Abstract
Pesticides are used extensively in residential settings for lawn maintenance and in homes to control household pests including application directly on pets to deter fleas and ticks. Pesticides are commonly detected in the home environment where people and pets can be subject to chronic exposure. Due to increased interest in using companion animals as sentinels for human environmental health studies, we conducted a comparative pesticide exposure assessment in 30 people and their pet dogs to determine how well silicone wristbands and silicone dog tags can predict urinary pesticide biomarkers of exposure. Using targeted gas chromatography–mass spectrometry analyses, we quantified eight pesticides in silicone samplers and used a suspect screening approach for additional pesticides. Urine samples were analyzed for 15 pesticide metabolite biomarkers. Several pesticides were detected in >70% of silicone samplers including permethrin, N,N-diethyl-meta-toluamide (DEET), and chlorpyrifos. Significant and positive correlations were observed between silicone sampler levels of permethrin and DEET with their corresponding urinary metabolites (rs = 0.50–0.96, p < 0.05) in both species. Significantly higher levels of fipronil were observed in silicone samplers from participants who reported using flea and tick products containing fipronil on their dog. This study suggests that people and their dogs have similar pesticide exposures in a home environment.
Keywords: exposure, wristband, silicone, biomonitoring, pesticide, dog
Graphical Abstract
1. INTRODUCTION
Pesticides, including herbicides, insecticides, and fungicides, constitute a diverse group of chemicals that are used in a variety of applications for pest control in homes, in agricultural practices, and in some medical treatments, to name a few. A United States Environmental Protection Agency (U.S. EPA) report on pesticide usage estimated that globally 5821 million pounds of pesticides was used at the producer level in 2012, with 20 % of that usage attributed to the United States.1 In the United States, the agricultural sector represents 90% of the total pesticide usage,1 with the remaining use attributed to residential applications and consumer products.
According to the U.S. EPA, approximately 88 million U.S. households use some type of pesticide. Pesticides are used in and around households in a wide variety of applications: elimination of weeds from lawns, protection of gardens from pests, and control of insects and rodents from indoors. Additionally, pesticides are applied directly to our bodies and/or our pets’ bodies to help prevent the transmission of vector-borne diseases. Despite the lower overall national share of pesticide use, private homeowners can use up to 10× more pesticide per acre than a typical agricultural application.2 While household exposures include the indoor and immediate outdoor (i.e., lawn and garden) application of pesticides, it is important to note that sources of exposures may include other local pesticide applications such as those from neighbors or shared neighborhood space, and municipal and nearby agricultural lands.
Exposure to pesticides in and around homes contributes to concerns about long-term health effects, particularly in children. Environmental exposures to various pesticides have been linked to many chronic diseases, including various cancers, diabetes, reproductive and neurological diseases, and birth defects.3-10 However, such exposures are not only a human health concern but they also present a veterinary health concern. Estimates suggest that between 38 and 50% of U.S. households have at least one pet dog.11,12 Pesticide exposure has been linked to several diseases in pet dogs including lymphoma,13-16 mammary,17 and bladder cancers.18,19 Pesticides used in lawn care, particularly the herbicide 2,4-D, have been reported to be associated with lymphoma in dogs.13-16 Dogs in India with mammary cancer had higher levels of total pesticides (which include fipronil and permethrin) measured in mammary tissues (β = 4.99).17 Also, two studies have reported that dogs diagnosed with bladder cancer (transitional cell carcinoma) were significantly more likely to experience greater exposure to household insecticides18,19 and lawn herbicides, particularly phenoxy herbicides.19 The value of investigating chronic diseases in dogs, particularly cancer, is increasingly being recognized by researchers to aid in both drug development and therapeutics and also to further our understanding of genomic (e.g., single-nucleotide polymorphisms) and environmental (e.g., exposures) risk factors.
There is considerable potential for including companion animals in long-term environmental health studies to investigate the links between shared environmental exposures and shared chronic diseases. In addition to sharing the domestic environment with their owners, pet dogs can develop chronic diseases that often reflect both histological and clinical aspects of the corresponding human diseases. Furthermore, there is a high degree of conservation of biological mechanisms across evolution in mammals.21-23 A major advantage of studying canines as a model for human health is that canines have a shorter life span, which in turn provides a shorter disease latency period compared to humans. In the current era of “omics” and big data, further research assessing associations between environmental exposures and chronic diseases across species could be invaluable.
Silicone samplers are being used increasingly as passive monitoring devices for assessing multipollutant exposures in a variety of settings including occupational,25-32 vulnerable populations (such as children33-42 and pregnant women43,44), pet cats45 and dogs,24 beehives,46 wild frogs,47 and their habitats.48 Furthermore, previous studies have demonstrated that chemicals measured on wristbands are significantly associated with human and canine exposure biomarkers, reinforcing their utility.24,37,39,43,49,50 Silicone wristbands are a useful tool for investigating personal exposures that occur via inhalation and through dermal routes.51 They are advantageous because they are noninvasive, relatively inexpensive to purchase, and convenient for participants to use as they can be mailed back and forth and do not require a clinical or research visit.
We have previously established a comparative environmental health framework from which these studies can move forward through the application of silicone wristbands as personal passive monitoring devices used on people and their pet dogs in tandem.24 We observed significant correlations between human and dog exposures across multiple classes of chemicals, including organophosphate esters, polybrominated diphenyl ethers, polychlorinated biphenyls, pesticides and phthalates. We also demonstrated that there were stronger correlations between organophosphate ester flame retardants and plasticizers levels in silicone dog tags and their urinary biomarkers in dogs compared to humans.24
This study builds on our previous comparative exposure assessment24 using the same cohort of people and their pet dogs and providing additional measurements of pesticides and new data on urinary biomarkers. Overall, we sought to examine pesticide exposures between people and their pet dogs living in the same household. Furthermore, the secondary aim was to validate the use of silicone wristbands for pesticide exposure by examining correlations between pesticide measurements in the silicone samplers with paired urinary metabolite data. Our hypothesis was that the use of silicone tags in dogs would provide a meaningful measurement of pesticide exposures, similar to what we observed previously for other classes of organic contaminants such as organophosphate esters in humans and dogs.24,49 To our knowledge, these data are the first that have been reported to integrate silicone wristbands and dog tag data with corresponding urinary biomarkers of exposure for any type of pesticide.
2. METHODS
2.1. Study Design.
Details on the study participants are reported in our previous publication.24 In brief, the participants were 30 pairs of people and their household dogs who were asked to wear a silicone personal monitoring device for 5 days throughout all daily activities (July/August, 2018). Humans wore wristbands, and a small piece of silicone band was affixed to the dogs’ collar. Three first morning void urine samples were collected from each human and their dog on days 1, 3, and 5 of the study and then pooled. Owners collected urine samples from their own dog using study-provided materials and stored them in standard urine collection containers. Questionnaire data were collected regarding details on the home and general daily routines of both the participants and their dogs. Owners were specifically asked to report on the current flea and tick products used on their dog. The study protocol was reviewed and approved by the Duke University Institutional Review Board. IACUC approval was not required for this study because all interaction with dogs and sample collection was between the owner and their own pet dog. All participants provided informed written consent to participate. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects’ research.
2.2. Urine Collection and Processing.
All urine samples were collected and pooled as previously described.24 After urine collection, the research participants stored the samples in their home freezers (−20 °C) until they were transferred to the laboratory. The samples remained frozen during transport and were stored at −20 °C up to 4 months prior to pooling and aliquoting for −80 °C long-term storage. The urine specific gravity (SG) was determined in each pooled urine sample using a digital handheld refractometer (Atago UG-α). An aliquot of the pooled urine, previously stored at −80 °C, was shipped overnight on dry ice to the CDC’s Environmental Health Laboratory at the Division of Laboratory Sciences for analysis using two previously published methods.52,53 We analyzed the pooled urine for 15 pesticide biomarkers: one phenoxy acid, three organophosphates, three pyrethroids, six neonicotinoids, and two N,N-diethyl-meta-toluamide (DEET) metabolites. Quality control and quality assurance involved maintaining tolerance limits for operational parameters using traceable calibration materials and using quality control samples in each analytical run to assess performance accuracy and precision. The various urinary metabolites quantified, their full names and acronyms, and their limits of detection (LODs) are reported in Table S1.
2.3. Wristband and Silicone Dog Tag Collection and Processing.
Precleaned personal passive silicone monitoring devices were worn by human and pet dog study participants (n = 30 each) and processed as previously described.24 In brief, the participants were provided precleaned wristbands and silicone dog tags and asked to wear them for five continuous days, after which they were removed, wrapped in precleaned (combusted) aluminum foil, and stored at −20 °C until analysis. Approximately ~1 g segments of each wristband and dog tag were cut, weighed, spiked with isotopically labeled internal standards, and extracted via sonication with 1:1 hexane/dichloromethane. Concentrated sample extracts (~1 mL) were cleaned with 8.0 g of water-deactivated Florisil (Acros Organics Florisil, 100–200 mesh, Fisher Scientific). The samples were then concentrated and spiked with a second set of isotopically labeled standards to measure the recovery of the internal standards. The samples were analyzed using a Q Exactive GC hybrid quadrupole-Orbitrap gas chromatography–mass spectrometry (GC–MS)/MS system (Thermo Scientific, Waltham, MA, USA) operated in the full-scan electron ionization mode. Field blanks (n = 8, four wristbands and four dog tags) and lab-processing blanks (n = 7) were analyzed alongside the participant samples for quality assurance and control. Additional information regarding the accuracy and precision of these methods, including the method detection limits, was previously published.24
2.4. Wristband Suspect Screening.
Mass spectrometry data files were screened against the NIST and Thermo Hi-Res Library mass spectral database to identify other putative pesticides in the samples. Suspect screening was conducted with Thermo’s Deconvolution plugin in Tracefinder. The features were filtered with a signal-to-noise threshold of 5, a total ion chromatography (TIC) threshold of 10,000, an ion overlap window of 95%, and an accurate mass tolerance of 5 ppm. The retention time (RT) alignment window was set to 5 s. Library searches were conducted using a hi-res reverse search approach with a reverse dot product threshold of 500. The peak list that was generated was then manually curated to identify high-quality pesticide matches. The manual curation was meant to identify true positives and remove false positives by considering a range of scoring metrics, including both forward and reverse search metrics. Due to the fact that the NIST spectral library is a low-resolution library while the data generated by the Q-Exactive GC–MS are high-resolution data, there was a potential for false positives. Each feature that had a library match was inspected to confirm that all spectral components had Gaussian peak shapes and that the sample spectrum was not missing any major spectral components when compared to the library match. Five pesticides were tentatively identified using this approach and included flamprop-methyl, promecarb, terbucarb, fipronil, and DEET. To determine if these identifications were correct, we purchased pure authentic analytical standards and compared their responses to the responses in the samples. After comparison, it was clear that flamprop-methyl, promecarb, and terbucarb were false positives, while fipronil and DEET were confirmed as positive identifications. To provide a semiquantitative estimate on the concentrations of fipronil and DEET in these samples, we took the area under the curve of the base peak for each suspect chemical and divided it by the area response of an isotopically labeled standard with the closest RT. Fipronil and DEET were thus evaluated using the area response of d10-chlorpyrifos. The same field blanks and laboratory processing blanks previously mentioned were analyzed alongside participant samples for quality control and quality assurance. Additionally, we conducted a matrix spike experiment to determine the recoveries of DEET and fipronil within our analytical method. DEET had 104% recovery and fipronil had 91% recovery, which are similar to the matrix spike recoveries of all pesticides examined in this study (Table S3).
2.5. National Health and Nutrition Examination Survey Data.
Urinary pesticide metabolite concentrations for the U.S. general population were obtained from the database for chemical exposures from the National Health and Nutrition Examination Survey (NHANES).54-56 The most recent available data were used for comparisons. Data from the survey years 2009–2010 were used for 3,5,6-trichloro-2-pyridinol (TCPY).54 Data from the survey years 2013–2014 were used for 2,4-dichlorophenoxyacetic acid (2,4-D), 3-phenoxybenzoic acid (3-PBA), 4-fluoro-3-phenoxybenzoic acid (4-F-3-PBA), trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (trans-DCCA), para-nitrophenol (PNP), and 2-isopropyl-4-methyl-6-hydroxypyrimidine (IMPY).55 Data from the survey years 2015–2016 were used for DEET metabolites and all neonicotinoids.55,56 Geometric mean (GM) and selected percentiles of urine concentrations reported as μg/L were used for comparison with NHANES data as our results were not adjusted for creatinine.
2.6. Statistical Analysis.
Data were analyzed using JMP PRO Statistical Discovery from SAS software (version 15.1.0, SAS Institute Inc., Cary, NC).57 The concentrations of urinary-metabolites were normalized using SG to account for differences in urine dilution based on previously published methods.58-61 We used the following formula
where [Analyte]normalized is the normalized concentration of the analyte in the sample, [Analyte]measured is the measured analyte concentration in the sample, SG refers to the measured specific gravity of the sample, and SGp is the average SG of the population. As there is variation between the SG across species, the population average used was species-specific. Analyses of urinary metabolites and associations with wristband or questionnaire data were carried out with both raw metabolite concentrations and SG-corrected values. In some samples, a signal interference on the mass spectrometer prevented an accurate detection of certain pesticide metabolites (n = 4), which were excluded from statistical analyses, as indicated in Table 1.
Table 1.
Descriptive Statistics of SG-Corrected Pesticide Biomarker Urinary Concentrations (μg/L) Measured in Human and Dog Samplesa
| Human |
Dog |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker | abbr. | LOD (μg/L) |
DF | n | GM | GM raw |
Med | 95th | 95th raw | DF | n | GM | GM raw |
Med | 95th | 95th raw |
rs |
| Organophosphates | |||||||||||||||||
| 3,5,6-trichloro-2-pyridinol | TCPY | 0.1 | 100 | 30 | 2.25 | 1.54 | 2.29 | 5.89 | 4.68 | 100 | 30 | 1.72 | 1.61 | 1.30 | 9.92 | 11.50 | 0.22 |
| para-nitrophenol | PNP | 0.1 | 100 | 30 | 1.76 | 1.21 | 1.54 | 8.63 | 7.40 | 100 | 30 | 2.91 | 2.72 | 2.86 | 6.52 | 7.59 | −0.14 |
| 2-isopropyl-4-methyl-6-hydroxypyrimidine | IMPY | 0.1 | 30 | 30 | N/A | N/A | 0.10 | 0.72 | 0.59 | 0 | 30 | N/A | N/A | N/A | N/A | N/A | N/A |
| Phenoxy acid | |||||||||||||||||
| 2,4-dichorophenoxyacetic acid | 2,4-D | 0.15 | 93 | 30 | 0.54 | 0.37 | 0.55 | 1.87 | 1.65 | 97 | 30 | 0.85 | 0.8 | 0.96 | 3.94 | 5.13 | −0.23 |
| Pyrethroids | |||||||||||||||||
| 3-ohenixybenzoic acid | 3-PBA | 0.1 | 100 | 30 | 3.39 | 2.32 | 3.47 | 21.22 | 13.49 | 20 | 30 | N/A | N/A | 0.05 | 1.53 | 0.88 | N/A |
| 4-fluoro-3-phenoxybenzoic acid | 4-F-3-PBA | 0.1 | 33 | 30 | N/A | N/A | 0.10 | 0.98 | 0.87 | 17 | 30 | N/A | N/A | 0.05 | 1.24 | 1.37 | N/A |
| trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid | trans-DCCA | 0.6 | 60 | 30 | 2.37 | 1.62 | 3.04 | 22.16 | 13.74 | 73 | 30 | 2.82 | 2.64 | 1.47 | 368.90 | 278.85 | 0.38* |
| Neonicotinoids | |||||||||||||||||
| 5-hydroxy imidacloprid‡ | 5-OH-IMI | 0.2 | 38 | 16‡ | 0.75 | 0.75 | 0.32 | 38.36 | 16.10 | 50 | 26‡ | 1.84 | 1.70 | 0.65 | 583.87 | 673.20 | N/A |
| acetamiprid | ACET | 0.2 | 3 | 30 | N/A | N/A | N/A | N/A | N/A | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| acetamiprid-N-desmethyl‡ | ACET-Ndes | 0.4 | 48 | 29‡ | 0.83 | 0.35 | 0.76 | 5.63 | 3.49 | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| clothianidin | CLOTH | 0.4 | 0 | N/A | N/A | N/A | N/A | N/A | N/A | 3 | 30 | N/A | N/A | N/A | N/A | N/A | N/A |
| imidacloprid‡ | IMI | 0.3 | 46 | 24‡ | 0.62 | 0.27 | 0.64 | 1.64 | 0.68 | 42 | 24‡ | 0.91 | 0.91 | 0.32 | 126.21 | 147.70 | N/A |
| thiacloprid | THIAC | 0.2 | 0 | N/A | N/A | N/A | N/A | N/A | N/A | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| DEET | |||||||||||||||||
| 3-(diethylcarbamoyl)ben zoic acid | DCBA | 0.03 | 100 | 30 | 51.06 | 21.73 | 43.16 | 21972.10 | 13874.50 | 100 | 30 | 16.82 | 15.77 | 16.21 | 255.87 | 205.00 | 0.82** |
| 3-(ethylcarbamoyl)benzoic acid‡ | ECBA | 0.2 | 100 | 29‡ | 20.98 | 8.96 | 20.87 | 8389.59 | 5287.80 | 83 | 30 | 4.67 | 4.38 | 5.28 | 98.33 | 71.60 | 0.87** |
p < 0.05
p < 0.0001; N/A: not applicable; LOD values are not SG-corrected. Detection frequency (DF) (%); geometric mean (GM); raw (i.e., uncorrected for specific gravity) values are also provided for comparison with NHANES data.
Some samples had mass spectrometry interferences that prevented accurate quantification of some biomarkers; these samples were excluded from statistical analyses. The “n” column indicates the number of urine samples for which data was collected and which there was no mass spectrometry interference. rs = the Spearman correlation coefficient estimated from human and dog urinary biomarkers. Analytes with a DF < 50% were not analyzed and entered as N/A.
Concentrations below the LOD (<LOD) were imputed a value equal to the LOD divided by two prior to adjusting for SG according to previously published best practices.62 Based on the threshold of our previous study and others, we only conducted Spearman’s correlations and reported GMs for analytes detected in >50% of the samples from each species.24,34,43
Wristband and urinary biomarkers concentrations were not normally distributed (p < 0.05, Shapiro–Wilk Test), and nonparametric statistical methods were used. The Wilcoxon-Rank sum test was used when comparing data from the questionnaires with silicone pesticide measurements, and Spearman’s correlations were used to assess the relationships between species and between matrices. The statistical significance was set at α = 0.05.
3. RESULTS AND DISCUSSION
3.1. Study Population.
Information on the demographics of the study population and data collected from the questionnaires are detailed by Wise et al.24 At the time of sample collection, the participants’ (n = 30) median age was 36.5 years (SD = 10.4), and the majority were women (63%). The dog population (n = 30) represented a variety of pure and mixed breed dogs with the majority being neutered males (57%) and a median age of 6 years (SD = 2.8). Eighty-three percent of owner–dog pairs were reported to be living in single detached homes. Known use of herbicides/pesticides on their property (outdoors) was reported by 47% of participants, whereas 30% of people reported known use inside their homes.
Eighty-three percent of the study participants reported routinely applying a flea and tick product (containing an insecticide) to their participating dog (Figure S1A). Only two dogs reportedly received no flea and tick preventatives containing pesticides within a year prior to sampling. Of the 28 dogs that had pesticides administered, their most recent applications were administered either orally (n = 12; 43%) or dermally (n = 16; 57%), with the latter through topical ointments or collars (Figure S1B). Thirty-two percent of the participating dogs had flea and tick preventatives administered during the 5 day sampling period (Figure S1C). According to the survey data, the most common active-ingredient pesticides in flea and tick preventatives used within the prior year were fipronil (n = 9) and imidacloprid (n = 9) (Figure S1D), both of which are typically used in topical applications. Two owners reportedly used flea and tick prevention products with permethrin listed as an active ingredient. All but one dog spent 13 h or more inside their home on a typical day, whereas only 47% of people spent 13 h or greater inside their home.
3.2. Pesticide Measurements.
3.2.1. Urine.
All urine samples (n = 60) were analyzed for 15 pesticide urinary metabolites. The summary statistics are shown in Table 1. In both humans and dogs, 7 of the 15 target pesticide metabolites were detected in more than 50% of the samples. A list of full chemical names and associated abbreviations is given in Table S1. The most abundant urinary pesticide metabolites detected in both humans and dogs were 3-(ethylcarbamoyl)benzoic acid (ECBA) and 3-(diethylcarbamoyl)benzoic acid (DCBA) (both metabolites of the insect repellent DEET). In humans, the next most abundant pesticide metabolite detected was 3-PBA, a nonspecific metabolite of several pyrethroid insecticides. In dogs, the second-most abundant pesticide metabolite was PNP, an organophosphate pesticide metabolite typically associated with exposure to parathion or methyl parathion.
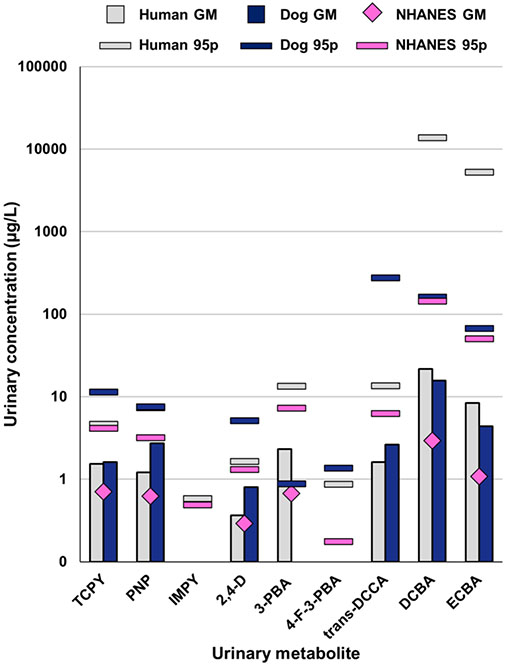
Urinary pesticide biomarker concentrations in human specimens were generally higher in our study compared to previous measurements from the U.S. adult general population (Figures 1 and S2). Both human and dog urine in this study had similar concentrations of pesticides with the exception of 2,4-D and PNP. Dogs had approximately 2 times higher concentrations of 2,4-D and PNP (GM = 0.8 and 2.7 μg/L, respectively) compared to humans (GM = 0.4 and 1.2 μg/L, respectively). These differences were statistically significant for both 2,4-D (p < 0.05) and PNP (p < 0.01), and these differences were observed in both the raw values and the SG-adjusted values (Figure S3). The GM concentration of 2,4-D in human urine measured in this study was similar to the GM from the general adult population for NHANES (0.3 μg/L).55 By contrast, the GM and 95th percentile concentrations of PNP and TCPY, metabolites of parathion and chlorpyrifos, respectively, were approximately 2 times higher in our study population compared to NHANES. The biggest differences in biomarker concentration were observed for DCBA and ECBA (GM = 21.7 and 8.4 μg/L, respectively) compared to the NHANES adult general population (GM = 2.9 and 1.1 μg/L, respectively).55
Figure 1.
Comparison of pesticide biomarker concentrations in urine samples from humans and dogs in this study compared with NHANES adult data. Comparisons are shown for the GM and 95p based on raw uncorrected concentrations of frequently detected metabolites measured in human and dog urine in our study compared to the corresponding and most recent biomonitoring data in NHANES for the general population. NHANES data are from adults 20 years and older in 2013/2014 except for DBCA and ECBA, which had data available from 2015/2016, and TCPY, which only had data from 2009/2010 limited to adults age 20–59. Comparisons were not included for analytes with a high proportion of samples with concentrations <LOD. IMPY was not detected in any dog urine samples. 95p for PNP are overlapping data points for human (7.40 μg/L) and dog (7.59 μg/L) samples from our study.
Neonicotinoid urinary biomarkers were detected in fewer than 50% of both human and dog urine samples. Neonicotinoids are commonly used insecticides around the globe for agriculture, landscaping, and flea and tick prevention products. Considering the relatively low detection frequency (DF) in our study and NHANES, we only compared the 95th percentiles (95p) for these biomarkers. However, considering our low sample size (n = 30), these values should be interpreted cautiously and are intended for relative comparison of the few samples with quantifiable data available. The samples from our study had much higher concentrations of imidacloprid metabolites, particularly the dog urine samples (Figure S2). Higher SG-corrected concentrations of urinary imidacloprid biomarkers, imidacloprid and 5-hydroxy imidacloprid, were found in dog urine (95p = 126.2 and 583.9 μg/L, respectively) compared to human urine (95p = 1.0 and 23.9 μg/L, respectively) in our study. The low DF of imidacloprid biomarkers resulted in insufficient power to statistically analyze any relationship between the use of imidacloprid flea products and urinary biomarker levels. While diet is considered to be a major route of exposure, flea and tick preventatives and household dust have been suggested as a factor for higher levels observed in young children.55
Seven study participants reported that the current flea and tick preventatives used on their participating dog contained imidacloprid as an active ingredient (Table S2). Urinary biomarkers of imidacloprid were detected in 16 of the 30 dog urine samples. When detected, higher concentrations of the urinary biomarkers were observed in dogs with a recent history of exposure to flea and tick products containing imidacloprid (n = 9, imidacloprid 3.8–158.3 μg/L, 5-hydroxy-imidacloprid 24.4–782.3 μg/L) compared to dogs that had not used these products (n = 7, imidacloprid 0.4–25.2 μg/L, 5-hydroxy imidacloprid 1.1–147.7 μg/L). The variations in biomarker concentrations observed may be a function of the time to sampling following the most recent product application, the dosage applied to the dog, or other potential sources of imidacloprid. Comparisons with the urine from the owners of those dogs are difficult to make because some samples showed a mass spectrometry interference for 5-hydroxy imidacloprid (14 human and 4 dog samples) or imidacloprid (6 samples for both humans and dogs), but the concentrations observed in dog urine were higher than in human urine (Table S2). Additionally, it is possible that the urinary levels of imidacloprid biomarkers in dogs may be the result of a combination of systemic absorption following topical cutaneous application and oral exposures through fur licking behaviors.
To assess the relationship between dog and human urinary biomarkers, Spearman correlation coefficients were calculated (Table 1). Two organophosphate pesticide metabolites, TCPY and PNP, were detected in all urine samples (human and dog); however, no statistically significant correlations were found between dog and human urinary organophosphate pesticide metabolites. The concentrations of the chlorpyrifos metabolite TCPY in human (GM = 1.54 μg/L) and dog (GM = 1.61 μg/L) urine were ~2× the levels reported in the general population (GM = 0.71 μg/L, as reported in the NHANES,54 despite the fact that chlorpyrifos use has declined since 2000, largely due to EPA restrictions and actions toward phasing-out approved usage.1,63 The fact that these two metabolites were not correlated in human and dog urine suggests that they have different primary sources of exposure, which seems likely to be dietary.64,65
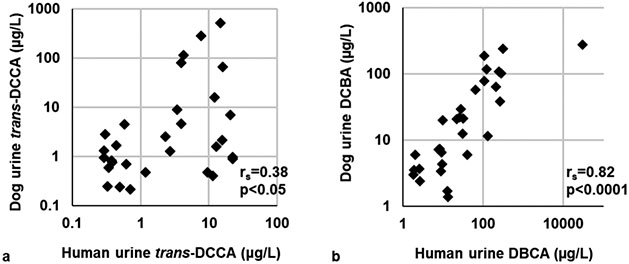
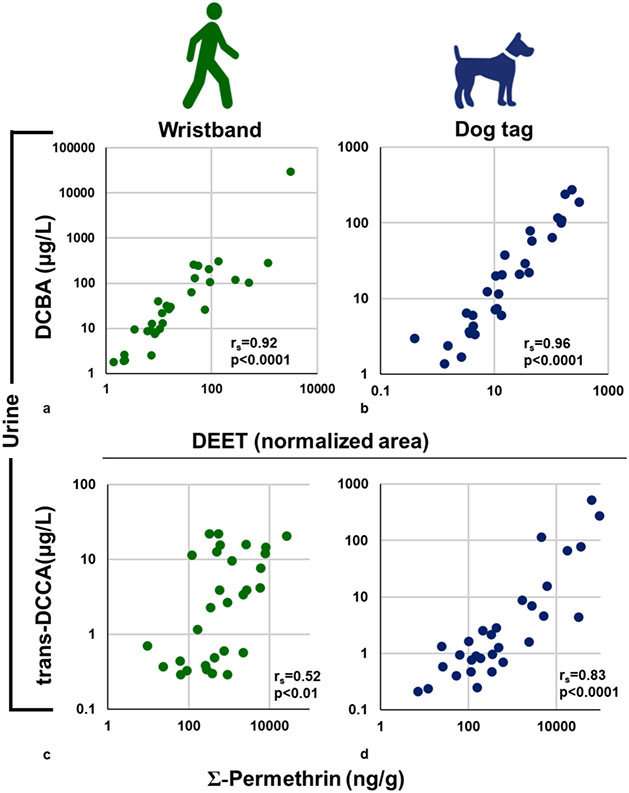
The only phenoxy acid measured in urine was 2,4-D, an herbicide used ubiquitously by homeowners and in agriculture, which was detected in nearly all urine samples (93 and 97% in human and dog urine, respectively). There was no statistically significant correlation between 2,4-D concentrations in human and dog urine. Three pyrethroid metabolites were detected in urine samples. trans-DCCA was detected in 60 and 73% of human and dog urine samples, respectively. Statistically significant correlations between human and dog urine were observed for trans-DCCA (rs = 0.38, p < 0.05, Figure 2a). 3-PBA was detected in 100% of human urine samples but only in 30% of dog urine samples. We detected two DEET metabolites, ECBA and DCBA, in almost all samples. Humans had higher concentrations (~2× times higher) of DEET biomarkers compared to their canine companions, although it was not statistically higher. Significant correlations between human and dog urinary ECBA and DCBA concentrations were observed (0.87 and 0.82, respectively, p < 0.0001; Figure 2b).
Figure 2.
Scatterplots and Spearman’s correlations (rs) between human and dog SG-corrected urinary concentrations of (a) trans-DCCA and (b) DCBA, which are metabolites of permethrin, cypermethrin, and cyfluthrin and DEET, respectively.
3.2.2. Previously Reported Wristband Pesticide Data.
In our previous publication, we reported on concentrations of eight pesticides measured in these silicone samplers,24 including azoxystrobin, chlorpyrifos, cypermethrin, lindane, cis-chlordane, trans-chlordane, cis-permethrin, and trans-permethrin. Several of these chemicals were detected in ≥50% of human wristbands (seven out of nine) and dog tags (seven out of nine). We demonstrated that pesticides could be quantified on the silicone samplers and that the concentrations were significantly correlated among dogs and their owners for all pesticides that had a high DF (>50%). Permethrin isomers were the most abundant pesticides in our previously published wristband data,24 and there was little difference in the GMs between species; however, the maximum concentrations were consistently higher in dogs.
There are several studies that have used silicone wristbands to detect exposure to a variety of pesticides in humans.24,41,42,44,66-70 Many of these may not be representative of our study population because they were focused on evaluating exposures in study participants that were either children or lived in agricultural areas.41,42,68,69 A recent study by our group used wristbands to measure exposure in an adult population with an overlapping geographic range of North Carolina counties over 7 days and also found that the most abundant pesticides were permethrin isomers.70
3.2.3. Suspect Pesticide Screening in Silicone Samplers.
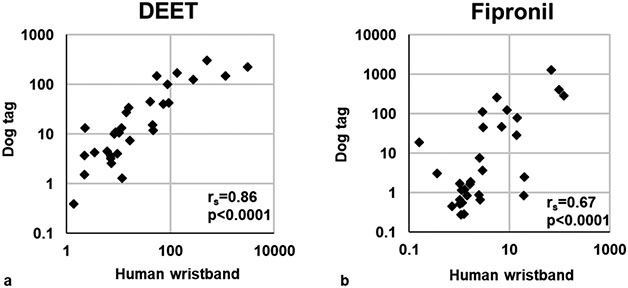
Two pesticides were positively identified using suspect screening with a high degree of confidence: DEET and fipronil. Both pesticides were detected in 100% of silicone wristbands and dog tags. The semiquantitative levels were compared between species to assess their correlations. DEET and fipronil both had statistically significant correlations between human wristbands and dog tags (rs = 0.86 and 0.67, respectively; p < 0.0001; Figure 3).
Figure 3.
Scatterplots and Spearman’s correlation coefficients (rs) for pesticides identified through suspect screening on human wristbands and silicone dog tags: (a) DEET, (b) fipronil. Data are semiquantitative and are based on area responses normalized to the nearest internal standard (by RT).
Five other studies used wristbands to assess exposure to the chemicals detected in our suspect screening.42,44,66-68 Detection of DEET in previous wristband studies is highly variable, ranging from detection in almost all wristbands worn by adults in Belgium (93%)67 and pregnant adult women in New Hampshire (83–95%)44 to detection frequencies below 50% in Peru66 and female Latina adolescents in California.68 None of these previous studies compared DEET wristbands levels with corresponding DEET urinary metabolites. DEET is one of the most widely used insect repellents in the United States.71 The variability in DEET detection on wristbands may be a result of seasonal or personal application choices at the time of sample collection (considering our samples were collected during summer) or in sampling and analysis differences among studies.
Fipronil is a broad-spectrum insecticide that is commonly used in flea and tick preventatives or treatments in addition to other household products, turf care (i.e., parks and golf courses), and structural treatments for termites. Fipronil has been infrequently detected (0–33%) on wristbands from other studies.42,66-68 Although two other wristband studies had higher detection frequencies for the transformation product fipronil sulfide (50 and 87%) but less than 50% DF for fipronil sulfone;42,68 neither of these breakdown products were investigated in our study. Previous studies have observed associations between the levels of fipronil in house dust and the presence of cats and dogs in the home.72,73 It is therefore not surprising that the nature of our study design would result in widespread detection (100%) of fipronil.
3.3. Relationship between Wristbands, Urine, and Questionnaire Results.
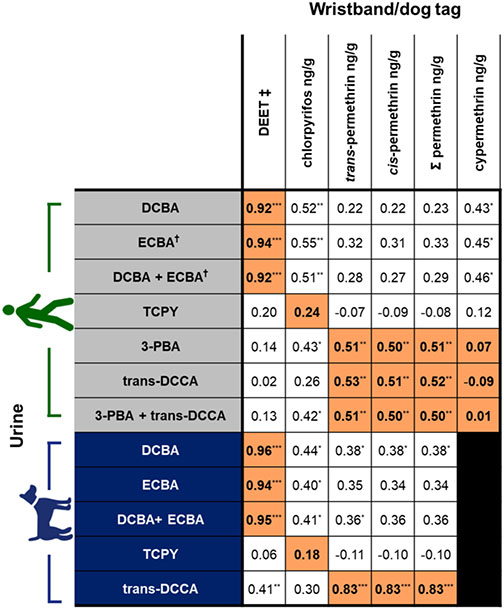
Correlations between pesticide levels on the silicone samplers and urinary biomarker concentrations were assessed for analytes commonly detected in urine (Figure 4 and Table S1). Not all pesticides measured on the silicone samplers have associated urinary biomarkers. In dogs, the concentrations of parent chemicals measured on silicone dog tags were significantly and positively correlated with their corresponding urinary metabolites for permethrin/trans-DCCA, DEET/DCBA, and DEET/ECBA (rs = 0.83–0.96, p < 0.0001; Figures 4 and 5). In humans, the concentrations of parent chemicals measured on silicone wristbands were significantly and positively correlated with their corresponding urinary metabolites for permethrin/trans-DCCA, permethrin/3-PBA, DEET/DCBA, and DEET/ECBA (rs = 0.51–0.94, p < 0.01; Figures 4 and 5). The above findings suggest that silicone samplers worn over a 5 day period are able to capture integrated exposures for some pesticides from the ambient environment.
Figure 4.
Spearman’s correlations for pesticides measured on silicone wristbands/dog tags with urinary biomarkers of exposure in each species. Analyses were restricted to chemicals with data available for known chemical/biomarker relationships. Direct relationships with parent chemicals (DEET, chlorpyrifos, total and individual isomers of permethrin, and cypermethrin) and their metabolites are highlighted in orange with bold text. Correlations were conducted using SG-corrected concentrations (μg/L). †Correlations coefficients for ECBA in human urine were done using n = 29. Detection frequencies were <50% for cypermethrin on silicone dog tags. 3-PBA was only detected in 20% of dog urine samples and therefore excluded. ‡DEET values are semiquantitative and are based on area responses normalized to the nearest internal standard (retention time). *p < 0.05; **p < 0.01; ***p < 0.0001.
Figure 5.
Scatterplots representing the relationships between pesticides detected on silicone wristband/dog tags and their urinary metabolites. DEET and total permethrin (sum of cis- and trans-isomers) on (a,c) wristbands and dog tags (b,d) were significantly correlated with the SG-corrected urinary concentrations of their biomarkers of exposure, DCBA and trans-DCCA, respectively. Values for DEET are semiquantitative.
Chlorpyrifos is the only organophosphate pesticide measured on silicone wristbands that had an associated urinary biomarker. In neither dogs nor humans was a significant correlation between chlorpyrifos measured on the silicone samplers and the urinary metabolite (TCPY) observed (Figure 4). This lack of correlation may be due to the abundance of chlorpyrifos exposure occurring through dietary exposures, which are not captured by the silicone wristbands. This is consistent with a study that evaluated exposures in people whose pet dogs wore chlorpyrifos-containing flea collars.74 In that study, no significant correlations were observed between chlorpyrifos residues on cotton t-shirts and gloves and urinary TCPY concentrations in adults or children. Furthermore, restrictions implemented on the residential uses of chlorpyrifos, including no longer use in pet collars, have likely reduced indoor inhalation or dermal exposure to chlorpyrifos.63,75,76 Dietary exposures to pesticide residues on food products, such as produce and grains, are likely the main contributors to urinary TCPY concentrations in the general population (i.e., in nonoccupational exposures).76-78 Interestingly, many of the same grains and produce are incorporated into commercial dog food and thus may be a source of exposure to chlorpyrifos.
Biomarkers of pyrethroids (such as permethrin and cypermethrin) are generally nonspecific with multiple distinct parent chemicals metabolized and excreted as common metabolites, such as 3-PBA and trans-DCCA.79 Total permethrin and each permethrin isomer were found to have significant and positive correlations between silicone sampler levels and trans-DCCA in both humans and dogs (rs = 0.52–0.83, p < 0.01; Figure 4). Human urinary 3-PBA concentrations were also significantly correlated with permethrin on wristbands (rs = 0.51, p < 0.01; Figure 4). Cypermethrin levels on human wristbands were not correlated with urinary 3-PBA (rs = 0.07, p = 0.73; Figure 4) or urinary trans-DCCA (rs = −0.09, p = 0.63; Figure 4). The detection frequencies of cypermethrin on the silicone dog tags and 3-PBA in dog urine were below 50% and therefore were not evaluated further. The relatively low DF of 3-PBA in dog urine may also suggest a different pattern of metabolism or excretion of synthetic pyrethroids in people compared to dogs.80 Alternatively, the poor correlations with urinary 3-PBA in dogs may suggest that other permethrin-based pesticide exposures occur in dogs that were not evaluated in our study. Coexposure to other chemicals, such as the organophosphate insecticide methyl parathion, can potentially result in an altered metabolism of pyrethroids due to the inhibition or competition of carboxylesterases in high-exposure scenarios.81-82 Considering that urinary concentrations of PNP, a metabolite of methyl parathion (and others), were twice as high in dog urine compared to human urine, it may reflect species differences in pyrethroid metabolism. Similarly, dietary exposures to pyrethroids may have a significant impact on the presence of TCPY, the chlorpyrifos urinary biomarker measured. Additionally, dog self-grooming behaviors, such as fur licking, may impact oral exposures in dogs which may lead to an increase in variability in the correlation between silicone dog tags and urinary metabolites.
Significant and strong positive correlations were observed between DEET levels in the silicone samplers and urinary biomarkers ECBA and DCBA in both humans and dogs (rs = 0.92–0.96, p < 0.0001; Figures 4 and 5). One person in our study reported self-applying an insect repellent containing DEET once during the study period. That individual had nearly a 100-fold higher concentration of ECBA and DCBA compared to the next highest measured concentration among the human urine samples analyzed here, as well as the highest semi-quantitative value for DEET on wristbands. This result suggests that pesticide exposures are highly individualized and could be influenced by the products in our environment and personal use, which can be detected in the wristbands, as was previously noted by Aerts et al.67 This much higher concentration of DEET metabolites with reported use was also evident in the samples from the dog living in the same home, which reinforces the notion that the choices we make also impact others who cannot make decisions about their own exposures (i.e., children and pets).
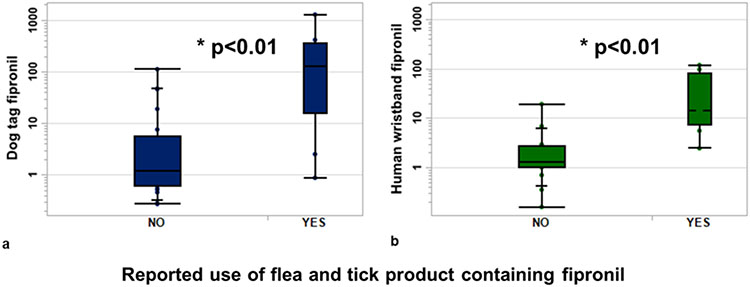
Based on the questionnaire data collected, we identified a statistically significant factor that was associated with participants’ exposure to fipronil. Participants who reported using flea and tick preventatives containing fipronil as an active ingredient on their dog (n = 9) had significantly higher fipronil levels on their silicone dog tags (~100×) and wristbands (~10×) compared to those who used other products or no flea and tick preventatives (Figure 6). In a previous study, 10 participant wristbands had detectable levels of fipronil, of which 3 participants reportedly used spot-on flea treatments and flea collars that contained fipronil.67 Together, these data suggest that the use of fipronil in veterinary products directly impacts human exposure. Flea and tick preventatives containing fipronil are recommended to be applied monthly on each pet and have the potential to be a significant source of the total mass contribution of fipronil exposure when the application frequency and quantity are considered. These types of products should be investigated more thoroughly for their environmental health impacts considering the continuous routine exposure and repeated application, especially in homes with multiple pets.
Figure 6.
Relative amount of fipronil measured on (a) silicone dog tags and (b) human wristbands based on the reported use of a fipronil containing flea and tick medication. Groupings are based on whether people reported using a flea and tick product containing fipronil on their dog (YES, n = 9) and those that did not (NO, n = 21).
Previous studies have suggested that human pesticide exposure was impacted by living in a household with pets that were treated with flea and tick prevention products, and the children in those households had significantly higher urinary pesticide metabolite concentrations than other children and adults.74,83-85 These elevated exposures could be from direct contact with the treated pet and/or secondary exposure sources (i.e., contact with contaminated house dust) within the household the treated pet resides. To the best of our knowledge, there have been no studies that assess chronic exposure occurring from repeated applications of pesticides for flea and tick prevention used throughout a dog’s life span or any additive exposure resulting from multiple pets all using the same preventatives. However, one recent study demonstrated that serum fipronil sulfone (a fipronil metabolite) placentally transfers to fetuses and was associated with adverse infant human health outcomes including thyroid function.86 Further studies assessing health outcomes related to long-term repeated exposures to veterinary flea and tick preventatives are warranted.
The results of the present study support our hypothesis and demonstrate that silicone wristbands and dog tags can detect and assess exposures to some pesticides. Importantly, a majority of the correlations between the pesticide levels in the silicone samplers and urinary concentrations of pesticide metabolites were stronger in dogs than in humans, similar to what we previously observed with organophosphate esters.24 Dogs normally spend more time inside the home compared to their owners, and it seems likely that their exposure profiles are consistent over time. These data further demonstrate that silicone passive samplers have the potential to be valuable tools for the cross-species assessment of exposures, showing high correlations between the exposures that people and their pet dogs share in their everyday environment. However, careful considerations need to be accounted for, particularly the potential differences in metabolism and excretion of certain chemicals that may mediate the causal pathway for disease among different species. A role for cross-species analyses that link environmental exposures with chronic diseases could, and should, be recognized. Silicone samplers could effectively be used as passive monitors to capture and document these exposures over time and investigate relationships with diseases. These results, in combination with our recent study investigating uptake rates of chemicals on wristbands, demonstrate that silicone samplers can be used to assess average integrated exposure over time (in this case over a week of exposure).87 Studies such as these could aid in identifying important health risks that could be mitigated in order to reduce the burden of these chronic diseases in both people and dogs.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank all the human and canine participants in this study. The authors would like to thank Sharon Zhang, Sam Baker, and Dickson Wambua for technical support. This study was supported by the NCSU Cancer Genomics Fund (M.B., NCSU), the Duke Cancer Institute (H.M.S., Duke), the Consortium for Canine Comparative Oncology (M.B., NCSU; H.M.S., Duke), and the V Foundation (M.B., NCSU; H.M.S., Duke), and the access to analytical support was provided by a Major Research Instrumentation grant from the National Science Foundation (CBET-1828257; H.M.S., Duke).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.1c06819.
Analytical details and questionnaire results (PDF)
The authors declare no competing financial interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Contributor Information
Catherine F. Wise, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States
Stephanie C. Hammel, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States; Present Address: The National Research Centre for the Working Environment, Lersø Park Allé 105, DK-2100 Copenhagen, Denmark
Nicholas J. Herkert, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States
Maria Ospina, Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, Georgia 30341, United States.
Antonia M. Calafat, Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, Georgia 30341, United States
Matthew Breen, Duke Cancer Institute, Durham, North Carolina 27710, United States; Department of Molecular Biomedical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, North Carolina 27607, United States; Comparative Medicine Institute and Center for Human Health and the Environment, North Carolina State University, Raleigh, North Carolina 27607, United States.
Heather M. Stapleton, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States; Duke Cancer Institute, Durham, North Carolina 27710, United States
REFERENCES
- (1).U.S. Environmental Protection Agency (EPA). Pesticide Industry Sales and Usage 2008–2012 Market Estimates; U.S. Environmental Protection Agency: Washington, DC, USA, 2017. https://www.epa.gov/sites/production/files/2017-01/documents/pesticides-industry-sales-usage-2016_0.pdf. [Google Scholar]
- (2).Md Meftaul I; Venkateswarlu K; Dharmarajan R; Annamalai P; Megharaj M Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ 2020, 711, 134612. [DOI] [PubMed] [Google Scholar]
- (3).Cox S; Niskar AS; Narayan KMV; Marcus M Prevalence of self-reported diabetes and exposure to organochlorine pesticides among Mexican Americans: Hispanic health and nutrition examination survey, 1982–1984. Environ. Health Perspect 2007, 115, 1747–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Harley KG; Huen K; Aguilar Schall R; Holland NT; Bradman A; Barr DB; Eskenazi B Association of organophosphate pesticide exposure and paraoxonase with birth outcome in mexican-american women. PLoS One 2011, 6, No. e23923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Koutros S; Lynch CF; Ma X; Lee WJ; Hoppin JA; Christensen CH; Andreotti G; Freeman LB; Rusiecki JA; Hou L; Sandler DP Heterocyclic aromatic amine pesticide use and human cancer risk: results from the U.S. Agricultural Health Study. Int. J. Cancer 2009, 124, 1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Montgomery MP; Kamel F; Saldana TM; Alavanja MCR; Sandler DP Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study. Am. J. Epidemiol 1993, 167, 1235–1246l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Mostafalou S; Abdollahi M Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol 2013, 268, 157–177. [DOI] [PubMed] [Google Scholar]
- (8).Ntzani EE; Chondrogiorgi C; Ntritsos G; Evangelou E; Tzoulaki I Literature review on epidemiological studies linking exposure to pesticides and health effects. EFSA Support. Publ 2013, 10, 497E. [Google Scholar]
- (9).Pardo LA; Beane Freeman LE; Lerro CC; Andreotti G; Hofmann JN; Parks CG; Sandler DP; Lubin JH; Blair A; Koutros S Pesticide exposure and risk of aggressive prostate cancer among private pesticide applicators. Environ. Health 2020, 19, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).von Ehrenstein OS; Ling C; Cui X; Cockburn M; Park AS; Yu F; Wu J; Ritz B Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: population based case-control study. BMJ 2019, 364, l962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).American Pet Products Association (APPA). 2019–2020 American Pet Products Association National Pet Owners Survey: Greenwich, CT, 2020. https://www.americanpetproducts.org. [Google Scholar]
- (12).American Veterinary Medical Association (AVMA). 2017–2018 Edition AVMA Pet Ownership and Demographics Sourcebook; American Veterinary Medical Association. 2018; ISBN: 978–1-882691-53-1 (PDF). [Google Scholar]
- (13).Schofield I; Stevens KB; Pittaway C; O’Neill DG; Fecht D; Dobson JM; Brodbelt DC Geographic distribution and environmental risk factors of lymphoma in dogs under primary-care in the UK. J. Small Anim. Pract 2019, 60, 746–754. [DOI] [PubMed] [Google Scholar]
- (14).Hayes HM; Tarone RE; Cantor KP; Jessen CR; McCurnin DM; Richardson RC Case-control study of canine malignant lymphoma: positive association with dog owner’s use of 2, 4-dichlorophenoxyacetic acid herbicides. J. Natl. Cancer Inst 1991, 83, 1226–1231. [DOI] [PubMed] [Google Scholar]
- (15).Takashima-Uebelhoer BB; Barber LG; Zagarins SE; Procter-Gray E; Gollenberg AL; Moore AS; Bertone-Johnson ER Household chemical exposures and the risk of canine malignant lymphoma, a model for human non-Hodgkin’s lymphoma. Environ. Res 2012, 112, 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Reynolds PM; Reif JS; Ramsdell HS; Tessari JD Canine exposure to herbicide-treated lawns and urinary excretion of 2, 4-dichlorophenoxyacetic acid. Cancer Epidemiol., Biomarkers Prev 1994, 3, 233–237. [PubMed] [Google Scholar]
- (17).Gautam S; Sood NK; Gupta K; Joshi C; Gill KK; Kaur R; Chauhan I Bioaccumulation of pesticide contaminants in tissue matrices of dogs suffering from malignant canine mammary tumors in Punjab, India. Heliyon 2020, 6, No. e05274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Luethcke KR; Ekena J; Chun R; Trepanier LA Glutathione S-transferase theta genotypes and environmental exposures in the risk of canine transitional cell carcinoma. J. Vet. Intern. Med 2019, 33, 1414–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Glickman LT; Raghavan M; Knapp DW; Bonney PL; Dawson MH Herbicide exposure and the risk of transitional cell carcinoma of the urinary bladder in Scottish Terriers. J. Am. Vet. Med. Assoc 2004, 224, 1290–1297. [DOI] [PubMed] [Google Scholar]
- (20).Shearin AL; Ostrander EA Leading the way: canine models of genomics and disease. Dis. Models Mech 2010, 3, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Berthelot C; Villar D; Horvath JE; Odom DT; Flicek P Complexity and conservation of regulatory landscapes underlie evolutionary resilience of mammalian gene expression. Nat. Ecol. Evol 2018, 2, 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Carroll SB Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 2008, 134, 25–36. [DOI] [PubMed] [Google Scholar]
- (23).Slodkowicz G; Goldman N Integrated structural and evolutionary analysis reveals common mechanisms underlying adaptive evolution in mammals. PNAS 2020, 117, 5977–5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Wise CF; Hammel SC; Herkert N; Ma J; Motsinger-Reif A; Stapleton HM; Breen M Comparative exposure assessment using silicone passive samplers indicates that domestic dogs are sentinels to support human health research. Environ. Sci. Technol 2020, 54, 7409–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Baum JL; Bakali U; Killawala C; Santiago KM; Dikici E; Kobetz EN; Solle NS; Deo S; Bachas L; Daunert S Evaluation of silicone-based wristbands as passive sampling systems using PAHs as an exposure proxy for carcinogen monitoring in firefighters: Evidence from the firefighter cancer initiative. Ecotoxicol. Environ. Saf 2020, 205, 111100. [DOI] [PubMed] [Google Scholar]
- (26).Caban-Martinez AJ; Louzado-Feliciano P; Santiago KM; Baum J; Solle NS; Rivera G; Miric M; Perez-Then E; Kobetz-Kerman EN; Daunert S Objective measurement of carcinogens among Dominican Republic firefighters using silicone-based wristbands. J. Occup. Environ. Med 2020, 62, e611–e615. [DOI] [PubMed] [Google Scholar]
- (27).Craig JA; Ceballos DM; Fruh V; Petropoulos ZE; Allen JG; Calafat AM; Ospina M; Stapleton HM; Hammel S; Gray R; Webster TF Exposure of nail salon workers to phthalates, di (2-ethylhexyl) terephthalate, and organophosphate esters: a pilot study. Environ. Sci. Technol 2019, 53, 14630–14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Hardos JE; Rubenstein M; Pfahler S; Sleight T Cholinesterase inhibition and exposure to organophosphate esters in aircraft maintenance workers. Aerosp. Med. Hum. Perform 2020, 91, 710–714. [DOI] [PubMed] [Google Scholar]
- (29).Nguyen LV; Gravel S; Labrèche F; Bakhiyi B; Verner MA; Zayed J; Jantunen LM; Arrandale VH; Diamond ML Can silicone passive samplers be used for measuring exposure of e-waste workers to flame retardants? Environ. Sci. Technol 2020, 54, 15277–15286. [DOI] [PubMed] [Google Scholar]
- (30).O’Connell SG; Kincl LD; Anderson KA Silicone wristbands as personal passive samplers. Environ. Sci. Technol 2014, 48, 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Poutasse CM; Poston WS; Jahnke SA; Haddock CK; Tidwell LG; Hoffman PD; Anderson KA Discovery of firefighter chemical exposures using military-style silicone dog tags. Environ. Int 2020, 142, 105818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Wang Y; Peris A; Rifat MR; Ahmed SI; Aich N; Nguyen LV; Urík J; Eljarrat E; Vrana B; Jantunen LM; Diamond ML Measuring exposure of e-waste dismantlers in Dhaka Bangladesh to organophosphate esters and halogenated flame retardants using silicone wristbands and T-shirts. Sci. Total Environ 2020, 720, 137480. [DOI] [PubMed] [Google Scholar]
- (33).Arcury TA; Chen H; Quandt SA; Talton JW; Anderson KA; Scott RP; Jensen A; Laurienti PJ Pesticide exposure among Latinx children: Comparison of children in rural, farmworker and urban, non-farmworker communities. Sci. Total Environ 2021, 763, 144233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Gibson EA; Stapleton HM; Calero L; Holmes D; Burke K; Martinez R; Cortes B; Nematollahi A; Evans D; Anderson KA; Herbstman JB Differential exposure to organophosphate flame retardants in mother-child pairs. Chemosphere 2019, 219, 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Hammel SC; Hoffman K; Phillips AL; Levasseur JL; Lorenzo AM; Webster TF; Stapleton HM Comparing the use of silicone wristbands, hand wipes, and dust to evaluate children’s exposure to flame retardants and plasticizers. Environ. Sci. Technol 2020, 54, 4484–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Kile ML; Scott RP; O’Connell SG; Lipscomb S; MacDonald M; McClelland M; Anderson KA Using silicone wristbands to evaluate preschool children’s exposure to flame retardants. Environ. Res 2016, 147, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Levasseur JL; Hammel SC; Hoffman K; Phillips AL; Zhang S; Ye X; Calafat AM; Webster TF; Stapleton HM Young children’s exposure to phenols in the home: Associations between house dust, hand wipes, silicone wristbands, and urinary biomarkers. Environ. Int 2021, 147, 106317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Lipscomb ST; McClelland MM; MacDonald M; Cardenas A; Anderson KA; Kile ML Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ. Health 2017, 16, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Quintana PJ; Hoh E; Dodder NG; Matt GE; Zakarian JM; Anderson KA; Akins B; Chu L; Hovell MF Nicotine levels in silicone wristband samplers worn by children exposed to secondhand smoke and electronic cigarette vapor are highly correlated with child’s urinary cotinine. J. Exposure Sci. Environ. Epidemiol 2019, 29, 733–741. [DOI] [PubMed] [Google Scholar]
- (40).Quintana PJ; Lopez-Galvez N; Dodder NG; Hoh E; Matt GE; Zakarian JM; Vyas M; Chu L; Akins B; Padilla S; Anderson KA Nicotine, cotinine, and tobacco-specific nitrosamines measured in children’s silicone wristbands in relation to secondhand smoke and E-cigarette vapor exposure. Nicotine Tob. Res 2021, 23, 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Travis SC; Aga DS; Queirolo EI; Olson JR; Daleiro M; Kordas K Catching flame retardants and pesticides in silicone wristbands: Evidence of exposure to current and legacy pollutants in Uruguayan children. Sci. Total Environ 2020, 740, 140136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Vidi PA; Anderson KA; Chen H; Anderson R; Salvador-Moreno N; Mora DC; Poutasse C; Laurienti PJ; Daniel SS; Arcury TA Personal samplers of bioavailable pesticides integrated with a hair follicle assay of DNA damage to assess environmental exposures and their associated risks in children. Mutat. Res 2017, 822, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Dixon HM; Scott RP; Holmes D; Calero L; Kincl LD; Waters KM; Camann DE; Calafat AM; Herbstman JB; Anderson KA Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods. Anal. Bioanal. Chem 2018, 410, 3059–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Doherty BT; Pearce JL; Anderson KA; Karagas MR; Romano ME Assessment of multipollutant exposures during pregnancy using silicone wristbands. Public Health Front. 2020, 8, 547239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Poutasse CM; Herbstman JB; Peterson ME; Gordon J; Soboroff PH; Holmes D; Gonzalez D; Tidwell LG; Anderson KA Silicone pet tags associate tris (1, 3-dichloro-2-isopropyl) phosphate exposures with feline hyperthyroidism. Environ. Sci. Technol 2019, 53, 9203–9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Bullock EJ; Schafsnitz AM; Wang CH; Broadrup RL; Macherone A; Mayack C; White HK Silicone wristbands as passive samplers in honey bee hives. Vet. Sci 2020, 7, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Yaw TJ; Swanson JE; Pierce CL; Muths E; Smalling KL; Vandever MW; Zaffarano BA Placement of intracoelomic radiotransmitters and silicone passive sampling devices in northern leopard frogs (Lithobates pipiens). J. Herpetol Med. Surg 2017, 27, 111–115. [Google Scholar]
- (48).Swanson JE; Muths E; Pierce CL; Dinsmore SJ; Vandever MW; Hladik ML; Smalling KL Exploring the amphibian exposome in an agricultural landscape using telemetry and passive sampling. Sci. Rep 2018, 8, 10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Hammel SC; Hoffman K; Webster TF; Anderson KA; Stapleton HM Measuring personal exposure to organophosphate flame retardants using silicone wristbands and hand wipes. Environ. Sci. Technol 2016, 50, 4483–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Hammel SC; Phillips AL; Hoffman K; Stapleton HM Evaluating the use of silicone wristbands to measure personal exposure to brominated flame retardants. Environ. Sci. Technol 2018, 52, 11875–11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Wang S; Romanak KA; Stubbings WA; Arrandale VH; Hendryx M; Diamond ML; Salamova A; Venier M Silicone wristbands integrate dermal and inhalation exposures to semi-volatile organic compounds (SVOCs). Environ. Int 2019, 132, 105104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Baker SE; Serafim AB; Morales-Agudelo P; Vidal M; Calafat AM; Ospina M Quantification of DEET and neonicotinoid pesticide biomarkers in human urine by online solid-phase extraction high-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem 2019, 411, 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Davis MD; Wade EL; Restrepo PR; Roman-Esteva W; Bravo R; Kuklenyik P; Calafat AM Semi-automated solid phase extraction method for the mass spectrometric quantification of 12 specific metabolites of organophosphorus pesticides, synthetic pyrethroids, and select herbicides in human urine. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci 2013, 929, 18–26. [DOI] [PubMed] [Google Scholar]
- (54).Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, Volume One, March 2018, DLS, NCEH, CDC https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Mar2018.pdf.
- (55).Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, Volume Two, March 2021, DLS, NCEH, CDC https://www.cdc.gov/exposurereport/.
- (56).Ospina M; Wong LY; Baker SE; Bishop Serafim A; Morales-Agudelo P; Calafat AM Exposure to Neonicotinoid Insecticides in the U.S. general population: Data from the 2015–2016 national health and nutrition examination survey. Environ. Res 2019, 176, 108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).JMP®. Version 15.1.0; SAS Institute Inc.: Cary, NC, 1989–2019. [Google Scholar]
- (58).Cooper EM; Covaci A; Van Nuijs ALN; Webster TF; Stapleton HM Analysis of the flame retardant metabolites bis (1, 3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem 2011, 401, 2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Meeker JD; Yang T; Ye X; Calafat AM; Hauser R Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ. Health Perspect 2011, 119, 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Sorahan T; Pang D; Esmen N; Sadhra S Urinary concentrations of toxic substances: an assessment of alternative approaches to adjusting for specific gravity. J. Occup. Environ. Hyg 2008, 5, 721–723. [DOI] [PubMed] [Google Scholar]
- (61).Xia Y; Wong LY; Bunker BC; Bernert JT Comparison of creatinine and specific gravity for hydration corrections on measurement of the tobacco-specific nitrosamine 4- (methylnitrosamino) -1- (3-pyridyl) -1-butanol (NNAL) in urine. J. Clin. Lab. Anal 2014, 28, 353–363l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Pleil J; Risby T; Herbig J Breath biomonitoring in national security assessment, forensic THC testing, biomedical technology and quality assurance applications: report from PittCon 2016. J. Breath Res 2016, 10, 029001. [DOI] [PubMed] [Google Scholar]
- (63).U.S. Environmental Protection Agency (EPA). Proposed Interim Registration Review Decision for Chlorpyrifos, EPA-HQ-OPP-2008-0850; U.S. Environmental Protection Agency: Washington, DC, USA, 2020. https://www.epa.gov/sites/production/files/2020-12/documents/chlorpyrifos_pid_signed_120320.pdf. [Google Scholar]
- (64).Griffin P; Mason H; Heywood K; Cocker J Oral and dermal absorption of chlorpyrifos: a human volunteer study. Occup. Environ. Med 1999, 56, 10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Nolan RJ; Rick DL; Freshour NL; Saunders JH Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol. Appl. Pharmacol 1984, 73, 8–15. [DOI] [PubMed] [Google Scholar]
- (66).Bergmann AJ; North PE; Vasquez L; Bello H; Ruiz MDCG; Anderson KA Multi-class chemical exposure in rural Peru using silicone wristbands. J. Exposure Sci. Environ. Epidemiol 2017 2017, 27, 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Aerts R; Joly L; Szternfeld P; Tsilikas K; De Cremer K; Castelain P; Aerts JM; Van Orshoven J; Somers B; Hendrickx M; Andjelkovic M Silicone wristband passive samplers yield highly individualized pesticide residue exposure profiles. Environ. Sci. Technol 2018, 52, 298–307. [DOI] [PubMed] [Google Scholar]
- (68).Harley KG; Parra KL; Camacho J; Bradman A; Nolan JE; Lessard C; Anderson KA; Poutasse CM; Scott RP; Lazaro G; Cardoso E Determinants of pesticide concentrations in silicone wristbands worn by Latina adolescent girls in a California farmworker community: The COSECHA youth participatory action study. Sci. Total Environ 2019, 652, 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Donald CE; Scott RP; Blaustein KL; Halbleib ML; Sarr M; Jepson PC; Anderson KA Silicone wristbands detect individuals’ pesticide exposures in West Africa. R. Soc. Open Sci 2016, 3, 160433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Kassotis CD; Herkert NJ; Hammel SC; Hoffman K; Xia Q; Kullman SW; Sosa JA; Stapleton HM Thyroid receptor antagonism of chemicals extracted from personal silicone wristbands within a papillary thyroid cancer pilot study. Environ. Sci. Technol 2020, 54, 15296–15312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).U.S. Environmental Protection Agency (EPA). DEET (N,N-Diethyl-meta-toluamide) Interim Registration Review Decision, EPA-HQ-OPP-2012-0162; U.S. Environmental Protection Agency: Washington, DC, USA, 2014. https://www.regulations.gov/document/EPA-HQ-OPP-2012-0162-0012. [Google Scholar]
- (72).Mahler BJ; Van Metre PC; Wilson JT; Musgrove M; Zaugg SD; Burkhardt MR Fipronil and its degradates in indoor and outdoor dust. Environ. Sci. Technol 2009, 43, 5665–5670. [DOI] [PubMed] [Google Scholar]
- (73).Testa C; Salis S; Rubattu N; Roncada P; Miniero R; Brambilla G Occurrence of Fipronil in residential house dust in the presence and absence of pets: a hint for a comprehensive toxicological assessment. J. Environ. Sci. Health, Part B 2019, 54, 441–448. [DOI] [PubMed] [Google Scholar]
- (74).Chambers JE; Boone JS; Davis MK; Moran JE; Tyler JW Assessing transferable residues from intermittent exposure to flea control collars containing the organophosphate insecticide chlorpyrifos. J. Exposure Sci. Environ. Epidemiol 2007, 17, 656–6661. [DOI] [PubMed] [Google Scholar]
- (75).Eaton DL; Daroff RB; Autrup H; Bridges J; Buffler P; Costa LG; Coyle J; McKhann G; Mobley WC; Nadel L; Neubert D Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol 2008, 38, 1–125. [DOI] [PubMed] [Google Scholar]
- (76).U.S. Food and Drug Administration (FDA). Pesticide Residue Monitoring Report and Data for Fiscal Year 2018 Pesticide Report; U.S. Food and Drug Administration. Washington D.C.: USA, 2018. https://www.fda.gov/food/chemicals-metals-pesticides-food/pesticides. [Google Scholar]
- (77).Lu C; Toepel K; Irish R; Fenske RA; Barr DB; Bravo R Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ. Health Perspect 2006, 114, 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).U.S, Department of Agriculture (USDA). Pesticide Data Program Annual Summary, Calendar Year 2019. U.S. Department of Agriculture: Washington D.C. Agricultural Marketing Service, Pesticide Data Program, 2019. https://www.ams.usda.gov/reports/pdp-annual-summary-reports. [Google Scholar]
- (79).Sudakin DL Pyrethroid insecticides: advances and challenges in biomonitoring. Clin. Toxicol 2006, 44, 31–37. [DOI] [PubMed] [Google Scholar]
- (80).Kaneko H; Izumi T; Matsuo M; Miyamoto J Metabolism of fenvalerate in dogs. J. Pestic. Sci 1984, 9, 269–274. [Google Scholar]
- (81).Leng G; Lewalter J; Röhrig B; Idel H The influence of individual susceptibility in pyrethroid exposure. Toxicol. Lett 1999, 107, 123–130. [DOI] [PubMed] [Google Scholar]
- (82).Ray DE; Ray D; Forshaw PJ Pyrethroid insecticides: poisoning syndromes, synergies, and therapy. J. Toxicol. Clin. Toxicol 2000, 38, 95–101. [DOI] [PubMed] [Google Scholar]
- (83).Cochran RC; Yu L; Krieger RI; Ross JH Postapplication fipronil exposure following use on pets. J. Toxicol. Environ. Health, Part A 2015, 78, 1217–1226. [DOI] [PubMed] [Google Scholar]
- (84).Driver JH; Ross JH; Holden LR; Selim S; Sharp JK; Carlson D; Nouvel L Cyphenothrin flea and tick squeeze-on for dogs: evaluation of potential health risks based on the results of observational biological monitoring. J. Toxicol. Environ. Health, Part A 2015, 78, 1105–1121. [DOI] [PubMed] [Google Scholar]
- (85).Dyk MB; Chen Z; Mosadeghi S; Vega H; Krieger R Pilot biomonitoring of adults and children following use of chlorpyrifos shampoo and flea collars on dogs. J. Environ. Sci. Health, Part B 2011, 46, 97–104. [DOI] [PubMed] [Google Scholar]
- (86).Kim YA; Yoon YS; Kim HS; Jeon SJ; Cole E; Lee J; Kho Y; Cho YH Distribution of fipronil in humans, and adverse health outcomes of in utero fipronil sulfone exposure in newborns. Int. J. Hyg. Environ. Health 2019, 222, 524–532. [DOI] [PubMed] [Google Scholar]
- (87).Hoffman K; Levasseur JL; Zhang S; Hay D; Herkert NJ; Stapleton HM Monitoring human exposure to organophosphate esters: comparing silicone wristbands with spot urine samples as predictors of internal dose. Environ. Sci. Technol. Lett 2021, 8, 805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.