Abstract
The abundance of docosahexaenoic acid (DHA) in brain membrane phospholipids has stimulated studies to explore its role in neurological functions. Upon released from phospholipids, DHA undergoes enzymatic reactions resulting in synthesis of bioactive docosanoids and prostanoids. However, these phospholipids are also prone to non-enzymatic reactions leading to more complex pattern of metabolites. A non-enzymatic oxidized product of DHA, 4(Rs)-4-F4t-Neuroprostane (44FNP), has been identified in cardiac and brain tissues. In this study, we examined effects of the 44FNP on oxidative and inflammatory responses in microglial cells treated with lipopolysaccharide (LPS). The 44FNP attenuated LPS-induced production of reactive oxygen species (ROS) in both primary and immortalized microglia (BV2). It also attenuated LPS-induced inflammation through suppressing NFκB-p65 and levels of iNOS and TNFα. In addition, 44FNP also suppressed LPS-induced mitochondrial dysfunction and upregulated the Nrf2/HO-1 antioxidative pathway. In sum, these findings with microglial cells demonstrated neuroprotective effects of this 44FNP and shed light into the potential of nutraceutical therapy for neurodegenerative diseases.
Keywords: 4(RS)-4-F4t-Neuroprostane; Microglia; Antioxidant, and anti-inflammatory
1. Introduction
Docosahexaenoic acid (DHA) is a polyunsaturated fatty acid (PUFA) abundant in the phospholipids in brain, and studies have shown a critical role for it to maintain brain health [1]. Intake of DHA has been correlated to lower cognitive impairment in Alzheimer’s disease [2,3]. There is growing evidence that some of the neuroprotective effects of DHA is due to its conversion to oxidative metabolites such as neuroprotectin D1, resolvins and maresins [4].
Besides enzymatic reactions, DHA is also vulnerable to non-enzymatic reactions to produce peroxidation products, such as neuroprostanoids [5–10]. Among these, 4(RS)-4-F4t-Neuroprostane (44FNP) is one of the most abundant [11–13], and has been proposed to be an oxidative damage biomarker of neurological diseases [14]. To date, studies have unveiled different health effects of 44FNP, e.g., on arrhythmia [15], decreasing infarct and ventricular tachycardia in ischemia reperfusion [16], inducing production of heme oxygenase-1 (HO-1) in SH-SY5Y cells [17], and proliferation of human breast cancer cells [18]. However, whether 44FNP can exert neuroprotective effects in microglia has not been investigated in detail.
In order to provide information to further our understanding of mechanism(s) underlying the neuroprotective effects of 44FNP, this study examined oxidative stress, inflammation, and mitochondrial membrane function in microglial cells stimulated with the bacteria toxin lipopolysaccharide (LPS). In addition, this study also evaluated whether 44FNP may confer antioxidation through the Nrf2/HO-1 pathway.
2. Materials and methods
2.1. Materials
Dulbecco’s modified Eagle’s medium (DMEM) and penicillin/streptomycin (P/S, 10,000 units/ml) were purchased from Life Technologies (Grand Island, NY), fetal bovine serum (FBS) and lipopolysaccharide (LPS) from Cayman Chemical (Ann Arbor, MI), dimethyl sulfoxide (DMSO), cOmplete™ protease inhibitor cocktail, PhosSTOP™ phosphatase inhibitor cocktail from Sigma-Aldrich (St. Louis, MO), radioimmunoprecipitation assay (RIPA) buffer, bicinchoninic acid (BCA) protein assay kit, SuperSignal™ West Pico plus chemiluminescent substrate, Restore™ PLUS Western blot stripping buffer, TNF-α mouse uncoated ELISA kit, and 5-(and-6)-chloromethyl-2’,7’-dichlorodihydro-fluorescein diacetate, acetyl ester (DCF) from Thermo Fisher Scientific (Waltham, MA). Primary antibodies against HO-1, phospho-NF-κB p65 (p-p65) was purchased from Cell Signaling (Beverly, MA), monoclonal anti-β-actin peroxidase antibody from Sigma-Aldrich (St. Louis, MO), anti-inducible nitric oxide synthase (iNOS) antibody and TMRM Assay Kit (Mitochondrial Membrane Potential) from Abcam (Cambridge, MA), and anti-Nrf2 antibody from GeneTex (Irvine, CA). Neural Tissue Dissociation kit (P), MACS buffer, and CD11b microbeads were purchased from Miltenyi Biotec (Auburn, California). 44FNP (1 mg/ml in methanol) was synthesized in Dr. T. Durand’s laboratory at IBMM, France [19].
2.2. Preparation of primary mouse microglia
Timed pregnant C57BL/6 mice were purchased from Charles River (Wilmington, MA). All animal care and experimental protocols were carried out with permission from the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Chicago. Preparation of primary mouse microglia from mouse pups (P1–P4) were accomplished using the Miltenyi Biotec MACS cells separation system. Briefly, cerebral cortices were dissected, and meninges removed. Brain tissue was homogenized using the Neural Tissue Dissociation Kit. Cells were collected and resuspended in ice-cold MACS buffer containing CD11b microbeads and then passed through the magnetized LS columns to harvest the primary microglia according to the manufacturer’s protocol. Microglial cells were collected and plated as previously described [20].
2.3. BV2 cell culture
BV2 cells were originally obtained from Dr. Rosario Donato (University of Perugia, Italy) and subsequently propagated and stored in Dr. Grace Sun’s laboratory (University of Missouri, MO, USA). BV2 mouse microglial cells were cultured in DMEM supplied with 5% FBS and 1% P/S. For experiments, cells were subcultured to 80% confluence in multiple well plates and serum starved for 3 h prior to treatment with 44FNP for 1 h and followed by stimulation with 50 ng/mL LPS. 44FNP was dissolved in DMSO diluted in DMEM to the indicated concentrations.
2.4. Measurement of ROS production
Cells were suspended in 96-well plates, pretreated with 44FNP for 1 h, and followed by LPS treatment for 12 h. After treatment, 10 μM DCF was added to the cells for 45 min. The fluorescent intensity of DCF in cells was measured using a Synergy H1 Plate Reader (BioTek, St. Louis, MO) with an excitation wavelength of 490 nm and an emission wavelength of 520 nm.
2.5. Western blot analysis
After treatment, the culture medium was removed, and cells were lysed in RIPA buffer containing protease and phosphatase inhibitors. Cell lysates were collected and centrifuged at 14,000×g for 15 min at 4 °C, and supernatants were collected. Protein concentration in cell lysate was determined using BCA assay. Samples were loaded onto SDS-PAGE for electrophoresis. Afterwards, proteins were transferred to 0.2-μm PVDF membranes at 100 V for 1 h at 4 °C. Membranes were blocked with 5% non-fat milk in Tris-buffered saline with 0.1% Tween 20 (TBS-T) for 1 h, and incubated with antibodies overnight at 4 °C. After washing with TBS-T, blots were incubated with secondary antibody for 1 h. Signals were developed using SuperSignal™ West Pico plus chemiluminescent substrate and captured with a myECL imager (Thermo Scientific). The optical density of bands was measured with the Image Studio Lite 5.2 (LI-COR Biotechnology, Lincoln, NE).
2.6. TNF-α ELISA assay
TNF-α in culture medium was determined by sandwich ELISA. Briefly, cells were treated, and medium was collected and centrifuged at 4000×g for 5 min. The levels of TNF-α were assessed using an ELISA kit following manufacturer’s instruction.
2.7. Mitochondrial membrane potential measurement
After treatment, cells were incubated with 200 nM TMRM in Hank’s balanced salt solution (HBSS) for 30 min at 37 °C. Fluorescence was analyzed use Synergy H1 Plate Reader with an excitation wavelength of 548 nm and an emission wavelength of 575 nm. For positive control, p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (FCCP), a mitochondrial uncoupler known to cause mitochondria depolarization, was used to validate our technique.
2.8. Statistical analysis
Data are expressed as mean ± standard deviation (SD) from at least three independent experiments. Statistical analysis between multiple groups was carried out using one-way ANOVA followed by Tukey’s post hoc HSD test in GraphPad Prism (version 8.10). A p value < 0.05 was considered statistically significant.
3. Results
3.1. Effects of 44FNP on LPS-induced ROS production
We examined the effects of 44FNP (2.5–20 μM) on ROS production in primary mouse microglia and BV2 cells stimulated with LPS (50 ng/ml). In both types of microglia, 44FNP inhibited LPS-stimulated ROS in a dose-dependent manner with a significant decrease at 5 μM (Fig. 1). To verify ROS production through NADPH oxidase, we used the gp91ds-tat peptide, known to block ROS production through disruption of NADPH subunits [21]. Addition of gp91ds-tat abolished LPS-induced ROS in these cells, suggesting that ROS production was mainly through NADPH oxidase. Our treatments in all experimental groups did not impose any ill effects on cell viability (data not shown).
Fig. 1.
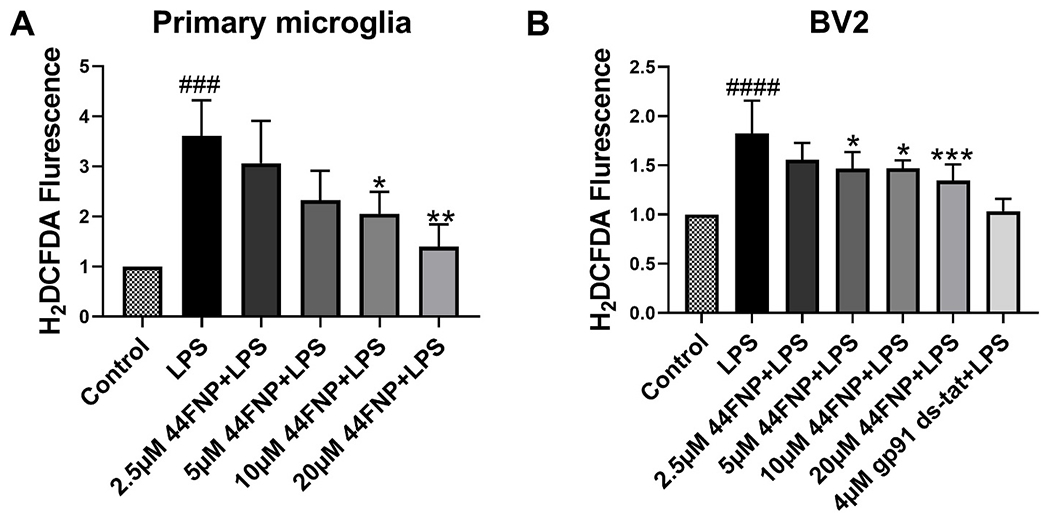
44FNP attenuated LPS-induced reactive oxygen species (ROS) production in primary mouse microglia and BV2 cells. Primary microglia (A), BV2 cells (B) were pretreated with 44FNP for 1 h, followed by stimulation with 50 ng/ml LPS for 12 h. Data are represented in mean ± standard deviation (SD) (n = 3). Statistical analysis was carried out with one way ANOVA followed by Bonferroni post-tests. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the LPS group; ###p < 0.001, ####p < 0.0001 compared with the control group.
3.2. Effects of 44FNP on LPS-induced inflammatory responses in BV2 cells
LPS has been reported to activate microglial inflammation through the NFκB-p65 pathway [20]. In this study, we examined effects of 44FNP on p65 and p-p65 expression in control and LPS-stimulated microglial cells. Results show that LPS-induced phosphorylation of p65 expression was attenuated by 44FNP in a dose-dependent manner (Fig. 2A).
Fig. 2.
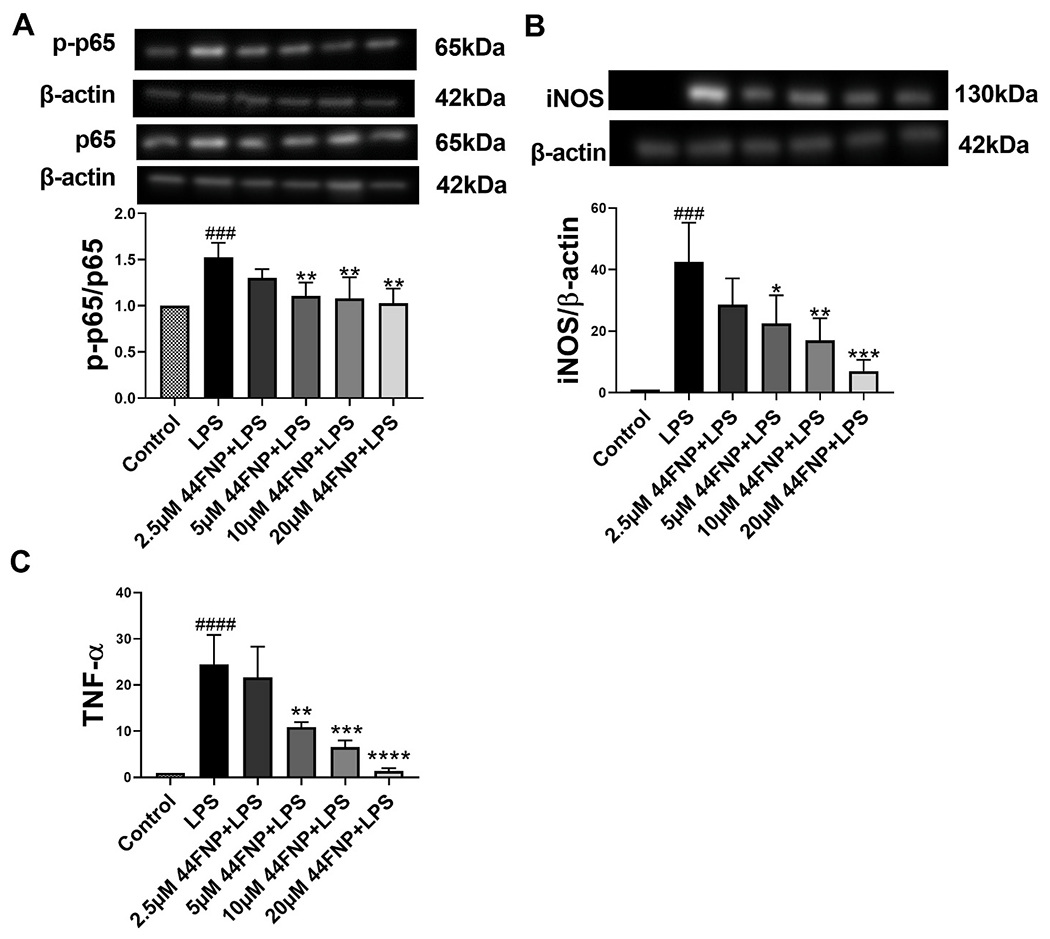
44FNP attenuated LPS-triggered the p65 pathway (A), iNOS (B), TNF-α (C) in BV2 cells. BV2 cells were pretreated with 44FNP for 1 h followed by stimulation with 50 ng/ml LPS for 1 h (A) or 18 h (B, C). Data are represented in mean ± SD (n = 3). Statistical analysis was carried out with one way ANOVA followed by Bonferroni post-tests. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the LPS group; ###p < 0.001, ####p < 0.0001 compared with the control group.
To further explore the anti-neuroinflammatory effects of 44FNP, results also showed that 44FNP was able to attenuate LPS-induced increase in iNOS (Fig. 2B) and TNFα secretion (Fig. 2C).
3.3. Effects of 44FNP on LPS-induced changes in mitochondrial membrane potential
A leaky mitochondrial membrane is a key characteristic of mitochondrial dysfunction. Loss of mitochondrial membrane potential (Δψm) is related to mitochondrial depolarization. To demonstrate the effects of 44FNP on LPS-induced mitochondrial dysfunction, we applied TMRM to monitor Δψm in BV2 cells. As shown in Fig. 3, exposing cells to LPS significantly decreased TMRM intensity, indicating the loss of Δψm. The loss of Δψm due to LPS was also attenuated by pretreatment of cells with 44FNP. Consequently, these results demonstrated the ability for 44FNP to suppress LPS-induced mitochondrial potential loss in microglial cells.
Fig. 3.
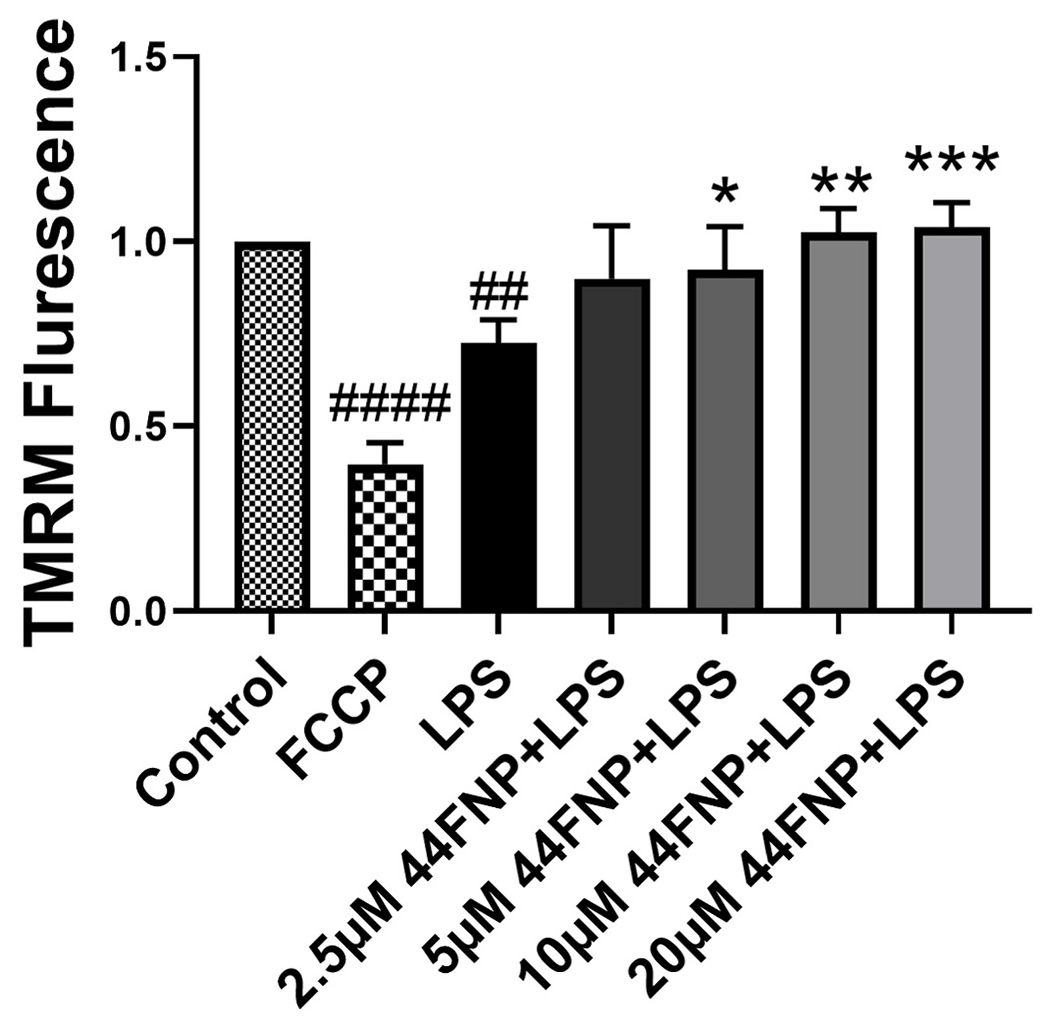
44FNP attenuated LPS-induced mitochondrial membrane potential loss in BV2 cells. Cells were pretreated with 44FNP for 1 h followed by stimulation with 50 ng/ml LPS for 24 h. Data are represented in mean ± SD (n = 3). Statistical analysis was carried out with one way ANOVA followed by Bonferroni post-tests. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the LPS group; ##p < 0.01, ####p < 0.0001 compared with the control group.
3.4. Effects of the 44FNP on Nrf2/HO-1
Our previous study demonstrated ability for exogenous DHA and its oxidative derivatives to upregulate the antioxidant pathway involving Nrf2/HO-1 in microglia. In this study, 44FNP dose-dependently upregulated Nrf2 and HO-1 in cells stimulated with LPS, but not in controls without stimulation with LPS (Fig. 4).
Fig. 4.
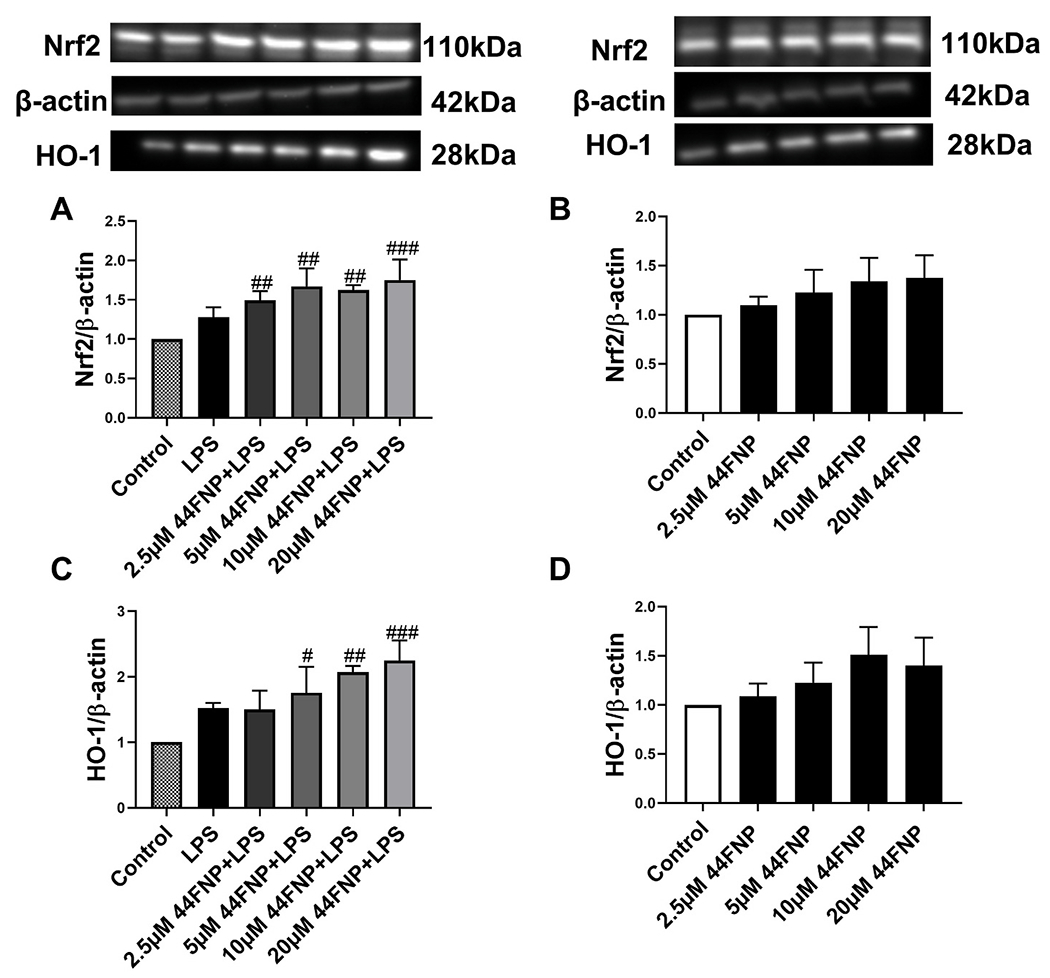
44FNP enhanced LPS-induced Nrf2/HO-1 pathway in BV2 cells. Cells were pretreated with 44FNP for 1 h followed by stimulation with 50 ng/ml LPS for 6 h. Data are represented in mean ± SD (n = 3). Statistical analysis was carried out with one way ANOVA followed by Bonferroni post-tests. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the control group.
4. Discussion
Free radical-induced peroxidation of arachidonic acid, another PUFA found in membrane phospholipids, results in the formation of isoprostanes [22]. These isoprostanes are elevated in brain and other body organs due to oxidant injury and are related to inflammatory mechanisms [23]. On the contrary, oxidative derivatives of DHA result in production of neuroprostanes which tend to show protective effects. In agreement with recent studies showing evidence for protective effects of DHA-derived 44FNP on the peripheral organs [16], results in this study demonstrated similar effects of this neuroprostane on microglial cells in the central nervous system.
In this study, we demonstrate the ability for 44FNP to suppress ROS production and inflammatory responses in both primary mouse microglia and immortalized microglial BV2 cells (Fig. 1A). By using gp91ds tat, results also demonstrated ROS production in microglia is mediated mainly through NADPH oxidase.
LPS is known to stimulate the Toll-like Receptor (TLR4) which is responsible for activation of the NF-κB transcriptional pathway [24]. Binding of the NF-κB dimer to the DNA consensus sequence of target genes leads to production of proinflammatory cytokines, such as TNFα [25]. Consistent with the anti-inflammatory effects of 44FNP demonstrated in human macrophage [26], our results with BV2 microglia also demonstrated the ability for 44FNP to suppress LPS-induced iNOS and TNFα through the NF-κB pathway (Fig. 2).
Mitochondrial membrane potential (Δψm) is generated from proton pump across the mitochondrial inner membrane and involves complexes I, III, and IV [27]. The stability of the Δψm is essential for many microglia functions [28]. In this study, we provided important new evidence showing that 44FNP was capable of suppressing LPS-induced loss of Δψm in microglia.
Nrf2 is an important transcription factor involving the maintenance of cellular redox state and alleviates expression of antioxidant genes such as HO-1. Some compounds that suppress NF-kB inflammation could also upregulate Nrf2/HO-1 [24,29]. In a recent study, 44FNP was found to elevate HO-1 mRNA expression in primary neurons [17]. However, study with microglia here showed effects of 44FNP on Nrf2/HO-1 only when cells were stimulated with LPS. These results suggest a role for 44FNP as an antioxidative agent in activated microglia.
In sum, this study demonstrated that 44FNP, a non-enzymatic peroxidation product of DHA, to exert neuroprotective effects on LPS-stimulated microglial cells through its abilities to attenuate oxidative stress, inflammatory responses, and mitochondrial dysfunction. Although future studies should include in vivo testing in animal models, this study has provided a better understanding on the mechanism(s) underlying the neuroprotective effects of 44FNP and better insights into its potential for the development of nutraceutical therapy for neurodegenerative diseases.
Acknowledgement
This work was supported by National Institutes of Health R01AG044404 (JCL).
References
- [1].Sun GY, Simonyi A, Fritsche KL, Chuang DY, Hannink M, Gu Z, Greenlief CM, Yao JK, Lee JC, Beversdorf DQ, Docosahexaenoic acid (DHA): an essential nutrient and a nutraceutical for brain health and diseases, Prostaglandins Leukot. Essent. Fatty Acids 136 (2018) 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J, Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease, Arch. Neurol 60 (7) (2003) 940–946. [DOI] [PubMed] [Google Scholar]
- [3].Chang Y-L, Chen S-J, Kao C-L, Hung S-C, Ding D-C, Yu C-C, Chen Y-J, Ku H-H, Lin C-P, Lee K-H, Docosahexaenoic acid promotes dopaminergic differentiation in induced pluripotent stem cells and inhibits teratoma formation in rats with Parkinson-like pathology, Cell Transplant. 21 (1) (2012) 313–332. [DOI] [PubMed] [Google Scholar]
- [4].Mallick R, Basak S, Duttaroy AK, Docosahexaenoic acid,22:6n-3: its roles in the structure and function of the brain, Int. J. Dev. Neurosci 79 (2019) 21–31. [DOI] [PubMed] [Google Scholar]
- [5].Milne GL, Dai Q, Roberts II LJ, The isoprostanes—25 years later, Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1851 (4) (2015) 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Leung K, Galano J, Durand T, Lee J-Y, Current development in non-enzymatic lipid peroxidation products, isoprostanoids and isofuranoids, in novel biological samples, Free Radic. Res 49 (7) (2015) 816–826. [DOI] [PubMed] [Google Scholar]
- [7].Reddy PH, Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease, J. Neurochem 96 (1) (2006) 1–13. [DOI] [PubMed] [Google Scholar]
- [8].Domenichiello AF, Sapio MR, Loydpierson AJ, Maric D, Goto T, Horowitz MS, Keyes GS, Yuan ZX, Majchrzak-Hong SF, Mannes AJ, Iadarola MJ, Ramsden CE, Molecular pathways linking oxylipins to nociception in rats, J. Pain 22 (3) (2021) 275–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Galano JM, Lee YY, Oger C, Vigor C, Vercauteren J, Durand T, Giera M, Lee JC, Isoprostanes, neuroprostanes and phytoprostanes: an overview of 25years of research in chemistry and biology, Prog. Lipid Res 68 (2017) 83–108. [DOI] [PubMed] [Google Scholar]
- [10].Ahmed OS, Galano JM, Pavlickova T, Revol-Cavalier J, Vigor C, Lee JC, Oger C, Durand T, Moving forward with isoprostanes, neuroprostanes and phytoprostanes: where are we now? Essays Biochem. 64 (3) (2020) 463–484. [DOI] [PubMed] [Google Scholar]
- [11].Galano J-M, Lee JC-Y, Gladine C, Comte B, Le Guennec J-Y, Oger C, Durand T, Non-enzymatic cyclic oxygenated metabolites of adrenic, docosahexaenoic, eicosapentaenoic and α-linolenic acids; bioactivities and potential use as biomarkers, Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1851 (4) (2015) 446–455. [DOI] [PubMed] [Google Scholar]
- [12].Roberts II LJ, Fessel JP, The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation, Chem. Phys. Lipids 128 (1–2) (2004) 173–186. [DOI] [PubMed] [Google Scholar]
- [13].Galano J-M, Mas E, Barden A, Mori TA, Signorini C, De Felice C, Barrett A, Opere C, Pinot E, Schwedhelm E, Isoprostanes and neuroprostanes: total synthesis, biological activity and biomarkers of oxidative stress in humans, Prostag. Other Lipid Mediat 107 (2013) 95–102. [DOI] [PubMed] [Google Scholar]
- [14].Peña-Bautista C, Vigor C, Galano JM, Oger C, Durand T, Ferrer I, Cuevas A, López-Cuevas R, Baquero M, López-Nogueroles M, Vento M, Hervás-Marín D, García-Blanco A, Cháfer-Pericás C, New screening approach for Alzheimer’s disease risk assessment from urine lipid peroxidation compounds, Sci. Rep 9 (1) (2019) 14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roy J, Oger C, Thireau J, Roussel J, Mercier-Touzet O, Faure D, Pinot E, Farah C, Taber DF, Cristol JP, Lee JC, Lacampagne A, Galano JM, Durand T, Le Guennec JY, Nonenzymatic lipid mediators, neuroprostanes, exert the antiarrhythmic properties of docosahexaenoic acid, Free Radic. Biol. Med 86 (2015) 269–278. [DOI] [PubMed] [Google Scholar]
- [16].Roy J, Fauconnier J, Oger C, Farah C, Angebault-Prouteau C, Thireau J, Bideaux P, Scheuermann V, Bultel-Poncé V, Demion M, Galano JM, Durand T, Lee JC, Le Guennec JY, Non-enzymatic oxidized metabolite of DHA, 4(RS)-4-F(4t)-neuroprostane protects the heart against reperfusion injury, Free Radic. Biol. Med 102 (2017) 229–239. [DOI] [PubMed] [Google Scholar]
- [17].Lee YY, Galano JM, Leung HH, Balas L, Oger C, Durand T, Lee JC, Nonenzymatic oxygenated metabolite of docosahexaenoic acid, 4(RS)-4-F(4t)-neuroprostane, acts as a bioactive lipid molecule in neuronal cells, FEBS Lett. 594 (11) (2020) 1797–1808. [DOI] [PubMed] [Google Scholar]
- [18].Roy J, Oliveira LT, Oger C, Galano J-M, Bultel-Poncé V, Richard S, Guimaraes AG, Vilela JMC, Andrade MS, Durand T, Polymeric nanocapsules prevent oxidation of core-loaded molecules: evidence based on the effects of docosahexaenoic acid and neuroprostane on breast cancer cells proliferation, J. Exp. Clin. Cancer Res 34 (1) (2015) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Oger C, Bultel-Poncé V, Guy A, Balas L, Rossi JC, Durand T, Galano JM, The handy use of Brown’s P2-Ni catalyst for a skipped diyne deuteration: application to the synthesis of a [D4]-labeled F4t-neuroprostane, Chemistry 16 (47) (2010) 13976–13980. [DOI] [PubMed] [Google Scholar]
- [20].Chuang DY, Simonyi A, Kotzbauer PT, Gu Z, Sun GY, Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway, J. Neuroinflammation 12 (2015) 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].He Y, Cui J, Lee JCM, Ding S, Chalimoniuk M, Simonyi A, Sun AY, Gu Z, Weisman GA, Wood WG, Sun GY, Prolonged exposure of cortical neurons to oligomeric amyloid-β impairs NMDA receptor function via NADPH oxidase-mediated ROS production: protective effect of green tea (−)-epigallocatechin-3-gallate, ASN neuro 3 (1) (2011), e00050 e00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Morrow AL, Pace JR, Purdy RH, Paul SM, Characterization of steroid interactions with gamma-aminobutyric acid receptor-gated chloride ion channels: evidence for multiple steroid recognition sites, Mol. Pharmacol 37 (2) (1990) 263–270. [PubMed] [Google Scholar]
- [23].Chen C, Agnès F, Gélinas C, Mapping of a serine-rich domain essential for the transcriptional, antiapoptotic, and transforming activities of the v-Rel oncoprotein, Mol. Cell Biol 19 (1) (1999) 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Giridharan S, Srinivasan M, Mechanisms of NF-κB p65 and strategies for therapeutic manipulation, J. Inflamm. Res 11 (2018) 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Albensi BC, What is nuclear factor kappa B (NF-κB) doing in and to the mitochondrion? Front. Cell Dev. Biol 7 (2019) 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bosviel R, Joumard-Cubizolles L, Chinetti-Gbaguidi G, Bayle D, Copin C, Hennuyer N, Duplan I, Staels B, Zanoni G, Porta A, Balas L, Galano JM, Oger C, Mazur A, Durand T, Gladine C, DHA-derived oxylipins, neuroprostanes and protectins, differentially and dose-dependently modulate the inflammatory response in human macrophages: putative mechanisms through PPAR activation, Free Radic. Biol. Med 103 (2017) 146–154. [DOI] [PubMed] [Google Scholar]
- [27].Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, Zorov DB, Mitochondrial membrane potential, Anal. Biochem 552 (2018) 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Agrawal I, Jha S, Mitochondrial dysfunction and alzheimer’s disease: role of microglia, Front. Aging Neurosci 12 (2020) 252, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang B, Li R, Michael Greenlief C, Fritsche KL, Gu Z, Cui J, Lee JC, Beversdorf DQ, Sun GY, Unveiling anti-oxidative and anti-inflammatory effects of docosahexaenoic acid and its lipid peroxidation product on lipopolysaccharide-stimulated BV-2 microglial cells, J. Neuroinflammation 15 (1) (2018) 202. [DOI] [PMC free article] [PubMed] [Google Scholar]


