Abstract
Background
Cerebral amyloid angiopathy is a common cause of subcortical hemorrhage in older adults. Although open hematoma removal may be performed for severe subcortical hemorrhage, its safety in patients with cerebral amyloid angiopathy has not been established, and postoperative rebleeding may occur. Therefore, this study aimed to investigate factors associated with postoperative rebleeding.
Methods
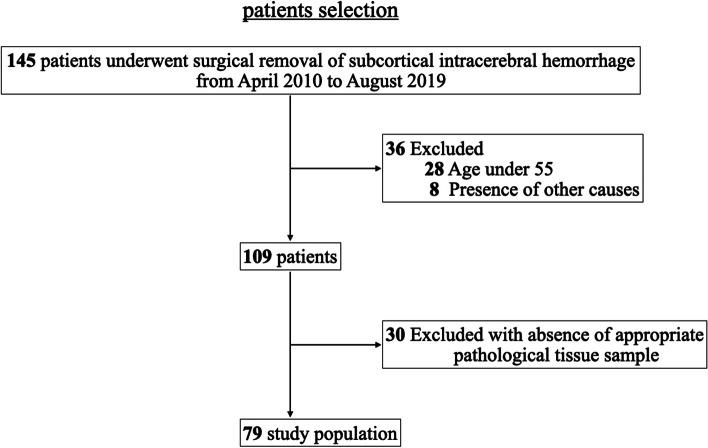
Out of 145 consecutive patients who had undergone craniotomy for surgical removal of subcortical intracerebral hemorrhage between April 2010 and August 2019 at a single institution in Japan, we examined 109 patients with subcortical hemorrhage who met the inclusion criteria. After excluding 30 patients whose tissue samples were unsuitable for the study, the final study cohort comprised 79 patients.
Results
Of the 79 patients, 50 (63%) were diagnosed with cerebral amyloid angiopathy (cerebral amyloid angiopathy group) and 29 (37%) were not diagnosed with noncerebral amyloid angiopathy (noncerebral amyloid angiopathy group). Postoperative rebleeding occurred in 12 patients (24%) in the cerebral amyloid angiopathy group and in 2 patients (7%) in the noncerebral amyloid angiopathy group. Preoperative prothrombin time–international normalized ratio and intraoperative bleeding volume were significantly associated with postoperative rebleeding in the cerebral amyloid angiopathy group (odds ratio = 42.4, 95% confidence interval = 1.14–1578; p = 0.042 and odds ratio = 1.005, 95% confidence interval = 1.001–1.008; p = 0.007, respectively).
Conclusions
Patients with cerebral amyloid angiopathy-related cerebral hemorrhage who are receiving antithrombotic therapy, particularly warfarin therapy, are at a high risk of postoperative rebleeding.
Trial registration
Registry and Registration Number of the study: 19–220, 2019/12/23, retrospectively registered.
Keywords: Amyloid-β, Antithrombotic therapy, Cerebral amyloid angiopathy, Postoperative rebleeding, Subcortical hemorrhage
Background
Cerebral amyloid angiopathy (CAA) is a well-known cause of cerebral subcortical hemorrhage in older adults and is characterized by the deposition of amyloid-ꞵ (Aβ) protein [1, 2]. Furthermore, CAA has been reported to be associated with Alzheimer’s disease [3, 4], as Aβ protein accumulation in the brain is thought to be involved in the development of both Alzheimer’s disease and CAA [5]. Because some patients with CAA develop cerebral subcortical hemorrhage, several studies on risk factors of cerebral subcortical hemorrhage other than Aβ accumulation have been conducted [6–9].
Craniotomy hematoma removal for CAA-related spontaneous intracerebral hemorrhage (CAA-ICH) has been reported [10, 11]. However, postoperative rebleeding and poor outcomes occurred in some patients, and scientific evidence to support the effectiveness of this treatment strategy is limited [10, 12]. Thus, the present study aimed to investigate factors associated with postoperative rebleeding and treatment outcomes of patients pathologically diagnosed with CAA-ICH who underwent craniotomy hematoma removal.
Methods
Study design
We retrospectively collected and analyzed data from consecutive patients who underwent craniotomy hematoma removal for subcortical hemorrhage at Saitama Medical University International Medical Center (SIMC), Japan. CAA was pathologically diagnosed using Aβ staining. Postoperative rebleeding factors were investigated in patients who were pathologically diagnosed with CAA.
Patient selection
Between April 2010 and August 2019, 145 consecutive patients underwent surgical removal of subcortical intracerebral hemorrhage at SIMC. Subcortical hemorrhage was determined based on radiological findings, and cases of basal ganglia hemorrhage were excluded. Patients who met the following Boston criteria were included in the study: (1) age > 55 years and (2) absence of other causes of hemorrhage. The phrase “other causes” was defined as excessive warfarin intake (i.e., international normalized ratio [INR] > 3.0), antecedent cranial trauma or ischemic stroke, central nervous system tumor, vascular malformation, vasculitis, blood dyscrasia, or coagulopathy [13, 14]. However, since this study aimed to evaluate the complications of antithrombotic medications, patients with excessive warfarin intake and those with coagulopathy were also included in this study. Furthermore, patients with Alzheimer’s disease, other dementias, and hypertension were included in this study. We excluded patients whose tissue samples did not contain any assessable vessels or parenchyma. The final study cohort comprised 79 patients (Fig. 1). Immunohistochemical staining for Aβ was performed with the tissue samples of all 79 patients to determine the presence or absence of CAA. Based on the results of the immunohistochemical staining, patients with the pathological diagnosis of CAA were classified as the CAA group and those without the pathological diagnosis of CAA were classified as the non-CAA group. Monoclonal antibodies specific for Aβ 11–28 (12B8, 1:100, IBL, Gunma, Japan) and Aβ 35–40 (1A10, 1:100, IBL) and polyclonal antibodies specific for Aβ 1–42 (1:100, IBL) were used in the immunohistochemical studies.
Fig. 1.
Details of patient selection. Of the 145 patients, 79 met the inclusion criteria. ICH: intracranial hemorrhage
Clinical and neuroimaging data collection
We retrospectively reviewed clinical and neuroimaging data of the enrolled patients from their electronic medical records. The following clinical data were systematically collected for each patient: age; sex; systolic and diastolic blood pressures on admission; hematoma volume; modified Rankin scale (mRS) score at the time of discharge; surgical duration; intraoperative bleeding volume; and history of hypertension, diabetes, dementia (defined as previous diagnosis or current use of antihypertensive, antidiabetic, or antidementia drugs, respectively), or antithrombotic therapy.
Hematoma volume was calculated using the ABC/2 technique, where A is the maximum diameter of the hemorrhagic area, B is the diameter at 90° to A, and C is the number of computed tomography (CT) slices indicating hemorrhage multiplied by the slice thickness [15].
We also assessed the presence or absence of rebleeding within 1 month after craniotomy hematoma removal. Rebleeding after surgery was ascertained by radiologists. Cases of hematoma of volume ≤ 10 mL localized in the resection cavity were excluded.
Statistical analysis
Age, hematoma volume, systolic and diastolic blood pressures on admission, surgical duration, and intraoperative bleeding volume were analyzed using Student t-test. Sex and history of hypertension, diabetes, dementia, and antithrombotic therapy were analyzed using Pearson χ2 test or Fisher exact test. To identify confounding factors, multiple logistic regression analysis was performed using forward stepwise selection methods, with postoperative rebleeding as the outcome. Statistical significance was set at p < 0.05. All analyses were performed using SPSS version 26 (IBM Corp., Armonk, NY).
Results
Patient characteristics
The clinical characteristics of the total cohort, CAA group, and non-CAA group are presented in Table 1. The median age of the patients included in the study was 76 years (range: 55–89 years). Of the total 79 patients, 41 (52%) were women. There were 40 patients (51%) with hypertension, 17 patients (22%) with dementia, and 30 patients (38%) consuming antithrombotic drugs. In total, 23 patients (29%) were on antiplatelet medications and 11 patients (14%) were on anticoagulants; none of them were on deep vein thrombosis prophylaxis. The mean surgical duration was approximately 185 min, and the mean intraoperative blood loss volume was approximately 308 mL (range: 10–1200 mL).
Table 1.
The clinical characteristics and postoperative rebleeding rate of patients classified by pathological diagnosis of CAA
| Total cohort (N = 79) | CAA (n = 50) | Non-CAA (n = 29) | P value | |
|---|---|---|---|---|
| Character | ||||
| Age, median (range), year | 76(55–89) | 78(57–89) | 73(55–84) | 0.001 |
| Female, No. (%) | 41(52) | 34 (68) | 7 (24) | < 0.001 |
| Hypertension, No. (%) | 40(51) | 21 (42) | 19 (66) | 0.062 |
| Diabetes, No. (%) | 7(9) | 2 (4) | 6 (21) | 0.025 |
| Dementia, No. (%) | 17(22) | 15 (30) | 2 (7) | 0.022 |
| Antithrombotic therapy, No. (%) | 30(38) | 16 (32) | 14 (48) | 0.229 |
| Antiplatelets, No. (%) | 23(29) | 11(22) | 12(41) | 0.078 |
| Anticoagulants, No. (%) | 11(14) | 5(10) | 6(21) | 0.162 |
| Combination, No. (%) | 4(5) | 0 | 4(14) | 0.016 |
| Postoperative rebleeding, No. (%) | 14(18) | 12(24) | 2(7) | 0.070 |
| Hematoma vol. (before)*1, Mean (SD), mL | 82(33) | 82(33) | 83(33) | 0.866 |
| Hematoma vol. (after)*2, Mean (SD), mL | 5.9(5.6) | 6.2(5.8) | 5.2(5.5) | 0.430 |
CAA Cerebral amyloid angiopathy
*1: Preoperative hematoma volume
*2: Residual hematoma volume immediately after surgery
Seven patients received intravenous vitamin K (vit. K) preoperatively, and they were on warfarin. Nine patients received fresh frozen plasma (FFP) transfusion preoperatively, and they were on antithrombotic drugs. Five patients received both vit. K and FFP transfusion (Table 2). The mean prothrombin time (PT)–INR on admission was 2.18 for the 11 patients who received vit. K or FFP and 1.03 for the 67 patients who received neither vit. K nor FFP (p = 0.003). Furthermore, the mean activated partial thromboplastin time (APTT) on admission was 42.6 for the patients who received vit. K or FFP and 28.8 for the patients who received neither vit. K nor FFP (p = 0.005). Because the surgery was an emergency surgery, few retests were performed after vit. K or FFP administration. In all cases, PT–INR and APTT normalized the day after surgery. All patients underwent standard craniotomy hematoma removal. Minimally invasive procedures, such as endoscopic hematoma removal, were not performed. The CAA group had higher proportions of older adults, women, and patients with dementia but lower proportions of patients with hypertension and those receiving antithrombotic therapy than the non-CAA group.
Table 2.
The postoperative rebleeding rates for patients who received preoperative Vit. K or FFP
| Total (N = 79) |
vit.K | FFP |
CAA (n = 50) |
vit.K | FFP |
non-CAA (n = 29) |
vit.K | FFP | |
|---|---|---|---|---|---|---|---|---|---|
| Antithrombotic therapy, No. (%) | 30(38) | 7(9) | 9(11) | 16 (32) | 2(4) | 3(6) | 14 (48) | 5(17) | 6(21) |
| Postoperative rebleeding, No. (%) | 14(18) | 3(4) | 5(6) | 12(24) | 2(4) | 3(6) | 2(7) | 1(3) | 2(7) |
CAA Cerebral amyloid angiopathy, vit.K Intravenous vitamin K, FFP Fresh Frozen Plasma
Rebleeding
Postoperative rebleeding occurred in 14 patients (18%), 12 (86%) of whom were in the CAA group. Rebleeding occurred in three of the seven patients who received intravenous vit. K preoperatively and in five of the nine patients who received FFP transfusion preoperatively (Table 2). Univariate analysis of the CAA group revealed that the proportion of patients on antithrombotic drugs and the volume of intraoperative bleeding were significantly higher in patients who had rebleeding (Table 3). Furthermore, multivariate analysis revealed that postoperative rebleeding was significantly associated with preoperative PT–INR and volume of intraoperative blood loss in the CAA group. Based on the adjusted odds ratio, preoperative PT–INR was strongly associated with postoperative rebleeding (Table 4). The results of Hosmer–Lemeshow test were good (p = 0.644), and the percentage of correct classifications was 84.0%.
Table 3.
Univariate analysis of patients with CAA-ICH classified by the presence of postoperative rebleeding
| Rebleeding( +) (n = 12) |
Rebleeding(-) (n = 38) |
P value | |
|---|---|---|---|
| Character | |||
| Age, median (range), year | 78.5 (71–86) | 78 (57–89) | 0.356 |
| Female, No. (%) | 10 (83) | 24 (63) | 0.172 |
| Hypertension, No. (%) | 6(50) | 15 (39) | 0.738 |
| Diabetes, No. (%) | 2 (17) | 0 | 0.054 |
| Dementia, No. (%) | 4 (33) | 11 (29) | 0.518 |
| Antithrombotic therapy, No. (%) | 7 (58) | 9 (24) | 0.032 |
| Anticoagulant therapy, No. (%) | 3 (25) | 2 (5) | 0.082 |
| Antiplatelet therapy, No. (%) | 4 (33) | 7 (18) | 0.240 |
| Systemic factor | |||
| Hematoma vol. (before)*1, Mean (SD), mL | 85 (36) | 81 (32) | 0.721 |
| Hematoma vol. (after)*2, Mean (SD), mL | 7 (6) | 6 (6) | 0.687 |
| Hematoma vol. (rebleed)*3, Mean (SD), mL | 28 (14) | 6 (6) | < 0.001 |
| Systolic blood pressure, Mean (SD), mmHg | 184 (25) | 173 (34) | 0.281 |
| Diastolic blood pressure, Mean (SD), mmHg | 88 (30) | 84 (22) | 0.605 |
| Preoperative PT-INR, Mean (SD) | 1.3(0.64) | 1.0(0.12) | 0.099 |
| Preoperative APTT, Mean (SD) | 29(8) | 28(4) | 0.521 |
| Surgeon’s experience, Mean (SD), year | 4.6(1.8) | 5.2(2.9) | 0.528 |
| Surgical time, Mean (SD), minute | 196 (60) | 188 (68) | 0.731 |
| Intraoperative bleeding volume, Mean (SD), ml | 508 (435) | 216 (160) | 0.042 |
| Outcome | |||
| Hospital stays, Mean (SD), day | 25(20) | 31(18) | 0.391 |
| mRS 0–3 at the discharge, No. (%) | 0 | 11 (28) | 0.032 |
| mRS 6 at the discharge, No. (%) | 5 (42) | 3 (8) | 0.014 |
CAA Cerebral amyloid angiopathy, ICH Intracerebral hemorrhage, SD Standard deviation, mRS Modified Rankin Scale, PT-INR Prothrombin Time-International Normalized Ratio, APTT Activated partial thromboplastin time
*1: Preoperative hematoma volume, *2: Residual hematoma volume immediately after surgery, *3: Hematoma volume after rebleeding
The mRS score 0 is no symptoms at all, 1 is no significant disability despite symptoms, 2 is slight disability, 3 is moderate disability, 4 is moderately severe disability, 5 is severe disability, and 6 is death
Table 4.
Multivariate analysis of factors associated with postoperative rebleeding in CAA-ICH group
| B | P value | Adjusted odds ratio (95%CI) | |
|---|---|---|---|
| Preoperative PT-INR | 3.746 | 0.042 | 42.369 (1.137–1578.683) |
| Intraoperative bleeding volume | 0.005 | 0.007 | 1.005 (1.001- 1.008) |
| Statistical Constant Term | -6.871 | 0.002 | 0.001 |
CAA Cerebral amyloid angiopathy, ICH Intracerebral hemorrhage, B Partial regression coefficient, PT-INR Prothrombin Time-International Normalized Ratio
In total, 10 patients in the CAA group and 2 patients in the non-CAA group presented with rebleeding immediately at the surgical site. Rebleeding was determined based on the comparison of CT images taken immediately after surgery with CT images taken the following day. Two patients in the CAA group had delayed rebleeding at sites distant from the surgical site.
Outcome
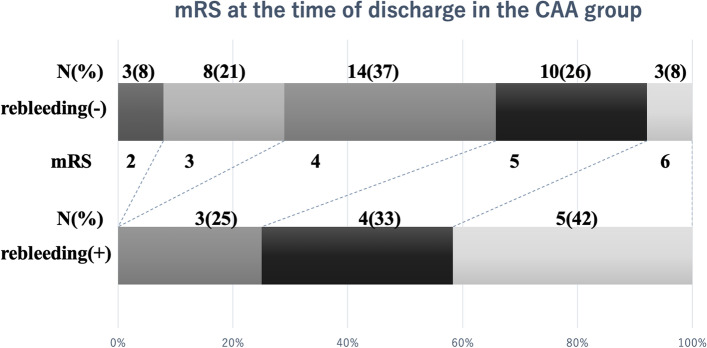
In the CAA group, the mRS score at the time of discharge was 0–3 in 11 of the 50 patients (22%), and the 1-month mortality rate was 16% (8 of the 50 patients). In patients with CAA who experienced rebleeding, the mRS score at the time of discharge was > 3 in all 11 patients, and the 1-month mortality rate was 45% (5 of the 11 patients). In contrast, in patients with CAA who did not have rebleeding, the mRS score at the time of discharge was 0–3 in 11 of the 39 patients (28%), and the 1-month mortality rate was 8% (3 of the 39 patients) (Fig. 2 and Table 3).
Fig. 2.
mRS scores of the CAA group at the time of discharge. mRS scores of patients in the CAA group with or without postoperative rebleeding at the time of discharge. An mRS score of 0 indicates no symptoms at all, a score of 1 indicates no significant disability despite symptoms, a score of 2 indicates mild disability, a score of 3 indicates moderate disability, a score of 4 indicates moderately severe disability, a score of 5 indicates severe disability, and a score of 6 indicates death. mRS: Modified Rankin scale
Discussion
This study aimed to identify the risk factors associated with rebleeding after craniotomy hematoma removal in patients with CAA-ICH. Although the results of neurosurgical evacuation for severe CAA-ICH have been reported, the effectiveness of surgical procedures remains unclear.
The incidence rate of postoperative rebleeding in patients pathologically diagnosed with CAA and subcortical hemorrhage varies from 22 to 50%, as reported in previous studies [12, 16, 17]. In our study, 12 out of 50 patients (24%) pathologically diagnosed with CAA had postoperative rebleeding. Petridis et al. showed that patients with CAA-ICH had a significantly higher rate of postoperative rebleeding than patients without CAA-ICH. They also observed that patients with CAA-ICH were significantly older and had a higher level of vascular vulnerability, which increases the risk of rebleeding, than patients without CAA-ICH [12].
In this study, preoperative PT–INR and intraoperative blood loss volume were significantly associated with postoperative rebleeding in the CAA group. The high intraoperative bleeding volume observed in this study (and in other studies) suggests that patients with CAA are older and have more fragile vessel walls than patients without CAA, which makes hemostasis more difficult [12, 18].
Although high preoperative PT–INR is naturally associated with a high risk of bleeding, all patients with high PT–INR received preoperative reversal therapy. It has been reported that early and aggressive anticoagulation recovery therapy and good blood pressure control lead to better outcomes in patients with ICH [19, 20]. However, in the CAA group, anticoagulation recovery therapy may have not been effective in preventing postoperative rebleeding. Due to the small number of cases, we could not comprehensively investigate this theory. Furthermore, the only antithrombotic antagonists used in this study were vit. K and FFP. Our results may have been different if other antagonists (such as four-factor prothrombin complex concentrates or platelet transfusions) had been used, but their efficacy against CAA-ICH remains unknown. In this study, no detection index of antithrombotic therapy other than PT–INR and APTT were evaluated, making the analysis difficult.
Furthermore, reports of the timing and location of postoperative rebleeding are rare. In this study, the 12 cases of immediate rebleeding at the same site highlight the difficulty of complete hemostasis at the surgical site in patients with CAA. In addition, in the two cases of delayed hemorrhage in other areas, postoperative stress and blood pressure fluctuations may have caused the collapse of cerebral vessels in areas that were originally vulnerable. Rebleeding of this type is difficult to prevent.
Our results showed that prognosis was significantly poor and mortality rate was high in patients with CAA when rebleeding occurred after craniotomy hematoma removal (Fig. 2). In other words, if a patient with CAA undergoing antithrombotic therapy has severe cerebral hemorrhage, craniotomy is unlikely to improve prognosis, and there is a relatively high chance that the patient will not survive. Hence, it is critical to consider the surgical strategy for hemostasis and carefully determine the surgical indications for hematoma removal in patients with CAA-ICH who are receiving antithrombotic therapy. Furthermore, antithrombotic therapy should be administered with caution to patients with CAA who are at a high risk of cerebral hemorrhage. For example, as much as possible, warfarin therapy should be avoided for patients with CAA. It is necessary to conduct additional research to develop a simple method for diagnosing patients with CAA and assessing their risk of cerebral hemorrhage.
This study has several limitations. First, this was a single-center study with a small number of patients. Therefore, the generalizability of our results for multiple institutions is limited, and studies with larger sample sizes should be conducted in the future to verify the findings of this study. Second, due to insufficient data, long-term outcomes could not be investigated. Nevertheless, the findings of this study are expected to contribute to a better understanding of the establishment of a treatment strategy for patients with CAA-ICH.
Conclusion
Craniotomy should be carefully considered for patients with CAA-ICH who are receiving antithrombotic medications and have a high PT–INR because they are at a high risk of postoperative rebleeding. Furthermore, antithrombotic therapy, particularly warfarin therapy, should be administered with caution to patients with CAA.
Acknowledgements
This work was supported by the Hidaka Research Projects [grant number: 01-D-1-07]. The authors would like to thank Enago (www.enago.jp) for the English language review. We are extremely grateful to the staff of the Department of Pathology at Saitama Medical University International Medical Center and the laboratory assistant, Ms. Watanabe.
Abbreviations
- CAA
Cerebral amyloid angiopathy
- Aβ
Amyloid-ꞵ protein
- ICH
Spontaneous intracerebral hemorrhage
- CAA-ICH
Cerebral amyloid angiopathy-related spontaneous intracerebral hemorrhage
- SIMC
Saitama Medical University International Medical Center
- mRS
Modified Rankin scale
Authors’ contributions
TY and HO: Conceptualization and Methodology. TY and HS: Resources, Investigation, Formal Analysis, Writing-Original draft preparation. MT and HK: Supervision. TY and KS: Writing-Reviewing and Editing. All authors have read and approved the final version of the manuscript.
Funding
The Hidaka Research Projects [grant number: 01-D-1–07].
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. SIMC Institutional Review Board, Approval No. 19–220.
Consent for publication
Informed consent was not obtained. This was a retrospective study that analyzed data obtained from the electronic medical records. The tissue samples obtained were used for diagnostic, and not for research, purposes.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Taro Yanagawa, Email: ta111-paper@yahoo.co.jp.
Hiroki Sato, Email: hirokihiroki1207@hotmail.com.
Kaima Suzuki, Email: ks7121@5931.saitama-med.ac.jp.
Hidetoshi Ooigawa, Email: towymickey@gmail.com.
Masaki Takao, Email: msktakaobrb@ncnp.go.jp.
Hiroki Kurita, Email: hk0836@5931.saitama-med.ac.jp.
References
- 1.Nelson T, Leung B, Bannykh S, Shah KS, Patel J, Dumitrascu OM. Cerebral amyloid angiopathy-related inflammation in the immunosuppressed: a case report. Front Neurol. 2019;10:1283. doi: 10.3389/fneur.2019.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamada M, Naiki H. Cerebral amyloid angiopathy. Prog Mol Biol Transl Sci. 2012;107:41–78. doi: 10.1016/B978-0-12-385883-2.00006-0. [DOI] [PubMed] [Google Scholar]
- 3.Boyle PA, Yu L, Nag S, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85:1930–1936. doi: 10.1212/WNL.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goos JD, Kester MI, Barkhof F, et al. Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke. 2009;40:3455–3460. doi: 10.1161/STROKEAHA.109.558197. [DOI] [PubMed] [Google Scholar]
- 5.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75:693–698. doi: 10.1212/WNL.0b013e3181eee40f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342:240–245. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 8.Westermark P, Benson MD, Buxbaum JN, et al. A primer of amyloid nomenclature. Amyloid. 2007;14:179–183. doi: 10.1080/13506120701460923. [DOI] [PubMed] [Google Scholar]
- 9.Yanagawa T, Takao M, Yasuda M, et al. Clinical and neuropathologic analysis of intracerebral hemorrhage in patients with cerebral amyloid angiopathy. Clin Neurol Neurosurg. 2019;176:110–115. doi: 10.1016/j.clineuro.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Leblanc R, Preul M, Robitaille Y, Villemure JG, Pokrupa R. Surgical considerations in cerebral amyloid angiopathy. Neurosurgery. 1991;29:712–718. doi: 10.1097/00006123-199111000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Wang X, Schultz C, Lanzino G, Rabinstein AA. Postoperative outcome of cerebral amyloid angiopathy-related lobar intracerebral hemorrhage: case series and systematic review. Neurosurgery. 2012;70:125–30. doi: 10.1227/NEU.0b013e31822ea02a. [DOI] [PubMed] [Google Scholar]
- 12.Petridis AK, Barth H, Buhl R, Hugo HH, Mehdorn HM. Outcome of cerebral amyloid angiopathic brain haemorrhage. Acta Neurochir (Wien) 2008;150:889–895. doi: 10.1007/s00701-008-0001-y. [DOI] [PubMed] [Google Scholar]
- 13.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the Boston criteria. Stroke. 2018;49:491–497. doi: 10.1161/STROKEAHA.117.016990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 16.Matkovic Z, Davis S, Gonzales M, Kalnins R, Masters CL. Surgical risk of hemorrhage in cerebral amyloid angiopathy. Stroke. 1991;22:456–461. doi: 10.1161/01.str.22.4.456. [DOI] [PubMed] [Google Scholar]
- 17.Hirohata M, Yoshita M, Ishida C, et al. Clinical features of non-hypertensive lobar intracerebral hemorrhage related to cerebral amyloid angiopathy. Eur J Neurol. 2010;17:823–829. doi: 10.1111/j.1468-1331.2009.02940.x. [DOI] [PubMed] [Google Scholar]
- 18.Dye JA, Rees G, Yang I, Vespa PM, Martin NA, Vinters HV. Neuropathologic analysis of hematomas evacuated from patients with spontaneous intracerebral hemorrhage. Neuropathology. 2014;34:253–260. doi: 10.1111/neup.12089. [DOI] [PubMed] [Google Scholar]
- 19.Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313:824–836. doi: 10.1001/jama.2015.0846. [DOI] [PubMed] [Google Scholar]
- 20.Hostettler IC, Seiffge DJ, Werring DJ. Intracerebral hemorrhage: an update on diagnosis and treatment. Expert Rev Neurother. 2019;19:679–694. doi: 10.1080/14737175.2019.1623671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.