Abstract
Purpose
Apolipoprotein E2 (ApoE2) gene therapy is a potential disease-modifying therapy for Alzheimer’s disease (AD). We investigated the potential of plasmid encoding ApoE2 loaded brain-targeted functionalized-liposomes for treatment of AD. This was achieved via systemic administration of liposomes entrapping therapeutic gene targeting the brain of mice.
Methods
Targeting and transfection efficiency of designed liposomes were determined in bEnd.3, primary glial and primary neuronal cells. The ability of liposomal formulations to translocate across in vitro blood-brain barrier (BBB) and, thereafter, transfect primary neuronal cells was investigated using in vitro triple co-culture BBB model. We quantified ApoE expression in the brain of mice after single intravenous injection of brain-targeted liposomes loaded with plasmid ApoE2.
Results
Dual surface modification enhanced the in vitro transfection efficiency of designed liposomes. Successful delivery of therapeutic gene overcoming BBB by Transferrin-Penetratin-modified liposomes was demonstrated both in vitro and in vivo. Significant (p < 0.05) increase in ApoE levels in the brain of mice was observed after intravenous administration of Tf-Pen-liposomes encasing plasmid ApoE2.
Conclusion
The results indicate that dual-ligand based liposomal gene delivery systems had both enhanced brain targeting and gene delivery efficiencies. Transferrin-Penetratin modified liposomes for delivery of plasmid ApoE2 has great potential for AD treatment.
Keywords: ApoE2, gene therapy, liposome, penetratin, transferrin
INTRODUCTION
Alzheimer’s disease (AD), specially the late onset form of AD (LOAD), is the common cause of dementia in individuals age 60 and older (1). This age-dependent neurological disorder is characterized by the presence of extracellular amyloid plaques composed of amyloid-β (Aβ) and intracellular neurofibrillary tangles (2,3). Mounting evidence supports the role of Aβ aggregation and accumulation as an early trigger of toxic cascade leading to synaptic dysfunction, neurodegeneration and cognitive impairment in the etiology of AD (4,5). Multiple genetic and environmental risk factors are involved in LOAD pathogenesis. Impairment of Aβ clearance is probably the major contributor to disease development. While the ε4 allele for APOE gene is the strongest genetic risk factor amongst its three polymorphic alleles (ε2, ε3 and ε4), the ε2 allele is protective (6-8). Many lines of evidence have shown that the risk factors of AD (aging and APOE ε4) accelerate accumulation of Aβ prior to the development of the disease. The apolipoprotein E (ApoE), in the central nervous system (CNS), transports cholesterol from astrocytes to neurons through cell surface receptors, including the low density lipo-protein receptor (LDLR) and LDLR-related protein 1 (LRP1) (9,10). Consequently, ApoE contributes to synaptic plasticity and neuronal function by controlling cholesterol homeostasis with the ApoE3 and ApoE2 alleles having superior function than ApoE4 (11).
A potential therapeutic approach in the development of disease-modifying therapies for AD considers the neuropro- tective functions of APOE ε2 allele. It has been suggested that ApoE2 isoform may differentially regulate Aβ clearance through neurons, microglia or delivery to blood brain barrier (BBB). These features of E2 isoform could be attributed to its higher conformational stability, lower binding affinity to LDLRs, and greater affinity to bind Aβ as compared to E3 and E4 isoforms (11-13). Therefore, ApoE2 isoform expression through targeted therapy may help establish gene based target therapy for AD prevention and treatment.
In this research, we investigated the potential of ApoE2 gene therapy to induce ApoE overexpression in the brain of mice using functionalized-liposomes with ligands-promoter of BBB crossing as gene delivery carriers. Since transferrin (Tf) receptors are expressed on brain capillary endothelial cells (14,15), liposomes were surface modified with transferrin ligand for brain-targeted gene delivery. Additionally, we employed the use of Penetratin (Pen) conjugated to DSPE-PEG-liposomes. Pen (RQIKIWFQNRRMKWKK) is a cell-penetrating peptide that plays important role on enhancement of translocation across cellular membrane of their associated cargo including Pen-associated liposomes (16,17). The current study shows the efficiency of Tf-Pen conjugated liposomes encapsulating chitosan-plasmid ApoE2 complexes to deliver pApoE2 to the brain of mice and induce the production of ApoE2 as a new therapeutic approach for treatment of AD.
MATERIAL AND METHODS
Synthesis of DSPE-PEG2000-Pen and DSPE-PEG2000-Tf
For preparation of liposomal formulations, first we coupled Pen and Tf to terminal NHS-activated DSPE-PEG2000 phospholipid, separately. Briefly, the Pen and DSPE-PEG2000-NHS (1:5 M ratio) were dissolved in anhydrous DMF, and adjusted the pH to 8.0–8.5 with triethylamine. After 120 h, uncoupled Pen was removed by dialysis (molecular weight cut-off of 3500 Da) against deionized water for 48 h. The resultant product was lyophilized and stored at −20°C until use. Holo-Tf (Sigma-Aldrich, St. Louis, MO, USA) and DSPE-PEG2000-NHS (125 μg Tf/μM phospholipid) were dissolved in anhydrous DMF, adjusted pH to 8.0–8.5 with triethylamine, and stirred for 24 h at room temperature. The product was passed through Sephadex G-100 column to removed unbound protein. Coupling efficiencies of both reactions were determined using BCA protein assay (Thermo Fisher Scientific, Waltham, MA, USA).
Preparation and Characterization of Liposomes
Pen-PEG-lipid (4 mol%), DOPE (45 mol%), DOTAP (45 mol%), and Cholesterol (2 mol%) were combined in chloroform:methanol (2:1, v/v) and dried to form a thin lipid film, which was thereafter hydrated using HEPES buffer (pH 7.4). Pen-liposomes were formed after sonication of the sample. Pen-liposomes were stirred overnight with Tf-micelles (4 mol%) to form Pen-Tf-liposomes. The liposomes were passed through Sephadex G-100 column to remove free Tf, and then, extruded through polycarbonate membrane (0.2 μm). For preparation of chitosan-pDNA complexes, 1% w/v chitosan (MW 30 kDa) was dissolved in 0.2 M acetate buffer (pH 4.5). The pDNA solution was added to chitosan at N/P ratio of 5 and stirred for 30 min. Thereafter, chitosan-pApoE2 polyplexes were added to the hydration buffer for incorporation into liposomes. Hydrodynamic size and zeta potential of the formulations were determined by dynamic light scattering using Zetasizer Nano ZS 90 (Malvern Instruments, Malvern, UK) at 25°C. Percent encapsulation of plasmid ApoE2 into liposomes was calculated using Hoechst 33342 (0.15 μg/mL), 354/458 nm excitation/emission wavelengths, respectively, considering absorbance in presence of 0.5% v/v Triton X-100 as 100%.
Chitosan-pDNA Binding Ability
The molar ratios of the amine groups in chitosan and phosphate groups in DNA, chitosan-pApoE2 N/P ratio, were monitored by ethidium bromide (EtBr) exclusion assay and agarose retardation assay. Free pApoE2 was used as a positive control. Different chitosan weight ratios (1-5, 10, 15 and 18) were complexed to pApoE2 (1 μg) and stained with EtBr (0.5 μg) for 5 min. Fluorescence intensity (excitation/emission wavelengths: 260/600 nm, respectively) was measured using a spectrophotometer. For agarose retardation assay, chitosan weight at different ratios (1,5,10,15 and 18) were complexed to pApoE2 (1 μg), then loaded in 0.8% w/v agarose gel stained with EtBr (0.5 μg/mL) and electrophoresed at 80 V in 0.5× Tris-acetate-EDTA (TAE, Bio-Rad, CA, USA) buffer for 80 min. The pDNA migration was thereafter recorded.
DNase Protection Assay
Liposomal formulations encapsulating pApoE2 (1 μg) were incubated for 60 min at 37°C with 1-unit DNase I. Addition of 5 μl of EDTA (100 mM) stopped the reaction. Subsequently, 20 μL of heparin (5 mg/mL) was added and the mixture incubated for 2 h at room temperature to release the pDNA from the complex. The released pDNA samples were subjected to agarose gel electrophoresis 0.8% (w/v) at 80 V for 80 min. Naked pApoE2 incubated with DNase I was used as a positive control.
Cell Culture and Animals
The mouse brain endothelial cell line bEnd.3, obtained from ATCC (Manassas, VA) were cultured in DMEM 10% v/v fetal bovine serum (FBS) and 1% v/v Penicillin Streptomycin (p/s) solution. Primary glial cells and primary neuronal cells were isolated from brain of 1-day old rats. Primary glial cells were cultured in DMEM with 10% v/v FBS and 1% v/v p/s. Glial fibrillary acidic protein (GFAP) immunostaining confirmed the purity of the culture. Primary neuronal cells were cultured in Neurobasal media with 10% v/v plasma-derived horse serum, B-27 supplements, L-Glutamate (25 mM) and 1% v/v p/s. Cytosine arabinoside (10 μM) was added on day 3 to remove non-neuronal cells. Anti-MAP2 antibody immunostaining confirmed the purity of the culture. Cells were incubated in atmosphere of 5% CO2 at 37°C.
Animal experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee (IACUC) at North Dakota State University. Rats (male/female) were purchased from Charles River Laboratories (Wilmington, MA, USA) and C57BL/6 (male/female) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The animals were housed maintained under standard conditions with free access to food and water and exposed to 12 h light-dark cycle.
In Vitro Transfection Efficiency
Evaluation of transfection efficiency of liposomal formulations containing chitosan-pApoE2 complexes were performed in bEnd.3, primary glial and primary neuronal cells. Cells (1 × 106 cells/well) were seeded in 6-well plates, 24 h prior to experiment. Liposomal formulations (100 nM) containing chitosan-pApoE2 complexes (1 μg) were added to the cells in serum-free medium. After 4 h, the media was replaced and cells incubated for a total of 48 h. ApoE expression was quantified using Apolipoprotein E ELISA kit (Thermo Fisher Scientific). Supernatant and cell lysates were incubated for 2.5 h in 96-well pre-coated plates. Biotinylated antibody was added and incubated for 1 h. HRP reagent was incubated 45 min. Subsequently, TMB substrate was incubated for 30 min, the reaction was stop with stop solution followed by colorimetric measurement at 450 nm and 550 nm. Subtraction of 550 nm values from 450 nm values corrected the optical imperfections in the plate. Measured values were normalized by total protein levels in the sample, which were determined by BCA protein assay.
Design of In Vitro Triple Co-Culture BBB Model
The in vitro triple co-culture BBB model was designed combining bEnd.3, primary glial and primary neuronal cells. Primary glial cells (1.5 × 104 cells/cm2) were seeded on the bottom side of culture inserts (0.4 μm pore size and 0.33 cm2 area) in DMEM with 20% v/v FBS, 1% v/v p/s, overnight incubation. Then, bEnd.3 cells (1.5 × 104 cells/cm2) were seeded on upper side of culture inserts that were placed in 24-well plates. Measurement of transendothelial electrical resistance (TEER) values using EVOM2 (World Precision Instruments, Sarasota, FL, USA) evaluated the formation of tight junctions. Primary neuronal cells were cultured in the bottom of 24-well plate.
Transfection Efficiency in Primary Neuronal Cells Following Liposome Transport across In Vitro BBB Model
Liposomal formulations (100 nM) encapsulating chitosan-pApoE2 complexes (1 μg) were added to the upper compartment of inserts and incubated for 8 h. Thereafter, the inserts were removed and the media was replaced for fresh media. After 48 h incubation, the amount ApoE produced by primary neuronal cells was determined by Apolipoprotein E ELISA kit, as described previously. Measured values were normalized by total protein levels in the samples, which were determined by BCA protein assay.
Blood Compatibility Study
In vitro biocompatibility of liposomal formulations was evaluated in freshly harvested erythrocytes from Sprague-Dawley rats. The blood was centrifuged at 1500 rpm for 10 min and washed three times with PBS, pH 7.4, 10 mM CaCl2. Erythrocyte solution containing 1.5 × 107 cells was incubated with negative control (PBS), positive control (Triton X-100 1% v/v) or liposomal formulations (31.25–1000 μM) for 1 h at 37°C. Thereafter, samples were centrifuged at 1500 rpm for 10 min. Absorbance of supernatants was measured at 540 nm. Percent hemolysis was calculated considering the absorbance in the presence of Triton X-100 as 100%.
In Vivo Transfection Efficiency and Biocompatibility
For study of transfection efficiency of liposomal formulations containing chitosan-pApoE2 complexes, seven C57BL/6 mice were injected with single dose of either PBS, or pApoE2 (1 mg pApoE2/kg body weight), or Plain-lip or PenTf-liposomes (~15.2 μ moles phospholipids/kg body weight) encapsulating 1 mg pApoE2/kg body weight. After 5 days of administration, different organs (brain, liver, kidneys, heart, lungs, spleen and blood) were removed, weighed, homogenized in RIPA buffer containing proteinase and phosphatase inhibitor cocktail and centrifuged at 4000 rpm, 4°C for 15 min. ApoE2 levels in the samples were quantified using Apolipoprotein E ELISA kit as previously described. Tissue samples from control mice (PBS administration) were similarly processed to quantify the ApoE expression of individual organs. Measured values were normalized by total protein levels in the samples, which were determined by BCA protein assay. Hematoxylineosin (H&E) staining were performed in tissue sections to analyze the tissue characteristics and the possible morphological changes after treatment.
RESULTS
Preparation and Characterization of Liposomes
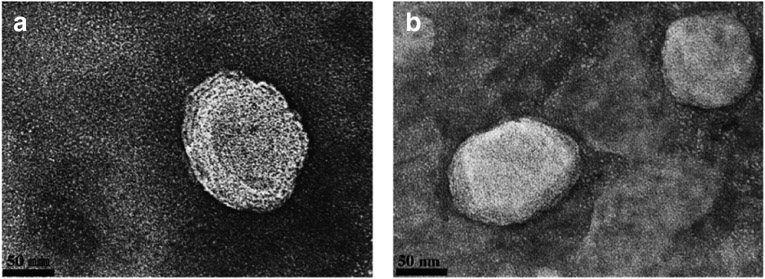
Conjugation of Pen and Tf to DSPE-PEG2000-NHS were performed as described previously. Coupling efficiency of Pen-PEG lipid reaction was 88.3 ± 1.7% and coupling efficiency of Tf-PEG lipid reaction was 83.3 ± 5.8%. Dynamic light scattering analysis showed particle size range from 144 nm to 166 nm with a relative small size distribution (PDI ≤ 0.3), Table I. We observed that dual modification of liposome enhanced the stability of nanoparticle as demonstrated by the smaller hydrodynamic particle size of Tf-Penliposome as compared to the other liposomal formulations and by the TEM images of unmodified-liposome (Fig. 1a) and Tf-Pen-liposome (Fig. 1b). The formulations showed analogous spherical shape and compacted structure. Liposome surface modification had direct influence on zeta potential. The positive charge of the phospholipid DOTAP contributed to positive zeta potential of Liposome (39.4 ± 3.22 mV). While zeta potential of Pen-liposomes was 30.6 ± 1.29 mV. The negative character of Tf significantly (p < 0.05) reduced the zeta potential as compared to that of control Liposome. For Tf-Pen-liposome, the positive charge of Pen balanced the negative charge of Tf leading to zeta potential of 23 ± 0.23 mV. Efficient encapsulations of plasmid ApoE2 into liposomes was obtained for all formulations (Table I), which were above 90%.
Table I.
Liposome-pApoE2: Particle Size, PDI, Zeta Potential and EE
| Liposomes | Particle size (nm) | PDIa | Zeta potential (mV) | EEb |
|---|---|---|---|---|
| Liposome | 166.1 ± 1.57 | 0.24 ± 0.02 | 39.4 ± 3.22 | 92.8 ± 0.54% |
| Tf-liposome | 156.8 ± 2.42 | 0.30 ± 0.03 | 17.3 ± 3.23 | 97.1 ± 1.20% |
| Pen-liposome | 147.9 ± 4.34 | 0.23 ± 0.01 | 30.6 ± 1.29 | 96.5 ± 0.43% |
| Tf-Pen-liposome | 144.9 ± 4.12 | 0.31 ± 0.04 | 23.0 ± 0.93 | 96.2 ± 1.04% |
PDI: Polydispersity index
EE: Encapsulation efficiency
Fig. 1.
Transmission electron microscopy (TEM) images of Plain-liposomes (a) and PenTf-liposomes (b) which was negatively stained with 0.1% phosphotungstic acid aqueous solution (Scale 50 nm).
Chitosan-pDNA Binding Affinity and pDNA Protection against Enzymatic Degradation
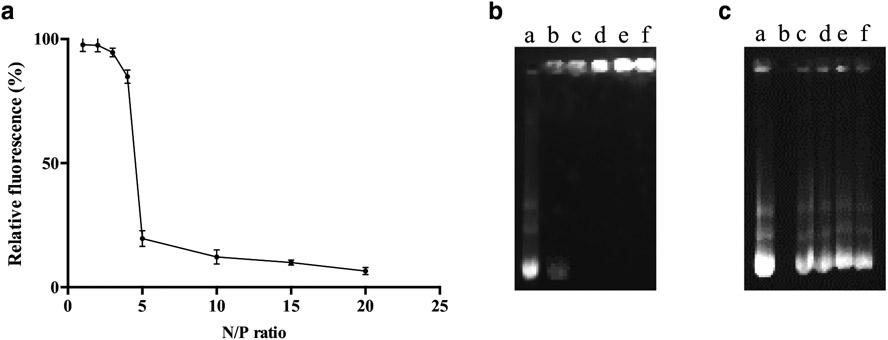
Chitosan was complexed to pDNA at different N/P ratios and the effect of chitosan on pDNA condensation was investigated using EtBr exclusion assay and gel retardation assay. The results of EtBr exclusion assay indicated that the N/P ratio of chitosan-pDNA greater than 5 led to tight binding of chitosan and pDNA. As depicted in Fig. 2a, the relative fluorescence intensity reduced significantly (p < 0.05) from N/P of 1 (97.7 ± 2.6%) to N/P of 5 (19.6 ± 3.2%), whereas we did not observe significant reduction of fluorescence intensity from N/P of 5 to N/P of 10, 15 and 20. This suggests that chitosan-plasmid ApoE2 interactions increased upon increasing N/P ratio. Gel retardation assay was used to determine any change in the electrophoretic mobility of complexed pDNA. Chitosan-pDNA complexes were created at N/P ratios of 1, 5, 10, 15 and 20. Uncomplexed pDNA was represented by the bright band in Fig. 2b lane a. Fig. 2 lane b showed the partial condensation of pDNA at N/P of 1. While Fig. 2b lanes c-f demonstrated complete pDNA complexation in chitosan condensates. The gel retardation assay indicates that chitosan has the ability to compact pDNA and the N/P ratio affects the binding degree. Based on our findings, N/P ratio of 5 was chosen for our experiments.
Fig. 2.
(a) Relative fluorescence of chitosan-pApoE2 complexes in different N/P ratios (1-5, 10, 15 and 20). Data are expressed as mean ± SD (n = 4). (b) Agarose gel electrophoresis of chitosan-pApoE2 complexes in different N/P ratios (1,5,10,15,20), lanes b-f, respectively). Naked pApoE2 (lane a) was used as control. (c) Protective effective of liposomal formulation containing chitosan-ApoE2 complexes (N/P 5) against nuclease degradation. Lane a, naked pApoE2; lane b, naked pApoE2 + DNase I; lanes c-f, Liposome, Tf-liposome, Pen-liposome, and Tf-Pen-liposomes containing chitosan-ApoE2 complexes, respectively + DNase I.
For effective gene expression, gene carriers must be able to overcome nuclease degradation and deliver the nucleic acid into the nucleus of cells (19). In order to evaluate the protective effect of liposomal nanoparticles on pDNA against enzymatic degradation, the formulations loaded chitosan-pApoE2 complexes were challenged in presence of DNase I. Nuclease digestion was assessed by comparing the signal intensity of the treated samples with the control. The integrity of pApoE2 was represented by Fig. 2c lane a. Upon incubation with DNase I, naked pDNA was completely degraded (Fig. 2c lane b). In contrast, DNase protection assay showed that all liposomal formulations protected pApoE2 from enzymatic degradation (Fig. 2c, lanes c-f) with signal intensities similar to control.
In Vitro Transfection Efficiency
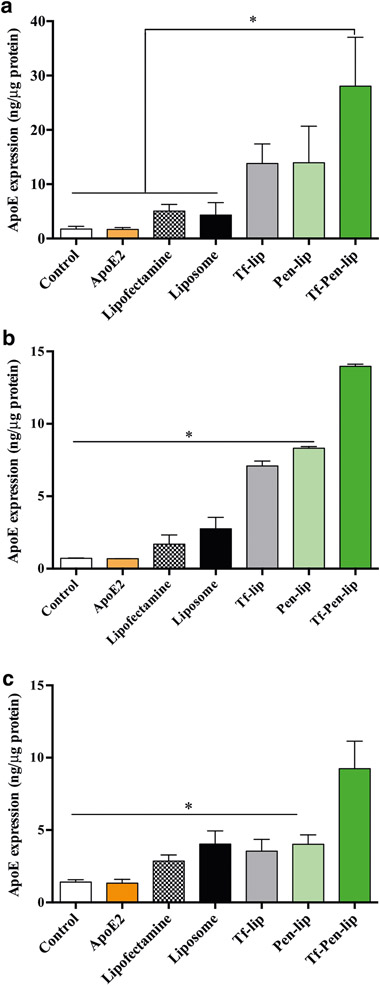
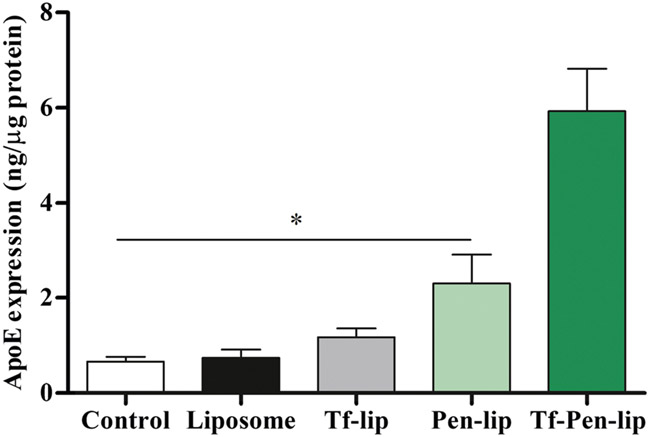
For evaluating in vitro transfection efficiency, bEnd.3 (Fig. 3a), primary glial (Fig. 3b) and primary neuronal cells (Fig. 3c) were treated with liposomes encapsulating chitosan-pApoE2 complexes. Quantification of ApoE levels after transfection showed the enhanced ability of Tf-Pen-liposomes to significantly (p < 0.05) increase ApoE levels as compared to Liposome, Tf-lip, Pen-lip, naked ApoE2 and Lipofectamine 3000/pApoE2 treatment in the aforementioned cells, and cellular ApoE levels (untreated cells).
Fig. 3.
ApoE expression levels 48 h after transfection of bEnd.3 (a), glial (b) and primary neuronal cells (c) with Liposome, Tf-lip, Pen-lip and Tf-Pen-liposomes containing chitosan-pApoE2 (1 μg) complexes. Data are expressed as mean ± SD (n = 4). Statistically significant (p < 0.05) differences are shown as (*).
In Vitro Co-Culture BBB Model
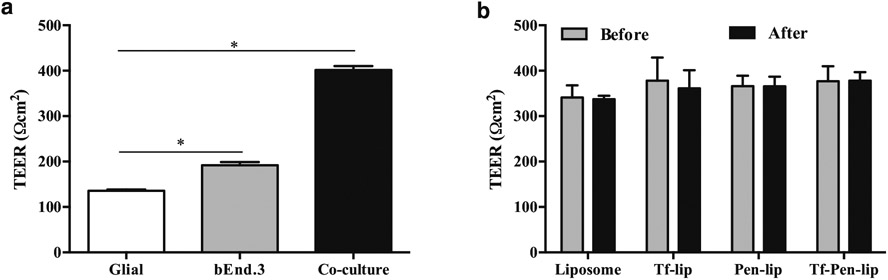
A significant number of potential drugs to brain diseases have failed mainly due to the restrictive permeability of BBB. The characterization of BBB penetration of therapeutic candidates during the development process can have significant impact for the success of the final product (20). Therefore, there is great interest for in vitro models that reflect the properties of the biological barrier. In attempt to mimic the BBB, we designed an in vitro co-culture model by culturing brain endothelial (bEnd.3) cells and glial cells on the upper and bottom side of culture inserts, respectively. This model showed significantly (p < 0.05) higher TEER (401.5 ± 8.5 Ωcm2) as compared to TEER of monolayer models of bEnd.3 (191.9 ± 7.1 Ωcm2) and glial cells (135.7 ± 2.8 Ωcm2), Fig. 4a. Glial cells are postulated to increase the tightness of endothelial monolayers as reflected by TEER values of co-culture model. Therefore, the in vitro co-culture BBB model represents a physiologically-relevant BBB model, which is adequate for functional studies of BBB and selection of brain-targeted formulations (18).
Fig. 4.
(a) Transendothelial electrical resistance (TEER, expressed as Ωcm−2) of different in vitro BBB model constructed using glial and bEnd.3 monolayers and co-culture of bEnd.3 and glial. Significantly (p < 0.05) higher statistical difference in the TEER values between the groups was noted (*). (b) TEER of co-cultured in vitro BBB model before and after 8 h of transport study upon incubation with Liposome, Tf-lip, Pen-lip and Tf-Pen-liposomes. All data are expressed as mean ± SD (n = 4).
Following the design of in vitro co-culture BBB model, we assessed the ability of liposomal formulations loading chitosan-pApoE2 complexes to cross the in vitro barrier layer and, subsequently, transfect primary neuronal cells. Liposomal formulations (100 nM) containing chitosan-pApoE2 complexes (1 μg) were added in the inserts, which were placed in the same well with primary neuronal cells cultured in the bottom, for 8 h. TEER values were measured before and after liposomal incubation to evaluate the potential cytotoxicity of formulations to the barrier layer. We did not observe significant differences between TEER values before and after incubation of liposomal formulations (Fig. 4b). This strongly indicates that liposomes did not cause membrane disruption or cellular damage after transport across the in vitro BBB model, therefore the integrity of the cellular barrier was maintained throughout the experiment.
Transfection Efficiency in Primary Neuronal Cells Following Liposome Transport across In Vitro BBB Model
After transcytosis across in vitro BBB model, we evaluated the functionality of designed liposomal formulations through quantification of transfection efficiency in primary neuronal cells. Tf-Pen-liposomes encapsulating chitosan-pApoE2 complexes demonstrated significantly (p < 0.05) higher ability to cross the in vitro BBB and induce ApoE expression in primary neuronal cells as compared to unmodified liposome, Tf-lip, Pen-liposomes and cellular ApoE levels (Fig. 5). The results suggested that the presence of both ligands on liposome surface determined the improved transport across BBB model as well as transfection in primary neuronal cells.
Fig. 5.
Transfection efficiency of Liposome, Tf-lip, Pen-lip and Tf-Pen-liposomes containing chitosan-pApoE2 (1 μg) complexes in primary neuronal cells after transport through the in vitro BBB co-culture model as determined by ApoE ELISA kit. Data are expressed as mean ± SD (n = 5). Statistically significant (p < 0.05) differences are shown as (*).
Blood Compatibility Study
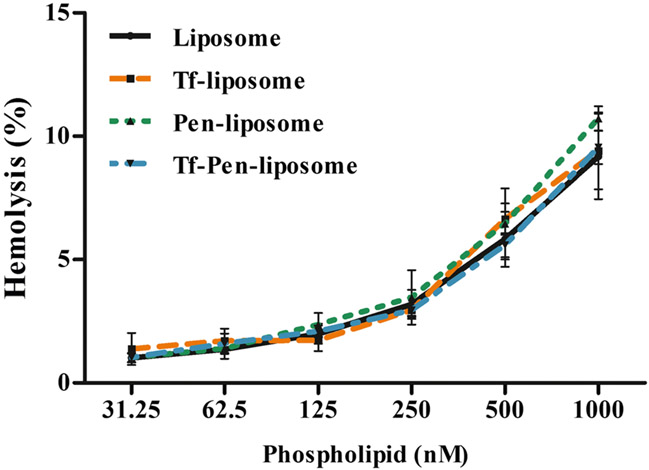
Blood compatibility study is an important parameter to evaluate the biological safety of formulations to be administered intravenously. In this study, we observed that the interaction of nanoparticles with red blood cells caused erythrocyte lysis dependent on liposome phospholipid concentration. Increasing liposomal concentration, the hemolysis percentage increased (Fig. 6). At 31.25 nM phospholipid concentration, we observed in average 1% hemolysis. While at 1000 nM phospholipid concentration, we observed in average 9.5% hemolysis for Liposome, Tf-liposome and Tf-Pen-liposome, and 10.7% hemolysis for Pen-liposome.
Fig. 6.
Hemolytic activity of Liposome, Tf-lip, Pen-lip and Tf-Pen-liposomes at different phospholipid concentrations (31.25–1000 μM). Hemolytic activity of 1% (v/v) Triton X-100 was considered as 100% hemolysis. Data are expressed as mean ± SD (n = 4).
In Vivo Transfection Efficiency and Biocompatibility
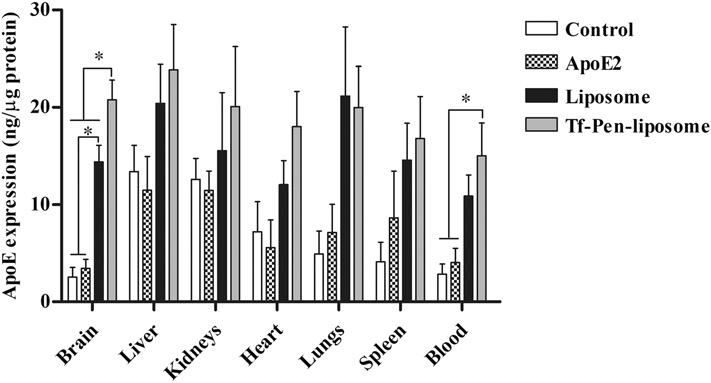
Our group has previously determined the biodistribution of liposomes modified with transferrin and penetratin in the brain of mice (21). The study compared the ability of liposome without surface modification, Tf-liposome, Pen-liposome and PenTf-liposome to penetrate the BBB, reach brain parenchyma and induce protein expression in brain cells. The results demonstrated that dual-functionalization of liposomes with Tf and Pen significantly (p < 0.05) enhanced the accumulation of liposomes in the brain of mice and induced protein expression as compared to the other liposomal formulations (21). Hence, dual-functionalization of liposome was important for brain-targeting. Following to these findings, next we evaluated whether single intravenous administration of Liposome or Tf-Pen-liposomes containing chitosan-pApoE2 (1 mg pApoE2/kg body weight) would significantly increase the levels of ApoE in the brain of mice. We observed that Tf-Pen-liposomes (20.8 ± 4.0 ng/μg of protein) significantly (p < 0.05) increased ApoE expression in the brain of mice as compared to unmodified liposomes encapsulating chitosan-pApoE2 complexes (14.4 ± 3.4 ng/μg of protein), naked plasmid ApoE2 (3.4 ± 1.8 ng/μg of protein) and endogenous ApoE levels (2.5 ± 2.0 ng/μg of protein), as depicted in Fig. 7. No significant differences in ApoE levels were observed in liver, kidneys, heart, lungs and spleen when comparing the endogenous ApoE levels and administration of naked ApoE2, Liposome and Tf-Pen-liposomes containing chitosan-pApoE2 complexes. Additionally, we observed that Tf-Pen-liposomes containing chitosan-pApoE2 significantly (p < 0.05) increased ApoE protein levels in the blood compared to endogenous protein levels (control group) and administration of plasmid ApoE2.
Fig. 7.
ApoE expression in C57BL/6 mice treated with liposomal formulations containing plasmid ApoE2 after 5 days of liposome administration. ApoE levels were quantified in different organs (brain, liver, kidneys, heart, lungs, spleen and blood) harvested from mice treated with plasmid ApoE2, Plain-liposome and Tf-Pen-liposomes containing chitosan-pApoE2 complexes (1 mg pDNA/kg body weight). Seven mice were used per group. Data are expressed as mean ± SD. Statistically significant differences (p < 0.05) are shown as (*).
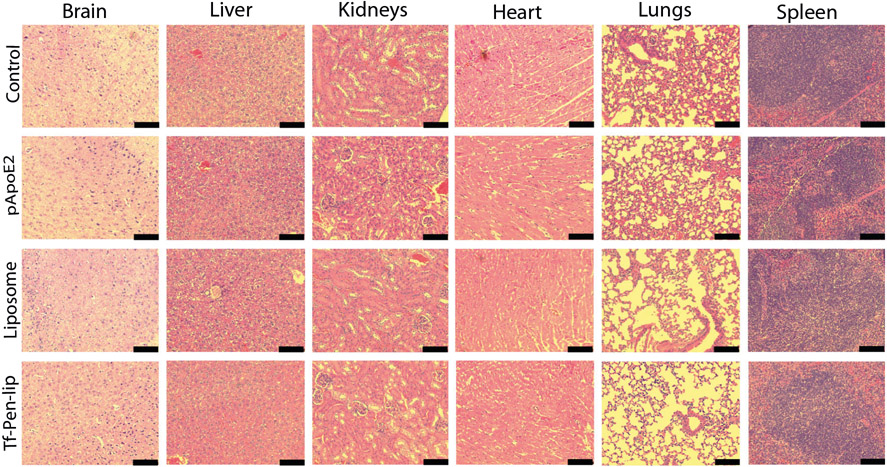
For evaluation of in vivo biocompatibility of liposomal formulations encapsulating chitosan-pApoE2 complexes, tissue sections of major organs such as brain, liver, kidneys, heart, lungs and spleen, were stained with hematoxylin and eosin (H&E), and compared to tissue sections of control (PBS administration). No obvious lesions, signs of inflammation or change in cell morphology were observed in the tissue sections (Fig. 8).
Fig. 8.
Representative tissue sections of mice stained with hematoxylin and eosin (H&E). Mice were administered with saline solution (control), plasmid ApoE2, Liposome encapsulating chitosan-pApoE2 complexes and Tf-Pen-liposome encapsulating chitosan-pApoE2 complexes through tail vein injection. Scale bar, 100 μm.
DISCUSSION
APOE gene has been established as the most important genetic risk factor for developing LOAD. The most common variants of ApoE are ApoE4, ApoE3 and ApoE2 (22). Increasing evidence shows that ApoE isoforms have differential roles on AD pathology and they might regulate AD-pathways through Aβ metabolism, however the mechanisms involved are still unclear. ApoE4 allele is known as the most important genetic risk factor for AD, whereas ApoE2 allele is protective (10). ApoE4 could predispose to AD by suppressing Aβ clearance in the brain, and enhancing Aβ oligomerization leading to increase of toxic Aβ oligomers (11,23). ApoE2 could modulate Aβ clearance, reduce Aβ levels and pathogenic plaques more efficiently as compared to ApoE4 (24,25). Despite the major safety concerns involving viral vectors (i.e. immunogenicity, cytotoxicity and insertional mutagenesis), viral gene delivery of ApoE2 through intracerebral administration has demonstrated to markedly reduce Aβ burden in AD mouse model (26). Therefore, ApoE2 gene therapy is a potential disease-modifying therapy for prevention and treatment of AD. In this study, we synthesized liposome surface modified with Tf and Pen for non-viral brain-targeted delivery of plasmid ApoE2 to the brain of mice using a non-invasive route of administration.
The tight regulation of movement of ions, molecules and cells between the blood and the brain by BBB limits the delivery of therapeutic agents into CNS. The development of properly designed nanocarrier that ensure BBB crossing and delivery of therapeutic gene into brain cells is expected to play important role on AD therapy (27). We assumed that Tf ligand mediates the transport of our designed brain-targeted liposome across BBB through receptor-mediated endocytosis and Pen peptide enhances liposome internalization into cells and assist in overcoming receptor saturation. Hence, we expected that our system would efficiently cross the BBB and deliver plasmid ApoE2 to brain cells followed by induction of protein expression. The rational synthesis of these cationic liposomes consisted of using equimolar ratio of DOPE and DOTAP phospholipids, which would provide enhanced cellular penetration and promotion of transfection (28). Cholesterol was used as stabilizer, controlling the fluidity of liposome bilayer and preventing vesicle aggregation (29). DSPE-PEG phospholipid contributed to improve carrier pharmacokinetics after intravenous administration by minimizing protein interaction, recognition by macrophage and, consequently, reducing nanoparticle clearance with ensuing prolonged systemic circulation (30,31). Finally, Tf ligands were conjugated to liposomes to provide brain-targeted properties, while conjugation of Pen peptide to liposomes would improve their internalization into cells and promote transfection.
Surface modification of liposomes improved the stability of nanoparticles leading to a lower particle size as compared to unmodified liposomes. Additionally, dual-functionalization enhanced this effect. In general, the designed liposomes were characterized by uniform size distribution and positive zeta potential. Plasmid DNA were complexed to the polycationic polymer chitosan as additional strategy for improvement of transfection. Chitosan positive charges electrostatically interact with the negative charges of DNA and spontaneously form chitosan-DNA complexes leading to DNA packing. As a consequence, nucleases are sterically prevented to access and degrade the condensed nucleic acid (32,33). However, a balance in chitosan-pDNA interaction needs to be adjusted to provide, simultaneously, DNA packing and protection against enzymatic degradation, as well as nucleic acid release from the complex once at the target site (33,34). Based on our analysis of chitosan-pDNA binding affinity using EtBr exclusion assay and gel retardation assay, the N/P ratio of 5 was chosen for preparation of chitosan-pApoE2 complexes. Furthermore, highest in vitro transfection efficiency has been demonstrated at N/P of 5 for chitosan/plasmid complexes (35,36). DNA molecules are vulnerable to DNase degradation particularly in vivo and during long storage. Therefore, to produce effective transfection gene carriers are required to prevent enzymatic degradation of the nucleic acid before delivering the genetic material into the cells. Our findings showed that the liposomal system were able to protect pApoE2 under the presence of nucleases.
Poor targeting property is a common limitation faced by liposome-based targeted gene delivery systems, which is demonstrated by negligible penetration at the target site and accumulation in the liver. The design of targeting ligands has been progressing and showing to improve the capability of carriers to deliver nucleic acids to desired cells and, consequently, enhance the transfection (19,21,37,38). Studying the transfection efficiency of designed liposomes loading chitosan-pApoE2 complexes, we observed that the combination of brain-targeted ligand (Tf) and cellular penetration enhancer (Pen) on liposome surface were actively involved in the successful in vitro transfection of bEnd.3, glial and primary neuronal cells.
The efficacy of treatment for AD is greatly hindered by the low brain specificity and low permeability across BBB of the therapeutic agents. We developed an in vitro triple co-culture BBB model to characterize the brain targeting properties as well as the ability of designed liposomal gene carrier to translocate across BBB and deliver pDNA into brain cells. The BBB model was constructed with co-culture of glial and bEnd.3 cells and the integrity of the barrier was characterized by measuring TEER. Primary neuronal cells cultured in the bottom of well plate completed the in vitro triple co-culture BBB model which was used to assist the selection of suitable formulations for brain-targeted gene delivery, i.e., the carriers should be able to overcome this barrier layer and still be able to deliver the therapeutic gene into neuronal cells. The targeting efficiency of dual modified liposomes, Tf-Pen-liposomes, revealed the superior ability of the system to translocate across in vitro BBB and, thereafter, transfect primary neuronal cells as compared to liposomes without surface modification, or single-modified liposomes. These results suggested the contribution of dual modification to promote transport of liposomes across BBB and transfection in primary neuronal cells. Therefore, considering our in vitro findings, Tf-Pen-liposome might be able to overcome in vivo BBB and deliver pApoE2 to brain cells.
Our liposomal formulations were designed for intravenous administration. Therefore, it is important to determine the blood compatibility of the system prior in vivo administration. The low hemolytic activity of liposomal formulations at low phospholipid concentration were suggestive to be safe for intravenous administration (39). Based on previous studies published by our group, Tf-Pen-liposomes demonstrated superior brain targeting properties, greater in vivo BBB permeability and transfection of brain cells as compared to unmodified liposomes and single modified liposomes (either Tf or Pen) (21). Therefore we opted to evaluate in vivo liposome-mediated pApoE2 transfection using unmodified and Tf-Pen-modified liposomal formulations. The engineered brain-targeted liposomes showed in vivo brain targeting and gene delivery efficiencies. The in vivo results demonstrated that Tf-Pen-liposomes encasing chitosan-pApoE2 was capable of increasing significantly (p < 0.05) ApoE levels in the brain of mice on single injection compared with unmodified liposomes encasing chitosan-pApoE2 complexes and equivalent dose of free plasmid ApoE2. Studies using AD mouse model have reported the potential clinical application of ApoE2 therapy for treatment of AD. Increasing ApoE2 levels in the brain of those animals decreased the endogenous Aβ as well as amyloid deposition which supported synaptic and neuritic plasticity (26,40,41). It is particularly encouraging to note that the current study demonstrated the effectiveness of designed brain-targeted liposomes-mediated ApoE2 gene therapy as a potential disease-modifying therapy for AD. Additional studies are needed to investigate the therapeutic potential of liposome-mediated ApoE2 gene transfer for treatment of AD using AD mouse model.
CONCLUSION
In this study, brain-targeted liposome for delivery of plasmid ApoE2 based on Tf receptor targeting with enhanced cellular penetration has been successfully developed as potential disease-modifying therapy for AD. The designed liposomal nanoparticles provided protection of plasmid ApoE2 against nuclease degradation. Liposome surface modified with Tf and Pen efficiently delivered pDNA and induced ApoE expression in vitro. Additionally, this system showed superior in vitro and in vivo BBB permeability. Tf-Pen-liposomes efficiently delivered the therapeutic gene into the brain of mice and increased ApoE expression, showing a better effect than the preparations without targeted-modifications. Therefore, the strategy for ApoE2 gene transfer across BBB using dual-modified liposomes for brain targeting and gene delivery has a great potential for prevention and treatment of AD. The therapeutic efficacy of the system will be further studied in AD mouse model.
ACKNOWLEDGMENTS AND DISCLOSURES
This research was supported by National Institutes of Health (Grant R01AG051574). B.S.R. is supported by doctoral fellowship from The Brazilian National Council for Scientific and Technological Development (CNPq, Brazil) with a scholarship for B.S.R (Full Doctorate Fellowship (GDE): 221327/2014–2). The authors report no conflict of interest.
ABBREVIATIONS
- AD
Alzheimer’s disease
- BBB
Blood brain barrier
- CPP
Cell-penetrating peptide
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DOTAP
1,2-dioleoyl-3-trimethylammonium-propane
- FBS
Fetal bovine serum
- H&E
Hematoxylin and eosin
- LOAD
Late onset form of Alzheimer’s disease
- MTT
(3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide)
- p/s
Penicillin and streptomycin
- Pen
Penetratin
- TEER
Transepithelial electrical resistance
- Tf
Transferrin
- TfR
Transferrin receptor
REFERENCES
- 1.Frozza RL, Lourenco MV, de Felice FG. Challenges for Alzheimer’s disease therapy: insights from novel mechanisms beyond memory defects. Front Neurosci. 2018;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frere S, Slutsky I. Alzheimer’s disease: from firing instability to homeostasis network collapse. Neuron. 2018;97(1):32–58. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henriques AG, Oliveira JM, Carvalho LP. da Cruz e Silva OAB. A?? Influences cytoskeletal signaling cascades with consequences to Alzheimer???S disease. Mol Neurobiol. 2014;52(3):1391–407. [DOI] [PubMed] [Google Scholar]
- 5.Holtzman D, Herz J. Apolipoprotein E and apolipoprotein receptors: normal biology and roles in Alzheimer’s disease. Cold Spring Harb Perspect Med. 2012;2(3):a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A, Singh A, Ekavali. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67(2): 195–203. [DOI] [PubMed] [Google Scholar]
- 7.Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J. Amyloid-beta: a crucial factor in Alzheimer’s disease. Med Princ Pract. 2015;24(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korolev IO. Alzheimer ‘s disease : a clinical and basic science review. Med Student Res J. 2014;04:24–33. [Google Scholar]
- 9.Sun X, Dong C, Levin B, Crocco E, Loewenstein D, Zetterberg H, et al. APOE ε4 carriers may undergo synaptic damage conferring risk of Alzheimer’s disease. Alzheimers Dement. 2016;12(11):1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu CC, Zhao N, Fu Y, Wang N, Linares C,Tsai CW, et al. ApoE4 accelerates early seeding of amyloid pathology. Neuron. 2017;96(5):1024–1032.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YWA, Zhou B, Wernig M, Südhof TC. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell. 2017;168(3):427–441.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Federoff HJ. Alzheimer’s disease: reducing the burden with ApoE2. Gene Ther. 2005;12(13):1019–29. [DOI] [PubMed] [Google Scholar]
- 13.Salomon-Zimri S, Glat MJ, Barhum Y, Luz I, Boehm-Cagan A, Liraz O, et al. Reversal of ApoE4-driven brain pathology by vascular endothelial growth factor treatment. J Alzheimers Dis. 2016;53(4):1443–58. [DOI] [PubMed] [Google Scholar]
- 14.Visser CC, Voorwinden LH, DJ a C, Danhof M, De Boer AG. Characterization and modulation of the transferrin receptor on brain capillary endothelial cells. Pharm Res. 2004;21(5):761–9. [DOI] [PubMed] [Google Scholar]
- 15.Johnsen KB, Moos T. Revisiting nanoparticle technology for blood-brain barrier transport: unfolding at the endothelial gate improves the fate of transferrin receptor-targeted liposomes. J Control Release. 2016;222:32–46. [DOI] [PubMed] [Google Scholar]
- 16.Chikh GG, Kong S, Bally MB, Meunier JC, Schutze-Redelmeier MP. Efficient delivery of Antennapedia homeodomain fused to CTL epitope with liposomes into dendritic cells results in the activation of CD8+ T cells. J Immunol. 2001;167(11):6462–70. [DOI] [PubMed] [Google Scholar]
- 17.Console S, Marty C, García-Echeverría C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem. 2003;278(37):35109–14. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelm I, Krizbai IA. In vitro models of the blood-brain barrier for the study of drug delivery to the brain. Mol Pharm. 2014;11(7):1949–63. [DOI] [PubMed] [Google Scholar]
- 19.Zylberberg C, Gaskill K, Pasley S, Matosevic S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 2017;24(8):441–52. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm I. In Vitro models of the blood – brain barrier for the study of drug delivery to the brain. 2014 [DOI] [PubMed] [Google Scholar]
- 21.dos Santos Rodrigues B, Oue H, Banerjee A, Kanekiyo T, Singh J. Dual functionalized liposome-mediated gene delivery across triple co-culture blood brain barrier model and specific in vivo neuronal transfection. J Control Release. 2018;286:264–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63(3):287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549(7673):523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conejero-Goldberg C, Gomar JJ, Bobes-Bascaran T, Hyde TM, Kleinman JE, Herman MM, et al. APOE2 enhances neuroprotection against Alzheimer’s disease through multiple molecular mechanisms. Mol Psychiatry. 2014;19(11):1243–50. [DOI] [PubMed] [Google Scholar]
- 25.Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE e2. Neurosci Biobehav Rev. 2013;37(10):2878–86. [DOI] [PubMed] [Google Scholar]
- 26.Dodart J-C, Marr RA, Koistinaho M, Gregersen BM, Malkani S, Verma IM, et al. Gene delivery of human apolipoprotein E alters brain Abeta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2005;102(4):1211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen MM, El-Salamouni NS, El-Refaie WM, Hazzah HA, Ali MM, Tosi G, et al. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: technical, industrial, and clinical challenges. J Control Release. 2017;245:95–107. [DOI] [PubMed] [Google Scholar]
- 28.Kim BK, Hwang GB, Seu YB, Choi JS, Jin KS, Doh KO. DOTAP/DOPE ratio and cell type determine transfection efficiency with DOTAP-liposomes. Biochim Biophys Acta Biomembr. 2015;1848(10):1996–2001. [DOI] [PubMed] [Google Scholar]
- 29.Briuglia M-L, Rotella C, McFarlane A, Lamprou DA. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv Transl Res. 2015;5(3):231–42. [DOI] [PubMed] [Google Scholar]
- 30.Mahendra A, James HP, Jadhav S. PEG-grafted phospholipids in vesicles: effect of PEG chain length and concentration on mechanical properties. Chem Phys Lipids. 2019;218:47–56. [DOI] [PubMed] [Google Scholar]
- 31.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1(3):297–315. [PMC free article] [PubMed] [Google Scholar]
- 32.Buschmann MD, Merzouki A, Lavertu M, Thibault M, Jean M, Darras V. Chitosans for delivery of nucleic acids. Adv Drug Deliv Rev. 2013;65(9):123–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62(1):12–27. [DOI] [PubMed] [Google Scholar]
- 34.Mansouri S, Lavigne P, Corsi K, Benderdour M, Beaumont E, Fernandes JC. Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: strategies to improve transfection efficacy. Eur J Pharm Biopharm. 2004;57(1):1–8. [DOI] [PubMed] [Google Scholar]
- 35.Ishii T, Okahata Y, Sato T. Mechanism of cell transfection with plasmid / chitosan complexes. Biochim Biophys Acta. 2001;1514:51–64. [DOI] [PubMed] [Google Scholar]
- 36.Sato T, Ishii T, Okahata Y. In vitro gene delivery mediated by chitosan. Effect of pH, serum, and molecular mass of chitosan on the transfection efficiency. Biomaterials. 2001;22(15):2075–80. [DOI] [PubMed] [Google Scholar]
- 37.Cheng P-W. Receptor ligand-facilitated gene transfer: enhancement of liposome-mediated gene transfer and expression by transferrin. Hum Gene Ther. 1996. Feb 10;7(3):275–82. [DOI] [PubMed] [Google Scholar]
- 38.Gupta B, Levchenko TS, Torchilin VP. TAT peptide-modified liposomes provide enhanced gene delivery to intracranial human brain tumor xenografts in nude mice. Oncol Res. 2007;16(8):351–9. [DOI] [PubMed] [Google Scholar]
- 39.Committee F04 Medical and surgical materials and devices SF 1. BTM. Standard practice for assessment of hemolytic properties of materials. Annu B ASTM Stand 2009;1–5. [Google Scholar]
- 40.Hudry E, Dashkoff J, Roe AD, Takeda S, Koffie RM, Hashimoto T, et al. Gene transfer of human Apoe isoforms results in differential modulation of amyloid deposition and neurotoxicity in mouse brain. Sci Transl Med. 2013;5(212):212ra161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu J, Liu CC, Chen XF, Zhang YW, Xu H, Bu G. Opposing effects of viral mediated brain expression of apolipoprotein E2 (apoE2) and apoE4 on apoE lipidation and Aβ metabolism in apoE4-targeted replacement mice. Mol Neurodegener. 2015;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]