Abstract
Genital antibody responses were compared in female mice immunized intravaginally (i.vag.) or intranasally (i.n.) with a bacterial protein antigen (AgI/II of Streptococcus mutans) coupled to the B subunit of cholera toxin. Serum and salivary antibodies were also evaluated as measures of disseminated mucosal and systemic responses. Although i.vag. immunization induced local vaginal immunoglobulin A (IgA) and IgG antibody responses, these were not disseminated to a remote secretion, the saliva, and only modest levels of serum antibodies were generated. In contrast, i.n. immunization was substantially more effective at inducing IgA and IgG antibody responses in the genital tract and in the circulation, as well as at inducing IgA antibodies in the saliva. Moreover, mucosal and systemic antibodies induced by i.n. immunization persisted for at least 12 months. Analysis of the molecular form of genital IgA indicated that the majority of both total IgA and specific IgA antibody was polymeric, and likely derived from the common mucosal immune system.
The continuing problem of sexually transmitted diseases and the spread of human immunodeficiency virus infection, especially by heterosexual transmission, have impelled efforts aimed at generating appropriate immune responses in the genital tract that would confer protection against these infections. Successful vaccination against genital infections will require not only the identification of suitable antigenic targets for each particular pathogen but also the development of strategies for inducing appropriate immune responses that are expressed at the sites of primary infection and invasion. Depending on the nature of the pathogen, such strategies might involve eliciting mucosal or circulating antibodies of the appropriate isotype to engage protective immune defense mechanisms, or inducing cell-mediated immunity, including cytotoxic T cells.
Conventionally, the genital tract has been considered a component of the common mucosal immune system (CMIS), and there is much evidence to sustain that concept, especially with respect to the female tract. The epithelium of the endocervix, fallopian tubes, and uterus, and the ectocervical glands, express polymeric immunoglobulin receptor (pIgR), and the underlying populations of plasma cells secrete predominantly polymeric immunoglobulin A (pIgA), which is transported into the luminal secretion by pIgR to form secretory immunoglobulin A (SIgA) (reviewed in reference 25). Less information is available for the male system, but IgA-secreting plasma cells and pIgR-expressing epithelial cells occur in the urethral glands of Littré, epididymis, prostate, and seminal vesicles of humans (1, 37, 39) and mice (36). The secretions of both male and female systems contain significant levels of SIgA, but these may be exceeded by IgG levels, at least in humans and other primates (3, 4, 6, 9, 18, 26, 27). Whereas SIgA is accepted as the distinct product of the CMIS, the source and means of delivery of IgG are less clear: both local production and transudation from the circulation have been implicated (reviewed in references 2 and 23).
These uncertainties as to the origins of genital antibodies have hampered efforts to define the most effective ways of actively immunizing the genital tracts. It seems clear that both male and female tracts lack true mucosal inductive sites, which are collectively known as mucosa-associated lymphoid tissues (MALT) and are typified by intestinal Peyer's patches and similar organized lymphoepithelial structures in the lower bowel and upper respiratory tract. Nevertheless, foci of lymphocytes and accessory cells, consisting of a core of B cells surrounded by T cells and an outer area of macrophage-like cells, have been described in the human vagina, cervix, and endometrium (54). However, the T cells are predominantly CD8+ CD4−, suggesting an immunoregulatory role. The same group has identified CD8+ cytotoxic T cells in the female genital tract (45, 46). CD4+ HLA class II+ Langerhans cells occur in the cervicovaginal epithelium (19) and might serve as antigen-presenting cells. Thus, at least in the female tract, there is an obvious potential to induce immune responses by the local application of immunogens, and the direct instillation of antigens into the vagina or uterus has been examined experimentally. Results, however, have been quite variable. As originally observed by Ogra and Ogra (33) with inactivated poliovirus vaccine, intravaginal or intrauterine immunization can induce a modest local antibody response (reviewed in reference 24), and more-recent findings with the well-known potent mucosal immunogen, cholera toxin B subunit (CTB), have confirmed this (22, 29, 44). However, there has been controversy over the effectiveness of local genital immunization in comparison to other routes of mucosal immunization, and the dissemination of responses to other mucosal sites or to the circulation. Because of anatomical proximity to the genital tract, shared lymphoid drainage, and the presence of lymphoid follicles resembling Peyer's patches, the rectum and colon have been considered likely sites for inducing genital antibody responses (12, 13, 21, 22, 32). However, variable results have been obtained, depending upon the species and upon the antigen, delivery system, and adjuvant used. In general, the most effective way of inducing mucosal immune responses is by exploiting the CMIS and administering antigens in an appropriate form to sites where organized MALT is present. We have extensively investigated mucosal immunization with antigens coupled to CTB (10, 16, 42) and have found that the intranasal (i.n.) route is especially effective at generating genital antibody responses (41, 49, 52). The present study is a direct comparison of the local genital, disseminated mucosal, and circulating antibody responses induced in mice by intravaginal (i.vag.) or i.n. administration of the same CTB-coupled immunogen.
MATERIALS AND METHODS
Animals, immunization, and sample collection.
Female BALB/c mice were purchased from Taconic (Germantown, N.Y.) through the University of Alabama at Birmingham (UAB) Animal Resources Program or were bred locally under pathogen-free barrier conditions from stock originally obtained from the National Cancer Institute (Frederick, Md.). Animals were maintained in microisolator cages on standard laboratory chow and water ad lib and were approximately 3 months old at the start of experiments. Animal use protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Because of minor differences in Ig levels in sera and secretions in the animals from the two sources, as well as possible genetic drift, all animals used in any one experiment were from the same source. However, essentially similar results were obtained with both sets of mice.
After preimmune samples of sera and secretions had been collected, groups of five mice were immunized either i.n. or i.vag. with Streptococcus mutans antigen I/II (AgI/II) conjugated to CTB (42). For i.n. immunization, a dose of 15 μg of AgI/II–CTB conjugate was administered in 15 μl of sterile Dulbecco phosphate-buffered saline (PBS) introduced into both nares with a pipettor fitted with a plastic tip. For i.vag. immunization, a dose of 15 μg of AgI/II–CTB conjugate plus 5 μg of CT adjuvant was given in 16 μl of Dulbecco PBS instilled into the vagina. Immunizations were repeated at intervals of 10 days, for a total of three doses by each route.
Samples of sera and secretions were collected from the mice prior to immunization and 7 days after the third immunization (53). Serum was separated from 50 to 100 μl of tail vein blood and stored at −20°C. Up to 100 μl of saliva was collected after intraperitoneal (i.p.) injection of carbachol (5 μg in 0.1 ml of sterile Dulbecco PBS) to stimulate flow and was stored at −20°C. Vaginal secretions were collected (after the collection of saliva) by washing three times with 50 μl of sterile Dulbecco PBS instilled into the vagina and withdrawn using a pipettor fitted with a plastic tip; the washes were combined and stored at −20°C.
Assay of Ig's and antibodies.
Antibodies to S. mutans AgI/II and to CT were assayed by enzyme-linked immunosorbent assay (ELISA) on microtiter plates coated with AgI/II (5 μg/ml) (40) or with GM1 ganglioside (2.5 μg/ml; Calbiochem, San Diego, Calif.) followed by CT (1.5 μg/ml; List Biological Laboratories, Campbell, Calif.), as described previously (42). Total IgM, IgG, and IgA concentrations were determined by ELISA on plates coated with unconjugated antibodies to mouse IgM, IgG, or IgA (Southern Biotechnology Associates, Birmingham, Ala.). Bound antibodies or Ig's were detected using peroxidase-conjugated antibodies to mouse IgM, IgG, or IgA, and the color developed with a substrate of o-phenylenediamine–H2O2 was measured in a Dynatech MRX microplate reader interfaced to a Macintosh computer for data retrieval and analysis. Antibody and Ig concentrations were calculated by interpolation on calibration curves constructed by a computer program using the four-parameter logistic model. Antibody levels in secretions were also expressed relative to the total corresponding Ig isotype concentrations.
Molecular form of IgA.
The molecular forms of IgA in serum, saliva, and vaginal-wash samples were analyzed by size exclusion chromatography on a 30- by 0.78-cm silica column (Biosep SEC-S3000; Phenomenex, Torrance, Calif.) connected to a Perkin-Elmer series 10 high-performance liquid chromatograph (HPLC) (41). The column was calibrated by chromatographing Mr standard proteins, as well as monomeric IgA, dimeric IgA, and SIgA. To ensure that apparently polymeric IgA was not simply aggregated or antigen-complexed IgA, samples were run under dissociating conditions (0.1 M acetate [pH 3.6] plus 0.05 M Na2SO4). Three-drop fractions (∼100 μl) were collected in tubes containing 1 M Tris (pH 9.5) to neutralize the acid and were assayed by ELISA to estimate IgA concentrations.
Statistics.
Antibody and Ig assay data were transformed to logarithms before statistical analysis in order to improve (“normalize”) the distribution and variance characteristics. For presentation of results, means ± standard deviations (SD) (of log data) were back-transformed into arithmetic values to generate geometric means ×/÷ SD, or were plotted on log10 scales. Student's t test was performed on log-transformed data to assess the significance of difference of means, and a P value of <0.05 was considered significant.
RESULTS
Comparison of antibody responses to immunization by the i.vag. versus the i.n. route.
Consistent with our previous observations, i.n. immunization of mice with three doses of AgI/II–CTB conjugate at 10-day intervals resulted in strong serum IgG antibody responses against both AgI/II and CT by day 7 after the last dose (Fig. 1). Serum IgM antibodies were not induced above preimmune levels (shown in Table 1), but serum IgA antibodies to both components of the immunogen were strongly elevated. i.vag. immunization with the same immunogen also elicited serum IgG and IgA antibodies (Fig. 1), though at substantially (10- to 100-fold) lower mean levels than those generated by i.n. immunization (P = 0.012 and P < 0.001 for IgG and IgA anti-AgI/II, respectively, and P < 0.001 for both IgG and IgA anti-CT). Particularly in the case of the serum IgG responses, i.vag. immunization resulted in much greater variability, as revealed by the SD. All mice displayed substantially higher total serum IgG concentrations after immunization by either route (7,052 ×/÷ 1.31 μg/ml for the i.n. group and 12,702 ×/÷ 1.49 μg/ml for the i.vag. group, compared with 308 ×/÷ 2.23 μg/ml for preimmune animals [Table 1]). This finding probably reflects low initial levels of IgG in immunologically naive young mice, which were elevated upon exposure to a potent immune stimulus.
FIG. 1.
Serum IgM, IgG, and IgA antibody responses to AgI/II and CT 7 days after the third i.n. or i.vag. immunization with AgI/II–CTB conjugate. Results are shown as geometric means and SD (n = 5 animals per group).
TABLE 1.
Preimmune levels of antibodies and total Ig concentrations in serum and secretions
| Fluid | Isotype | Concna (μg/ml)
|
||
|---|---|---|---|---|
| Anti-AgI/II | Anti-CT | Total | ||
| Serum | IgM | 3.42 ×/÷ 1.49b | 0.20 ×/÷ 1.38c | 239 ×/÷ 1.15c |
| IgG | 0.25 ×/÷ 2.31 | 0.14 ×/÷ 1.53 | 308 ×/÷ 2.23 | |
| IgA | 1.42 ×/÷ 1.38 | 1.05 ×/÷ 1.13 | 593 ×/÷ 1.26 | |
| Saliva | IgA | 0 | 0 | 7.41 ×/÷ 2.45 |
| Vaginal wash | IgA | 0.13 ×/÷ 2.26 | 0.20 ×/÷ 2.94 | 5.29 ×/÷ 5.81 |
| IgG | 0 | 0 | 0.42 ×/÷ 4.72 | |
Data are geometric means ×/÷ SD; n = 10.
n = 8.
n = 9.
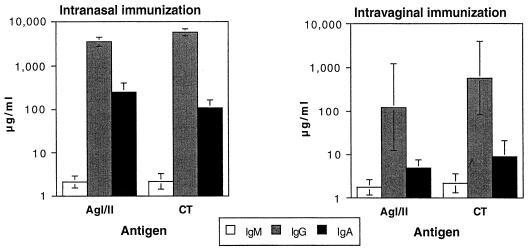
Likewise, i.n. immunization was very effective at generating salivary IgA antibodies to AgI/II and CT (Fig. 2), whereas no IgA antibodies to AgI/II were detectable above the assay background in the saliva of i.vag. immunized animals, and only two of five animals in this group developed low levels of salivary IgA antibodies to CT (Fig. 2). As noted previously, i.n. immunization resulted in an overall increase in total salivary IgA concentrations, whereas i.vag. immunization had a lesser effect (Table 1 and Fig. 2). Allowing for this difference by expressing salivary IgA antibody levels relative to total salivary IgA concentrations showed that i.n. immunization resulted in substantial salivary IgA antibody responses, whereas i.vag. immunization did not (Table 2).
FIG. 2.
Mucosal antibody responses to AgI/II and CT 7 days after the third i.n. or i.vag. immunization with AgI/II–CTB conjugate. Results are shown as geometric means and SD (n = 5 animals per group).
TABLE 2.
Antibody responses in secretions 7 days after the third i.n. or i.vag. immunization with AgI/II–CTB
| Fluid and isotype | Antibody responsea after:
|
|||
|---|---|---|---|---|
| i.n. immunization
|
i.vag. immunization
|
|||
| Anti-AgI/II | Anti-CT | Anti-AgI/II | Anti-CT | |
| Salivary IgA | 48.2 ×/÷ 1.3 | 15.3 ×/÷ 1.9 | <0.7b | <1.0c |
| Vaginal IgA | 38.2 ×/÷ 2.4 | 3.8 ×/÷ 5.2 | 2.7 ×/÷ 2.0 | 4.9 ×/÷ 2.3 |
| Vaginal IgG | 30.5 ×/÷ 1.3 | 53.5 ×/÷ 1.3 | 4.7 ×/÷ 3.4 | 10.6 ×/÷ 2.9 |
Expressed as a percentage of the total corresponding Ig isotype concentration. Data are geometric means ×/÷ SD; n = 5.
Antibodies not detectable.
Antibodies detectable in only two of five mice.
i.vag. immunized mice developed vaginal IgA antibodies to AgI/II (Fig. 2), but these were consistently and significantly lower (P = 0.025) than those induced by i.n. immunization. i.vag. immunization was as effective at inducing vaginal IgA antibodies to CT as i.n. immunization, but these were at a much lower level than the response to AgI/II induced by i.n. immunization (Fig. 2). In other words, the vaginal IgA response to CT was low, whether induced by i.n. or i.vag. immunization. Total vaginal IgA concentrations in vaginal washes were highly variable both prior to and after immunization by either route, possibly reflecting varying efficiency in sampling as well as inherent variations in the animals. When vaginal IgA antibodies were expressed relative to the total IgA concentration in each animal, the difference with respect to antibodies to AgI/II induced by i.vag. and i.n. immunization was sustained, and antibodies to CT remained comparable and at lower levels (Table 2). There was no consistent effect of immunization by either route on the total concentration of IgA recovered in vaginal washes (Table 1 and Fig. 2).
Vaginal IgG antibodies were also detectable in both immunized groups of mice, but at much lower levels than IgA antibodies to both components of the immunogen (Fig. 2). i.vag. immunized mice showed a greater elevation of total IgG concentrations in vaginal washes than i.n. immunized mice (Table 1 and Fig. 2), so that when specific IgG antibody responses were expressed relative to total IgG concentration, it again became evident that i.n. immunization resulted in a stronger vaginal IgG antibody response to both AgI/II and CT than i.vag. immunization (Table 2).
Duration of responses.
In another experiment, mice that had been immunized i.n. with AgI/II–CTB (as previously) were sampled at 4, 8, and 12 months after the last immunization. Antibodies to AgI/II persisted in serum, saliva, and vaginal wash samples throughout this period (Table 3), although levels declined from the initial high values. Salivary IgA and vaginal IgG antibody levels expressed as a proportion of total corresponding IgA or IgG appeared to increase at 12 months, but this reflects a decrease in the total IgA or IgG concentration recorded in those secretions at this time point. Overall, these results show that mucosal antibodies induced by i.n. immunization can persist for prolonged periods.
TABLE 3.
Persistence of antibody responses against AgI/II after i.n. immunization with AgI/II–CTB
| Time after immunization (no. of mos) | Antibody responsea in:
|
||||
|---|---|---|---|---|---|
| Serum
|
Saliva (IgA)b | Vaginal wash
|
|||
| IgG (μg/ml) | IgA (μg/ml) | IgAb | IgGb | ||
| 4 | 571 ×/÷ 1.84 | 43.1 ×/÷ 1.50 | 60.5 ×/÷ 1.41 | 14.6 ×/÷ 1.79 | 11.0 ×/÷ 1.72 |
| 8 | 175 ×/÷ 1.58 | 16.1 ×/÷ 1.69 | 13.6 ×/÷ 1.76 | 14.8 ×/÷ 1.51 | 9.96 ×/÷ 1.65 |
| 12 | 136 ×/÷ 1.83 | 24.2 ×/÷ 1.40 | 21.6 ×/÷ 1.54 | 9.48 ×/÷ 1.75 | 19.8 ×/÷ 3.10 |
Data are geometric means ×/÷ SD; n = 5.
Expressed as a percentage of the total corresponding Ig isotype concentration.
Origins of vaginal antibodies.
To examine the molecular forms of IgA as evidence of the origins of vaginal antibodies, two sets of experiments were performed. In the first, the molecular form of IgA and of IgA antibody to AgI/II present in vaginal wash was examined by HPLC on a calibrated size-exclusion column and was compared with the molecular profiles of IgA in serum and saliva (Fig. 3). Serum IgA occurred in both monomeric and polymeric forms as expected in mice (data not shown). Salivary IgA and IgA antibody developed after i.n. immunization were predominantly polymeric, with a minor monomeric peak (Fig. 3A). Vaginal-wash IgA, and IgA antibody induced by either i.n. or i.vag. immunization, also resembled salivary IgA in having a main peak corresponding to polymeric (dimeric) IgA and a minor peak of monomeric IgA (Fig. 3B and C). These findings indicate that the majority of IgA in vaginal washes was likely to be polymeric SIgA derived from the mucosal immune system. Moreover, vaginal IgA antibodies induced by either common mucosal or local immunization were also predominantly polymeric, and therefore probably mostly SIgA.
FIG. 3.
HPLC size exclusion analysis of total IgA and IgA antibody to AgI/II in saliva (A) and vaginal wash (B and C) from mice immunized i.n. (A and B) or i.vag. (C) with AgI/II–CTB conjugate. Fractions were assayed by ELISA, and results are shown as optical densities (OD) which are not comparable among panels A, B, and C or between total IgA and specific antibody curves, since different sample dilutions and substrate development times were used to reveal the distribution of molecular forms in each sample. Monomeric IgA peaked at approximately fraction 18, and dimeric IgA peaked at approximately fraction 10; higher polymers eluted in earlier fractions.
We also took advantage of the effect of carbachol, primarily used to stimulate salivary flow, as it enhances the flow of other secretions, including those of the genital tract. We first washed the vaginas of mice without administering carbachol (wash 1). Forty minutes later, the vaginas were washed again, also without carbachol stimulation (wash 2). Then carbachol was injected i.p., and after a further 40 min the vaginas were washed again (wash 3). As a control, a similar group of mice was sampled, but without the administration of carbachol between the second and third vaginal washes. IgA and IgG concentrations were assayed in these washes, and some wash samples were subjected to HPLC size exclusion analysis. We expected that wash 1 would contain most of the IgA that had accumulated over the previous indeterminate period, whereas wash 2 would contain much lower levels of residual IgA; wash 3 from carbachol-treated mice would contain higher levels of IgA in vaginal fluid that had been newly secreted under the influence of carbachol. This was indeed the case (Table 4), and IgG in vaginal washes followed a similar pattern, although at a much lower level. Moreover, HPLC analysis showed that the newly secreted IgA in the third wash was predominantly polymeric (Fig. 4A), and if the washes were taken from an immunized animal, newly secreted IgA antibodies were also polymeric (Fig. 4B).
TABLE 4.
Effect of carbachol treatment on concentrations of IgA and IgG in sequential vaginal washes collected at 40-min intervals
| Wash no. | IgA concn (μg/ml)a
|
IgG concn (μg/ml)a
|
||
|---|---|---|---|---|
| Without carbachol | With carbacholb | Without carbachol | With carbacholb | |
| 1 | 50.3 ×/÷ 2.86 | 63.7 ×/÷ 3.33 | 1.63 ×/÷ 2.70 | 2.67 ×/÷ 3.32 |
| 2 | 29.0 ×/÷ 3.48 | 13.2 ×/÷ 3.24 | 0.72 ×/÷ 2.61 | 0.70 ×/÷ 2.98 |
| 3 | 17.8 ×/÷ 2.07 | 28.4 ×/÷ 3.85 | 0.53 ×/÷ 3.36 | 1.38 ×/÷ 3.53 |
Data are geometric means ×/÷ SD; n = 5.
Administered i.p. after the second wash.
FIG. 4.
HPLC size exclusion analysis of total IgA (A) and IgA antibody to AgI/II (B) in three sequential vaginal washes taken at 40-min intervals; carbachol was administered i.p. after the second wash. IgA concentrations in fractions were assayed by ELISA and are shown as optical densities (OD) which are not comparable between panels A and B, as different sample dilutions and substrate development times were used for total IgA and specific antibody assays. Monomeric IgA peaked at approximately fraction 18, and dimeric IgA peaked at approximately fraction 10; higher polymers eluted in earlier fractions.
DISCUSSION
We find that mucosal immunization using the i.n. route is very effective for generating antibody responses in the female genital tract. Most likely, this depends on the operation of the CMIS, since we have previously shown that i.n. immunization stimulates cells in the nasal lymphoid tissue (NALT) and its draining cervical lymph nodes (50, 51). We have also shown previously that NALT serves as an inductive site for the CMIS, especially with respect to the salivary glands as a representative effector site of the head and neck region (49), as well as the respiratory and genital tracts (52). Numerous other reports have described the effectiveness of i.n. immunization for inducing genital-tract antibody responses in humans as well as experimental animals (5, 11, 14, 15, 17, 28, 34, 41, 43, 47). The compartmentalization of the CMIS (7, 31), such that the gut-associated lymphoid tissues preferentially supply α4β7-expressing pIgA-secreting cell precursors to the lamina propria of the gut (and also lactating mammary glands), reflects the distribution of the mucosal addressin MAdCAM-1 on venular endothelial cells mainly within these tissues (8). In distinction, the NALT also supplies antibody-secreting precursor cells to extraintestinal effector sites, including the genital tract, where the venules apparently do not express MAdCAM-1, and where the receptor-addressin systems responsible for lymphocyte homing are not as well defined. Our finding, however, that i.vag. immunization does induce antibodies in the genital tract, albeit at a lower level than those generated by stimulation of the CMIS through one of its inductive sites, demonstrates the ability of mucosal tissues to mount local immune responses that are not generally distributed to remote mucosal effector sites (30).
Our results, however, differ markedly from recently published findings on immune responses to herpes simplex virus given i.n. or i.vag. in mice (35), in which it was shown that i.vag. immunization was superior both for generating antibodies in the vagina and for protecting against genital viral infection. It is therefore pertinent to consider why this should be the case. We suggest that a major factor in these divergent results is that Parr and Parr used a live infectious agent, whereas we used a nonviable protein, as an immunogen. Infection of the genital epithelial cells with herpesvirus was clearly capable of inducing a strong local immune response manifested in terms of both antibodies and gamma interferon secretion by T cells, as well as a serum IgG antibody response that was approximately twofold higher than that obtained after i.n. immunization. These authors, however, did not report on total Ig concentrations in the genital secretions, which we have found to be variable between individuals and strongly influenced by local immunization, possibly as a result of inflammatory changes induced by the immunogen. It would be interesting to know if the immune response to herpesvirus given i.vag. extends to remote sites of the CMIS. Our findings suggest that responses would not be disseminated, since although we detected IgG and IgA antibodies in vaginal washes after local immunization with AgI/II–CTB, these antibodies were not expressed in the saliva. Furthermore, Parr and Parr's findings of protection against herpesvirus could involve additional cellular mechanisms of immunity.
Parr and Parr also treated their animals with hormones in order to stabilize the estrous cycle, which is known to influence immune responses, especially within the genital tract (35). However, they report essentially no difference in the response to i.vag. administered herpesvirus, whether the animals were treated with progestin or estradiol. We chose not to resort to hormone treatments because they have complex effects on genital immune responses. For example, estradiol enhances the expression of pIgR (and hence transport of SIgA) in the uterus but decreases it in the vagina (48). Moreover, antigen-presenting activity is highest at proestrus and lowest at estrus (38), and progesterone treatment has been found to enhance responses especially to local vaginal immunization (20). Thus, overall antibody responses can fluctuate depending on the particular hormone treatment or stage of the cycle, and on the particular site of study within the genital tract. However, the mouse estrous cycle lasts approximately 4 days (compared to the 10-day cycle of immunizations given in these studies), and female mice kept together in the absence of males tend to synchronize their cycles or even become anestrous, thereby diminishing the variation due to asynchronous cycles between animals. By reporting vaginal antibody responses relative to total corresponding Ig isotype concentrations, we could apply a correction for cycle-dependent (or other) variations in Ig secretion rates as well as for differences in the efficiency of vaginal washing. In spite of the potential for hormone-dependent effects to enhance or suppress Ig secretion in the genital tract, we could show that i.n. immunization with a CTB-coupled protein immunogen was more effective for the generation of specific antibodies, especially of the IgA isotype, in the genital tract than i.vag. immunization. This effect was evident even though i.vag. immunization was supplemented with the potent mucosal adjuvant CT, which we previously found to be necessary for obtaining significant responses by this route (29; H.-Y. Wu, unpublished data), whereas addition of CT to AgI/II–CTB conjugates delivered i.n. is not necessary and furthermore tends to promote responses to itself rather than to the AgI/II component of the conjugate (53).
The origins of Ig's and antibodies in the genital tract are important with respect to the routes of immunization, because IgG is a major component of genital-tract immunity, at least in humans, where it may predominate over IgA (26), and is probably derived from the circulation as well as local synthesis, though the mechanisms of transport are uncertain. Mice are clearly different in that the major isotype of Ig in female secretions, as collected by vaginal washing, is IgA. The findings that total IgA and specific IgA antibodies were predominantly polymeric and that IgA antibodies were preferentially induced by remote mucosal (i.n.) immunization suggested that these were mainly SIgA derived from the CMIS, but no antibody against a murine secretory component was available for direct confirmation of this. Nevertheless, the high specific activity of the anti-AgI/II IgA antibody relative to the total IgA concentration in vaginal washes after i.n. immunization (mean, 38.2% [Table 2]), comparable with the specific activity in the saliva of the same animals (mean, 48.2%), further suggests that it is of mucosal rather than circulatory origin. The higher ratio of IgA to IgG in vaginal washes compared with serum is also consistent with the selective transport of IgA into the secretions. Both IgA and IgG antibody-secreting cells have been found in the genital mucosa after i.vag. or i.n. immunization (20).
The immune responsiveness of the female genital tract must be seen in relation to its physiological functions in reproduction. The lower tract (vagina) is colonized with a specialized microbiota and has a partially keratinized pseudostratified epithelium. The upper tract (uterus and fallopian tubes) is normally sterile but must permit the passage of allogeneic sperm and support the development of a semiallogeneic fetus engrafted into the endometrium for a prolonged period. Standing guard between these two regions is the cervix, which possesses a population of subepithelial plasma cells secreting IgG and pIgA, and a pIgR-expressing epithelium that can transport SIgA (23, 25). Although it lacks organized MALT, it is clear that the female genital tract can mount immune responses to histoincompatible fetal antigens, sperm acrosomal antigens, and certain infectious agents as well as experimental vaccines. However, infectious agents clearly differ in their abilities to induce responses in the genital tract: herpes simplex virus can induce responses that may confer protection against further infection (35), whereas Neisseria gonorrhoeae does not (18). Different routes and strategies of immunization against sexually transmitted diseases may therefore be required depending on the particular pathogen and vaccine antigen concerned. However, we find that the most effective strategy for inducing SIgA and IgG antibodies in the female genital tract is by stimulating the CMIS through one of its inductive sites with an antigen coupled to CTB.
ACKNOWLEDGMENT
This study was supported by US-PHS grant DE06746 from the National Institute for Dental and Craniofacial Research.
REFERENCES
- 1.Anderson D J, Pudney J. Mucosal immune defense against HIV-1 in the male urogenital tract. Vaccine Res. 1992;1:143–150. [Google Scholar]
- 2.Anderson D J, Pudney J. Human male genital tract immunity and experimental models. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. San Diego, Calif: Academic Press; 1999. pp. 1411–1422. [Google Scholar]
- 3.Bélec L, Dupré T, Prazuck T, Tévi-Bénissan C, Kanga J M, Pathey O, Lu X S, Pillot J. Cervicovaginal overproduction of specific IgG to human immunodeficiency virus (HIV) contrasts with normal or impaired IgA local response in HIV infection. J Infect Dis. 1995;172:691–697. doi: 10.1093/infdis/172.3.691. [DOI] [PubMed] [Google Scholar]
- 4.Bélec L, Tévi-Bénissan C, Lu X-S, Prazuck T, Pillot J. Local synthesis of IgG antibodies to HIV within the female and male genital tracts during asymptomatic and pre-AIDS stages of HIV infection. AIDS Res Hum Retrovir. 1995;11:719–729. doi: 10.1089/aid.1995.11.719. [DOI] [PubMed] [Google Scholar]
- 5.Bergquist C, Johansson E L, Lagergård T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandtzaeg P. Mucosal immunity in the female genital tract. J Reprod Immunol. 1997;36:23–50. doi: 10.1016/s0165-0378(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg P, Baekkevold E S, Farstad I N, Jahnsen F L, Johansen F E, Nilsen E M, Yamanaka T. Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunol Today. 1999;20:141–151. doi: 10.1016/s0167-5699(98)01413-3. [DOI] [PubMed] [Google Scholar]
- 8.Butcher E C. Lymphocyte homing and intestinal immunity. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. San Diego, Calif: Academic Press; 1999. pp. 507–522. [Google Scholar]
- 9.Crowley-Nowick P A, Bell M, Edwards R P, McCallister D, Gore H, Kanbour-Shakir A, Mestecky J, Partridge E E. Normal uterine cervix: characterization of isolated lymphocyte phenotypes and immunoglobulin secretion. Am J Reprod Immunol. 1995;34:241–247. doi: 10.1111/j.1600-0897.1995.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 10.Czerkinsky C, Russell M W, Lycke N, Lindblad M, Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989;57:1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, De Magistris M T. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996;64:974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson K, Quiding-Järbrink M, Osek J, Möller Å, Björk S, Holmgren J, Czerkinsky C. Specific-antibody-secreting cells in the rectums and genital tracts of nonhuman primates following vaccination. Infect Immun. 1998;66:5889–5896. doi: 10.1128/iai.66.12.5889-5896.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forrest B D, Shearman D J C, LaBrooy J T. Specific immune responses in humans following rectal delivery of live typhoid vaccine. Vaccine. 1990;8:209–212. doi: 10.1016/0264-410x(90)90047-p. [DOI] [PubMed] [Google Scholar]
- 14.Gallichan W S, Rosenthal K L. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589–1595. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 15.Gizurarson S, Jónsdóttir V M, Heron I. Intranasal administration of diphtheria toxoid. Selecting antibody isotypes using formulations having various lipophilic characteristics. Vaccine. 1995;13:617–621. doi: 10.1016/0264-410x(94)00066-v. [DOI] [PubMed] [Google Scholar]
- 16.Hajishengallis G, Hollingshead S K, Koga T, Russell M W. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154:4322–4332. [PubMed] [Google Scholar]
- 17.Hashigucci K, Ogawa H, Ishidate T, Yamashita R, Kamiya H, Watanabe K, Hattori N, Sato T, Suzuki Y, Nagamine T, Aizawa C, Tamura S, Kurata T, Oya A. Antibody responses in volunteers induced by nasal influenza vaccine combined with Escherichia coli heat-labile enterotoxin B subunit containing a trace amount of the holotoxin. Vaccine. 1996;14:113–119. doi: 10.1016/0264-410x(95)00174-y. [DOI] [PubMed] [Google Scholar]
- 18.Hedges S R, Mayo M S, Mestecky J, Hook E W, Russell M W. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect Immun. 1999;67:3937–3946. doi: 10.1128/iai.67.8.3937-3946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain L A, Kelly C G, Fellowes R, Hecht E-M, Wilson J, Chapman M, Lehner T. Expression and gene transcript of Fc receptors for IgG, HLA class II antigens and Langerhans cells in human cervico-vaginal epithelium. Clin Exp Immunol. 1992;90:530–538. doi: 10.1111/j.1365-2249.1992.tb05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson E-L, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantele A, Häkkinen M, Moldoveanu Z, Lu A, Savilahti E, Alvarez R D, Michalek S, Mestecky J. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infect Immun. 1998;66:5630–5635. doi: 10.1128/iai.66.12.5630-5635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozlowski P A, Cu-Uvin S, Neutra M R, Flanigan T P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutteh W H. Mucosal immunity in the human female reproductive tract. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. San Diego, Calif: Academic Press; 1999. pp. 1423–1434. [Google Scholar]
- 24.Kutteh W H, Edwards R P, Menge A C, Mestecky J. IgA immunity in female reproductive tract secretions. In: Griffin P D, Johnson P M, editors. Local immunity in reproduction tract tissues. Oxford, United Kingdom: Oxford University Press; 1993. pp. 229–243. [Google Scholar]
- 25.Kutteh W H, Mestecky J. Secretory immunity in the female reproductive tract. Am J Reprod Immunol. 1994;31:40–46. doi: 10.1111/j.1600-0897.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 26.Kutteh W H, Prince S J, Hammond K R, Kutteh C C, Mestecky J. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin Exp Immunol. 1996;104:538–542. doi: 10.1046/j.1365-2249.1996.36742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lü F X, Ma Z, Rourke T, Srinivasan S, McChesney M, Miller C J. Immunoglobulin concentrations and antigen-specific antibody levels in cervicovaginal lavages of rhesus macaques are influenced by the stage of the menstrual cycle. Infect Immun. 1999;67:6321–6328. doi: 10.1128/iai.67.12.6321-6328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubeck M D, Natuk R J, Chengalvala M, Chanda P K, Murthy K K, Murthy S, Mizutani S, Lee S G, Wade M S, Bhat B M, et al. Immunogenicity of recombinant adenovirus-human immunodeficiency virus vaccines in chimpanzees following intranasal administration. AIDS Res Hum Retrovir. 1994;10:1443–1449. doi: 10.1089/aid.1994.10.1443. [DOI] [PubMed] [Google Scholar]
- 29.Menge A C, Michalek S M, Russell M W, Mestecky J. Immune response of the female rat genital tract after oral and local immunization with keyhole limpet hemocyanin conjugated to the cholera toxin B subunit. Infect Immun. 1993;61:2162–2171. doi: 10.1128/iai.61.5.2162-2171.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mestecky J, McGhee J R, Michalek S M, Arnold R R, Crago S S, Babb J L. Concept of the local and common mucosal immune response. Adv Exp Med Biol. 1978;107:185–192. doi: 10.1007/978-1-4684-3369-2_22. [DOI] [PubMed] [Google Scholar]
- 31.Moldoveanu Z, Russell M W, Wu H-Y, Mestecky J. Compartmentalization within the mucosal immune system. Adv Exp Med Biol. 1995;371A:97–101. doi: 10.1007/978-1-4615-1941-6_17. [DOI] [PubMed] [Google Scholar]
- 32.Nardelli-Haefliger D, Kraehenbuhl J-P, Curtiss R, Schödel F, Potts A, Kelly S, de Grandi P. Oral and rectal immunization of adult female volunteers with a recombinant attenuated Salmonella typhi vaccine strain. Infect Immun. 1996;64:5219–5224. doi: 10.1128/iai.64.12.5219-5224.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogra P L, Ogra S S. Local antibody response to polio vaccine in the female genital tract. J Immunol. 1973;110:1307–1311. [PubMed] [Google Scholar]
- 34.Pal S, Peterson E M, de la Maza L M. Intranasal immunization induces long-term protection in mice against a Chlamydia trachomatis genital challenge. Infect Immun. 1996;64:5341–5348. doi: 10.1128/iai.64.12.5341-5348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parr E L, Parr M B. Immune responses and protection against vaginal infection after nasal or vaginal immunization with attenuated herpes simplex virus type-2. Immunology. 1999;98:639–645. doi: 10.1046/j.1365-2567.1999.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parr M B, Ren H P, Russell L D, Prins G S, Parr E L. Urethral glands of the male mouse contain secretory component and immunoglobulin A plasma cells and are targets of testosterone. Biol Reprod. 1992;47:1031–1039. doi: 10.1095/biolreprod47.6.1031. [DOI] [PubMed] [Google Scholar]
- 37.Perra M T, Turno F, Sirigu P. Human urethral epithelium: immunohistochemical demonstration of secretory IgA. Arch Androl. 1994;32:227–233. doi: 10.3109/01485019408987790. [DOI] [PubMed] [Google Scholar]
- 38.Prabhala R H, Wira C R. Sex hormone and IL-6 regulation of antigen presentation in the female reproductive tract mucosal tissues. J Immunol. 1995;155:5566–5573. [PubMed] [Google Scholar]
- 39.Pudney J, Anderson D J. Immunobiology of the human penile urethra. Am J Pathol. 1995;147:155–165. [PMC free article] [PubMed] [Google Scholar]
- 40.Russell M W, Bergmeier L A, Zanders E D, Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980;28:486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S M. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell M W, Wu H-Y. Distribution, persistence, and recall of serum and salivary antibody responses to peroral immunization with protein antigen I/II of Streptococcus mutans coupled to the cholera toxin B subunit. Infect Immun. 1991;59:4061–4070. doi: 10.1128/iai.59.11.4061-4070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staats H F, Nichols W G, Palker T J. Mucosal immunity to HIV-1. Systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A) J Immunol. 1996;157:462–472. [PubMed] [Google Scholar]
- 44.Wassén L, Schön K, Holmgren J, Jertborn M, Lycke N. Local intravaginal vaccination of the female genital tract. Scand J Immunol. 1996;44:408–414. doi: 10.1046/j.1365-3083.1996.d01-320.x. [DOI] [PubMed] [Google Scholar]
- 45.White H D, Crassi K M, Givan A L, Stern J E, Gonzalez J L, Memoli V A, Green W R, Wira C R. CD3+CD8+ CTL activity within the human female reproductive tract. Influence of stage of the menstrual cycle and menopause. J Immunol. 1997;158:3017–3027. [PubMed] [Google Scholar]
- 46.White H D, Yeaman G R, Givan A L, Wira C R. Mucosal immunity in the human female reproductive tract: cytotoxic T lymphocyte function in the cervix and vagina of premenopausal and postmenopausal women. Am J Reprod Immunol. 1997;37:30–38. doi: 10.1111/j.1600-0897.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 47.Winchell J M, Van Kruiningen H J, Silbart L K. Mucosal immune response to an HIV C4/V3 peptide following nasal or intestinal immunization of rabbits. AIDS Res Hum Retrovir. 1997;13:881–889. doi: 10.1089/aid.1997.13.881. [DOI] [PubMed] [Google Scholar]
- 48.Wira C R, O'Mara B, Richardson J, Prabhala R. The mucosal immune system in the female reproductive tract: influence of sex hormones and cytokines on immune recognition and responses to antigen. Vaccine Res. 1992;1:151–167. [Google Scholar]
- 49.Wu H-Y, Nguyen H, Russell M W. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46:506–513. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- 50.Wu H-Y, Nikolova E B, Beagley K W, Eldridge J H, Russell M W. Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunization. Infect Immun. 1997;65:225–235. doi: 10.1128/iai.65.1.227-235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu H-Y, Nikolova E B, Beagley K W, Russell M W. Induction of antibody-secreting cells and T helper and memory cells in murine nasal lymphoid tissue. Immunology. 1996;88:493–500. doi: 10.1046/j.1365-2567.1996.d01-690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu H-Y, Russell M W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu H-Y, Russell M W. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine. 1998;16:286–292. doi: 10.1016/s0264-410x(97)00168-0. [DOI] [PubMed] [Google Scholar]
- 54.Yeaman G R, Guyre P M, Fanger M W, Collins J E, White H D, Rathbun W, Orndorff K A, Gonzalez J, Stern J E, Wira C R. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol. 1997;61:427–435. [PubMed] [Google Scholar]