Abstract
Chlorpyrifos (CPF) is an organophosphate pesticide that can inhibit endocannabinoid (eCB) metabolizing enzymes in animal models at levels that do not significantly alter acetylcholinesterase (AChE) in the central nervous system. Previous studies indicated that repeated low-level CPF exposure in developing rats increased the levels of eCBs in the brain. Because eCBs play a role in immune homeostasis through their engagement with cannabinoid receptors, we investigated the role of cannabinoid receptor 1 (CB1, encoded by the Cnr1 gene) on the CPF-mediated effects in the spleen and lung of neonatal and adult female mice. We treated neonatal and adult female Cnr1−/− mice with 2.5 mg/kg oral CPF or vehicle for 7 days. Tissues were harvested 4 h after the last CPF dose to evaluate eCB metabolic enzyme activity, levels of eCBs, and tissue immunophenotype. There were a small number of genotype-dependent alterations noted in the endpoints following CPF treatment that were specific to age and tissue type, and differences in eCB metabolism caused by CPF treatment did not correlate to changes in eCB levels. To explore the role of CB1 in CPF-mediated effects on immune endpoints, in vitro experiments were performed with WT murine splenocytes exposed to chlorpyrifos oxon (CPO; oxon metabolite of CPF) and challenged with lipopolysaccharide (LPS). While CPO did not alter LPS-induced pro-inflammatory cytokine levels, inactivation of CB1 by the antagonist SR141716A augmented LPS-induced IFN-γ levels. Additional experiments with WT and Cnr1−/− murine splenocytes confirmed a role for CB1 in altering the production of LPS-induced pro-inflammatory cytokine levels. We conclude that CPF-mediated effects on the eCB system are not strongly dependent on CB1, although abrogation of CB1 does alter LPS-induced cytokine levels in splenocytes.
Keywords: chlorpyrifos, organophosphates, endocannabinoids, cannabinoid receptor 1
Introduction
The organophosphate (OP) pesticide chlorpyrifos (CPF) has been registered for agricultural purposes in the United States since 1965, although its residential use was revoked in 2000 (Centner 2018; Grube et al. 2011). In California alone, 601,173 pounds of chlorpyrifos was utilized in 2018; the greatest percentage applied to almonds and cotton (Regulation 2021). California banned CPF in 2020 (Regulation 2019), and the EPA recently revoked all food residue tolerances effective February 28th 2022 (EPA 2021). Although it has been fully banned for food and feed, it is still approved for multiple non-food commodities in the US and will also continue to be used internationally on food crops. Thus, CPF remains a potential health risk for individuals, including children, living in agricultural communities in proximity to where CPF is used (Tamaro et al. 2018; Arcury et al. 2007; Koch et al. 2002). Exposure in children is of particular concern because CPF has been linked to developmental neurotoxicity, including decreased IQ and motor skill deficits (Rauh et al. 2012; Gunier et al. 2017; Ruckart et al. 2004).
The canonical toxic mechanism of action following acute exposures of CPF and other OPs is through inhibition of acetylcholinesterase (AChE) in the central nervous system, causing a subsequent accumulation of acetylcholine and ultimately a cholinergic crisis (Pope, Karanth, and Liu 2005). However, when administered to rodents at doses that are lower than the threshold needed to significantly inhibit brain AChE, CPF and other Ops have been shown to inhibit non-cholinergic serine hydrolases such as the enzymes responsible for metabolizing endocannabinoids (eCBs) (Quistad, Liang, et al. 2006; Quistad, Klintenberg, et al. 2006; Nomura and Casida 2011; Xie et al. 2010). In previous work from our labs, oral exposures of rats to CPF during their development caused eCB metabolizing enzymes to be significantly inhibited and increased the levels of eCBs in brain, spleen, serum, and liver of rat neonates at doses too low to inhibit brain AChE activity (Carr et al. 2013; Carr, Borazjani, and Ross 2011; Carr et al. 2014; Robert et al. 2017). This exposure also resulted in decreased anxiety-like behavior and altered exploratory and social behaviors in male and female rats during the early postweaning period (Carr et al. 2017).
The eCB system is comprised of enzymes involved in the biosynthesis and degradation of 2-arachidonoylglycerol and anandamide (endogenous lipid ligands) and their cognate G-protein coupled receptors (CB1 and CB2) (Rom and Persidsky 2013; Rodríguez de Fonseca et al. 2005; Sido, Nagarkatti, and Nagarkatti 2015). In addition to its role in brain development, the eCB system has been shown to play an important role in immune homeostasis, particularly through the anti-inflammatory effects of eCBs (Gallily, Breuer, and Mechoulam 2000; Facchinetti et al. 2003; Panikashvili et al. 2006; Lu et al. 2014). eCBs modulate immunity in part through their engagement with cannabinoid receptors. In rodent brain, CB1 mRNA expression and its ligand-binding capacity increase progressively from birth until postnatal day (PND) 60, at which point its expression and binding capacity plateaus (Fride and Mechoulam 1996; Belue et al. 1995). CB1 is the main cannabinoid receptor in the central nervous system and is responsible for the psychotropic effects of cannabinoids. On the other hand, CB2 is the main peripheral cannabinoid receptor, due to its expression in immune cells. Despite this CB1 has also been found to play an active role in peripheral tissues (Kaplan 2013; Rom and Persidsky 2013). Interestingly, CB1 was shown to be a target of covalent modification by the bioactive form of CPF, chlorpyrifos oxon (CPO) (Quistad et al. 2002). This suggests that CPF might alter CB1-mediated immune functions following either direct binding of CPO or through alterations in the local levels of eCBs due to inactivation of catabolic enzymes.
In a recent study from our group, several carboxylesterase (Ces) isoforms in lungs of wildtype (WT) neonatal and adult mice were inactivated by CPF treatment (2.5 mg/kg, p.o., 7 d) (Szafran et al. 2021). When lipopolysaccharide (LPS; 1.25 mg/kg, i.p.) was administered following the CPF dosing period, little to no differences in lung immune responses (cytokines and immunophenotyping) were noted between the CPF and vehicle groups. However, a CPF-dependent increase in the percent of dendritic cells and amounts of certain lipid mediators in female lung following LPS challenge was observed. Thus, the goals of the current study were to determine whether CB1 plays a role in CPF-mediated effects on the eCB system in immune tissues of neonatal and adult female mice. Previous work from our lab have identified a role for some CPF-inhibited enzymes in immune responses of the spleen and lung (Szafran et al. 2015; Szafran et al. 2021; Szafran et al. 2022) so those tissues were our main focus but we also conducted some studies in brain and/or liver. Cnr1−/− and WT neonatal and adult female mice were treated with 2.5-mg/kg CPF for 7 days. The effects of CPF on serine hydrolase activities, lipid mediators, and immunophenotypes in tissues were examined. Most of the results from the WT mice have been previously published (Szafran et al., 2021); however, they are presented again alongside data from the Cnr1−/− mice to extend our previous findings and provide context (i.e., a WT comparator group). Results that were previously reported are clearly indicated in the figure legends. It is important to point out that the study with the Cnr1−/− mice was performed in conjunction with that of the WT mice (i.e., animal housing conditions, batches of CPF dosing solutions, and assay reagents were the same for both WT and knockout mice). This ensures that valid comparisons between the different genotypes can be made. Furthermore, in vitro experiments were performed by treating mouse splenocytes with CPO in the presence or absence of LPS. The role of CB1 was addressed by using a CB1 antagonist and Cnr1−/− littermate splenocytes. These mechanistic experiments allowed us to determine whether CB1 plays a role in CPF-mediated effects on the eCB system, and whether these effects can alter immunity.
Materials and Methods
Materials
Chlorpyrifos (CPF; >99% purity, determined by HPLC-photodiode array analysis) was a generous gift from DowElanco Chemical Company (currently known as Corteva, Inc., Indianapolis, IN). Chlorpyrifos oxon (CPO; ≥95% purity) was a generous gift from the late Dr. Howard Chambers from Mississippi State University. p-Nitrophenyl valerate (pNPVa) was from Sigma (St. Louis, MO, USA). Antibodies for ELISAs and flow cytometry were purchased from BioLegend (San Diego, CA). Solvents for LC-MS/MS and ELISA kits were purchased from Invitrogen/Thermo Fisher (Waltham, MA). Authentic standards of lipid mediators were purchased from Cayman Chemical (Ann Arbor, MI). LPS (E. coli 055:B5) was obtained from Sigma (St. Louis, MO).
Treatment of Mice
Animals. Adult C57BL/6 WT mice from Jackson Laboratories (Bar Harbor, ME) and Cnr1−/− mice on a C67BL/6 background from the National Institutes of Health were used to establish a breeding colony. All mice were housed in temperature- and humidity-controlled AAALAC-approved facilities (20–25 °C and 40–60% humidity) under a 12-h light cycle and used in accordance with the Mississippi State University Institutional Animal Care and Use Committee (protocols 17–296 to RLC and 17–342 to BLFK). In Vivo Studies: For neonates, the day of birth was designated as postnatal day 0 (PND 0). CPF dissolved in research-grade corn oil (CO, Sigma, C8267, Lot #MKBS6944V) or vehicle (CO) was administered in a volume of 0.5 mL/kg body weight to Cnr1−/− neonatal mice or 1 mL/kg to Cnr1−/− adult mice (7–8 weeks). Neonates (mixed sex) at PND 10 (n=3–5) and adult female mice (n=5) received either CO or CPF orally every day for 7 days. The 2.5-mg/kg dose was based on a previous study in which no effect on body weight was noted (Szafran et al., 2021). Mice were sacrificed by cervical dislocation 4 h after the final CPF dose. Tissues (lung, liver, spleen, and brain) were harvested and flash frozen in liquid nitrogen; however, in some cases prior to flash freezing, a portion of the liver, lung, and spleen was removed and immediately processed for flow cytometry staining.
In Vitro Studies:
Adult male and female WT mice (n=3) were sacrificed, and harvested spleens were stored in 1x RPMI medium at 4° C for immediate processing. For the first set of endpoints, splenocytes were pooled into a single cell suspension by the mechanical disruption of spleens through a 70 μm filter and were seeded at 2 × 107 cells/well in 2 mL of complete media (1x RPMI containing 1% w/v bovine calf serum, 1% w/v penicillin-streptomycin, and 50 μM 2-mercaptoethanol; 6-well plates). Splenocytes received one of the following treatments (n=3 wells/treatment): Vehicle (0.1% EtOH), CPO (1 μM in EtOH), LPS (1 μg/mL) or a combination of CPO and LPS. Cells were incubated for 3.5 h, after which cells were pelleted by centrifugation and lysed in icecold 50 mM Tris-HCl (pH 7.4) buffer by sonification. Lysates and supernatants were stored at −80° C until further use.
For endpoints that investigated the role of CB1, splenocytes were collected by processing the spleen through a 70-μm filter and seeding cells at 1×107 cells per well in one 24-well plate per mouse. The culture medium (1 mL/well) for these experiments consisted of 1x RPMI supplemented with 5% v/v bovine calf serum (HyClone, Logan, UT), 1% w/v penicillin/streptomycin (Gibco, Gaithersburg, MD), and 50 μM 2-mercaptoethanol (2-ME; Gibco). One half of the wells in the 24-well plate were treated with DMSO (0.05% v/v) and the other half with SR141716A (CB1 antagonist; 1 or 10 μM) in DMSO. Each plate was further divided into 4 treatment groups ± SR141716A (n=3 replicate wells/treatment): Vehicle (0.1% EtOH), CPO (1 μM in EtOH), Veh/LPS (0.1% EtOH, 1 μg/mL LPS), or CPO/LPS (1 μM CPO, 1 μg/mL LPS). Cells were incubated for 24 hr at 37°C prior to collection of the supernatants, which were stored at-80° C prior to ELISA. For the second part of this investigation, F1 breeding to produce WT and Cnr1−/− littermates was initiated. This was to ensure immune effects were due to genotype differences and not influenced by separate breeding. Adult male and female WT and Cnr1−/− littermate mice were sacrificed and splenocytes processed and plated into 24-well plates (n=3 mice per sex and genotype) as described above. Splenocytes from each mouse were divided into 3 treatment groups (n=4 replicates/treatment group): Untreated and one of two LPS concentrations (0.1 μg/mL or 1 μg/mL). Cells were incubated for 24, 48, or 72 hr at 37°C prior to collection of the supernatants. Supernatants were stored at −80° C prior to ELISA.
AChE Activity in Brain Tissue
A portion of the brain (half of the brain after sagittal cut minus cerebellum, medulla, and pons) was homogenized as previously described (Buntyn et al. 2017). An aliquot of homogenate was diluted in cold 50 mM Tris-HCl buffer (pH 7.4 at 37°C) to a final tissue concentration of 0.625 mg/mL. AChE activity was measured spectrophotometrically using acetylthiocholine as the substrate (1 mM final concentration) and 5,5-dithiobis(nitrobenzoic acid) as the chromogen. Protein concentrations were quantified by the Lowry procedure and enzyme activity was normalized by the protein concentration.
Preparation of Tissue Homogenates for eCB Hydrolysis and Ces Activity
A portion of the lung, liver, spleen, and the brain were used to prepare tissue homogenate by homogenizing them at approximately 20% w/v in 50 mM Tris-HCl (pH 7.4) buffer using a Dounce homogenizer on ice. A low-speed centrifugation was done to remove debris (4°C, 20 min, 1,000 × g) from the crude homogenates. All homogenates were stored at −80°C until analysis. Protein concentrations were determined using the BCA reagent (ThermoPierce) following the manufacturer’s instructions. Following completion of the reaction, all enzyme activities were normalized by the protein concentrations.
Evaluation of 2-AG & AEA Hydrolytic Activities
Lung, liver, spleen, or brain homogenate protein (50 μg) was added to 100 μL of 50 mM Tris-HCl (pH 7.4) buffer. Samples were pre-incubated at 37°C for 5 min followed by the addition of either 2-AG or AEA to a final concentration of 50 μM. Following a 20-min incubation at 37°C, the reactions were quenched with 200 μL of cold acetonitrile containing an internal standard (2.5 μM of arachidonic acid-d8) and rested on ice for 10 min. The samples were centrifuged (4°C, 10 min, 16,100 × g) and the resulting supernatant transferred to volume-reducing inserts in an LC vial. The samples were stored at −20°C until analysis. Non-enzymatic (no tissue homogenate) and ‘blank’ (no added 2-AG or AEA) samples were prepared as negative controls.
Evaluation of Ces Activity
Tissue homogenates were diluted in 50 mM Tris-HCl (pH 7.4) buffer and pre-incubated for 5 min at 37°C before adding pNPVa to a final concentration of 750 μM. The release of para-nitrophenol (the hydrolytic product of pNPVa) was monitored at 405 nm for a period of 5 min. Following completion of the reaction, enzyme activities were normalized by the protein concentrations.
Analysis of Lipid Mediators
Another portion of the liver, lung, spleen, and brain were weighed and homogenized on ice in 1:1 v/v hexane:ethyl acetate (containing 0.1% acetic acid and the deuterated standards 2-AG-d8, PEA-d4, OEA-d4, and AEA-d8) using a Dounce homogenizer. The crude homogenates were centrifuged at low speed to remove debris (4°C, 30 min, 2,000 × g). HPLC-grade water (1 mL) was added to the resulting supernatants, which were then vigorously vortexed, followed by centrifugation to separate the two layers (RT, 5 min, 800 × g). The top organic layer was transferred to a clean test tube, and an additional 1 mL of 1:1 hexane:ethyl acetate was added to re-extract the aqueous layer. After centrifugation, the top layer was transferred to the tube containing the organic fraction and the combined organic layers dried under nitrogen gas. MeOH (100 μL) was added to the dried sample and the reconstituted sample transferred to an LC vial with volume-reducing insert for analysis by LC-MS/MS as previously described (Wang et al. 2013). The signal for each lipid mediator in the sample was normalized by the response of its corresponding internal standard and tissue weight used for extraction, except for the adult lungs, which are presented as the ratio of lipid mediator to internal standard.
Evaluation of the Lung Immunophenotype by Flow Cytometry
A fresh, unfrozen portion of the liver, lung, and spleen was homogenized in serum-free RPMI and strained through a 70 μm sieve. Individual cells were stained with extracellular antibodies for CD4 (PECy5; BioLegend clone GK1.5; 0.12 μg/ml), CD8 (PECy7; BioLegend clone 53–6.7; 0.12 μg/ml ), and CD19 (BV650: BioLegend clone 6D5; 0.6 μg/ml). Cells were then fixed in Cytofix™ (BD Biosciences) and an ACEA Novocyte Flow Cytometer was used to analyze the stained and fixed cells. Compensation was set utilizing antibody capture beads and gates were established utilizing fluorescent-minus one controls. Adaptive immune cells, quantified by percent parent lymphocyte, were identified as cytotoxic T cells (CD8+), T helper cells (CD4+), or B cells (CD19+).
Cytokine Measurement by ELISA
Cytokine levels were measured as previously described (Szafran et al. 2018; Szafran et al. 2021) using the appropriate anti-mouse antibodies (IL-6, IFN-γ; BioLegend) or kit (IL-1β, TNF-α; Invitrogen).
Statistical Analysis
GraphPad Prism (Version 7, San Diego, CA, USA) was utilized to perform all statistical analysis and results are presented as mean +/− standard deviation (SD). Differences between groups were assessed utilizing a student’s t-test, one-way analysis of variance, or two-way analysis of variance as appropriate. Outliers were identified utilizing Grubb’s outlier test. p-values <0.05 were considered significant. Statistics for the various interactions from in vivo studies can be found in Supplemental Table 1 grouped by endpoint.
Results
The goal of these studies was to examine a role for CB1 in CPF-mediated inhibition of various enzymes (i.e., AChE, endocannabinoid metabolizing enzymes, Ces) in immune tissues and determine whether CB1 has any impact on CPF effects in immune cells. Moreover, the effects of CPF in neonatal versus adult animals was determined. While most of the data are focused on spleen and lung for immune tissues, some endpoints were also determined in brain and liver for comparison to other work on these two tissues from our labs (Sette et al. 2022; Robert et al. 2017; Szafran et al. 2021).
CPF Did Not Inhibit Brain AChE Activity
First it was important to establish whether the absence of CB1 altered CPF’s effect on AChE activity since it is the canonical target of OP pesticides. Previous data showed that CPF has different effects on293 various enzymes depending on dose (Szafran et al. 2021). However, CPF treatmentat 2.5 mg/kg for 7 days did not inhibit brain AChE activity in neonate and adult mice of either genotype (Figure 1).
Figure 1.
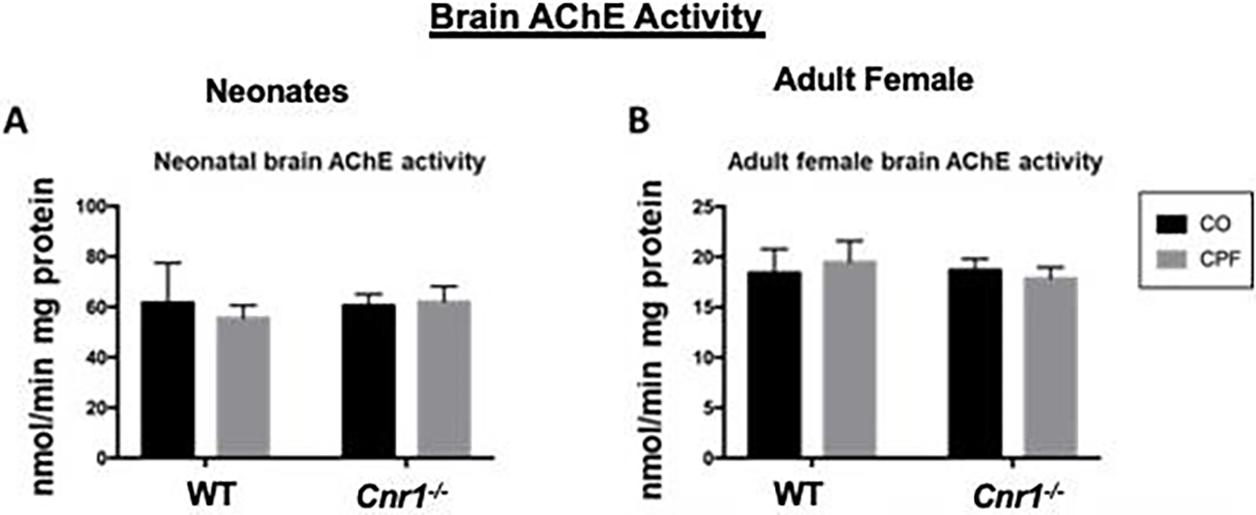
AChE activities of brain tissue. AChE activity was measured in the brain of mixed-sex neonatal (A) and adult female (B) WT and depending on dose Cnr1−/− mice treated with either CO or CPF. Specific activities are given as nmoles of product min−1 mg protein−1. Data are expressed as mean ± SD (n=3–5 mice per condition).
CPF Induces Minimal Genotype-dependent Differences in Endocannabinoid Hydrolysis
Next the effects of CPF on endocannabinoid metabolism were determined. Because different eCB metabolizing enzymes are primarily responsible for 2AG and AEA hydrolysis and this hydrolysis differs between tissues, both 2AG and AEA hydrolysis was measured. CPF treatment did not alter the 2-AG hydrolytic activities in spleen (Figure 2A, C) and liver (Supplemental Figure 1A, B) of WT or Cnr1−/− mice regardless of age. It also did not alter the 2-AG hydrolytic activity in the brain of WT and Cnr1−/− adult mice (Supplemental Figure 1C). There was a 311 significant decrease in 2-AG hydrolytic activity by CPF in adult female WT lung by t-test, as we previously reported (Szafran et al. 2021), but not in adult female Cnr1−/− lung (Figure 2D). 2-AG hydrolysis activity was ~2-fold higher in CPF-treated neonatal lungs from Cnr1−/− mice compared to the corresponding WT mice (Figure 2B, p=0.0093), while it was lower in neonatal Cnr1−/− spleens than in WT spleens regardless of treatment (Figure 2A, p=0.0125 CO, p=0.0067 CPF).
Figure 2.
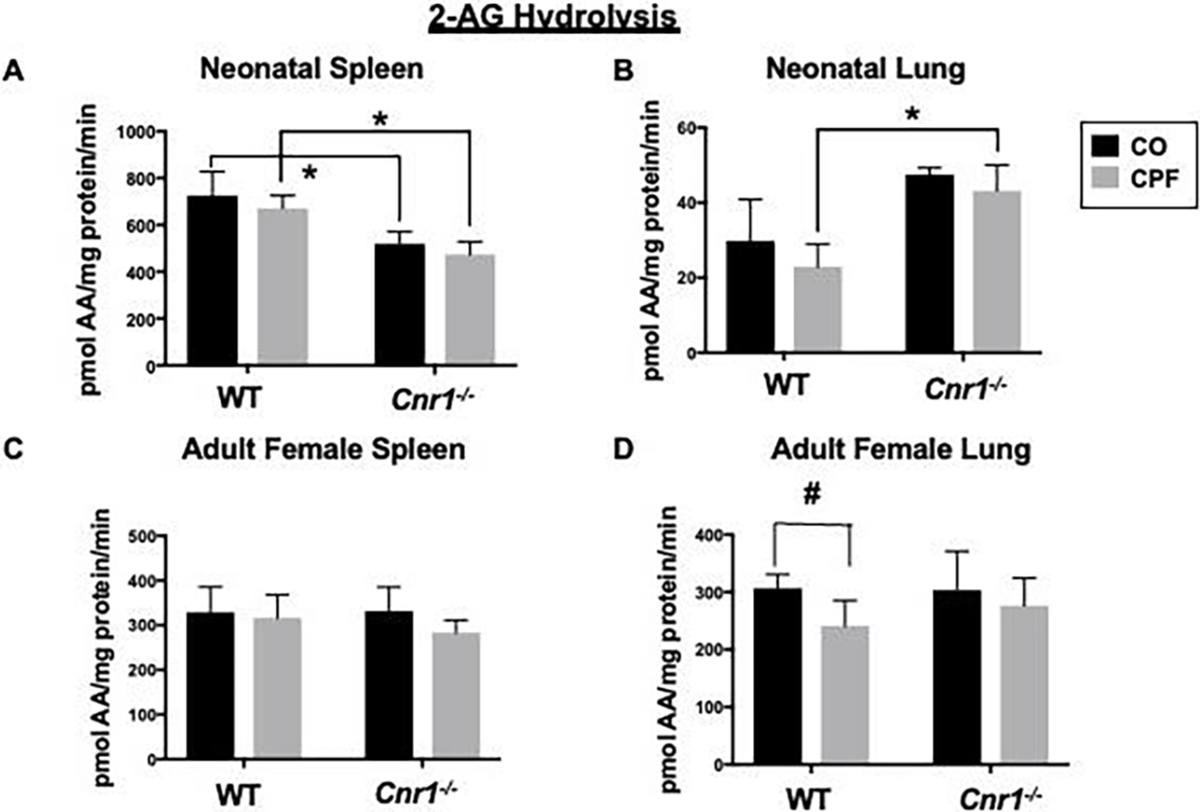
2-AG hydrolysis activity of tissue homogenates. 2-AG hydrolysis activities in whole-tissue homogenates of 314 spleen (A, C) and lung (B, D) from mixed-sex neonatal (A, B) and adult female (C, D) WT and Cnr1−/− mice. Differences between genotypes were significant in neonatal spleen tissue (A) and neonatal lung tissue (B). Data from WT mice were previously published (Szafran et al. 2021) but are presented here as comparative controls for the Cnr1−/− mice. Data are expressed as mean ± SD (n=3–5 mice per condition). *p<0.05 317 for indicated comparison. #p<0.05 by t-test for indicated comparison.
There was no genotype-dependent difference in the AEA hydrolysis activity of the lung, spleen (figure 3A, B), or brain (Supplemental Figure 1E), in adult mice or differences caused by CPF except for the slight CPF-dependent inhibition noted in Cnr1−/− adult lung when analyzed by t-test (Figure 3B, p=0.0235). CPF induced the level of AEA hydrolysis activity in Cnr1−/− neonatal brain by nearly twofold (Supplemental Figure 1D, p=0.0247), whereas it had no effect in WT neonatal brain (Supplemental Figure 1D and (Szafran et al. 2021)). Cnr1−/− neonatal mice had lower brain AEA hydrolysis activity compared to control between the CPF-treated WT and Cnr1−/− neonate brains (Supplemental Figure 1D, p=0.6828).
Figure 3.
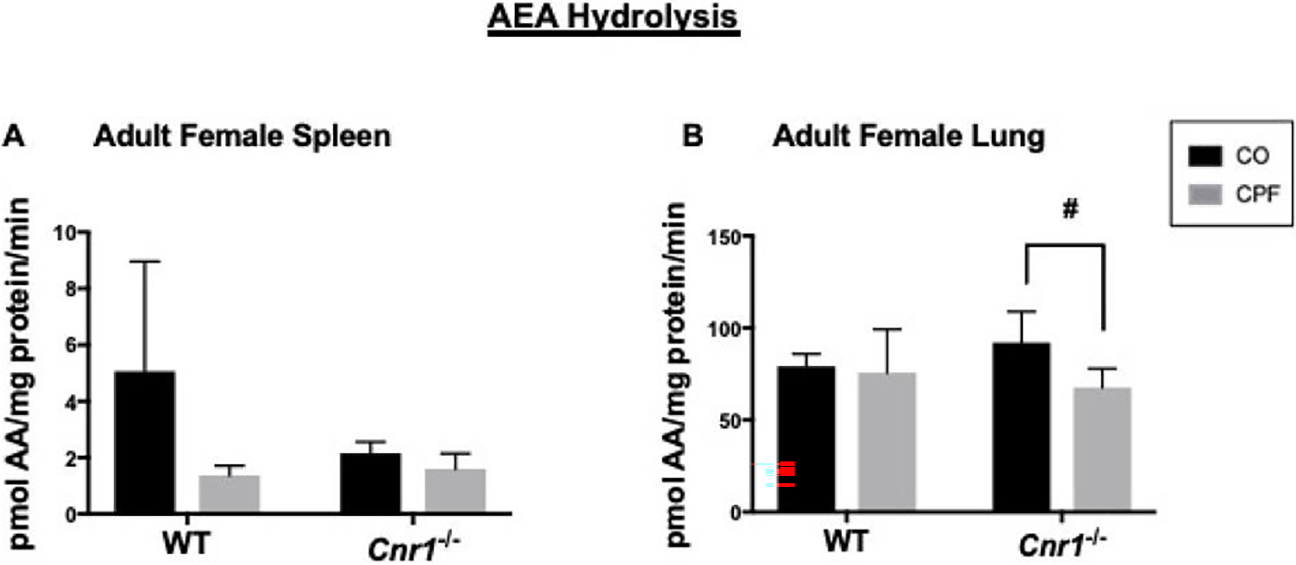
AEA hydrolysis activity of tissue homogenates. AEA hydrolysis activities in whole-tissue homogenates of spleen (A) WT mice (p=0.0155), and lung (B) brain from adult female WT and Cnr1−/− mice. Data from WT mice were previously published (Szafran et al. 2021) but but there was no are presented here as comparative controls for the Cnr1−/− mice. Data are presented as mean ± SD (n=3–5 mice per condition). difference in this activity *p<0.05 for indicated comparison. #p<0.05 by t-test for indicated comparison.
CPF Inhibits Ces Activity in WT and Cnr1−/− Mice
Ces is also a target of inhibition by CPF, and our previous work has focused on the role of this enzyme in a mouse model of inflammation (Szafran et al. 2022; Szafran et al. 2021). These enzymes are highly expressed in multiple tissues and play a role in the hydrolytic metabolism of pesticides and lipids (Ross, Streit, and Herring 2010).
Although Magl is the primary 2-AG hydrolytic enzyme in multiple tissues (Szafran et al.2021), Ces can, to some extent, hydrolyze 2-AG (Wang et al. 2013). CPF treatment reduced the Ces activity in neonatal spleen and lung and adult lung of WT and Cnr1−/− neonatal and adult mice (Figure 4A, B, D), whereas it did not alter Ces activity in brains from mice of either genotype or developmental age (Supplemental Figure 2), or spleens from adult mice (Figure 4C). There was no genotype-dependent difference in the Ces activity of adult tissues (Figure 4C, D), but there was a genotype difference noted in neonatal lung and spleen (Figure 4A, B). Specifically, Ces activity was higher in lung (Figure 4B, p=0.0032) and lower in spleen (Figure 4A, p=0.0028) of WT control neonates compared to their corresponding Cnr1−/− neonates. However, this difference was not observed in the CPF-treated neonatal mice (Figure 4A, B, p≥0.9999 lung, p=0.9996 spleen).
Figure 4.
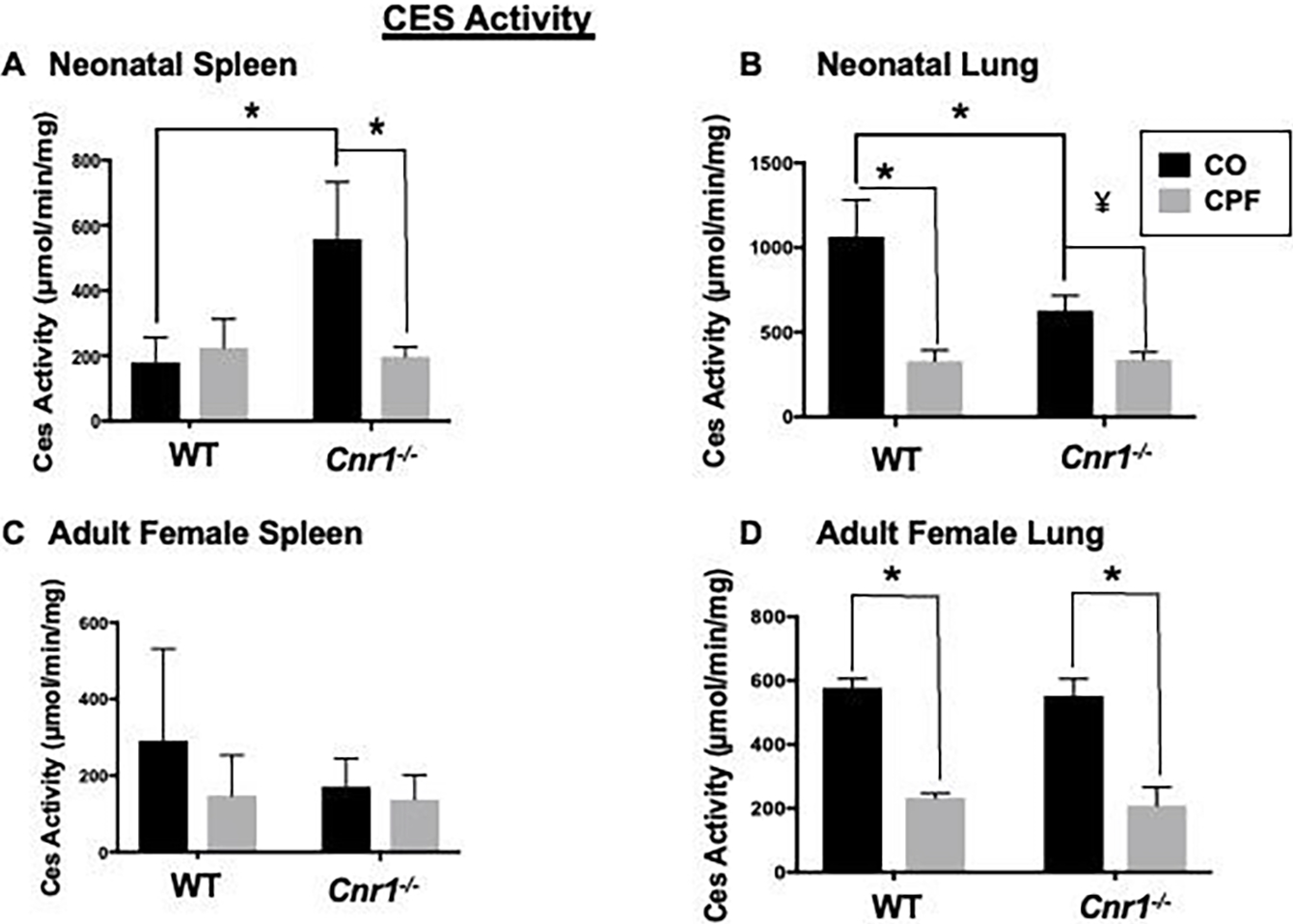
Ces activity of tissue homogenates. Whole-tissue homogenates of spleen (A, C) and lung (B, D) from mixed-sex neonatal (A, B) and adult female (C, D) WT and Cnr1−/− mice. Differences between genotypes were significant in neonatal spleen tissue (A) and neonatal lung tissue (B). Data from WT mice were previously published (Szafran et al. 2021) but are presented here as comparative controls for the Cnr1−/− mice. Data are presented as mean ± SD (n=3–5 mice per condition). *p<0.05 for indicated comparison, ¥p=0.0533 relative to CO control.
CPF and Genotype had Minimal Impact on Endocannabinoid Levels
To determine whether any of the observations of CPF on eCB hydrolysis resulted in changes in eCBs, levels were assessed. CPF treatment had no impact on 2-AG, AEA, and OEA levels in adult lung and spleen regardless of genotype, whereas PEA was decreased or trended downward in lungs of Cnr1−/− mice compared to WT mice (Figure 5A, B). In spleens of adult mice, CPF decreased PEA levels in WT mice but not Cnr1−/− mice, while WT mice treated with CO had higher PEA than their Cnr1−/− counterparts (Figure 5A). CPF treatment also did not alter 2-AG and AEA levels in neonatal lung (Figure 5C), liver, and brain (Supplemental Figure 3A, B) regardless of genotype. There were also no alterations in oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) levels in neonate brain and lung; however, CPF increased these lipid mediators in neonate livers of both genotypes (Supplemental Figure 3A, B).
Figure 5.
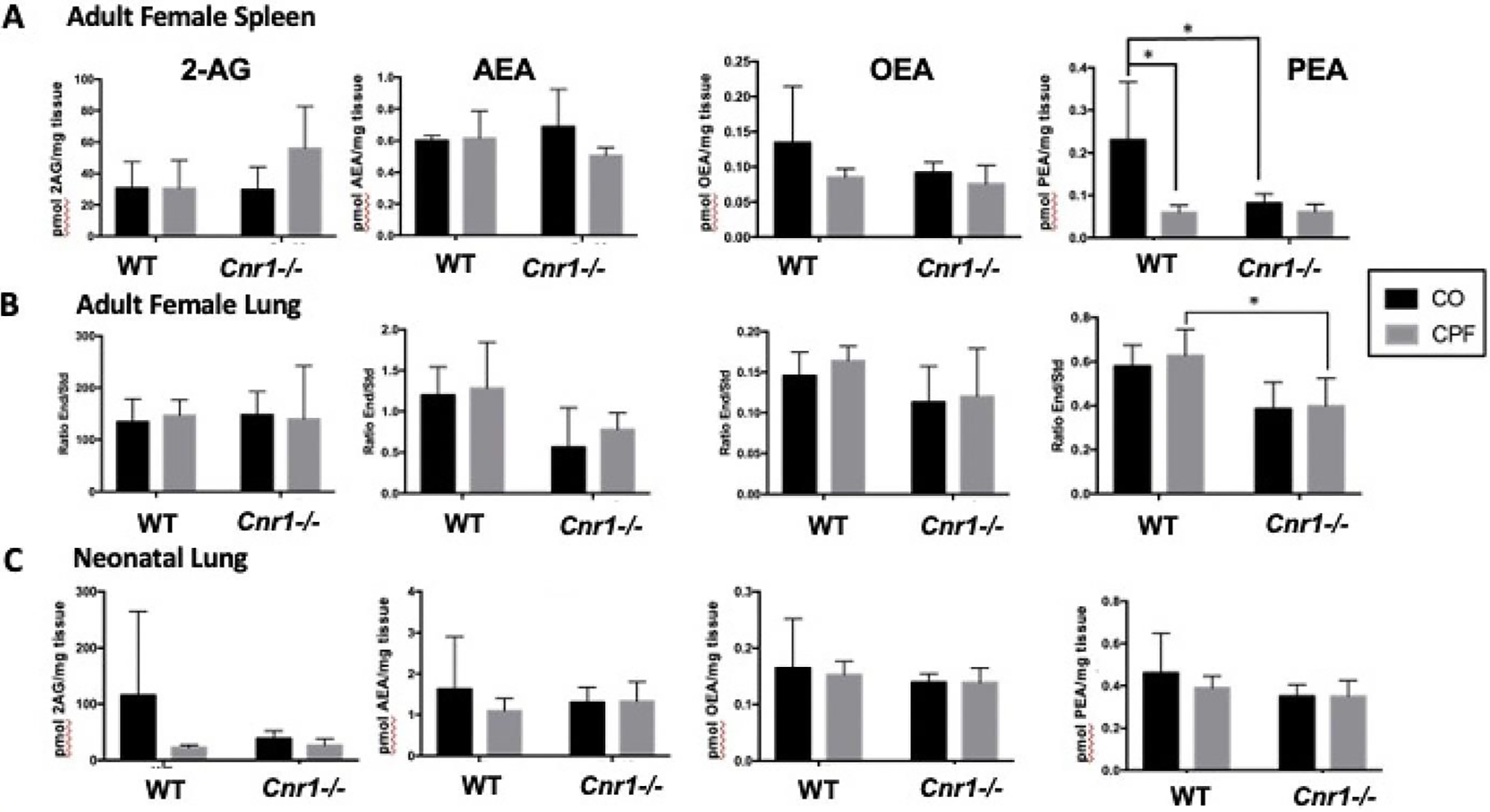
eCB levels as measured by LC-MS/MS. One half of the spleen (A) and lung (B, C) from mixed-sex neonatal (C) and adult female (A, B) WT and Cnr1−/− mice was extracted for eCBs and quantified using deuterated standards (n=3–5). The resulting values were standardized by tissue weight except in the adult lungs. Adult lung values are expressed as the ratio of eCB to deuterated standard. Data are expressed as mean ± SD, *p<0.05 for indicated comparison.
CPF Did Not Alter the Adaptive Immunophenotype
Having established that CPF can alter some some endocannabinoid levels, CPF effects on immune cell populations and the potential role for CB1 was examined. CPF treatment did not alter the distribution of adaptive immune cells in the spleens of neonatal and adult mice regardless of genotype (Figure 6A, B). Similar results were observed in lungs (Figure 6C) and livers (Supplemental Figure 4) of adult mice; no treatment- or genotype-dependent differences were noted.
Figure 6.
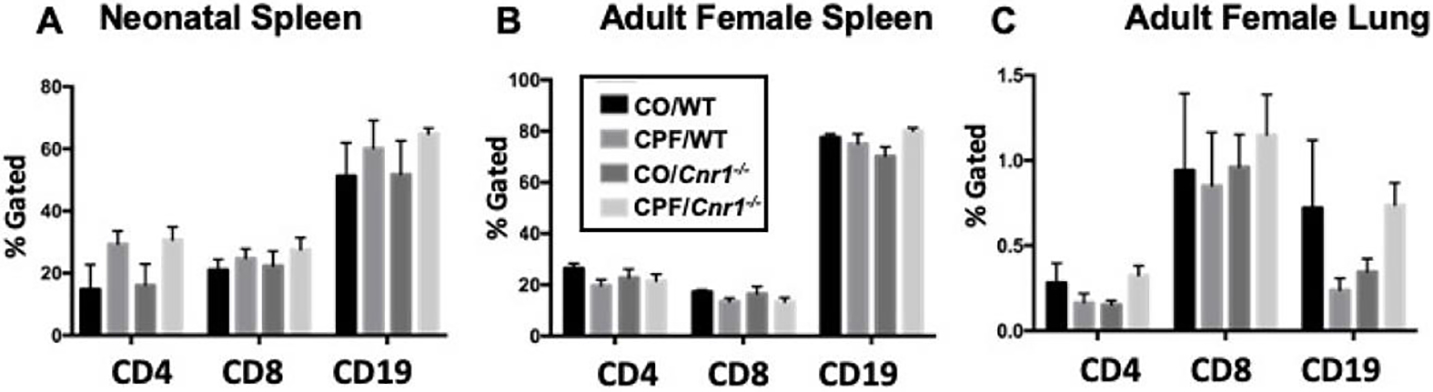
Adaptive immune cell phenotypes by flow cytometry. Cells 379 immune cell from WT and Cnr1−/− mixed-sex neonatal spleens (A, n=3–5) were stained for extracellular markers to identify T helper cells (CD4), 380 populations and the cytotoxic T cells (CD8), and B cells (CD19). Additionally, cells from WT and Cnr1−/− adult female spleens (B, n=4–9), and lung (C, n=4–5) potential role for CB1 were similarly stained. Data are expressed as the mean of percent lymphocyte gated ± SD. None were significant within cell surface 382 was examined. CPF marker group.
CPO Inhibits 2-AG Hydrolytic Activity in Splenocytes
Next, the effect of the active metabolite of CPF, CPO, was examined on eCB metabolism and cytokine response to LPS in vitro in murine splenocytes. Due to the limitations of the in vivo studies, such as the small sample size, these in vitro assays were used to complement the in vivo studies and determine if direct treatment with CPO of splenocytes uncovered any CPF-mediated effects. Initially, only splenocytes from adult WT mice were utilized. CPO treatment significantly inhibited the 2-AG hydrolytic activity in WT splenocytes independent of LPS stimulation (3.5 hr treatment with LPS; Figure 7A); however, CPO did not affect the levels of IL-6 produced by LPS-stimulated splenocytes (Figure 7B).
Figure 7.
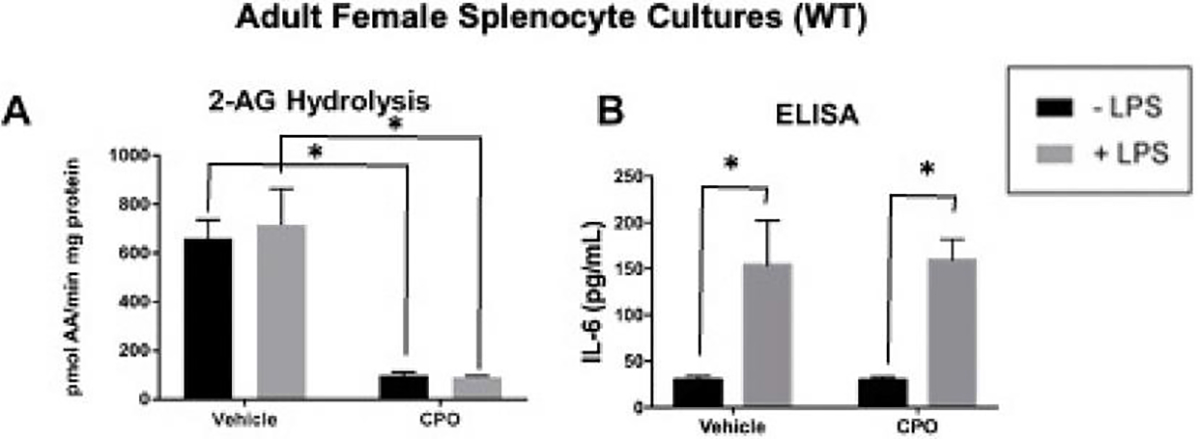
2-AG hydrolytic activity and IL-6 ELISA of treated adult splenocytes. Murine splenocytes were following a 24 h incubation isolated from naïve mice and treated with vehicle, LPS (1 μg/mL), CPO (1 μM), or a combination of LPS and period with LPS. To explore the CPO. After 3.5 hr, the media and cells were harvested together to measure 2-AG hydrolytic activity by LC-MS/MS (A) and IL-6 levels by ELISA (B), respectively. Data are expressed as mean ± SD (n=3 mice & 3 were performed with and without technical replications), *p<0.05 for indicated comparison.
Antagonism of CB1 Enhances LPS-Induced IFN-γ Production Independent of CPO
To evaluate possible effects of CPO with longer-term stimulation, the levels of additional pro-inflammatory cytokines were determined following a 24 h incubation period with LPS. To explore the role of CB1, these experiments were performed with and without the CB1 receptor antagonist SR141716A. While no CPO-mediated effects were seen, there was a difference in IFN-γ between the control cells (DMSO vehicle) and those treated with SR141716A.
Specifically, treatment with the CB1 receptor antagonist significantly augmented LPS-induced IFN-γ levels in female splenocytes (Figure 8A). An increased trend was noted in male splenocytes; however, it did not reach statistical significance (Figure 8B). No significant differences were detected for the other cytokines measured (IL-1β, IL-6, TNFα; Figure 8A, B).
Figure 8.
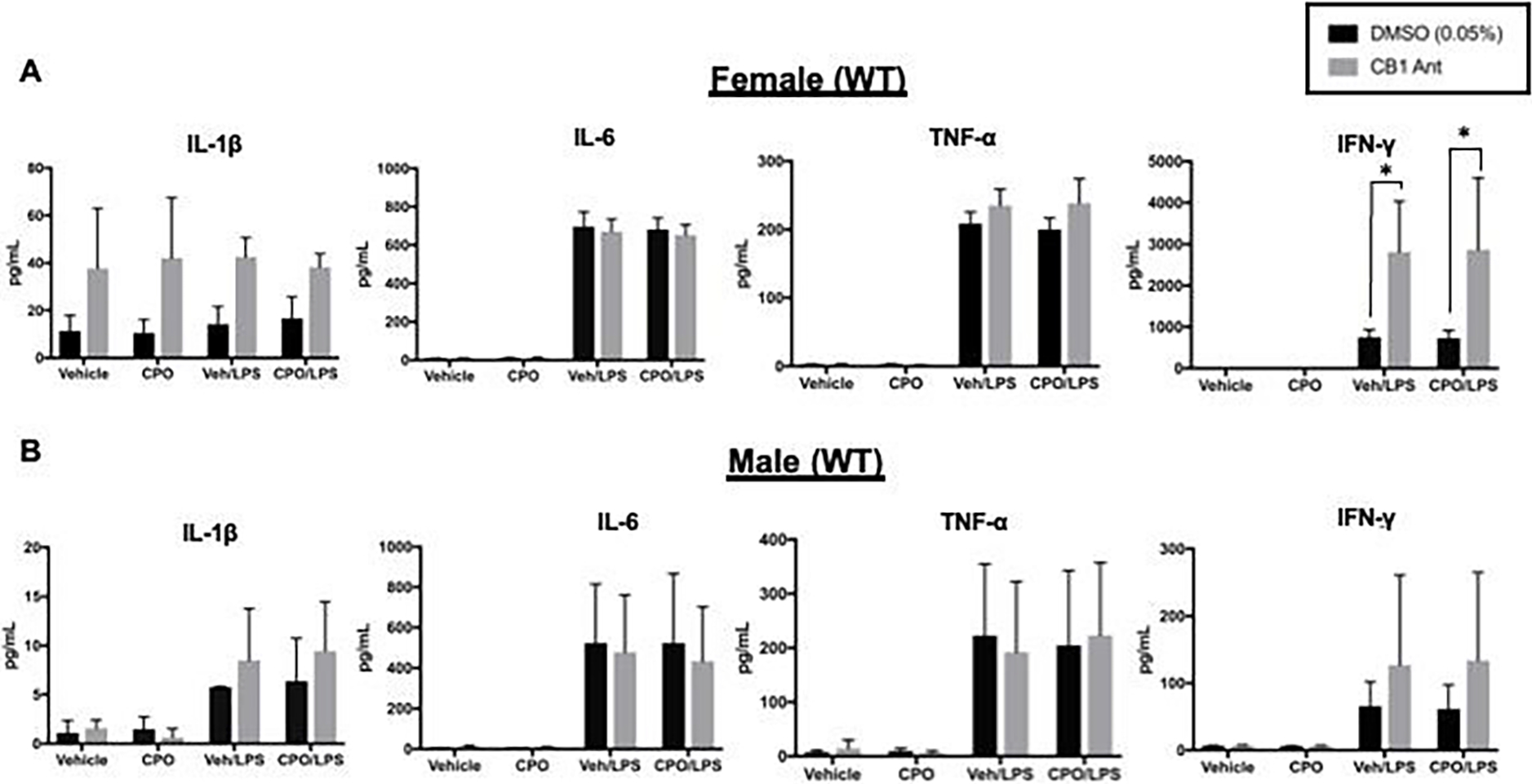
Cytokine ELISA results for splenocytes treated with DMSO or a CB1 antagonist. Splenocytes were isolated from naïve adult female (A) and male (B) mice. Cells from each mouse were first divided in half and treated with SR141716A (CB1 Ant) in DMSO or DMSO. Once plated, cells were treated additionally with CPO (1 μM) in EtOH or EtOH (Veh) ± LPS (1 μg/mL) for 24 hr at 37°C. IL-1β, IL-6, TNF-α, and IFN-γ levels were measured in the serum by ELISA. Data are expressed as mean ± SD (n=3), *p<0.05 for indicated comparison.
Splenocytes from Cnr1−/− Mice Respond More Robustly to LPS than those from WT Littermates
To confirm that the observed differences were due to antagonism of CB1, isolated splenocytes from WT and Cnr1−/− adult male and female littermates were treated with LPS at two concentrations over several days. Naïve and LPS-induced IL-1β levels did not differ between genotypes or incubation periods (Figure 9A). In male (but not female) splenocytes, LPS-induced IL-6 levels were trending upwards or higher in Cnr1−/− splenocytes than those in WT splenocytes after 24, 48, and 72 h of incubation with 0.1 and 1 μg/mL LPS (Figure 9B). In female (but not male) splenocytes, the LPS-induced TNF-α levels in Cnr1−/− cells were significantly higher than those in WT cells after 24-h incubation with 1 μg/mL LPS (Figure 9C). There was also an increased level of IFN-γ in male Cnr1−/− splenocytes compared to male WT splenocytes after a 72-h incubation with 1 μg/mL LPS (Figure 9D).
Figure 9.
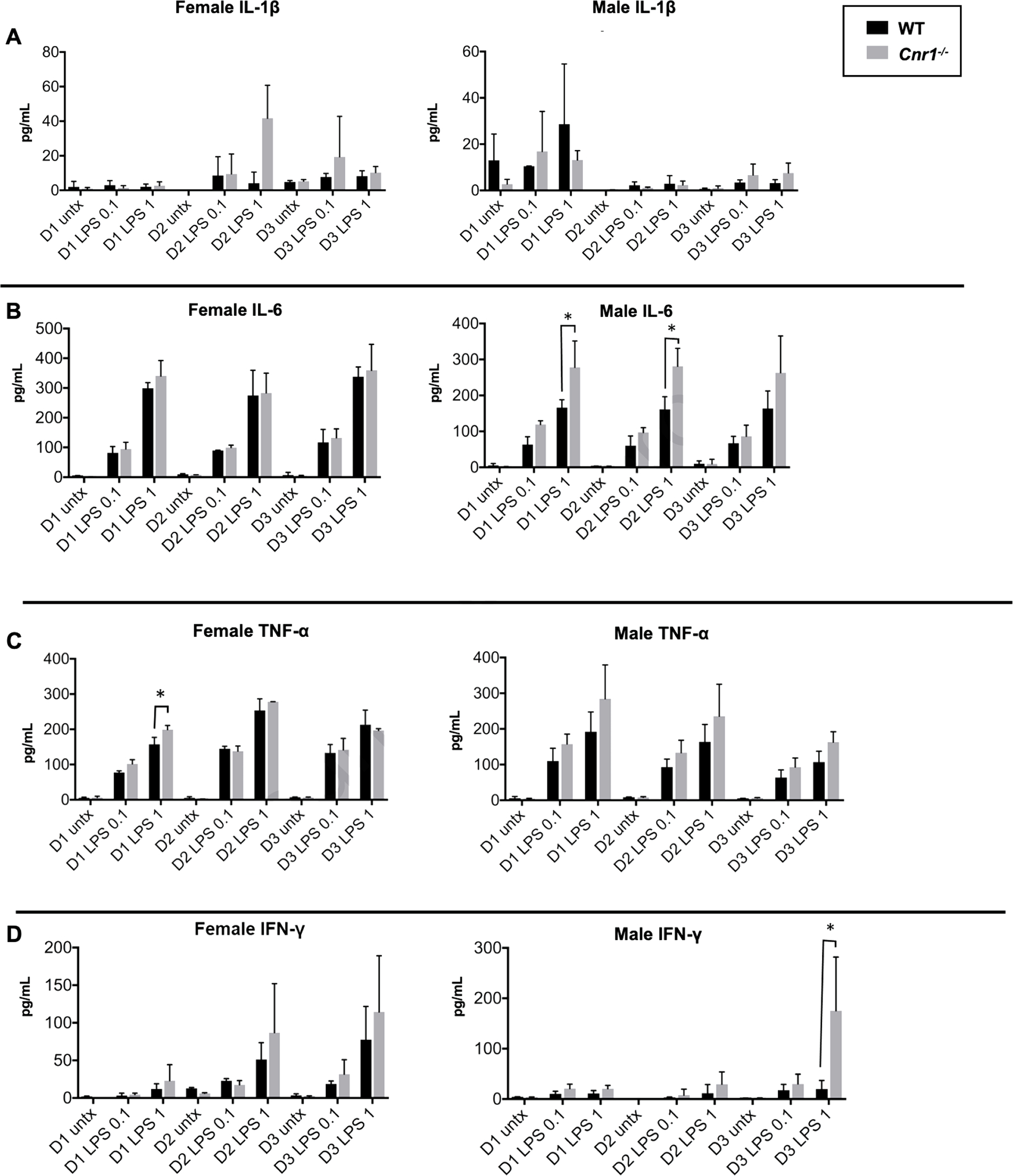
Cytokine ELISA results for splenocytes from WT and Cnr1−/− littermates. Splenocytes were isolated from wild-type (WT) and Cnr1−/− female and male murine littermates (n=3). Cells were left untreated or treated with 0.1 μg/mL or 1 μg/mL LPS for 24 h (D1), 48 h (D2), or 72 h (D3). IL-1β (A), IL-6 (B), TNF-α (C), and IFN-γ (D) levels were measured in the serum by ELISA. Data are expressed as mean ± SD (n=4 technical replications), *p<0.05 for indicated comparison.
Discussion
CPF was once a commonly used pesticide in agriculture despite concerns about its neurotoxic effects in children (Gunier et al. 2017; Centner 2018; Grube et al. 2011; Regulation 2021; Rauh et al. 2012; Ruckart et al. 2004). The EPA recently revoked all tolerances for CPF in food (EPA 2021), but there are still opportunities for exposure. Exposure to OPs such as CPF can inhibit eCB metabolizing enzymes in animal models at dose levels that do not inhibit brain AChE, thus causing subsequent increases in eCB levels (Carr et al. 2013; Carr et al. 2017; Carr et al. 2014; Howell et al. 2018; Buntyn et al. 2017). A mechanism by which eCBs can alter immune homeostasis is via their engagement with cannabinoid receptors, such as CB1 (Kaplan 2013; Rodríguez de Fonseca et al. 2005; Lu and Mackie 2016). Here, we investigated the role of CB1 on CPF-mediated effects on the eCB system in the spleen and lung of neonatal and adult female mice using WT and Cnr1−/− mice. This was followed by in vitro studies of murine splenocytes to investigate immune effects. In general, CPF-mediated effects on the eCB system in vivo were not dependent on the presence of functional CB1. However, inactivation of CB1 by genetic and pharmacologic approaches enhanced the levels of some pro-inflammatory cytokines following LPS stimulation of splenocytes, although CB1 abrogation did not modulate the effects caused by CPO exposures in this model system.
Although a limitation is the small sample size (n=3–5), the results of the current study are consistent with some other studies exploring the effects of CPF exposure in mice. A previous study that compared the acute effects of CPF (up to 300 mg/kg subcutaneously) in WT and Cnr1−/− mice found that there were no major differences in toxicity between the two genotypes (Baireddy et al. 2011). The only minor difference was that the WT mice were more sensitive to the inhibitory effects of CPF on AChE in specific brain regions (Baireddy et al. 2011). In the current study, we observed no differences in brain AChE activity following CPF treatments of neonates and adults of both genotypes. There were also no CPF-dependent differences in 2-AG hydrolysis activity in the examined tissues of the WT and Cnr1−/− neonatal and adult female mice [(Szafran et al. 2021); and data shown here]. This contrasts with previous studies in neonatal rats (dosed at 1 mg/kg of CPF for 7 days), which showed a significant inhibition of 2-AG and AEA hydrolysis activities in brain, liver, and spleen (Buntyn et al. 2017). This suggests a marked difference in the sensitivity of neonatal rats and mice toward CPF (rats being more susceptible to toxicity than mice), at least for these endpoints. This could be due to species-specific differences in the toxicokinetic behavior of CPF, differences in the intrinsic sensitivity of rat and mouse eCB hydrolytic enzymes towards reactive CPO, and/or because of redundancies in the types of enzymes that metabolize eCBs in mice (i.e., expression of ‘moonlighting enzymes’ that help to maintain eCB homeostasis). Indeed, a recent study from our laboratories indicated species-specific difference in the toxicokinetic behavior of CPF between neonatal rats and mice with mice exhibiting higher protection against CPF exposure as compared to rats (Sette et al. 2022).
In the current study, CPF decreased AEA hydrolysis in the Cnr1−/− adult female lung, whereas it increased this activity in Cnr1−/− neonate brain. These effects on AEA hydrolysis activity were not observed in WT mice. In addition, some small genotype-dependent differences in 2-AG, AEA, and Ces hydrolytic activities were noted. 2-AG and AEA are agonists of CB1 (Kaplan 2013) and Cnr1−/− mice do not have CB1 receptors to engage with these ligands, thus alterations in eCB metabolic activities in Cnr1−/− mice could be a mechanism to compensate for the lack of CB1. For example, the lower 2-AG and AEA hydrolysis activities in some tissues of the Cnr1−/− mice relative to those in WT mice could be due to the absence of activating signals on monoacyl glycerol lipase and fatty acid amide hydrolase (MAGL and FAAH, respectively; 2 major eCB hydrolases that regulate levels of CB1 endogenous ligands), which are evoked when CB1 is engaged by agonists. However, such an adaptive mechanism would appear to be age- and tissue-dependent, because eCB hydrolysis activity was not uniformly lower in the Cnr1−/− mice compared to WT mice.
Previous research showed that CPF-mediated inhibition of eCB hydrolysis activity in the juvenile rat brain led to concomitant increases in eCB levels (Carr et al. 2013), thus there is a tight regulatory linkage between this enzymatic activity and ligand concentration. In the present study, CPF treatment exerted minimal effects on eCB hydrolysis activities and eCB levels in mouse tissues. This discrepancy could be explained by the lower sensitivity of neonatal mice to CPF exposure as compared to rats (Sette et al. 2022). The increase of AEA hydrolytic activity in brains of neonatal Cnr1−/− mice following CPF treatment was unexpected, but it was not associated with a decrease in AEA level. Similarly, the CPF-dependent decrease in lung AEA hydrolysis in adult female Cnr1−/− mice did not cause an increase in lung AEA. The only effect on eCB levels caused by CPF in neonatal mice (of both genotypes) was an increase in liver OEA and PEA levels. OEA and PEA are both eCB-like substances but have a low affinity for the cannabinoid receptors (Ramer, Schwarz, and Hinz 2019). OEA and PEA (and other N-acyl-ethanolamines) have been termed “entourage” lipid mediators of AEA and are regulated in a similar manner by FAAH. For example, downregulation of AEA levels was correlated with lower levels of both OEA and PEA in human meningiomas and gliomas (Ramer, Schwarz, and Hinz 2019; Maccarrone et al. 2001). In the present study, however, AEA levels were not decreased by CPF in the same way that OEA and PEA were (Figure 4), suggesting that the regulation of these lipid amide mediators is complex.
Although CB2 is the most abundant CB receptor in immune cells, CB1 is also present, and its activation can alter tissue immunophenotype (Kaplan 2013). One mechanism by which eCBs can influence immune responses is through their engagement with cannabinoid receptors expressed on T and B cells, which leads to altered immune cell function and cytokine release (Kaplan 2013). In the present study, we used flow cytometry to quantify the percentages of T helper cells, cytotoxic T cells, and B cells in the spleen, liver (adult only), and lung (adult only) of WT and Cnr1−/− mice following exposure to CPF. Neither CPF treatment nor genotype affected the immunophenotypes of these tissues. Previous studies had shown that CPF could alter immune endpoints in WT rats or mouse pups, but these studies utilized immune stimulants such as concanavalin A, phytohemagglutin, or LPS (Blakley et al. 1999; Navarro et al. 2001; Singh et al. 2013). Our previous study also examined the effects of an LPS challenge in WT mice exposed to CPF, yet no widespread alterations in immune responses were identified except for a small increase in LPS-induced lung dendritic cells in adult female, but not male, mice (Szafran et al. 2021). We suggested this alteration was due to the sex-specific inhibition of Ces1b by CPF in the lungs of female but not male mice; however, more work is still needed.
The in vitro CPO treatment of murine splenocytes significantly reduced 2-AG hydrolysis activity. Of note here, CPF did not inhibit 2-AG hydrolysis activity in-vivo in the spleen, but this discrepancy is most likely due to an inability of the CPO metabolite to reach the spleen at high enough levels to inhibit splenic 2-AG hydrolytic activity. Although CPO reduced splenocyte 2-AG hydrolytic activity, it did not alter LPS-induced cytokine levels. This differs somewhat to a previous study in human THP-1 cells that found an upregulation of pro-inflammatory cytokine mRNAs following CPO exposure (Proskocil et al. 2019). However, the knockout or pharmacologic inactivation of CB1 did modulate LPS-induced cytokine levels in a sex- and time-dependent manner that was independent of CPO treatment. It is possible the sex difference is due to different cannabinoid receptor expression in males versus females in untreated cells and in response to LPS. Alternatively, there could be differences in eCB tone as a result of sex-dependent differences in eCB biosynthesis or degradative enzymes. However, there are limited data available from direct comparisons between male and female splenocytes for CB1 expression or these various components of the eCB system. Other studies have reported altered cytokine responses resulting from CB1 silencing. For example, our previous work showed an increased level of IFN-γ production by ex vivo restimulated splenocytes from Cnr1−/− mice compared to those from WT controls in a mouse model of experimental autoimmune encephalomyelitis (Nichols and Kaplan 2021). In another study, a murine model of EAE was found to exhibit increased levels of IFN-γ, TNF-α, and IL-6 in the brains and spinal cords following administration of a CB1 antagonist (Lou, Zhao, and Xiao 2012). On the other hand, there are some studies that have observed anti-inflammatory effects caused by CB1 antagonism (Croci et al. 2003; Sugamura et al. 2009); however, they did not examine splenocytes, CNS, or use an EAE model, suggesting that these particular findings were tissue and/or model dependent.
Conclusion
Our results showed that the CPF-dependent effects on the eCB system in neonatal and adult female mice was minimally affected by genotype (WT versus Cnr1−/−). The effects on eCB metabolizing enzymes in the spleen and lung of Cnr1−/− mice did not correlate with changes in eCB levels in vivo. Although CPO strongly inhibited splenocyte 2-AG hydrolysis activity in vitro, it did not alter LPS-induced pro-inflammatory cytokine levels regardless of genotype. However, inactivation of CB1 enhanced LPS-induced IFN-γ levels of female-derived splenocytes independent of CPO treatment. In Cnr1−/− mouse splenocytes, LPS-induced IL-6, TNF-α, and IFN-γ levels were enhanced in a sex- and time-dependent manner. Our study did not identify widespread alterations in eCB metabolic activity and eCBs levels following CPF treatment in Cnr1−/− mice; however, the splenocyte experiments provide further evidence that supports a role for CB1 in maintaining immune homeostasis.
Supplementary Material
Highlights.
CB1 did not play a big role in CPF-mediated changes in the lung or spleen
In WT female SPLC, the CB1 antagonist enhanced LPS-induced IFN-γ
In WT male SPLC, the CB1 antagonist modestly enhanced LPS-induced IFN-γ
In male SPLC, there was enhanced LPS-stimulated IL-6 and IFN-γ in Cnr1−/− cells
ACKNOWLEDGEMENTS
We thank Dr. Shirley Guo-Ross for her technical assistance with the animal studies. Funding was provided by the Mississippi State University College of Veterinary Medicine and by NIH R15GM128206 (to M.K.R.).
Abbreviations:
- 2-AG
2-arachidonylglycerol
- 2-ME
2-mercaptoethanol
- AChE
acetylcholinesterase
- AEA
anandamide
- Ant
antagonist
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CD4+
T helper cells
- CD8+
cytotoxic T cells
- CD19+
B cells
- Ces
carboxylesterase
- Cnr1
cannabinoid receptor 1 (gene)
- CNS
central nervous system
- CO
corn oil
- CPF
chlorpyrifos
- CPO
chlorpyrifos oxon
- DMSO
dimethyl sulfoxide
- EAE
experimental autoimmune encephalomyelitis
- eCB
endocannabinoid
- EtOH
ethyl alcohol
- FAAH
fatty acid amide hydrolase
- IFN-γ
interferon gamma
- IL-1β
interleukin 1 beta
- IL-6
interleukin 6
- LPS
lipopolysaccharide
- MAGL
monoacylglycerol lipase
- MeOH
methanol
- OEA
oleoylethanolamide
- OP
organophosphate
- PEA
palmitoylethanolamide
- PND
postnatal day
- pNPVa
p-Nitrophenyl valerate
- SD
standard deviation
- TNF-α
tumor necrosis factor alpha
- Veh
vehicle
- WT
wild type
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arcury TA, Grzywacz JG, Barr DB, Tapia J, Chen H, and Quandt SA. 2007. “Pesticide urinary metabolite levels of children in eastern North Carolina farmworker households.” Environ Health Perspect 115 (8):1254–60. doi: 10.1289/ehp.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baireddy P, Liu J, Hinsdale M, and Pope C. 2011. “Comparative effects of chlorpyrifos in wild type and cannabinoid Cb1 receptor knockout mice.” Toxicol Appl Pharmacol 256 (3):324–9. doi: 10.1016/j.taap.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belue RC, Howlett AC, Westlake TM, and Hutchings DE. 1995. “The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats.” Neurotoxicol Teratol 17 (1):25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Blakley BR, Yole MJ, Brousseau P, Boermans H, and Fournier M. 1999. “Effect of chlorpyrifos on immune function in rats.” Veterinary and human toxicology 41 (3):140–144. [PubMed] [Google Scholar]
- Buntyn RW, Alugubelly N, Hybart RL, Mohammed AN, Nail CA, Parker GC, Ross MK, and Carr RL. 2017. “Inhibition of Endocannabinoid-Metabolizing Enzymes in Peripheral Tissues Following Developmental Chlorpyrifos Exposure in Rats.” Int J Toxicol 36 (5):395–402. doi: 10.1177/1091581817725272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Adams AL, Kepler DR, Ward AB, and Ross MK. 2013. “Induction of endocannabinoid levels in juvenile rat brain following developmental chlorpyrifos exposure.” Toxicol Sci 135 (1):193–201. doi: 10.1093/toxsci/kft126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Armstrong NH, Buchanan AT, Eells JB, Mohammed AN, Ross MK, and Nail CA. 2017. “Decreased anxiety in juvenile rats following exposure to low levels of chlorpyrifos during development.” Neurotoxicology 59:183–190. doi: 10.1016/j.neuro.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Graves CA, Mangum LC, Nail CA, and Ross MK. 2014. “Low level chlorpyrifos exposure increases anandamide accumulation in juvenile rat brain in the absence of brain cholinesterase inhibition.” Neurotoxicology 43:82–89. doi: 10.1016/j.neuro.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr Russell L., Borazjani Abdolsamad, and Ross Matthew K.. 2011. “Effect of Developmental Chlorpyrifos Exposure, on Endocannabinoid Metabolizing Enzymes, in the Brain of Juvenile Rats.” Toxicological Sciences 122 (1):112–120. doi: 10.1093/toxsci/kfr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centner Terence J. 2018. “Cancelling pesticide registrations and revoking tolerances: The case of chlorpyrifos.” Environmental Toxicology and Pharmacology 57:53–61. doi: 10.1016/j.etap.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Croci Tiziano, Landi Marco, Galzin Anne-Marie, and Marini Pietro. 2003. “Role of cannabinoid CB1 receptors and tumor necrosis factor-α in the gut and systemic anti-inflammatory activity of SR 141716 (Rimonabant) in rodents.” British journal of pharmacology 140 (1):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. 2021. “Tolerance Revocations: Chlorpyrifos.” https://www.regulations.gov/document/EPA-HQ-OPP-2021-0523-0001.
- Facchinetti F, Del Giudice E, Furegato S, Passarotto M, and Leon A. 2003. “Cannabinoids ablate release of TNFalpha in rat microglial cells stimulated with lypopolysaccharide.” Glia 41 (2):161–8. doi: 10.1002/glia.10177. [DOI] [PubMed] [Google Scholar]
- Fride E, and Mechoulam R. 1996. “Developmental aspects of anandamide: ontogeny of response and prenatal exposure.” Psychoneuroendocrinology 21 (2):157–72. doi: 10.1016/0306-4530(95)00039-9. [DOI] [PubMed] [Google Scholar]
- Gallily R, Breuer A, and Mechoulam R. 2000. “2-Arachidonylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-alpha production in murine macrophages, and in mice.” Eur J Pharmacol 406 (1):R5–7. [DOI] [PubMed] [Google Scholar]
- Grube Arthur, Donaldson David, Kiely Timothy, and Wu La. 2011. “Pesticides industry sales and usage.” US EPA, Washington, DC. [Google Scholar]
- Gunier Robert B., Bradman Asa, Harley Kim G., Kogut Katherine, and Eskenazi Brenda. 2017. “Prenatal Residential Proximity to Agricultural Pesticide Use and IQ in 7-Year-Old Children.” Environmental health perspectives 125 (5):057002–057002. doi: 10.1289/EHP504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GE 3rd, Kondakala S, Holdridge J, Lee JH, and Ross MK. 2018. “Inhibition of cholinergic and non-cholinergic targets following subacute exposure to chlorpyrifos in normal and high fat fed male C57BL/6J mice.” Food Chem Toxicol 118:821–829. doi: 10.1016/j.fct.2018.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan BL 2013. “The role of CB1 in immune modulation by cannabinoids.” Pharmacol Ther 137 (3):365–74. doi: 10.1016/j.pharmthera.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Koch D, Lu C, Fisker-Andersen J, Jolley L, and Fenske RA. 2002. “Temporal association of children’s pesticide exposure and agricultural spraying: report of a longitudinal biological monitoring study.” Environ Health Perspect 110 (8):829–33. doi: 10.1289/ehp.02110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou ZY, Zhao CB, and Xiao BG. 2012. “Immunoregulation of experimental autoimmune encephalomyelitis by the selective CB1 receptor antagonist.” J Neurosci Res 90 (1):84–95. doi: 10.1002/jnr.22721. [DOI] [PubMed] [Google Scholar]
- Lu HC, and Mackie K. 2016. “An Introduction to the Endogenous Cannabinoid System.” Biol Psychiatry 79 (7):516–25. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Peng F, Dong M, and Yang H. 2014. “Endocannabinoid 2-arachidonylglycerol protects primary cultured neurons against LPS-induced impairments in rat caudate nucleus.” J Mol Neurosci 54 (1):49–58. doi: 10.1007/s12031-014-0246-2. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Attinà M, Cartoni A, Bari M, and Finazzi-Agrò A. 2001. “Gas chromatography-mass spectrometry analysis of endogenous cannabinoids in healthy and tumoral human brain and human cells in culture.” J Neurochem 76 (2):594–601. doi: 10.1046/j.1471-4159.2001.00092.x. [DOI] [PubMed] [Google Scholar]
- Navarro HA, Basta PV, Seidler FJ, and Slotkin TA. 2001. “Neonatal chlorpyrifos administration elicits deficits in immune function in adulthood: a neural effect?” Brain Res Dev Brain Res 130 (2):249–52. doi: 10.1016/s0165-3806(01)00254-1. [DOI] [PubMed] [Google Scholar]
- Nichols JM, and Kaplan BLF. 2021. “The CB(1) Receptor Differentially Regulates IFN-γ Production In Vitro and in Experimental Autoimmune Encephalomyelitis.” Cannabis Cannabinoid Res 6 (4):300–314. doi: 10.1089/can.2020.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Daniel K., and Casida John E.. 2011. “Activity-based protein profiling of organophosphorus and thiocarbamate pesticides reveals multiple serine hydrolase targets in mouse brain.” Journal of agricultural and food chemistry 59 (7):2808–2815. doi: 10.1021/jf101747r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikashvili D, Shein NA, Mechoulam R, Trembovler V, Kohen R, Alexandrovich A, and Shohami E. 2006. “The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines.” Neurobiol Dis 22 (2):257–64. doi: 10.1016/j.nbd.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Pope C, Karanth S, and Liu J. 2005. “Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action.” Environ Toxicol Pharmacol 19 (3):433–46. doi: 10.1016/j.etap.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Proskocil BJ, Grodzki ACG, Jacoby DB, Lein PJ, and Fryer AD. 2019. “Organophosphorus Pesticides Induce Cytokine Release from Differentiated Human THP1 Cells.” Am J Respir Cell Mol Biol 61 (5):620–630. doi: 10.1165/rcmb.2018-0257OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Caboni P, Liang SN, and Casida JE. 2006. “Monoacylglycerol lipase inhibition by organophosphorus compounds leads to elevation of brain 2-arachidonoylglycerol and the associated hypomotility in mice.” Toxicol Appl Pharmacol 211 (1):78–83. doi: 10.1016/j.taap.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Quistad Gary B., Liang Shannon N., Fisher Karl J., Nomura Daniel K., and Casida John E.. 2006. “Each Lipase Has a Unique Sensitivity Profile for Organophosphorus Inhibitors.” Toxicological Sciences 91 (1):166–172. doi: 10.1093/toxsci/kfj124. [DOI] [PubMed] [Google Scholar]
- Quistad Gary B., Nomura Daniel K., Sparks Susan E., Segall Yoffi, and Casida John E.. 2002. “Cannabinoid CB1 receptor as a target for chlorpyrifos oxon and other organophosphorus pesticides.” Toxicology Letters 135 (1):89–93. doi: 10.1016/S0378-4274(02)00251-5. [DOI] [PubMed] [Google Scholar]
- Ramer Robert, Schwarz Rico, and Hinz Burkhard. 2019. “Modulation of the Endocannabinoid System as a Potential Anticancer Strategy.” Frontiers in pharmacology 10:430–430. doi: 10.3389/fphar.2019.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA, and Peterson BS. 2012. “Brain anomalies in children exposed prenatally to a common organophosphate pesticide.” Proc Natl Acad Sci U S A 109 (20):7871–6. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulation, California Department of Pesticide. 2019. “Chlorpyrifos cancellation.” https://www.cdpr.ca.gov/docs/chlorpyrifos/index.htm.
- Regulation, California Department of Pesticide. 2021. “Pesticide Use Reporting - 2018 Summary Data.” https://www.cdpr.ca.gov/docs/pur/pur18rep/18_pur.htm.
- Robert W. Buntyn, Navatha Alugubelly, Rachel L. Hybart, Afzaal N. Mohammed, Carole A. Nail, Greta C. Parker, Matthew K. Ross, and Russell L. Carr. 2017. “Inhibition of Endocannabinoid-Metabolizing Enzymes in Peripheral Tissues Following Developmental Chlorpyrifos Exposure in Rats.” International Journal of Toxicology 36 (5):395–402. doi: 10.1177/1091581817725272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Del Arco I, Bermudez-Silva FJ, Bilbao A, Cippitelli A, and Navarro M. 2005. “The endocannabinoid system: physiology and pharmacology.” Alcohol Alcohol 40 (1):2–14. doi: 10.1093/alcalc/agh110. [DOI] [PubMed] [Google Scholar]
- Rom S, and Persidsky Y. 2013. “Cannabinoid receptor 2: potential role in immunomodulation and neuroinflammation.” J Neuroimmune Pharmacol 8 (3):608–20. doi: 10.1007/s11481-013-9445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MK, Streit TM, and Herring KL. 2010. “Carboxylesterases: Dual roles in lipid and pesticide metabolism.” J Pestic Sci 35 (3):257–264. doi: 10.1584/jpestics.R10-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckart Perri Zeitz, Kakolewski Kirsten, Bove Frank J., and Kaye Wendy E.. 2004. “Long-term neurobehavioral health effects of methyl parathion exposure in children in Mississippi and Ohio.” Environmental health perspectives 112 (1):46–51. doi: 10.1289/ehp.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette KN, Alugubelly N, Glenn LB, Guo-Ross SX, Parkes MK, Wilson JR, Seay CN, and Carr RL. 2022. “The mechanistic basis for the toxicity difference between juvenile rats and mice following exposure to the agricultural insecticide chlorpyrifos.” Toxicology 480:153317. doi: 10.1016/j.tox.2022.153317. [DOI] [PubMed] [Google Scholar]
- Sido JM, Nagarkatti PS, and Nagarkatti M. 2015. “Role of Endocannabinoid Activation of Peripheral CB1 Receptors in the Regulation of Autoimmune Disease.” Int Rev Immunol 34 (5):403–14. doi: 10.3109/08830185.2014.921165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Parashar A, Singh AK, and Singh R. 2013. “Pre-natal/juvenile chlorpyrifos exposure associated with immunotoxicity in adulthood in Swiss albino mice.” J Immunotoxicol 10 (2):141–9. doi: 10.3109/1547691X.2012.700653. [DOI] [PubMed] [Google Scholar]
- Sugamura Koichi, Sugiyama Seigo, Nozaki Toshimitsu, Matsuzawa Yasushi, Izumiya Yasuhiro, Miyata Keishi, Nakayama Masafumi, Kaikita Koichi, Obata Toru, and Takeya Motohiro. 2009. “Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages.” Circulation 119 (1):28–36. [DOI] [PubMed] [Google Scholar]
- Szafran B, Borazjani A, Lee JH, Ross MK, and Kaplan BL. 2015. “Lipopolysaccharide suppresses carboxylesterase 2g activity and 2-arachidonoylglycerol hydrolysis: A possible mechanism to regulate inflammation.” Prostaglandins Other Lipid Mediat 121 (Pt B):199–206. doi: 10.1016/j.prostaglandins.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafran BN, Borazjani A, Seay CN, Carr RL, Lehner R, Kaplan BLF, and Ross MK. 2021. “Effects of Chlorpyrifos on Serine Hydrolase Activities, Lipid Mediators, and Immune Responses in Lungs of Neonatal and Adult Mice.” Chem Res Toxicol. doi: 10.1021/acs.chemrestox.0c00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafran BN, Lee JH, Borazjani A, Morrison P, Zimmerman G, Andrzejewski KL, Ross MK, and Kaplan BLF. 2018. “Characterization of Endocannabinoid-Metabolizing Enzymes in Human Peripheral Blood Mononuclear Cells under Inflammatory Conditions.” Molecules 23 (12). doi: 10.3390/molecules23123167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafran Brittany N, Borazjani Abdolsamad, Scheaffer Hannah L, Crow J Allen, McBride Ann Marie, Adekanye Oluwabori, Wonnacott Caitlin B, Lehner Richard, Kaplan Barbara LF, and Ross Matthew K. 2022. “Carboxylesterase 1d Inactivation Augments Lung Inflammation in Mice.” ACS Pharmacology & Translational Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaro CM, Smith MN, Workman T, Griffith WC, Thompson B, and Faustman EM. 2018. “Characterization of organophosphate pesticides in urine and home environment dust in an agricultural community.” Biomarkers 23 (2):174–187. doi: 10.1080/1354750X.2017.1395080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Borazjani A, Matthews AT, Mangum LC, Edelmann MJ, and Ross MK. 2013. “Identification of palmitoyl protein thioesterase 1 in human THP1 monocytes and macrophages and characterization of unique biochemical activities for this enzyme.” Biochemistry 52 (43):7559–74. doi: 10.1021/bi401138s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Borazjani A, Hatfield MJ, Edwards CC, Potter PM, and Ross MK. 2010. “Inactivation of lipid glyceryl ester metabolism in human THP1 monocytes/macrophages by activated organophosphorus insecticides: role of carboxylesterases 1 and 2.” Chem Res Toxicol 23 (12):1890–904. doi: 10.1021/tx1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


