Abstract
Sulbactam-durlobactam is a pathogen-targeted β-lactam/β-lactamase inhibitor combination in late-stage development for the treatment of Acinetobacter infections, including those caused by multidrug-resistant strains. Durlobactam is a member of the diazabicyclooctane class of β-lactamase inhibitors with broad-spectrum serine β-lactamase activity. Sulbactam is a first-generation, narrow-spectrum β-lactamase inhibitor that also has intrinsic antibacterial activity against Acinetobacter spp. due to its ability to inhibit penicillin-binding proteins 1 and 3. The clinical utility of sulbactam for the treatment of contemporary Acinetobacter infections has been eroded over the last decades due to its susceptibility to cleavage by numerous β-lactamases present in this species. However, when combined with durlobactam, the activity of sulbactam is restored against this problematic pathogen. The following summary describes what is known about the molecular drivers of activity and resistance as well as results from surveillance and in vivo efficacy studies for this novel combination.
Keywords: Acinetobacter, sulbactam, durlobactam, β-lactamase inhibitor, diazabicyclooctane
Sulbactam was designed and synthesized by Pfizer in the late 1970s with the objective of inhibiting β-lactamases when given in combination with ampicillin [1]. This penicillanic acid sulfone–based β-lactamase inhibitor, with a limited inhibition spectrum toward certain class A β-lactamases (eg, SHV and CTX-M), was found to lower minimum inhibitory concentrations (MICs) of ampicillin when tested in combination against gram-positive and gram-negative pathogens [1]. By the early 1980s, the intrinsic antimicrobial activity of sulbactam against Acinetobacter species was first observed and confirmed later against contemporary clinical isolates of Acinetobacter species [2,3]. Although sulbactam alone was not available for clinical use, the ampicillin-sulbactam combination was (and is still) used in the treatment of infections due to Acinetobacter species [4–7]. In initial studies using membrane preparations from Acinetobacter species, sulbactam was found to inhibit penicillin-binding protein 2 (PBP2), which was suggested to be its primary mechanism of intrinsic activity toward Acinetobacter species [8,9]. However, a subsequent study conducted with purified PBPs from Acinetobacter species revealed that the half maximal inhibitory concentration for sulbactam was 55 µM and 4 µM for PBP1a and PBP3, respectively; thus, these PBPs are likely targets for sulbactam [10]. Moreover, the inhibition of PBP3, and PBP1 to a lesser extent, by sulbactam was further confirmed in another study by the determination of in vitro acylation rates as well as microscopy that revealed the classical filamentation morphology observed due to PBP3 inhibition of Acinetobacter species when treated with sulbactam [11]. Thus, PBP3 is likely the main target for sulbactam in Acinetobacter species.
However, resistance to β-lactam-sulbactam combinations in Acinetobacter species, especially multidrug- and carbapenem-resistant strains, has increased [12–15]; in one study from Detroit, Michigan, resistance increased from 10% to 60% from 2003 to 2008 [16]. Resistance has been linked to increased expression of TEM-1, ADC-30, and metallo-β-lactamases in Acinetobacter species [11,17–19]. A more in-depth analysis of spontaneous resistance in Acinetobacter species was conducted by Penwell et al, who found that frequency of resistance to sulbactam is low (1.39 × 10−9 to 4.15 × 10−10) and is typically associated with mutations in the gene that encodes PBP3 [11]. Additional sulbactam resistance mechanisms identified included those involved in cell wall metabolism or stress responses [11]. Resistance to sulbactam appears to confer a fitness cost as revealed by in vitro growth rates [11]. The ability of sulbactam to be hydrolyzed by different β-lactamases was also assessed and class A, C, and D serine β-lactamases (eg, TEM-1, KPC-2, ADC-7, OXA-23, OXA-24) as well as class B metallo-β-lactamases (eg, New Delhi metallo-β-lactamase [NDM]–1) were able to turn over sulbactam with varying degrees of activity; of the enzymes tested, only SHV-5 was potently inactivated by sulbactam (ie, kinact/KI >100 000 M−1s−1) [19]. Increased resistance to approved β-lactam–sulbactam combinations as well as other anti-Acinetobacter agents has led to increased mortality and the need for novel treatment strategies [20,21]. Because the main mechanism for resistance to sulbactam is the production of β-lactamases, combining sulbactam with a potent β-lactamase inhibitor that covers common class A, C, and D β-lactamases produced by Acinetobacter species seemed necessary to preserve its clinical effectiveness.
DURLOBACTAM IS A DIAZABICYCLOOCTANE β-LACTAMASE INHIBITOR WITH BROAD-SPECTRUM INHIBITION OF SERINE β-LACTAMASES AND PBPs
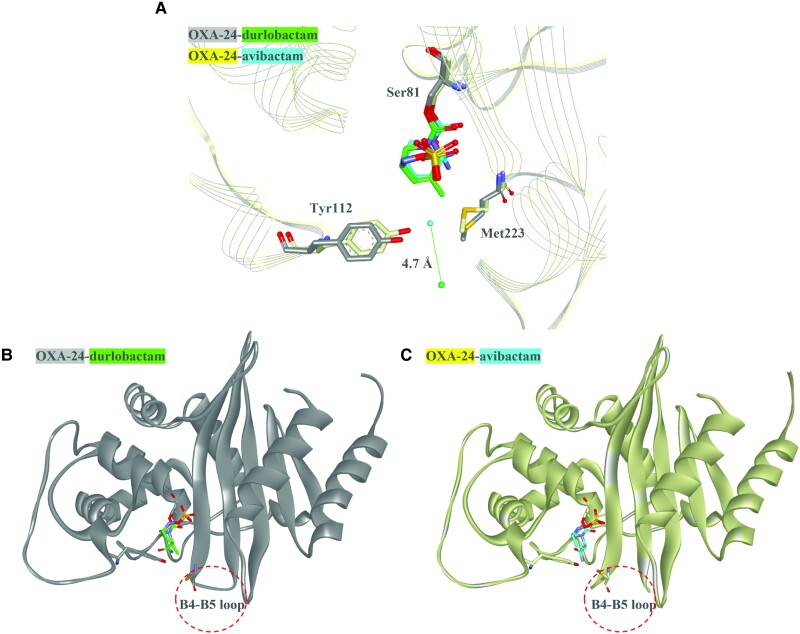
Commercially available β-lactamase inhibitors (eg, avibactam, relebactam, vaborbactam) partnered with different β-lactams demonstrate activity versus class A and C β-lactamases in vitro [22]. Moreover, they are effective antimicrobials against Enterobacterales and Pseudomonas aeruginosa with class A and C β-lactamases, but not against most/all class D β-lactamases or Acinetobacter species. Thus, durlobactam (formerly ETX2514), a novel diazabicyclooctane (DBO) β-lactamase inhibitor, was rationally designed and synthesized by Entasis Therapeutics to maintain potent class A and C inactivation; however, in addition, using structure–activity relationships, durlobactam's inhibition profile was expanded to include class D β-lactamases (eg, OXA-23, OXA-24, and OXA-58) often produced by Acinetobacter species (Table 1) [23,24]. The expanded profile of durlobactam was achieved by modifying the compound for size and polarity, which also enhanced penetration into the bacterial cell and was subsequently found to transverse OmpA in Acinetobacter species [23,26]. Also, the addition of the double bond enhanced the reactivity of durlobactam [23]. Crystallography and molecular modeling of class D β-lactamases with durlobactam and other durlobactam-like DBOs, as well as another similar β-lactamase inhibitor, avibactam, which lacks the double bond and methyl side chain, were performed. These studies revealed that durlobactam and durlobactam-like compounds did not disrupt the hydrophobic bridge (Met 223:Tyr112) and blocked solvent from accessing the covalent bond with the nucleophilic serine present in class D β-lactamases, thus likely leading to the improved potency and decreased deacylation, respectively (Figure 1A ) [23]. By evaluating the structures published in 2 other studies, the loop between B4 and B5 β-strand of OXA-24 is not resolved in the OXA-24–avibactam structure (Protein Data Bank [PDB]: 4WM9) compared to the OXA-24–durlobactam structure (PDB: 6MPQ), suggesting that the loop is flexible in the former structure; this flexibility may slow the acylation of avibactam compared to durlobactam (Figure 1B and 1C ) [24,27]. A quantum mechanics/molecular modeling study comparing avibactam and durlobactam versus OXA-24 further revealed that protonation states of Lys84 and Lys218 are crucial for the rapid chemical reactivity of durlobactam over that of avibactam [28]. Durlobactam rapidly inactivates class A, C, and D β-lactamases and is slow to recyclize (ie, reform active inhibitor), with koff rates (ie, how quickly durlobactam dissociates from the enzyme) being the lowest for class D enzymes (Table 1) [24,25]. Minor hydrolysis of durlobactam was observed with KPC-2 after a 2-hour incubation; however, these conditions were not considered physiologically relevant [25]. Moreover, durlobactam was also found to be an inhibitor of PBPs, particularly PBP2 (Table 1) [23]. Thus, sulbactam-durlobactam not only inactivates PBP3 and PBP2, respectively, but also inhibits class A, C, and D β-lactamases found in Acinetobacter species. The multiple targets of sulbactam-durlobactam likely contribute to its antimicrobial potency as described below.
Table 1.
Inhibitory Kinetic Parameters for Durlobactam Against Representative β-lactamases and Penicillin-Binding Proteins
| β-lactamase/PBP | k 2/K (M−1s−1) | k off (s−1) |
|---|---|---|
| TEM-1 (class A) | 1.4 ± 0.6 × 107 | 1.4 ± 0.2 × 10−3 |
| KPC-2 (class A) | 9.3 ± 0.6 × 105 | 1.0 ± 0.1 × 10−3 |
| ADC-7 (class C) | 1.0 ± 0.1 × 106 | 8.0 ± 0.1 × 10−4 |
| OXA-24 (class D) | 9.0 ± 0.2 × 103 | 1.7 ± 0.1 × 10−5 |
| Acinetobacter baumannii PBP1a | 18 ± 0.6 × 101 | Not determined |
| A. baumannii PBP2 | 1.8 ± 0.6 × 103 | Not determined |
| A. baumannii PBP3 | 3.37 ± 0.06 | Not determined |
Source: [23–25]. The k2/K value represents the acylation rate, or how quickly durlobactam gets bound to a β-lactamase or PBP; the higher the number, the faster the reaction. The koff value indicates how quickly durlobactam dissociates from the β-lactamase; the lower the number, the slower durlobactam comes off the β-lactamase.
Abbreviation: PBP, penicillin-binding protein.
Figure 1.
Co-crystal structures of durlobactam or avibactam and OXA-24. A, The crystal structures of OXA-24 (gray) with durlobactam (green) (Protein Data Bank [PDB]: 6MPQ) and OXA-24 (yellow) with avibactam (cyan) (PDB: 4WM9) show that the water molecule in the OXA-24–durlobactam structure (green ball) is 4.7Å away from the hydrophobic bridge formed by Tyr112 and Met223 compared to the OXA-24–avibactam structure (cyanball). This difference is likely due to the methyl side chain present on durlobactam that is lacking on avibactam. B, The crystal structure of OXA-24 (gray) with durlobactam (green) (PDB: 6MPQ) reveals that the B4-B5 loop is resolved in the structure. C, The crystal structure of OXA-24 (yellow) with avibactam (cyan) (PDB: 4WM9) lacks resolution of the B4–B5 loop, suggesting that this region is flexible.
FREQUENCY AND MECHANISMS OF RESISTANCE TO SULBACTAM-DURLOBACTAM
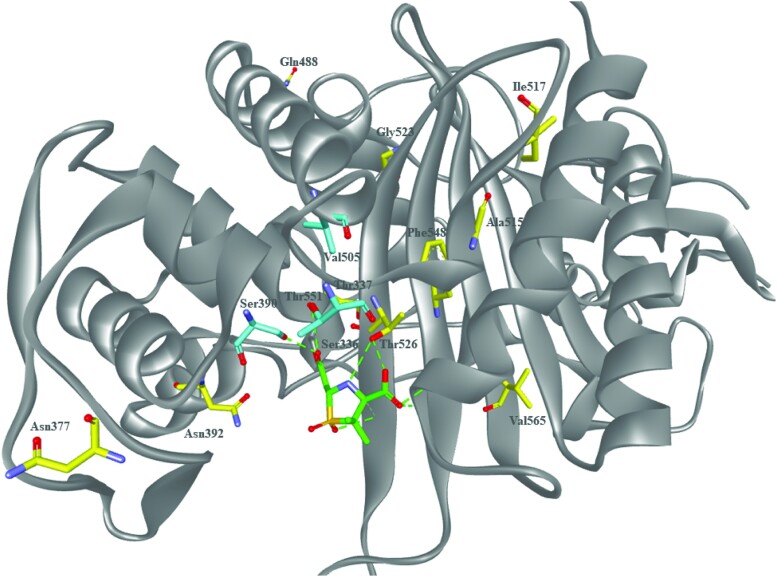
Sulbactam-durlobactam was evaluated for its ability to lead to the in vitro emergence of resistance in clinical isolates of Acinetobacter species [29]. The frequency of resistance was determined to be 7.6 × 10−10 to <9.0 × 10−10 at 4 × MIC [29]. Stable mutants were whole genome sequenced to identify the mechanisms leading to sulbactam-durlobactam resistance. Most of the mutations mapped to ftsI, the gene that encodes PBP3, the target for sulbactam [29]. The corresponding amino acid substitutions identified, S390T, V505L, and T511A were near the active site serine (Ser336) (Figure 2), and the S390T variant was found to be approximately 140-fold less inhibited by sulbactam compared to wild-type PBP3 [29]. Mutations in tRNA synthetases, aspS and gltX were also identified in sulbactam-durlobactam–nonsusceptible Acinetobacter isolates. Mutations in these genes have been linked to induction of the stringent response, which is a cellular stress response that renders PBP2 dispensable [29]. In a separate study of 72 well-characterized A. baumannii isolates from the Walter Reed Army Medical Center, 4 isolates were found to have elevated sulbactam-durlobactam MICs (≥8 µg/mL) [24]. These isolates with elevated sulbactam-durlobactam MIC values encode either the A326V or S1010R substitutions in AdeJ, an efflux pump membrane transporter subunit, or for the H370Y or A578T amino acid substitutions in PBP3; however, these PBP3 residues sit further away from the active site and their impact on the ability of sulbactam to inhibit PBP3 has yet to be defined (Figure 2) [24]. In addition, results from another study suggested that durlobactam could be a substrate for select efflux pumps within Acinetobacter species as efflux pump knockout strains (ΔadeB, ΔadeJ, and ΔadeB/ΔadeJ) were more susceptible to sulbactam-durlobactam, but not sulbactam alone [24]. Overall, several studies have shown that in vitro spontaneous resistance to sulbactam-durlobactam is low. Notably, some strains that were resistant had a compensatory loss in fitness in vitro [29].
Figure 2.
Molecular model of sulbactam bound to Acinetobacter baumannii penicillin-binding protein 3 (PBP3). A molecular model of the acyl-enzyme complex of PBP3 (Protein Data Bank: 3UE3) (gray) with sulbactam (green) bound showing residues that were found to acquire substitutions during frequency of resistance determinations (cyan) as well as those amino acid substitutions identified in PBP3 from clinical isolates of Acinetobacter species (yellow) that had elevated sulbactam-durlobactam minimum inhibitory concentrations. The amino acids Ser390 and Thr526 are within hydrogen bonding distance of sulbactam.
IN VITRO ACTIVITY OF SULBACTAM-DURLOBACTAM AGAINST CONTEMPORARY ISOLATES OF THE ACINETOBACTER BAUMANNII–CALCOACETICUS COMPLEX
Several reports describe the in vitro antibacterial activity of sulbactam-durlobactam and comparator antibacterial agents against contemporary clinical isolates of the Acinetobacter baumannii–calcoaceticus complex (ABC) [30–35]. ABC is a closely related group of bacterial species that are often hard to differentiate from one another and are comprised of several species including A. baumannii, A. calcoaceticus, A. nosocomialis, and A. pittii [36]. The largest study to date evaluated 5032 ABC isolates collected between 2016 and 2021 from community- and hospital-associated infections in 33 countries across Asia/South Pacific (13.6%), Europe (42.1%), Latin America (12.6%), the Middle East (Israel only) (1.7%), and North America (United States only) (29.9%) [35]. Since A. baumannii is the ABC species most often associated with nosocomial outbreaks and high levels of antibacterial resistance, 80.2% of the isolates selected for this study were A. baumannii, followed by 12.7% A. pittii, 5.9% A. nosocomialis, and 1.1% A. calcoaceticus. Isolates were collected from 5 common infection sources: respiratory tract (54.3%), bloodstream (20.2%), urinary tract (16.5%), skin and soft tissue (4.5%), and intra-abdominal (4.3%). These distributions of isolates across species, geographic regions, and infection types were consistent for all 6 years of the study [35]. Against all 5032 ABC isolates, the addition of durlobactam to sulbactam lowered the MIC90 by 32-fold, compared to that of sulbactam alone, from 64 µg/mL to 2 µg/mL (Table 2).
Table 2.
In Vitro Activity of Sulbactam-durlobactam and Comparator Antibacterial Agents Against Global Acinetobacter baumannii–calcoaceticus Complex Clinical Isolates Collected From 2016 to 2021
| Category | Antibacterial Agent | No. | MIC50, µg/mL | MIC90, µg/mL | CLSI Interpretive Criteriaa | ||
|---|---|---|---|---|---|---|---|
| % Susceptible | % Intermediate | % Resistant | |||||
| All isolates | Sulbactam-durlobactam | 5032 | 1 | 2 | 98.3 | NA | 1.7 |
| Sulbactam | … | 8 | 64 | 46.9 | 8.0 | 45.1 | |
| Amikacin | … | 4 | >64 | 58.6 | 3.3 | 38.1 | |
| Cefepime | … | 16 | >16 | 44.6 | 7.9 | 47.4 | |
| Ciprofloxacin | … | >4 | >4 | 44.4 | 0.7 | 54.9 | |
| Colistin | … | 0.5 | 1 | NA | 95.9 | 4.1 | |
| Imipenem | … | 8 | >64 | 48.9 | 0.6 | 50.5 | |
| Meropenem | … | 16 | >64 | 47.9 | 1.1 | 51.0 | |
| Minocycline | … | 0.5 | 16 | 78.3 | 10.1 | 11.6 | |
| Tigecycline | … | 0.5 | 2 | NA | NA | NA | |
| Acinetobacter species | |||||||
| ȃA. baumannii | Sulbactam-durlobactam | 4038 | 1 | 2 | 98.0 | NA | 2.0 |
| Imipenem | … | 32 | >64 | 37.7 | 0.6 | 61.6 | |
| ȃA. calcoaceticus | Sulbactam-durlobactam | 55 | 0.5 | 1 | 100 | NA | 0.0 |
| Imipenem | … | 0.25 | 0.25 | 100 | 0.0 | 0.0 | |
| ȃA. nosocomialis | Sulbactam-durlobactam | 296 | 0.5 | 1 | 99.7 | NA | 0.3 |
| Imipenem | … | 0.25 | 0.5 | 92.2 | 0.0 | 7.8 | |
| ȃA. pittii | Sulbactam-durlobactam | 636 | 0.5 | 2 | 99.4 | NA | 0.6 |
| Imipenem | … | 0.25 | 0.5 | 95.1 | 0.3 | 4.5 | |
| Region | |||||||
| ȃAsia/South Pacific | Sulbactam-durlobactam | 685 | 1 | 2 | 98.4 | NA | 1.6 |
| Imipenem | … | 32 | >64 | 43.9 | 0.4 | 55.6 | |
| ȃEurope | Sulbactam-durlobactam | 2121 | 1 | 4 | 98.6 | NA | 1.4 |
| Imipenem | … | 32 | >64 | 43.9 | 0.5 | 55.5 | |
| ȃLatin America | Sulbactam-durlobactam | 632 | 1 | 2 | 95.3 | NA | 4.7 |
| Imipenem | … | 64 | >64 | 28.8 | 0.2 | 71.0 | |
| ȃMiddle East (Israel) | Sulbactam-durlobactam | 88 | 1 | 2 | 97.7 | NA | 2.3 |
| Imipenem | … | 32 | 64 | 28.4 | 0 | 71.6 | |
| ȃNorth America (US) | Sulbactam-durlobactam | 1506 | 1 | 2 | 99.2 | NA | 0.8 |
| Imipenem | … | 0.25 | 64 | 67.9 | 0.9 | 31.3 | |
| Infection type | |||||||
| ȃRespiratory tract | Sulbactam-durlobactam | 2731 | 1 | 2 | 98.1 | NA | 1.9 |
| Imipenem | … | 32 | >64 | 43.3 | 0.6 | 56.1 | |
| ȃBloodstream | Sulbactam-durlobactam | 1015 | 1 | 2 | 98.4 | NA | 1.6 |
| Imipenem | … | 2 | >64 | 50.9 | 0.6 | 48.5 | |
| ȃUrinary tract | Sulbactam-durlobactam | 832 | 1 | 2 | 98.9 | NA | 1.1 |
| Imipenem | … | 0.5 | 64 | 62.7 | 0.6 | 39.7 | |
| ȃIntra-abdominal | Sulbactam-durlobactam | 217 | 1 | 2 | 97.7 | NA | 2.3 |
| Imipenem | … | 32 | >64 | 41.9 | 0.0 | 58.1 | |
| ȃSkin and soft tissue | Sulbactam-durlobactam | 227 | 1 | 2 | 99.1 | NA | 0.9 |
| Imipenem | … | 0.5 | >64 | 63.0 | 0.4 | 36.6 | |
Source: [35].
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; MIC50, The antibiotic concentration that inhibits the growth of 50% of the tested isolates; MIC90, The antibiotic concentration that inhibits the growth of 90% of the tested isolates; NA, not available; US, United States.
As published by CLSI M100 (2021) [39]. Sulbactam-durlobactam MICs were interpreted using the preliminary breakpoint of susceptible ≤4 µg/mL and resistant ≥8 µg/mL. Sulbactam MICs were interpreted using the sulbactam component of the ampicillin-sulbactam breakpoints (≤8/4 µg/mL [susceptible], 16/8 [intermediate], ≥32/16 [resistant]) [39].
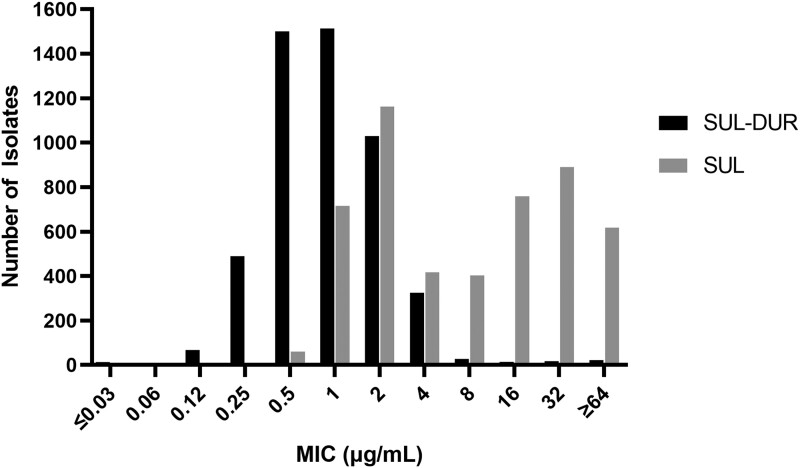
Using the preliminary sulbactam-durlobactam breakpoint of 4 µg/mL [37], 98.3% of the isolates were susceptible to sulbactam-durlobactam (Figure 3). Conversely, more than half of these isolates were nonsusceptible to carbapenems (51.1% and 52.1% for imipenem and meropenem, respectively). The colistin and tigecycline MIC90 values had similar potency to sulbactam-durlobactam; however, the in vitro susceptibilities of these agents often do not correlate with efficacy due to toxicities and poor pharmacokinetic properties [38].
Figure 3.
Minimum inhibitory concentration (MIC) distribution for sulbactam-durlobactam (SUL-DUR) and sulbactam (SUL) against 5032 Acinetobacter baumannii–calcoaceticus complex global isolates collected from 2016 to 2021 [35].
The activity of sulbactam-durlobactam was consistent across the ABC species tested, despite large variations in carbapenem susceptibility (∼38% for A. baumannii isolates and ≥92% for the other species tested) (Table 2) [35]. The activity of sulbactam-durlobactam was also consistent across the geographical regions, with isolates from Europe having a MIC90 value 1 doubling dilution higher compared to the entire set (4 µg/mL vs 2 µg/mL). Across each region, the percentage of isolates with a sulbactam-durlobactam MIC ≤4 µg/mL was >95%. In addition, the potency of sulbactam-durlobactam was similar for each year (2016–2021) and different infection sources. Sulbactam-durlobactam was found to be active against phenotypically different resistant subsets, including carbapenem-nonsusceptible, colistin-resistant, multidrug-resistant (MDR), and extensively drug-resistant (XDR) isolates, each with an MIC50 of 1 or 2 µg/mL and an MIC90 of 4 µg/mL (Table 3). The percentage of isolates with a sulbactam-durlobactam MIC ≤4 µg/mL was >96% across these different antibiotic-resistant groups [35].
Table 3.
In Vitro Activity of Sulbactam-durlobactam Against Phenotypically Antibiotic-Nonsusceptible Acinetobacter baumannii–calcoaceticus Complex Clinical Isolates Collected Globally From 2016 to 2021
| Resistance Phenotypea | No. | Sulbactam-durlobactam | ||
|---|---|---|---|---|
| MIC50, µg/mL | MIC90, µg/mL | % Susceptibleb | ||
| All isolates | 5032 | 1 | 2 | 98.3 |
| Sulbactam nonsusceptible | 2670 | 1 | 4 | 96.9 |
| Imipenem nonsusceptible | 2570 | 1 | 4 | 96.7 |
| Colistin resistant | 204 | 2 | 4 | 98.0 |
| Ciprofloxacin nonsusceptible | 2796 | 1 | 4 | 97.5 |
| Amikacin nonsusceptible | 2083 | 2 | 4 | 96.9 |
| Minocycline nonsusceptible | 1092 | 2 | 4 | 97.7 |
| Multidrug-resistantc | 2680 | 1 | 4 | 96.9 |
| Extensively drug-resistantc | 2116 | 2 | 4 | 97.2 |
Source: [35].
Abbreviations: MIC50, The antibiotic concentration that inhibits the growth of 50% of the tested isolates; MIC90, The antibiotic concentration that inhibits the growth of 50% of the tested isolates.
Nonsusceptibility or resistance as determined by Clinical and Laboratory Standards Institute M100 (2021) [39].
Sulbactam and sulbactam-durlobactam MICs were interpreted using the preliminary breakpoint of susceptible ≤4 µg/mL.
Multidrug-resistant isolates were defined as nonsusceptible to 1 agent from ≥3 different antimicrobial classes and extensively drug-resistant isolates were defined as not susceptible to at least 5 of the following 7 agents (classes): cefepime (extended-spectrum cephalosporins), imipenem (carbapenems), amikacin (aminoglycosides), ciprofloxacin (fluoroquinolones), minocycline (tetracyclines), sulbactam (penicillin/β-lactamase inhibitor; sulbactam is the active component of ampicillin-sulbactam against Acinetobacter spp), and colistin (polymyxins), based on proposed international guidelines [40]. For colistin, only colistin-resistant isolates were used in these determinations as only intermediate and resistant interpretive criteria exist for colistin [39].
In addition to the 6-year global surveillance study [35], the in vitro activity of sulbactam-durlobactam was evaluated in a surveillance of 982 A. baumannii clinical isolates collected during 2016–2018 from 22 sites distributed across mainland China [32]. Isolates were mostly from hospital-associated infection types (89.5%) and 72.8% were from lower respiratory tract, 17.3% from intra-abdominal, 6.0% from urinary tract, 3.6% from skin and soft tissue, and 0.3% from bloodstream infections. For these isolates, susceptibility was low for levofloxacin (14%), imipenem (15.2%), and amikacin (26.2%). The MIC50 and MIC90 values of 1 and 2 µg/ml, respectively, for sulbactam-durlobactam, were 32-fold lower than those observed for sulbactam alone. This level of activity was consistent across Chinese sites and most infection types; however, the urinary tract isolates were more susceptible to sulbactam-durlobactam (MIC50 and MIC90 values of 0.5 and 1 µg/mL, respectively).
In 4 separate studies, sulbactam-durlobactam was profiled against collections of molecularly characterized, carbapenem-resistant A. baumannii clinical isolates from different geographical regions, which further demonstrates that durlobactam can restore sulbactam activity against MDR isolates from around the globe. These isolates represent a wide breadth of international clonal groups, presence of class D carbapenemases (eg, OXA-23–like, OXA-24/40–like, OXA-143–like, and OXA-58), and antibiotic resistance phenotypes. Nodari and colleagues tested 112 A. baumannii clinical isolates from Brazil that represent the major South American clones (mostly IC5, IC1, IC4, and IC7) for susceptibility to sulbactam-durlobactam [33]. All isolates were carbapenem-resistant and 92.8%, 86.6%, and 18.8% were amikacin-resistant, gentamicin-resistant, and polymyxin-resistant, respectively. Sulbactam-durlobactam MIC values ranged from ≤0.25 µg/mL to 4 µg/mL with MIC50 and MIC90 values of 1 µg/mL each, which was consistent across the different international clones and isolates expressing different class D carbapenemases. In Petropoulou et al, 190 unique carbapenem-resistant A. baumannii isolates collected from 11 hospitals in Greece during 2015 were assayed for susceptibility to sulbactam-durlobactam [30]. This collection displayed high levels of resistance to antibiotics with MIC90 values of 16 µg/mL for colistin, 32 µg/mL for minocycline, >64 µg/mL for imipenem, and >128 µg/mL for amikacin. Sulbactam-durlobactam had an 8-fold lower MIC50/MIC90 value of 4/8 µg/mL compared to sulbactam alone, with 87% of isolates with a sulbactam-durlobactam MIC value of ≤4 µg/mL.
One collection curated by researchers at the University of Fribourg, Switzerland, was comprised of 100 clinical A. baumannii isolates of worldwide origin and included producers of OXA-23 OXA-40–like, OXA-58, OXA-72, and NDM as well as 9 colistin-resistant isolates [34]. This set had a high degree of antibiotic resistance with susceptibility of only 18% for amikacin, 5% for cefepime, 0% for imipenem, 46% for minocycline, and 8% for sulbactam-cefoperazone. In contrast, 71% of isolates had a sulbactam-durlobactam MIC ≤4 µg/mL, the preliminary breakpoint [37]. Sulbactam-durlobactam showed little activity against the NDM-producing isolates, consistent with the finding that durlobactam does not inhibit metallo-β-lactamases [23].
The activity of sulbactam-durlobactam was also measured against 246 carbapenem-resistant A. baumannii isolates collected by researchers at the University of Cologne, Germany, between 2012 and 2016 from patients from 37 countries in 6 world regions (Africa, Asia/South Pacific, Europe, Latin America, Middle East, and North America) [31]. In addition to being resistant to carbapenems, 69.5% of isolates were resistant to amikacin, 24.4% resistant to minocycline, and 4.1% resistant to colistin. Sulbactam alone had MIC50/MIC90 values of 16/64 µg/mL. Conversely, sulbactam-durlobactam had MIC50 and MIC90 values of 1 and 2 µg/mL, respectively. There was no correlation between the sulbactam-durlobactam MICs and class D β-lactamases, other serine carbapenemases, or clonal strain type.
EFFICACY OF SULBACTAM-DURLOBACTAM IN PRECLINICAL MODELS OF A. BAUMANNII INFECTION
Sulbactam-durlobactam has shown robust in vivo efficacy in multiple preclinical models of A. baumannii infection [23, 24]. The MDR and XDR isolates tested in these models had established drug resistance phenotypes (specifically class D β-lactamase production), with sulbactam-durlobactam MIC values ranging from 0.5 to 4 µg/mL. Treatment with sulbactam-durlobactam resulted in a dose-dependent reduction in XDR A. baumannii bacterial counts in both neutropenic mouse thigh abscess and pneumonia infection models, resulting in >1-log reduction of bacterial burden compared to the initial inoculum and multiple logs as compared to the growth control [41].
CONCLUSIONS
Taken together, these results demonstrate that the biochemical inhibition of β-lactamases by durlobactam, as well as the restoration of in vitro susceptibility of sulbactam by durlobactam that was observed in multiple surveillance studies, is consistent with the robust in vivo efficacy of the combination seen in standard preclinical murine models of infection [41]. If approved, sulbactam-durlobactam may address an urgent unmet medical need for patients with serious infections caused by Acinetobacter species, including MDR strains.
Contributor Information
Krisztina M Papp-Wallace, Research Service, Veterans Affairs Northeast Ohio Healthcare System, USA; Departments of Biochemistry and Medicine, Case Western Reserve University, Cleveland, OH, USA.
Sarah M McLeod, Entasis Therapeutics, Waltham, MA, USA.
Alita A Miller, Entasis Therapeutics, Waltham, MA, USA.
Notes
Acknowledgments. The effort for this review was supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs (VA Merit Review Award Number 1I01 BX002872 to K. M. P.-W. from the Biomedical Laboratory Research and Development Service of the VA Office of Research and Development).
Financial support. This work was sponsored by Entasis Therapeutics, Inc., a wholly owned subsidiary of Innoviva, Inc. The authors did not receive any fees for authorship.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs.
Supplement sponsorship. This article appears as part of the supplement “Sulbactam-durlobactam, a Targeted β-lactam/β-lactamase Inhibitor, for MDR Acinetobacter,” sponsored by Entasis Therapeutics Inc., a wholly owned subsidiary of Innoviva, Inc.
References
- 1. English AR, Retsema JA, Girard AE, et al. . CP-45,899, a β-lactamase inhibitor that extends the antibacterial spectrum of β-lactams: initial bacteriological characterization. Antimicrob Agents Chemother 1978; 14:414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones RN, Wilson HW, Thornsberry C, Barry AL. In vitro antimicrobial activity of cefoperazone-sulbactam combinations against 554 clinical isolates including a review and β-lactamase studies. Diagn Microbiol Infect Dis 1985; 3:489–99. [DOI] [PubMed] [Google Scholar]
- 3. Jones RN, Barry AL, Thornsberry C, Wilson HW. The cefoperazone-sulbactam combination. In vitro qualities including β-lactamase stability, antimicrobial activity, and interpretive criteria for disk diffusion tests. Am J Clin Pathol 1985; 84:496–504. [DOI] [PubMed] [Google Scholar]
- 4. Jimenez-Mejias ME, Pachon J, Becerril B, Palomino-Nicas J, Rodriguez-Cobacho A, Revuelta M. Treatment of multidrug-resistant Acinetobacter baumannii meningitis with ampicillin/sulbactam. Clin Infect Dis 1997; 24:932–5. [DOI] [PubMed] [Google Scholar]
- 5. Levin AS, Levy CE, Manrique AE, Medeiros EA, Costa SF. Severe nosocomial infections with imipenem-resistant Acinetobacter baumannii treated with ampicillin/sulbactam. Int J Antimicrob Agents 2003; 21:58–62. [DOI] [PubMed] [Google Scholar]
- 6. Zalts R, Neuberger A, Hussein K, et al. . Treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia: retrospective comparison between intravenous colistin and intravenous ampicillin-sulbactam. Am J Ther 2016; 23:e78––85.. [DOI] [PubMed] [Google Scholar]
- 7. Gkentzi D, Tsintoni A, Christopoulou I, et al. . Extensively-drug resistant Acinetobacter baumannii bacteremia in neonates: effective treatment with the combination of colistin and ampicillin/sulbactam. J Chemother 2020; 32:103–6. [DOI] [PubMed] [Google Scholar]
- 8. Labia R, Morand A, Lelievre V, Mattioni D, Kazmierczak A. Sulbactam: biochemical factors involved in its synergy with ampicillin. Rev Infect Dis 1986; 8(Suppl 5):S496–502. [DOI] [PubMed] [Google Scholar]
- 9. Urban C, Go E, Mariano N, Rahal JJ. Interaction of sulbactam, clavulanic acid and tazobactam with penicillin-binding proteins of imipenem-resistant and -susceptible Acinetobacter baumannii. FEMS Microbiol Lett 1995; 125(2–3):193–7. [Google Scholar]
- 10. Papp-Wallace KM, Senkfor B, Gatta J, et al. . Early insights into the interactions of different β-lactam antibiotics and β-lactamase inhibitors against soluble forms of Acinetobacter baumannii PBP1a and Acinetobacter spp. PBP3. Antimicrob Agents Chemother 2012; 56:5687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Penwell WF, Shapiro AB, Giacobbe RA, et al. . Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrob Agents Chemother 2015; 59:1680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wadl M, Heckenbach K, Noll I, et al. . Increasing occurrence of multidrug-resistance in Acinetobacter baumannii isolates from four German university hospitals, 2002–2006. Infection 2010; 38:47–51. [DOI] [PubMed] [Google Scholar]
- 13. Sengstock DM, Thyagarajan R, Apalara J, Mira A, Chopra T, Kaye KS. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin Infect Dis 2010; 50:1611–6. [DOI] [PubMed] [Google Scholar]
- 14. Punpanich W, Munsrichoom A, Srisarang S, Treeratweeraphong V. In vitro activities of colistin and ampicillin/sulbactam against Acinetobacter baumannii. J Med Assoc Thai 2011; 94(Suppl 3):S95–100. [PubMed] [Google Scholar]
- 15. Dafopoulou K, Tsakris A, Pournaras S. Changes in antimicrobial resistance of clinical isolates of Acinetobacter baumannii group isolated in Greece, 2010–2015. J Med Microbiol 2018; 67:496–8. [DOI] [PubMed] [Google Scholar]
- 16. Reddy T, Chopra T, Marchaim D, et al. . Trends in antimicrobial resistance of Acinetobacter baumannii isolates from a metropolitan Detroit health system. Antimicrob Agents Chemother 2010; 54:2235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krizova L, Poirel L, Nordmann P, Nemec A. TEM-1 β-lactamase as a source of resistance to sulbactam in clinical strains of Acinetobacter baumannii. J Antimicrob Chemother 2013; 68:2786–91. [DOI] [PubMed] [Google Scholar]
- 18. Kuo SC, Lee YT, Yang Lauderdale TL, et al. . Contribution of Acinetobacter-derived cephalosporinase-30 to sulbactam resistance in Acinetobacter baumannii. Front Microbiol 2015; 6:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shapiro AB. Kinetics of sulbactam hydrolysis by β-lactamases, and kinetics of β-lactamase inhibition by sulbactam. Antimicrob Agents Chemother 2017; 61:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang Y, Fu Y, Lan P, et al. . Molecular epidemiology and mechanism of sulbactam resistance in Acinetobacter baumannii isolates with diverse genetic backgrounds in China. Antimicrob Agents Chemother 2018; 62:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chopra T, Marchaim D, Awali RA, et al. . Epidemiology of bloodstream infections caused by Acinetobacter baumannii and impact of drug resistance to both carbapenems and ampicillin-sulbactam on clinical outcomes. Antimicrob Agents Chemother 2013; 57:6270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papp-Wallace KM. The latest advances in β-lactam/β-lactamase inhibitor combinations for the treatment of gram-negative bacterial infections. Expert Opin Pharmacother 2019; 20:2169–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Durand-Reville TF, Guler S, Comita-Prevoir J, et al. . ETX2514 Is a broad-spectrum β-lactamase inhibitor for the treatment of drug-resistant gram-negative bacteria including Acinetobacter baumannii. Nat Microbiol 2017; 2:17104. [DOI] [PubMed] [Google Scholar]
- 24. Barnes MD, Kumar V, Bethel CR, et al. . Targeting multidrug-resistant Acinetobacter spp. sulbactam and the diazabicyclooctenone β-lactamase inhibitor ETX2514 as a novel therapeutic agent. mBio 2019; 10:e00159–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shapiro AB, Gao N, Jahic H, Carter NM, Chen A, Miller AA. Reversibility of covalent, broad-spectrum serine β-lactamase inhibition by the diazabicyclooctenone ETX2514. ACS Infect Dis 2017; 3: 833–44. [DOI] [PubMed] [Google Scholar]
- 26. Iyer R, Moussa SH, Durand-Reville TF, Tommasi R, Miller A. Acinetobacter baumannii OmpA is a selective antibiotic permeant porin. ACS Infect Dis 2018; 4:373–81. [DOI] [PubMed] [Google Scholar]
- 27. Lahiri SD, Mangani S, Jahic H, et al. . Molecular basis of selective inhibition and slow reversibility of avibactam against class D carbapenemases: a structure-guided study of OXA-24 and OXA-48. ACS Chem Biol 2015; 10:591–600. [DOI] [PubMed] [Google Scholar]
- 28. Lizana I, Pecchi G, Uribe EA, Delgado EJ. A rationale for the unlike potency of avibactam and ETX2514 against OXA-24 β-lactamase. Arch Biochem Biophys 2022; 727:109343. [DOI] [PubMed] [Google Scholar]
- 29. McLeod SM, Shapiro AB, Moussa SH, et al. . Frequency and mechanism of spontaneous resistance to sulbactam combined with the novel β-lactamase inhibitor ETX2514 in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 2018; 62:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petropoulou D, Siopi M, Vourli S, Pournaras S. Activity of sulbactam-durlobactam and comparators against a national collection of carbapenem-resistant Acinetobacter baumannii isolates from Greece. Front Cell Infect Microbiol 2021; 11:814530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seifert H, Muller C, Stefanik D, Higgins PG, Miller A, Kresken M. In vitro activity of sulbactam/durlobactam against global isolates of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 2020; 75:2616–21. [DOI] [PubMed] [Google Scholar]
- 32. Yang Q, Xu Y, Jia P, et al. . In vitro activity of sulbactam/durlobactam against clinical isolates of Acinetobacter baumannii collected in China. J Antimicrob Chemother 2020; 75:1833–9. [DOI] [PubMed] [Google Scholar]
- 33. Nodari CS, Santos FF, Kurihara MNL, Valiatti TB, Cayo R, Gales AC. In vitro activity of sulbactam/durlobactam against extensively drug-resistant Acinetobacter baumannii isolates belonging to South American major clones. J Glob Antimicrob Resist 2021; 25:363–6. [DOI] [PubMed] [Google Scholar]
- 34. Findlay J, Poirel L, Bouvier M, Nordmann P. In-vitro activity of sulbactam-durlobactam against carbapenem-resistant Acinetobacter baumannii and mechanisms of resistance. J Glob Antimicrob Resist 2022; 30:445–50. [DOI] [PubMed] [Google Scholar]
- 35. Karlowsky JA, Hackel MA, McLeod SM, Miller AA. In vitro activity of sulbactam-durlobactam against global isolates of Acinetobacter baumannii–calcoaceticus complex collected from 2016 to 2021. Antimicrob Agents Chemother 2022; 66:e0078122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ayoub Moubareck C, Hammoudi Halat D. Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics (Basel) 2020; 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rodvold KA, Gotfried MH, Isaacs RD, O’Donnell JP, Stone E. Plasma and intrapulmonary concentrations of ETX2514 and sulbactam following intravenous administration of ETX2514SUL to healthy adult subjects. Antimicrob Agents Chemother 2018; 62:e01089-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Isler B, Doi Y, Bonomo RA, Paterson DL. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 2019; 63:e01110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clinical and Laboratory Standards Institute (CLSI) . Performance standards for antimicrobial susceptibility testing. M100. 31st ed. Wayne, PA: CLSI, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Magiorakos AP, Srinivasan A, Carey RB, et al. . Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. [DOI] [PubMed] [Google Scholar]
- 41. O’Donnell JP, Bhavnani SM. The pharmacokinetics/pharmacodynamic relationship of durlobactam in combination with sulbactam in in vitro and in vivo infection model systems versus Acinetobacter baumannii–calcoaceticus complex.Clin Infect Dis 2023; 76(Suppl 2):S202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]