Abstract
Atrial fibrillation (AF) is a very common cardiac arrhythmia with an estimated prevalence of 33.5 million patients globally. It is associated with an increased risk of death, stroke and peripheral embolism. Although genetic studies have identified a growing number of genes associated with AF, the definitive impact of these genetic findings is yet to be established. Several mechanisms, including electrical, structural and neural remodelling of atrial tissue, have been proposed to contribute to the development of AF. Despite over a century of exploration, the molecular and cellular mechanisms underlying AF have not been fully established. Current antiarrhythmic drugs are associated with a significant rate of adverse events and management of AF using ablation is not optimal, especially in cases of persistent AF. This review discusses recent advances in our understanding and management of AF, including new concepts of epidemiology, genetics and pathophysiological mechanisms. We review the current status of antiarrhythmic drug therapy for AF, new potential agents, as well as mechanism-based AF ablation.
This article is part of the theme issue ‘The heartbeat: its molecular basis and physiological mechanisms’.
Keywords: atrial fibrillation, genetics, pharmacology, atrial myopathy, electrophysiology, thrombosis
1. Introduction
Atrial fibrillation (AF) is one of the most common cardiac arrhythmias worldwide. Its prevalence is estimated at 0.4% in the general population (33.5 million people), increasing to approximately 6% in older individuals over 65 years as age is an independent risk factor [1,2]. The number of patients afflicted with AF is projected to increase to 50 million worldwide by 2030 [1,3,4]. The prevalence of AF varies among geographical regions, socioeconomic status, ethnicity, age and sex. It is the number one cause of hospitalization for arrhythmias and is also associated with an unfavourable prognosis [5–7]. AF is characterized by uncoordinated electrical activity in the atria without discrete P waves and an irregular ventricular response on the surface electrocardiogram. This causes the heart beat to become fast and irregular and increases the risk of stroke and sudden death [8].
The pathophysiology of AF is complex, involving dynamic interactions among several factors, including substrates, triggers, modulators and perpetuators. The therapeutic approaches/strategies are informed by the disease progression from initiation of the abnormal electrical rhythm to its maintenance. Owing to the potential side effects of antiarrhythmic drugs and recurrence following ablation of persistent AF, the current therapies for AF are suboptimal. This article is focused on recent advances in the epidemiology, genetics and pathophysiological mechanisms of AF. We discuss the status of antiarrhythmic drug therapy for AF today, review molecular mechanisms and the possible clinical use of some new agents, as well as mechanism-based ablation of AF.
2. Definitions of atrial fibrillation
According to the recent expert consensus documents, paroxysmal AF is defined as recurrent AF episodes that terminate spontaneously or with intervention within 7 days of onset; persistent AF is defined as continuous AF that is sustained beyond 7 days; long-standing persistent AF is defined as continuous AF of greater than 12-month duration. The first diagnosed AF refers to AF that has not been diagnosed before, irrespective of the duration of the arrhythmia or the presence and severity of AF-related symptoms [9–12]. From a clinical point of view, the latter is important as more than 50% of patients with a first diagnosed AF episode will not experience recurrences over long-time follow up in the absence of antiarrhythmic drugs, cardiac structural abnormalities and significant comorbidities [13]. The term permanent AF is defined as AF in which the presence of the AF is accepted by the patient and physician, and no further attempts will be made either to restore or maintain sinus rhythm [10].
Atrial myopathy is characterized by atrial fibrotic, electrical and autonomic remodelling, facilitates the development of both AF and stroke through the mechanism of inflammation and oxidative stress, which in turn leads to a worsening myopathy. Emerging evidence suggests that targeting this myopathy might shed light on the best therapy to decrease the occurrence and burden of AF [14–17]. Various animal models of atrial myopathy, such as a pacing-induced heart failure (HF) canine model with massive atrial fibrosis, a transforming growth factor (TGF)-β1 overexpressed mouse model, a rat model of obesity and obstructive apnea, proliferator-activated receptor-γ coactivator-1β deficient aged mouse model, and a crossbred mouse model expressing NaV1.5-F1759A and mitochondrial catalase, have helped decipher the complex interplay between atrial myopathy and AF [18–23]. Also, human clinical data show that individuals with markers of atrial myopathy have an elevated risk of developing both AF and stroke [24].
3. Genetics of atrial fibrillation
(a) . Familial atrial fibrillation and linkage analysis
Approximately 15% of patients with AF are familial. The incidence of AF increases together with the numbers of affected individuals with early onset AF in the family [7]. A family history of AF is associated with a 40% increased risk for new-onset AF, suggesting a genetic predisposition [25,26]. AF is commonly associated with other inherited syndromes including dilated or hypertrophic cardiomyopathies, long-QT syndromes, short QT syndromes, Brugada syndrome, familial amyloidosis, congenital cardiac abnormalities and preexcitation syndromes [27–35]. Genetic variants contributing to ion channel function, cell–cell coupling, fibrosis, nuclear structure and transcript factor, have been linked to the development and maintenance of AF over the last decade. Chen and colleagues discovered the first genetic variant, KCNQ1-S140G, linked to familial AF in the Chinese population. This variant generates a gain-of-function in the slowly activating delayed rectifier potassium current (IKs) leading to abbreviation of the effective refractory period (ERP), thus increasing susceptibility for development of AF [36]. NPPA encodes the atrial natriuretic peptide, which is highly expressed in heart. A frameshift variant in this gene, c.456–457delAA, has been identified in a distinct AF family, which leads to abbreviation of ERP in an isolated heart model facilitating the occurrence of AF [37]. Somatic variants in the GJA5 gene, encoding the connexin40 protein, have been associated with AF as well [38]. Causative variants in genes including KCNQ1, SCN5A, KCNH2, GJA5, GJA1, NPPA, TTN, TBX5, KCN5A, LMNA and MYL4, have been implicated in AF (table 1). Whereas, the causative association of AF with variants in other genes remains to be proved [2]. For example, research has recently illustrated that a variant in the RYR2 gene (RYR2-P238S) is involved in the pathogenesis of AF through interrupting the Ca2+ homeostasis [39–42].
Table 1.
Causative genes involved in atrial fibrillation. (AF, atrial fibrillation; BrS, Brugada syndrome; DCM, dilated cardiomyopathy; ERS, early repolarization syndrome; HCM, hypertrophic cardiomyopathy; JLNS, Jervell and Lange–Nielsen syndrome; LGMD, limb girdle muscular dystrophy; LQT, long-QT syndrome type; MEPPC, multifocal ectopic Purkinje-related premature contraction; SND, sinus node dysfunction; SQT, short QT syndrome type; TMD, tibial muscular dystrophy; VF, ventricular fibrillation.)
| gene | protein | other phenotypes | effect on function |
|---|---|---|---|
| SCN5A | Na+ channel α subunit 5 (Nav1.5) | heart block, BrS1, VF, SND, LQT3, DCM, ERS, MEPPC | loss-of-function, gain-of-function |
| KCNQ1 | K+ voltage-gated channel (subfamily Q1, Kv7.1) | SQT2, LQT1, JLNS | gain-of-function |
| KCNH2 | K+ voltage-gated channel (subfamily H2, Kv11.1) | SQT1, LQT2 | gain-of-function |
| KCNA5 | K+ voltage-gated channel (subfamily A5) (Kv1.5) | none | loss-of-function |
| GJA5 | connexin 40 | atrial standstill, digenic (GJA5/SCN5A) | ↓ electrical cell-to-cell coupling |
| GJC1 | connexin 45 | none | ↓ electrical cell-to-cell coupling |
| TTN | titin | DCM, HCM, LGMD, TMD, Salih myopathy | disruption of sarcomeres, fibrosis |
| MYL4 | atrial myosin alkali light chain | conduction disease | disruption of sarcomeric structure, atrial enlargement and electrical abnormalities |
| LMNA | lamin A/C | DCM1A, Emery-Dreifuss muscular dystrophy 2/3, Charcot-Marie-Tooth disease, type 2B1, familial lipodystrophy type 2, Hutchinson–Gilford progeria, congenital muscular dystrophy, Malouf syndrome |
↓INa, impaired interaction between lamin A/C and NUP155 |
| NPPA | atrial natriuretic peptide | atrial standstill | gain-of-function, loss of interaction with the atrial natriuretic peptide receptor |
| TBX5 | T-box transcription factor 5 | Holt–Oram syndrome | loss-of-function, reduction in the transcriptional activity of TBX5 |
(b) . Genome-wide association studies
Genome-wide association studies (GWAS) involve testing genetic variants across the genomes of large individuals with or without related diseases to identify genotype–phenotype associations. Although linkage analysis and genotyping are able to identify causative rare variants associated with AF, the use of GWAS has enabled identification and analysis of hundreds of thousands of genetic variants or single nucleotide polymorphisms (SNPs). A recent GWAS study demonstrates that the risk for AF conferred by genomic variation is substantial and that established AF loci only explain a moderate proportion of disease risk, suggesting that further genetic discovery with an emphasis on common variation is warranted [43]. Since the first GWAS for AF performed in 2007, GWAS have identified over 260 SNPs in 166 loci associated with pathogenesis of AF, including 4q25 near the PITX2, 16q22 close to the ZFHX3,1q21 close to the KCNN3, 12q24 near the TBX5, 6q22 near the GJA1, and 15q24 close to HCN4 [1]. Variants identified by GWAS tend to locate in non-coding regions, which are presumed to alter the activity of transcriptional regulatory elements, affecting the expression of a nearby gene. PITX2 belongs to the homeobox transcription factor family PITX, which is crucial for left-right asymmetry and pulmonary vein (PV) development. PITX2c is the major cardiac isoform [44]. Atrial-specific PITX2-deficient mice show a gain-of-function of IK1 favouring abbreviation of ERP and the development of re-entrant activity [45]. In a PITX2c-deficient zebrafish model, changes in sarcomere length, increased amount of fibrosis and increased expression of HCN4 have been linked to AF [46]. TBX5 belongs to the T-box family of transcription factors. In a murine model, conditional deletion of TBX5 in the heart leads to spontaneous AF [47]. This research also demonstrates that TBX5 is a direct activator of PITX2c, with TBX5 and PITX2c antagonistically regulating the expression of ion channel genes, such as SCN5A, GJA1, RYR2 and ATP2A2 [48]. TBX5 deficient mice show a predisposition to development of AF. Interestingly, the phenotype is rescued by PITX2c [48]. Furthermore, the cooperative interactions between the transcription factors TBX5, GATA4 and NKX2-5 is key for proper binding and cooperative regulation of the down-stream target genes and of cardiac development linking to AF [49].
(c) . Polygenic risk score
Many common variants and SNPs have been identified in large populations using GWAS, together with rare causative variants in family, pointing to AF as a complex trait. Using genetic data to identify high-risk individuals so as to provide preventative treatment or lifestyle modification is a strategy that is being developed. Khera and colleagues develop an AF polygenic risk score (PRS) containing more than 6.6 million variants. The top 6.1% individuals with a high PRS have a more than threefold increased risk for AF [50]. Weng and colleagues combine PRS with a clinical risk factor burden [51]. Individuals in the highest tertile of polygenic risk have a higher lifetime risk for AF (43.6%) compared with the individuals in the lowest tertile (22.3%). When clinical risk increases to the highest tertile, the lifetime risk of AF continues to augment irrespective of the polygenic risk. Numerous studies have recently focused on the PRS predictive value for post-operative AF, recurrence of AF in patients undergoing ablation, recurrence in patients after direct current cardioversion and after and ischaemic stroke [52–57]. Marston and colleagues show that AF PRS is a strong independent predictor of incident AF in patients with cardiovascular conditions and provides strong complementary predictive value when combined with clinical risk factors and amino-terminal pro-B-type natriuretic peptide [58].
4. Pathophysiology of atrial fibrillation
(a) . Electrophysiology
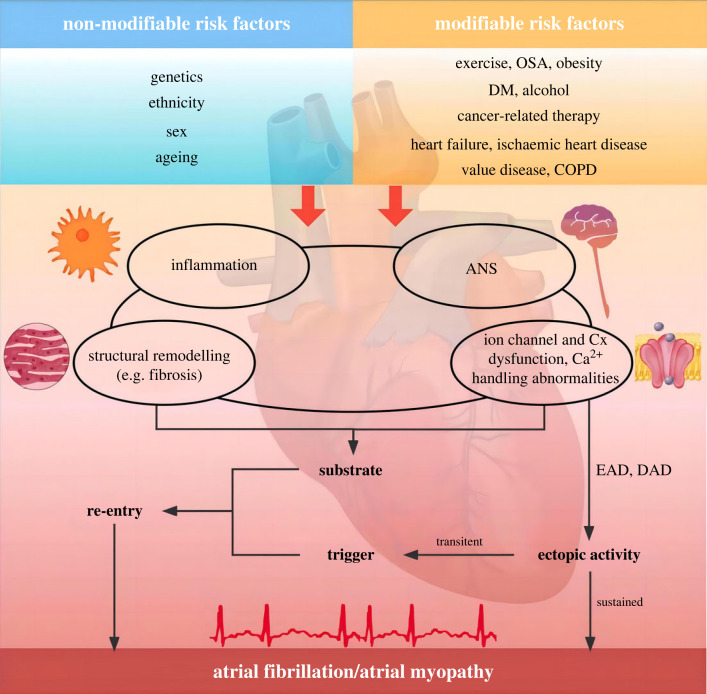
The initiation and maintenance of AF involve both triggers and substrates (figure 1). PV has a smaller IK1, ICa,L and larger IKr and IKs reducing resting potentials and action potential duration (APD). Furthermore, PV possesses a unique anatomical structure consisting of branching fibres with limited lateral coupling and abrupt fibre-orientation transitions, which increases their ability to generate spontaneous activity. These properties facilitate the development of ectopic activity via a re-entrant mechanism, serving as a trigger of AF [59,60]. Delayed afterdepolarizations (DADs) are believed to be responsible for ectopic activity arising from other parts of the atrium. Dysfunction of the ryanodine receptor 2 (RyR2) Ca2+-release channels or elevated SR Ca2+ load leads to increased diastolic Ca2+ concentration, predisposing to the development of DADs [5]. Even sub-threshold DADs can be proarrhythmic by promoting dispersion of excitability with conduction block owing to heterogeneous voltage-dependent Na+-channel inactivation [61]. An alternative mechanism generating ectopic activity is early afterdepolarization (EAD). EAD-induced triggered activity is commonly associated with prolongation of the atrial APD [62]. Mice with long-QT syndrome 3 show excessive atrial APD-prolongation and EADs [63]. Similarly, acute Calcium-calmodulin dependent protein kinase II (CaMKII) activation initiates AF through EAD-mediated triggered activity in old rats, which can be suppressed by inhibition of INa,late [64].
Figure 1.
Summary of mechanisms underlying the initiation and maintenance of atrial fibrillation. Atrial fibrillation can be maintained by either re-entry or sustained ectopic activity. Trigger (commonly from an ectopic beat) combined with vulnerable substrate facilitate the development of re-entry. Inflammation, ANS dysfunction, structural remodelling, ion channel, connexin dysfunction and Ca2+ handling abnormalities underline the generation and progression of vulnerable substrate. Risk factors (modifiable and non-modifiable) are the basal component closely associated with atrial fibrillation. OSA, obstructive sleep apnea; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; ANS, autonomic nerve system; Cx, connexin; EAD, early afterdepolarization; DAD, delayed afterdepolarization (Online version in colour.).
The maintenance of AF generally requires a vulnerable substrate which may be a result of both age/disease-related remodelling as well as AF-related electrical and structural remodelling [5]. AF leads to downregulation of Ca2+ current via reduced expression of α-subunit of the Ca2+ channel which underlies the abbreviation of ERP [65]. Additional changes in ion channels that contribute to abbreviation of ERP include increased expression of IKs, IKACh and IK1. All of these changes in ion channels abbreviate ERP, stabilize the re-entry circuits and increase AF vulnerability and sustainability [66]. Meanwhile, several research articles suggest that the expression of the small-conductance Ca2+-activated K+ channel (SK) subunit and SK open probability are augmented in AF, promoting repolarization shortening [5,67]. Moreover, the decreased expression of connexins and relocalization of connexins to lateral margins, promote conduction abnormalities facilitating development of re-entry [68]. Structural remodelling includes progressive atrial dilatation at the macro level, as well as abnormalities of the atrial cardiomyocyte, fibrotic changes, and alterations in the interstitial matrix at the cellular level [69,70]. Atrial fibrosis plays a pivotal role in the maintenance of AF and the transition from paroxysmal to persistent AF. A wide range of molecular mechanism are involved in the pathogenesis of AF via atrial fibrosis [71]. The detailed mechanisms are elaborated below. There are four hypotheses for AF maintenance: multiple wavelet theory, focal source(s), rotor theory, and the double layer hypothesis [72–74]. Although these hypotheses can explain some phenomena, the mechanisms underlying the development of AF are diverse and still not fully understood.
(b) . Inflammation
Accumulating evidence indicates that inflammation is implicated in various AF-related pathological processes including structural and electrophysiological remoulding [75,76]. Recent investigations have shown that the tumour necrosis factor (TNF)-α, macrophage migration inhibitory factor, interleukin (IL)-1β and IL-6 facilitate the progression of AF [77–79]. TNF-α administration to PV cardiomyocytes produces a variety of ionic changes including a smaller ICa,L, larger transient outward K+ current (Ito), elevated systolic Ca2+ transients (larger diastolic intracellular calcium), enhanced inward sodium-calcium exchange, and decreased sarcoplasmic reticulum (SR) ATPase expression, facilitating the development of DADs-induced triggered activity in PV [80]. In addition, TNF-α has the ability to alter the expression and distribution of connexin43 and connexin40 [81]. Moreover, TNF-α is also involved in the pathogenesis of atrial fibrosis via activation of the TGF-β signalling pathway and increased secretion of matrix metalloproteinases [81]. Clinical research suggests that macrophages are increased in AF patients. Moreover, pro-inflammatory macrophages (M1) participate the generation of AF in mouse and canine models through secreting IL-1β [82]. Our research further demonstrate that Ang-II-treated human cardiac myocyte-derived exosomal plasmacytoma variant translocation 1 facilitates extracellular-matrix remodelling of atrial fibroblasts by inducing macrophage to M1 polarization via upregulation of the expression of IL-16 [83]. In turn, activated macrophages enhance atrial fibroblast proliferation and differentiation. JUN N-terminal kinases (JNKs) orchestrate cellular stress responses and regulate cell differentiation, survival and migration, involved in the development of AF in humans and animal models, especially JNK2 [84–86]. JNK2 increases diastolic Ca2+ leak from the SR through stimulating activity of sarco/endoplasmic reticulum ATPase type 2 and activating CaMKII [87,88]. Moreover, JNK2 is implicated in both impaired cell-to-cell communication and slowed atrial conduction velocity via reduced expression of connexin43 [88]. Activation of NACHT, LRR and PYD domains-containing protein-3 (NLRP3) releases IL-1β and IL-18 through active caspase-1, mediating inflammatory response. NLRP3-inflammasome enhances aberrant RyR2-mediated SR Ca2+ release during diastole owing to increased RyR2 protein expression or CaMKII-dependent hyperphosphorylation of RyR2. Yao et al. show that activation of NLRP3 signalling enhances the protein expression of RyR2, leading to increased RyR2-mediated arrhythmic SR Ca2+ release events, which ultimately cause ectopic firing via the generation of DADs [89]. Furthermore, NLRP3-inflammasome augments IKur and IKACh current and increases atrial hypertrophy and fibrosis, participating in the electrical and structural remodelling of atrium [1,90].
(c) . Fibrosis
Atrial fibrosis is a common feature in AF patients, yet how and if atrial fibrosis is involved in AF generation is poorly understood [91]. Ramos et al. reported recently that the amount of atrial fibrosis is not different in patients with various stages of AF and controls [92]. It is atrial structural remodelling rather than atrial fibrosis that can be causal for AF [92]. Interestingly, mild and moderate atrial fibrosis has been reported to be associated with an increase of AF burden and advanced atrial fibrosis (encountered in patients with severe HF) is associated with a reduction of AF burden [93,94]. Advanced atrial fibrosis is accompanied by a rate-dependent depression of atrial excitability, reducing the occurrence of rapid activation (i.e. AF). Mild and moderate rather than severe atrial fibrosis may be causative for AF. In addition to structural remodelling in AF itself, atrial fibrosis also results from a broad range of factors, including mechanical stretch, inflammation, neurohumoral activation, oxidative stress, ageing and so on. Fibroblasts play a pivotal role in the pathogenesis of fibrosis. Ca2+ entry is important for fibroblast responsiveness and fibrosis generation. Owing to lacking of voltage-gated Ca2+ channels, transient receptor potential (TRP) channels are mediators of Ca2+ signalling in cardiac fibroblasts [95]. TRP channels, both TRPC3 in canine, goat and human models and TRPM7 in human AF, are significantly upregulated in AF-fibroblasts and enhance fibroblast Ca2+ entry [96–98]. Moreover, IK1 in fibroblast is upregulated, which hyperpolarizes the resting potential, increases the driving force for Ca2+ entry, and promotes fibroblast proliferation [99]. Pro-fibrotic cell membrane receptors including connective tissue growth factor, angiotensin-II (particularly type 1 receptors), platelet derived growth factor and TGF-β through down-stream signalling, such as mitogen-activated protein kinases (MAPK), Janus kinase (JAK) and NLRP3, modify the messenger RNA-transcription of proteins like procollagen, fibronectin, matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases that govern extracellular-matrix remodelling and lead to fibrosis [100]. Reactive oxygen species coupled to type-1 Ang-II receptors and TGF-β via down-stream systems like MAPK and NLRP3-inflammasome formation and pro-fibrotic inflammatory signalling play a prominent role in AF [101–103]. M1 macrophages participate in the pathogenesis of AF through a pro-inflammatory effect, whereas non-classical M2 macrophages promote AF via pro-fibrotic effect [104]. In the animal models of acute ischaemic injury, hypertension and chronic HF, M2 macrophages facilitate the development of fibrosis [105–107]. Accordingly, compared to non-AF individuals, patients with AF show significantly higher pro-fibrotic M2 macrophage level in atrial tissue [108]. The increased extracellular-matrix disrupts myocardial bundle continuity and disorganizes gap-junction formation. These effects lead to slowed conduction velocity and increased heterogeneous conduction [109], which promotes re-entrant substrates for the maintenance and perpetuation of AF [109,110]. Also, cardiomyocyte–fibroblast electrical interactions have particular significance for the electrical remodelling in AF. Fibroblasts are essentially non-excitable cells. However, they have a typical resting potential of about −30 mV and couple with cardiomyocytes and transfer current between cardiomyocytes through connexins. Therefore, cardiomyocyte–fibroblast coupling has been suggested to slow conduction, abbreviate APD, alter myocyte excitability by depolarizing cardiomyocyte resting membrane potential (RMP) and induce spontaneous phase 4 depolarization [95,99,111,112].
(d) . Neural mechanisms
The heart is richly innervated by the autonomic nervous system (ANS), including extrinsic and intrinsic nervous systems, which plays a significant role in the occurrence and maintenance of AF [113,114]. Activation of sympathetic nerves enhances automaticity via reducing IK1 and increasing If current. Meanwhile, diastolic RyR2 Ca2+ leak and increased RyR2 open probability, owing to protein kinase A (PKA)/CaMKII phosphorylation, induced by β-adrenergic enhancement result in DADs. Furthermore, β-adrenergic activation can augment ICa,L through PKA/CaMKII phosphorylation, increasing EAD likelihood, particularly when IKs is dysfunctional, such as long-QT syndrome type 1 [115,116]. Additionally, β-adrenergic activation increases Ikur, IKs and IKACh, and facilitates ERP shortening, while, ɑ-adrenoceptor stimulation displays the opposite effect through inhibiting Ito [66,117]. Proximal renal sympathetic activation induces shortening of ERP, significantly increasing ERP dispersion and AF inducibility in dogs [118]. Renal sympathetic denervation in canines could suppress AF and reduce the increasing trend of TNF-α and IL-6 induced by rapid atrial pacing [119]. Acute middle cerebral artery occlusion in canines leads to an increase in left stellate ganglion activity, atrial β1-AR expression, atrial macrophage infiltration and AF vulnerability, while ablation of the left stellate ganglion reverses these changes [120]. Although the definitive mechanism is not very clear, it is possible that the effect is related to sympathetic nerve-regulated immune remodelling [114,121].
In general, vagal nerve activation can trigger the release of the neurotransmitter ACh, leading to the repression of TNF-α, IL-1β and IL-6 in macrophages by α7nAChR and then producing a cascade effect to reduce systemic inflammation via the cholinergic anti-inflammatory pathway. This pathway has been identified through myriad research on AF. Previous research of canines with rapid atrial pacing uncovers low-level vagus nerve stimulation significantly suppresses atrial electrical remodelling and AF inducibility, accompanied by low levels of TNF-α and IL-6 in the left atria [122–124]. Spinal cord stimulation facilitates the effect of vagus nerve stimulation and reduces the induction of AF via increasing nerve growth factor and repressing SK2 [125]. We further demonstrate that median nerve stimulation can heighten cardiac vagal tone and atrial ACh levels, and reverse the enhanced inflammation and AF inducibility by short-term rapid atrial pacing [126]. It cannot be ignored that vagal stimulation strongly abbreviates atrial refractoriness by augmenting IKACh [117]. Moreover, vagal stimulation increases the AF inducibility and duration in a dog model via abbreviating atrial refractoriness and increasing spatial dispersion of APD restitution [127].
Extensive studies involving both AF patients and animal models indicate that onset of AF is associated with combined sympatho-vagal activation rather than alterations in vagal or sympathetic drive alone [128,129]. Cholinergic stimulation is commonly the main factor for AF initiation in animal models, but adrenergic tone regulates the initiation and maintenance of cholinergically mediated AF [117]. Atrial sympathetic hyperinnervation and extracardiac nerve remolding have been reported in experimental animals and humans [130–133]. Sympathetic hyperinnervation may lead to spatial heterogeneity through heterogeneous β-adrenoceptor activation, increasing the risk of focal triggers and creating gradients of repolarization and increasing the susceptibility to re-entry [66]. This suggests a bidirectional relationship between the ANS and AF.
5. Special conditions and atrial fibrillation
(a) . Sleep apnea
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of partial or complete upper airway obstruction and results in hypoxia, hypercapnia, oxidative stress, inflammation, intrathoracic pressure changes and hyperactivity of the ANS [134]. Patients with OSA are two to four times more likely to develop AF. Acute apneic episodes result in sympatho-vagal co-activation, shortening atrial ERP and promoting the initiation of AF [135]. Repetitive OSA may lead to atrial structural and electrical remodelling characterized by atrial dilation, fibrosis, connexin remodelling, and dysfunction of NaV1.5, all of which may predispose to AF [136,137].
(b) . Obesity
Obesity has been increasing globally over the past 40 years and is an independent risk factor for AF as every 1 kg m−2 increment in body mass index is associated with a 4% increase in the incidence of new-onset AF [136,138]. Left atrial enlargement and diastolic dysfunction are known consequences of obesity. Furthermore, recent studies have confirmed the correlation between increased epicardial adipose tissue and onset, severity and recurrence of AF [139,140]. Epicardial adipose tissue promotes atrial fibrosis and its associated electrical substrate through the effect of adipokines, inflammatory cytokines, reactive oxygen species and ANS dysfunction [135]. In addition, hypertension and sleep apnea which are considered to be associated with obesity, are deemed indirect mechanisms for obesity-induced AF. Weight loss through lifestyle changes or bariatric surgery could reverse atrial remodelling and result in a significant reduction of AF burden [141]. Weight fluctuations may offset this benefit by reducing energy expenditure and increasing appetite, adipocytokines and metabolic dysfunction [142].
(c) . Ageing
Age is the most important risk factor for the development of AF. Older age is associated with increased AF burden, with a sharp incline after the age of 65 years. Ageing-related atrial remodelling shares common mechanisms with AF, including atrial fibrosis, inflammation, oxidative stress, gap-junction remodelling and changes in Ca2+ homeostasis [136]. Increased fibrosis can disrupt the coupling between individual myocytes and result in conduction abnormalities, which can lead to re-entry. Age-related calcium handling abnormalities are characterized by increasing spontaneous diastolic Ca2+ leak, which promotes afterdepolarizations and spontaneous atrial ectopic activity [143]. In addition, ageing carries other AF risk factors including HF, hypertension, and obesity. These indirect ageing effects may increase AF occurrence through atrial remodelling. Furthermore, age-dependent changes in systemic regulators may contribute to AF, such as the imbalance between sympathetic and parasympathetic, reduction in testosterone levels and elevation of blood inflammatory markers [110,144].
(d) . Exercise and diabetes mellitus
Moderate physical exercise is beneficial for cardiovascular health and to decrease AF risk. However, long-term vigorous exercise increases the risk of AF [145]. Potential underlying mechanisms may include atrial dilation, fibrosis, hypertrophy, excessive ANS stimulation, and chronic inflammation, occurring as a response to increased cardiac output and atrial wall stretch during exercise [136]. Lately, Aschar-Sobbi et al. and colleagues illustrated that intense exercise induces TNF-α dependent activation of the signalling pathway, prompts atrial structural remodelling and AF vulnerability [146].
Diabetes mellitus (DM) is associated with about a 40% increase in risk for AF. This association has not yet been comprehensively elucidated. There seems to be a multifactorial, complex relationship comprising mechanisms such as oxidative stress, inflammation, insulin resistance, endothelium dysfunction, atrial fibrosis and connexin remodelling, which leads to mechanical and electrical left atrial remodelling [143]. Recent researche demonstrate that glycemic fluctuations, rather than hyperglycaemia alone, contribute to the development of AF in diabetes [147]. Glucose fluctuations are associated with increased atrial fibrosis, oxidative stress and susceptibility to AF in a rat model of DM. Therefore, management of diabetes should be focused on not only reducing blood glucose levels, but also preventing glycaemic fluctuations [148].
(e) . Alcohol
Alcohol consumption is prevalent worldwide and excessive binge drinking has been closely associated with the development of cardiac arrhythmia, often known as ‘holiday heart syndrome'. AF is the most frequently diagnosed arrhythmia in patients with ‘holiday heart syndrome' [88,136]. In a recent clinical trial on secondary prevention of AF, alcohol abstinence reduces AF recurrence [149]. The mechanisms by which alcohol excess increases the risk of AF are multifactorial [150]. First, alcohol could directly affect atrial electrophysiological properties. Both binge and frequent drinking decrease atrial conduction velocity and atrial ERP. Second, alcohol could cause the hyperactivation of the ANS and exaggerate cardiac arrhythmia. Furthermore, chronic alcohol consumption is associated with larger left atrial size and impairments in atrial mechanical and reservoir function. Additionally, alcohol is also associated with inflammation, hypertension, sleep apnea and left ventricular dysfunction, which all increase the risk of AF.
(f) . Cancer and related therapy
Cancer increases the risk of AF. The incidence of AF in cancer patients is up to 20%, peaking in the first three months after diagnosis [136]. In a Danish nationwide study, an increase in AF occurrence in cancer patients is observed for all major types of cancer, with lung cancer patients having the highest AF incidence [151]. The potential mechanisms underlying the association between cancer and increased AF risk remain unclear [152]. Shared risk factors (e.g. advanced age, obesity, diabetes and inflammation) may explain this association. In addition, cancer treatments, including surgery, radiation, chemotherapy, targeted therapies and high-dose corticosteroids, increase the risk of AF [153]. The most notable chemotherapeutics associated with AF are alkylating agents. For example, the incidence of AF with cisplatin is 15–32% [154]. Recent research reports that AF incidence rates may reach 38% in patients treated with ibrutinib [155]. Proposed mechanisms for treatment-associated AF include the exacerbated inflammatory response, atrial fibrosis, oxidative stress, neurohormonal dysregulation and calcium homeostasis disorder.
6. Drug and ablation development of atrial fibrillation
(a) . Status of antiarrhythmic drugs
The key mechanisms for initiation and maintenance of AF are combined with triggers and substrates. Therefore, removal of ectopic triggers, prevention of the formation and progression of vulnerable substrates and disruption of re-entry are the key strategies for terminating AF [156]. RyR2 channels play a major role in the Ca2+-handling abnormalities which underlie DADs. Targeting abnormal RyR2 channels either by using direct RyR2-channel blockers or RyR2 function modulators would be an alternative. Because of the RyR2-Ca2+ channel blocking effect, flecainide, a classic Ic Na+ channel blocker, can also been viewed as a IVb antiarrhythmic drug according to the new classification criteria of antiarrhythmic drugs [157]. According to the leading circle concept, re-entry circuits can be destabilized when conduction velocity is slowing and/or refractoriness is prolonged, so that the re-entrant wavefront will reach tissue that is still in the refractory state [158]. K+ blockers and multichannel blockers destabilize re-entry through prolonging ERP, while, Na+ channel blockers reduce excitability and delay conduction leading to collapse of the re-entry wavelet [159]. According to updated classification of current antiarrhythmic drugs, molecular mechanisms influencing longer-term changes upstream of the electrophysiological processes and tissue structure remodelling also constitute novel potential therapeutic antiarrhythmic targets. As for AF, inflammatory signalling (JNK2, NLRP3), pro-fibrotic signalling (renin–angiotensin–aldosterone system, TGF-β, JAK-STAT), mitochondrial dysfunction (NAD+/NADH level) and sarcomeric cytoskeleton damage (HDAC) are involved in the structural and electrical remodelling of atrium. Moreover, clinical studies have provided supportive data for the potential value of such ‘upstream therapy' with the renin–angiotensin–aldosterone system targetted, for primary prevention of AF [160,161].
Antiarrhythmic drugs are widely used for cardioversion and prevention of AF. The current clinically approved anti-AF agents are: (i) the rapidly activating delayed rectifier potassium current (IKr) inhibitors (dofetilide, ibutilide, etc.); (ii) sodium channel current (INa) blockers (flecainide, propafenone, etc.); and (iii) potent multiple channel blockers (amiodarone, quinidine, etc.). Anti-AF efficacy of these agents is relatively high against paroxysmal AF and relatively low against persistent AF.
A major limitation of anti-AF agents is their safety. The use of INa blockers having slowly unbinding kinetics (flecainide and propafenone) is associated with an increased risk of developing ventricular arrhythmias in patients with significant structural heart diseases. The risk of IKr inhibitors is a potential induction of long-QT-mediated Torsade-de-Pointe arrhythmias. The use of amiodarone is rarely associated with ventricular arrhythmias. However, there is a relatively high risk of extracardiac side effects with long-term application (such as pulmonary toxicity), significantly limiting long-term use of amiodarone. The annual AF recurrence rate is still high, ranging from 40% to 70% under the use of antiarrhythmic drugs, therefore new drugs with improved efficacy, safety and tolerability are urgently needed [162].
(b) . New drug uses and new molecular mechanisms
Numerous novel pharmacological targets and approaches for rhythm control of AF have been suggested over the past 30 years [163]. Most prominent have been atrial-specific or selective inhibition of the IKur, SK, IKACh and INa, intending to cause atrial-specific or selective prolongation of the ERP (table 2). Li et al. report acacetin, a natural flavone, inhibits Ito, IKur and IKACh, without significantly influencing other major cardiac ion channel currents in atria. It prevents AF without prolonging the corrected QT interval in an in vivo canine model [164]. Later on, they further synthesize highly water-soluble acacetin prodrug effectively terminating acute AF in the canine model [165]. Although inhibition of IKur and SK channels is shown to promote AF termination in animal models, these agents were not effective in terminating AF in humans [166]. This failure is predicted on the basis of studies conducted in coronary-perfused canine atria exposed to a low concentration (50 μM) of 4-aminopyridine, which is selective for inhibition of IKur. Inhibition of IKur abbreviates the atrial action potential owing to recruitment of IKr secondary to a positive shift of the action potential plateau. The abbreviation of atrial APD permits induction of AF with premature stimulation [167]. IKACh is constitutively upregulated during AF. Activation of IKACh by vagal stimulation decreases ERP, and contributes to maintenance of AF. Selective block of IKACh seems a potential target for AF treatment. It is important to note that selective IKACh blockers for AF must avoid significant vagolytic adverse effects at therapeutic doses [166]. BMS914392, a potent and selective oral inhibitor of IKACh, fails to maintain sinus rhythm in patients with paroxysmal AF when it is administered at the doses required to avoid neurologic adverse effects [168]. Although new drugs blocking IKACh have not emerged for clinical use, inhibition of IKACh remains a promising therapeutic target for the management of AF. Atrial-selective inhibition of INa is shown by us and others to be valid in terms of anti-AF efficacy and safety in both experimental and clinical studies [169–174]. Atrial-selective prolongation of ERP and anti-AF efficacy of IKur and SK channel blockers have turned out to be largely or exclusively owing to concomitant atrial-selective inhibition of INa [171,175]. Ranolazine effectively terminate AF owing to an atrial-selective sodium channel blocking effect combined with Ikr blocking in various AF models [169,176–178]. De Ferrari et al. show higher doses of ranolazine (500 and 750 mg) are associated with an approximately 35% reduction of recurrence in persistent AF patients. Furthermore, Reiffel et al. demonstrate that ranolazine combined with dronedarone significantly decrease the AF burden in paroxysmal AF patients. Ranolazine displays tolerance and safety well in both trials [166,170,179]. It appears that atrial-selective inhibition of INa is a reasonable and practical approach in the search for novel anti-AF agents so far. WenXin KeLi is a Chinese herb extract capable of atrial-selective inhibition of INa owing to more negative voltage for steady-state inactivation, less negative RMP, and shorter diastolic intervals in atria. Besides, it has more complex effects prompting the management of AF, including inhibition of ICa,L and Ito, regulating the CaMKII signal pathway, suppressing the atrial substrate remodelling and anticholinergic action [173,180]. The Shensong Yangxin (SSYX) capsule is another traditional Chinese herbal medicine potentially a drug for AF. SSYX reduces AF susceptibility and prevents the progression of AF, via extending the atrial ERP and reducing the dispersion of the atrial ERP, partially through regulating the imbalance of autonomic nerve activity, reactive oxygen species production and the cholinergic anti-inflammatory pathway in various animal models including metabolic syndrome-induced AF mouse models, long-term intermittent pacing-induced AF mouse models and post-myocardial infarction HF mouse models [181–183].
Table 2.
Experimental drugs for the management of atrial fibrillation. (AF, atrial fibrillation; SK, small-conductance Ca2+-activated K+ channel; SAC, stretch-activated nonselective cation channel; JNK, JUN N-terminal kinase.)
| agent | mechanism of action | description |
|---|---|---|
| MK-0448 | IKur inhibition | effective in preclinical studies, well tolerated in phase 1 clinical trials |
| XEN-D0101 | IKur inhibition | suppresses AF in preclinical studies, does not prolong QT interval in phase1 clinical trials |
| xention-D0103/S66913 | IKur inhibition | effective in preclinical studies, no effect in phase 2 clinical trials, but safe and well tolerated |
| acacetin | multi K+ current blocker: Ito, IKur and IKACh | effective in preclinical studies |
| AP14145 | SK inhibition | effective in preclinical studies and does not prolong QT interval, no clinical trial |
| AP30663 | SK inhibition | effective in preclinical studies, safe and well tolerated in phase 2 clinical trials |
| gadolinium | SAC blocker | effective in preclinical studies |
| ranolazine | multichannel blocker: INa, IKr | atrial selective INa inhibition, significantly reduces clinical AF burden in combination with dronedarone |
| WenXin KeLi | multichannel blocker: INa, Ito, ICa,L | effective in preclinical and clinical studies |
| BMS914392 | IKACh inhibition | ineffective in phase 2 clinical trials, well tolerated and does not affect QTc |
| budiodarone | multichannel blocker: IKur, INa, IKACh,IKr, IKs | effective in preclinical and phase 2 clinical trials, few adverse events |
| ShenSong YangXin capsule | prolongs the atrial effective refractory period, inhibits atrial fibrosis, decreases intracellular iron overload | effective in preclinical and clinical studies |
| vanoxerine | multichannel blocker: INa, IKr, ICa,L | effective in preclinical and phase 2 clinical trials, QT prolongation but no effect on transmural dispersion of repolarization, increased risk of ventricular proarrhythmia in patients with structural heart disease, needs additional studies |
| moxonidine | centrally acting sympathoinhibitory agent | reduces AF burden in hypertensive patients and postablation AF recurrences in hypertensive patients in phase 2 clinical trials, needs additional studies |
| rotigaptide | connexin modulator, prevents the uncoupling of connexin 43-mediated gap-junction communication and normalizes cell-to-cell communication | improvement of conduction velocity does not correlate with AF vulnerability in preclinical studies |
| SP600125 | JNK inhibitor | under preclinical studies |
| dantrolene | RyR2 stabilizer | effective in preclinical studies, no clinical study |
| JTV-519 | multi-ion channel blocker: INa, ICa, IKACh, IKr, IKs; promotes RyR2-FKBP12.6 (calstabin2) binding | effective in preclinical studies, undergoing phase 2 trials for restoration and maintenance of sinus rhythm, and terminates, no data available |
A combination of atrial-selective inhibition of INa with potassium channel blockers may enhance the safety and efficacy of anti-AF drugs. Among the potential combinations are agents that atrial-selectively inhibit INa and IKr. Such a combination may cause synergistic atrial-predominant prolongation of ERP with little or no risk of ventricular arrhythmias [184]. Atrial selectivity of INa blockers essentially eliminates the risk of conduction-related ventricular arrhythmias, and concomitant inhibition of late INa reduces the risk of long-QT-mediated arrhythmias. A combined inhibition of IK1 and INa may enhance the efficiency of INa blockers to cardiovert AF and prevent its recurrence (particularly persistent AF) [185]. IK1 is augmented in persistent AF, bringing the atrial RMP to more negative voltages, and, therefore, reducing INa blocking efficiency of INa blockers. Restoration of atrial RMP with reduction of IK1 should augment the inhibition of the sodium channel by INa blockers, which, in turn, is expected to increase anti-AF efficiency of the blockers [185]. Owing to the well-known antiarrhythmic efficacy of amiodarone, multichannel blockade drugs are being developed, which are expected to have strong antiarrhythmic effect and broad cardiac safety and less extracardiac adverse effects. Budiodarone, an analogue of amiodarone, undergoes rapid metabolism by plasma and tissue esterases [186]. In preliminary randomized trials in patients with paroxysmal AF, budiodarone significantly reduces AF burden after 12 weeks of treatment compared with placebo [187]. In addition, vanoxerine developed as a drug for Parkinson's disease is shown to potently inhibit IKr, INa and ICa,L [188]. In a multicentre, phase IIb trial, involving 104 patients with recent-onset AF, vanoxerine is statistically more effective in cardioverting AF compared with placebo, with better safety [189]. Inhibition of the RyR2 and CaMKII are among the preclinical experimental pharmacological anti-AF approaches [163,190]. Flecainide reduces the open probability of RyR2 in vitro via an open-state blocking mechanism and eliminates the formation of Ca2+-dependent DADs. Hence, it prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans caused by RYR2 mutation [191,192]. Salvage et al. illustrate that flecainide rescues the atrial arrhythmogenic phenotype by increasing atrial wavelength and INa in a homozygous RYR2-P2328S mouse model [191,193,194]. Dantrolene, a clinical drug to treat malignant hyperthermia, has shown to stabilize cardiac RyR2 channels by improving the interaction between the channel N-terminal and central domains. Research has shown that dantrolene could suppress the susceptibility of AF in a left atrial myocardial infarction sheep model, a CaMKIIδC overexpressing mouse model and an electrically maintained AF dog model [156,195]. There are also a number of factors that have been associated with AF generation, including inflammation, oxidative stress and ANS [190]. Their feasibility as targets for pharmacological rhythm control management of AF is under investigation (moxonidine, MCC950, SP600125, nicotinamide riboside, 4-phenylbutyrate, ACY-1215) [84,196,197].
(c) . Mechanism-based atrial fibrillation ablation
Catheter ablation has become an important treatment for rhythm control of AF. Haissaguerre and colleagues demonstrate that local elimination of PV triggers using radiofrequency energy is highly effective in terminating AF [198]. Pulmonary vein isolation (PVI) is currently the cornerstone for ablation of AF [11,199]. While this approach is often successful in cases of paroxysmal AF, it is less so in cases of persistent and long-standing persistent AF, in which recurrence of AF is high [200]. When AF persists, electrophysiological and structural changes predispose patients to development of non-PV triggers, by creating the substrate for sustained AF. Other regions participating in AF maintenance, often need to be ablated as well, including the posterior left atrial wall, the vein of Marshall, coronary sinus and the left atrial appendage [201–203]. Many of these regions are empirically targeted for ablation without underlying mechanistic insights. The lack of target specifity can result in an extensive area of ablation [204]. The widely accepted mechanism underlying AF is that it is maintained by high-frequency drivers establishing a spatial hierarchy gradient of progressively slowing activation rates as the wavefronts propagate centrifugally from the source. Numerous studies have emerged focused on a mechanism-based approach for mapping drivers [205–209]. In a prospective, multicentre, single-arm study, Seitz and colleagues conclude that clusters of electrograms with spatial dispersion at a minimum of three adjacent bipoles is indicative of the presence of drivers. Compared with conventional ablation approach (PVI and stepwise), patients undergoing ablation of spatio-temporal dispersion areas, have a higher arrhythmia-free survival rate as well as shorter radiofrequency times [207]. A meta-analysis shows that AF driver ablation alone or added to PVI has a freedom from AF ratio of 3 : 1, freedom from all arrhythmias ratio of 1 : 8 and predicts a favourable outcome compared with controls [210]. However, the evidence comes mainly from uncontrolled and non-randomized studies, with substantial heterogeneity in reported outcomes. Further research is needed to confirm the results. Looking to the future, new technologies, including three-dimensional mapping, a combination of high-resolution ultrasound and optical mapping, as well as advanced signal processing algorithms, are expected to significantly advance the concept of mechanism-based ablation of AF.
7. Conclusion
AF is the most common cardiac arrhythmia with a complex genetic basis. Both rare variants and common SNPs are with increased predisposition of AF. The mechanisms underlying AF are not fully understood. The initiation and maintenance of AF involve both trigger and substrate, that are accentuated by electrical and structural remodelling of atrium. Inflammation, fibrosis and ANS dysfunction form a complex network participating in the pathogenesis of AF. Environmental factors, including alcohol, ageing, obesity, exercise, DM, OSA and cancer, can significantly increase the susceptibility to development of AF. Current pharmacologic and ablation approaches to therapy of AF are suboptimal, calling for innovative new safe and effective drugs as well as mechanism-based ablation leading to higher success rates and fewer recurrences.
Contributor Information
Dan Hu, Email: hudan0716@hotmail.com.
Hong Jiang, Email: hongj0505@126.com.
Data accessibility
This article has no additional data.
Authors' contributions
D.H.: conceptualization, funding acquisition, project administration, supervision, writing—original draft, writing—review, editing and validation; H.B.-M.: conceptualization, funding acquisition, writing—original draft, writing—review, editing and validation; C.A.: funding acquisition, writing—original draft, writing—review, editing and validation; Z.-H.Z., H.-Y.D., Q.-Y.Z., A.B., and M.M.M.: writing—original draft, writing—review, editing and validation; M.-W.B., Y.-M.D. and C.P.: validation, writing—original draft, writing—review and editing; C.-X.H.: supervision, writing—original draft, writing—review, editing and validation; H.J.: conceptualization, supervision, writing—original draft, writing—review, editing and validation.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported by Grants from the National Natural Science Foundation Project of China (grant nos. 82270332 and 81670304 to D.H.); Fundamental Research Funds for the Central Universities of China (grant no. 2042022kf1217 to D.H.); National Institutes of Health of USA, NIH R56 (grant no. HL47678 to C.A. and H.B.-M.); National Institutes of Health of USA, NIH R01 (grant nos. HL138103 and HL152201 to C.A. and H.B.-M.); W.W. Smith Charitable Trust and the Wistar and Martha Morris Funds (C.A. and H.B.-M.) and Sharpe-Strumia Research Foundation (H.B.-M.).
References
- 1.Brundel B, Ai X, Hills MT, Kuipers MF, Lip GYH, de Groot NMS. 2022. Atrial fibrillation. Nat. Rev. Dis. Primers 8, 21. ( 10.1038/s41572-022-00347-9) [DOI] [PubMed] [Google Scholar]
- 2.Wilde AAM, et al. 2022. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases. Europace 24, 1307-1367. ( 10.1093/europace/euac030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. 2013. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am. J. Cardiol. 112, 1142-1147. ( 10.1016/j.amjcard.2013.05.063) [DOI] [PubMed] [Google Scholar]
- 4.Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. 2014. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 6, 213-220. ( 10.2147/clep.S47385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heijman J, Voigt N, Nattel S, Dobrev D. 2014. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ. Res. 114, 1483-1499. ( 10.1161/CIRCRESAHA.114.302226) [DOI] [PubMed] [Google Scholar]
- 6.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna W, Seward JB, Iwasaka T, Tsang TS. 2006. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community-based study over two decades. Eur. Heart J. 27, 936-941. ( 10.1093/eurheartj/ehi694) [DOI] [PubMed] [Google Scholar]
- 7.Roberts R. 2006. Mechanisms of disease: genetic mechanisms of atrial fibrillation. Nat. Clin. Pract. Cardiovasc. Med. 3, 276-282. ( 10.1038/ncpcardio0509) [DOI] [PubMed] [Google Scholar]
- 8.Zhao QY, et al. 2020. Contemporary characteristics, management, and outcomes of patients hospitalized for atrial fibrillation in China: results from the real-world study of Chinese atrial fibrillation registry. Chin. Med. J. 133, 2883-2884. ( 10.1097/cm9.0000000000001151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirchhof P, et al. 2017. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Revista espanola de cardiologia (English ed.) 70, 50. ( 10.1016/j.rec.2016.11.033) [DOI] [PubMed] [Google Scholar]
- 10.Calkins H, et al. 2017 2017. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J. Arrhythm. 33, 369-409. ( 10.1016/j.joa.2017.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.January CT, et al. 2019. 2019 AHA/ACC/HRS Focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 140, e125-e151. ( 10.1161/cir.0000000000000665) [DOI] [PubMed] [Google Scholar]
- 12.Kumbhani DJ, et al. 2021. 2020 ACC expert consensus decision pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease: a report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 77, 629-658. ( 10.1016/j.jacc.2020.09.011) [DOI] [PubMed] [Google Scholar]
- 13.Pappone C, et al. 2008. Atrial fibrillation progression and management: a 5-year prospective follow-up study. Heart Rhythm 5, 1501-1507. ( 10.1016/j.hrthm.2008.08.011) [DOI] [PubMed] [Google Scholar]
- 14.Shen MJ, Arora R, Jalife J. 2019. Atrial myopathy. JACC Basic Transl. Sci. 4, 640-654. ( 10.1016/j.jacbts.2019.05.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brambatti M, et al. 2014. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 129, 2094-2099. ( 10.1161/circulationaha.113.007825) [DOI] [PubMed] [Google Scholar]
- 16.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, Miller C, Qi D, Ziegler PD. 2009. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ. Arrhythm. Electrophysiol. 2, 474-480. ( 10.1161/circep.109.849638) [DOI] [PubMed] [Google Scholar]
- 17.Martin DT, et al. 2015. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur. Heart J. 36, 1660-1668. ( 10.1093/eurheartj/ehv115) [DOI] [PubMed] [Google Scholar]
- 18.Li D, Fareh S, Leung TK, Nattel S. 1999. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 100, 87-95. ( 10.1161/01.cir.100.1.87) [DOI] [PubMed] [Google Scholar]
- 19.Everett THt, Olgin JE. 2007. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm 4, S24-S27. ( 10.1016/j.hrthm.2006.12.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwasaki YK, Shi Y, Benito B, Gillis MA, Mizuno K, Tardif JC, Nattel S. 2012. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm 9, 1409-1416.e1401. ( 10.1016/j.hrthm.2012.03.024) [DOI] [PubMed] [Google Scholar]
- 21.Valli H, Ahmad S, Fraser JA, Jeevaratnam K, Huang CL. 2017. Pro-arrhythmic atrial phenotypes in incrementally paced murine Pgc1β(-/-) hearts: effects of age. Exp. Physiol. 102, 1619-1634. ( 10.1113/ep086589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valli H, Ahmad S, Chadda KR, Al-Hadithi A, Grace AA, Jeevaratnam K, Huang CL. 2017. Age-dependent atrial arrhythmic phenotype secondary to mitochondrial dysfunction in Pgc-1β deficient murine hearts. Mech. Ageing Dev. 167, 30-45. ( 10.1016/j.mad.2017.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avula UMR, et al. 2021. Attenuating persistent sodium current-induced atrial myopathy and fibrillation by preventing mitochondrial oxidative stress. JCI Insight 6, e147371. ( 10.1172/jci.insight.147371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaihov-Teper O, et al. 2021. Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation 143, 2475-2493. ( 10.1161/circulationaha.120.052009) [DOI] [PubMed] [Google Scholar]
- 25.Tucker NR, Clauss S, Ellinor PT. 2016. Common variation in atrial fibrillation: navigating the path from genetic association to mechanism. Cardiovasc. Res. 109, 493-501. ( 10.1093/cvr/cvv283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubitz SA, et al. 2010. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA 304, 2263-2269. ( 10.1001/jama.2010.1690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sébillon P, et al. 2003. Expanding the phenotype of LMNA mutations in dilated cardiomyopathy and functional consequences of these mutations. J. Med. Genet. 40, 560-567. ( 10.1136/jmg.40.8.560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohler PJ, et al. 2003. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421, 634-639. ( 10.1038/nature01335) [DOI] [PubMed] [Google Scholar]
- 29.Hong K, et al. 2005. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc. Res. 68, 433-440. ( 10.1016/j.cardiores.2005.06.023) [DOI] [PubMed] [Google Scholar]
- 30.Hu D, et al. 2017. The phenotypic spectrum of a mutation hotspot responsible for the short QT syndrome. JACC Clin. Electrophysiol. 3, 727-743. ( 10.1016/j.jacep.2016.11.013) [DOI] [PubMed] [Google Scholar]
- 31.Morita H, et al. 2002. Atrial fibrillation and atrial vulnerability in patients with Brugada syndrome. J. Am. Coll. Cardiol. 40, 1437-1444. ( 10.1016/s0735-1097(02)02167-8) [DOI] [PubMed] [Google Scholar]
- 32.Gillmore JD, Booth DR, Pepys MB, Hawkins PN. 1999. Hereditary cardiac amyloidosis associated with the transthyretin Ile122 mutation in a white man. Heart (British Cardiac Society) 82, e2. ( 10.1136/hrt.82.3.e2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez-Roelens I, De Roy L, Ovaert C, Sluysmans T, Devriendt K, Brunner HG, Vikkula M. 2006. A novel CSX/NKX2-5 mutation causes autosomal-dominant AV block: are atrial fibrillation and syncopes part of the phenotype? Eur. J. Hum. Genet. 14, 1313-1316. ( 10.1038/sj.ejhg.5201702) [DOI] [PubMed] [Google Scholar]
- 34.Gollob MH, Seger JJ, Gollob TN, Tapscott T, Gonzales O, Bachinski L, Roberts R. 2001. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation 104, 3030-3033. ( 10.1161/hc5001.102111) [DOI] [PubMed] [Google Scholar]
- 35.Hu D, et al. 2020. Identification, clinical manifestation and structural mechanisms of mutations in AMPK associated cardiac glycogen storage disease. EBioMedicine 54, 102723. ( 10.1016/j.ebiom.2020.102723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YH, et al. 2003. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science (New York, N.Y.) 299, 251-254. ( 10.1126/science.1077771) [DOI] [PubMed] [Google Scholar]
- 37.Hodgson-Zingman DM, et al. 2008. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl. J. Med. 359, 158-165. ( 10.1056/NEJMoa0706300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gollob MH, et al. 2006. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl. J. Med. 354, 2677-2688. ( 10.1056/NEJMoa052800) [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, et al. 2013. Conduction slowing contributes to spontaneous ventricular arrhythmias in intrinsically active murine RyR2-P2328S hearts. J. Cardiovasc. Electrophysiol. 24, 210-218. ( 10.1111/jce.12015) [DOI] [PubMed] [Google Scholar]
- 40.Salvage SC, Gallant EM, Beard NA, Ahmad S, Valli H, Fraser JA, Huang CL, Dulhunty AF. 2019. Ion channel gating in cardiac ryanodine receptors from the arrhythmic RyR2-P2328S mouse. J. Cell Sci. 132, jcs229039. ( 10.1242/jcs.229039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goddard CA, Ghais NS, Zhang Y, Williams AJ, Colledge WH, Grace AA, Huang CL. 2008. Physiological consequences of the P2328S mutation in the ryanodine receptor (RyR2) gene in genetically modified murine hearts. Acta Physiol. (Oxf.) 194, 123-140. ( 10.1111/j.1748-1716.2008.01865.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King JH, Zhang Y, Lei M, Grace AA, Huang CL, Fraser JA. 2013. Atrial arrhythmia, triggering events and conduction abnormalities in isolated murine RyR2-P2328S hearts. Acta Physiol. (Oxf.) 207, 308-323. ( 10.1111/apha.12006) [DOI] [PubMed] [Google Scholar]
- 43.Weng LC, et al. 2017. Heritability of atrial fibrillation. Circul. Cardiovasc. Genet. 10, e001838. ( 10.1161/CIRCGENETICS.117.001838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirchhof P, et al. 2011. PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circul. Cardiovasc. Genet. 4, 123-133. ( 10.1161/circgenetics.110.958058) [DOI] [PubMed] [Google Scholar]
- 45.Chinchilla A, et al. 2011. PITX2 insufficiency leads to atrial electrical and structural remodeling linked to arrhythmogenesis. Circul. Cardiovasc. Genet. 4, 269-279. ( 10.1161/circgenetics.110.958116) [DOI] [PubMed] [Google Scholar]
- 46.Collins MM, et al. 2019. Early sarcomere and metabolic defects in a zebrafish pitx2c cardiac arrhythmia model. Proc. Natl Acad. Sci. USA 116, 24 115-24 121. ( 10.1073/pnas.1913905116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley G, Syeda F, Kirchhof P, Fabritz L. 2012. An introduction to murine models of atrial fibrillation. Front. Physiol. 3, 296. ( 10.3389/fphys.2012.00296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nadadur RD, et al. 2016. Pitx2 modulates a Tbx5-dependent gene regulatory network to maintain atrial rhythm. Sci. Translat. Med. 8, 354ra115. ( 10.1126/scitranslmed.aaf4891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Ouwerkerk AF, et al. 2020. Epigenetic and transcriptional networks underlying atrial fibrillation. Circ. Res. 127, 34-50. ( 10.1161/CIRCRESAHA.120.316574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khera AV, et al. 2018. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 50, 1219-1224. ( 10.1038/s41588-018-0183-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weng LC, et al. 2018. Genetic predisposition, clinical risk factor burden, and lifetime risk of atrial fibrillation. Circulation 137, 1027-1038. ( 10.1161/CIRCULATIONAHA.117.031431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shoemaker MB, et al. 2020. Genetic susceptibility for atrial fibrillation in patients undergoing atrial fibrillation ablation. Circ. Arrhythm. Electrophysiol. 13, e007676. ( 10.1161/circep.119.007676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vogel S, Rudaka I, Rots D, Isakova J, Kalējs O, Vīksne K, Gailīte L. 2021. A higher polygenic risk score is associated with a higher recurrence rate of atrial fibrillation in direct current cardioversion-treated patients. Medicina (Kaunas, Lithuania) 57, 1263. ( 10.3390/medicina57111263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Sullivan JW, et al. 2021. Combining clinical and polygenic risk improves stroke prediction among individuals with atrial fibrillation. Circul. Genomic Prec. Med. 14, e003168. ( 10.1161/circgen.120.003168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kertai MD, Mosley JD, He J, Ramakrishnan A, Abdelmalak MJ, Hong Y, Shoemaker MB, Roden DM, Bastarache L. 2021. Predictive accuracy of a polygenic risk score for postoperative atrial fibrillation after cardiac surgery. Circul. Genom. Precis. Med. 14, e003269. ( 10.1161/circgen.120.003269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khurshid S, et al. 2021. Predictive accuracy of a clinical and genetic risk model for atrial fibrillation. Circul. Genomic Precision Med. 14, e003355. ( 10.1161/circgen.121.003355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Börschel CS, et al. 2021. Risk prediction of atrial fibrillation in the community combining biomarkers and genetics. Europace 23, 674-681. ( 10.1093/europace/euaa334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marston NA, et al. 2022. A polygenic risk score predicts atrial fibrillation in cardiovascular disease. Eur. Heart J. 44, 221-231. ( 10.1093/eurheartj/ehac460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehrlich JR, Cha TJ, Zhang L, Chartier D, Melnyk P, Hohnloser SH, Nattel S. 2003. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J. Physiol. 551, 801-813. ( 10.1113/jphysiol.2003.046417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hocini M, et al. 2002. Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation 105, 2442-2448. ( 10.1161/01.cir.0000016062.80020.11) [DOI] [PubMed] [Google Scholar]
- 61.Liu MB, de Lange E, Garfinkel A, Weiss JN, Qu Z. 2015. Delayed afterdepolarizations generate both triggers and a vulnerable substrate promoting reentry in cardiac tissue. Heart Rhythm 12, 2115-2124. ( 10.1016/j.hrthm.2015.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fozzard HA. 1992. Afterdepolarizations and triggered activity. Basic Res. Cardiol. 87(Suppl. 2), 105-113. ( 10.1007/978-3-642-72477-0_10) [DOI] [PubMed] [Google Scholar]
- 63.Avula UMR, et al. 2019. Heterogeneity of the action potential duration is required for sustained atrial fibrillation. JCI Insight 5, e128765. ( 10.1172/jci.insight.128765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pezhouman A, Cao H, Fishbein MC, Belardinelli L, Weiss JN, Karagueuzian HS. 2018. Atrial fibrillation initiated by early afterdepolarization-mediated triggered activity during acute oxidative stress: efficacy of late sodium current blockade. J. Heart Health 4, 2379-769x.146. ( 10.16966/2379-769x.146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qi XY, et al. 2008. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ. Res. 103, 845-854. ( 10.1161/circresaha.108.175463) [DOI] [PubMed] [Google Scholar]
- 66.Schotten U, Verheule S, Kirchhof P, Goette A. 2011. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol. Rev. 91, 265-325. ( 10.1152/physrev.00031.2009) [DOI] [PubMed] [Google Scholar]
- 67.Qi XY, et al. 2014. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation 129, 430-440. ( 10.1161/circulationaha.113.003019) [DOI] [PubMed] [Google Scholar]
- 68.Papathanasiou KA, et al. 2021. Molecular insights in atrial fibrillation pathogenesis and therapeutics: a narrative review. Diagnostics (Basel, Switzerland) 11, 1584. ( 10.3390/diagnostics11091584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA, Weyman AE. 1990. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 82, 792-797. ( 10.1161/01.cir.82.3.792) [DOI] [PubMed] [Google Scholar]
- 70.Goette A, et al. 2016. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 18, 1455-1490. ( 10.1093/europace/euw161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li CY, Zhang JR, Hu WN, Li SN. 2021. Atrial fibrosis underlying atrial fibrillation (Review). Int. J. Mol. Med. 47, 1-1. ( 10.3892/ijmm.2020.4842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moe GK, Abildskov JA. 1959. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am. Heart J. 58, 59-70. ( 10.1016/0002-8703(59)90274-1) [DOI] [PubMed] [Google Scholar]
- 73.Jalife J, Berenfeld O, Mansour M. 2002. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc. Res. 54, 204-216. ( 10.1016/s0008-6363(02)00223-7) [DOI] [PubMed] [Google Scholar]
- 74.de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H, Allessie MA. 2010. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation 122, 1674-1682. ( 10.1161/circulationaha.109.910901) [DOI] [PubMed] [Google Scholar]
- 75.Scott L Jr, Li N, Dobrev D. 2019. Role of inflammatory signaling in atrial fibrillation. Int. J. Cardiol. 287, 195-200. ( 10.1016/j.ijcard.2018.10.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Y, Ding HS, Song T, Chen YT, Wang T, Tang YH, Barajas-Martinez H, Huang CX, Hu D. 2021. Abrogation of CC chemokine receptor 9 ameliorates ventricular electrical remodeling in mice after myocardial infarction. Front. Cardiovasc. Med. 8, 716219. ( 10.3389/fcvm.2021.716219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, Darbar D. 2010. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm 7, 438-444. ( 10.1016/j.hrthm.2009.12.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luan Y, et al. 2010. Interleukin-18 among atrial fibrillation patients in the absence of structural heart disease. Europace 12, 1713-1718. ( 10.1093/europace/euq321) [DOI] [PubMed] [Google Scholar]
- 79.Liu Y, Shi Q, Ma Y, Liu Q. 2018. The role of immune cells in atrial fibrillation. J. Mol. Cell. Cardiol. 123, 198-208. ( 10.1016/j.yjmcc.2018.09.007) [DOI] [PubMed] [Google Scholar]
- 80.Lee SH, Chen YC, Chen YJ, Chang SL, Tai CT, Wongcharoen W, Yeh HI, Lin CI, Chen SA. 2007. Tumor necrosis factor-alpha alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 80, 1806-1815. ( 10.1016/j.lfs.2007.02.029) [DOI] [PubMed] [Google Scholar]
- 81.Liew R, Khairunnisa K, Gu Y, Tee N, Yin NO, Naylynn TM, Moe KT. 2013. Role of tumor necrosis factor-α in the pathogenesis of atrial fibrosis and development of an arrhythmogenic substrate. Circul. J. Offic. J. Jap. Circ. Soc. 77, 1171-1179. ( 10.1253/circj.cj-12-1155) [DOI] [PubMed] [Google Scholar]
- 82.Sun Z, Zhou D, Xie X, Wang S, Wang Z, Zhao W, Xu H, Zheng L. 2016. Cross-talk between macrophages and atrial myocytes in atrial fibrillation. Basic Res. Cardiol. 111, 63. ( 10.1007/s00395-016-0584-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cao F, Li Z, Ding W, Yan L, Zhao Q. 2021. Angiotensin II-treated cardiac myocytes regulate M1 macrophage polarization via transferring exosomal PVT1. J. Immunol. Res. 2021, 1994328. ( 10.1155/2021/1994328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yan J, et al. 2018. The stress kinase JNK regulates gap junction Cx43 gene expression and promotes atrial fibrillation in the aged heart. J. Mol. Cell. Cardiol. 114, 105-115. ( 10.1016/j.yjmcc.2017.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan J, et al. 2021. JNK2, a newly-identified SERCA2 enhancer, augments an arrhythmic [Ca(2+)](SR) leak-load relationship. Circ. Res. 128, 455-470. ( 10.1161/circresaha.120.318409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yan J, Kong W, Zhang Q, Beyer EC, Walcott G, Fast VG, Ai X. 2013. c-Jun N-terminal kinase activation contributes to reduced connexin43 and development of atrial arrhythmias. Cardiovasc. Res. 97, 589-597. ( 10.1093/cvr/cvs366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yan J, et al. 2018. Stress signaling JNK2 crosstalk with CaMKII underlies enhanced atrial arrhythmogenesis. Circ. Res. 122, 821-835. ( 10.1161/circresaha.117.312536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yan J, et al. 2018. Role of stress kinase JNK in binge alcohol-evoked atrial arrhythmia. J. Am. Coll. Cardiol. 71, 1459-1470. ( 10.1016/j.jacc.2018.01.060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yao C, et al. 2018. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation 138, 2227-2242. ( 10.1161/circulationaha.118.035202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heijman J, et al. 2020. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ. Res. 127, 1036-1055. ( 10.1161/circresaha.120.316710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marrouche NF, et al. 2014. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 311, 498-506. ( 10.1001/jama.2014.3) [DOI] [PubMed] [Google Scholar]
- 92.Ramos S, et al. 2022. Degree of fibrosis in human atrial tissue is not the hallmark driving AF. Cells 11, 427. ( 10.3390/cells11030427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burashnikov A, Antzelevitch C. 2018. Is extensive atrial fibrosis in the setting of heart failure associated with a reduced atrial fibrillation burden? Pacing Clin. Electrophysiol. 41, 1289-1297. ( 10.1111/pace.13474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burashnikov A, et al. 2014. A temporal window of vulnerability for development of atrial fibrillation with advancing heart failure. Eur. J. Heart Fail. 16, 271-280. ( 10.1002/ejhf.28) [DOI] [PubMed] [Google Scholar]
- 95.Yue L, Xie J, Nattel S. 2011. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc. Res. 89, 744-753. ( 10.1093/cvr/cvq329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nattel S, Heijman J, Zhou L, Dobrev D. 2020. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ. Res. 127, 51-72. ( 10.1161/circresaha.120.316363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, Liang B, Yue L. 2010. TRPM7-mediated Ca2 + signals confer fibrogenesis in human atrial fibrillation. Circ. Res. 106, 992-1003. ( 10.1161/circresaha.109.206771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harada M, et al. 2012. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 126, 2051-2064. ( 10.1161/circulationaha.112.121830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nattel S. 2017. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin. Electrophysiol. 3, 425-435. ( 10.1016/j.jacep.2017.03.002) [DOI] [PubMed] [Google Scholar]
- 100.Sohns C, Marrouche NF. 2020. Atrial fibrillation and cardiac fibrosis. Eur. Heart J. 41, 1123-1131. ( 10.1093/eurheartj/ehz786) [DOI] [PubMed] [Google Scholar]
- 101.Qiu H, Liu W, Lan T, Pan W, Chen X, Wu H, Xu D. 2018. Salvianolate reduces atrial fibrillation through suppressing atrial interstitial fibrosis by inhibiting TGF-β1/Smad2/3 and TXNIP/NLRP3 inflammasome signaling pathways in post-MI rats. Phytomed. Int. J. Phytotherapy Phytopharmacol. 51, 255-265. ( 10.1016/j.phymed.2018.09.238) [DOI] [PubMed] [Google Scholar]
- 102.Qiu H, et al. 2018. Chronic kidney disease increases atrial fibrillation inducibility: involvement of inflammation, atrial fibrosis, and connexins. Front Physiol 9, 1726. ( 10.3389/fphys.2018.01726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simon JN, Ziberna K, Casadei B. 2016. Compromised redox homeostasis, altered nitroso-redox balance, and therapeutic possibilities in atrial fibrillation. Cardiovasc. Res. 109, 510-518. ( 10.1093/cvr/cvw012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shahid F, Lip GYH, Shantsila E. 2018. Role of monocytes in heart failure and atrial fibrillation. J. Amer. Heart Assoc. 7, e007849. ( 10.1161/jaha.117.007849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Glezeva N, Voon V, Watson C, Horgan S, McDonald K, Ledwidge M, Baugh J. 2015. Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: evidence of M2 macrophage activation in disease pathogenesis. J. Card. Fail. 21, 167-177. ( 10.1016/j.cardfail.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 106.Moore JP, et al. 2015. M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Amer. J. Physiol. Heart Circul. Physiol. 309, H906-H917. ( 10.1152/ajpheart.00821.2014) [DOI] [PubMed] [Google Scholar]
- 107.Ben-Mordechai T, et al. 2013. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J. Am. Coll. Cardiol. 62, 1890-1901. ( 10.1016/j.jacc.2013.07.057) [DOI] [PubMed] [Google Scholar]
- 108.Watson CJ, et al. 2020. Atrial tissue pro-fibrotic M2 macrophage marker CD163+, gene expression of procollagen and B-type natriuretic peptide. J. Amer. Heart Assoc. 9, e013416. ( 10.1161/jaha.119.013416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sagris M, Vardas EP, Theofilis P, Antonopoulos AS, Oikonomou E, Tousoulis D. 2021. Atrial fibrillation: pathogenesis, predisposing factors, and genetics. Int. J. Mol. Sci. 23, 6. ( 10.3390/ijms23010006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Heijman J, Linz D, Schotten U. 2021. Dynamics of atrial fibrillation mechanisms and comorbidities. Annu. Rev. Physiol. 83, 83-106. ( 10.1146/annurev-physiol-031720-085307) [DOI] [PubMed] [Google Scholar]
- 111.Zlochiver S, Muñoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J. 2008. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophys. J. 95, 4469-4480. ( 10.1529/biophysj.108.136473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nattel S. 2018. Electrical coupling between cardiomyocytes and fibroblasts: experimental testing of a challenging and important concept. Cardiovasc. Res. 114, 349-352. ( 10.1093/cvr/cvy003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Y, et al. 2013. The increase in sympathetic nerve density in the atrium facilitates atrial fibrillation in patients with rheumatic heart disease. Int. J. Cardiol. 165, 174-178. ( 10.1016/j.ijcard.2011.08.058) [DOI] [PubMed] [Google Scholar]
- 114.Wang J, Dai M, Cao Q, Yu Q, Luo Q, Shu L, Zhang Y, Bao M. 2019. Carotid baroreceptor stimulation suppresses ventricular fibrillation in canines with chronic heart failure. Basic Res. Cardiol. 114, 41. ( 10.1007/s00395-019-0750-1) [DOI] [PubMed] [Google Scholar]
- 115.Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. 2014. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ. Res. 114, 1500-1515. ( 10.1161/CIRCRESAHA.114.303772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shen MJ, Choi EK, Tan AY, Lin SF, Fishbein MC, Chen LS, Chen PS. 2011. Neural mechanisms of atrial arrhythmias. Nat. Rev. Cardiol. 9, 30-39. ( 10.1038/nrcardio.2011.139) [DOI] [PubMed] [Google Scholar]
- 117.Linz D, et al. 2019. Role of autonomic nervous system in atrial fibrillation. Int. J. Cardiol. 287, 181-188. ( 10.1016/j.ijcard.2018.11.091) [DOI] [PubMed] [Google Scholar]
- 118.Yu L, et al. 2017. Impacts of renal sympathetic activation on atrial fibrillation: the potential role of the autonomic cross talk between kidney and heart. J. Amer. Heart Assoc. 6, e004716. ( 10.1161/jaha.116.004716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang X, Zhao Q, Huang H, Tang Y, Xiao J, Dai Z, Yu S, Huang C. 2013. Effect of renal sympathetic denervation on atrial substrate remodeling in ambulatory canines with prolonged atrial pacing. PLoS ONE 8, e64611. ( 10.1371/journal.pone.0064611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y, et al. 2019. A brain-stellate ganglion-atrium network regulates atrial fibrillation vulnerability through macrophages in acute stroke. Life Sci. 237, 116949. ( 10.1016/j.lfs.2019.116949) [DOI] [PubMed] [Google Scholar]
- 121.Dai M, et al. 2016. Low-level carotid baroreflex stimulation suppresses atrial fibrillation by inhibiting left stellate ganglion activity in an acute canine model. Heart Rhythm 13, 2203-2212. ( 10.1016/j.hrthm.2016.08.021) [DOI] [PubMed] [Google Scholar]
- 122.Chen M, Yu L, Zhou X, Liu Q, Jiang H, Zhou S. 2015. Low-level vagus nerve stimulation: an important therapeutic option for atrial fibrillation treatment via modulating cardiac autonomic tone. Int. J. Cardiol. 199, 437-438. ( 10.1016/j.ijcard.2015.07.083) [DOI] [PubMed] [Google Scholar]
- 123.Ptito A, Zatorre RJ. 1988. Impaired stereoscopic detection thresholds after left or right temporal lobectomy. Neuropsychologia 26, 547-554. ( 10.1016/0028-3932(88)90111-x) [DOI] [PubMed] [Google Scholar]
- 124.Yu L, et al. 2012. Interactions between atrial electrical remodeling and autonomic remodeling: how to break the vicious cycle. Heart Rhythm 9, 804-809. ( 10.1016/j.hrthm.2011.12.023) [DOI] [PubMed] [Google Scholar]
- 125.Wang S, Zhou X, Huang B, Wang Z, Zhou L, Chen M, Yu L, Jiang H. 2016. Spinal cord stimulation suppresses atrial fibrillation by inhibiting autonomic remodeling. Heart Rhythm 13, 274-281. ( 10.1016/j.hrthm.2015.08.018) [DOI] [PubMed] [Google Scholar]
- 126.Zhao Q, et al. 2018. Median nerve stimulation prevents atrial electrical remodelling and inflammation in a canine model with rapid atrial pacing. Europace 20, 712-718. ( 10.1093/europace/eux003) [DOI] [PubMed] [Google Scholar]
- 127.Lu Z, Cui B, He B, Hu X, Wu W, Wu L, Huang C, Po SS, Jiang H. 2011. Distinct restitution properties in vagally mediated atrial fibrillation and six-hour rapid pacing-induced atrial fibrillation. Cardiovasc. Res. 89, 834-842. ( 10.1093/cvr/cvq334) [DOI] [PubMed] [Google Scholar]
- 128.Piccirillo G, et al. 2009. Power spectral analysis of heart rate variability and autonomic nervous system activity measured directly in healthy dogs and dogs with tachycardia-induced heart failure. Heart Rhythm 6, 546-552. ( 10.1016/j.hrthm.2009.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tan AY, et al. 2008. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation 118, 916-925. ( 10.1161/circulationaha.108.776203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gould PA, Yii M, McLean C, Finch S, Marshall T, Lambert GW, Kaye DM. 2006. Evidence for increased atrial sympathetic innervation in persistent human atrial fibrillation. Pacing Clin. Electrophysiol. PACE 29, 821-829. ( 10.1111/j.1540-8159.2006.00447.x) [DOI] [PubMed] [Google Scholar]
- 131.Jayachandran JV, Sih HJ, Winkle W, Zipes DP, Hutchins GD, Olgin JE. 2000. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation 101, 1185-1191. ( 10.1161/01.cir.101.10.1185) [DOI] [PubMed] [Google Scholar]
- 132.Chang CM, et al. 2001. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation 103, 22-25. ( 10.1161/01.cir.103.1.22) [DOI] [PubMed] [Google Scholar]
- 133.Nguyen BL, Fishbein MC, Chen LS, Chen PS, Masroor S. 2009. Histopathological substrate for chronic atrial fibrillation in humans. Heart Rhythm 6, 454-460. ( 10.1016/j.hrthm.2009.01.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yeghiazarians Y, et al. 2021. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 144, e56-e67. ( 10.1161/cir.0000000000000988) [DOI] [PubMed] [Google Scholar]
- 135.Huang B, et al. 2021. Atrial fibrillation in obstructive sleep apnea: neural mechanisms and emerging therapies. Trends Cardiovasc. Med. 31, 127-132. ( 10.1016/j.tcm.2020.01.006) [DOI] [PubMed] [Google Scholar]
- 136.Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. 2020. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ. Res. 127, 4-20. ( 10.1161/circresaha.120.316340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lebek S, et al. 2020. Enhanced CaMKII-dependent late I(Na) induces atrial proarrhythmic activity in patients with sleep-disordered breathing. Circ. Res. 126, 603-615. ( 10.1161/circresaha.119.315755) [DOI] [PubMed] [Google Scholar]
- 138.Korantzopoulos P, Kolettis TM. 2005. Obesity and the risk of new-onset atrial fibrillation. JAMA 293, 1974. Author reply 1975. ( 10.1001/jama.293.16.1974-a) [DOI] [PubMed] [Google Scholar]
- 139.Kim MS, Kim WJ, Khera AV, Kim JY, Yon DK, Lee SW, Shin JI, Won HH. 2021. Association between adiposity and cardiovascular outcomes: an umbrella review and meta-analysis of observational and Mendelian randomization studies. Eur. Heart J. 42, 3388-3403. ( 10.1093/eurheartj/ehab454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hatem SN, Redheuil A, Gandjbakhch E. 2016. Cardiac adipose tissue and atrial fibrillation: the perils of adiposity. Cardiovasc. Res. 109, 502-509. ( 10.1093/cvr/cvw001) [DOI] [PubMed] [Google Scholar]
- 141.Al-Kaisey AM, Kalman JM. 2021. Obesity and atrial fibrillation: epidemiology, pathogenesis and effect of weight loss. Arrhythmia Electrophysiol. Rev. 10, 159-164. ( 10.15420/aer.2021.36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee HJ, Choi EK, Han KD, Lee E, Moon I, Lee SR, Cha MJ, Oh S, Lip GYH. 2020. Bodyweight fluctuation is associated with increased risk of incident atrial fibrillation. Heart Rhythm 17, 365-371. ( 10.1016/j.hrthm.2019.09.029) [DOI] [PubMed] [Google Scholar]
- 143.Hamilton S, Terentyev D. 2019. Altered intracellular calcium homeostasis and arrhythmogenesis in the aged heart. Int. J. Mol. Sci. 20, 2386. ( 10.3390/ijms20102386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Laredo M, Waldmann V, Khairy P, Nattel S. 2018. Age as a critical determinant of atrial fibrillation: a two-sided relationship. Can. J. Cardiol. 34, 1396-1406. ( 10.1016/j.cjca.2018.08.007) [DOI] [PubMed] [Google Scholar]
- 145.Newman W, Parry-Williams G, Wiles J, Edwards J, Hulbert S, Kipourou K, Papadakis M, Sharma R, O'Driscoll J. 2021. Risk of atrial fibrillation in athletes: a systematic review and meta-analysis. Br. J. Sports Med. 55, 1233-1238. ( 10.1136/bjsports-2021-103994) [DOI] [PubMed] [Google Scholar]
- 146.Aschar-Sobbi R, et al. 2015. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFα. Nat. Commun. 6, 6018. ( 10.1038/ncomms7018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang A, Green JB, Halperin JL, Piccini JP Sr. 2019. Atrial fibrillation and diabetes mellitus: JACC review topic of the week. J. Am. Coll. Cardiol. 74, 1107-1115. ( 10.1016/j.jacc.2019.07.020) [DOI] [PubMed] [Google Scholar]
- 148.Saito S, Teshima Y, Fukui A, Kondo H, Nishio S, Nakagawa M, Saikawa T, Takahashi N. 2014. Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc. Res. 104, 5-14. ( 10.1093/cvr/cvu176) [DOI] [PubMed] [Google Scholar]
- 149.Voskoboinik A, et al. 2020. Alcohol abstinence in drinkers with atrial fibrillation. N. Engl. J. Med. 382, 20-28. ( 10.1056/NEJMoa1817591) [DOI] [PubMed] [Google Scholar]
- 150.Voskoboinik A, Prabhu S, Ling LH, Kalman JM, Kistler PM. 2016. Alcohol and atrial fibrillation: a sobering review. J. Am. Coll. Cardiol. 68, 2567-2576. ( 10.1016/j.jacc.2016.08.074) [DOI] [PubMed] [Google Scholar]
- 151.Jakobsen CB, Lamberts M, Carlson N, Lock-Hansen M, Torp-Pedersen C, Gislason GH, Schou M. 2019. Incidence of atrial fibrillation in different major cancer subtypes: a Nationwide population-based 12 year follow up study. BMC Cancer 19, 1105. ( 10.1186/s12885-019-6314-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Menichelli D, Vicario T, Ameri P, Toma M, Violi F, Pignatelli P, Pastori D. 2021. Cancer and atrial fibrillation: epidemiology, mechanisms, and anticoagulation treatment. Prog. Cardiovasc. Dis. 66, 28-36. ( 10.1016/j.pcad.2021.04.004) [DOI] [PubMed] [Google Scholar]
- 153.Burashnikov A. 2022. Atrial fibrillation induced by anticancer drugs and underling mechanisms. J. Cardiovasc. Pharmacol. 80, 540-546. ( 10.1097/fjc.0000000000001182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Madnick DL, Fradley MG. 2022. Atrial fibrillation and cancer patients: mechanisms and management. Curr. Cardiol. Rep. 24, 1517-1527. ( 10.1007/s11886-022-01769-3) [DOI] [PubMed] [Google Scholar]
- 155.Baptiste F, et al. 2019. High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart 6, e001049. ( 10.1136/openhrt-2019-001049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saljic A, Heijman J, Dobrev D. 2022. Emerging antiarrhythmic drugs for atrial fibrillation. Int. J. Mol. Sci. 23, 4096. ( 10.3390/ijms23084096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lei M, Wu L, Terrar DA, Huang CL. 2018. Modernized classification of cardiac antiarrhythmic drugs. Circulation 138, 1879-1896. ( 10.1161/CIRCULATIONAHA.118.035455) [DOI] [PubMed] [Google Scholar]
- 158.Lip GY, et al. 2016. Atrial fibrillation. Nat. Rev. Dis. Primers 2, 16016. ( 10.1038/nrdp.2016.16) [DOI] [PubMed] [Google Scholar]
- 159.Ravens U. 2010. Antiarrhythmic therapy in atrial fibrillation. Pharmacol. Ther. 128, 129-145. ( 10.1016/j.pharmthera.2010.06.004) [DOI] [PubMed] [Google Scholar]
- 160.Hindricks G, et al. 2021. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42, 373-498. ( 10.1093/eurheartj/ehaa612) [DOI] [PubMed] [Google Scholar]
- 161.Savelieva I, Kakouros N, Kourliouros A, Camm AJ. 2011. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace 13, 308-328. ( 10.1093/europace/eur002) [DOI] [PubMed] [Google Scholar]
- 162.Hanley CM, Robinson VM, Kowey PR. 2016. Status of antiarrhythmic drug development for atrial fibrillation: new drugs and new molecular mechanisms. Circ. Arrhythm Electrophysiol. 9, e002479. ( 10.1161/CIRCEP.115.002479) [DOI] [PubMed] [Google Scholar]
- 163.Nattel S, Sager PT, Hüser J, Heijman J, Dobrev D. 2021. Why translation from basic discoveries to clinical applications is so difficult for atrial fibrillation and possible approaches to improving it. Cardiovasc. Res. 117, 1616-1631. ( 10.1093/cvr/cvab093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li GR, et al. 2008. Acacetin, a natural flavone, selectively inhibits human atrial repolarization potassium currents and prevents atrial fibrillation in dogs. Circulation 117, 2449-2457. ( 10.1161/CIRCULATIONAHA.108.769554) [DOI] [PubMed] [Google Scholar]
- 165.Liu H, Wang YJ, Yang L, Zhou M, Jin MW, Xiao GS, Wang Y, Sun HY, Li GR. 2016. Synthesis of a highly water-soluble acacetin prodrug for treating experimental atrial fibrillation in beagle dogs. Sci. Rep. 6, 25743. ( 10.1038/srep25743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Calvo D, Filgueiras-Rama D, Jalife J. 2018. Mechanisms and drug development in atrial fibrillation. Pharmacol. Rev. 70, 505-525. ( 10.1124/pr.117.014183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Burashnikov A, Antzelevitch C. 2008. Can inhibition of IKur promote atrial fibrillation? Heart Rhythm 5, 1304-1309. ( 10.1016/j.hrthm.2008.05.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Podd SJ, Freemantle N, Furniss SS, Sulke N. 2016. First clinical trial of specific IKACh blocker shows no reduction in atrial fibrillation burden in patients with paroxysmal atrial fibrillation: pacemaker assessment of BMS 914392 in patients with paroxysmal atrial fibrillation. Europace 18, 340-346. ( 10.1093/europace/euv263) [DOI] [PubMed] [Google Scholar]
- 169.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. 2007. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation 116, 1449-1457. ( 10.1161/circulationaha.107.704890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Reiffel JA, Camm AJ, Belardinelli L, Zeng D, Karwatowska-Prokopczuk E, Olmsted A, Zareba W, Rosero S, Kowey P. 2015. The HARMONY trial: combined ranolazine and dronedarone in the management of paroxysmal atrial fibrillation: mechanistic and therapeutic synergism. Circ. Arrhythm. Electrophysiol. 8, 1048-1056. ( 10.1161/circep.115.002856) [DOI] [PubMed] [Google Scholar]
- 171.Burashnikov A. 2020. Investigational anti-atrial fibrillation pharmacology and mechanisms by which antiarrhythmics terminate the arrhythmia: where are we in 2020? J. Cardiovasc. Pharmacol. 76, 492-505. ( 10.1097/fjc.0000000000000892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Burashnikov A, Petroski A, Hu D, Barajas-Martinez H, Antzelevitch C. 2012. Atrial-selective inhibition of sodium-channel current by Wenxin Keli is effective in suppressing atrial fibrillation. Heart Rhythm 9, 125-131. ( 10.1016/j.hrthm.2011.08.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hu D, Barajas-Martínez H, Burashnikov A, Panama BK, Cordeiro JM, Antzelevitch C. 2016. Mechanisms underlying atrial-selective block of sodium channels by Wenxin Keli: experimental and theoretical analysis. Int. J. Cardiol. 207, 326-334. ( 10.1016/j.ijcard.2016.01.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Meng Z, et al. 2015. Wenxin Keli versus sotalol for paroxysmal atrial fibrillation caused by hyperthyroidism: a prospective, open label, and randomized study. Evidence-based Compl. Altern. Med. eCAM 2015, 101904. ( 10.1155/2015/101904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wettwer E, et al. 2013. The new antiarrhythmic drug vernakalant: ex vivo study of human atrial tissue from sinus rhythm and chronic atrial fibrillation. Cardiovasc. Res. 98, 145-154. ( 10.1093/cvr/cvt006) [DOI] [PubMed] [Google Scholar]
- 176.Burashnikov A, Di Diego JM, Barajas-Martínez H, Hu D, Cordeiro JM, Moise NS, Kornreich BG, Belardinelli L, Antzelevitch C. 2014. Ranolazine effectively suppresses atrial fibrillation in the setting of heart failure. Circ. Heart Fail. 7, 627-633. ( 10.1161/circheartfailure.114.001129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zygmunt AC, Nesterenko VV, Rajamani S, Hu D, Barajas-Martinez H, Belardinelli L, Antzelevitch C. 2011. Mechanisms of atrial-selective block of Na⁺ channels by ranolazine: I. Experimental analysis of the use-dependent block. Amer. J. Physiol. Heart Circul. Physiol. 301, H1606-H1614. ( 10.1152/ajpheart.00242.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barajas-Martínez H, Hu D, Goodrow RJ Jr, Joyce F, Antzelevitch C. 2013. Electrophysiologic characteristics and pharmacologic response of human cardiomyocytes isolated from a patient with hypertrophic cardiomyopathy. Pacing Clin. Electrophysiol. PACE 36, 1512-1515. ( 10.1111/pace.12227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.De Ferrari GM, et al. 2015. Ranolazine in the treatment of atrial fibrillation: results of the dose-ranging RAFFAELLO (Ranolazine in Atrial Fibrillation Following An ELectricaL CardiOversion) study. Heart Rhythm 12, 872-878. ( 10.1016/j.hrthm.2015.01.021) [DOI] [PubMed] [Google Scholar]
- 180.Liu Y, Zhang Z, Yang Y, Zhang N, Li G, Liu T. 2016. The Chinese herb extract Wenxin Keli: a promising agent for the management of atrial fibrillation. Int. J. Cardiol. 203, 614-615. ( 10.1016/j.ijcard.2015.10.211) [DOI] [PubMed] [Google Scholar]
- 181.Ma J, Yin C, Ma S, Qiu H, Zheng C, Chen Q, Ding C, Lv W. 2018. Shensong Yangxin capsule reduces atrial fibrillation susceptibility by inhibiting atrial fibrosis in rats with post-myocardial infarction heart failure. Drug Des. Dev. Therapy 12, 3407-3418. ( 10.2147/dddt.S182834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang HJ, Kong B, Shuai W, Zhang JJ, Huang H. 2022. Shensong Yangxin attenuates metabolic syndrome-induced atrial fibrillation via inhibition of ferroportin-mediated intracellular iron overload. Phytomed. Int. J. Phytotherapy Phytopharmacol. 101, 154086. ( 10.1016/j.phymed.2022.154086) [DOI] [PubMed] [Google Scholar]
- 183.Zhao HY, et al. 2017. Effect of Shensong Yangxin on the progression of paroxysmal atrial fibrillation is correlated with regulation of autonomic nerve activity. Chinese Med. J. 130, 171-178. ( 10.4103/0366-6999.197997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Burashnikov A, Belardinelli L, Antzelevitch C. 2015. Inhibition of IKr potentiates development of atrial-selective INa block leading to effective suppression of atrial fibrillation. Heart Rhythm 12, 836-844. ( 10.1016/j.hrthm.2014.12.033) [DOI] [PubMed] [Google Scholar]
- 185.Burashnikov A. 2021. Depolarization of the atrial resting membrane potential as an approach to enhance the anti-atrial fibrillation efficacy of sodium channel blockers. Heart Rhythm 18, 1221-1222. ( 10.1016/j.hrthm.2021.03.039) [DOI] [PubMed] [Google Scholar]
- 186.Morey TE, Seubert CN, Raatikainen MJ, Martynyuk AE, Druzgala P, Milner P, Gonzalez MD, Dennis DM. 2001. Structure-activity relationships and electrophysiological effects of short-acting amiodarone homologs in guinea pig isolated heart. J. Pharmacol. Exp. Ther. 297, 260-266. [PubMed] [Google Scholar]
- 187.Ezekowitz MD, et al. 2012. A randomized trial of budiodarone in paroxysmal atrial fibrillation. J. Interv. Card. Electrophysiol. 34, 1-9. ( 10.1007/s10840-011-9636-3) [DOI] [PubMed] [Google Scholar]
- 188.Lacerda AE, Kuryshev YA, Yan GX, Waldo AL, Brown AM. 2010. Vanoxerine: cellular mechanism of a new antiarrhythmic. J. Cardiovasc. Electrophysiol. 21, 301-310. ( 10.1111/j.1540-8167.2009.01623.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Piccini JP, et al. 2016. Randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of a single oral dose of vanoxerine for the conversion of subjects with recent onset atrial fibrillation or flutter to normal sinus rhythm: RESTORE SR. Heart Rhythm 13, 1777-1783. ( 10.1016/j.hrthm.2016.04.012) [DOI] [PubMed] [Google Scholar]
- 190.Dobrev D, Wehrens XHT. 2017. Calcium-mediated cellular triggered activity in atrial fibrillation. J. Physiol. 595, 4001-4008. ( 10.1113/jp273048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Salvage SC, King JH, Chandrasekharan KH, Jafferji DI, Guzadhur L, Matthews HR, Huang CL, Fraser JA. 2015. Flecainide exerts paradoxical effects on sodium currents and atrial arrhythmia in murine RyR2-P2328S hearts. Acta Physiol. (Oxf.) 214, 361-375. ( 10.1111/apha.12505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Watanabe H, et al. 2009. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat. Med. 15, 380-383. ( 10.1038/nm.1942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Salvage SC, Huang CL, Fraser JA, Dulhunty AF. 2022. How does flecainide impact RyR2 channel function? J. Gen. Physiol. 154, e202213089. ( 10.1085/jgp.202213089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Salvage SC, Chandrasekharan KH, Jeevaratnam K, Dulhunty AF, Thompson AJ, Jackson AP, Huang CL. 2018. Multiple targets for flecainide action: implications for cardiac arrhythmogenesis. Br. J. Pharmacol. 175, 1260-1278. ( 10.1111/bph.13807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu T, Xiong F, Qi XY, Xiao J, Villeneuve L, Abu-Taha I, Dobrev D, Huang C, Nattel S. 2020. Altered calcium handling produces reentry-promoting action potential alternans in atrial fibrillation-remodeled hearts. JCI Insight 5, e133754. ( 10.1172/jci.insight.133754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Deftereos S, et al. 2013. Effectiveness of moxonidine to reduce atrial fibrillation burden in hypertensive patients. Am. J. Cardiol. 112, 684-687. ( 10.1016/j.amjcard.2013.04.049) [DOI] [PubMed] [Google Scholar]
- 197.Coll RC, et al. 2015. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248-255. ( 10.1038/nm.3806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haïssaguerre M, et al. 1998. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 339, 659-666. ( 10.1056/nejm199809033391003) [DOI] [PubMed] [Google Scholar]
- 199.Guo F, et al. 2022. Effects of pulsed field ablation on autonomic nervous system in paroxysmal atrial fibrillation: a pilot study. Heart Rhythm 20, 329-338. ( 10.1016/j.hrthm.2022.11.013) [DOI] [PubMed] [Google Scholar]
- 200.Buist TJ, Zipes DP, Elvan A. 2021. Atrial fibrillation ablation strategies and technologies: past, present, and future. Clin. Res. Cardiol. 110, 775-788. ( 10.1007/s00392-020-01751-5) [DOI] [PubMed] [Google Scholar]
- 201.La Rosa G, Quintanilla JG, Salgado R, González-Ferrer JJ, Cañadas-Godoy V, Pérez-Villacastín J, Jalife J, Pérez-Castellano N, Filgueiras-Rama D. 2021. Anatomical targets and expected outcomes of catheter-based ablation of atrial fibrillation in 2020. Pacing Clin. Electrophysiol. PACE 44, 341-359. ( 10.1111/pace.14140) [DOI] [PubMed] [Google Scholar]
- 202.Valderrábano M, et al. 2020. Effect of catheter ablation with vein of marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: the VENUS randomized clinical trial. JAMA 324, 1620-1628. ( 10.1001/jama.2020.16195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Di Biase L, et al. 2016. Left atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation: BELIEF Trial. J. Am. Coll. Cardiol. 68, 1929-1940. ( 10.1016/j.jacc.2016.07.770) [DOI] [PubMed] [Google Scholar]
- 204.Quintanilla JG, Shpun S, Jalife J, Filgueiras-Rama D. 2021. Novel approaches to mechanism-based atrial fibrillation ablation. Cardiovasc. Res. 117, 1662-1681. ( 10.1093/cvr/cvab108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Atienza F, et al. 2014. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial. J. Am. Coll. Cardiol. 64, 2455-2467. ( 10.1016/j.jacc.2014.09.053) [DOI] [PubMed] [Google Scholar]
- 206.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. 2012. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J. Am. Coll. Cardiol. 60, 628-636. ( 10.1016/j.jacc.2012.05.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Seitz J, et al. 2017. AF ablation guided by spatiotemporal electrogram dispersion without pulmonary vein isolation: a wholly patient-tailored approach. J. Am. Coll. Cardiol. 69, 303-321. ( 10.1016/j.jacc.2016.10.065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Haissaguerre M, et al. 2014. Driver domains in persistent atrial fibrillation. Circulation 130, 530-538. ( 10.1161/circulationaha.113.005421) [DOI] [PubMed] [Google Scholar]
- 209.Honarbakhsh S, Schilling RJ, Finlay M, Keating E, Hunter RJ. 2020. Prospective STAR-guided ablation in persistent atrial fibrillation using sequential mapping with multipolar catheters. Circ. Arrhythm. Electrophysiol. 13, e008824. ( 10.1161/circep.120.008824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Baykaner T, et al. 2018. Clinical implications of ablation of drivers for atrial fibrillation: a systematic review and meta-analysis. Circ. Arrhythm. Electrophysiol. 11, e006119. ( 10.1161/circep.117.006119) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.