Abstract
Recombinant beta-toxin from Clostridium perfringens type C was found to increase the conductance of bilayer lipid membranes (BLMs) by inducing channel activity. The channels exhibited a distribution of conductances within the range of 10 to 380 pS, with the majority of the channels falling into two categories of conductance at 110 and 60 pS. The radii of beta-toxin pores found for the conductance states of 110 and 60 pS were 12.7 and 11.1 Å, respectively. The single channels and the steady-state currents induced by beta-toxin across the BLMs exhibited ideal monovalent cation selectivity. Addition of divalent cations (Zn2+, Cd2+, or Mg2+) at a concentration of 2 mM increased the rate of beta-toxin insertion into BLMs and the single-channel conductance, while application of 5 mM Zn2+ to a beta-toxin-induced steady-state current decreased the inward current by approximately 45%. The mutation of arginine 212 of beta-toxin to aspartate, previously shown to increase the 50% lethal dose of beta-toxin for mice nearly 13-fold, significantly reduced the ability of beta-toxin to form channels. These data support the hypothesis that the lethal action of beta-toxin is based on the formation of cation-selective pores in susceptible cells.
Beta-toxin is produced by Clostridium perfringens type B and C strains and is the primary lethal factor in the type C strains. No molecular mechanism has been elucidated for beta-toxin which could be used as a basis for investigating its role in the pathogenesis of these clostridial pathogens. It has been suggested that beta-toxin may be a pore-forming toxin on the basis of weak similarities (10% identity) between the primary structure of beta-toxin and those of the pore-forming alpha-hemolysin and gamma-hemolysin and the leukocidin from Staphylococcus aureus (9). Whether or not beta-toxin is cytotoxic remains unclear; only a single report has suggested that beta-toxin is weakly cytotoxic on intestinal 407 cells (6). However, a previous study suggested that the cytotoxicity associated with beta-toxin preparations was not linked to the beta-toxin itself, but to minor contaminants in the toxin preparation from C. perfringens (11). Recently, Steinthorsdottir et al. demonstrated that beta-toxin could induce the release of arachidonic acid and inositol from human umbilical vein endothelial cells (HUVECs) (32). No cytolytic effects were reported, suggesting that beta-toxin may not be necessarily lethal to these cells. Several other cell types were also tested by these investigators, but they were unresponsive to beta-toxin.
C. perfringens type C strains cause necrotic enteritis primarily in pigs, chickens, cattle, sheep, and goats. Although adult animals can contract this disease, it most frequently occurs in the young of these species (34). Piglets are particularly susceptible to type C infections (5, 10, 18, 33), although a similar infection occurs in neonatal calves (7), lambs (8), and goats. During a type C infection, necrosis of the intestine can be extensive; death appears to be the result of toxemia with beta-toxin (reviewed in reference 29). Acute and peracute deaths frequently occur in these animals, suggesting that systemic effects of the toxin are important. In a C. perfringens type C disease of adult sheep, termed “struck,” the animals succumb to the infection so rapidly that they appear to have been struck by lightning. Prior to death, nervous signs such as tetani and opisthotonus have been observed in these animals (reviewed in reference 29), suggesting neurological involvement. Infection of humans by type C strains appears to be largely restricted to certain tribal populations in Papua New Guinea, although infrequent cases of type C infection have occurred in humans throughout the world. Type C infections result in necrotizing enterocolitis (“pigbel”) in these individuals after consumption of undercooked pork during certain ritualistic practices (13). Typically, type C necrotizing enterocolitis in humans resembles the disease in animals. The importance of beta-toxin in both animal and human disease has been demonstrated by immunization studies using a toxoid of beta-toxin. When immunized with the toxoid of beta-toxin, the Papua New Guinea tribespeople experienced a fivefold reduction in the incidence of necrotic enteritis (13), whereas a beta-toxin toxoid administered to infant pigs during an outbreak of necrotizing enterocolitis reduced mortality by approximately 30% (30). In the case of agriculturally important animals, vaccination against type C infections is universally advocated in order to avoid devastating losses. Therefore, beta-toxin plays a key role in the lethal outcome of type C infections, yet we know very little about its mechanism or the cell types it affects.
The results presented below demonstrate that beta-toxin is an efficient pore-forming toxin which generates potential-dependent, cation-selective channels in membranes. The channels formed by beta-toxin exhibit characteristics that may provide some insight into the lethal activity of this toxin.
MATERIALS AND METHODS
Bacterial strains, plasmids, and chemicals.
The gene for beta-toxin was cloned from an isolate from a piglet that succumbed to C. perfringens type C necrotic enteritis. Escherichia coli BLR/DE3 was used as the host for the expression of recombinant beta-toxin and its derivatives as previously described by Nagahama et al. (17). Beta-toxin and its derivatives were expressed as inactive glutathione S-transferase (GST) fusion proteins in the pGex4T-2 expression vector (Pharmacia, Piscataway, N.J.). Beta-toxin was cloned from the type C strain described above by PCR amplification of the gene using primers Beta-BamHI (5′-GGATCCGATATAGGTAAAACTACTACTAT) and Beta-EcoRI (5′-GGATCCGATATAGGTAAAACTACTACTAT). These primers placed a BamHI site at the 5′ end of the coding sequence of the mature toxin (without the signal peptide) and an EcoRI site at the 3′ end of the gene immediately downstream of the stop codon for the beta-toxin gene. Fusion of GST to the downstream beta-toxin was achieved between the carboxyl terminus of the GST and Asp29 of beta-toxin. The conversion of R212 of beta-toxin to aspartic acid was achieved using PCR overlap mutagenesis as previously described by Shepard et al. (27) for the mutation of the perfringolysin O gene. All chemicals were obtained from Sigma Chemical Co. (St. Louis, Mo.), and all lipids and sterols were purchased from Avanti Polar Lipids (Alabaster, Ala.).
Purification of recombinant beta-toxin.
Large-scale growth of the beta-toxin–GST fusion protein was initiated by inoculating one 8-liter carboy of sterile terrific broth (TB) (25), containing 200 μg of ampicillin/ml and 1 ml of sterile antifoam, with a 1:33 inoculum of an overnight culture (grown at 30°C) of E. coli BLR/DE3 expressing the GST–beta-toxin fusion protein. The large culture was incubated at 37°C with constant aeration by way of an air dispersion tube blowing sterile filtered air into the medium. Expression of the fusion protein was induced by the addition of isopropyl β-d-thiogalactopyranoside (IPTG; Gold Biochemicals, St. Louis, Mo.) to a final concentration of 0.2 mM when the A600 of the culture reached 1.0. The induced culture was grown for 3 h, and the cells were harvested by centrifugation.
The cell pellets from an 8-liter culture were suspended in 160 ml of 10 mM 2-(N-morpholino)ethanesulfonic acid (MES; Research Organics, Cleveland, Ohio)–150 mM NaCl (pH 6.5) (buffer A). The cells were lysed by passage through a French pressure cell at 20,000 lb/in2 (Aminco, Silver Spring, Md.). The cell debris was removed by centrifugation at 40,000 × g for 15 min. The beta-toxin-containing supernatant was loaded onto a column of glutathione Sepharose (Pharmacia) that had been equilibrated with buffer A. The GST–beta-toxin fusion bound to this column, while most other proteins passed through without binding. The bound fusion protein was eluted (1 ml/min) with 30 ml of buffer A containing 50 mM glutathione in 10 mM Tris-HCl (pH 8.0). The fractions containing the bulk of the beta-toxin were pooled, concentrated, and dialyzed overnight at 4°C in 2 liters of 10 mM Tris-HCl (pH 8) containing 100 mM NaCl. The fusion protein was then incubated overnight at room temperature with a 1:100 ratio (wt/wt) of thrombin to toxin. The next day, the mixture was treated with an excess of phenylmethylsulfonyl fluoride (PMSF) to inhibit the thrombin and was again passed over the glutathione Sepharose column, with the free beta-toxin collected as the flowthrough from the column. The fractions containing the beta-toxin were pooled and concentrated in an Amicon pressurized stirred cell equipped with a 10-kDa cutoff membrane. Glycerol was added to the toxin to a final concentration of 10%, and it was then quick-frozen in liquid N2 and stored at −80°C until used. All chromatography was performed with a Rainin (Woburn, Mass.) titanium high-pressure liquid chromatography system and Dynamax software.
Protein was measured by a rapid colorimetric protein assay (Pierce Chemical Co., Rockford, Ill.) according to the manufacturer's instructions. Bovine serum albumin was used as the protein standard.
LD50 determination.
Adult (approximately 10-week-old) female outbred ICR mice (approximately 28 g) were used to determine the 50% lethal dose (LD50) for the recombinant beta-toxin. The mice were injected intraperitoneally with 0.1 ml of various toxin dilutions and then monitored for 24 h. The time of death was recorded for each animal. Doses of beta-toxin varied from 6 to 0.5 μg per mouse, and a total of eight mice per group were used. The LD50 was calculated based on the method of Reed and Muench (19).
Planar bilayer experiments.
The various experiments to examine pore formation by beta-toxin were carried out using a planar bilayer system. Planar membranes were formed by painting a phosphotidylcholine (PC)-cholesterol mixture in heptane across a 180-μm-diameter hole in a Delrin cup held within a Warner bilayer chamber (Warner Instruments Corporation, Hamden, Conn.). The membrane separated equal volumes of buffer (1 ml) containing 10 mM HEPES (pH 7.4) and the various salts as required for each experiment. The PC and cholesterol were maintained at a ratio of 50.2 mol% PC and 49.8 mol% cholesterol, and the total lipid concentration in heptane solution was 20 mg/ml. The bilayer membrane capacitance was monitored until it stabilized, usually with a capacitance of 100 to 120 pF. The buffer solutions in both chambers could be stirred as required. Membrane formation was also visually monitored by using reflected light with a monocular microscope. Voltage clamp recordings of transmembrane current were made by using silver chloride electrodes immersed in a 0.2 M KCl solution with 0.2 M KCl agar bridges. Silver electrodes were used to connect the high-resolution amplifier headstage (Warner Instrument Corporation) with the bath solution in both compartments of the membrane chamber. The polarization potential between the electrodes did not exceed 1 to 1.5 mV. Voltage was clamped and controlled via the bilayer amplifier with a 1-kHz bandwidth. The Delrin cup is referred to as the trans side, and the outer polystyrene chamber is referred to as the cis side. The potential difference was referenced to the trans side of the membrane, which was defined as zero. Membrane currents were recorded on an XY recorder (Kipp and Zonnen; Fisher Scientific, Dallas, Tex.) or a digital tape drive (A. R. Vetter Co., Rebersburg, Pa.).
The radii of the channels formed by beta-toxin were determined by the rapid method of Sabirov et al. (20). This method is based on the observation that the addition of nonelectrolytes with different hydrodynamic radii to a 100 mM NaCl solution alters the current passing through the channel. The relationship between ionic channel conductance and the conductivity of a 100 mM NaCl solution containing different concentrations of nonelectrolytes defines parameter ν. Parameter ν is dependent on the hydrodynamic radius of the nonelectrolyte molecule. When the size of the nonelectrolyte is similar to the size of the channel, the value for parameter ν is zero. Low-molecular-weight nonelectrolytes, including ethylene glycol, glycerin, glucose, sucrose (Aldrich, Milwaukee, Wis.), and polyethylene glycols (PEGs) with average molecular weights of 300, 400, 1,000, 1,500, 2,000, 3,000, 4,000, 6,000, and 20,000 (Fluka, Milwaukee, Wis.), were mixed with a 100 mM solution of NaCl in 10 mM HEPES (pH 7.4) and used as membrane-bathing fluids at a final concentration of 20%. All electrophysiology experiments were carried out at room temperature (20 to 24°C).
RESULTS
Characterization of the recombinant beta-toxin.
Recombinant beta-toxin was produced as a GST fusion protein, and the GST was subsequently removed as described above. The resulting recombinant beta-toxin was tested for lethality in the mouse model and exhibited an LD50 of approximately 25 μg/kg of body weight (intraperitoneal administration), which falls into the range reported for beta-toxin isolated from C. perfringens (21, 22). Beta-toxin which had been heated to 100°C for 10 min did not exhibit lethal activity at the highest dose (160 μg/kg). It had been previously reported by Gibert et al. (6) that beta-toxin isolated from C. perfringens was cytotoxic on intestinal 407 cells at a concentration of 0.4 μg/ml. We did not detect cytotoxic activity for the recombinant toxin on this cell line at a maximum concentration of 67 μg/ml (not shown), which is approximately 168 times the concentration used by Gibert et al. in their studies. Given that the LD50 of the recombinant toxin is similar to that of the native toxin (and certainly no more than 10-fold less toxic), it seems unlikely that the cytotoxic effect would be completely abrogated in the recombinant toxin. Also, at least two other labs using independently isolated native or recombinant beta-toxin have not observed any cytotoxic effects when beta-toxin was applied to this cell line (J. G. Songer, personal communication; R. Titball, personal communication). This discrepancy may be linked to impurities in the beta-toxin isolated from C. perfringens. Knight et al. (11) had previously reported that preparations of beta-toxin from C. perfringens appeared to exhibit cytotoxic activity, but that this activity was not linked to beta-toxin.
Formation and characteristics of beta-toxin-dependent channels in planar bilayers.
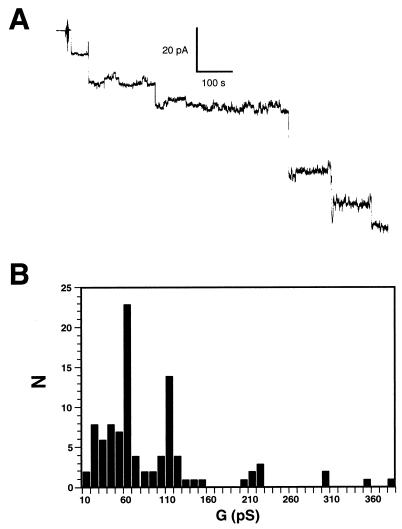
Purified recombinant beta-toxin was applied to a planar bilayer comprised of 50.2 mol% PC and 49.8 mol% cholesterol (Fig. 1). Within 30 s, channels were detected under a cis-negative potential (Fig. 1A). The conductance of beta-toxin channels varied within the range of 10 to 380 pS, with two major peaks at 60 and 110 pS (Fig. 1B). To rule out any channel-forming activity from an E. coli protein that may have contaminated the beta-toxin, we purified the GST expressed in E. coli under the same conditions as those used for the GST–beta-toxin fusion protein and then tested it for channel-forming activity. The purified GST was added to the cis compartment of the bilayer system at 18 times the concentration used for the formation of channels by beta-toxin. The voltage was clamped at −60 mV, and membrane conductance was monitored for more than 30 min. During this time no channel formation was detected, although the addition of beta-toxin to the same membrane resulted in the formation of channels (data not shown).
FIG. 1.
Beta-toxin induces channels in planar lipid bilayers. (A) Stepwise increase in current, driven by a constant voltage, after the introduction of beta-toxin. Channel openings are shown as downward deflections. Beta-toxin was added to the cis side of the membrane in a symmetric solution of 100 mM NaCl. (B) The distribution of single-channel conductance states is represented in an amplitude histogram. For these analyses, current increases on the membrane were monitored at the holding potential of −60 mV. Toxin was added to the cis side at a concentration of 129 nM.
It had been previously shown by Steinthorsdottir et al. (31) that the conversion of arginine 212 of beta-toxin to aspartate by in vitro mutagenesis of the beta-toxin gene increased the LD50 of the recombinant beta-toxin 12-fold for mice. The channel-forming activity of this beta-toxin derivative was significantly decreased; only two channels were detected over a time frame of 2.2 h (data not shown). By comparison, native toxin formed hundreds of channels within this time frame. Thus, the R212E mutation results in concomitant decreases in both the lethal activity of beta-toxin (31) and the rate of channel formation. This observation indicates that the channel-forming activity of beta-toxin is intimately linked to its lethal effect in animals.
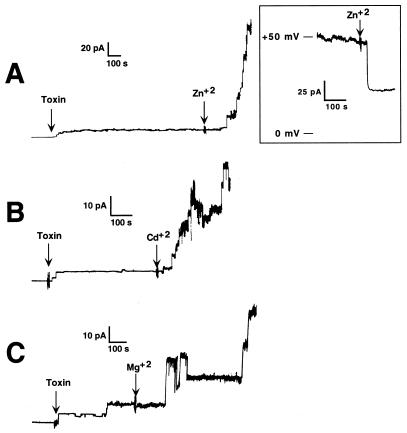
The addition of divalent cations such as Zn2+, Cd2+, or Mg2+ to a final concentration of 2 mM to the same compartment as the beta-toxin increased both the rate of insertion and the conductance of single channels in a 100 mM NaCl bathing solution (Fig. 2). Therefore, divalent cations appear to facilitate the insertion of beta-toxin into the planar bilayer.
FIG. 2.
Effects of divalent cations on beta-toxin insertion and channel conductance. The influence of Zn2+ (A), Cd2+ (B), and Mg2+ (C) on Na+ current through a BLM treated with beta-toxin is shown. In all experiments, toxin was added to the cis side of the membrane at a concentration of 10.8 nM. This concentration of beta-toxin was 12-fold lower than that used to generate the channels shown in Fig. 1 and typically did not produce significant numbers of channels for long periods of time. The divalent cations were added to the cis compartment at a final concentration of 2 mM at the times indicated. For these experiments the holding potential was −60 mV. Channel openings appear as upward deflections. Each panel represents a different membrane. (Inset) Blocking of the steady-state current, produced by multiple beta-toxin channels, by Zn2+ ions. Zn2+ was added to the cis side of the membrane to a final concentration of 5 mM. For this experiment the membrane was bathed in a symmetric solution of 100 mM NaCl, and the holding potential was maintained at +50 mV.
Although Zn2+ increased the rate of channel formation, it also decreased the channel conductance when it was added to preformed beta-toxin channels. When the concentration of Zn2+ was increased to 5 mM in the cis compartment after the channels had been formed, it decreased the steady-state inward current by 45% ± 5% (Fig. 2A, inset) in a 100 mM Na+ solution. The nature of the zinc-dependent closure of the preformed beta-toxin channels is unknown, but this phenomenon has been observed for other pore-forming toxins (1, 2, 4, 14–16). Other divalent cations, such as Ca2+, Cd2+, or Mg2+, at a concentration of 5 mM did not block the transmembrane current of 100 mM Na+.
Ionic selectivity and potential dependence of the beta-toxin channel.
The current-voltage (I/V) relationship of the beta-toxin channel was determined as conductance reached a steady-state level (Fig. 3A). Current-voltage curves were determined by the application of different consecutive voltage pulses (each lasting about 10 s). In order to diminish the effect of membrane capacity, extra time was allowed before recording the induced current when only one or a few channels were used in this analysis. Beta-toxin applied to the cis side of the membrane induced a membrane current that showed a voltage-dependent rectification (i.e., potential dependence) in all of the salt solutions tested (10 or 100 mM Na+, 10 or 100 mM K+, and 10 mM Ca2+). The current rectified at positive potentials, and approximately 2.1 times more current passed through the beta-toxin channel at −60 mV than at +60 mV (Fig. 3A). Therefore, the application of a positive potential decreased the probability of the open state for single channels (shown for a single channel in Fig. 3B). A potential of −80 to −100 mV also induced long-lasting closures for single channels and decreased the steady-state current for the membranes containing many channels after the application of holding potentials for several minutes or longer (data not shown). Beta-toxin incorporated from the trans side inverted the voltage dependence of the transmembrane current, i.e., more current passed at positive potentials (Fig. 3A, inset). These data indicate that beta-toxin molecules insert into the lipid bilayer in an oriented manner and that the beta-toxin channel remains in the open state when the negative potential is applied to the side of the membrane to which the toxin is added.
FIG. 3.
Ion selectivity of the beta-toxin channel. (A) Current-voltage (I/V) curves of a BLM modified with beta-toxin under bi-ionic conditions (i.e., the concentration of NaCl is held constant in the trans side and varied in the cis compartment, or vice versa). In all cases the trans side of the membrane was maintained at 100 mM NaCl whereas the cis side was consecutively washed with 100 mM NaCl (circles), 10 mM NaCl (triangles), and finally 100 mM NaCl (squares). Beta-toxin was introduced to the cis compartment and allowed to form channels prior to changing of the bathing solution for the cis compartment. The reversal potential was calculated from the intercept between the I/V plots obtained with NaCl concentrations of 100 mM (circles) and 10 mM (triangles) on the cis side, with the trans side maintained at 100 mM NaCl. At zero current the reversal potential was +57 mV. (Inset) Current-voltage relationships elicited after the addition of beta-toxin to the trans side of a membrane in a symmetric solution of 100 mM NaCl. Each panel represents a different membrane. For both experiments the final concentration of beta-toxin was 129 nM. (B) A single-channel record was obtained, and the membrane was held at a potential of −60 mV (lower trace) or +60 mV (upper trace). The bilayer was bathed in a symmetric bathing solution of 100 mM NaCl, and the beta-toxin was added to the cis side of the membrane to a final concentration of 10.8 nM.
The ion selectivity of the beta-toxin channel was determined by the examination of the reversal potential of the channel under different ionic conditions. The reversal potentials of the beta-toxin-induced currents under bi-ionic conditions were measured as the membrane conductance reached its steady state in a symmetric solution of 100 mM NaCl (Fig. 3A). To determine the selectivity of beta-toxin for cations and anions, the composition of the membrane-bathing solution was then changed on the cis side of the bilayer lipid membrane (BLM) in order to create bi-ionic conditions. Placing 10 mM NaCl on the cis side of the membrane and 100 mM NaCl on the trans side resulted in a 57 ± 1-mV shift of zero current potential (Fig. 3A). This coincides favorably with the value predicted by the Nernst equation (+58 mV) for ideal cation selectivity by the channel. Therefore, the current within the beta-toxin channel is comprised mainly of cations. For several preparations of beta-toxin (each obtained from a separate purification) utilizing other monovalent cations, we were able to estimate a specific permeability sequence. If the permeability of Na+ is assigned a value of 1, then the relative permeabilities for K+, Li+, Rb+, and Cs+ were 0.72, 0.59, 0.51, and 0.45, respectively. Therefore the beta-toxin channel was weakly selective for Na+ compared to other monovalent cations. Assuming that, in vivo, divalent cations may compete for the cation-selective site(s) of beta-toxin, we placed 10 mM CaCl2 on the cis side of a BLM against 10 mM NaCl on the trans side to compare the permeability of the channel for monovalent cations versus divalent cations. Under these bi-ionic conditions, the inward current reversed at +25 mV. The relative permeability calculated at this bi-ionic potential was 7.8-fold lower for Ca2+ then for Na+. This coincides favorably with the relative permeability for ideal monovalent cation selectivity predicted by the Goldman-Hodgkin-Katz equation (26). Thus, these data indicate that the beta-toxin channel current is comprised mainly of monovalent cations with a weak preference for Na+ and K+.
Beta-toxin pore radius.
The purified preparations of beta-toxin were observed to generate two major pools of ion channels in PC-cholesterol BLMs, with conductances of 110 and 60 pS (Fig. 1). As described by Sabirov et al. (20), the addition of the nonelectrolytes with different hydrodynamic radii into the bilayer bathing solution lowers the conductance of a channel as the hydrodynamic radius of the nonelectrolyte approaches that of the channel radius in a bilayer experiment. Parameter ν, which depends on the hydrodynamic radius of the nonelectrolytes, was defined as (G0 − G20)/(k0 − k20)/(G0/k0), where k20 and k0 are the electric conductivities of the 100 mM NaCl solution with and without nonelectrolytes (final concentration, 20% [wt/vol]) and G20 and G0 are the ion channel conductances in the same solutions, respectively (20). Assuming that the effective radius of the ion channel pore is close to the minimal size of an impermeable nonelectrolyte molecule, the radius of the beta-toxin pore was determined within the transition zone from limited permeation of a nonelectrolyte to impermeability at ν = 0. The radii of beta-toxin channels with conductances of 110 and 60 pS were determined to be 12.7 and 11.1 Å, respectively (Fig. 4).
FIG. 4.
The beta-toxin channel radius. Parameter ν (see Results for the definition of ν) was calculated as a function of the hydrodynamic radii of the nonelectrolyte PEG. The radius of the beta-toxin pore was determined within the transition zone from limited permeation of a nonelectrolyte to impermeability at ν = 0 (at the intersection between the dotted line at zero and linear curve fits). The radii of beta-toxin channels with conductances of 110 and 60 pS were determined to be 12.7 and 11.1 Å, respectively. Open circles represent the parameter ν calculated for the channels with a conductance of 110 pS in a PEG-free solution. Solid circles represent the parameter ν calculated for the channels with a conductance of 60 pS in a nonelectrolyte-free solution. The membrane-bathing solution contained 100 mM NaCl (pH 7.4). The holding potential applied to the membrane was −60 mV. The addition of low-molecular-weight nonelectrolyte molecules with hydrodynamic radii of less than 6 Å to the membrane-bathing solution did not decrease transmembrane current sufficiently, and therefore these were not included in this analysis.
DISCUSSION
Until now a mechanism for C. perfringens beta-toxin had not been directly demonstrated, although on the basis of weak similarities between its primary structure and those of pore-forming toxins from S. aureus it was proposed by others (9, 31) that it may be a pore-forming toxin. The elucidation of the mechanism of beta-toxin has proved difficult, since no cell types have been reported to be susceptible to this toxin, until recently (32). Steinthorsdottir et al. (32) showed that beta-toxin formed oligomeric complexes on the membranes of HUVECs and induced the leakage of inositol from these cells, although it was not shown if this release was a direct or an indirect effect of the toxin. Several other cell types were tested by these investigators, but none were affected by beta-toxin. Although beta-toxin induced the release of inositol and arachidonic acid from the HUVECs, there was no evidence that beta-toxin was cytolytic or lethal to these cells. The data presented here clearly show that beta-toxin readily forms ion-conductive channels in planar membranes, a finding consistent with those of Steinthorsdottir et al. (32).
The variability in beta-toxin channel conductance suggests that the channel formed by beta-toxin may have more than one conductance state. The basis for this variability is currently unknown. Two major peaks of conductance were identified for beta-toxin channels (60 and 110 pS), and each exhibited a slightly different pore size. It has been shown that other pore-forming toxins can exhibit some flexibility in their oligomer size, which would impact their single-channel conductance. For example, S. aureus alpha-hemolysin is apparently capable of forming both hexameric and heptameric oligomers (3, 28). The larger conductances observed for BLMs modified with beta-toxin (up to 380 pS) are probably the result of simultaneous insertion of several channels. Although the formation of beta-toxin channels in the lipid bilayer occurred at both positive and negative potentials, the channels were more likely to close under a cis-positive potential. The application of positive potentials to open channels changed the gating kinetics such that the beta-toxin channel exhibited long-lasting closures.
Channel formation by beta-toxin in the presence of monovalent cations, such as NaCl, appears to be enhanced by the presence of divalent cations. The increase in transmembrane current observed after the addition of divalent cations to the same side of the membrane as the toxin occurred because of an increased rate of incorporation and the increased conductance of single ion-conductive structures in the lipid bilayer. No activating effect was observed when divalent cations were added to the toxin-free side of the planar bilayer. However, it is still possible that an interaction between the divalent cations and the lipid bilayer, when on the same side as the toxin, increases the rate of insertion and conductance of beta-toxin channels. In certain cases, this property of divalent cations has been shown to facilitate the fusion of pore-forming proteins with lipid bilayers (12). Alternatively, the change in the actual conductance of the channels induced by the divalent cations may be the result of a structural change that is induced in the channel by these compounds; however, we do not have direct evidence of such a structural effect. Even though the reasons for the enhancement of insertion and conductance of the single channels by divalent cations remain obscure, we suggest that the presence of physiologically significant divalent cations on the same side of the membrane as beta-toxin may be important in its pore-forming activity in vivo. Steinthorsdottir et al. (32) showed that Ca2+ exhibited a positive effect on the beta-toxin-dependent release of arachidonic acid from HUVECs. Our results suggest that their observation may be at least partially attributed to the stimulatory effect that divalent cations have on the insertion and conductance of the beta-toxin channel.
Beta-toxin in which R212 had been converted to aspartate formed pores at a significantly reduced rate compared with the parent toxin. These data are consistent with a previous study that showed that the LD50 of beta-toxin for mice was significantly increased by this mutation (31). Also, the more recent study of Steinthorsdottir et al. (32) showed that this mutation significantly decreased the beta-toxin-induced release of arachidonic acid from HUVECs, as well as decreasing the extent to which it oligomerized on the membranes of these cells. Hence, these data correlate the essential role of pore formation with the activity of beta-toxin on cells and in vivo in animal models.
Whether beta-toxin can induce cell death remains unknown; however, the characteristics of the beta-toxin channel described herein may suggest an alternative role that does not require a cytotoxic effect. The characteristics of the beta-toxin channel and the in vivo neuromuscular perturbations induced by beta-toxin suggest that the autonomic and peripheral neuromuscular junctions may be primary targets of beta-toxin. The opening of beta-toxin-induced cation-selective channels could potentially trigger the rapid transport of Na+ and K+ across the nerve membrane, which would induce its rapid, and possibly irreversible, depolarization. The ion selectivity exhibited by the beta-toxin channel is consistent with the effects of beta-toxin observed in animals. Intraperitoneal administration of beta-toxin at several times the LD50 for mice results in sudden spastic muscle contractions immediately prior to the death of the animal (J. G. Songer, personal communication). Purified beta-toxin has also been shown to exhibit a variety of neuromuscular effects in rats. Sakuri et al. (24) showed that several physiological parameters in rats are dramatically affected by the intraperitoneal administration of beta-toxin. These effects included increased blood pressure and a decreased heart rate. Subsequently, it was shown by Sakurai et al. that beta-toxin induced arterial constriction and that the rise in blood pressure could be substantially reduced in rats that were treated with guanethidine or that had an adrenal medullectomy (23). Thus, it was concluded that beta-toxin had a direct effect on the autonomic nervous system.
In view of the recent findings of Steinthorsdottir et al. (32) and the results presented here, we propose that beta-toxin forms cation-conducting channels in susceptible cells but that it is not necessarily cytolytic. Hence, the lethal activity of beta-toxin may result from a direct perturbation of the cation distribution across the membranes of cells associated with the nervous system.
ACKNOWLEDGMENT
This research was supported by a grant from the United States Department of Agriculture (96-35204-37B8).
REFERENCES
- 1.Alder G M, Arnold W M, Bashford C L, Drake A F, Pasternak C A, Zimmermann U. Divalent cation-sensitive pores formed by natural and synthetic melittin and by Triton X-100. Biochim Biophys Acta. 1991;1061:111–120. doi: 10.1016/0005-2736(91)90275-d. [DOI] [PubMed] [Google Scholar]
- 2.Bashford C L, Rodrigues L, Pasternak C A. Protection of cells against membrane damage by haemolytic agents: divalent cations and protons act at the extracellular side of the plasma membrane. Biochim Biophys Acta. 1989;983:56–64. doi: 10.1016/0005-2736(89)90380-5. [DOI] [PubMed] [Google Scholar]
- 3.Czajkowsky D M, Sheng S, Shao Z. Staphylococcal alpha-hemolysin can form hexamers in phospholipid bilayers. J Mol Biol. 1998;276:325–330. doi: 10.1006/jmbi.1997.1535. [DOI] [PubMed] [Google Scholar]
- 4.Finck-Barbancon V, Duportail G, Meunier O, Colin D A. Pore formation by a two-component leukocidin from Staphylococcus aureus within the membrane of human polymorphonuclear leukocytes. Biochim Biophys Acta. 1993;1182:275–282. doi: 10.1016/0925-4439(93)90069-d. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald G R, Barker T, Welter M W, Welter C J. Diarrhea in young pigs: comparing the incidence of the five most common infectious agents. Vet Med. 1988;83:80–86. [Google Scholar]
- 6.Gibert M, Jolivet-Renaud C, Popoff M R. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene. 1997;203:65–73. doi: 10.1016/s0378-1119(97)00493-9. [DOI] [PubMed] [Google Scholar]
- 7.Griner L A, Bracken K F. Clostridium perfringens (type C) in acute hemorrhagic enteritis in calves. J Am Vet Med Assoc. 1953;122:99–102. [PubMed] [Google Scholar]
- 8.Griner L A, Johnson H W. Clostridium perfringens type C in hemorrhagic enterotoxemia of lambs. J Am Vet Med Assoc. 1954;125:125–127. [PubMed] [Google Scholar]
- 9.Hunter S E, Brown J E, Oyston P C, Sakurai J, Titball R W. Molecular genetic analysis of beta-toxin of Clostridium perfringens reveals sequence homology with alpha-toxin, gamma-toxin, and leukocidin of Staphylococcus aureus. Infect Immun. 1993;61:3958–3965. doi: 10.1128/iai.61.9.3958-3965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson M W, Fitzgerald G R, Welter M W, Welter C J. The six most common pathogens responsible for diarrhea in newborn pigs. Vet Med. 1992;87:382–386. [Google Scholar]
- 11.Knight P A, Queminet J, Blanchard J H, Tilleray J H. In vitro tests for the measurement of clostridial toxins, toxoids and antisera. II. Titration of Clostridium perfringens toxins and antitoxins in cell culture. Biologicals. 1990;18:263–270. doi: 10.1016/1045-1056(90)90028-x. [DOI] [PubMed] [Google Scholar]
- 12.Knoll W, Apell H J, Eibl H, Miller A. Direct evidence for Ca++-induced lateral phase separation in black membranes of lipid mixtures by the analysis of gramicidin A single channels. Eur Biophys J. 1986;13:187–193. doi: 10.1007/BF00542562. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence G W, Lehmann D, Anian G, Coakley C A, Saleu G, Barker M J, Davis M W. Impact of active immunisation against enteritis necroticans in Papua New Guinea. Lancet. 1990;336:1165–1167. doi: 10.1016/0140-6736(90)92776-e. [DOI] [PubMed] [Google Scholar]
- 14.Mahadevan D, Ndirika A, Vincent J, Bashford L, Chambers T, Pasternak C. Protection against membrane-mediated cytotoxicity by calcium and zinc. Am J Pathol. 1990;136:513–520. [PMC free article] [PubMed] [Google Scholar]
- 15.Menestrina G. Ionic channels formed by Staphylococcus aureus alpha-toxin: voltage-dependent inhibition by divalent and trivalent cations. J Membr Biol. 1986;90:177–190. doi: 10.1007/BF01869935. [DOI] [PubMed] [Google Scholar]
- 16.Menestrina G, Bashford C L, Pasternak C A. Pore-forming toxins: experiments with S. aureus alpha-toxin, C. perfringens theta-toxin and E. coli haemolysin in lipid bilayers, liposomes and intact cells. Toxicon. 1990;28:477–491. doi: 10.1016/0041-0101(90)90292-f. [DOI] [PubMed] [Google Scholar]
- 17.Nagahama M, Kihara A, Miyawaki T, Mukai M, Sakaguchi Y, Ochi S, Sakurai J. Clostridium perfringens beta-toxin is sensitive to thiol-group modification but does not require a thiol group for lethal activity. Biochim Biophys Acta. 1999;1454:97–105. doi: 10.1016/s0925-4439(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 18.Pace L W, Kreeger J M, Miller M A, Turnquist S E, Fischer J R, Johnson G C, Turk J R, Pittman L L, Fales L H, Maddox C W, Rottinghaus A A, Gosser H S. Necropsy findings from Vietnamese potbellied pigs, 33 cases. J Vet Diagn Investig. 1992;4:351–352. doi: 10.1177/104063879200400325. [DOI] [PubMed] [Google Scholar]
- 19.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 20.Sabirov R Z, Krasilnikov O V, Ternovsky V I, Merzliak P G. Relation between ionic channel conductance and conductivity of media containing different nonelectrolytes: a novel method of pore size determination. Gen Physiol Biophys. 1993;12:95–111. [PubMed] [Google Scholar]
- 21.Sakurai J, Duncan C L. Some properties of beta-toxin produced by Clostridium perfringens type C. Infect Immun. 1978;21:678–680. doi: 10.1128/iai.21.2.678-680.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai J, Fujii Y. Purification and characterization of Clostridium perfringens beta toxin. Toxicon. 1987;25:1301–1310. doi: 10.1016/0041-0101(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 23.Sakurai J, Fujii Y, Dezaki K, Endo K. Effect of Clostridium perfringens beta toxin on blood pressure of rats. Microbiol Immunol. 1984;28:23–31. doi: 10.1111/j.1348-0421.1984.tb02944.x. [DOI] [PubMed] [Google Scholar]
- 24.Sakurai J, Fujii Y, Matsuura M, Endo K. Pharmacological effect of beta toxin of Clostridium perfringens type C on rats. Microbiol Immunol. 1981;25:423–432. doi: 10.1111/j.1348-0421.1981.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Shamoo A E, Goldstein D A. Isolation of ionophores from ion transport systems and their role in energy transduction. Biochim Biophys Acta. 1977;472:13–53. doi: 10.1016/0304-4157(77)90013-2. [DOI] [PubMed] [Google Scholar]
- 27.Shepard L A, Heuck A P, Hamman B D, Rossjohn J, Parker M W, Ryan K R, Johnson A E, Tweten R K. Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an α-helical to β-sheet transition identified by fluorescence spectroscopy. Biochemistry. 1998;37:14563–14574. doi: 10.1021/bi981452f. [DOI] [PubMed] [Google Scholar]
- 28.Song L Z, Hobaugh M R, Shustak C, Cheley S, Bayley H, Gouaux J E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 29.Songer J G. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Springer S, Selbitz H J. The control of necrotic enteritis in sucking piglets by means of a Clostridium perfringens toxoid vaccine. FEMS Immunol Med Microbiol. 1999;24:333–336. doi: 10.1111/j.1574-695X.1999.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinthorsdottir V, Fridriksdottir V, Gunnarsson E, Andresson O S. Site-directed mutagenesis of Clostridium perfringens beta-toxin—expression of wild-type and mutant toxins in Bacillus subtilis. FEMS Microbiol Lett. 1998;158:17–23. doi: 10.1111/j.1574-6968.1998.tb12794.x. [DOI] [PubMed] [Google Scholar]
- 32.Steinthorsdottir V, Halldorsson H, Andresson O S. Clostridium perfringens beta-toxin forms multimeric transmembrane pores in human endothelial cells. Microb Pathog. 2000;28:45–50. doi: 10.1006/mpat.1999.0323. [DOI] [PubMed] [Google Scholar]
- 33.Taylor D J. Causes of enteritis in young piglets. Proc Pig Vet Soc. 1984;11:56–66. [Google Scholar]
- 34.Timoney J F, Gillespie J H, Scott F W, Barlough J E. Hagan and Bruner's microbiology and infectious diseases of domestic animals. New York, N.Y: Comstock Publishing Associates; 1988. [Google Scholar]