Abstract
J wave syndrome (JWS) is an inherited cardiac channelopathy associated with malignant ventricular arrhythmias and sudden cardiac death (SCD), which comprises early repolarization syndrome and Brugada syndrome. Here, we explore the association between variants in the L-type calcium channel gene subunits, α1C (CACNA1C) and β2b (CACNB2b), and the JWS phenotype. Using next-generation genetic sequencing of 402 JWS probands and their family members, we identified a CACNA1C-G37R (p.Gly37Arg) mutation in five individuals in four families, two of which had a family history of SCD as well as a CACNB2b-S143F (p.Ser143Phe) mutation in seven individuals in three families, two of which had a family history of SCD. The variants were located in exon 2 in CACNA1C and exon 5 in CACNB2b; both were in highly conserved amino acid residues. Whole-cell patch-clamp results showed that compared with the wild-type group, calcium current density of CACNB2b-S143F and CACNA1C-G37R were significantly lower displaying a dominant-negative effect. Our findings provide further support for the hypothesis that variants in CACNA1C and CACNB2b are associated with JWS. The results suggest that mutations in these two genes lead to loss-of-function of the cardiac calcium channel current warranting their inclusion in genetic screening protocols.
This article is part of the theme issue ‘The heartbeat: its molecular basis and physiological mechanisms’.
Keywords: Brugada syndrome, early repolarization syndrome, genetics, electrophysiology, sudden cardiac death
1. Introduction
In recent years, the J wave and J wave syndrome (JWS) have received much attention because of its association with life-threatening ventricular arrhythmias and sudden cardiac death (SCD), the latter is comprised of Brugada syndrome (BrS) and early repolarization syndrome (ERS) [1]. SCD has been recognized as one of the leading causes of death accounting for up to 20% of all-cause deaths in developed countries, with an annual toll of about 50 – 100/100 000 people [2,3]. In BrS, three ECG patterns are recognized. The ECG pattern diagnostic of BrS is a Type 1 ST-segment elevation characterized by a coved-type ST-segment elevation in the right precordial leads (V1–V3). A Type 2 BrS pattern is characterized by a ‘saddle-back’ configuration with an ST-segment elevation of greater than or equal to 1 mm in right precordial leads, whereas a Type 3 is characterized by a similar shape with ST-segment elevation of less than 1 mm [4,5]. Early repolarization pattern (ERP) is a relatively common variant of the normal ECG, which is observed in approximately 5% of the population [6]. This pattern is defined by J-point elevation of greater than or equal to 0.1 mV in greater than or equal to two inferior/lateral leads. When patients with ERP are resuscitated from otherwise unexplained syncope or polymorphic ventricular tachycardia and ventricular fibrillation (pVT/VF), the clinical condition is referred to as ERS [4]. In patients diagnosed as ERS, ERP in the lateral leads is referred to as ERS Type 1, in inferolateral leads is Type 2, and with a global pattern (inferolateral + anterior or right ventricular leads) is Type 3. Type 3 ERS is associated with the highest mortality rate, followed by Type 2; Type 1 had the lowest mortality rate [7].
The cellular mechanism underlying JWS has long been a matter of controversy. Two hypotheses have been advanced in the case of BrS: (i) the repolarization hypothesis proposed by Antzelevitch and co-workers. The repolarization hypothesis, is based on the observation that non-homogeneous repolarization in the different areas of right ventricular epicardium gives rise to phase 2 re-entry, leading to the development of closely coupled premature beats capable of inducing VT/VF [8,9]; (ii) the depolarization hypothesis proposed by Wilde and co-workers maintains that delayed conduction in the right ventricular outflow tract (RVOT) plays a principal role in the development of the arrhythmic and electrocardiographic manifestations due delayed conduction into the RVOT [10]. These hypotheses are not mutually exclusive and may be synergistic.
JWS has a clear familial predisposition, and mutations in the CACNA1C and CACNB2b genes have attracted recent attention [11–15]. Variants in the α1C, β2b and α2δ subunits of the L-type calcium channel (LTCC) have previously been linked to a combined BrS and/or short QT phenotype. We present here a strong association of loss-of-function (LOF) mutations in the α1C (CACNA1C) and β2b (CACNB2b) subunits with JWS. We use next-generation sequencing technology, cell transfection and whole-cell patch-clamp experiments to explore the potential relationship between calcium channel genetic variants and JWS, possible pathogenic mechanisms and possible approaches to therapy of patients carrying these variants.
2. Methods
(a) . Analysis of clinical characteristics
Clinical and genetic studies were performed on 402 patients diagnosed with JWS and 420 healthy controls with no family history of arrhythmia, after approval of the ethics committee of the Renmin Hospital of Wuhan University and informed consent of the enrolled subjects. According to the most recent expert consensus statement, BrS is diagnosed when a value of greater than or equal to 3.5 is calculated using the Shanghai BrS scoring system. Diagnosis of ERS is made when a value of greater than or equal to 5.0 is obtained using the Shanghai ERS scoring system [16]. For each patient, we collected information on age at time of diagnosis, gender, clinical presentation, family history and therapy. P wave duration, PR interval, QT interval, QTc interval, QRS duration and Tp-e were measured from 12-lead ECGs. Patients with structural heart disease were excluded from the study.
(b) . Genetic screening
Genomic DNA was extracted from peripheral blood leucocytes of patients according to standard protocols. Exons and exon–intron junction sequences of candidate genes were amplified by polymerase chain reaction (PCR). PCR products were purified with reagent (ExoSAPIT, USB, Cleveland, OH, USA) and the purified PCR products were sequenced in a loop on an ABI 3730 Genetic Analyzer (Applied Biosystem, Foster City, CA, USA). Sequencing results were confirmed by Mutation Surveyor v.4.0.8 software (Softgenetics, USA), and the above procedures were repeated for reconfirmation. According to the guidelines of the American College of Medical Genetics and Genomics (ACMG), the use of variant classifiers required variants to meet the criteria set out before they can be classified as pathogenic.
(c) . Site-directed mutagenesis and transfection of cells
For the patch-clamp study, full-length human CACNA1C (wild-type, WT, or mutant) with enhanced yellow fluorescence protein (EYFP), CACNB2b (WT or mutant) cDNA, together with CACNA2D1-WT were cloned in the pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA) using site-directed mutagenesis. TSA201 cells were transfected with CACNA1C, CACNB2b and CACNA2D1 plasmids used for electrophysiological studies [12,17]. cDNAs of the three LTCC subunits were co-transfected with a 1 : 1 : 1 molar ratio using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). Electrophysiological studies were performed after 48–72 h of incubation.
(d) . Cellular electrophysiology experiments
L-type calcium currents (ICaL) were measured at room temperature (20–24°C) using an Axon-700B membrane clamp amplifier and PclamP10.4 software (Axon Instruments, San Francisco, CA, USA). Macroscopic whole-cell Ca2+ current was recorded by using bath solution perfusion containing (in mmol l–1) 2 CaCl2, 1 MgCl2, 150 TEA, 10 HEPES and 10 glucose (PH 7.35 with CsOH). Patch pipettes were fabricated from borosilicate glass capillaries (1.5 mm O.D., Fisher Scientific, Pittsburgh, PA, USA), which were filled with the perfusion containing (in mmol l–1) 110 CsCl, 0.1 CaCl2, 10 HEPES, 10 EGTA, 2 MgATP and 10 TEA (PH7.35 with CsOH), with uncompensated access resistances of 1.0–2.8 MΩ. Recorded currents were filtered with an eight-pole Bessel filter at 5 kHz and digitized at 50 kHz. Series resistance was electronically compensated at 70–85%. Data were recorded and analysed with Pclamp v.10.4 (Axon Instruments, Sunnyvale, CA, USA), Excel (Microsoft, Redmond, WA, USA) and Origin 7.5 (Microcal Software, Northampton, MA, USA). The voltage-dependent steady-state activation (SSA) curve of ICaL used a dual-pulse protocol in which the conditioned pulse was holding potential of −90 mV. The command potential was −50 to +60 mV in 10 mV step increments from the holding potential with 400 ms pulses. A Boltzmann function was fitted to the activation or inactivation curves with the pulse voltage as the horizontal axis and the whole-cell conductance as the vertical axis.
(e) . Statistical analysis
Data were presented as mean ± s.d. unless otherwise noted. Comparisons between the two and multiple groups were performed with the Student's t-test or one-way ANOVA with Bonferroni correction, as appropriate. All data involving statistics were analysed using GraphPad software v.8.0. Differences were considered statistically significant at a value of p < 0.05.
3. Results
(a) . Clinical characteristics of the probands and family members with mutations
We uncovered four probands carrying CACNA1C-G37R mutations and three carrying CACNB2b-S143F mutations. The clinical characteristics of JWS patients displaying calcium channel mutations are shown in table 1. The main symptoms at the time of diagnosis included syncope, SCD/VT/VF, premature ventricular contraction (PVC) and bradycardia.
Table 1.
Clinical characteristics of J wave syndrome probands carrying calcium channel mutations.
| index | CACNA1C-G37R (n = 4) | CACNB2b-S143F (n = 3) |
|---|---|---|
| age (years) | 36.0 ± 11.0 | 52.3 ± 25.1 |
| male, n (%) | 4 (100.0) | 2 (66.7) |
| symptom, n (%) | ||
| syncope | 2 (50.0) | 3 (100.0) |
| SCD/VT/VF | 1 (25.0) | 0 |
| PVC | 1 (25.0) | 0 |
| bradycardia | 1 (25.0) | 0 |
| asymptomatic | 1 (25.0) | 0 |
| family history of SCD | 2 (50.0) | 2 (66.7) |
Among all 402 JWS patients, the CACNA1C-G37R was identified in five cases from four families, two with a family history of SCD. Among the probands carrying the CACNA1C-G37R variant (four males, 100%; mean age 36.0 ± 11.0 years), two were diagnosed with BrS and two with ERS. One suffered from SCD/VF, PVC, bradycardia (25.0%) and two (50.0%) presented with syncope.
The CACNB2b-S143F mutation was uncovered in seven patients from three families, two with a family history of SCD. Among the probands carrying the CACNB2b-S143F variant, two presented with a phenotype of spontaneous Type 1 BrS and one with ERS (two males, 66.7%; mean age 52.3 ± 25.1 years). All three suffered from syncope (100.0%).
Compared with healthy controls, heart rate (HR) was strikingly slower in probands carrying CACNB2b-S143F; P wave duration was significantly longer in both mutant groups, and QTc interval was significantly shorter in CACNA1C-G37R carriers when compared with WT (table 2). Other variables, including HR in CACNA1C-G37R, QTc interval in CACNB2b-S143F and PR interval, QRS duration, Tp-e, and Tp-e/QT in both CACNA1C-G37R and CACNB2b-S143F, did not differ significantly between control and the two mutation groups (table 2). Table 3 shows the ECG parameters of JWS patients carrying the calcium channel mutations. HR, P wave duration and QTc interval but not Tp-e were significantly different between CACNA1C-G37R carriers and healthy controls.
Table 2.
ECG parameters in J wave syndrome probands carrying calcium channel gene mutations. If p < 0.05, it is shown in italics (mean ± s.d.). p-value indicates the statistical difference between study group versus healthy controls.
| index | healthy control (n = 420) | CACNA1C-G37R (n = 4) | p-value | CACNB2b-S143F (n = 3) | p-value |
|---|---|---|---|---|---|
| HR (bpm) | 72.7 ± 8.9 | 69.0 ± 11.7 | 0.410 | 62.5 ± 11.4 | 0.049 |
| P wave duration (ms) | 87.6 ± 9.1 | 99.0 ± 22.9 | 0.015 | 113.3 ± 5.8 | <0.001 |
| PR interval (ms) | 170.7 ± 18.7 | 175.0 ± 19.1 | 0.647 | 178.7 ± 35.9 | 0.464 |
| QRS duration (ms) | 89.4 ± 14.6 | 100.5 ± 17.2 | 0.131 | 98.0 ± 13.1 | 0.310 |
| QTc interval (ms) | 408.2 ± 21.4 | 377.0 ± 23.4 | 0.004 | 393.2 ± 40.1 | 0.230 |
| Tp-e | 82.3 ± 9.9 | 90.0 ± 8.2 | 0.122 | 80.0 ± 0.0 | 0.643 |
| Tp-e/QT | 0.22 ± 0.05 | 0.25 ± 0.02 | 0.232 | 0.20 ± 0.01 | 0.489 |
Table 3.
ECG parameters of J wave syndrome patients carrying calcium channel gene mutations. If p < 0.05, it is shown in italics (mean ± s.d.). p-value indicates the statistical difference between study group versus healthy controls.
| index | healthy control (n = 420) | CACNA1C-G37R (n = 5) | p-value | CACNB2b-S143F (n = 7) | p-value |
|---|---|---|---|---|---|
| HR (bpm) | 72.7 ± 8.9 | 69.8 ± 10.2 | 0.470 | 65.8 ± 10.9 | 0.043 |
| P wave duration (ms) | 87.6 ± 9.1 | 103.2 ± 21.9 | <0.001 | 108.0 ± 16.4 | <0.001 |
| PR interval (ms) | 170.7 ± 18.7 | 176.0 ± 16.7 | 0.529 | 165.2 ± 33.2 | 0.448 |
| QRS duration (ms) | 89.4 ± 14.6 | 98.4 ± 15.6 | 0.172 | 92.8 ± 12.2 | 0.541 |
| QTc interval (ms) | 408.2 ± 21.4 | 378.7 ± 20.6 | 0.002 | 396.6 ± 29.0 | 0.158 |
| Tp-e | 82.3 ± 9.9 | 92.0 ± 8.4 | 0.030 | 82.0 ± 11.0 | 0.937 |
| Tp-e/QT | 0.22 ± 0.05 | 0.26 ± 0.02 | 0.075 | 0.22 ± 0.02 | 1.000 |
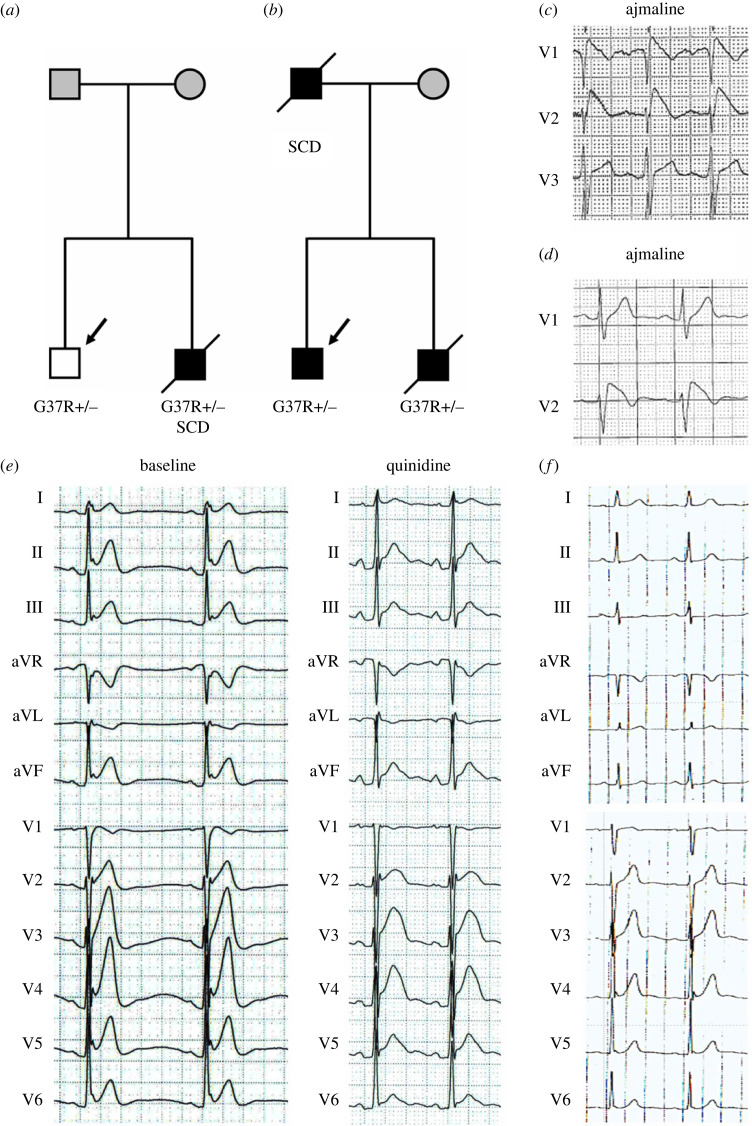
The younger brother of proband 1, who carried CACNA1C-G37R, had ERS and died at age of 25 suddenly during vagal circumstances (figure 1a). At 23 years of age, his ECG showed typical Type 3 ERP and bradycardia. Because of his positive family history and genetic results, quinidine was administrated. Quinidine led to a dramatic increase of QTc from 343.5 to 447.8 ms, and a significant decrease of the J wave in inferior and lateral leads (figure 1e). Proband 2, a 31-year-old man (figure 1b) presented with an ECG displaying ERP in anterior leads as well as multiple PVC (figure 1f). His deceased brother had the same pattern on his ECG and his father died of SCD at age 50 years. Type 1 Brugada ECG was observed after ajmaline provocation in probands 3 and 4 (figure 1c,d). Both presented with syncope but had a negative family history. Their ECGs both showed significant elevation of a coved-type ST-segment (greater than or equal to 0.2 mm) in the right precordial leads V1–V2.
Figure 1.
Pedigrees of representative families and ECGs of JWS patients carrying CACNA1C-G37R. (a) The family members included proband 1 and his deceased younger brother with a history of SCD. (b) Proband 2 and his deceased younger brother both presented with ERS/SQT, and the father died of SCD at age of 50 years. (c) The ECG of proband 3 shows an ajmaline-induced Type 1 pattern in leads V1 and V2. (d) After ajmaline provocation, ECG of proband 4 is characterized by a coved ST-segment elevation in leads V1 and V2. (e) ECG of proband 1 is presented at baseline and after treatment with quinidine. He shows significant decrease of ST-segment in leads V2–V6 and remained asymptomatic on quinidine. (f) ECG of proband 2 showing spontaneous ERP in anterior leads. Squares indicate male subjects, circles female subjects and symbols with a slash mark deceased individuals. The arrows indicate the probands and pointed triangle indicates twins. −/− wild-type (WT); +/− heterozygous for the mutation. The symptomatic subjects are labelled by black. The asymptomatic subjects are shown as white and unaffected subjects are labelled by dark grey. (Online version in colour.)
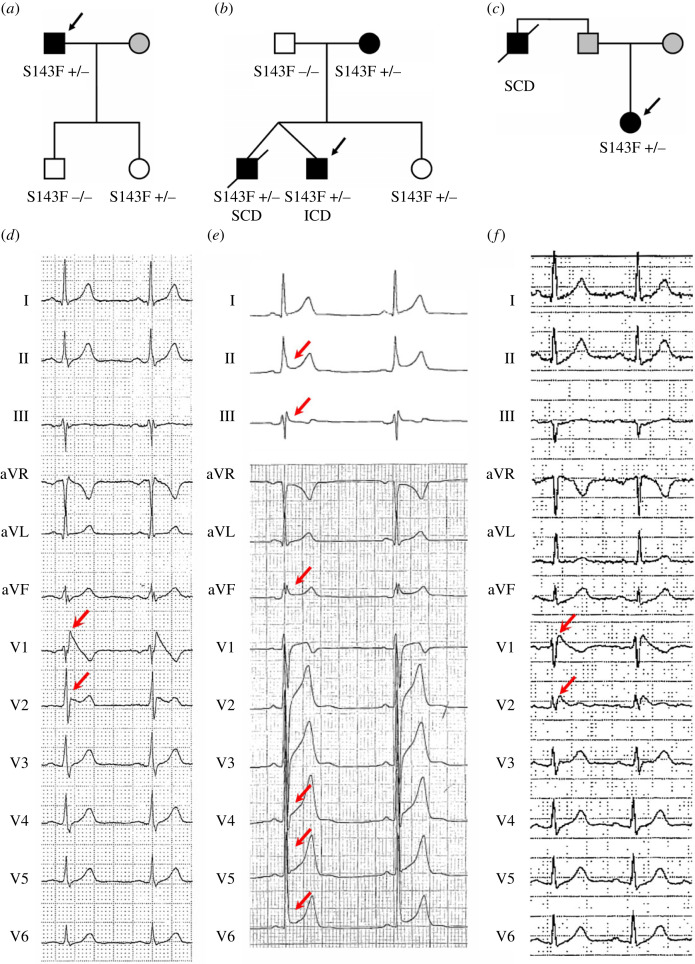
Among CACNB2b-S143F carriers, probands 5 and 7 both exhibited spontaneous Type 1 ECG patterns in the right precordial leads (figure 2d,f), whereas proband 6 presented with an ERS phenotype and a history of syncope (figure 2e). As shown in figure 2, QTc interval was shorter than normal in probands 5 and 6 (QTc = 375 and 365 ms). Proband 7 had normal QT (QTc = 439 ms), likely due to additional genetic variants (see below).
Figure 2.
Pedigrees of representative families and ECGs of JWS patients carrying CACNB2b-S143F. (a) Family members with a BrS phenotype include a father and his daughter; the genetic result of his son is negative. (b) Proband 6, his mother and his twin brother present mutation-positive with a ERS phenotype, whereas his sister is a silent carrier. He eventually received implantable cardioverter–defibrillator (ICD) therapy. (c) Proband 7 exhibits a BrS phenotype; his father died of SCD. (d) The ECG of proband 5 shows a spontaneous Type 1 ECG pattern in lead V1–V2. (e) ECG of proband 6 is characterized by J-point elevation greater than or equal to 1 mm in contiguous inferior and lateral leads. (f) ECG of proband 7 shows significant ST-segment elevation (Type 1) in leads V1–V2. (Online version in colour.)
(b) . Genetic discovery of calcium mutations in J wave syndrome
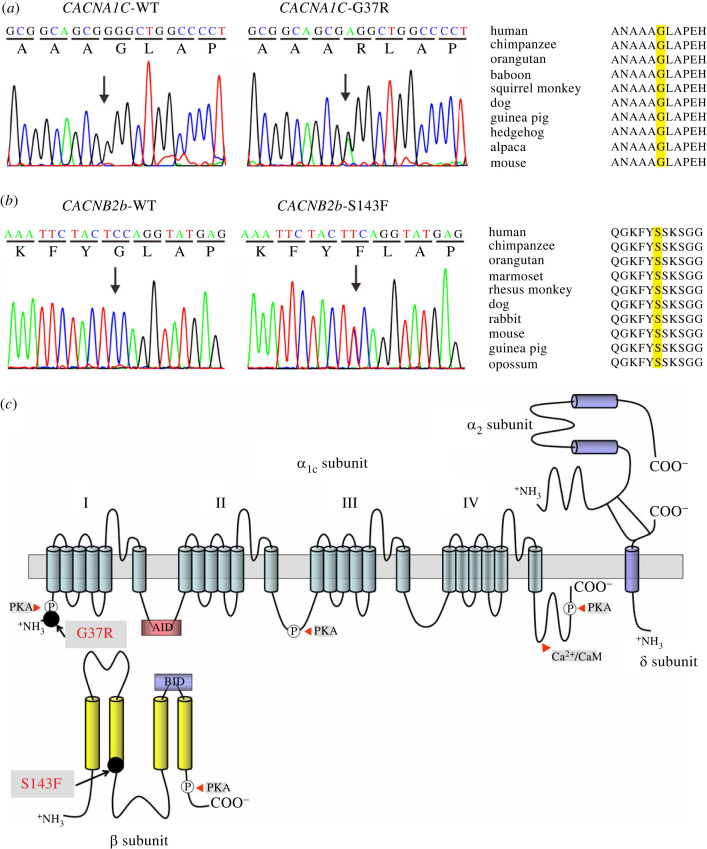
Genetic analysis revealed two heterozygous missense mutations in the α1C (CACNA1C) and β2b (CACNB2b) subunits of LTCC in seven probands (figure 3), five (71.4%) of which harboured additional genetic variants, such as CACNB2B-D601E, SCN1B-L210P, SCN5A-H558R, KCNH2-K897T, KCNE1-G38S, KCNH2-R1047L. These calcium channel variants were not present in 450 reference control alleles. The CACNA1C gene was mutated from a G to A substitution at location 109 in exon 2, resulting in an amino acid change from glycine to arginine at position 37 (G37R, figure 3a). The CACNB2b gene was mutated with substitution of T for C at position 428 in exon 5, resulting in an amino acid change from serine to phenylalanine at position 143 (S143F, figure 3b). Additionally, we evaluated the pathogenicity of both missense substitutions using multiple prediction tools, including Mutation Taster, PolyPhen-2, SIFT, REVEL, MetaLR and ClinVar (table 4). The majority of prediction tools predicted ‘disease causing’ or ‘damaging’ variants with a global minor allele frequency (MAF) < 0.001 for each in the 1000 Genomes database.
Figure 3.
Genetic analysis of CACNA1C-G37R and CACNB2b-S143F. (a) Electropherogram of CACNA1C-WT and CACNA1C-G37R and amino acid sequence alignment of CACNA1C-G37R. (b) Electropherogram of CACNB2b-WT and CACNB2b-S143F and amino acid sequence alignment of CACNB2b-S143F. (c) Schematic of the CaV1.2 channel pore-forming α1C subunit and the auxiliary α2δ and β subunits. The CACNA1C-G37R mutation is in the N-terminus close to the PKA binding site (black circle). The CACNB2b-S143F mutation is located at the Hook region. Phosphorylation sites by PKA and calcineurin (CaN) binding sites located in CaV1.2 and β subunits (white cycles with P). BID, β subunit interacting domain and AID, α subunit interacting domain. (Online version in colour.)
Table 4.
Summary of J wave syndrome probands carrying calcium channel gene mutations G37R and S143F.
| CACNA1C-G37R | CACNB2b-S143F | |
|---|---|---|
| reported ID | rs34534613 | rs150528041 |
| variant type | missense | missense |
| nucleotide change | c.109G > A | c.428C > T |
| exon location | 2 | 5 |
| MAF | ||
| GnomAD | 0.003464 | 0.000510 |
| ExAC | 0.007385 | 0.000509 |
| 1000 Genomes | 0.000399 | 0.000399 |
| SIFT | ||
| score | 0.000 | 0.010 |
| prediction | deleterious | deleterious |
| PolyPhen-2 | ||
| score | 0.967 | 0.730 |
| prediction | probably damaging | possibly damaging |
| Mutation Taster | ||
| score | 1 | 1 |
| prediction | disease causing | disease causing |
| MetaLR | ||
| score | 0.903 | 0.703 |
| prediction | damaging | damaging |
| REVEL | ||
| score | 0.655 | 0.611 |
| prediction | likely disease causing | likely disease causing |
| ClinVar | ||
| score | two stars | a star |
| prediction | benign/likely benign | uncertain significance/likely benign |
(c) . Functional expression of CACNA1C-G37R and CACNB2b-S143F
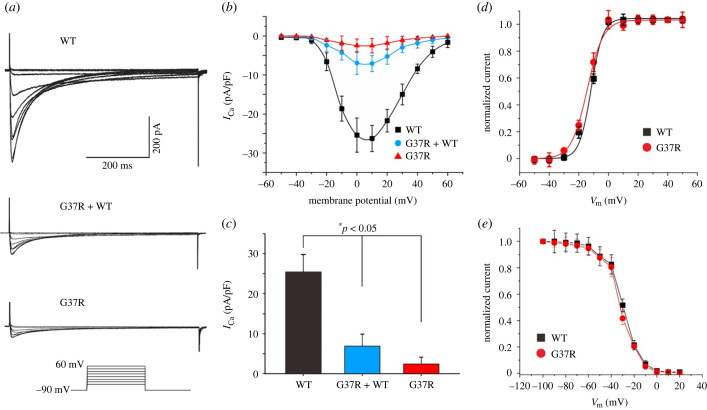
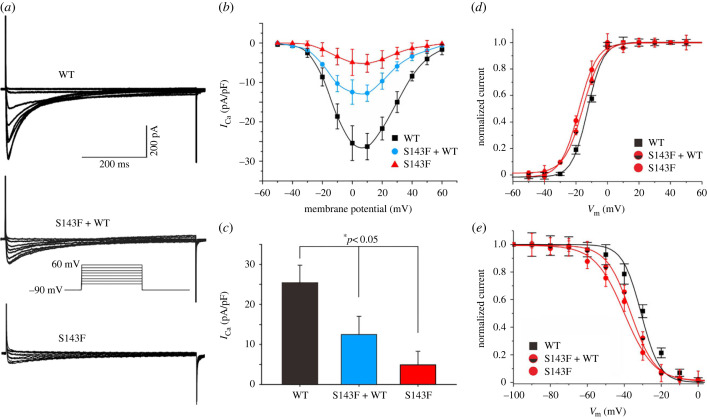
Expression studies using whole-cell patch-clamp techniques to evaluate the effect of CACNA1C-G37R and CACNB2b-S143F mutations were performed. Representative ICaL tracings of voltage-dependent activation are shown in figures 4a and 5a. From a holding potential of −90 mV voltage was depolarized to various potentials until +10 mV (figures 4b and 5b). Current–voltage relationships (I–V curves) showed that homozygous expression of CACNA1C-G37R reduced ICaL by 90.3% (25.4 ± 4.4 pA/pF versus 2.5 ± 1.7, n = 12, 13; *p < 0.05), and by 72.7% in the case of heterozygous expression (25.4 ± 4.4 pA/pF versus 6.9 ± 3.0, n = 12, 12; *p < 0.05) when compared to WT (figure 4c). Homozygous expression of CACNB2b-S143F reduced ICaL by 80.7% (25.4 ± 4.4 pA/pF versus 4.9 ± 3.4 pA/pF, n = 12, 8; *p < 0.05), and by 51.0% in the case of heterozygous expression (25.4 ± 4.4 pA/pF versus 12.5 ± 4.5 pA/pF, n = 12, 9; *p < 0.05) at 0 mV when compared to WT (figure 5c).
Figure 4.
Functional expression of CACNA1C-G37R on ICaL. (a) Representative ICaL recordings from TSA201 cells expressing CACNA1C-WT, CACNA1C-G37R + WT and CACNA1C-G37R (n = 12, 12, 13, respectively). (b) Current density–voltage relationships for CACNA1C-WT, CACNA1C-G37R + WT and CACNA1C-G37R. (c) Bar graph depicting peak ICaL density at 0 mV for CACNA1C-WT, CACNA1C-G37R + WT and CACNA1C-G37R channels. Data are expressed as mean + s.e.m. (*p < 0.05, compared with CACNA1C-G37R + WT. *p < 0.05, compared with CACNA1C-G37R). (d) Steady-state activation curve of ICaL for CACNA1C-WT and CACNA1C-G37R. (e) Steady-state inactivation curve of ICaL for CACNA1C-WT and CACNA1C-G37R. (Online version in colour.)
Figure 5.
Functional expression of CACNB2b-S143F on ICaL. (a) Representative ICaL recordings from TSA201 cells expressing CACNB2b-WT, CACNB2b-S143F + WT and CACNB2b-S143F (n = 12, 9, 8, respectively). (b) Current density–voltage relationships for CACNB2b-WT, CACNB2b-S143F + WT and CACNB2b-S143F. (c) Bar graph depicting peak ICaL density at 0 mV for CACNB2b-WT, CACNB2b-S143F + WT and CACNB2b-S143F channels. Data are expressed as mean + s.e.m. (*p < 0.05, compared with CACNA1C-S143F + WT. *p < 0.05, compared with CACNA1C-S143F). (d) Steady-state activation curve of ICaL for CACNB2b-WT and CACNB2b-S143F. (e) Steady-state inactivation curve of ICaL for CACNB2b-WT and CACNB2b-S143F. (Online version in colour.)
The half-activation voltage (V1/2) was obtained by fitting the activation conductance variables (I/Imax), and there was no significant difference observed between CACNA1C-WT and CACNA1C-G37R groups (CACNA1C-WT versus CACNA1C-G37R: −11.91 ± 0.45 mV versus −14.51 ± 1.43 mV, n = 12, 13; p > 0.05; figure 4d). Similarly, no significant difference in steady-state inactivation was found between the two groups (CACNA1C-WT versus CACNA1C-G37R: −30.90 ± 1.45 mV versus −32.65 ± 1.87 mV, n = 12, 6; p > 0.05; figure 4e). However, there was a significant acceleration in steady-state activation and inactivation for CACNB2b-S143F compared with CACNB2b-WT (CACNB2b-WT versus CACNB2b-S143F: −11.91 ± 0.45 mV versus −18.02 ± 1.47 mV, n = 12, 8 for activation, p < 0.01; CACNB2b-WT versus CACNB2b-S143F: −30.90 ± 1.45 mV versus −39.83 ± 1.74 mV, n = 12, 6 for inactivation, p < 0.01, respectively; figure 5d,e). The results showed a significant negative shift in steady-state activation and even larger negative shifts in steady-state inactivation when the mutant β2b-subunit were expressed. In summary, the results of the patch clamp experiments revealed significant LOF in the cardiac calcium channel activity of both CACNA1C-G37R and CACNB2b-S143F mutations.
4. Discussion
Age and gender of our JWS probands carrying CACNA1C-G37R, are similar to that reported previously, 74.1–92.0% of JWS patients were male, with a mean age at time of onset of 30–42 years [16,18,19]. These classic features were not observed in the JWS probands carrying CACNB2b-S143F. Average age at time of onset was much older (52.3 ± 25.1) and male predominance was lower (66.7%). This may not be representative due to the small sample size, selection bias or due to factors such as female gender or genetic variants that predispose to long QT syndrome [12]. BrS patients are known to develop symptoms between 20 and 65 years of age. ERS tends to occur in younger people, especially in men, possibly due to high levels of testosterone, and higher vagal tone [16]. In recent work from our group, ERS probands were 7.5 years younger than BrS probands [20]. In the present study, we observed a similar disparity among BrS and ERS probands with same mutation (CACNA1C-G37R or CACNB2b-S143F). The difference likely depends on the distinctions between the electrophysiological mechanisms underlying BrS and ERS.
Most arrhythmic events and SCD typically occur during episodes of vagal predominance and/or bradycardia which is normally associated with the appearance of accentuated J waves and ST-segment elevation [16,21,22]. Notably, the younger brother of proband 1 died suddenly during episodes of vagal predominance. This is consistent with the results of Viskin et al. who proposed that both a history of syncope at rest and bradycardia have a strong association with risk in cases of ERS, contributing to pause-dependent augmentation of ST-segment elevation leading to VF [23]. These findings support the notion that changes in vagal tone increase arrhythmic risk. It is for this reason that, sympathetic mimic drug is an option in the approach to therapy.
Family history of SCD is an important risk factor in clinical practice. JWS is related to vulnerability for the development of SCD, pVT and VF in patients with structurally normal hearts [24,25]. A meta-analysis by Rattanawong et al. [26] found that a family history of SCD in BrS patients less than 40 years of age doubled the risk of a major arrhythmic event (MAE). Previous reports also suggested the family history of SCD in ERS ranges from 13% to 18% [27,28]. Interestingly, Hu et al. recently reported that approximately 34% of SCN5A+ JWS probands have a family history of unexplained SCD, suggesting a worse outcome in this scenario [20]. Our results show that 50.0% of CACNA1C-G37R and 66.7% of CACNB2b-S143F have a family history of SCD among JWS probands. These findings call for close follow-up of survivors who have a family history of unexplained SCD at a young age.
We assume that carriers of calcium mutations with LOF will have a shorter QTc interval. A report by Antzelevitch et al. [13], is the first to associate LTCC mutations with a combined BrS phenotype and shorter than normal QT interval. Napolitano and co-workers confirmed that CACNA1C is an infrequent but definitive cause of BrS, typically associated with a short QTc interval (371 ms) [11]. Our team identified a short QTc interval not only in ERS with single calcium channel mutation (387 ms), but also in a hypertrophic cardiomyopathy patient carrying a calcium channel mutation [14,29]. Here, we observed that QT/QTc intervals are typically shorter than normal in JWS probands carrying CACNA1C-G37R when compared with healthy controls, but QT/QTc intervals are normal in JWS probands carrying CACNB2b-S143F. We believe that this is due to the complex genetic background in our CACNB2b-S143F probands. For example, proband 7 has a normal QTc interval most likely resulting from additional genetic variants (CACNB2b-D601E, KCNH2-R1047L and K897T), which are known to prolong QTc interval by augmenting ICaL or reducing IKr [17,30]. The opposing influence of these additional gene variants can account for the appearance of a longer QT interval in JWS patients [17].
The ST–T wave morphology changes in JWS are thought to be attributable to genetically mediated alterations in the interplay between depolarizing or repolarizing cardiac currents, which includes genes regulating the sodium current (INa), the L-type calcium current (ICaL) or the transient outward potassium current (Ito) [31]. Specifically, an increase in INa contributes to a reduction in the depolarization of the cardiac action potential (AP), while ICaL is responsible for the plateau phase of the cardiac AP, both of which can promote early repolarization. Additionally, Ito contributes to the phase 1 of AP. At the ion channel level, a reduction of inward currents (INa or ICaL) or increase in outward delayed rectifier potassium currents (IKr or IK-ATP) gives the Ito the possibility to accentuate phase 1 repolarization. Ito is a prominent repolarizing current that partially repolarizes the membrane in physiological conditions, determining the rapid repolarization of the AP and setting the amplitude of the plateau, which gives rise to the spike-and-dome AP morphology and presents as prominent J wave in ECG [32].
Quinidine is the only currently recognized antiarrhythmic drug with inhibition on the outward potassium currents (Ito, IKr, etc.), which can effectively prevent spontaneous or induced VF in JWS [28,33]. The responsiveness to quinidine by BrS carrying calcium channel mutations was first reported by Antzelevitch et al. [13]. We provide additional evidence for the responsiveness of JWS patients to quinidine. The ability of quinidine to prevent induction of VT/VF and its effect to prolong QTc interval in proband 1 is consistent with earlier reports [5,33,34]. Unfortunately, the use of quinidine is currently limited due to a lack of drug availability [35]. Furthermore, electrical storms can be suppressed with β-adrenergic agents, such as isoproterenol capable of augmenting the LTCC [36]. Additional pharmacological therapy includes cilostazol and milrinone, a phosphodiesterase III inhibitor, which have a significant role in the improvement of JWS by augmenting ICaL as well as reducing Ito [37,38]. However, large clinical studies are still needed to further demonstrate their safety and benefit to patients with JWS. Recently, a new discovery from Antzelevitch and Ackerman showing that acacetin, a natural flavonoid, has a potent blocking effect on Ito in canine ventricular myocytes as well as human iPS-derived cardiomyocytes and capable of preventing the development of pVT in experimental wedge and whole-heart models of BrS and ERS, including models induced by calcium blocker [8,39,40]. In fact, we first observe quinidine is highly effective in our ERS case carried CACNA1C-G37R mutation. However, the full extent of quinidine's effect on the properties of calcium channels in patients with mutations is not yet fully understood and warrants further investigation. It should be noted that further experiments of transgenic models are needed to confirm this mechanism.
Variants in genes encoding the calcium channels including CACNA1C (Cav1.2), CACNB2b (Cavβ2b) and CACNA2D1 (Cavα2δ) have been reported in up to 13% of probands [12,13,41]. Antzelevitch and co-workers first identified nine mutations of the CACNA1C gene in patients diagnosed with BrS, ERS and idiopathic VF, including A39V, G490R, V2014I, E1829-Q1833dup, E850del, R1880Q, D2130N, E1115K and C1837Y, of which the first four cause functional deletion of ICa [12,13]. Subsequently, Napolitano et al., identified nine additional potentially pathogenic mutations in CACNA1C including Q428E, A1648T, T320M, E850D, N1255S, A1717G, R1880Q, E850del, and G2084E. It is noteworthy that Fukuyama et al. study pathogenic mutations of LTCC-related genes in 312 probands with a diagnosis of BrS, ERS and short QT syndrome, consequently discovering six gene mutations: CACNA1C, N547S, R632R, R858H, R1780H, C1855Y and R1910Q [42]. Among them, five were potentially pathogenic CACNA1C mutations reported twice, including T320M, A1648T, A1717G, R1880Q and E850del. We sequenced 402 JWS patients and their families and identified four JWS probands carrying CACNA1C-G37R mutation and three carrying CACNB2b-S143F mutation. Functional evidence of those two mutation groups both show significant reduction of ICaL density. Their kinetics were also significantly accelerated, displaying a LOF of ICaL in both. Our results support the conclusion that CACNA1C-G37R and CACNB2b-S143F are two hotspots among the rare mutations in the cardiac calcium channels associated with a JWS phenotype.
(a) . Study limitations
The small number of affected individuals in our study is susceptible to selection, and referral biases. Secondly, this work lacks long-term follow-up with these patients, especially those with a high risk of malignant arrhythmias. Finally, our functional study would be ideal if detailed assessment of other variants contributing to the complex genetic background were presented.
5. Conclusion
Genetic and functional studies identify two high-frequency LOF mutations, including CACNA1C-G37R and CACNB2b-S143F, which are definitive causes of JWS with a family history of SCD. We also provide additional evidence for the effectiveness of quinidine in this setting. JWS patients carrying these two common pathogenic mutations are clinically characterized by prolonged P wave duration. However, there are some clinical differences between CACNA1C-G37R and CACNB2b-S143F mutations. The former is associated with significantly shorter QTc intervals, while the latter is linked to a significant slowing of HR.
Acknowledgements
We are grateful to Dr Michel Springer, a cardiology specialist in Louisville, KY, USA, for providing clinical information; Dr Sami Viskin, from Department of Cardiology, Tel Aviv Sourasky Medical Center and Sackler School of Medicine, Tel Aviv University, Israel, for providing clinical information and Ryan Pfeiffer from the Masonic Medical Research Institute, USA, for technical assistance.
Contributor Information
Dan Hu, Email: hudan0716@hotmail.com.
Hector Barajas-Martinez, Email: barajash69@hotmail.com.
Ethics
This study is approved by the ethics committee of the Renmin Hospital of Wuhan University.
Data accessibility
All datasets generated for this study are available from the corresponding author upon reasonable request.
Authors' contributions
B.Z.: conceptualization, data curation, formal analysis, investigation, methodology, software, visualization, writing—original draft, writing—review and editing; X.Z.: conceptualization, data curation, investigation, methodology, visualization, writing—original draft, writing—review and editing; R.S.: data curation, investigation, resources, writing—review and editing; A.P.: data curation, funding acquisition, resources, writing—review and editing; M.G.: formal analysis, methodology, resources, writing—review and editing; C.A.: conceptualization, funding acquisition, project administration, resources, validation, writing—review and editing; D.H.: conceptualization, formal analysis, funding acquisition, methodology, project administration, software, supervision, validation, visualization, writing—original draft, writing—review and editing; H.B.-M.: conceptualization, data curation, formal analysis, funding acquisition, project administration, resources, supervision, validation, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
C.A. served as a consultant and received grant funds from Novartis and Trevena Inc. All other authors report no relationships to disclose.
Funding
This work was supported by the National Natural Science Foundation Project of China (grant nos. 82270332 and 81670304, to D.H.), the Fundamental Research Funds for the Central Universities of China (grant no. 2042022kf1217 to D.H.), the National Institutes of Health of USA (NIH R56 (HL47678), NIH R01 (HL138103), NIH R01 (HL152201) to C.A. and H.B.-M.), the W.W. Smith Charitable Trust and the Wistar and Martha Morris Fund to C.A., the W.W. Smith Charitable Trust and Sharpe-Strumia Research Foundation to H.B.-M.
References
- 1.Antzelevitch C, Yan GX. 2010. J wave syndromes. Heart Rhythm. 7, 549-558. ( 10.1016/j.hrthm.2009.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Heart Rhythm A . 2006. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J. Am. Coll. Cardiol. 48, e247-e346. ( 10.1016/j.jacc.2006.07.010) [DOI] [PubMed] [Google Scholar]
- 3.Fishman GI, et al. 2010. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 122, 2335-2348. ( 10.1161/CIRCULATIONAHA.110.976092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antzelevitch C, et al. 2005. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 111, 659-670. ( 10.1161/01.CIR.0000152479.54298.51) [DOI] [PubMed] [Google Scholar]
- 5.Priori SG, et al. 2015. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 36, 2793-2867. ( 10.1093/eurheartj/ehv316) [DOI] [PubMed] [Google Scholar]
- 6.Antzelevitch C, et al. 2017. J-wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Europace 19, 665-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Priori SG, et al. 2013. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 10, 1932-1963. ( 10.1016/j.hrthm.2013.05.014) [DOI] [PubMed] [Google Scholar]
- 8.Di Diego JMA, et al. 2020. Acacetin suppresses the electrocardiographic and arrhythmic manifestations of the J wave syndromes. PLoS ONE 15, e0242747. ( 10.1371/journal.pone.0242747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz PJ, et al. 2020. Inherited cardiac arrhythmias. Nat. Rev. Dis. Primers 6, 58. ( 10.1038/s41572-020-0188-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morita H, Zipes DP, Wu J. 2009. Brugada syndrome: insights of ST elevation, arrhythmogenicity, and risk stratification from experimental observations. Heart Rhythm. 6, S34-S43. ( 10.1016/j.hrthm.2009.07.018) [DOI] [PubMed] [Google Scholar]
- 11.Novelli V, et al. 2022. Role of CACNA1C in Brugada syndrome: prevalence and phenotype of probands referred for genetic testing. Heart Rhythm. 19, 798-806. ( 10.1016/j.hrthm.2021.12.032) [DOI] [PubMed] [Google Scholar]
- 12.Burashnikov E, et al. 2010. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 7, 1872-1882. ( 10.1016/j.hrthm.2010.08.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antzelevitch C, et al. 2007. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation 115, 442-449. ( 10.1161/CIRCULATIONAHA.106.668392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, et al. 2021. Clinical and functional genetic characterization of the role of cardiac calcium channel variants in the early repolarization syndrome. Front. Cardiovasc. Med. 8, 680819. ( 10.3389/fcvm.2021.680819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutphin BS, et al. 2016. Molecular and functional characterization of rare CACNA1C variants in sudden unexplained death in the young. Congenit. Heart Dis. 11, 683-692. ( 10.1111/chd.12371) [DOI] [PubMed] [Google Scholar]
- 16.Antzelevitch C, et al. 2016. J-wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Heart Rhythm. 13, e295-e324. ( 10.1016/j.hrthm.2016.05.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu D, et al. 2010. Dual variation in SCN5A and CACNB2b underlies the development of cardiac conduction disease without Brugada syndrome. Pacing Clin. Electrophysiol. 33, 274-285. ( 10.1111/j.1540-8159.2009.02642.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voskoboinik A, et al. 2020. The many faces of early repolarization syndrome: a single-center case series. Heart Rhythm. 17, 273-281. ( 10.1016/j.hrthm.2019.09.013) [DOI] [PubMed] [Google Scholar]
- 19.Kamakura T, et al. 2020. Long-term prognosis of patients with J-wave syndrome. Heart 106, 299-306. ( 10.1136/heartjnl-2019-315007) [DOI] [PubMed] [Google Scholar]
- 20.Zhang ZH, et al. 2021. Distinct features of probands with early repolarization and Brugada syndromes carrying SCN5A pathogenic variants. J. Am. Coll. Cardiol. 78, 1603-1617. ( 10.1016/j.jacc.2021.08.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aizawa Y, et al. 2012. Dynamicity of the J-wave in idiopathic ventricular fibrillation with a special reference to pause-dependent augmentation of the J-wave. J. Am. Coll. Cardiol. 59, 1948-1953. ( 10.1016/j.jacc.2012.02.028) [DOI] [PubMed] [Google Scholar]
- 22.Kalla H, Yan GX, Marinchak R. 2000. Ventricular fibrillation in a patient with prominent J (Osborn) waves and ST segment elevation in the inferior electrocardiographic leads: a Brugada syndrome variant? J. Cardiovasc. Electrophysiol. 11, 95-98. ( 10.1111/j.1540-8167.2000.tb00743.x) [DOI] [PubMed] [Google Scholar]
- 23.Rosso R, et al. 2008. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J. Am. Coll. Cardiol. 52, 1231-1238. ( 10.1016/j.jacc.2008.07.010) [DOI] [PubMed] [Google Scholar]
- 24.Haissaguerre M, et al. 2008. Sudden cardiac arrest associated with early repolarization. N. Engl. J. Med. 358, 2016-2023. ( 10.1056/NEJMoa071968) [DOI] [PubMed] [Google Scholar]
- 25.Nam GB, Kim YH, Antzelevitch C. 2008. Augmentation of J waves and electrical storms in patients with early repolarization. N. Engl. J. Med. 358, 2078-2079. ( 10.1056/NEJMc0708182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rattanawong P, et al. 2021. Does the age of sudden cardiac death in family members matter in Brugada syndrome? J. Am. Heart Assoc. 10, e019788. ( 10.1161/JAHA.120.019788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe H, et al. 2012. Clinical characteristics and risk of arrhythmia recurrences in patients with idiopathic ventricular fibrillation associated with early repolarization. Int. J. Cardiol. 159, 238-240. ( 10.1016/j.ijcard.2012.05.091) [DOI] [PubMed] [Google Scholar]
- 28.Haissaguerre M, et al. 2009. Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy. J. Am. Coll. Cardiol. 53, 612-619. ( 10.1016/j.jacc.2008.10.044) [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, et al. 2017. Novel trigenic CACNA1C/DES/MYPN mutations in a family of hypertrophic cardiomyopathy with early repolarization and short QT syndrome. J. Transl. Med. 15, 78. ( 10.1186/s12967-017-1180-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Z, et al. 2004. Role of a KCNH2 polymorphism (R1047 L) in dofetilide-induced torsades de pointes. J. Mol. Cell. Cardiol. 37, 1031-1039. ( 10.1016/j.yjmcc.2004.09.001) [DOI] [PubMed] [Google Scholar]
- 31.Calo L, et al. 2016. A new electrocardiographic marker of sudden death in Brugada syndrome: the S-wave in lead I. J. Am. Coll. Cardiol. 67, 1427-1440. ( 10.1016/j.jacc.2016.01.024) [DOI] [PubMed] [Google Scholar]
- 32.Antzelevitch C. 2006. Brugada syndrome. Pacing Clin. Electrophysiol. 29, 1130-1159. ( 10.1111/j.1540-8159.2006.00507.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belhassen B, Glick A, Viskin S. 2004. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation 110, 1731-1737. ( 10.1161/01.CIR.0000143159.30585.90) [DOI] [PubMed] [Google Scholar]
- 34.Andorin A, et al. 2017. The QUIDAM study: hydroquinidine therapy for the management of Brugada syndrome patients at high arrhythmic risk. Heart Rhythm. 14, 1147-1154. ( 10.1016/j.hrthm.2017.04.019) [DOI] [PubMed] [Google Scholar]
- 35.Viskin S, Wilde AA, Krahn AD, Zipes DP. 2013. Inaccessibility to quinidine therapy is about to get worse. J. Am. Coll. Cardiol. 62, 355. ( 10.1016/j.jacc.2013.04.009) [DOI] [PubMed] [Google Scholar]
- 36.Watanabe A, et al. 2006. Low-dose isoproterenol for repetitive ventricular arrhythmia in patients with Brugada syndrome. Eur. Heart J. 27, 1579-1583. ( 10.1093/eurheartj/ehl060) [DOI] [PubMed] [Google Scholar]
- 37.Shinohara T, et al. 2014. Combination therapy of cilostazol and bepridil suppresses recurrent ventricular fibrillation related to J-wave syndromes. Heart Rhythm. 11, 1441-1445. ( 10.1016/j.hrthm.2014.05.001) [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa K, et al. 2014. Long-term pharmacological therapy of Brugada syndrome: is J-wave attenuation a marker of drug efficacy? Intern. Med. 53, 1523-1526. ( 10.2169/internalmedicine.53.1829) [DOI] [PubMed] [Google Scholar]
- 39.Koncz I, et al. 2014. Mechanisms underlying the development of the electrocardiographic and arrhythmic manifestations of early repolarization syndrome. J. Mol. Cell. Cardiol. 68, 20-28. ( 10.1016/j.yjmcc.2013.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye D, et al. 2022. Acacetin, a potent transient outward current blocker, may be a novel therapeutic for KCND3-encoded Kv4.3 gain-of-function-associated. Circ. Genom. Precis. Med. 15, e003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurnett CA, De Waard M, Campbell KP. 1996. Dual function of the voltage-dependent Ca2+ channel alpha 2 delta subunit in current stimulation and subunit interaction. Neuron 16, 431-440. ( 10.1016/S0896-6273(00)80061-6) [DOI] [PubMed] [Google Scholar]
- 42.Fukuyama M, et al. 2013. L-type calcium channel mutations in Japanese patients with inherited arrhythmias. Circ. J. 77, 1799-1806. ( 10.1253/circj.CJ-12-1457) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are available from the corresponding author upon reasonable request.