biomimetic nanoparticles deliver mRNAs encoding costimulatory receptors and enhance T cell mediated cancer immunotherapy. Nat Commun. 2021;12:7264. doi: 10.1038/s41467-021-27434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sabnis S., Kumarasinghe E.S., Salerno T., Mihai C., Ketova T., Senn J.J., et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol Ther. 2018;26:1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hewitt S.L., Bailey D., Zielinski J., Apte A., Musenge F., Karp R., et al. Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin Cancer Res. 2020;26:6284–6298. doi: 10.1158/1078-0432.CCR-20-0472. [DOI] [PubMed] [Google Scholar]
- 61.Hewitt S.L., Bai A., Bailey D., Ichikawa K., Zielinski J., Karp R., et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aat9143. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H., You X., Wang X., Cui L., Wang Z., Xu F., et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2005191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oberli M.A., Reichmuth A.M., Dorkin J.R., Mitchell M.J., Fenton O.S., Jaklenec A., et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rybakova Y., Kowalski P.S., Huang Y., Gonzalez J.T., Heartlein M.W., DeRosa F., et al. mRNA delivery for therapeutic anti-HER2 antibody expression in vivo. Mol Ther. 2019;27:1415–1423. doi: 10.1016/j.ymthe.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burris H.A., III, Patel M.R., Cho D.C., Clarke J.M., Gutierrez M., Zaks T.Z., et al. A phase 1, open-label, multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in subjects with resected solid tumors and in combination with pembrolizumab in subjects with unresectable solid tumors. J Glob Oncol. 2019;5:93. [Google Scholar]
- 66.Finn J.D., Smith A.R., Patel M.C., Shaw L., Youniss M.R., van Heteren J., et al. A single administration of CRISPR/Cas9 lipid manoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 2018;22:2227–2235. doi: 10.1016/j.celrep.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 67.Rosenblum D., Gutkin A., Kedmi R., Ramishetti S., Veiga N., Jacobi A.M., et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci Adv. 2020;6 doi: 10.1126/sciadv.abc9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramishetti S., Hazan-Halevy I., Palakuri R., Chatterjee S., Naidu Gonna S., Dammes N., et al. A combinatorial library of lipid nanoparticles for RNA delivery to leukocytes. Adv Mater. 2020;32 doi: 10.1002/adma.201906128. [DOI] [PubMed] [Google Scholar]
- 69.Liu J., Chang J., Jiang Y., Meng X., Sun T., Mao L., et al. Fast and efficient CRISPR/Cas9 genome editing in vivo enabled by bioreducible lipid and messenger RNA nanoparticles. Adv Mater. 2019;31 doi: 10.1002/adma.201902575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X., Li B., Luo X., Zhao W., Jiang J., Zhang C., et al. Biodegradable amino-ester nanomaterials for Cas9 mRNA delivery in vitro and in vivo. ACS Appl Mater Interfaces. 2017;9:25481–25487. doi: 10.1021/acsami.7b08163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller J.B., Zhang S., Kos P., Xiong H., Zhou K., Perelman S.S., et al. Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew Chem Int Ed Engl. 2017;56:1059–1063. doi: 10.1002/anie.201610209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Islam M.A., Reesor E.K.G., Xu Y., Zope H.R., Zetter B.R., Shi J. Biomaterials for mRNA delivery. Biomater Sci. 2015;3:1519–1533. doi: 10.1039/c5bm00198f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Badwaik H.R., Kumari L., Nakhate K., Verma V.S., Sakure K. In: Studies in natural products chemistry. Rahman A.U., editor. Elsevier; Amsterdam: 2019. Phytoconstituent plumbagin: chemical, biotechnological and pharmaceutical aspects; pp. 415–460. [Google Scholar]
- 74.Devulapally R., Paulmurugan R. Polymer nanoparticles for drug and small silencing RNA delivery to treat cancers of different phenotypes. WIREs Nanomed Nanobiotechnol. 2014;6:40–60. doi: 10.1002/wnan.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang F., Parayath N.N., Ene C.I., Stephan S.B., Koehne A.L., Coon M.E., et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun. 2019;10:3974. doi: 10.1038/s41467-019-11911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parayath N.N., Stephan S.B., Koehne A.L., Nelson P.S., Stephan M.T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat Commun. 2020;11:6080. doi: 10.1038/s41467-020-19486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haabeth O.A.W., Blake T.R., McKinlay C.J., Waymouth R.M., Wender P.A., Levy R. mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice. Proc Natl Acad Sci U S A. 2018;115:E9153–E9161. doi: 10.1073/pnas.1810002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McKinlay C.J., Vargas J.R., Blake T.R., Hardy J.W., Kanada M., Contag C.H., et al. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc Natl Acad Sci U S A. 2017;114:E448–E456. doi: 10.1073/pnas.1614193114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilk A.J., Weidenbacher N.L.-B., Vergara R., Haabeth O.A.W., Levy R., Waymouth R.M., et al. Charge-altering releasable transporters enable phenotypic manipulation of natural killer cells for cancer immunotherapy. Blood Adv. 2020;4:4244–4255. doi: 10.1182/bloodadvances.2020002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McKinlay C.J., Benner N.L., Haabeth O.A., Waymouth R.M., Wender P.A. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc Natl Acad Sci U S A. 2018;115:E5859–E5866. doi: 10.1073/pnas.1805358115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abbasi S., Uchida S., Toh K., Tockary T.A., Dirisala A., Hayashi K., et al. Co-encapsulation of Cas9 mRNA and guide RNA in polyplex micelles enables genome editing in mouse brain. J Control Release. 2021;332:260–268. doi: 10.1016/j.jconrel.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 82.Yan Y., Xiong H., Zhang X., Cheng Q., Siegwart D.J. Systemic mRNA delivery to the lungs by functional polyester-based carriers. Biomacromolecules. 2017;18:4307–4315. doi: 10.1021/acs.biomac.7b01356. [DOI] [PubMed] [Google Scholar]
- 83.Mandal B., Bhattacharjee H., Mittal N., Sah H., Balabathula P., Thoma L.A., et al. Core–shell-type lipid–polymer hybrid nanoparticles as a drug delivery platform. Nanomed Nanotechnol Biol Med. 2013;9:474–491. doi: 10.1016/j.nano.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 84.Kong N., Tao W., Ling X., Wang J., Xiao Y., Shi S., et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53 -deficient cancers to mTOR inhibition. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aaw1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Islam M.A., Xu Y., Tao W., Ubellacker J.M., Lim M., Aum D., et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat Biomed Eng. 2018;2:850–864. doi: 10.1038/s41551-018-0284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Persano S., Guevara M.L., Li Z., Mai J., Ferrari M., Pompa P.P., et al. Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials. 2017;125:81–89. doi: 10.1016/j.biomaterials.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uchida S., Kinoh H., Ishii T., Matsui A., Tockary T.A., Takeda K.M., et al. Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety. Biomaterials. 2016;82:221–228. doi: 10.1016/j.biomaterials.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 88.Zhao W., Zhang C., Li B., Zhang X., Luo X., Zeng C., et al. Lipid polymer hybrid nanomaterials for mRNA delivery. Cell Mol Bioeng. 2018;11:397–406. doi: 10.1007/s12195-018-0536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiong H., Liu S., Wei T., Cheng Q., Siegwart D.J. Theranostic dendrimer-based lipid nanoparticles containing PEGylated BODIPY dyes for tumor imaging and systemic mRNA delivery in vivo. J Control Release. 2020;325:198–205. doi: 10.1016/j.jconrel.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeong W., Bu J., Kubiatowicz L.J., Chen S.S., Kim Y., Hong S. Peptide–nanoparticle conjugates: a next generation of diagnostic and therapeutic platforms? Nano Converg. 2018;5:38. doi: 10.1186/s40580-018-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kübler H., Scheel B., Gnad-Vogt U., Miller K., Schultze-Seemann W., vom Dorp F., et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J Immunother Cancer. 2015;3:26. doi: 10.1186/s40425-015-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kallen K.J., Heidenreich R., Schnee M., Petsch B., Schlake T., Thess A., et al. A novel, disruptive vaccination technology: self-adjuvanted RNActive® vaccines. Hum Vaccines Immunother. 2013;9:2263–2276. doi: 10.4161/hv.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sebastian M., Schröder A., Scheel B., Hong H.S., Muth A., von Boehmer L., et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol Immunother. 2019;68:799–812. doi: 10.1007/s00262-019-02315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Udhayakumar V.K., De Beuckelaer A., McCaffrey J., McCrudden C.M., Kirschman J.L., Vanover D., et al. Arginine-rich peptide-based mRNA nanocomplexes efficiently instigate cytotoxic T cell immunity dependent on the amphipathic organization of the peptide. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201601412. [DOI] [PubMed] [Google Scholar]
- 95.Mai Y., Guo J., Zhao Y., Ma S., Hou Y., Yang J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell Immunol. 2020;354 doi: 10.1016/j.cellimm.2020.104143. [DOI] [PubMed] [Google Scholar]
- 96.Lei S., Zhang X., Men K., Gao Y., Yang X., Wu S., et al. Efficient colorectal cancer gene therapy with IL-15 mRNA nanoformulation. Mol Pharm. 2020;17:3378–3391. doi: 10.1021/acs.molpharmaceut.0c00451. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X., Men K., Zhang Y., Zhang R., Yang L., Duan X. Local and systemic delivery of mRNA encoding survivin-T34A by lipoplex for efficient colon cancer gene therapy. Int J Nanomed. 2019;14:2733–2751. doi: 10.2147/IJN.S198747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y., Su H., Yang Y., Hu Y., Zhang L., Blancafort P., et al. Systemic delivery of modified mRNA encoding Herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Mol Ther. 2013;21:358–367. doi: 10.1038/mt.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao Y., Men K., Pan C., Li J., Wu J., Chen X., et al. Functionalized DMP-039 hybrid nanoparticle as a novel mRNA vector for efficient cancer suicide gene therapy. Int J Nanomed. 2021;16:5211–5232. doi: 10.2147/IJN.S319092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deehan M., Garcês S., Kramer D., Baker M.P., Rat D., Roettger Y., et al. Managing unwanted immunogenicity of biologicals. Autoimmun Rev. 2015;14:569–574. doi: 10.1016/j.autrev.2015.02.007. [DOI] [PubMed] [Google Scholar]