Abstract
Each of the four epidermal growth factor (EGF)-like domains of the Plasmodium falciparum sexual-stage antigen Pfs25 has been individually expressed as a yeast-secreted recombinant protein (yEGF1 through yEGF4). All four are recognized by the immune sera of animals and humans vaccinated with TBV25H (the corresponding yeast-secreted full-length recombinant form of Pfs25), with antibody titers to yEGF1 and yEGF2 weakly correlating with the ability of the sera to block the transmission of parasites to the mosquito host. All four proteins are poorly immunogenic in mice vaccinated with aluminum hydroxide-absorbed formulations. However, all four successfully primed the mice to mount an effective secondary antibody response after a single boost with TBV25H. Sera from mice vaccinated with yEGF2-TBV25H completely block the development of oocysts in mosquito midguts in membrane-feeding assays. Further, of the four proteins, only the depletion of antibodies to yEGF2 from the sera of rabbits vaccinated with TBV25H consistently abolished the ability of those sera to block oocyst development. Thus, antibodies to the second EGF-like domain of Pfs25 appear to mediate a very potent blocking activity, even at low titers. Vaccination strategies that target antibody response towards this domain may improve the efficacy of future transmission-blocking vaccines.
Plasmodium falciparum, the etiologic agent of lethal malaria, continues to confound control efforts, due in part to parasite drug resistance and a decline in the effectiveness of control programs against both the vector and the parasite. Concerted campaigns to develop vaccines as a component of control or even eradication strategies have produced numerous candidate antigens. These are aimed against various stages of the parasite, and they include transmission-blocking vaccines.
Transmission-blocking vaccines are designed to specifically prevent parasites ingested by female Anopheles mosquitoes from undergoing sexual and sporogonic development. They thus utilize the widespread coverage provided by vaccination to target the parasite during the vulnerable transition from vertebrate host to vector (6). A number of antigens expressed during the sexual stage of the P. falciparum life cycle are the target of antibodies capable of preventing the transmission of the parasite from human to mosquito (1–3, 14, 20). A leading candidate is Pfs25 (11), an antigen expressed mainly on the surface of P. falciparum zygotes and ookinetes (23). Pfs25 is a cysteine-rich 25-kDa antigen composed of four tandem epidermal growth factor (EGF)-like domains putatively anchored to the parasite's surface through a glycosylphosphatidylinositol moiety (24). At least in ex vivo membrane-feeding assays, antibodies to Pfs25 completely prevent mosquitoes from becoming infected (1, 9, 12, 23).
Vaccine development of Pfs25 is quite advanced, with a recombinant form of the molecule, TBV25H, secreted by Saccharomyces cerevisiae at high concentrations and purified to near homogeneity, having been in human phase I clinical trials (D. C. Kaslow et al., unpublished data). Vaccination of mice, rabbits, and monkeys with TBV25H adsorbed to aluminum hydroxide (a formulation suitable for use in humans) can induce complete transmission-blocking antibodies (1, 8, 12, 15).
However, two developmental problems have been found with TBV25H. Although it is known to be antibody mediated, transmission-blocking activity often poorly correlates with total immunogen-specific antibody titers (8). Fine-specificity epitope mapping of the antibody response has been complicated by the intricate secondary structure of a molecule with 22 cysteines. Second, when TBV25H was adsorbed to aluminum hydroxide and administered to humans, antibody titers were low and complete blocking was difficult to achieve (D. C. Kaslow, unpublished results). In an attempt to overcome these developmental problems, we sought to further characterize the immune response generated to TBV25H. To this end, each of the four EGF-like domains of the TBV25H form of Pfs25 was expressed as a yeast-secreted recombinant protein. These recombinant proteins were then used to analyze the results of previous TBV25H studies and used as immunogens themselves. This work has unexpectedly revealed the potency of antibodies directed against the second EGF-like domain of Pfs25.
MATERIALS AND METHODS
Recombinant protein production. (i) EGF-like domain constructs.
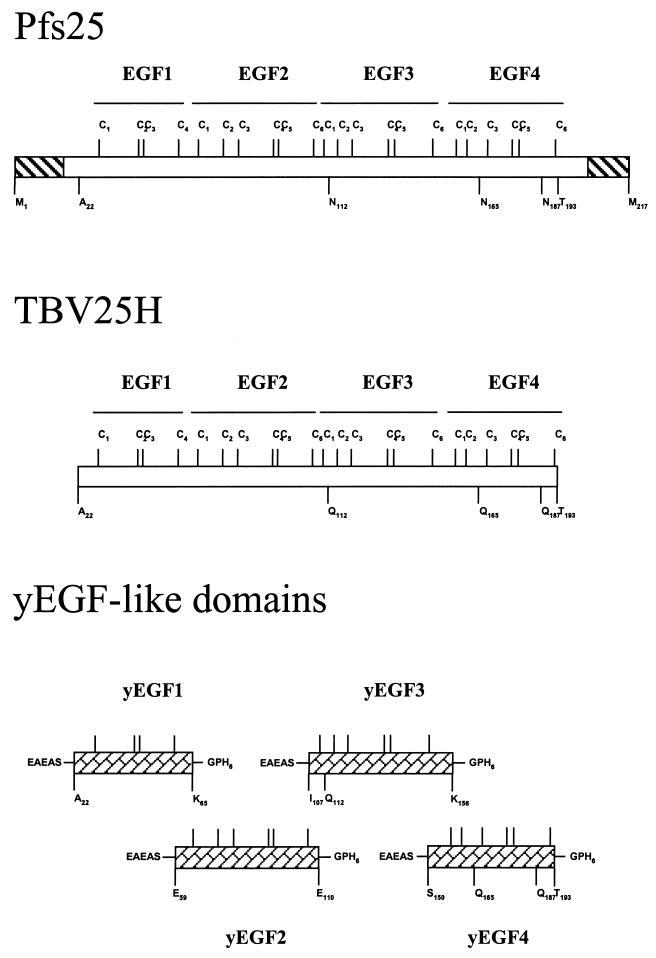
All constructs were based on the sequence of TBV25H, in which codon usage was optimized for yeast expression (12). The amino acid sequence of TBV25H is identical to Ala22 to Thr193 of Pfs25 from the 3D7 strain, with the substitution of Gln for Asn at positions 112, 165, and 187 (Fig. 1). Each domain was amplified by PCR using TBV25H as a template, with primers designed to flank the desired sequence with 5′ NheI and 3′ ApaI restriction endonuclease sites. yEGF1 contains amino acids Ala22 to Lys65, yEGF2 contains amino acids Glu59 to Glu110, yEGF3 contains amino acids Ile107 to Lys156, and yEGF4 contains amino acids Ser150 to Thr193. Thus, yEGF3 has one Gln-for-Asn substitution, while yEGF4 has two.
FIG. 1.
Production of each of the four EGF-like domains of Pfs25 as an individual recombinant protein secreted by S. cerevisiae. Shown are the differences between the parasite Pfs25 and the recombinant TBV25H which were inherited by the EGF-like recombinant proteins.
(ii) Yeast secretion vector.
Yeast episomal plasmid YEpRPEU3 is a variant plasmid of that used previously (4, 10). The gene of interest is cloned into the 5′ NheI site that follows the secretory alpha-factor sequence, cleaved by the enzyme KEX2. Flanking the 3′ ApaI site is a sequence encoding a six-histidine tag and a stop codon. Any expressed protein thus has the sequence EAEAS…GPHHHHHH (underlined amino acids from the restriction site, EAE, are vector sequences thought to aid the KEX2 cleavage of the prepro secretory sequence [17]). Gene expression is under the control of the ADH2 promoter for ethanol-induced production, and plasmid selection is coded by TRP1 downstream of the gene.
(iii) Host cells and fermentation.
The plasmids were used to transform the S. cerevisiae VK1 cell line (haploid, trp1 Δ lys2-801 pep4−:ura). Protein production was essentially as described previously (4, 12).
(iv) Protein purification.
Fermentation culture supernatants were recovered by microfiltration (0.1-mm-pore-size Amicon hollow fiber). The supernatant was then concentrated by ultrafiltration and diafiltered with a 3-kDa-cutoff spiral-fiber filter (Amicon) into 2× phosphate-buffered saline, pH 7.4 (PBS). The protein was purified from the supernatant by Ni-nitrotriacetic acid (Ni-NTA) chromatography (Qiagen) followed by size exclusion chromatography and buffer exchange into PBS using a Superdex 75 column (Pharmacia Biotech). Amino acid sequencing by automated Edman degradation and electron spray mass spectroscopy were performed on liquid samples, or on samples transferred to polyvinylidene difluoride membranes after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), by the Biological Resources Branch, National Institute of Allergy and Infectious Diseases. Protein concentrations were determined by bicinchoninic acid protein assay (Pierce).
Secondary-structure analysis.
Recombinant protein was incubated in iodoacetyl-LC-biotin (4 mM, dissolved in dimethyl sulfoxide [Pierce]) in 100 mM Tris–10 mM EDTA (pH 8.0) for 90 min in the dark at room temperature. A 10-fold molar excess of iodoacetyl-LC-biotin over cysteine residues was used. Negative control reactions were performed by incubating the samples without the alkylating reagent. Positive control reactions were performed by incubating the samples in a fivefold molar excess of dithiothreitol (Sigma) over cysteine residues for 60 min at 37°C before incubating with a 10-fold molar excess of iodoacetyl-LC-biotin over dithiothreitol as before. Protein samples were size fractionated by SDS-PAGE (4 to 20% polyacrylamide) (Novex Experimental Technology, San Diego, Calif.) and electrophoretically transferred onto nitrocellulose membranes. The membranes were blocked for 1 h at room temperature in blocking buffer (5% [wt/vol] nonfat powdered milk in PBS–0.05% Tween 20). The blots were then incubated with alkaline phosphatase-conjugated streptavidin (Kirkegaard & Perry Laboratories, Inc.) diluted (1:1,500) in blocking buffer for 1 h at room temperature and then washed three times. The protein bands were visualized by incubation with Western blue (substrate for alkaline phosphatase) (Promega). Alternately, samples were sent directly for electron spray mass spectroscopy.
Animals and vaccinations.
All animal studies were done in compliance with National Institutes of Health guidelines and under the auspices of an Animal Care and Use Committee-approved protocol. CAF1 mice, 6 to 8 weeks old, were used for all studies. Mice received 25 μg of each yEGF-like recombinant protein per dose or 100 μg of TBV25H per dose (to give a molar equivalency). Protein was adsorbed to aluminum hydroxide (Alhydrogel; Superfos Biosector lot no. 2179) at 800 μg/0.5-ml dose for 30 min at room temperature with continuous rocking. Vaccinations were performed at 0, 3, and 6 weeks by the intraperitoneal route. All mice received an additional boost of 25 μg of TBV25H at 12 weeks.
Primary serum sources.
For measurements involving the antigenicity of the yEGF-like recombinant proteins, mouse and rabbit sera were used. The mice were vaccinated as described above with three immunizations of 50 μg of TBV25H absorbed to aluminum hydroxide (alum). The rabbit serum was obtained from previous studies. The sera of 24 rabbits were used (Kaslow, unpublished data); 4 of these rabbits received 50 μg of TBV25H adsorbed to alum per dose, 12 received 250 μg of TBV25H adsorbed to alum per dose, 4 received 50 μg of TBV25H adsorbed to alum-QS21 per dose, and four received 250 μg of TBV25H adsorbed to alum-QS21 per dose. All rabbits received three vaccinations, and the sera from day 70 were analyzed.
ELISA.
Serum antibodies to recombinant proteins were assayed as described previously (4). Immulon-4 96-well plates (Dynatech) were coated for 16 h at 4°C with 100 μl of a 1-μg/ml dilution of recombinant protein in coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) per well. The plates were blocked with 5% (wt/vol) nonfat milk powder (Difco) in PBS for 1 h at room temperature. Serum samples were serially diluted in blocking buffer and incubated in the coated plates for 2 h at room temperature. The plates were washed extensively with PBS–0.05% Tween 20 and incubated with the appropriate secondary antibody for 1 h at room temperature. The secondary antibodies were 1:1,000 dilutions in blocking buffer of goat anti-mouse, anti-rabbit, or anti-human immunoglobulin G conjugated to alkaline phosphatase (Kirkegaard & Perry Laboratories, Inc.). After the washing step was repeated, the plates were given an additional wash in Tris-buffered saline, pH 7.4. Detection was performed using 100 μl of p-nitrophenyl disodium phosphate solution (Sigma 104 phosphatase substrate; 1 tablet per 5 ml of coating buffer) per well. After a 20-min incubation, absorbance was read at 405 nm with a Dynatech MR500 enzyme-linked immunosorbent assay (ELISA) plate reader. Serum dilutions that gave an absorbance value of 0.5 U above background were designated the end point of the serum ELISA titer.
Inhibition-competition ELISAs were performed as described above, but prior to use the serum was preincubated for 2 h at room temperature in blocking buffer containing a 5-μg/ml concentration of either one of the recombinant proteins described above or a yeast-secreted form of glutathione S-transferase.
Serum antibody depletion.
To deplete a serum sample of antibodies to a particular recombinant protein, the serum sample was passed over an Ni-NTA column with the protein bound to it. A 340-μl portion of Ni-NTA (50% [vol/vol] slurry preequilibrated with 2× PBS, pH 8.0) was added to 0.4 mg of recombinant protein (or PBS for negative-depletion control) and incubated with mixing for 2 h at 4°C. An equal volume of 5% (wt/vol) bovine serum albumin (BSA) (fraction V; Sigma) was then added, and a 1-h incubation at 4°C was performed. The Ni-NTA was centrifuged at 500 × g for 5 min, and the pellet was washed three times with 1 ml of PBS. A 200-μl portion of rabbit serum (collected from each of four rabbits, each receiving three vaccinations of 250 μg of TBV25H adsorbed to aluminum hydroxide) was then added to the prepared Ni-NTA, and the sample was incubated for 16 h at 4°C with mixing. Unbound antibodies were separated from the Ni-NTA slurry by centrifugation. The depletion of antibodies to the recombinant protein of interest was then confirmed by ELISA.
Transmission-blocking assays.
Transmission-blocking assays were performed on the sera of mice vaccinated with recombinant proteins and on the sera of rabbits vaccinated with TBV25H and subsequently depleted of antibodies to individual recombinant proteins. Assays were performed as described previously (14). Briefly, test sera were mixed with mature in vitro-cultured P. falciparum gametocytes and fed to mosquitoes through a membrane-feeding apparatus consisting of an artificial membrane stretched across the base of a water-jacketed glass cylinder. Mosquitoes were kept for 6 to 8 days after feeding to allow parasites to develop into mature oocysts. Infectivity was measured by dissecting midguts, staining with mercurochrome, and counting the number of oocysts per midgut for at least 20 mosquitoes. The data were analyzed for statistical significance as previously described (7).
RESULTS
Recombinant protein production.
The yeast-expressed vaccine candidate molecule TBV25H is derived from the P. falciparum major surface target antigen, Pfs25. Modifications include the deletion of the C-terminal glycosylphosphatidylinositol anchor attachment sequence to allow secretion and the substitution of glutamine for asparagine at three putative N-linked glycosylation sites to eliminate glycosylation (12). The four recombinant proteins expressed here are themselves derived from the TBV25H sequence. Each includes a single EGF-like domain of TBV25H, encompassing all amino acids from, but not including, the last cysteine residue of the preceding domain to, but not including, the first cysteine residue of the succeeding domain (Fig. 1).
After immobilized metal affinity chromatography and size exclusion chromatography, the yield and N-terminal sequence of each purified recombinant protein were determined. All N termini were as predicted, and yields were 4.5, 53.4, 15.2 and 9.8 mg/liter for yEGF1 through yEGF4, respectively. The lack of specific reagents for each domain allowed only indirect determination of protein conformation (except for yEGF3; see below). By reducing and nonreducing SDS-PAGE, all proteins appeared to be greater than 95% pure by scanning densitometry and showed an electrophoretic mobility shift between reducing and nonreducing conditions. However, some evidence of differences in structural confirmation was observed (A. W. Stowers, submitted for publication). Using the reagent iodoacetyl-LC-biotin, which methylates the sulfhydryl groups of free cysteines and adds a biotin group, we assayed for the presence of free sulfhydral groups. None were detected by Western blotting (data not shown), indicating that all cysteine residues were involved in disulfide-bond formation. These results were confirmed for yEGF2 by mass spectroscopy (Table 1). The observed mass for yEGF2, with or without iodoacetyl-LC-biotin, was six atomic mass units less than the predicted mass for the amino acid sequence (for the six protons presumably lost during the formation of three disulfide bridges). Two monoclonal antibodies (MAbs) raised against Pfs25 (16) were predicted to bind to the third EGF-like domain (unpublished observation). These are 1D2 (conformation dependent) and 4B7 (conformation independent). By ELISA, both MAbs recognized yEGF3 specifically with no reaction to the other recombinant proteins (data not shown), and this provided a further assurance of correct secondary structure.
TABLE 1.
Secondary structure of yEGF2 by mass spectroscopy
| Protein sample | Predicted mass (Da) | Observed mass (Da) |
|---|---|---|
| yEGF2 | 7,134.03 with no disulfide bonds, 7,128.03 with no free sulfhydryls (minus six protons) | 7,127.98 (6990.94a; Δ = 137.04) |
| yEGF2, alkylated (plus iodoacetyl-LC-biotin) | 7,134.03 (plus 382.5 for every free sulfhydryl alkylated [maximum, 9,429.03]) | 7,128.25 (6990.97a; Δ = 137.28)* |
| yEGF2 reduced and alkylated (plus dithiothreitol and iodoacetyl-LC-biotin) | 7,134.03 (plus 382.5 for each of six alkylated sulfhydryls = 9,429.03) | 9,428.2b |
A minor peak also observed; molecular weight of histidine when in a peptide chain = 137.2.
Unassignable additional peaks were also seen.
Serology performed on transmission-blocking serum.
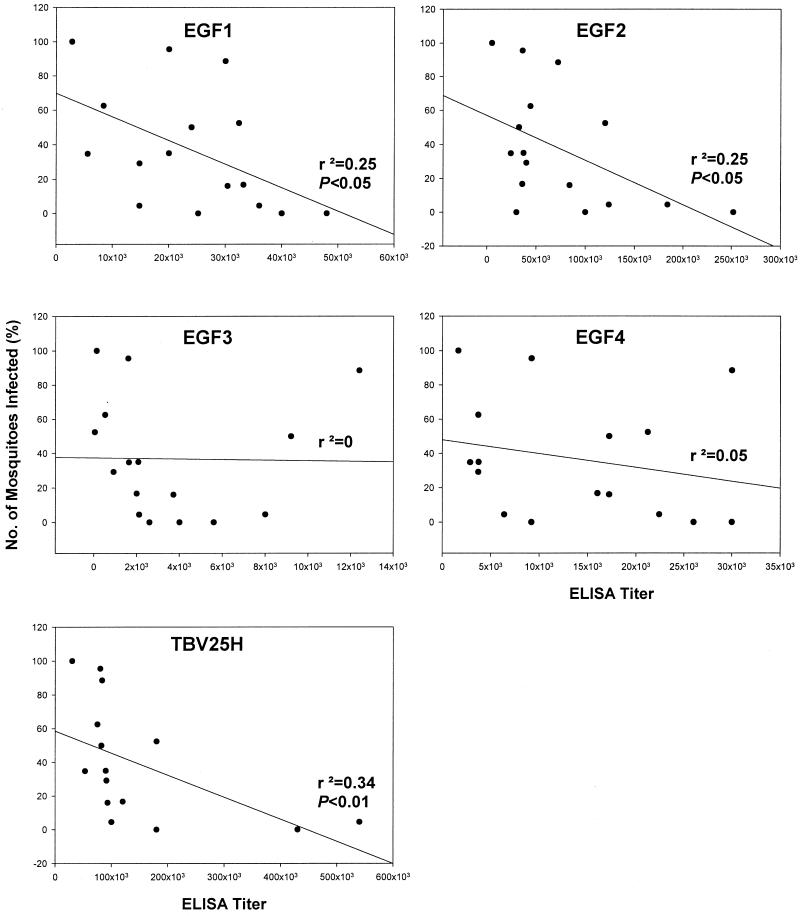
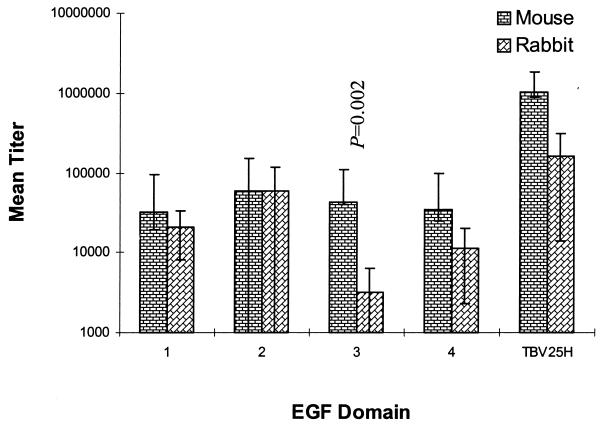
In our previous studies, vaccination with TBV25H generated serum with the ability to block the development of the P. falciparum parasite in the mosquito vector. Such transmission-blocking sera have been generated in mice (12), rabbits (4), primates (8), and humans (Kaslow, unpublished data). We analyzed representative samples of that serum for its reactivity by direct ELISA to the four recombinant proteins produced here. For these studies, the entire serum collection from a previous study with mice or rabbits (19; M. M. Gozar, submitted for publication) was used (rather than selecting a subset of serum). The reactivities of rabbit serum raised against TBV25H to all four individual yEGF domains and to full-length TBV25H were measured by direct ELISA, and end point dilution titers were established. Reactivities to yEGF1 and yEGF2 showed weak but significant r2 values in correlation with transmission-blocking activity, although those were slightly below that for full-length TBV25H (Fig. 2). Compared to mouse serum also raised against TBV25H, a species-specific difference in the reactivity to yEGF3 was observed, with mice more likely to make a significant portion of their anti-TBV25H response to this domain than rabbits (Fig. 3). In either species, yEGF2 was the most immunogenic domain.
FIG. 2.
Correlation between rabbit antibody titers to recombinant proteins and transmission-blocking activity. Rabbits were immunized with TBV25H, and their sera were assayed for reactivity to five recombinant proteins by ELISA. Plotted is the ELISA titer versus transmission-blocking activity as measured by percentage of mosquitoes infected compared to those dissected. The regression line is shown for each assay, along with r2 values and P values (where significant).
FIG. 3.
Mean antibody titers of the sera of mice (n = 9) and rabbits (n = 24) vaccinated with TBV25H to the individual yEGF-like domains. Shown is the mean antibody titer (and standard deviation) as measured by direct ELISA directed against each of the individual domains. The titers of antibodies to the third EGF-like domain are significantly different between the mice and rabbits.
Individual domains as immunogens.
Each of the four individual yEGF proteins was absorbed to aluminum hydroxide (alum) and used to vaccinate a group of six CAF1 mice. As controls, mice were also vaccinated with alum alone or TBV25H absorbed to alum. After three vaccinations, the sera were assayed for antibody titer and transmission-blocking ability in membrane-feeding assays. All groups of mice were then boosted with a vaccination of full-length TBV25H absorbed to aluminum hydroxide and further assayed.
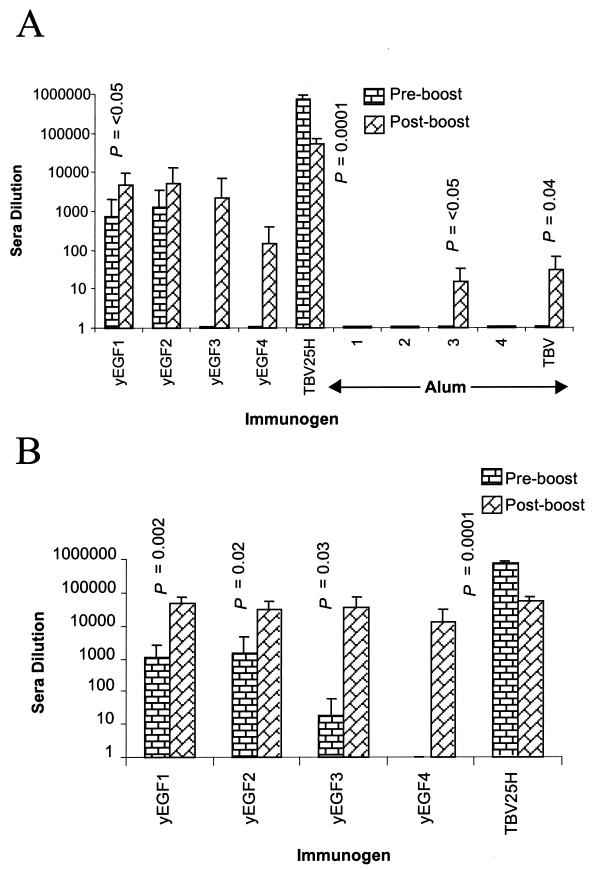
Antibody titers to individual domains are shown in Fig. 4. All four individual yEGF domains are poorly immunogenic in alum, and antibody titers varied considerably between mice (for the yEGF1-vaccinated group there were four seroconverters of six, with three, zero, and one of six, respectively, for yEGF2 to -4). The subsequent vaccination with TBV25H significantly increased the antibody response to TBV25H for all of the domain-immunized groups as determined by the Student t test (and all now seroconverted to TBV25H) but not necessarily that to the individual domain with which the mice were originally immunized (six, five, six, and four of six seroconverted to the individual domain of immunization within each group). Before the boost with TBV25H, the mean titers to the individual domains used for vaccination matched those to TBV25H (e.g., for yEGF1, preboost anti-yEGF1 titer = 744 and preboost anti-TBV25H titer = 1,026). Thus, all antibodies produced apparently recognize the full-length molecule. However, after boosting with TBV25H, there was nearly a 10-fold difference between the mean antibody titer to the vaccine priming domain (e.g., yEGF1 titer to yEGF1 = 4,724, a sixfold increase) and the antibody titer to the full-length boosting immunogen (e.g., yEGF1 titer to TBV25H = 50,696, a 50-fold increase). Immunizing with a single vaccination of TBV25H did not produce these high titers to the individual domains or to the full-length molecule (alum postboost group in Fig. 4). This is evidence that vaccination with the individual domains, even in the cases of yEGF3 and yEGF4, which produced very low antibody titers, has presumably primed helper T cells that mediate subsequent antibody responses to all domains.
FIG. 4.
Antibody titers raised in mice after vaccination with yEGF-like domains. Mice were vaccinated three times with a single yEGF-like domain (preboost) and then given a subsequent boost with TBV25H (postboost). Control mice were vaccinated three times with alum alone (preboost) and then boosted with TBV25H (postboost). The x axes are labeled with the antigen used for preboost vaccinations. (A) The ELISA plate capture antigen was in each case the yEGF-like domain used for preboost vaccination (except in the case of the control alum group, where it was each of the yEGF domains and full-length TBV25H [1 to 4 and TBV]). (B) The ELISA plate capture antigen was the postboost vaccination antigen, TBV25H. Serum titers reported are the average dilution (and standard deviation) for a group of six mice at which the ELISA optical density at 405 nm was 0.5 reported absorbance unit. Statistically significant differences between pre- and postboost titers are also shown.
Transmission-blocking activity of antisera raised to recombinant yEGFs.
By membrane-feeding transmission-blocking assays, vaccination with the individual yEGF proteins (i.e., pre-TBV25H boost) proved incapable of eliciting antibodies with significant transmission-blocking activity (Table 2); however, following boosting with TBV25H, the yEGF2-vaccinated mouse serum pool completely blocked the formation of oocysts in the mosquito midgut, while the control alum-TBV25H serum did not (Table 2). This assay was confirmed by repetition, and then the sera from individual mice were assayed for transmission-blocking activity. Sera from two of the six mice completely blocked oocyst formation, sera from two allowed a single oocyst to form in the gut of 1 out of 31 or 23 mosquitoes, and serum from one allowed the formation of a single oocyst in 2 out of 38 mosquitoes. One of the mice failed to develop antibody that blocked transmission. This mouse also failed to develope any anti-yEGF2 antibody response.
TABLE 2.
Transmission-blocking activity of mouse sera raised against recombinant proteins
| Immunogen(s)a | Mean no. of oocysts (range) | No. of mosquitoes infected/no. dissected (%) | z-test P value | Mann-Whitney significanceb | Infectivity (% of control)c |
|---|---|---|---|---|---|
| Alum | 2.16 (0–8) | 22/25 (88.0) | 0.500 | Not significant | 100 |
| TBV25H | 0.00 (0–0) | 0/24 (0) | 0.000 | Significant | 0 |
| yEGF1 | 1.70 (0–8) | 20/25 (80.0) | 0.205 | Not significant | 79.1 |
| yEGF2 | 1.91 (0–10) | 22/26 (84.6) | 0.270 | Not significant | 88.4 |
| yEGF3 | 1.89 (0–6) | 20/24 (83.3) | 0.352 | Not significant | 87.6 |
| yEGF4 | 1.90 (0–9) | 20/25 (80.0) | 0.338 | Not significant | 88.3 |
| Alum-TBV25H | 1.34 (0–9) | 20/32 (62.5) | 0.500d | Not significant | 100 |
| yEGF1-TBV25H | 0.55 (0–7) | 9/22 (40.9) | 0.032 | Not significant | 41.4 |
| yEGF2-TBV25H | 0.00 (0–0) | 0/32 (0) | 0.000 | Significant | 0 |
| yEGF3-TBV25H | 0.68 (0–6) | 17/28 (60.7) | 0.051 | Not significant | 50.8 |
| yEGF4-TBV25H | 0.73 (0–4) | 14/25 (56.0) | 0.071 | Not significant | 54.7 |
For combination vaccinations, mice received three vaccinations of the first protein, followed by one vaccination of the second.
A stringent significance level of 0.01 is applied to all transmission-blocking assays.
Calculated as the geometric mean oocyst count for the test group divided by the geometric mean oocyst count for the alum group times 100. For combination vaccinations, the control group was the alum-TBV25H group.
P value compared to the original alum control group (for comparison purposes) = 0.079.
In an attempt to reduce the number of immunizations, these experiments were repeated for the yEGF2 protein. Groups of mice received two vaccinations of 25 μg of yEGF2 adsorbed to alum, while control mice received two vaccinations of either 100 μg of TBV25H or alum alone. All mice then received a third vaccination of 25 μg of TBV25H adsorbed to alum (or alum alone for negative controls). Although the mean antibody titers of the mice receiving yEGF2-TBV25H were lower than those of the mice receiving TBV25H, the pooled sera from both the yEGF2-TBV25H and the TBV25H groups had significant transmission-blocking activity (Table 3). As previously, in the EGF2-immunized mice, proportionally a larger component of the response was directed to EGF-like domain 2.
TABLE 3.
Activity of mouse sera raised against yEGF2 and TBV25H recombinant proteins
| Immunogen(s)a | Mean ELISA titer to:
|
No. of mosquitoes infected/no. dissected (P) | |
|---|---|---|---|
| yEGF2 | TBV25H | ||
| EGF2-TBV25H | 1,530.3 | 2,859.0 | 6/18 (0.0001) |
| TBV25H-TBV25H | 3,956.6 | 62,377.2 | 0/18 (0.0001) |
| Adjuvant | 0 | 0 | 18/25 (Not significant) |
Mice in each group received two vaccinations of 25 μg (yEGF2) or 100 μg (TBV25H) of the first antigen adsorbed to alum, followed by a third vaccination of 25 μg of TBV25H adsorbed to alum.
In the original experiment, the mice vaccinated with yEGF1-TBV25H both developed the highest anti-TBV25H titers and had anti-yEGF1 domain titers similar to the anti-yEGF2 domain titers of the yEGF2-TBV25H-vaccinated mice, but this serum did not block transmission. This suggests that the transmission-blocking ability of the yEGF2-TBV25H antiserum is not simply a result of the highest titers but that antibodies developed towards EGF-like domain 2 are qualitatively more effective at blocking oocyst formation. This hypothesis was tested using immunodepleted hyperimmune rabbit serum.
Transmission-blocking assays using rabbit sera depleted of yEGF2 antibodies.
The serum from each of four rabbits previously vaccinated with three vaccinations of 250 μg of alum-absorbed TBV25H was obtained (4). These sera were then depleted of antibodies to each of the individual yEGF proteins. The depletion was confirmed by ELISA (data not shown). All four rabbits had relatively low titers to yEGF2 predepletion as measured by end point ELISA (titers for rabbits A through D, 3,826, 4,485, 479, and 569, respectively).
When used in membrane-feeding transmission-blocking assays, the unadulterated sera showed varying activity, from complete blocking (rabbit A) to near complete blocking (rabbit B) to moderate or poor blocking (rabbits C and D). These sera were then reassayed for transmission-blocking activity after the antibody depletion (Table 4). Despite the relatively low titers to yEGF2, depleting three of the rabbit sera of antibodies to yEGF2 significantly reduced the sera's blocking ability and allowed oocyst formation. Only depletion of antibodies to full-length TBV25H had a similar consistent effect. For rabbit B, depletion of yEGF1 antibodies also resulted in a significant increase in transmission.
TABLE 4.
Reduction in transmission-blocking ability of rabbit sera depleted of antibodies to EGF-like domains
| Rabbit Seruma | Mean no. of oocysts (range) | No. of mosquitoes infected/no. dissected (%) | z-test P valueb | Mann-Whitney significancec | Infectivity (% of value for TBV25H-depleted serum)d |
|---|---|---|---|---|---|
| A | 0.00 (0–0) | 0/36 (0) | 0.068 | Not significant | 0 |
| A-TBV25H | 6.16 (0–16) | 33/34 (97.1) | 0.000 | Significant | 100 |
| A-yEGF1 | 0.3 (0–2) | 8/24 (33.3) | 0.285 | Not significant | 4.9 |
| A-yEGF2 | 1.24 (0–6) | 18/28 (64.3) | 0.001 | Significant | 20.1 |
| A-yEGF3 | 0.57 (0–3) | 8/20 (40.0) | 0.103 | Not significant | 9.3 |
| A-yEGF4 | 0.13 (0–2) | 5/31 (16.1) | 0.321 | Not significant | 2.1 |
| B | 0.04 (0–1) | 2/33 (6.1) | 0.336 | Not significant | 1.2 |
| B-TBV25H | 3.29 (0–17) | 19/21 (90.5) | 0.000 | Significant | 100 |
| B-yEGF1 | 0.54 (0–4) | 12/25 (48.0) | 0.007 | Significant | 16.4 |
| B-yEGF2 | 3.57 (0–23) | 17/24 (70.8) | 0.000 | Significant | 108.5 |
| B-yEGF3 | 0.11 (0–3) | 2/20 (10.0) | 0.463 | Not significant | 3.3 |
| B-yEGF4 | 0.13 (0–3) | 3/23 (13.0) | 0.464 | Not significant | 4.0 |
| C | 1.06 (0–6) | 16/24 (66.7) | 0.019 | Not significant | 17.6 |
| C-TBV25H | 6.03 (0–21) | 21/22 (95.5) | 0.000 | Significant | 100 |
| C-yEGF1 | 0.44 (0–3) | 12/27 (44.4) | 0.394 | Not significant | 7.3 |
| C-yEGF2 | 1.63 (0–7) | 29/35 (82.9) | 0.000 | Significant | 27.0 |
| C-yEGF3 | 0.24 (0–4) | 7/29 (24.1) | 0.163 | Not significant | 4.0 |
| C-yEGF4 | 0.13 (0–2) | 4/26 (15.4) | 0.063 | Not significant | 2.2 |
| D | 1.11 (0–11) | 19/30 (63.3) | NDe | ND | 43.9 |
| D-TBV25H | 2.53 (0–14) | 18/25 (72.0) | ND | ND | 100 |
| D-yEGF1 | 5.61 (0–20) | 20/21 (95.2) | ND | ND | 221.7 |
| D-yEGF2 | 3.24 (0–10) | 25/29 (86.2) | ND | ND | 128.1 |
| D-yEGF3 | 1.66 (0–11) | 21/27 (77.8) | ND | ND | 65.6 |
| D-yEGF4 | 3.83 (0–14) | 26/28 (92.9) | ND | ND | 151.4 |
Sera were generated in rabbits by vaccination with TBV25H depleted of antibodies to the protein shown.
For statistics, the control serum used for a group was that rabbit's serum depleted of antibodies in a sham reaction using BSA (data for sham sera not shown).
A stringent significance level of 0.01 is applied to all transmission-blocking assays.
Calculated as the geometric mean oocyst count of the test group divided by the geometric mean oocyst count of the TBV25H-depleted sera times 100.
ND, not determined. The control reaction to deplete antibodies to BSA for rabbit D appeared to have a nonspecific effect, significantly reducing transmission-blocking activity. Hence, statistical analysis was not performed, as depletion with neither TBV25H nor any of the domains resulted in a further significant reduction in transmission-blocking activity.
The serum from rabbit D had the poorest transmission-blocking activity, and the control reaction to deplete antibodies to BSA appeared to have a nonspecific effect, further reducing transmission-blocking activity. Thus, depletion with neither TBV25H nor any of the domains resulted in a further significant reduction in transmission-blocking activity.
DISCUSSION
Previous studies have proven TBV25H to be a potent inducer of P. falciparum transmission-blocking immunity (12). Vaccination of mice, rabbits, and monkeys can result in complete blocking of the parasite's ability to form mature oocysts in the mosquito midgut (1, 4, 8). However, when translated into human trials, the bugbear of malaria vaccine development strikes: the inability to duplicate results in humans due to antibody titers orders of magnitude lower than those in the animal models (Kaslow, unpublished data). This problem plagues malaria vaccine development in the sporozoite, asexual-stage, and transmission-blocking areas.
The general approach to overcoming low titers is to search for the appropriate combination of adjuvant and immunogen that will raise antibody titers to the biologically active levels seen in the animal models. Such an approach can be highly successful but runs the risk of also increasing the reactogenicity of the vaccine and the number of adverse events (21). An alternate strategy suggested by the data presented here is to target the immune response more effectively to biologically active components of the molecule. This is part of the approach with peptide-based vaccines, although the complex disulfide-bridge structure for each of the four EGF-like domains of TBV25H makes it seem an unlikely target for such an approach. However, the production of the individual domains of TBV25H as yeast-secreted recombinant proteins with appropriate secondary structures has opened up this avenue of research. Better targeting of the immune response to critical B-cell epitopes may thus lower the antibody titers required for transmission blocking to levels achievable with adjuvants currently approved for use in humans.
The antibody titers to the protein yEGF2, when it is adsorbed to aluminum hydroxide and delivered to mice, are very low (mean titer, 1/5,265; titers of responding individual mice, 1/77 to 1/18,588) yet are very effective in blocking transmission (Tables 2 and 3). That such low titers can have biological activity is surprising, but this nevertheless was confirmed by immunodepletion experiments with rabbit sera. The anti-yEGF2 titers of the sera of four rabbits ranged from 1/400 to 1/4,000, and depleting those sera of anti-yEGF2 antibodies significantly reduced the ability of those sera to block transmission (Table 4).
This is not to say that antibodies to other portions of TBV25H do not play any role in generating transmission-blocking antibodies, just that the anti-yEGF2 antibodies clearly are extremely effective. Previously observed poor correlations between anti-TBV25H antibody titers and transmission-blocking ability (8) may thus be explained if a small portion of the antibody is playing a significant biological role. This may explain in part the discrepancies observed previously between the transmission-blocking activities of anti-Pfs25 MAbs and anti-TBV25H polyclonal serum (8). It has been previously noted that higher concentrations of MAbs 1D2 and 4B7 than of polyclonal serum are required to block transmission (9). Further, the stages of parasite development affected by monoclonal and polyclonal sera appear to differ (8). MAbs 4B7 and 1D2 appear to interfere with the parasite's development sometime between the ookinete's penetration of the peritrophic matrix and midgut epithelium and the parasite's formation of an oocyst (19). Polyclonal serum against TBV25H appears to act earlier, blocking the transformation of zygotes to ookinetes (8). A plausible explanation for this can now be hypothesized. The transmission-blocking efficacy of polyclonal serum may be due to an anti-EGF-like domain 2 activity, which we know from the present study is highly potent and hence effective at lower antibody concentrations. We also know from the present work that MAbs 4B7 and 1D2 are directed towards EGF-like domain 3. Hence, a higher concentration of MAb may be required, as antibody to EGF-like domain 3 may be less effective and/or may not be accessible for antibody binding until much later in the parasite's development in the mosquito (possibly when there has been some loss of antibody quantity or potency).
The recombinant yEGF2 produced in this study is clearly poorly immunogenic in mice by itself. However, we believe that it may still be a useful molecule as a vaccine. The vaccinations were performed in aluminum hydroxide, an adjuvant that is noted for being less effective than many others (1, 8, 15) but that is already approved for human use. Thus, we have shown that (at least in mice) the yEGF2-alum combination successfully primes an immune response, presumably by activating helper T cells. Thus, following a boost with TBV25H, highly efficacious antibodies are produced not only to EGF-like domain 2 but also to the full-length TBV25H and the other domains as well. The ability to focus the immune response may be important, as it is apparent that there are some species-specific differences in the proportion of antibodies made to each domain of TBV25H (Fig. 3).
It is of course possible to elicit very good transmission-blocking antibodies in animal models using full-length TBV25H alone. However, in the one phase I study performed to date in humans (Kaslow, unpublished data), full-length TBV25H adsorbed to alhydrogel gave relatively poor levels of antibody response and of transmission-blocking activity. Alternate forms of Pfs25 have also given no transmission-blocking activity in humans (13, 22). The outcome of this study is not to show higher titer transmission-blocking antibodies through vaccination with EGF2-TBV25H compared to TBV25H alone (in mice, TBV25H alone gives very-high-titer transmission-blocking antibodies). Rather, where titers are much lower than normal (for example, 1/30,000 compared to 1/760,000), we can still get effective transmission blocking by using EGF2-TBV25H. This may result from a more significant proportion of the antibody response being generated to the EGF2 region, compensating for the overall decline in antibody levels.
We suggest, then, that an effective vaccination strategy for the next clinical trial of TBV25H may well involve one or more priming vaccinations with EGF2 delivered by one of the modalities that elicit a potent helper T-cell response (e.g., yEGF2 protein, peptide-based vaccines [18], or DNA-based vaccines [5]) before vaccination with TBV25H.
ACKNOWLEDGMENTS
We are pleased to acknowledge the excellent technical assistance of Richard Shimp, Yanling Zhang, and Roseanne Hearn in the production of the recombinant proteins used in this study.
REFERENCES
- 1.Barr P J, Green K M, Gibson H L, Bathurst I C, Quakyi I A, Kaslow D C. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter R, Graves P M, Keister D B, Quakyi I A. Properties of epitopes of Pfs 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol. 1990;12:587–603. doi: 10.1111/j.1365-3024.1990.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 3.Duffy P E, Pimenta P, Kaslow D C. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1993;177:505–510. doi: 10.1084/jem.177.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gozar M M, Price V L, Kaslow D C. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infect Immun. 1998;66:59–64. doi: 10.1128/iai.66.1.59-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman S L, Doolan D L, Sedegah M, Aguiar J C, Wang R, Malik A, Gramzinski R A, Weiss W R, Hobart P, Norman J A, Margalith M, Hedstrom R C. Strategy for development of a pre-erythrocytic Plasmodium falciparum DNA vaccine for human use. Vaccine. 1997;15:842–845. doi: 10.1016/s0264-410x(96)00273-3. [DOI] [PubMed] [Google Scholar]
- 6.Kaslow D C, Bathurst I C, Barr P J. Malaria transmission-blocking vaccines. Trends Biotechnol. 1992;10:388–391. doi: 10.1016/0167-7799(92)90280-9. [DOI] [PubMed] [Google Scholar]
- 7.Kaslow D C, Bathurst I C, Isaacs S N, Keister D B, Moss B, Barr P J. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant Pfs25. Mem Inst Oswaldo Cruz. 1992;87:175–177. doi: 10.1590/s0074-02761992000700028. [DOI] [PubMed] [Google Scholar]
- 8.Kaslow D C, Bathurst I C, Lensen T, Ponnudurai T, Barr P J, Keister D B. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect Immun. 1994;62:5576–5580. doi: 10.1128/iai.62.12.5576-5580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaslow D C, Isaacs S N, Quakyi I A, Gwadz R W, Moss B, Keister D B. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant vaccinia virus. Science. 1991;252:1310–1313. doi: 10.1126/science.1925544. [DOI] [PubMed] [Google Scholar]
- 10.Kaslow D C, Kumar S. Expression and immunogenicity of the C-terminus of a major blood-stage surface protein of Plasmodium vivax, Pv200(19), secreted from Saccharomyces cerevisiae. Immunol Lett. 1996;51:187–189. doi: 10.1016/0165-2478(96)02570-9. [DOI] [PubMed] [Google Scholar]
- 11.Kaslow D C, Quakyi I A, Syin C, Raum M G, Keister D B, Coligan J E, McCutchan T F, Miller L H. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 12.Kaslow D C, Shiloach J. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Biotechnology. 1994;12:494–499. doi: 10.1038/nbt0594-494. [DOI] [PubMed] [Google Scholar]
- 13.Ockenhouse C F, Sun P F, Lanar D E, Wellde B T, Hall B T, Kester K, Stoute J A, Magill A, Krzych U, Farley L, Wirtz R A, Sadoff J C, Kaslow D C, Kumar S, Church L W, Crutcher J M, Wizel B, Hoffman S, Lalvani A, Hill A V, Tine J A, Guito K P, de Taisne C, Anders R, Ballou W R, et al. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J Infect Dis. 1998;177:1664–1673. doi: 10.1086/515331. [DOI] [PubMed] [Google Scholar]
- 14.Quakyi I A, Carter R, Rener J, Kumar N, Good M F, Miller L H. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139:4213–4217. [PubMed] [Google Scholar]
- 15.Rawlings D J, Kaslow D C. Adjuvant-dependent immune response to malarial transmission-blocking vaccine candidate antigens. J Exp Med. 1992;176:1483–1487. doi: 10.1084/jem.176.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rener J, Carter R, Rosenberg Y, Miller L H. Anti-gamete monoclonal antibodies synergistically block transmission of malaria by preventing fertilization in the mosquito. Proc Natl Acad Sci USA. 1980;77:6797–6799. doi: 10.1073/pnas.77.11.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romanos M A, Scorer C A, Clare J J. Foreign gene expression in yeast: a review. Yeast. 1992;8:423–488. doi: 10.1002/yea.320080602. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y P, Hasnain S E, Sacci J B, Holloway B P, Fujioka H, Kumar N, Wohlhueter R, Hoffman S L, Collins W E, Lal A A. Immunogenicity and in vitro protective efficacy of a recombinant multistage Plasmodium falciparum candidate vaccine. Proc Natl Acad Sci USA. 1999;96:1615–1620. doi: 10.1073/pnas.96.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieber K P, Huber M, Kaslow D, Banks S M, Torii M, Aikawa M, Miller L H. The peritrophic membrane as a barrier: its penetration by Plasmodium gallinaceum and the effect of a monoclonal antibody to ookinetes. Exp Parasitol. 1991;72:145–156. doi: 10.1016/0014-4894(91)90132-g. [DOI] [PubMed] [Google Scholar]
- 20.Snewin V A, Premawansa S, Kapilananda G M, Ratnayaka L, Udagama P V, Mattei D M, Khouri E, Del Giudice G, Peiris J S, Mendis K N, et al. Transmission blocking immunity in Plasmodium vivax malaria: antibodies raised against a peptide block parasite development in the mosquito vector. J Exp Med. 1995;181:357–362. doi: 10.1084/jem.181.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 22.Tine J A, Lanar D E, Smith D M, Wellde B T, Schultheiss P, Ware L A, Kauffman E B, Wirtz R A, De Taisne C, Hui G S, Chang S P, Church P, Hollingdale M R, Kaslow D C, Hoffman S, Guito K P, Ballou W R, Sadoff J C, Paoletti E. NYVAC-Pf7: a poxvirus-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. Infect Immun. 1996;64:3833–3844. doi: 10.1128/iai.64.9.3833-3844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermeulen A N, Ponnudurai T, Beckers P J, Verhave J P, Smits M A, Meuwissen J H. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985;162:1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermeulen A N, van Deursen J, Brakenhoff R H, Lensen T H, Ponnudurai T, Meuwissen J H. Characterization of Plasmodium falciparum sexual stage antigens and their biosynthesis in synchronised gametocyte cultures. Mol Biochem Parasitol. 1986;20:155–163. doi: 10.1016/0166-6851(86)90027-7. [DOI] [PubMed] [Google Scholar]