Abstract
Background:
The pathology of primary osteoarthritis (OA) begins with structural cartilage damage, which initiates a self-propagating inflammatory pathway that further exacerbates cartilage deterioration. Current standard of care for knee primary OA involves treating the inflammatory symptoms to manage pain, which includes intra-articular (IA) injections of cortisone, an anti-inflammatory steroid, followed by a series of joint-cushioning hyaluronic acid gel injections. However, these injections do not delay the progression of primary OA. More focus on the underlying cellular pathology of OA has prompted researchers to develop treatments targeting the biochemical mechanisms of cartilage degradation.
Purpose:
Researchers have yet to develop a United States Food and Drug Administration (FDA)–approved injection that has been demonstrated to significantly regenerate damaged articular cartilage. This paper reviews the current research on experimental injections aimed at achieving cellular restoration of the hyaline cartilage tissue of the knee joint.
Study Design:
Narrative review.
Methods:
The authors conducted a narrative literature review examining studies on primary OA pathogenesis and a systematic review of non–FDA-approved IA injections for the treatment of primary OA of the knee, described as “disease-modifying osteoarthritis drugs” in phase 1, 2, and 3 clinical trials.
Conclusion:
New treatment approaches for primary OA investigate the potential of genetic therapies to restore native cartilage. It is clear that the most promising IA injections that could improve treatment of primary OA are bioengineered advanced-delivery steroid-hydrogel preparations, ex vivo expanded allogeneic stem cell injections, genetically engineered chondrocyte injections, recombinant fibroblast growth factor therapy, injections of selective proteinase inhibitors, senolytic therapy via injections, injectable antioxidant therapies, injections of Wnt pathway inhibitors, injections of nuclear factor–kappa β inhibitors, injections of modified human angiopoietin-like–3, various potential viral vector–based genetic therapy approaches, and RNA genetic technology administered via injections.
Keywords: osteoarthritis, injections, articular cartilage, regeneration
Osteoarthritis (OA) is the most prevalent form of arthritis, affecting over 30 million Americans annually, approximately one-fourth of the total adult population of the United States (US).17,34 This disease frequency is greatly increased among elderly cohorts, as studies show over 80% of people older than 75 years in Western countries and over 70% of Americans between the ages of 55 and 70 years are affected by primary OA. 1 OA is also one of the most economically costly diseases, as it accounted for over $16.5 billion in hospital costs in the US in 2013. 17 The knee joint is the most common joint affected by OA. 17 The Global Burden of Disease 2020 report revealed an 8% to 9% increase in the prevalence of OA from 1990 to 2017. 49 The prevalence of this condition among the national and global populations is expected to substantially increase as obesity rates rise.35,36
OA of the knee joint is characterized by the progressive loss of articular cartilage at the lateral and medial femoral condyles, tibial plateau, and patellofemoral surface. 77 This cartilage degradation is associated with chronic pain, knee joint inflammation, and loss of mobility. 82 The primary symptom of primary OA is worsening joint pain, leading to loss of joint function and physical disability. 18
Our understanding of primary OA has advanced beyond the initial “wear and tear” paradigm of disease pathology. Modern medical technology has unveiled that primary OA is a multifactorial, heterogenous, whole-joint degenerative disease influenced by both genetic and environmental factors.17,35,39,45 The pathogenesis of primary OA involves interactions of mechanical, cellular, and biochemical processes within the joint, synovium, periosteum, and underlying bone, ultimately perpetuating a malicious cycle of inflammation, chondrotoxicity, and osteochondral modification.17,39
As a result of the disease’s complexity, developing a universal cure suitable for all patients is incredibly challenging. 49 The treatment of knee OA is further complicated due to relative avascular composition of adult hyaline cartilage. Limited blood flow to the articular cartilage permits tissue strength and durability, but limits its regenerative capacity due to reduced perfusion of growth factors, nutrients, and other compounds that would otherwise enhance chondrogenesis. 17 Surgical treatment options are expensive, pose many risks to the patient’s health, and are not guaranteed to eliminate pain or completely restore function. OA of the knee joint is incredibly prevalent and economically costly throughout much of the developed world. Given these facts, it is clear why such a robust focus has been placed on developing a less invasive treatment modality that can modify the pathology of the disease.
Due to the local immune and paracrine dysregulation in the joint, paired with the avascular nature of articular cartilage, oral medications are unlikely to achieve adequate perfusion into the necessary tissues in order to modify cellular function. Therefore, the majority of cellular therapies currently being investigated for the treatment of OA are intra-articular (IA) injections of the knee. An IA injection allows the drug direct access to the affected tissue with efficient delivery, and further poses less risks to the patient than a surgical procedure. A pharmaceutical agent capable of altering disease progression by arresting cartilage loss is referred to as a “disease-modifying osteoarthritis drug” (DMOAD). 49 Currently, the United States Food and Drug Administration (FDA) and the European Medicines Agency have not yet approved any medication as an effective DMOAD. 49
STUDY OBJECTIVES
The purpose of this article was to review the most recent literature on new developments in human trials involving experimental, injectable DMOADs. Through evaluation and comparison of this literature, we aimed to elucidate the most promising therapeutic mechanisms and targets for DMOADs. This information will hopefully guide future researchers as to where to invest resources when developing new DMOADs. Furthermore, through such a robust literature review, this article will also illustrate our current understanding of the multifactorial nature of primary OA of the knee.
PATHOLOGY OF OA
OA is a complex polygenic disease, now recognized as a clinical syndrome that is not yet fully understood.39,47,49,53,66 The pathophysiology of OA involves metabolic, genetic, epigenetic, cellular senescence, and environmental components that all contribute to disease advancement.35,43 Genetic predisposition, determined by multiple “risk loci,” plays a large role in one’s susceptibility to local immune dysregulation within the knee joint as a result of initial tissue injury.43,49 OA is further understood to be a multitissue disease, involving nearly all tissues within the joint. In knee OA, meniscal degeneration, subchondral bone sclerosis, synovitis, and more all contribute to the metabolic and immune dysfunction that underlie OA pathology. 52 Subchondral bone changes in OA involve macrophage infiltration, aberrant osteoclast proliferation, osteoblast activation, and environmental acidification. 49
Initial cartilage tissue loss from wear and tear mechanisms may initiate inflammatory and cell regulatory cascades, ultimately inducing aberrant cell-cell signaling and gene expression.17,39,55 Cartilage degeneration from shear stress has been shown to stimulate chondrocytes’ production of extracellular matrix (ECM)–degrading enzymes. In a healthy knee, chondrocytes maintain the integrity of the hyaline cartilage through genetic expression of proteins, signaling molecules, and enzymes that sustain the ECM.34,45,49 In OA, chondrocyte maintenance of the ECM is impaired, causing alterations in gene expression, leading to excess production of pro-inflammatory signaling molecules, catabolic enzymes, and other disruptive paracrine mediators.34,55,66,70 Inflammatory cytokines overexpressed in OA have been demonstrated to upregulate adhesion molecules including E-selectin, intracellular adhesion molecule–1 (ICAM-1), vascular cell adhesion molecule–1 (VCAM-1), and P-selectin on endothelial vessels, enhancing lymphocyte migration. 62
Numerous cell signaling pathways, such as Wnt, nuclear factor–kappa β (NF-κβ), transforming growth factor–β (TGF-β), Notch, autophagy, and the cell cycle, have all been implicated in OA pathogenesis. 52 Recent studies have implicated inflammation-induced loss of proliferative capacity of CD271+ native, joint-resident mesenchymal stem cells (MSCs) in OA pathogenesis. 43 Ultimately, the destroyed hyaline cartilage is replaced by fibrocartilage, which is inadequate to handle the role of innate articular cartilage, leading to joint stiffness and pain. 62
Cell-cell interactions between chondrocytes, fibroblasts, T and B cells, neutrophils, mast cells, and endothelial cells trigger increased expression of pro-inflammatory cytokines and interleukins, aberrant macrophage recruitment, increased extracellular protease expression, chondrocyte metabolic dysfunction and apoptosis, and other inflammatory pathways.12,17,34,35,49,53 This is seen as degeneration of the collagen ECM, chondrocyte apoptosis, osteophyte production, and bone remodeling of the femoral condyles/tibial plateau.12,17 This chondral loss advances slowly and symptoms generally appear late in the progression to primary OA. 12
OA TREATMENT AND MANAGEMENT
Currently, there are no FDA-approved DMOADs that can halt or reverse the progression of the OA.3,17,18 Due to the lack of available curative therapies, the current standard of care focuses on pain management.3,17,18 Conventional approaches currently use IA injections to target inflammation, while encouraging lifestyle changes to strengthen and protect the joint from further damage (physical therapy, exercise, diet, etc).3,8,17 However, these strategies do not address the underlying disease pathology. 8 Pain is typically treated with anti-inflammatory therapies via both oral and IA routes. Anti-inflammatory drugs are limited in efficacy and further pose the risk of expensive and serious adverse events including immunosuppression, along with gastrointestinal, cardiovascular, and renal complications.2,69
Official recommendations for the treatment of OA are divided into nonpharmacological (exercise, physical therapy, dietary changes, etc), pharmacological (oral medications, IA injections, etc), and surgical (autologous chondrocyte implantation, microfracture, total knee arthroplasty, etc) interventions. 71 The most severe stages of arthritis require total joint arthroplasty, a costly operation that poses many risks and ultimately fails to permanently alleviate pain in 20% of patients. 17
Since primary OA is a very localized condition, IA injections pose an attractive strategy to target the affected tissue while minimizing adverse side effects. 18 However, the efficacy of intra-articularly injected substances such as corticosteroids and hydrogels is limited by factors such as lymphatic clearance, synovial vascular absorption, proteinase degradation, and more. 18 IA glucocorticoid injections provide effective short-term pain relief and have been shown to temporarily improve joint function.78,79 However, these effects are limited by the short half-life of glucocorticoids in the joint and subsequent relatively brief duration anti-inflammatory and analgesic effects. 82 Furthermore, there are conflicting data as to whether long-term use of IA corticosteroid injections is safe in OA, as animal and in vivo studies have demonstrated cytotoxicity to articular cartilage, although this has not been definitively proven in large human trials. 33 Many authors agree that more long-term prospective studies are required to elucidate all the risks and benefits of chronic IA glucocorticoid injections. 33
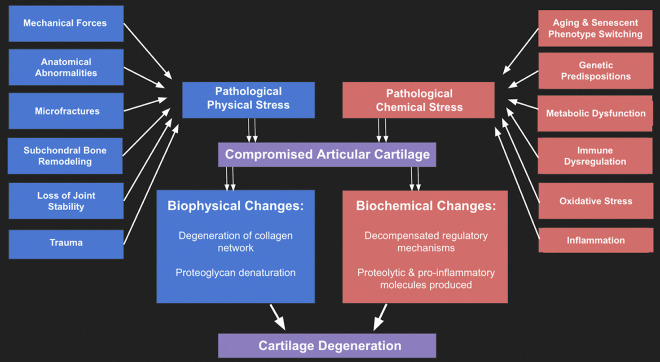
Currently, there are various DMOADs undergoing investigation, with a variety of molecular, genetic, and cellular targets. In order to achieve true modification of the primary OA disease progression, new therapies must be able to induce changes in gene expression, cell metabolism, and/or intracellular signaling.4,9,16,17,59,60 (Figure 1). To this end, significant effort has gone into generating cell-based therapies that could potentially halt or reverse OA.59,62
Figure 1.
Flowchart of factors involved in primary osteoarthritis. 4
HYDROGEL INJECTIONS
Hyaluronic Acid Biochemistry in Primary OA
Hyaluronic acid (HA) is a glycosaminoglycan (GAG) that is one of the main components of both the ECM of articular cartilage tissue and the knee joint synovial fluid, which further plays a vital role in several physiological processes that regulate cell migration, proliferation, differentiation, and inflammation. 41 One of the most important functional properties of HA is its ability to bind water up to 10,000 times its own weight, which is a key factor in its ability to cushion the joint and protect the native hyaline cartilage.41,49 Decreases in joint HA have been implicated in the pathology of OA, as HA concentrations have been found to be reduced in knees of patients with OA. 17 Additionally, the synovial concentration of HA is a determining factor in the production of hyaline cartilage and is associated with the expression of chondrogenic genes. 41 HA is known to interact with CD44 glycoprotein receptors on chondrocytes to enhance chondrocyte metabolism and function. 54 Furthermore, HA may exert chondroprotective effects through scavenging free oxygen radicals and improving regulation of immune complex interactions. 55
Due to its excellent biocompatibility, HA is FDA approved in its native form as a viscosupplementation IA injection for the treatment of primary OA of the knee joint.41,49 Exogenous HA supplementation into the knee joint reinforces the ECM of the native cartilage, improves synovial fluid viscoelasticity, and helps to provide temporary joint lubrication and cushioning.17,39 A series of 3 once-weekly IA HA injections have been demonstrated in several clinical trials to provide significant short-term OA pain reduction over several months. However, this relief is temporary and additional triplet HA injection series are typically required either biannually or annually.17,39,49 Additionally, common side effects of IA HA injection include musculoskeletal pain, joint swelling, joint stiffness, injection site pain, erythema, and more, according to the Mayo Clinic website. 44 HA is currently one of the most widespread therapies for primary OA. However, HA is not a DMOAD, and there is no evidence that HA delays or prevents the need for knee joint replacements.17,41,42,49,50
Advancements in Viscosupplementation
While HA is not considered a DMOAD, advancements in HA and hydrogel drug delivery systems have improved its capability to reduce symptoms of OA. Recent innovations that may increase the therapeutic efficacy of HA include cross-linked hydrogels, modified-structure HA derivatives, living HA derivatives, combination therapies using HA and platelet-rich plasma (PRP), and the use of HA derivatives to deliver MSCs.17,18,41,49,57
HA can be cross-linked with itself and with other polysaccharide polymers to form hydrogels, enhancing its chemical stability while retaining biocompatibility. 55 This increases the retention time of HA in the joint, providing more lasting pain relief.54,55 One hybridized cross-linked hydrogel being studied for primary knee OA is HA cross-linked with polyethylene glycol (PEG). 55 Injectable hydrogels derived from 2 parent polymers are known to integrate properties of both parents.54,55 PEG is a synthetic water-binding gel that, when hybridized with HA via covalent interconnected polymer chains, can improve PEG bioactivity while enhancing HA stability within the joint.18,41,49 The cross-linking of HA polymer chains is also theorized to improve in situ gelation in the joint, increasing its therapeutic potential.54,55 Through cross-linking HA derivatives, researchers have developed potentially superior hydrogels for IA injection in the management of knee OA.18,41,49
Another strategy is to introduce hydrophobic groups into the chain matrix to promote amphipathic properties. An example of one of these products is HYAFF (Haemo Pharma), which consists of hyaluronan esters with a uniquely modifiable structure whose microporosity is directly correlated with the degree of esterification, allowing for fine-tuning of viscosity and opening the potential for usage as a scaffold for delivery of stem cells. 41 Another example is HYADD (Fidia Pharma USA), an elastic hydrogen with incorporation of hexadecylamine into the polymer, which has been demonstrated to have increased joint residence time compared with traditional HA.21,41 According to the official website for HYADD, side effects are comparable to those of traditional HA injections, including arthralgia, transient pain, and swelling. 21
Researchers have also covalently modified HA with additional biologically relevant molecules, such as ECM proteins. 41 Adding ECM proteins to the hydrogel permits entrapment of antibodies within HA, which enhance its delivery to target immune cells. 41 This has been demonstrated with the addition of fibronectin, a cell adhesion protein; various GAGs; and heparin-like molecules, which are theorized to have further therapeutic potential through recruiting key factors for chondrogenesis. 41
Kartogenin
Hydrogels have been further improved through integrating a recently discovered chondrogenic molecule, kartogenin (KGN), into the hydrogel matrix.20,40,79,81 This has been shown to provide HA with improved chondroprotective properties.20,55 While still largely being evaluated in preclinical models, there is resoundingly positive evidence for the efficacy of KGN-hydrogel IA injections to potentially act as a DMOAD.40,55,81 KGN is known to be chondrogenic, as confirmed in multiple ex vivo cultures and animal models of hyaline cartilage where KGN was shown to stimulate proliferation of cartilage stem cell progenitors.55,72,79,81 In vitro studies of human chondrocyte OA models have demonstrated that KGN administration results in increased collagen 2:1 expression and decreased matrix metalloproteinase (MMP) expression, promoting the innate properties that support the function of articular cartilage.40,72 These studies have also shown increases in gene expression of interleukin-6 (IL-6) and its receptor, revealing an anti-inflammatory mechanism. 40 This study also observed chondrogenesis in the KGN-treated model with an increased concentration of CD44+/CD105+ HSCs, potentially stimulated via the IL-6/Stat3 pathway. 40
The precise molecular pathways involved in KGN’s promotion of chondrocyte differentiation are still being investigated. One 2019 study revealed that KGN is hydrolyzed in the joint to produce 4-aminobiphenyl (4-ABP), which is later found to be incorporated into chondrocytes in a rat OA model. 81 OA-induced rats treated exclusively with IA 4-ABP isolate injections showed reduced OA compared with both KGN-injected and control models. 81 Transcriptional profiling suggested that 4-ABP upregulates the PRS6KA2 and PI3K-Akt pathways to promote MSC proliferation and repair. 81 Since most published studies regarding KGN involve animal models, more information regarding potential adverse effects in humans is required. Currently, a KGN-derived molecule, KA34, is in phase 1 human clinical trials as an injectable DMOAD (ClinicalTrials.gov identifier: NCT03133676).
Hydrogels and HA Injection: Future Directions
While hydrogels themselves do not reverse the progression of primary OA, they represent one of the most popular and efficacious tools for symptom management for OA. Given the success of HA in providing temporary symptom relief in a portion of patients with knee OA, new research in this area aims to enhance and improve the mechanisms of HA within the joint.
PRP INJECTION
Overview
PRP is a blood product concentrated to contain mostly activated platelets through the process of centrifugation. These activated platelets drive the secretion of many beneficial growth factors such as insulin-like growth factor–1 (IGF-1), hepatocyte growth factor, and platelet-derived growth factor that mediate tissue healing and regeneration. 17 IA injections of PRP have been shown in some studies to improve the dysfunctional homeostasis in joint tissues seen in knee osteoarthritic patients.17,63 This improvement has been theorized to be achieved primarily through chondroprotective, anabolic, anti-inflammatory, and immunomodulatory effects, leading to the reduction of pain and the improvement of physical function.17,63 Similarly, PRP injections have been shown to reduce OA pain and lead to improved prognosis in OA when compared with age-matched patients receiving IA HA. 23 However, in many cases, IA injections alone are not sufficient to adequately reduce the somatic pain associated with OA. 60 Supplementing IA injections with intraosseous PRP injections is an interesting strategy that has been shown to have more potent anti-inflammatory effects and tissue regeneration. 60 However, this is more invasive and requires anesthesia.41,60 Furthermore, one variable that may affect the efficacy of PRP treatment for OA is the concentration of leukocytes in the plasma.17,23,47,60 Studies have been inconsistent on the advantages and drawbacks of leukocyte-rich (LR) versus leukocyte-poor (LP) PRP.6,17,23,47,60 Regarding adverse effects, research with PRP has described knee joint pain, swelling, and stiffness, but not any serious events. 6 A clearer elucidation of this topic should drastically improve the understanding of the efficacy and consistency of PRP treatment.
Pharmacodynamics of PRP
Most studies on PRP focus on its ability to suppress cytokines to reduce inflammation in patients with OA, leading to pain relief.12,17,60 PRP is hypothesized to exert additional chondroprotective and potentially regenerative effects through various cellular mechanisms.6,12,17,60 Specifically, PRP contains many growth factors (eg, TGF-β, IGF-1, bone morphogenic proteins, platelet-derived growth factor, vascular endothelial growth factor, epidermal growth factor, fibroblast growth factor [FGF], and hepatocyte growth factor) that increase the proliferation and differentiation of chondrocytes leading to tissue regeneration and reducing the tissue damage in OA.12,17,60 However, such effects have proved to be temporary, with most advanced treatments lasting up to 12 months before regression. 17 The current belief is that PRP could potentially lead to the creation of new, permanent cartilage cells, but this has not yet been definitively proven in human subjects.
Evidence From Clinical Trials: PRP IA Injections
Many published clinical trials agree on the superiority of PRP compared with other IA injections for early-stage knee OA. Additionally, PRP therapeutic effects are believed to possibly last longer, usually up to a year. 47 In terms of late-stage knee OA, a 2017 clinical trial publication by Nayana 47 confirmed that even a single PRP IA injection was successful in reducing knee OA pain and improving quality of life. However, this study did not find that PRP IA injections were superior to other treatment options. In elderly patients (age, ≥67 years), it was demonstrated that a single PRP IA injection had similar effects to a single shot of corticosteroid. 47 Despite good evidence from many human trials, additional clinical trials are needed to determine if PRP is superior to other available IA injections for knee OA.
Intraosseous PRP Injections
Traditionally, PRP is delivered via IA injections. However, previous studies have shown that this strategy does not target the subchondral bone.60,63 Since PRP has poor penetration to the subchondral bone, it is likely unable to alter the pathological degradation, fibrosis, and sclerosis of the bone. 63 In a 2016 study by Sánchez et al, 60 intraosseous injections were used to supplement the conventional IA treatment to pinpoint the subchondral bone in severe knee OA. Fourteen patients with OA underwent 3 weekly treatments of PRP. The first treatment included one PRP IA injection and 2 PRP intraosseous infiltrations (femoral condyle and tibial plateau). The next 2 treatments were conventional IA injections. Patient-reported qualitative data revealed substantially decreased knee pain, improved mobility, and better overall quality of life.17,23,60,63
Co-administration of PRP With HA
Co-administration of PRP and HA IA may provide improved symptomatic relief and better chondrogenic potential compared with either product individually.41,57 This simultaneous injection is theorized to be effective through delivering a potent combination of therapeutic effects, with anti-inflammatory properties from both drugs, chondrogenic potential from PRP, and reduction of joint friction from HA.41,57 One 2016 study showed that in vitro OA models treated with an HA and PRP combination revealed higher chondrocyte GAG concentrations and increased chondrocyte proliferation compared with a culture treated only with HA.41,57
LR-PRP vs LP-PRP
In the process of PRP generation, the centrifugation process can produce plasma that differs in leukocyte concentration, yielding LR and LP products. 17 Typically, in generating LP-PRP, the patient’s blood sample is centrifuged once and the buffy coat is discarded from the final injectable product. 17 In LR-PRP, the sample is often run through 2 centrifuge spins to better isolate the white blood cell–rich layer, which is then included in the final product. 17 However, studies have not yet found a consensus as to which is superior for the clinical treatment of knee OA. The primary argument for LR-PRP is that leukocytes are important for the production of pro-healing factors such as cytokines and key enzymes. 17 However, the push against this therapy stems from leukocytes being pro-inflammatory, meaning that LR-PRP can potentially further exacerbate OA pain in the acute, postinjection setting.6,17,47 As for LP-PRP, studies have been very encouraging in terms of less adverse effects, 6 but not superior long-term outcomes compared with LR-PRP.17,23 However, LP-PRP therapy has been shown to have superior short-term OA symptom relief as reported by patients. 17 This has been linked to a markedly reduced threat of pro-inflammatory effects compared with LR-PRP. 6 The available literature suggests that LP-PRP is currently more commonly utilized in outpatient clinics to minimize the risk of an acute inflammatory response. 6
Future Directions for PRP Injections
The future for PRP injections is exciting and encouraging. Further studies should look to find the most effective form of PRP delivery in terms of LP versus LR. Although IA injections are the norm, there are some studies that show that intraosseous injections have superior tissue specificity and improved DMOAD effectiveness. Nonetheless, due to the demonstrated efficacy and relative simplicity of administering IA PRP, this treatment modality is likely to remain in clinical practice until another option is determined to be unequivocally superior.
ADVANCED DRUG DELIVERY SYSTEMS
Inflammation in Primary OA and Corticosteroids
Chronic inflammation of the knee joint is a hallmark of primary OA and is a strong contributor to OA-associated joint pain.34,49,55 In clinical orthopaedics, glucocorticoid injections are one of the most commonly administered IA injections for symptom management of OA.5,17,59 Corticosteroids, such as glucocorticoids, exert anti-inflammatory effects extracellularly by blocking receptors for pro-inflammatory signaling molecules (cytokines, chemokines, etc), and intracellularly by binding to glucocorticoid receptors, and activating transcriptional regulatory pathways to decrease production of pro-inflammatory signalers and increase production of anti-inflammatory substances. 5 However, these changes are temporary and have not been shown to permanently alter the progression of primary OA.3,5,59
Utilization of advanced drug delivery systems aims to prolong the medicinal activity of current OA treatments, including glucocorticoids. The goal is to create a drug delivery system that takes advantage of regulated biochemical aspects of the injected environment for controlled and sustained release of the drug. These systems leverage biochemical properties that allow for reactions only to occur at specific pHs and temperatures and in reaction with certain species, 82 designed to deliver the drug directly to the target cells. 18 Recent studies have aimed at extending the activity of glucocorticosteroids in the joint, since IA injections of glucocorticosteroids are known to be a largely effective short-term pain and inflammation reliever.18,82 These injections fail to provide long-term efficacy due to the short half-life of glucocorticoids in the joint, along with their brief anti-inflammatory effects. 82
ProGel-Dex
Dexamethasone is a glucocorticoid that has anti-inflammatory properties. 82 ProGel-Dex (Ensign Pharmaceuticals) is a dexamethasone pro-drug solution with specific thermoresponsive properties. 82 ProGel-Dex has the ability to exist as a liquid at 4°C, while becoming a hydrogel when the temperature reaches around 30°C (body temperature). 82 This thermoresponsive ability allows for selective uptake and slower dissolution of the drug. Upon injection, ProGel-Dex assumes a gel form in the joint, which has been shown to provide slow release of individual ProGel-Dex molecules that are in turn predominantly internalized by synoviocytes, which are pathogenic in knee OA. 82 This provides the pro-drug IA injection a unique cell selectivity mechanism for targeted drug delivery. In rodent models, ProGel-Dex was demonstrated to provide sustained relief of pain and inflammation without noticeable adverse effects. The absence of negative side effects can be attributed to the low molecular weight of ProGel-Dex, which allows for less systemic absorption of the drug due to rapid renal clearance. 82 While these authors predict a lower occurrence of side effects compared with traditional glucocorticoid injections, this is still to be proven in human clinical trials. 82 ProGel-Dex is currently still in animal trials while showing impressive efficacy and safety in rats. 82
Liposomes
Liposomes are effective vehicles for IA drug delivery since they can transport drugs across plasma membranes while acting autonomously to lubricate the joint.18,30 Even when not loaded with anti-inflammatory drug cargo, one liposomal product, MM-II (Moebius Medical and Sun Pharma), was demonstrated to provide statistically significant improvements in knee joint pain relief compared with HA injection and placebo groups (n = 40). 30 Specifically, MM-II liposomes are constructed in concentric lipid layers designed to improve biomechanisms for extended delivery of drugs intra-articularly. 30 Researchers have found that cell membrane crossing of liposomes can be further enhanced through conjugating “cell-permeable proteins” attached to the liposome; however, so far none have yet to receive FDA approval. 62 Another promising concept is conjugating specific antigens onto the liposomes to allow selective binding to pathologic phagocytic cells in the joint. 55 So-called immunoliposomes have already been FDA approved in specific cancer therapies, and the MM-II liposome is currently concluding phase 2 human trials according to the US National Library of Medicine’s ClinicalTrials website (www.clinicaltrials.gov).
Disadvantages of liposome drug delivery systems include high production cost, relatively short half-life, and risk of immune rejection. 62 Due to the exogenous and drug-free nature of the liposome vehicles, side effects specific to MM-II have not yet been described, and potential side effects would likely depend on the potential drug cargo. While larger human trials are necessary to unambiguously confirm the efficacy of MM-II, the results are nonetheless very promising.
Nanoparticles
Nanoparticle delivery systems aim to increase the bioavailability of anti-inflammatory medications by altering their liberation, absorption, distribution, metabolism, and excretion. 55 The technological characteristics of these nanomedicines improve on the unfavorable physicochemical properties of anti-inflammatory medications, as these drugs tend to have poor water solubility and are susceptible to enzymatic inactivation or degradation.41,49,59 Nanoparticles prolong circulation of the drug-vector complex in the joint, enhance drug release at the cellular level, and allow for the drug to cross biological barriers for optimal effects.34,49,59 One potential drawback in nanoparticle formulations is that without tight binding to cellular or ECM structures, the nanoparticles can be easily cleared by lymphatics. 18 While the nanoparticle delivery systems have demonstrated advantageous delivery, they have been predominantly limited to animal trials, as confirmed by the ClinicalTrials website.
Polymeric micelles are a commonly studied platform for IA drug delivery.18,49 These micelles are composed of amphipathic polymers that self-assemble into “nanospheres” or “nanoparticles,” providing the capability to encapsulate a wide range of therapeutics.1,41 This provides efficient delivery for poorly soluble compounds and protects the active components from in vivo degradation and clearance.49,55 Synthetic polymeric particles such as poly-lactic co-glycolic acid (PLGA) are currently the only FDA-approved nanoparticle-based drug delivery systems, which are being used in various medical applications. This is likely attributed to its demonstrated safety, as PLGA particles naturally biodegrade into joint-native metabolites that are fully reabsorbed. 55 Therefore, side effects specific to PLGA have not yet been identified. Despite being FDA approved, PLGA is not currently widely used in orthopaedics for IA delivery of OA therapeutic medications. 55
KGN and Nanoparticles
The previously mentioned chondrogenic molecule, KGN, is also being investigated in nanoparticle delivery systems. In a 2018 study, amphiphilic polyurethanes were formulated with pendant amino groups bound to KGN, the drug of interest. 20 These structures were shown to autoassemble into KGN-conjugated nanoparticles, (PN-KGN), spherical in shape and approximately 25 nm in diameter, which provide sustained release of KGN to the pathologic tissue. 20 This has been demonstrated in vitro, as a human OA culture model that administered PN-KGN over 12 weeks showed an improved collagen 2:1 ratio and much delayed cartilage degeneration.20,79,81 One 2021 study demonstrated that co-administration of KGN and bone marrow–derived stem cells intra-articularly through a polyethylene glycol and branched polyethyleneimine (PEI) nanoparticle, both in vitro and in animal models, delayed OA progression, with chondrogenesis observed histologically, and decreased joint space narrowing, seen in animal models. 79
Zilretta
Zilretta (Pacira Biosciences) is an FDA-approved IA injection that extends the release time of triamcinolone acetonide (TCA) in the knee joint. This synthetic glucocorticoid has anti-inflammatory properties. By binding to glucocorticoid receptors, TCA downregulates pro-inflammatory cytokines while also upregulating transcription factors that are anti-inflammatory, such as increasing PLA2 (phospholipase A2) inhibitors.13,14,59 In Zilretta, TCA is contained in a PLGA spherical particle that extends the release of the active form of TCA in synovium. This lessens the amount in systemic blood flow and achieves a higher active form at the site of concentration.13,14,59 Another added benefit is that PLGAs can be degraded safely into joint native metabolites for reabsorption.52,59 A phase 3 clinical trial (24 weeks, randomized) in 2019 demonstrated how Zilretta injections mitigated pain in patients with OA.13,14,52 The clinical trial utilized the Western Ontario and McMaster Universities Arthritis Index (WOMAC) scoring for pain, and Zilretta showed significant relief compared with placebo.13,14,52 A phase 3b study (52 weeks, n = 208) demonstrated that a repeat injection was as effective as the first injection without any negative effects observed on the joint. 52 This suggests that additive injections can be beneficial. In 2021, a rodent colony study with Zilretta showed its possible effect in reducing the difficulty of weightbearing load on the joint. 72 While literature has described a lesser risk for adverse events with Zilretta compared with traditional glucocorticoids, the website for Zilretta (www.zilretta.com) lists potential side effects as “joint pain, headache, joint swelling, back pain, sore throat and runny nose, upper respiratory tract infection, and bruising.” 51 With success in these studies, as well as being FDA approved, it is clear that Zilretta is a product that shows promise for knee OA. However, it is still unclear whether Zilretta has any permanent disease-modifying effects. 55
Future of IA Drug Delivery
The future of IA drug delivery is promising. It aims to improve current therapies already available by extending active release within the joint. There are a variety of options for IA drug delivery. The gamut of delivery techniques also introduces the idea of using products in combination. Researchers can look at the success and drawbacks of Zilretta, ProGel-Dex, nanoparticles, and other IA drug delivery methods in order to investigate more effective formulations for the treatment of OA.
STEMPEUCEL AND ELIXCYTE INJECTION
Allogeneic MSCs
MSCs have been widely investigated for the treatment of degenerative diseases. The anti-inflammatory and immunomodulatory properties of MSCs suggest that these cells can reduce inflammation in the knee.18,22,29,31,34,41,55,70 Concurrently, MSCs may initiate the repair process of the damaged cartilage by differentiating into chondrocytes, by inducing proliferation and maturation of the healthy chondrocytes, or by inducing differentiation of chondroprogenitors.18,41,42 A whole host of growth factors, biological modulators, and ECM proteins produced by MSCs may play a pivotal role in enhancing neocartilage formation.18,34,41,49,70 It appears that the bioactive paracrine factors secreted by MSCs play some role, if not all, to provide beneficial effects in modulating the microenvironment of the damaged tissue, leading to more favorable conditions for tissue regeneration.18,34,41,42,49,70
MSCs secrete a spectrum of paracrine factors, collectively termed “secretome,” consisting of various proteins for diverse biological functions, including immune regulation, angiogenesis, antiapoptotic, antioxidative, cell homing, and promotion of cell differentiation.22,29,34,37,42 Specifically, MSCs release cytokines to initiate cartilage repair, which is followed by chondrogenic proliferation with secretion of ECM proteases and growth factors such as TGF-β, IGF-1, and FGF.22,29,34,37 These factors collectively comprise an important part of the MSC secretome and stimulate cartilage repair. A recent study revealed that MSCs secrete various chemokine (C-X-C motif) ligands and chemokine (C-C motif) ligands, vascular endothelial growth factor A, and IL-6 under the exposure of synovial fluid from patients with early- and late-stage OA. 37 Such cell-based therapy has led to short-term improvement in symptoms and may reduce or delay arthroplasty; however, long-term data are still lacking. 55 In certain studies, IA injections of MSCs were shown to provide improved pain relief, better quality of life, and significantly improved cartilage quality, with less need for hospitalization or surgery.17,50
Stempeucel
Stempeucel (Stempeutics) is a bone marrow–derived, ex vivo–expanded, pooled, allogeneic human MSC (hMSC) population that has been characterized previously. 24 A prior human trial study of 60 patients showed that Stempeucel differentiated into chondrocytes and synthesized significant amounts of sulfated GAG (sGAG) compared with that produced by the undifferentiated cells.24,65 These data suggest that the pooled bone marrow MSC (bmMSC) samples efficiently differentiated into the chondrogenic lineage, confirming the presence of mature chondrocytes after differentiation. 24 Intra-articularly administered MSCs most likely play an important role in attenuating the inflammation-induced pain by secreting a wide range of anti-inflammatory cytokines and analgesic peptides, and Stempeucel may have also contributed to pain reduction through a similar mechanism.24,65 Stempeucel displayed promising results in its phase 2 trials but has not yet entered into phase 3 trials in the US, according to the ClinicalTrials website; however, phase 3 trials are approved in China. There is currently a robust phase 3 trial evaluating the efficacy of autologous MSC injections, the results of which will be pivotal to permit future Stempeucel human trials.
Elixcyte
Elixcyte (UnicoCell Biomed) is an injectable allogeneic adipose stem cell product that has undergone a phase 1/2 human clinical trial for the treatment of primary OA of the knee joint. 9 In a clinical trial of Elixcyte for OA, the stem cell product showed good immune regulation, anti-inflammatory properties, and the characteristics of promoting chondrocyte proliferation and protecting chondrocytes. 69 Another study evaluated the changes in the WOMAC pain score from baseline to week 24 after treatment with Elixcyte. 9 All Elixcyte groups had a significant reduction in WOMAC total score, stiffness, and functional limitation score from baseline to posttreatment at weeks 12 to 48. 9 Other research showed that Elixcyte has good safety in the treatment of arthritis and demonstrated the potential and trends for positive response and efficacy. 69 Data published from Elixcyte’s phase 1/2 US clinical trials show promising efficacy and safety; however, phase 3 US trials have not yet begun.
Allogeneic MSCs: Future Directions
Elixcyte and Stempeucel have both completed phase 1/2 human trials and have displayed an impressive safety profile with promising efficacy as potential DMOADs. The allogeneic nature of these MSCs provides a major advantage over autologous MSCs as they do not require surgical harvesting of donor cells from the patient. While autologous MSC harvesting is FDA approved and is being performed with good results, the harvesting of adipose tissue or bone marrow requires anesthesia and concurrently poses other risks to the patient. Currently, the Multicenter Trial of Stem Cell Therapy for Osteoarthritis (MILES) project is underway to test the effects of IA injection of bone marrow–derived, adipose tissue–derived, and umbilical cord–derived MSCs on the progression of knee OA versus glucocorticoid injections. Side effects for MSC injections are described predominantly as knee swelling, which reportedly resolves in 6 weeks for most participants, and no major adverse events have been reported yet.7,31,50 Completion of the MILES project will likely provide more information on potential side effects.
Promising results from the MILES trials will likely pave the path for future phase 3 trials with allogeneic MSCs, including Elixcyte and Stempeucel. Adipose tissue is an attractive source of therapeutic MSCs not only because of its less invasive procurement for harvest but also because it provides a higher MSC concentration than bone marrow, has a smaller expression of MHC class 1 antigens, and has greater replicative and secretory potential of MSCs. 9 Additionally, adipose tissue MSC harvesting, also known as “lipogems,” can be performed at boutique orthopaedic offices with the assistance of a plastic surgeon or liposuction specialist. Furthermore, MSCs from bone marrow appear to have a higher propensity to undergo chondrocyte hypertrophy and bone formation, and thus may not be as ideal for the repair of articular cartilage. 9
TissueGene-C IA INJECTION
TGF-β
TGF-β is an anti-inflammatory cytokine that when expressed controls chondrocyte proliferation and differentiation, as well as ECM accumulation. 11 As a result of this process, TGF-β has been identified as a major player in maintaining the structural integrity and overall health of articular cartilage. 35 Therefore, TGF-β is considered as a primary cytokine in restoring articular cartilage that has been damaged. However, it has been difficult to overcome the pharmacokinetics of growth factors due to low bioavailability when directly administered. In order to bypass this problem and to allow for maximal activity of TGF-β, cell-based delivery strategies have been developed, one being TissueGene-C.19,35
Generation of TissueGene-C Allogeneic Chondrocytes
TissueGene-C (TissueGene) is an IA injection genetic therapy developed for the treatment of primary OA. 11 Its components include allogeneic chondrocytes and irradiated GP2-293 cells overexpressing TGF-β, mixed in a 3:1 ratio. The irradiation process prevents postinjection proliferation of the modified TGF-β–expressing chondrocytes and maintains the 3:1 ratio by preventing proliferation of the transplanted cells.19,37
Evidence From Clinical Trials
In phase 2 trials of TissueGene-C, patients with primary knee OA treated by TissueGene-C were shown to attain improved functionality with decreased pain, compared with patients either at the start of treatment or given a placebo.35,37 In a double-blinded study (n = 263), a single 3-mL IA injection of TissueGene-C, consisting of 2 parts nontransduced human allogeneic chondrocytes and transduced human allogeneic chondrocyte, was tested against a saline placebo injection. The results revealed that when compared with the placebo, the patients treated with TissueGene-C showed significantly greater improvement in International Knee Documentation Committee (IKDC) score and visual analog scale for pain at various time frames. 35 The patients who received this treatment also demonstrated improvement in the WOMAC categories of pain, stiffness, and physical function compared with those in the placebo group at all 3 time points. 35 Furthermore, compared with the placebo group, patients treated with TissueGene-C showed increased scores on each of the Knee injury and Osteoarthritis Outcome Score (KOOS) subscales (pain, symptoms, activities of daily living, function in sports and recreation, and knee-related quality of life). 35 Via imaging, when observing the changes in joint space narrowing as well as those in bone marrow edema lesions, cartilage defect surface areas, joint fluid, meniscal structures, meniscal signals, periarticular inflammation, synovial thickening and inflammation, and sizes of major lesions through either radiograph or MRI, statistical differences were not observed. However, an overall pain reduction of 25% was achieved with TissueGene-C treatment at 52 weeks compared with just 10% with placebo. 35
TissueGene-C Outlook
While there were significantly more minor adverse effects reported in TissueGene-C groups compared with placebo, the potential dramatic benefits from this therapy seem to outweigh the low risk of negative side effects.35,37 The rates of adverse events were 63% in the TissueGene-C treatment group and 44% in the placebo, with arthralgia, peripheral edema, joint swelling, and injection site pain being the most prevalent. In all cases but 3, these effects resolved during the study period. Studies thus far have found slower OA pathological progression with TissueGene-C treatment. 35 Although more data are needed, the results have shown clear promise in decreasing pain and increasing functionality. Combined with the possibility of reducing disease progression, TissueGene-C may represent a key player in IA injections for primary OA in years to come. Two large-scale phase 3 trials evaluating IA TissueGene-C injections on the progression of OA are currently ongoing in the US, according to the TissueGene website (www.tissuegene.com).
SPRIFERMIN INJECTION
Human FGF–18
Human FGF–18 (hFGF18) is a gene that encodes for a protein of the FGF pathway. 49 The FGF family of proteins is known to possess widespread mitogenic and cell regulatory properties, and is involved in processes such as cell growth and tissue repair.4,62,64 The ability of FGF to regenerate various tissues is currently being studied in several contexts.62,64 The pharmaceutical company Merck has recently patented AS902330 (sprifermin), an IA injection of recombinant hFGF18 (rhFGF18), as a potential DMOAD for primary OA of the knee. While human trials are still underway, preliminary evidence shows that biyearly injections of sprifermin decrease the rates of tibiofemoral cartilage loss compared with placebo, 78 reduce OA-related knee pain,49,55,64,78 and may increase total tibiofemoral cartilage volume.49,62,64
Sprifermin (AS902330)
In ex vivo cultures of human hyaline cartilage extracted from the knee joint, application of sprifermin was shown to increase type 2 collagen production, stimulate chondrocyte proliferation, activate proteoglycan production and SOX9 expression in chondrocytes, and increase overall ECM proteoglycan content.49,55,78 Rodent model studies later confirmed the drug’s ability to stimulate proliferation of articular chondrocytes, suppress proteinase activity, induce hyaline cartilage ECM synthesis, and repair cartilage defects.26,49,55 These findings were also demonstrated in a 2018 study utilizing bovine models. These studies have confirmed the capability of rhFGF18 to stimulate chondrogenesis and upregulate cartilage matrix production through acting on fibroblast growth receptors 2 and 3. 64 However, not all the studies conducted on sprifermin show such promising results. Other documented studies using human participants failed to identify biological tibiofemoral cartilage changes with sprifermin administration over 6 months and 12 months.26,64,78
Recently, more powerful human trials, such as the FORWARD (FGF-18 Osteoarthritis Randomized Trial with Administration of Repeated Doses) study, a 5-year phase 2 study with over 500 patients and extending at least 2 years, found that 3 once-weekly injections of 100 µg sprifermin provided a significant improvement in tibiofemoral cartilage thickness when administered every 6 months, compared with placebo.26,49,78 An exploratory analysis of the same study via MRI found, at the 3-year follow-up (n = 442), improvements in total tibiofemoral cartilage thickness in patients receiving IA sprifermin injections compared with those receiving placebo injections.26,49 Adverse events documented in the FORWARD trials were present in more than 90% of participants and described as mostly mild or moderate, but as unrelated to the treatment per the site investigators. The most frequent side effects were arthralgia, back pain, upper respiratory infections, nasopharyngitis, hypertension, and headache. 26 A 2020 study that applied post hoc analysis of the same data obtained from the FORWARD study found that patients receiving thrice-weekly sprifermin injections demonstrated increases in cartilage thickness that were approximately twice those experienced in patients receiving placebo injections.26,49
Sprifermin: Future Directions
Current studies on sprifermin show good efficacy; however, there is a concern regarding the frequency of reported adverse events in the FORWARD trials.26,49 The use of a recombinant growth factor as a cellular therapy still appears to be a promising treatment strategy for the modification of primary OA. The positive findings presented in both preclinical and clinical trials thus far warrant more investigation into the therapeutic potential of rhFGF18, and further future recombinant growth factors for cellular therapy in general. Phase 3 clinical trials are required to determine its safety and degree of disease modification in patients with primary knee OA.49,64 Sprifermin phase 3 trials have not yet begun recruiting participants, according to the ClinicalTrials website.
PROTEINASE INHIBITOR INJECTION
Wnt Signaling Pathway
At a molecular level, the Wnt signaling pathway is responsible for chondrocyte and osteoblast lineage specification in differentiation. 49 Research has shown that increased Wnt signaling predisposes MSCs to an osteogenic lineage and further induces production of MMPs that play a role in the pathogenesis of OA. 49 Wnt signaling activation in osteoblasts promotes bone formation and sclerosis, as seen in the pathologic subchondral bone of OA. 49 Increased expression and activation of the Wnt signaling pathway has been seen in chondrocytes of articular cartilage affected by primary OA. Given the following evidence, it is likely that Wnt signaling activation in the synovium and joint tissues plays a key role in the pathogenesis of OA. 49
Role of Proteinases in Primary OA
Proteases play the role of matrix-degrading enzymes in the joint and include collagenases and aggrecanases, which degrade cartilage 2 and aggrecan, respectively. Specific proteases such as MMP13 and ADAMTS5 are involved in joint degradation from progressive cartilage loss in OA models.27,49
Lorecivivint (SM04690)
Lorecivivint (Biosplice) is an injection of a small molecule (CLK/DYRK1A) that acts as an inhibitor of the Wnt/β-catenin pathway, downregulating transcription of ECM metalloprotease genes.27,49,58 IA injections of lorecivivint demonstrated induction of chondrogenesis and reduction of cartilage loss in preclinical OA models.49,58,75,76 Similarly in human trials, lorecivivint has also fared well. A 52-week, multicenter phase 2 trial of 455 patients showed a significant improvement in OA pain relief (WOMAC score) compared with placebo IA saline injection.58,75,76 This study also demonstrated a statistically significant improvement in medial knee joint space width on radiographs in patients with primary knee OA.49,58,75,76 The preliminary phase 1/2 results of this drug show good efficacy and impressive safety as a potential IA DMOAD.49,58,75,76 Thus far, the literature documents that the most common adverse event experienced by participants is arthralgia; however, more data on this will come out with new trials.49,58 Phase 3 clinical trials are currently underway according to the ClinicalTrials website.
Outlook for Protease- and Wnt-Targeted Therapy
Due to the recently elucidated role of the Wnt signaling pathway and its associated MMPs in the pathogenesis of primary OA, it is understandable why much effort has gone toward trying to inhibit this pathway in a safe manner. By blocking the function of 2 key pathologic enzyme classes directly, collagenases and aggrecanases, the Wnt signaling pathway is a promising target for the future of OA therapeutics.
SENOLYTIC THERAPIES
Role of Senolysis in Primary OA
Research in the pathophysiology of OA has uncovered that cellular senescence of articular chondrocytes plays a key role in OA disease progression.15,43,45,71,74 Senescent cells (SnCs) within the cartilage are a newly implicated agent in the pathology of the disease because they promote cartilage deterioration via production of pro-inflammatory cytokines, chemokines, proteases, and growth factors.15,43,45 This chondrocyte phenotype has been labeled the “senescence-associated secretory phenotype” (SASP).43,45,71 Isolated articular cartilage from patients with OA compared with age-matched donors without OA revealed a significantly greater prevalence of SASP chondrocytes with premature telomere shortening, accumulation of DNA damage, and an increase in expression of MMPs.43,45 These SnCs are known to accumulate in the cartilage of OA-afflicted joints and secrete pro-inflammatory mediators, produce matrix-degrading enzymes, and disrupt local intercellular homeostasis.15,43,45,71 Therefore, selective removal of these cells is a potentially efficacious strategy to treat the underlying pathology of primary OA.
Senotherapeutic agents target the SnCs in the joint by either inducing apoptosis directly or reversing the phenotype of younger cells by blocking SASP, or via immune-mediated clearance of SnCs.15,45,71 Through selective removal of senescent, pathologic chondrocytes, senolytic agents should help to protect the local environment within the articular cartilage.15,43,45,49 There is also evidence that the removal of these cells can be chondrogenic in itself, as surrounding chondrocytes compensate for the death of the SnCs by enhancing their proliferation and synthesis of key matrix proteins. 15
UBX0101
UBX0101 (Unity Biotech) is a senolytic agent that is a small-molecule inhibitor of the MDM2/p53 protein interaction, which plays a key role in modulating cellular senescence.45,49 UBX0101 was validated as a potent antisenescent in mouse models for a variety of degenerating conditions, including OA, degenerative disc disease, osteoporosis, pulmonary fibrosis, and more.45,49 UBX0101 was shown to alleviate primary OA in a mouse model and increase collagen 2 expression while reducing MMP and pro-inflammatory cytokine expression.45,49 In human chondrocyte models of primary OA, UBX0101 was shown to reduce the expression of senescence-associated genes and further increased the rate of proliferation of remaining chondrocytes. 45
A phase 1 human clinical trial (n = 48) revealed that 1 IA injection of UBX0101 demonstrated impressive safety and dose-dependent, clinically significant improvements in knee pain according to the total WOMAC score. However, the results from the most recent, 12-week human trial published in 2021 revealed no significant improvement in WOMAC scores after UBX0101 IA injection protocol. 49 It is theorized that, by eliminating the pathologic SASP seen in primary OA, UBX0101 removes a key causative factor in primary OA and potentially establishes an environment favorable toward chondrogenesis.15,43,45 UBX010 is also studied in the context of cancer treatment, and these studies have reported adverse effects, including thrombocytopenia and gastrointestinal distress; however, it is unclear if this transfers over to IA injections. 15 A phase 1 trial is currently underway evaluating the efficacy and safety of UBX0101 injection for the treatment of painful knee OA (ClinicalTrials.gov identifier: NCT03513016).
Navitoclax (ABT263)
Navitoclax (ABT263) is a well-established, injectable antisenescent therapeutic drug used in cancer therapy that inhibits Bcl2 and Bcl xL, 2 key antiapoptotic factors that SnCs rely on to survive despite persistent stress. 74 In human chondrocyte OA models and rodent models, IA delivery of navitoclax was shown to selectively eliminate SnCs by inducing apoptosis and promoting enhanced function of the remaining chondrocytes. 74 Navitoclax delivery was also demonstrated to reduce expression of pro-inflammatory cytokines in both models. 74 In rodent models, IA navitoclax was shown to reduce cartilage loss and subchondral bone changes associated with primary OA pathology. 74 Similar to UBX0101, the side effects of navitoclax are thus far only described in cancer treatment trials, including thrombocytopenia and gastrointestinal distress, although it has not been determined if these are present when injected intra-articularly. 15 These effects were described in 2020, but navitoclax injections as a senolytic DMOAD are still in the phase 1/2 stage, according to the ClinicalTrials website.
Antioxidation, NRF2, and Trichostatin A
New research into the pathology of OA has implicated oxidation as a key factor in cartilage loss and disease propagation.45,49 Nrf2 is a transcription factor that regulates the expression of several antioxidant genes that maintain cellular redox balance within the joint. These genes include heme oxygenase–1, nicotinamide adenine dinucleotide phosphate (NADPH), quinone oxidoreductase–1 (NQQ-1), among others. 49 Studies in a mouse model of primary OA revealed that treatment with a targeted histone deacetylase inhibitor, trichostatin A, which upregulates Nrf2, reduced the severity of OA. Analysis on the cartilage postmortem confirmed increased expression of Nrf2 antioxidant genes and revealed reduced catabolic cytokine and metalloprotease production.45,49 Adverse effects have yet to be characterized in humans for this therapy, however. Nrf2 signaling as a therapeutic target is in phase 1 clinical trials for the prevention of general age-related conditions, but it is not currently being studied as an IA injection for primary OA in humans, according to the ClinicalTrials website.
Senolytic Therapy: Future Outlook
SnCs are clearly the driving force behind the degenerative pathology of OA, and therefore therapeutics that can selectively eliminate these cells while sparing competent cells are enticing potential DMOADs.28,74 The implication of SnCs in the pathophysiology of OA is relatively recent, and human trials utilizing these agents have not entered into phase 3 longitudinal studies. Nonetheless, as this new evidence becomes incorporated into the widespread clinical paradigm of primary OA, it seems likely that increased effort will go into exploring therapeutic drugs that can prevent the pathologic actions of SnCs.
NF-κβ INHIBITOR INJECTION
Role of NF-κβ in Primary OA
NF-κβ is a key transcription factor for regulating the expression of immune and inflammatory molecules, including IL-1, IL-7, and tumor necrosis factor–α (TNF-α), in a variety of cells. Dysregulation of the NF-κβ signaling pathway is implicated in human oncogenesis in some cancers and contributes to the chronic inflammation seen in diseases such as rheumatoid arthritis and multiple sclerosis. 62 In OA, the NF-κβ pathway is associated with the expression of pro-inflammatory cytokines, adhesion molecules, matrix-degrading MMPs, and other mediators that are critical to the progressive pathology of primary OA. 53 Multiple in vivo studies have confirmed that inflamed synovial cells and OA-afflicted chondrocytes have been shown to have greatly increased expression of NF-κβ.49,62
In pathologic chondrocytes and joint endothelial cells (perhaps senescent or otherwise affected by OA), dysregulation of NF-κβ results in excessive production of pro-inflammatory mediators that propagate the disease’s progression.49,55,62 However, in healthy chondrocytes, NF-κβ signaling is key to the maintenance of joint homeostasis, and thus NF-κβ inhibitors serving as potential DMOADs must be able to selectively inhibit NF-κβ in targeted cells.55,62 Preliminary research in mice models supported the role of NF-κβ in primary OA and showed that small interfering RNA (siRNA)–mediated silencing of this pathway resulted in decreased joint inflammation, synovitis, and OA-associated cartilage loss.49,55,62
Sneaking Ligand Construct
Sneaking ligand construct (SLC1) is a novel sneaking ligand fusion protein (SFLP), a recombinant protein with a selective binding domain specifically for the inhibition of the NF-κβ signaling pathway.43,49 This protein targets and binds to endothelial cells that express E-cadherin, and thus have a pro-inflammatory phenotype, and is then activated (through binding of a specific “activation domain”) to exert its inhibitory effects on NF-κβ.43,49 The specific mechanism of SLC1 is described as binding endothelial cells via E-cadherin–selective endocytosis and then is transported into the endoplasmic reticulum, where the recombinant protein is cleaved into activated subunits, which are then released into the cytoplasm and provide a “blockade” to the assembly of the IKK complex to inhibit NF-κβ.43,49 In vitro studies and mice models have demonstrated that SLC1 is selectively engulfed by E-selectin–expressing human endothelial cells and effectively reduces transcription of pro-inflammatory cytokines. Although SLC1 has not yet reached the human clinical trial phase, it is nonetheless a promising therapeutic agent that deserves consideration in future phase 1/2 trials for primary OA, according to the ClinicalTrials website.
Ampion
Ampion (Ampio Pharmaceuticals) is a unique small molecule. This drug is a nonsteroidal, injectable compound derived from 2 amino acids found in human serum albumin (HSA).32,46 Due to its construction from ultrafiltered HSA, which has already been used therapeutically in other conditions (hypovolemia, hypoalbuminemia, etc), Ampion is being seen as highly favorable by government-pharmaceutical regulatory bodies. Ultrafiltered HSA has known anti-inflammatory properties, and Ampion aims to harness and utilize those effects in the context of primary OA.32,53 This small molecule has been found to modulate cell signaling by reducing pro-inflammatory cytokines and blocking transcription of the NF-κβ pathway. 46 Specifically, Ampion is known to reduce levels of TNF-α and IL-12 (associated with pain) and CXCL10 (immunogenic), as well as IL-1β and IL-6 (which are directly correlated with OA severity). 32 Preliminary human trials have shown that Ampion injections may reduce OA-associated knee pain. 46
In 2021, Ampio announced the results from its phase 3 clinical trial of 585 patients with knee OA, which found statistically significant pain reduction with 3 IA Ampion injections (at 2, 10, and 12 weeks) compared with placebo. 32 Ampio has additional phase 3 trials underway, although the company reports that these have been delayed due to the COVID-19 pandemic. Results from several of Ampio’s human trials confirm the safety of Ampion injections, and thus far no drug-related serious adverse events have been reported. 32 Reports from this study have revealed that the majority of side effects are unrelated to treatment. The study also found the incidence of side effects to be similar between Ampion and saline control, indicating that adverse event incidence may be far lower than what is reported for current therapies. 32 However, it seems that more data on potential side effects of this drug would be of value. Ampion may be on track to become a novel OA drug with a unique mechanism of action. If additional phase 3 human trials confirm the efficacy of Ampion, this could be a drug to receive FDA approval in the upcoming years.
Outlook on NF-κβ Inhibitors for Treatment of OA
While NF-κβ is implicated in a number of age-related inflammatory conditions including primary OA, it has been a challenge for researchers to develop selective NF-κβ inhibitors that spare healthy cells within the joint. As a key contributing factor to the pathologic phenotype of OA-afflicted cells, it is reasonable for pharmaceutical researchers to focus on NF-κβ as a potential target for future DMOADs.
DNA-TARGETED GENETIC THERAPY
Overview of CRISPR-Cas9 and Viral Vector–Based Gene Therapy
There is a clear strong genetic influence that directs the pathology of OA, and dysfunctional gene regulation is a hallmark of the aberrant pro-inflammatory molecule production that contributes to the symptoms and progression of OA.16,37,50 For this reason, modulation in gene expression through known strategies, including CRISPR-Cas9, other viral vector strategies, and nonviral vector modalities, is of high interest to researchers in developing DMOADs. Specifically, scientists aim to silence genes involved in the expression of pro-inflammatory cytokines and production of matrix proteases, while enhancing the expression of anti-inflammatory mediators.16,50,71 Viral vectors commonly utilized include adenovirus, lentivirus, and retroviruses for transferring genes into OA-affected tissues. 71
In rodent studies, researchers have employed CRISPR-Cas9 to ablate genes for MMP13, IL-1β, and nerve growth factor (NGF), and found that silencing these genes provided improved pain management, joint structure, and mobility for the OA-induced mice.55,71 The results have demonstrated that IL-1β and MMP13 ablation reduces the progression of OA, while the suppression of NGF significantly reduces OA-associated pain.55,62 However, one negative issue noted with many viral-vector based gene delivery systems is the tendency for stimulation of an inflammatory host response, and potential spread of viral vectors to other tissues. 71
YAP, FOXD1, and CBX4
Yes-associated protein (YAP) was first identified as a major effector of the Hippo transcriptional pathway, and it plays a key role in the regulation of genes that determine cellular senescence and aging. 80 One team of researchers produced YAP-deficient hMSCs and discovered that YAP regulates the expression of FOXD1, a substance termed a “gero-activate” protein. 77 These scientists found that lentivirus-mediated gene transfer of YAP and/or FOXD1 “rejuvenates” aged hMSCs and simultaneously reduces primary OA in mice models. 80 Lentivirus-mediated gene transfer of YAP and/or FOXD1 via IA injection has not yet entered the human clinical trial phase according to the ClinicalTrials website.
αKLOTHO and sTGFβR2
Both αKLOTHO and sTGFβR2 genes are implicated in the pathology of OA; however, their exact mechanisms largely remain to be elucidated, although they are known to play a role in the regulation of inflammation. 80 Experiments in rodents and ex vivo human chondrocyte models revealed that adenovirus-mediated transduction αKLOTHO and sTGFβR2 plasmid genes resulted in improved function of cartilage tissue, downregulation of the overactive immune response, and enhanced chondrocyte cell proliferation. 80 Despite this efficacy demonstrated in preclinical models, human trials involving IA injections of recombinant αKLOTHO and sTGFβR2 are still not yet underway, according to the ClinicalTrials website.
XT150
IL-10 is an anti-inflammatory cytokine that has been shown to repress expression of MMPs, pro-inflammatory cytokines, and major signalers of chondrocyte apoptosis. 16 XT150 is an injectable therapeutic containing plasmid DNA with a variant of the human IL-10 transgene. 16 XT-150 is currently in phase 2 human trials as an injectable therapeutic for patients in knee OA, and the trials were completed late in April of 2022. 16 In theory, inducing the expression of IL-10 in human chondrocytes afflicted with OA should establish a strong anti-inflammatory environment that is unfavorable for the progression of OA and favorable for chondrogenesis at most and OA symptomatic relief at the least. 16 While some human trials have been completed and do demonstrate good safety and moderate efficacy, human phase 2 trials are still ongoing, according to the ClinicalTrials website. While adverse effects for this drug have not been definitively described, these findings are expected upon completion of the trials, with publication of the phase 2 results.
Overview of Nonviral Gene Therapy Techniques
Some experts in the field believe that, due to ongoing safety concerns of the use of viral vectors in vivo, the development of nonviral therapies is gaining some interest. 71 Delivery systems for nonviral therapies currently being investigated include liposomes, hydrogels, DNA conjugates, and more, which are relatively cost-effective and theoretically pose a lower risk of adverse events. 71
Hydrogel and Nanoparticle Gene Delivery
Researchers have had some success in using HA-embedded PLGA nanoparticles to deliver exogenous plasmid DNA encoding TGF-β for in vitro human chondrocytes, a major innovation in nonviral gene therapy as a potential DMOAD. 41 Superior nonviral TGF-β gene delivery to human chondrocytes in vivo has been shown with HA-coated PLGA nanoparticles compared with nanoparticles delivered in isolation.41,71 Similarly, researchers have tested other hydrogel polymer-based systems for nonviral gene delivery, including poly-l-lysine, PEI, and PEG. 71
The theorized efficacy of hydrogel-based nonviral gene delivery systems is due to the tendency of the polymers to form strong electrostatic interactions with nucleic acids, facilitating the delivery of such genes to target cells. 71 It is known that HA interacts with the MSC CD44 receptor, which could be critical in nonviral gene transfer to target cells. One study using chitosan-conjugated HA nanoparticles for transfection with GDF-5 gene (growth factor and anti-inflammatory mediator of the TGF-β superfamily) via IA injection to OA-induced rabbits found low cytotoxicity and improved ECM production in vivo.41,71
DNA Genetic Therapy: Future Directions
Genetic therapies targeting DNA clearly present an efficacious treatment strategy that could modulate the pathophysiology of primary OA. There are various target genes of interest, including YAP, FOXD1, TFG-β, IL-10, NGF, and more. One challenge that must be addressed in the investigation of injectable DNA therapeutic agents is successful delivery to target cells. Gene modulation that can alleviate symptoms of OA without causing adverse side effects requires perfect selectivity of pathologic cells and efficient delivery. Several strategies are being investigated as drug delivery vehicles for gene therapeutics, including liposomes, hydrogels, and nanoparticles.16,41,71 Several of these measures show promise, and as human trials continue for various gene targets and gene delivery vectors, researchers should pay close attention as to which results reveal the most efficacy and safety, which warrants further investigation.
RNA-TARGETING GENETIC THERAPY
Overview
Despite the fact that OA is a major health problem in the elderly population, no DMOAD has been made available for clinical use. 1 This has prompted investigations into the direct delivery of messenger RNA (mRNA) into cells, as this seems to directly modulate the expression of proteins of interest. RNA interference (RNAi) is a mechanism through which mRNA is degraded by short double-stranded RNA. 56 siRNAs are base-pair duplex oligonucleotides that are complementary to the target RNA; the siRNA’s selective degradation provides a means to limit expression of proteins that are involved in OA. 56 Similar to siRNAs, microRNAs are noncoding strands that are also important in gene regulation. Available evidence from the literature suggests that RNA genetic therapy is efficacious in the treatment of some aspects of OA. 56
MicroRNA-455-3p
Studies have shown that microRNA-455-3p is a key regulator of chondrogenesis and that the expression of this RNA is upregulated in adipose-derived MSCs during chondrogenesis. 10 MicroRNA-455-3p works by promoting the expression of genes such as Col2a1 and Comp while repressing Runx2, which is theorized to promote early chondrogenic differentiation.10,68 However, the exact mechanism by which these genes promote chondrogenesis is unknown. In addition, microRNA-455-3p plays a vital role in regulating histone acetylation by controlling the expression of class I HDACs, which likely underly the upregulation of chondrogenic genes.10,68 In one experiment, the expression pattern of microRNA-455-3p, HDAC2/HDAC3/HDAC8, and SOX9 during chondrogenesis was monitored in cell lines derived from mouse teratocarcinoma cells (ATDC5) during stimulated in vitro differentiation into chondrocytes. The results found a significant upregulation of microR-455-3p in chondrogenic ATDC5 cells. 10 Furthermore, there was also a significant upregulation of HDAC2 and HDAC8 when microRNA-455-3p levels were decreased, and a decrease in HDAC2 and HDAC8 levels when microRNA-455-3p levels were increased. 10 This trend suggested an inverse correlation between microR-455-3p and HHDAC2/HDAC8 expression. In a subsequent study, microRNA-455-3p was shown to decrease mRNA expression of HDAC2 and HDAC8 while increasing the expression of H3 histones. Therefore, the study showed that microRNA-455-3p promotes chondrogenesis by enhancing histone acetylation. 10 As of now, therapy with microRNA-455-3p is not in human clinical trials as yet, and experiments have only been performed on animal models.
MicroRNA-140
MicroRNA-140 is one microRNA that plays a protective role for chondrocytes and holds promise for the treatment of OA 77 microRNA; 140 is specifically expressed in articular chondrocytes and plays a role in cartilage development and metabolic balance within the cartilage matrix by inhibiting ADAMTS-5 and MMP-13. 77 Utilizing exosomes that are engineered to specifically target chondrocytes, microR-140 can be effectively delivered into chondrocytes deep in articular tissues, without noticeable diffusion to other tissues. 77 A previously published study on OA rodent models determined that targeted exosome-mediated delivery of microR-140 enables intervention of MMP-13 concomitantly, which is translated into significant suppression of OA progression. 77 This treatment was also found to be nontoxic to major organs. 77 The benefits of targeted delivery include improved treatment efficacy, lower toxicity to other tissues, and reduced therapeutic costs. 77 In addition, encapsulating microRNA within the nanoscale structure of exosomes can avoid phagocytosis by monocytes and subsequent degradation by enzymes in the cell matrix. 77 However, thus far microR-140 has not yet been investigated in human trials, according to the ClinicalTrials website.
RUNX1
RUNX1, a cartilage-anabolic transcription factor, has been shown to regulate chondrogenesis in embryos and adults. Additionally, previous in vivo data have shown that RUNX1 activates transcription of the Col2a1 (collagen type 2 alpha 1) gene. 1 In a mouse model evaluating the effects of RUNX1 on OA, the RUNX1-injected group showed a trend toward suppression of the OA phenotypes compared with the control. 1 Immunohistochemistry revealed that the expression of RUNX1 proteins was enhanced in the articular cartilage of the RUNX1-injected group. 1 The enhancement was more prominent in osteophyte-like regions than in other areas, suggesting a link between the suppressed OA phenotypes and RUNX1 proteins derived from the delivered mRNA. 1 Further histological analyses revealed that OA progression was suppressed in the RUNX1-injected group compared with the control group, in terms of both cartilage degeneration and osteophyte formation. 1 RUNX1 is currently being studied as a target for the treatment of acute myeloid leukemia (AML), but no human trials involving IA RUNX1 injections for OA are currently underway, according to the ClinicalTrials website.
siRNA Nanotherapy
siRNA technology works by selectively suppressing the expression of a gene product and potentially shows promise in the treatment of arthritic diseases. 56 However, there is currently not an efficient mechanism to deliver these potential therapeutic modalities to the pathologic tissue. 56 Once siRNA is bound to its complementary sequence mRNA, an RNA-induced silencing complex is assembled. These complexes function to silence targeted gene products. 56 Recent research has revealed a self-assembling, 55-nm peptide-siRNA nanocomplex that penetrates cartilage and silences NF-kB, a signaling molecule that controls the expression of several matrix-degrading enzymes involved in cartilage remodeling. 56 Furthermore, other features of siRNA nanotherapy, such as siRNA multiplexing, which allows for the targeting of 3 or more pathways concurrently, are clinically relevant and promising. 56 Delivery can be achieved with lipid-soluble nanoparticles and liposomes that permit transport across cell membranes. Rai et al 56 advocate for more research to be done in the basic sciences in order to obtain a more comprehensive understanding of siRNAs before siRNA nanotherapy becomes a possible treatment method for OA. This technology is still in preclinical phases but represents an interesting potential future method for selective gene silencing.
RNA Gene Therapy: Future Directions
The literature reviewed suggest that siRNA-based treatment for OA seems efficacious in treating some aspects of this disease. Nanoplatforms are effective in delivery when injected intra-articularly; however, they lack specificity for a certain cell or tissue type. This creates the need to develop nanoplatforms that are able to target specific tissues within the joint.
BIOPRINTING AND SCAFFOLDING TECHNIQUES
Overview
Bioprinting and scaffolding refer to the process of engineering injectable matrices with favorable local environments for chondrogenesis, especially with regard to IA delivery of MSCs.41,45 Until recently, hydrogels were solely 2D, uniform structures that conferred weak mechanical and anabolic properties to passenger MSCs, leading to decreased efficiency and in vivo viability.41,45 This led to the emergence of 3D hydrogels as cell carriers that offer superior host tissue integration. 41 Cartilage regeneration has been studied to become more effective in 3D hydrogel systems as compared with 2D models. 45
Scaffolding is a technique that utilizes these 3D systems to guide supportive cells for cartilage repair as well as protecting the graft against invasive fibroblasts. 25 Polysaccharides have emerged as important polymers in the generation of scaffolds and bioprinted hydrogels.45,54 This is due to their capability to undergo the solute-hydrogel transition and their resemblance to the glycan constituent of native ECM. 54 Moreover, the bioprint-scaffold–based approach to cartilage regeneration has demonstrated various advantages to cell-based techniques such as improved integration into tissues, decreased deterioration of healthy cartilage, and more accurate cell delivery.41,45 Along with these advantages come potential drawbacks such as inappropriate distribution of cells, cell leakage, and poor differentiation, all of which remain to be addressed. 45
Evidence From Preliminary Studies
Scaffolding and bioprinting techniques for OA treatments are currently in preclinical trials, as verified by the ClinicalTrials website. In preliminary studies thus far, there have been some promising conclusions on the efficacy of these techniques. One study that combined transcription factors such as SOX5, SOX6, and SOX9 with a porous polylactide-co-glycolide (PGLA) scaffold yielded enhanced adipose stem cell chondrogenesis leading to preferable OA treatment outcomes. 71 The main focus of current preliminary studies seems to involve finding the best vehicle for stem cell scaffolds, especially those that display both strong safety and efficacy profiles. 25 Regarding passenger stem cells, researchers have identified adipose- and bone marrow–derived MSCs as having the greatest viability in chondrogenesis due to their capability of differentiating into both cartilage and bone tissues in vitro and in vivo.25,71
Bioprinting and Scaffolding in OA: Outlook
The future of bioprinting and scaffolding in OA treatment is certainly auspicious.41,45,54 The development of bioprinting and scaffolding techniques in OA therapy is a significant advancement in therapeutics. It has set a foundation in finding a solution to historically challenging facets of OA stem cell–based therapy, including lack of rigidity, long-term durability, and deposition of collagen 2 and aggrecan.41,54 Looking to the future, an emphasis should be placed on surpassing current accomplishments through more patient-specific therapies, greater cell-to-cell communication between native and injected cartilage tissues, and biocompatibility. As of now, these techniques are still being assessed in preclinical trials. 54
INDUCED PLURIPOTENT STEM CELLS
Overview
Induced pluripotent stem cells (iPSCs) were brought into existence to resolve the ethical controversy associated with the use of embryonic stem cells, as well as to provide a less invasive method of harvesting stem cells compared with bone marrow and/or adipose tissue harvesting.34,73 Professor Shinya Yamanaka, an orthopaedic surgeon, famously won the 2012 Nobel Prize in Physiology for his discovery that mature cells could be reprogrammed to iPSCs.67,73 The iPSC technique represents an area of interest in many fields due to the pluripotency of iPSCs—the ability to differentiate into any cell lineage. The intrigue of iPSC usage in OA is that they can be differentiated into chondrogenic cells and ultimately mature chondrocytes. This treatment holds promise due to their increased potential for proliferation compared with chondrocyte implantation, and possible ease of harvesting.48,67,73 Another difficulty in treatment is that the phenotype and function of cartilage chondrocytes depend on their location in the joint.45,48 As such, iPSCs demonstrate improved bioavailability and expansion potential compared with autologous, well-differentiated chondrocytes.45,48,67 The hope is that IA injection of iPSCs will restore the ECM and potentially regenerate articular cartilage. 48
Advancements in iPSC Feasibility
In 1 study using rodent primary OA models, chondrocytes derived from iPSCs were injected intra-articularly and analyzed after 15 weeks. Results confirmed increased proliferating chondrocytes in the rodent articular cartilage, suggesting that cartilage regeneration took place.45,83 In a 2015 study, Yamashita et al 73 created cartilaginous nodules that were derived from iPSCs and then implanted these into immunodeficient rodents. This experiment showed repair of osteochondral defects over the course of 4 weeks. 45 These same nodules were then placed in cartilage defects of mini pigs and remained viable after 1 month while displaying integration with the hosts’ cartilage, confirming the nodules’ capability to stimulate cartilage regeneration even under heavy weightbearing conditions.45,73 The success in these animal trials is promising and will lay the foundation for future phase 1 human clinical trials. However, cost, inefficiency, and time are all barriers that need to be overcome before progressing with iPSC investigation for OA therapy. 45
Future Directions for iPSC Therapy in OA
The potential of iPSCs in OA therapy is enticing, as seen by the success and regenerative potential displayed in the aforementioned animal studies. Yet, challenges remain to be overcome, including aberrant DNA methylation, epigenetic variations, and genetic instabilities. 34 Contrarily, autologous MSCs may have less possibility of becoming malignant after infusion compared with iPSCs. 34 Human autologous MSC stem cell injections are still being studied in phase 3 trials (MILES project), which are laying the foundation for future trials with human iPSC IA injections, according to the ClinicalTrials website.
MODIFIED HUMAN ANGIOPOIETIN-LIKE–3 (LNA043)
Overview
Modified human angiopoietin-like–3 (LNA043) is a protein that stimulates joint-resident MSCs to develop through the chondrocyte lineage. This protein LNA043 is intended to repair cartilage damage and decrease inflammation in patients with primary knee OA.36,61 In preclinical OA models, LNA043 has been shown to induce chondrogenesis and cartilage repair. 36 According to the FDA website (FDA.gov), in 2021 the pharmaceutical company Novartis received fast-track approval for phase 3 human trials using LNA043 as a treatment for primary knee OA, which are still underway, as verified by the ClinicalTrials website. According to publications available on Novartis’ website (www.novartis.com), LNA043 is a novel protein that was discovered within the Novartis Institutes for BioMedical Research.
LNA043: Evidence From Clinical Trials
In the first human clinical trial (n = 30), LNA043 displayed a favorable safety profile while demonstrating a pro-chondrogenic effect as determined through modulations in RNA.36,61 Altered RNA levels in articular chondrocytes of participants demonstrated augmentation of several genes involved in cartilage homeostasis and repair. 61 Immunohistochemical examination results published in this study confirmed that the LNA043 protein penetrated the articular cartilage 2 hours after injection. 61 In a following clinical trial, researchers aimed to test the chondroregenerative effects of LNA043 on the donor site in patients undergoing autologous chondrocyte implantation procedures. 36 This study revealed that a single IA injection of LNA043 was found to induce a mean of 65% refilling of the donor site compared with 38% in the placebo group over 12 weeks. 36 The study also found that the LNA043-treated group experienced longer-lasting and more permanent filling of the cartilage defect, versus the placebo group demonstrating further cartilage loss by week 12. 36 The same study also found significantly increased GAG content in the LNA043 group. Cartilage regeneration was confirmed via MRI, and no serious adverse events were reported. 36
LNA043: Future Directions
The future of LNA043 in the therapy of primary knee OA will largely depend on the results from Novartis’ FDA fast-tracked phase 3 clinical trial. Nonetheless, from the first 2 published clinical trials by Novartis, LNA043 appears to be an efficacious and relatively safe IA injection that could potentially alter the progression of primary OA. Due to its FDA fast-tracked status, LNA043 could represent a major advancement in the development of DMOADs.
Discussion
With a complex, multifactorial, heterogenous, degenerative disease such as primary knee OA, there are a variety of genetic, cellular, and enzymatic targets for potential DMOADs. The FDA-approved injection products currently available in the US include HA hydrogels, PRP, and Zilretta; however, none of these are classified as DMOADs. These products have shown efficacy in reducing the symptoms of primary OA, although evidence suggests that these effects are not permanent.
HA hydrogels reduce pain in some patients by physically cushioning the joint, decreasing friction, protecting the cartilage (to an extent), and potentially providing anti-inflammatory effects.41,54,55,57 Various HA derivatives are available for clinicians, including Euflexxa, Hyalgan, Orthovisc, and others, all of which have gained FDA approval due to the joint-native HA composition and subsequent excellent safety profile. PRP is known to provide an anti-inflammatory and potentially pain-reducing effect in patients with knee OA.11,23,27,49,63 The presence of various pro-chondrogenic growth factors suggests it may have a potential to regenerate cartilage, but this has not been proven to consistently or significantly occur in an all patients.6,12,17,47,57 Zilretta, an FDA-approved, extended-release TCA, may also provide a superior reduction in pain and inflammation compared with traditional glucocorticoid injections.13,14,49,52,55,59 However, it has not been proven that Zilretta provides permanent disease-modifying effects.49,52,55
The DMOADs that are still in the preclinical phase include DNA plasmid vectors containing genes αKLOTHO and sTGFβR2; YAP/FOXD1, a selective NF-κβ inhibitor (SLC1); trichostatin A; a RUNX1 injection; microRNA 140 and 455-3p injections; bioprinted-scaffolded hydrogel injections; ProGel-Dex; and iPSCs. Viral vector gene transfer of antisenolytic genes YAP and FOXD, as well as anti-inflammatory genes αKLOTHO and sTGFβR, has shown promise in rodent models.76,77 The SLC1 “sneaking ligand fusion” protein has also shown efficacy as an NF-κβ inhibitor in mice. 62 Upregulation of Nrf2 via injectable trichostatin A, an HDAC inhibitor, was demonstrated to reduce inflammation and cartilage loss in rodents. 49 RUNX1, the injectable chondrogenic transcription factor, may upregulate matrix production. 1 MicroRNAs 140 and 455-3p have both been shown to enhance expression of pro-chondrogenic genes and suppress certain pro-inflammatory, OA-associated genes in rodents.10,68,71,77 Bioprinted-scaffolded hydrogel injections will continue to be an area of investigation for the development of injectable hydrogel matrices with favorable local environments for chondrogenesis with regard to IA delivery of MSCs.26,39,41,42,45,73 ProGel-Dex, the thermoresponsive injectable dexamethasone pro-drug, continues to be investigated as a selective, extended-release anti-inflammatory therapy. 82 iPSCs could potentially act as a chondrogenic stimulator; however, cost and technological barriers remain restrictive toward widespread application.45,52,55,67,73,83
The injections in phase 1 of clinical trials include KGN and UBX0101. KGN is currently in phase 1 trials as a KA34, an injectable, pro-chondrogenic KGN derivative.20,40,79,81 UBX0101 will continue to be investigated as a senolytic agent for knee OA.43,49 Navitoclax (ABT263), another senolytic agent and a cross-linked HA derivative hydrogel, have both advanced past phase 1 and are currently in between (conducting phase 1/2 trials) with hopes of fully launching large-scale phase 2 trials.41,49,55,74
XT150 and MM-II liposomes are currently in phase 2 trials, while Elixcyte and Stempeucel (pooled donor MSC products) have recently completed phase 2 human trials and are awaiting the results of the MSC MILES trials before entering phase 3. XT150 is an injectable DNA plasmid containing recombinant human IL-10 transgene, which promotes expression of this anti-inflammatory cytokine. 16 MM-II liposomes are an endogenous, therapeutic IA vehicle that can be loaded with a drug to provide sustained, targeted release.18,62
In phase 3, closest to hitting the market, there are lorecivivint, TissueGene-C, LNA043, sprifermin, autologous MSC injections, and Ampion. Lorecivivint (SM04690), the Wnt inhibitor, blocks MMP expression.49,56,64,78 TissueGene-C increases expression of the TGF-β anti-inflammatory cytokine. 34 LNA043 can potentially stimulate native MSCs to differentiate into chondrocytes.36,61 Sprifermin (AS902330) may be able to increase endogenous collagen production, stimulate cartilage repair, promote expression of anti-inflammatory genes, and downregulate expression of pro-inflammatory genes.49,64,78 Autologous MSCs must be harvested from the patient’s adipose or bone marrow tissue, and their efficacy is still being studied. ‡ Ampion appears to be a potent anti-inflammatory endogenous molecule with a highly favorable safety profile.32,45,53
Conclusion
The field of orthopaedics is rapidly evolving in terms of its nonsurgical treatment approaches for primary OA of the knee joint. While there are currently no disease-modifying primary OA drugs available, there seem to be several on the cusp of entering the US healthcare market. This is exciting news for physicians and patients alike, as primary knee OA is one of the most economically costly, widespread, age-related degenerative and potentially disabling diseases in the US. New evidence suggests that efficacious drug targets for primary knee OA include molecular inhibition and/or downregulation of pro-inflammatory cellular signaling, upregulation of anti-inflammatory gene expression, removal of senescent chondrocyte phenotypes, regulation of local immune function, translocation of anti-inflammatory genes, delivery of pro-chondrogenic molecules, and administration of compatible MSCs. These novel drug targets should provide promising treatment avenues for future DMOADs. The results of additional human trials will be pivotal to determine which drug targets are most deserving of future clinical research.
Acknowledgment
We would like to thank Wooyoung Chang for his contribution toward this article.
Footnotes
Final revision submitted October 18, 2022; accepted November 9, 2022.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1.Aini H, Itaka K, Fujisawa A, et al. Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment. Sci Rep. 2016;6:18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bąkowski P, Kaszyński J, Wałecka J, et al. Autologous adipose tissue injection versus platelet-rich plasma (PRP) injection in the treatment of knee osteoarthritis: a randomized, controlled study—study protocol. BMC Musculoskelet Disord. 2020;21(1):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PJ.How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol. 2006;148(3):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basic bone biology. Medtronic Sofamor Danek; 2002. https://www.healio.com/news/orthopedics/20120331/etiology-and-pathophysiology-of-osteoarthritis
- 5.Bastos R, Mathias M, Andrade R, et al. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2018;26(11):3342–3350. [DOI] [PubMed] [Google Scholar]
- 6.Bennell KL. Effect of intra-articular platelet-rich plasma vs. placebo on pain and cartilage volume in knee osteoarthritis. JAMA. 2021;326(20):2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bistolfi A, Roato I, Fornelli G, et al. Treatment of knee osteoarthritis by intra-articular injection of concentrated autologous adipose tissue: a twenty-four-month follow-up study. Int Orthop. 2021;45(3):627–633. [DOI] [PubMed] [Google Scholar]
- 8.Block TJ, Garza JR. Regenerative cells for the management of osteoarthritis and joint disorders: a concise literature review. Aesthet Surg J. 2017;37(3):S9–S15. [DOI] [PubMed] [Google Scholar]
- 9.Chen CF, Hu CC, Wu CT, et al. Treatment of knee osteoarthritis with intra-articular injection of allogeneic adipose-derived stem cells (ADSCs) ELIXCYTE®: a phase I/II, randomized, active-control, single-blind, multiple-center clinical trial. Stem Cell Res Ther. 2021;12(1):562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Chen L, Zhang Z, et al. MicroRNA-455-3p modulates cartilage development and degeneration through modification of histone H3 acetylation. Biochim Biophys Acta Mol Cell Res. 2016;1862(12):2881–2891. [DOI] [PubMed] [Google Scholar]
- 11.Choi K, Lee H, Kim D, et al. Invossa™ (TISSUEGENE-C) induces an anti-inflammatory environment in the arthritic knee joints via macrophage polarization. Osteoarthritis Cartilage. 2020;28(5):1237–1252. [Google Scholar]
- 12.Cobianchi BF, De Marino L, Arrigoni F, et al. T2-mapping MRI evaluation of patellofemoral cartilage in patients submitted to intra-articular platelet-rich plasma (PRP) injections. La Radiol Med. 2021;126(8):1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conaghan PG, Cohen SB, Berenbaum F, et al. Brief report: a phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intra-articular injection in knee osteoarthritis. Arthritis Rheumatol. 2018;70(2):204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Joint Surg Am. 2018;100(8):666–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins JA, Diekman BO, Loeser RF, et al. Targeting aging for disease modification in osteoarthritis. Curr Opin Rheumatol. 2018;30(1):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins SD.Efficacy and safety of XT-150 in osteoarthritis of the knee. Accessed January 28, 2023. https://clinicaltrials.gov/ct2/show/NCT04124042
- 17.Cook CS, Smith PA. Clinical update: why PRP should be your first choice for injection therapy in treating osteoarthritis of the knee. Curr Rev Musculoskelet Med. 2018;11(4):583–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeJulius CR, Gulati S, Hasty KA, et al. Recent advances in clinical translation of intra-articular osteoarthritis drug delivery systems. Adv Ther. 2021;4(1):2000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans CH, Ghivizzani SC, Robbins PD, et al. Arthritis gene therapy approved in Korea. J Am Acad Orthop Surg. 2018;26(2):e36–e38. [DOI] [PubMed] [Google Scholar]
- 20.Fan W, Li J, Yuan L, et al. Intra-articular injection of kartogenin-conjugated polyurethane nanoparticles attenuates the progression of osteoarthritis. Drug Deliv. 2018;25(1):1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fidia Pharma USA Inc. Hymovis pain & side effects. Accessed January 11, 2023. https://www.hymovis.com/oa-your-treatment/pain-side-effects
- 22.Garay-Mendoza D, Villarreal-Martínez L, Garza-Bedolla A, et al. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int J Rheum Dis. 2018;21(1):140–147. [DOI] [PubMed] [Google Scholar]
- 23.Görmeli G, Görmeli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958–965. [DOI] [PubMed] [Google Scholar]
- 24.Gupta PK, Chullikana A, Rengasamy M, et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison-Brown M, Scholes C, Hafsi K., et al. Efficacy and safety of culture-expanded, mesenchymal stem/stromal cells for the treatment of knee osteoarthritis: a systematic review protocol. J Orthop Surg Res. 2019;14(1):14–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochberg MC, Guermazi A, Guehring H, et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis. JAMA. 2019;322(14):1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imbert O, Deckx H, Bernard K, et al. The design of a randomized, placebo-controlled, dose-ranging trial to investigate the efficacy and safety of the ADAMTS-5 inhibitor S201086/GLPG1972 in knee osteoarthritis. Osteoarthr Cartil Open. 2021;3(4):100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeon OH, Kim C, Laberge R, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jo CH, Chai JW, Jeong EC., et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med. 2017;45(12):2774–2783. [DOI] [PubMed] [Google Scholar]
- 30.Kandell L, Dolev Y, Rivkind G, et al. Safety and efficacy of MM-II, an intra-articular injection of liposomes, in moderate knee osteoarthritis. Prospective randomized double-blinded study. Osteoarthritis Cartilage. 2014;22:S57–S489. [Google Scholar]
- 31.Kasir R, Vernekar VN. Regenerative engineering of cartilage using adipose-derived stem cells. Regen Eng Transl Med. 2015;1(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy K. Ampio provides an update on osteoarthritis of the knee (OAK) program, reiterates compelling data in earlier phase III trials of Ampion in severe OAK. Orthopedics. 2018;41(1):e77–e83.29156068 [Google Scholar]
- 33.Kijowski R. Risks and benefits of intra-articular corticosteroid injection for treatment of osteoarthritis: what radiologists and patients need to know. Radiology. 2016;293(3):664–665. [DOI] [PubMed] [Google Scholar]
- 34.Kim HJ, Park JS. Usage of human mesenchymal stem cells in cell-based therapy: advantages and disadvantages. Dev Reprod. 2017;21(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MK, Ha CW, In Y., et al. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum Gene Ther Clin Dev. 2018;29(1):48–59. [DOI] [PubMed] [Google Scholar]
- 36.Laurent D, Scotti C, Schreiner M, et al. Regeneration of hyaline cartilage in response to a single injection of LNA043, an ANGPTL3 mimetic, in the knee of patients with a focal cartilage defect. OARS J. 2021;1(29):S220–S221. [Google Scholar]
- 37.Lee H, Kim H, Seo J, et al. et al. TissueGene-C promotes an anti-inflammatory micro-environment in a rat monoiodoacetate model of osteoarthritis via polarization of M2 macrophages leading to pain relief and structural improvement. Inflammopharmacology. 2020;28(5):1237–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee WY. Cartilage repair by mesenchymal stem cells: clinical trial update and perspectives. J Orthop Translat. 2017;1(9):17–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C, Wang K, Zhou X, et al. Controllable fabrication of hydroxybutyl chitosan/oxidized chondroitin sulfate hydrogels by 3D bioprinting technique for cartilage tissue engineering. Biomed Mater. 2019;14(2):025006. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Liu T, Wang T, et al. Kartogenin mediates cartilage regeneration by stimulating the IL-6/Stat3-dependent proliferation of cartilage stem/progenitor cells. Biochem Biophys Res Commun. 2020; 532(3):385–192. [DOI] [PubMed] [Google Scholar]
- 41.López-Ruiz E, Jiménez G, de Cienfuegos A, et al. Advances of hyaluronic acid in stem cell therapy and tissue engineering, including current clinical trials. Eur Cells Mater. 2019;37(1):186–213. [DOI] [PubMed] [Google Scholar]
- 42.Lu L, Dai C, Zhang Z, et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther. 2019;10(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malaise O, Tachikart Y, Brondello JM, et al. Senolytic treatments applied to osteoarthritis: a step towards the end of orthopedic surgery? AME Med J. 2017;10(1):21037. [Google Scholar]
- 44.Mayo Foundation for Medical Education and Research. Hyaluronic acid (injection route): side effects. November 1, 2022. Accessed January 28, 2023. https://www.mayoclinic.org/drugs-supplements/hyaluronic-acid-injection-route/side-effects/drg-20074557
- 45.Nam Y, Rim YA, Lee JJ, et al. Current therapeutic strategies for stem cell-based cartilage regeneration. Stem Cells Int. 2018;2018:8490489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Institute for Health and Care Research. DMI-9523 (Ampion) for moderate to severe osteoarthritis of the knee. NIHR Horizon Scanning Research & Intelligence Centre; 2016. Accessed January 28, 2023. https://database.inahta.org/article/17300
- 47.Nayana JJ. Platelet-rich plasma injections for advanced knee osteoarthritis: a prospective, randomized, double-blinded clinical trial. Orthop J Sports Med. 2017;5(2):2325967116689386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okano H, Nakamura M, Yoshida K, et al. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112(3):523–533. [DOI] [PubMed] [Google Scholar]
- 49.Oo WM, Little C, Duong V, et al. The development of disease-modifying therapies for osteoarthritis (DMOADs): the evidence to date. Drug Des Devel Ther. 2021;15(1):2921–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95(12):1535–1541. [DOI] [PubMed] [Google Scholar]
- 51.Pacira Pharmaceuticals Inc. Highlights of prescribing information, ZILRETTA® (triamcinolone acetonide extended-release injectable suspension), for intra-articular use. Accessed January 28, 2023. https://www.zilrettalabel.com/PI
- 52.Paik J, Duggan ST, Keam SJ, et al. Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the knee. Drugs. 2019;79(4):455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phillips MJ, McGrath B. A randomized, placebo-controlled, double-blind study to evaluate the efficacy and safety of two doses of intra-articular injection of Ampion™ in adults with pain due to osteoarthritis of the knee. Accessed January 28, 2023. https://clinicaltrials.gov/ct2/show/NCT01839331
- 54.Radhakrishnan J, Subramanian A, Sethuraman S, et al. Injectable and 3D bioprinted polysaccharide hydrogels: from cartilage to osteochondral tissue engineering. Biomacromolecules. 2017;18(1):1–26. [DOI] [PubMed] [Google Scholar]
- 55.Rai MF. Intra-articular drug delivery systems for joint diseases. Curr Opin Pharmacol. 2018;40(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rai MF, Pan H, Yan H, et al. Applications of RNA interference in the treatment of arthritis. Transl Res. 2019;214(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russo F, D’Este M, Vadal G, et al. Platelet rich plasma and hyaluronic acid blend for the treatment of osteoarthritis: rheological and biological evaluation. PLoS One. 2016;11(6):e0157048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sabha M, Siaton BC, Hochberg MC, et al. Lorecivivint, an intra-articular potential disease-modifying osteoarthritis drug. Expert Opin Investig Drugs. 2020;19(12):1339–1346. [DOI] [PubMed] [Google Scholar]
- 59.Sánchez A, Calpena AC, Soriano JL, et al. Anti-inflammatory nanomedicines: what does the future hold? Nanomedicine. 2020;15(14):1357–1360. [DOI] [PubMed] [Google Scholar]
- 60.Sánchez M, Delgado D, Sánchez P, et al. Combination of intra-articular and intraosseous injections of platelet rich plasma for severe knee osteoarthritis: a pilot study. Biomed Res Int. 2016;2016:4868613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scotti C, Gimbel M, Dider L, et al. LNA043, a novel cartilage regenerative treatment for osteoarthritis: results from a first-in-human trial in patients with knee osteoarthritis. Arthritis Rheumatol. 2020;72(suppl 10):1485. [Google Scholar]
- 62.Sehnert B, Burkhardt H, Dübel S, et al. Cell-type targeted NF-kappaB inhibition for the treatment of inflammatory diseases. Cells. 2020;9(7):1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Şen Eİ, Yıldırım MA, Yeşilyurt T, et al. Effects of platelet-rich plasma on the clinical outcomes and cartilage thickness in patients with knee osteoarthritis. J Back Musculoskelet Rehabil. 2020;33(4):597–605. [DOI] [PubMed] [Google Scholar]
- 64.Sennett ML, Meloni GR, Farran AJE, et al. Sprifermin treatment enhances cartilage integration in an in vitro repair model. J Orthop Res. 2018;36(10):2648–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shapiro SA, Kazmerchek SE, Heckman MG, et al. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med. 2017;45(1):82–90. [DOI] [PubMed] [Google Scholar]
- 66.Sun Q, Zhen G, Li TP, et al. Parathyroid hormone attenuates osteoarthritis pain by remodeling subchondral bone in mice. Elife. 2021;10(1):e66532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140(12):2457–2461. [DOI] [PubMed] [Google Scholar]
- 68.Tokyo Medical and Dental University. A possible new treatment for osteoarthritis joint issues. MedicalXpress. August 25, 2021. Accessed January 28, 2023. https://medicalxpress.com/news/2021-08-treatment-osteoarthritis-joint-issues.html
- 69.Tsai CC, Tsai J. Development of an allogeneic stem cell product “ELIXCYTE” for the treatment of osteoarthritis and chronic kidney disease. Cytotherapy. 2020;22(5):S110. [Google Scholar]
- 70.University of Southampton. Power of stem cells harnessed to create cartilage tissue. MedicalXpress. September 28, 2021. Accessed January 28, 2023. https://medicalxpress.com/news/2021-09-power-stem-cells-harnessed-cartilage.html
- 71.Uzieliene I, Kalvaityte U, Bernotiene E, et al. Non-viral gene therapy for osteoarthritis. Front Bioeng Biotechnol. 2021;8(1):618399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Westhof A, Kleinschmidt-Doerr K, Michaelis M, et al. Dynamic weight-bearing test during jumping: a sensitive outcome measure of chronic osteoarthritis pain in rats. Heliyon. 2021;7(9):e07906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamashita A, Morioka M, Yahara Y. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Rep. 2015;4(3):404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang H, Chen C, Chen H, et al. Navitoclax (ABT263) reduces inflammation and promotes chondrogenic phenotype by clearing senescent osteoarthritic chondrocytes in osteoarthritis. Aging. 2020;12(13):12750–12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yazici Y, McAlindon TE, Gibofsky A, et al. Lorecivivint, a novel intra-articular CDC-like kinase 2 and dual-specificity tyrosine phosphorylation-regulated kinase 1A inhibitor and Wnt pathway modulator for the treatment of knee osteoarthritis: a phase II randomized trial. Arthritis Rheumatol. 2020;72(10):1694–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yazici Y, McAlindon TE, Gibofsky A, et al. Efficacy and safety from a phase 2B trial of SM04690, a novel, intra-articular, WNT pathway inhibitor for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2019;27(1):S503. [Google Scholar]
- 77.Yujie L, Xu X, Li X, et al. Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12(33):36938–36947. [DOI] [PubMed] [Google Scholar]
- 78.Zeng N, Chen XY, Yan ZP, et al. Efficacy and safety of sprifermin injection for knee osteoarthritis treatment: a meta-analysis. Arthritis Res Ther. 2021;23(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeng WN, Zhang Y, Wang D, et al. Intra-articular injection of kartogenin-enhanced bone marrow-derived mesenchymal stem cells in the treatment of knee osteoarthritis in a rat model. Am J Sports Med. 2021;49(10):2795–2809. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J, Liu GH, Qu J, et al. Treating osteoarthritis via gene therapy with rejuvenation factors. Nat News. 2020;27(1):309–311. [DOI] [PubMed] [Google Scholar]
- 81.Zhang S, Hu P, Liu T, et al. Kartogenin hydrolysis product 4-aminobiphenyl distributes to cartilage and mediates cartilage regeneration. Theranostics. 2019;9(24):7108–7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao G, Ren R, Wei X, et al. Thermoresponsive polymeric dexamethasone prodrug for arthritis pain. J Control Release. 2021;339(1):484–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu Y, Wu X, Liang Y, et al. Repair of cartilage defects in osteoarthritis rats with induced pluripotent stem cell derived chondrocytes. BMC Biotechnol. 2016;16(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]