Abstract
Invasion of human erythrocytes by Plasmodium falciparum merozoites is a multistep process. For many strains of the parasite, part of this process requires that the erythrocyte binding antigen 175 (EBA-175) of the merozoite binds to sialic acid residues of glycophorin A on the erythrocyte surface, a receptor-ligand interaction which represents a potential target for inhibition by antibodies. This study characterizes the reactivity of naturally acquired human antibodies with four recombinant proteins representing parts of EBA-175 (region II, regions III to V, and the dimorphic C and F segment region) in populations in which the organism is endemic. Serum immunoglobulin G (IgG) recognizing the recombinant proteins is predominantly of the IgG1 and IgG3 subclasses, and its prevalence increases with age. In a large population study in The Gambia, serum positivity for IgG or IgG1 and IgG3 subclass antibodies to each of the EBA-175 recombinant antigens was not significantly associated with subsequent protection from clinical malaria. However, there was a trend indicating that individuals with high levels of IgG to region II may have some protection.
A vaccine is needed to protect against malaria, a disease affecting millions of people in the tropical and subtropical regions of the world. Plasmodium falciparum, the most lethal of the human malaria parasites, is responsible for more than 90% of malaria cases in Africa and accounts for approximately one million deaths annually. One option for vaccine research is to evaluate the protective potential of antigens which have conserved function among different Plasmodium species (21). Alternatively, if particular polymorphic regions of P. falciparum antigens can be shown to be targets of protective immunity, it may be logical to develop a multicomponent vaccine incorporating the different allelic forms (5). In the parasite's merozoite stage, several antigens have both conserved and polymorphic regions in their sequences, including the P. falciparum erythrocyte binding antigen 175 (EBA-175).
EBA-175 is located in the apical micronemes of merozoites and appears to mediate parasite invasion of host erythrocytes, as a cysteine-rich region (region II) binds to sialic acid residues on glycophorin A (31). This region is fairly highly conserved in P. falciparum (18), and its homologue has been isolated in other Plasmodium species (1, 11, 16, 23). Some preliminary evidence suggests that the initial binding may be followed by proteolytic cleavage of EBA-175 and subsequent binding of a dimorphic region, encoding the C and F segments (36), to the glycophorin A peptide backbone (15). However, no association has been seen between these dimorphic alleles and the degree of dependence of parasites on neuraminidase- or trypsin-sensitive receptors (which include glycophorin A) in erythrocyte invasion (3).
The interaction between EBA-175 and glycophorin A represents a potential target for inhibition by vaccine-induced antibodies. It has been shown that recombinant fragments of EBA-175 are recognized by antibodies in pooled human sera from areas where malaria is endemic (7). Antibodies raised in mice against a 42-amino-acid peptide of EBA-175, a conserved sequence (30) within regions III to V termed EBA peptide 4, blocked binding of native EBA-175 to human erythrocytes and inhibited merozoite invasion in vitro (32). The cysteine-rich region II and EBA peptide 4 were recognized by antibodies eluted from immune clusters of merozoites (29), thus confirming the accessibility of these domains on the surface of merozoites. A 12-amino-acid peptide (amino acids 1085 to 1096) within EBA peptide 4 may be involved in secondary binding to glycophorin A, although this peptide was only weakly recognized by human antibodies acquired during natural infections (14).
The present study characterizes the reactivities of naturally acquired human antibodies against different parts of EBA-175, namely, the cysteine-rich region II, the dimorphic C and F segments, and regions III to V. The serum immunoglobulin G (IgG) subclass specificities, age dependencies, and potential protective associations of these antibodies were investigated.
MATERIALS AND METHODS
Sera from adults.
Sera were obtained from 38 Nigerian volunteers (age 18 to 60 years) who accompanied their children at the Massey Street Children's Hospital, Lagos Island, Nigeria, in August and September 1997. These adults consented to donate 20 ml of venous blood, under approval from the ethical committee of the National Institute for Medical Research, Lagos, Nigeria. Twenty control sera were obtained in the United Kingdom from European donors with no previous exposure to malaria.
Sera from children.
A population-based study was carried out in rural communities near Farafenni, on the north bank of the Gambia river, as described previously (28), with approval granted by the Medical Research Council/Gambian Government Ethical Committee. Malaria transmission in The Gambia is seasonal, with most P. falciparum infections occurring from July through November. A total of 284 children age 3 to 9 years, with data on incidence of malaria throughout the 1988 transmission season, were chosen for the purpose of this study. Plasma samples had been obtained from each child in May 1988, prior to the malaria transmission season, and each child was followed up once a week to assess parasitological and clinical status for a 5-month period (June to October). The outcomes were categorized as at least one episode of clinical malaria (fever of >37.5°C [axillary temperature] plus P. falciparum parasitemia of >5,000 parasites/μl of blood), asymptomatic infection (parasitemia in the absence of clinical symptoms), or no malaria infection.
Recombinant EBA-175 antigens expressed in Escherichia coli.
PCR amplifications of regions of the eba-175 gene were performed with high-fidelity Pfu polymerase (Stratagene Europe, Gebouw California, The Netherlands). The dimorphic rF-seg-INT, corresponding to codons 761 to 1017 of the FCR-3 sequence (36), was amplified from the K1 clone, while rINT-C-seg, corresponding to codons 761 to 990 of the Camp Malaysia sequence (32), was amplified from the T9/96 clone using the following pairs of oligonucleotide primers (the portions of each primer in lowercase contain restriction endonuclease sites, BamHI on the forward primer and NotI on the reverse primer, for directional insertion of the fragments into the expression vector pGEX 4T-3): 5′-tct gct gga tcc CAA GAA GCA GTT CCT GAG G-3′ and 5′-cga gta gcg gcc gcA TGT TCT TTC ACC TCT TCA TG-3′ (using an initial hot start of 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, 52°C for 3 min, and 72°C for 2 min, with a final extension at 72°C for 7 min). INT is a conserved sequence of 115 amino acids present in both P. falciparum strains, intervening between the positions of the F and C segments (36). The rReg III-V, corresponding to codons 983 to 1272 of the Camp Malaysia sequence, was amplified using oligonucleotide primers 5′-att gac gga tcc AAT CAT GAA GAG GTG AAA GAA-3′ and 5′-gtc aga gcg gcc gcA TCC CCA GAA TTT CCC CC-3′ (using an initial hot start of 94°C for 5 min, followed by 30 cycles of 94°C for 1 min, 48°C for 2 min, and 72°C for 3 min, with a final extension at 72°C for 7 min). DNA products were purified, ligated into the pGEX 4T-3 vector (33), and transformed into competent JM109 E. coli cells (Promega, Southampton, United Kingdom) for propagation and storage, and plasmid inserts were sequenced using a Thermo Sequenase dye terminator cycle sequencing premix kit (Amersham Life Science, Inc., Cleveland, Ohio) and ABI 377 DNA sequencer. Plasmids containing the expected sequences were transformed into E. coli BL-21 cells (Pharmacia Biotech), and recombinant EBA-175 fragments were expressed as fusion proteins fused to the C terminus of glutathione S-transferase (GST) of Schistosoma japonicum (33). The expressed soluble fusion proteins were purified on glutathione agarose beads (Pharmacia Biotech) and cleaved with thrombin protease (33).
Recombinant baculovirus-derived EBA-175 protein.
DNA containing the region II domain-coding sequences (amino acid residues 144 to 753) (RII) was amplified by PCR from P. falciparum strain 3D7 genomic DNA and cloned into the baculovirus transfer vector plasmid pBSV-8His (17). This plasmid contains DNA sequences encoding the human FHL-1 amino-terminal secretion signal sequence and a carboxy-terminal polyhistidine (8 residues) tag. The correct structure of the insert and cloning junctions was confirmed by DNA sequencing, and the resulting plasmid transfer vector was used to construct a recombinant EBA-175 RII-expressing baculovirus using methods previously described (7). The RII-His8 polypeptide was expressed as a secreted protein and purified by Ni2+ agarose affinity chromatography. The purified protein was resuspended in 1× phosphate-buffered saline (PBS) and stored at −70°C until use. Further details on the cloning, expression, purification, and functional characterization of this recombinant RII polypeptide will be reported elsewhere (C. F. Ockenhouse et al., unpublished data).
Rabbit immunization with recombinant EBA-175 antigens.
Two New Zealand White rabbits were immunized with 500 μg of either rF-seg-INT or rC-seg-INT mixed with MPL/TDM/CWS adjuvant (Sigma Chemical Co., St. Louis, Mo.). The rabbits were boosted at week 4, week 10, and week 14 with another 500 μg of antigen. Serum samples were collected for analysis before each immunization and 4 weeks after the final immunization.
Western blot analysis of rEBA-175 proteins.
Recombinant proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions and electrophoretically transferred onto Protran BA 85 nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Molecular mass standards (Amersham Pharmacia Biotech) were visualized with 0.2% Ponceau S (Sigma Chemical Co.) in 0.3% trichloroacetic acid, and the membrane was blocked overnight at 4°C in blocking buffer (5% bovine serum albumin in PBS). Sera or horseradish peroxidase (HRP)-conjugated secondary antibodies (1/1,000) were incubated with the membrane for 2 h (all reactions and washes were carried out at room temperature). Blots were developed with diaminobenzidine tetrahydrochloride dihydrate (Bio-Rad, Hercules, Calif.).
ELISA.
Sera were tested by enzyme-linked immunosorbent assay (ELISA) for the presence of IgG antibodies reactive with the recombinant EBA-175 fragments. Ninety-six-well plates (Immulon 4; Dynatech, Chantilly, Va.) were coated with 2 μg of rReg II ml−1 or 0.5 μg each of rINT-C-seg, rF-seg-INT, and rReg III-V recombinant antigens ml−1 (determined by a checkerboard titration) in 100 μl of coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.3) for 2 h at 37°C. The wells were washed three times in washing buffer (0.05% Tween 20 in PBS). Unoccupied protein binding sites were blocked with 150 μl of blocking buffer (5% [wt/vol] skim milk powder in PBS) per well overnight at 4°C. The plates were washed three times in washing buffer. Human sera diluted 1/200 in 0.5% skim milk powder (100 μl per well) were added to duplicate antigen-coated wells and incubated for 2 h at room temperature. Antibodies against rReg II antigen were tested at a 1/400 serum dilution. Plates were washed three times and incubated for a further 2 h at room temperature with 100 μl of HRP-conjugated rabbit anti-human IgG diluted 1/2,000 (Dako, Ely, United Kingdom) per well. The plates were again washed three times before incubation for 5 min at room temperature with 100 μl of substrate (0.1 mg of o-phenylenediamine [Sigma Chemical Co.], 0.012% H2O2) in development buffer (24.5 mM citric acid monohydrate, 52 mM Na2HPO4, pH 5.0). The reaction was stopped by the addition of 50 μl of 8 N H2SO4, and the optical density (OD) was measured at 490 nm. The cutoff values at which the binding of antibodies from malaria-exposed individuals was regarded as significantly above background were calculated as the mean plus two standard deviations of the OD readings obtained from the sera of a panel of 20 European donors without a history of exposure to malaria.
Human IgG subclass analysis was carried out with HRP-conjugated affinity-purified anti-IgG1, -IgG2, -IgG3, and -IgG4 antibodies (codes AP006 to AP009; Binding Site, Birmingham, United Kingdom) used at a 1/1,000 dilution (except for anti-IgG2, which was used at 1/500). Rabbit IgGs against rF-seg-INT and rINT-C-seg were tested by ELISA at serial dilutions of 100 to 3,200 and with antigens applied at 0.5 μg ml−1. Plates were further incubated with 100 μl of HRP-conjugated swine anti-rabbit IgG diluted 1/2,000 (Dako) per well for 2 h at room temperature and developed as described above.
Statistical analysis.
Antibody and epidemiological data were entered into Microsoft Excel worksheets and imported into SPSS version 8.0 and Epi-Info 6.0 for statistical analysis. Correlations between OD values for antibody reactivities with pairs of individual antigens were calculated as Spearman's rank correlation coefficients. Chi-square tests and multiple logistic regression analyses were used to determine the significance of associations between the presence of detectable IgG to each antigen, or of OD levels of reactivity to each antigen, and subsequent incidence of clinical malaria.
RESULTS
EBA-175 recombinant proteins.
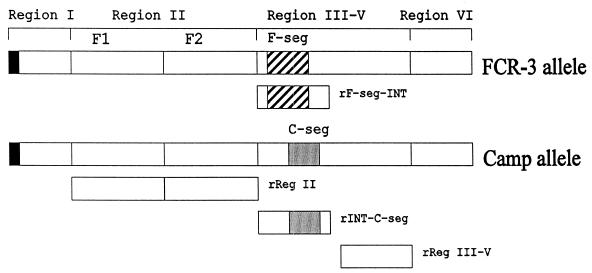
The recombinant constructs rINT-C-seg, rF-seg-INT, and rReg III-V (Fig. 1) were expressed as GST fusion proteins. The proteins were bound to glutathione agarose beads and cleaved with thrombin protease to yield the P. falciparum antigen fragments (without GST) for antibody studies. Proteins were checked for purity and removal of the GST portion by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot assays. The nucleotide sequences of rINT-C-seg and rReg III-V were identical to the corresponding sequences in the Malaysian Camp strain (32), whereas the rF-seg-INT sequence was identical to the allelic sequence in the FCR-3 strain (36). The baculovirus-derived EBA-175 rReg II (Fig. 1) had a sequence identical to that expected from the 3D7 strain of P. falciparum, as described for a previously constructed recombinant protein (7).
FIG. 1.
Schematic diagram of the exon 1-encoded EBA-175 extracellular domain showing the recombinant expression constructs studied. F-seg and C-seg are dimorphic allelic segments of EBA-175 present in the FCR-3 (accession number L07755) and Camp (accession number X52524) strains, respectively, of P. falciparum. INT is an intervening conserved sequence present in both dimorphic proteins (C terminal to F-seg and N-terminal to C-seg). Full details of the sequences expressed are given in Materials and Methods.
Anti-EBA-175 IgG and IgG subclass reactivities in adult Nigerian sera.
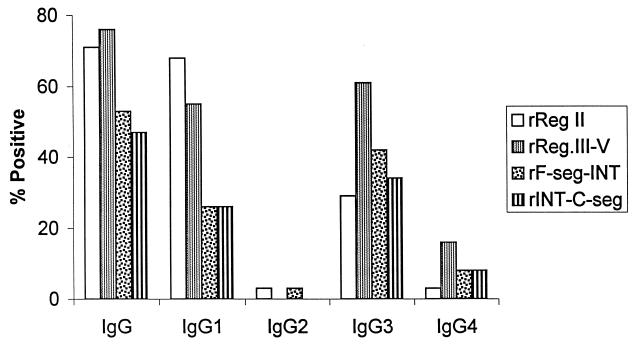
The proportion of adult Nigerians with detectable IgG antibodies, and each of the IgG subclasses, against the recombinant antigens as determined by ELISA is shown in Fig. 2. Against each of the four recombinant proteins, the highest prevalence of subclass antibodies was seen for IgG1 and IgG3. Against three of the recombinant antigens (rINT-C-seg, rF-seg-INT, and rReg III-V), IgG3 antibodies were more prevalent than IgG1, but IgG1 antibodies were more prevalent than IgG3 against rReg II (Fig. 2). Only a small minority of sera contained IgG2 or IgG4 to any of the recombinant antigens. Qualitatively similar results were obtained by assaying antibody reactivities to the antigens on Western blots (not shown).
FIG. 2.
Proportions of Nigerian donors (n = 38) with serum IgG and IgG subclass antibodies against recombinant antigens of EBA-175, tested by ELISA.
Antibodies recognize C and F segment allele-specific epitopes.
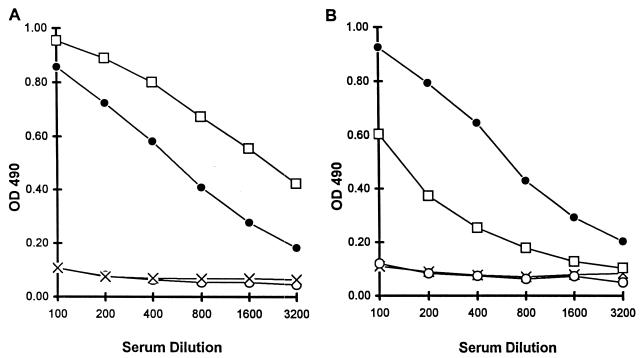
Rabbits which were immunized with soluble preparations of the allelic rINT-C-seg and rF-seg-INT recombinant antigens produced serum IgG which had higher reactivity against the homologous antigen than against the heterologous antigen (Fig. 3). The only sequence differences between these antigens were in the allelic C and F segments, and thus the differences in reactivity indicate that allele-specific F and C segment epitopes exist on these proteins. However, a significant reactivity to the heterologous protein in each case indicates the presence also of conserved epitopes, presumably in the intervening (INT) sequence.
FIG. 3.
Demonstration of the presence of allele-specific and conserved epitopes in rF-seg-INT and rINT-C-seg recombinant antigens. ELISA analysis of serum IgG in a rabbit immunized with rF-seg-INT (×, preimmunization; □, postimmunization) and in a rabbit immunized with rINT-C-seg (○, preimmunization; ●, postimmunization) is shown. (A) ELISA plate coated with rF-seg-INT; (B) ELISA plate coated with rINT-C-seg. Antigens were applied at 0.5 μg ml−1. Assay details are given in Materials and Methods.
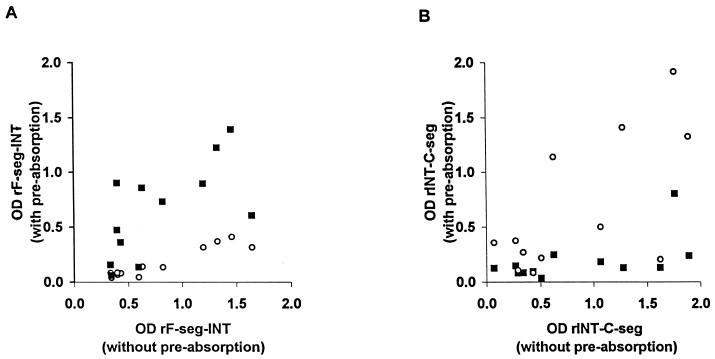
To determine whether human antibodies recognize allele-specific epitopes in the C and F segments, 12 human sera which contained IgG against either or both recombinant antigens were selected for preabsorption experiments. These sera were preincubated with PBS or 10 μg of soluble rINT-C-seg or rF-seg-INT antigen per ml prior to testing and then were tested at a 1/400 dilution against each of the two antigens by ELISA (100 μl of coating antigen at 0.5 μg ml−1). Thus, homologous and heterologous antigen preabsorption was performed both ways. A few sera showed recognition of conserved epitopes, as the OD was high without preabsorption but was reduced when sera were preabsorbed with either the homologous or heterologous antigen. In contrast, antibodies to allele-specific epitopes were detected in most of the sera, as preabsorption had a marked effect only with the homologous antigen (Fig. 4).
FIG. 4.
Effects of antigen preabsorption on serum IgG reactivity (ELISA OD values) in 12 Nigerian donors show the presence of allele-specific F-seg and C-seg epitopes. Sera were absorbed with homologous and heterologous recombinant antigens prior to being tested against each of the antigens by ELISA. ■, absorption with rINT-C-seg; ○, absorption with rF-seg-INT. IgG reactivities after preabsorption with each antigen (y axes) are plotted against the reactivities without antigen preabsorption (x axes). (A) ELISA plate coated with rF-seg-INT; (B) ELISA plate coated with rINT-C-seg.
Serum IgG reactivity to recombinant EBA-175 proteins in a large population sample of children in The Gambia.
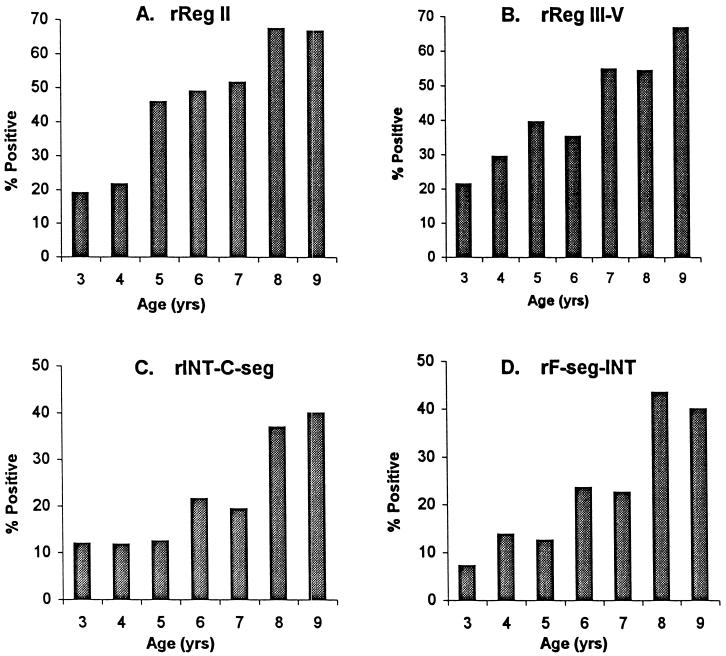
IgG antibodies against rEBA-175 antigens were detected by ELISA in sera collected before the malaria transmission season from 284 children age 3 to 9 years. The overall proportion of children with detectable serum IgG antibodies to the recombinant proteins rReg II, rReg III-V, rF-seg-INT, and rINT-C-seg were 43, 40, 22, and 20%, respectively. The antibody prevalences to each of the antigens increased steadily with age (Fig. 5).
FIG. 5.
Proportions of Gambian children with serum IgG against recombinant proteins of EBA-175. The numbers (n) in each age class were as follows: 3 years, n = 42; 4 years, n = 51; 5 years, n = 48; 6 years, n = 51; 7 years, n = 31; 8 years, n = 46; 9 years, n = 15.
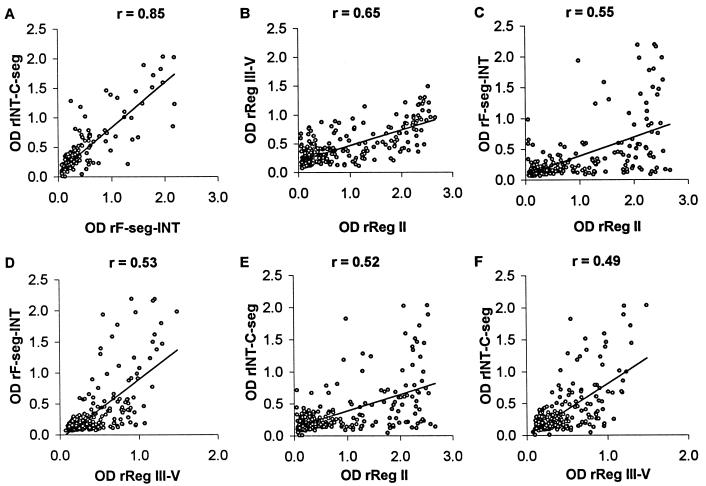
The pairwise correlations between OD values of IgG reactivity to the different recombinant antigens are shown in Fig. 6. The strongest correlation was between IgG levels to allelic rF-seg-INT and rINT-C-seg (Spearman's correlation coefficient r value = 0.85; P < 0.001), which may be due to the conserved intervening INT sequence as noted above. All of the other pairs of antigens were structurally unrelated at the primary sequence level, so the lower correlations of OD values (r values of 0.49 to 0.65) were expected as merely reflecting differences in prior exposure to malaria among the subjects.
FIG. 6.
Correlations between serum IgG reactivities to different recombinant antigens of EBA-175 in Gambian children. (A) rINT-C-seg versus rF-seg-INT; (B) rReg III-V versus rReg II; (C) rF-seg-INT versus rReg II; (D) rF-seg-INT versus rReg III-V; (E) rINT-C-seg versus rReg II; (F) rINT-C-seg versus rReg III-V.
The association between total serum IgG and IgG subclasses to the recombinant EBA-175 antigens prior to the transmission season and clinical malaria outcome between June and October was analyzed (Table 1). The proportions of individuals who had clinical malaria were not significantly different between those who had and those who did not have serum IgG, IgG1, or IgG3 antibodies against each of the recombinant proteins prior to the transmission season. Consequently, the relative risk values were not significantly different from 1.0 (Table 1). There was still no association when individuals' ages (in years) were adjusted for by multiple logistic regression analysis.
TABLE 1.
Association between total IgG and IgG subclass antibodies to recombinant proteins of EBA-175 in May and malaria outcome (June to October) in a cohort of 284 Gambian children
| EBA-175 recombinant antigen and antibody reactivity | % of children (no./total) acquiring clinical malaria
|
Relative risk (95% confidence interval) | P valuea | |
|---|---|---|---|---|
| Antibody positive | Antibody negative | |||
| rReg II | ||||
| Total IgG | 37.4 (46/123) | 39.1 (63/161) | 0.96 (0.71–1.29) | 0.77 |
| IgG1 | 33.7 (33/98) | 40.9 (76/186) | 0.82 (0.59–1.14) | 0.24 |
| IgG3 | 35.2 (19/54) | 39.1 (90/230) | 0.90 (0.60–1.34) | 0.59 |
| rReg III-V | ||||
| Total IgG | 34.8 (39/112) | 40.7 (70/172) | 0.86 (0.63–1.17) | 0.32 |
| IgG1 | 33.3 (6/18) | 38.7 (103/266) | 0.86 (0.44–1.68) | 0.65 |
| IgG3 | 38.9 (14/36) | 38.3 (95/248) | 1.02 (0.65–1.57) | 0.95 |
| rINT-C-seg | ||||
| Total IgG | 31.6 (18/57) | 40.1 (91/227) | 0.79 (0.52–1.19) | 0.24 |
| IgG1 | 55.0 (11/20) | 37.1 (98/264) | 1.48 (0.97–2.27) | 0.11 |
| IgG3 | 33.3 (8/24) | 38.8 (101/260) | 0.86 (0.48–1.54) | 0.60 |
| rF-seg-INT | ||||
| Total IgG | 32.8 (20/61) | 39.9 (89/223) | 0.82 (0.55–1.22) | 0.31 |
| IgG1 | 36.4 (8/22) | 38.5 (101/262) | 0.94 (0.53–1.67) | 0.84 |
| IgG3 | 32.1 (9/28) | 39.1 (100/256) | 0.82 (0.47–1.44) | 0.47 |
Determined by univariate chi-square tests; inclusion of age in multiple regression analyses had no major effect on P values.
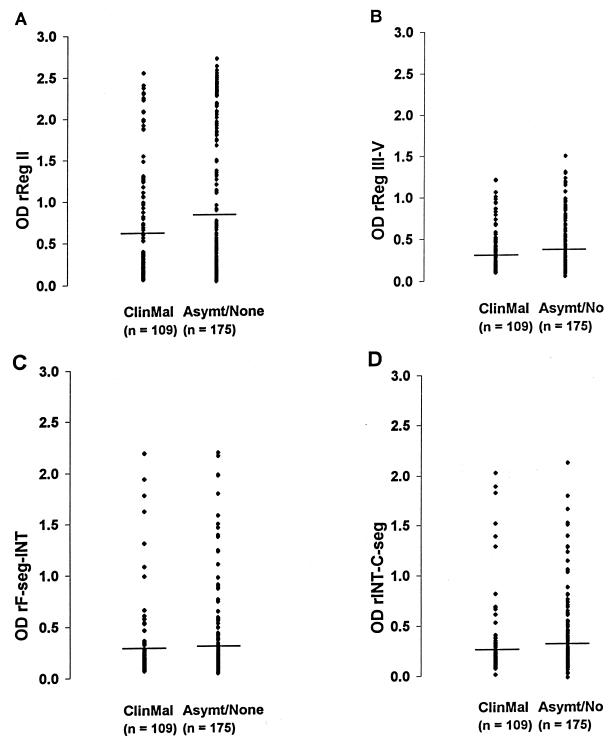
To examine whether preseason antibody levels (rather than simple antibody positivity above the cutoff value) correlated with malaria outcome, OD values were analyzed. Overall, there were no significant differences in the distribution of IgG levels (OD) between those who subsequently had clinical malaria and those who did not (Fig. 7). However, the trend in the data indicated that higher OD values against rReg II may have been more common among those who did not subsequently have malaria. To explore this, it was decided to test whether a very high OD value against rReg II (above an arbitrary OD cutoff value of 1.5) was associated with malaria outcome. Sixteen (27.1%) of the 59 individuals with anti-rReg II IgG OD values of >1.5 subsequently had malaria, in comparison with 93 (41.3%) of the 225 individuals who had OD values of <1.5. In a univariate analysis, this apparent protective effect was just significant (P = 0.046; relative risk and 95% confidence limits = 0.60 and 0.35 to 1.01). After adjustment for individuals' ages in years by multiple logistic regression, this association was not quite significant (P = 0.086).
FIG. 7.
Distribution of levels of serum IgG (OD values) in May against recombinant proteins of EBA-175 in Gambian children who subsequently had clinical malaria (ClinMal) versus levels in those who had an asymptomatic infection or none (Asymt/None) during follow-up (June to October). (A) rReg II; (B) rReg III-V; (C) rF-seg-INT; (D) rINT-C-seg. The horizontal lines indicate the mean levels. There were no significant differences in the overall distributions of IgG levels between the two groups of children (Mann-Whitney U tests, P > 0.05 for each comparison).
DISCUSSION
Recombinant proteins representing different parts of P. falciparum EBA-175 were used to study naturally acquired human antibodies to the protein. The results demonstrated that IgG antibodies recognize epitopes in the dimorphic C and F segment sequences of EBA-175 as well as conserved epitopes in this part of the molecule and in the adjacent region II and regions III to V. There is a high prevalence of serum IgG to all four recombinant proteins of EBA-175 among the Nigerian adults and Gambian children studied, and this prevalence increases with age among the children.
Human antibodies to EBA-175 are here shown to be predominantly of the IgG1 and IgG3 subclasses. A similar subclass distribution has been observed in studies on other merozoite antigens (6, 35). Antibody-dependent cellular inhibition of P. falciparum-infected erythrocytes has been associated with cytophilic IgG1 and IgG3 isotypes (4). Also, only the IgG1 and IgG3 subclasses are capable of mediating opsonizing phagocytosis (12). This is because IgG1 and IgG3 bind to receptors on monocytes, macrophages, and neutrophils, the cells involved in antibody-dependent cellular inhibition and phagocytosis. Some evidence suggests that IgG3 antibodies against merozoite surface proteins MSP2 and MSP3 are associated with immunity to clinical malaria (22, 34).
The presence of serum IgG or the IgG1 and IgG3 subclasses against the recombinant EBA-175 antigens was not significantly associated with protection from clinical malaria in the cohort of Gambian children studied here. Thus, serum positivity to these antigens does not apparently result in an ability to resist natural challenge infections. However, a high level of IgG reactivity (OD value of >1.5) to the rReg II protein was marginally associated with protection. This may indicate a real protective association of antibodies to region II of EBA-175 and justifies further studies to see if it is repeatable in other study populations. Region II contains the domain which binds to glycophorin A on the erythrocyte surface, and it may be that a high concentration of high-affinity antibodies is required to inhibit this binding. Using the same cohort of Gambian children as in the present study, a significant association had been observed between antibodies to the C terminus of P. falciparum MSP1 (MSP119) and a lower risk of clinical malaria (10, 28). Many factors may account for variation in the level of antibody responses in an area of seasonal endemicity like The Gambia where the longitudinal cohort study was conducted, and it is worth noting that variation in apparent protection may be seen between different cohort studies, as illustrated by studies on MSP119 (2, 5, 8, 10, 28).
In vitro assays show that not all P. falciparum parasites are dependent on EBA-175 recognition of glycophorin A for erythrocyte invasion, but there is heterogeneity in the receptors used in erythrocyte invasion (3, 9, 13, 19, 20, 25). It has been shown that the use of alternative invasion pathways by P. falciparum merozoites is not an artifact of long-term in vitro culture but commonly occurs in the field (24). It is also important to note that, in one in vitro clone, targeted disruption of the eba-175 gene has caused switching to an alternative invasion pathway (27). The parasite ligands that mediate the alternative invasion pathways are yet to be identified, and it is interesting that other eba-175-like genes exist in P. falciparum (26). A more complete understanding of the role of EBA-175 and putative alternative parasite ligands as targets of naturally acquired immune responses will be relevant to the design of vaccines aimed to inhibit erythrocyte invasion by merozoites.
ACKNOWLEDGMENTS
We thank Jamiu Ogunbanwo and Chimere Agomo for assistance during sample collection in Nigeria and S. J. Allen and B. M. Greenwood for their role in the longitudinal cohort study in The Gambia. Peter Zipfel kindly provided the baculovirus His tag transfer plasmid.
This work was funded by the Wellcome Trust (grant no. 050352/Z/97, Travelling Research Fellowship for D.M.N.O.). The longitudinal cohort study in The Gambia was supported by WHO/TDR.
REFERENCES
- 1.Adams J H, Sim K L B, Dolan S A, Fang X, Kaslow D C, Miller L H. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA. 1992;89:7085–7089. doi: 10.1073/pnas.89.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Yaman F, Genton B, Kramer K J, Chang S P, Hui G S, Baisor M, Alpers M P. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am J Trop Med Hyg. 1996;54:443–448. doi: 10.4269/ajtmh.1996.54.443. [DOI] [PubMed] [Google Scholar]
- 3.Binks R H, Conway D J. The major allelic dimorphisms in four Plasmodium falciparum merozoite proteins are not associated with alternative pathways of erythrocyte invasion. Mol Biochem Parasitol. 1999;103:123–127. doi: 10.1016/s0166-6851(99)00115-2. [DOI] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chougsuphajaisid-dhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conway D J, Cavanagh D R, Tanabe K, Roper C, Mikes Z S, Sakihama N, Bojang K A, Oduola A M J, Kremsner P G, Arnot D E, Greenwood B M, McBride J S. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat Med. 2000;6:689–692. doi: 10.1038/76272. [DOI] [PubMed] [Google Scholar]
- 6.Da Silveira L A, Dorta M L, Kimura E A S, Katzin A M, Kawamoto F, Tanabe K, Ferreira M U. Allelic diversity and antibody recognition of Plasmodium falciparum merozoite surface protein 1 during hypoendemic malaria transmission in the Brazilian Amazon region. Infect Immun. 1999;67:5906–5916. doi: 10.1128/iai.67.11.5906-5916.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daugherty J R, Murphy C I, Doros-Richert L A, Barbosa A, Kashala L O, Ballou W R, Snellings N J, Ockenhouse C F, Lanar D E. Baculovirus-mediated expression of Plasmodium falciparum erythrocyte binding antigen 175 polypeptides and their recognition by human antibodies. Infect Immun. 1997;65:3631–3637. doi: 10.1128/iai.65.9.3631-3637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodoo D, Theander T G, Kurtzhals J A, Koram K, Riley E, Akanmori B D, Nkrumah F K, Hviid L. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect Immun. 1999;67:2131–2137. doi: 10.1128/iai.67.5.2131-2137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolan S A, Proctor J L, Alling D W, Okubo Y, Wellems T E, Miller L H. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol Biochem Parasitol. 1994;64:55–63. doi: 10.1016/0166-6851(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 10.Egan A F, Morris J, Barnish G, Allen S, Greenwood B M, Kaslow D C, Holder A A, Riley E M. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis. 1996;173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 11.Fang X, Kaslow D C, Adams J H, Miller L H. Cloning of the Plasmodium vivax Duffy receptor. Mol Biochem Parasitol. 1991;44:125–132. doi: 10.1016/0166-6851(91)90228-x. [DOI] [PubMed] [Google Scholar]
- 12.Groux H, Gysin J. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Res Immunol. 1990;141:529–542. doi: 10.1016/0923-2494(90)90021-p. [DOI] [PubMed] [Google Scholar]
- 13.Hadley T J, Klotz F W, Pasvol G, Haynes J D, McGinniss M H, Okubo Y, Miller L H. Falciparum malaria parasites invade erythrocytes that lack glycophorin A and b (MkMk). Strain differences indicate receptor heterogeneity and two pathways for invasion. J Clin Invest. 1987;80:1190–1193. doi: 10.1172/JCI113178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakobsen P H, Heegaard P M, Koch C, Wasniowska K, Lemnge M M, Jensen J B, Sim B K. Identification of an erythrocyte binding peptide from erythrocyte binding antigen, EBA-175, which blocks parasite multiplication and induces peptide-blocking antibodies. Infect Immun. 1998;66:4203–4207. doi: 10.1128/iai.66.9.4203-4207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kain K C, Orlandi P A, Haynes J D, Sim B K L, Lanar D E. Evidence for two-stage binding by the 175 kDa erythrocyte binding antigen of Plasmodium falciparum. J Exp Med. 1993;178:1497–1505. doi: 10.1084/jem.178.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kappe S H I, Noe A R, Fraser T S, Blair P L, Adams J H. A family of chimeric erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA. 1998;95:1230–1235. doi: 10.1073/pnas.95.3.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn S, Zipfel P F. The baculovirus expression vector pBSV-8His directs secretion of histidine-tagged proteins. Gene. 1995;162:225–229. doi: 10.1016/0378-1119(95)00360-i. [DOI] [PubMed] [Google Scholar]
- 18.Liang H, Sim B K L. Conservation of structure and function of the erythrocyte-binding domain of Plasmodium falciparum EBA-175. Mol Biochem Parasitol. 1997;84:241–244. doi: 10.1016/s0166-6851(96)02791-0. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell G H, Hadley T J, McGinniss M H, Klotz F W, Miller L H. Invasion of erythrocytes by Plasmodium falciparum malaria parasites. Evidence for receptor heterogeneity and two receptors. Blood. 1986;67:1519–1521. [PubMed] [Google Scholar]
- 20.Narum D L, Haynes J D, Fuhrmann S, Moch K, Liang H, Hoffman S L, Sim B K L. Antibodies against the Plasmodium falciparum receptor binding domain of EBA-175 block invasion pathways that do not involve sialic acids. Infect Immun. 2000;68:1964–1966. doi: 10.1128/iai.68.4.1964-1966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Donnell R A, Saul A, Cowman A F, Crabb B S. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nat Med. 2000;6:91–95. doi: 10.1038/71595. [DOI] [PubMed] [Google Scholar]
- 22.Oeuvray C, Bouharoun-Tayoun H, Gras-Masse H, Bottis E, Kaidoh T, Aikawa M, Filgueira M C, Tartar A, Druilhe P. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood. 1994;84:1594–1602. [PubMed] [Google Scholar]
- 23.Okenu D M N, Malhotra P, Lalitha P V, Chitnis C E, Chauhan V S. Cloning and sequence analysis of a gene encoding an erythrocyte binding protein from Plasmodium cynomolgi. Mol Biochem Parasitol. 1997;89:301–306. doi: 10.1016/s0166-6851(97)00118-7. [DOI] [PubMed] [Google Scholar]
- 24.Okoyeh J N, Pillai C R, Chitnis C E. Plasmodium falciparum field isolates commonly use erythrocyte invasion pathways that are independent of sialic acid residues of glycophorin A. Infect Immun. 1999;67:5784–5791. doi: 10.1128/iai.67.11.5784-5791.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins M E, Holt E H. Erythrocyte receptor recognition varies in Plasmodium falciparum isolates. Mol Biochem Parasitol. 1988;27:23–34. doi: 10.1016/0166-6851(88)90021-7. [DOI] [PubMed] [Google Scholar]
- 26.Peterson D S, Miller L H, Wellems T E. Multiple sequences in the Plasmodium falciparum genome encode conserved domains homologous to those in erythrocyte binding proteins. Proc Natl Acad Sci USA. 1995;92:7100–7104. doi: 10.1073/pnas.92.15.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed M B, Caruana S R, Batchelor A H, Thompson J K, Crabb B S, Cowman A F. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid-independent pathway of invasion. Proc Natl Acad Sci USA. 2000;97:7509–7514. doi: 10.1073/pnas.97.13.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley E M, Allen S J, Wheeler J, Blackman M J, Bennet S, Takacs B, Shonfeld H, Holder A A, Greenwood B M. Naturally acquired cellular and humoral immune responses to the major surface antigen in Plasmodium falciparum associated with reduced malaria morbidity. Parasite Immunol. 1992;14:321–338. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 29.Sim B K L. Delineation of functional regions on Plasmodium falciparum EBA-175 by antibodies eluted from immune complexes. Mol Biochem Parasitol. 1998;95:183–192. doi: 10.1016/s0166-6851(98)00078-4. [DOI] [PubMed] [Google Scholar]
- 30.Sim B K L. Sequence conservation of structure and function of the erythrocyte binding domain of Plasmodium falciparum EBA-175. Mol Biochem Parasitol. 1990;41:293–296. doi: 10.1016/s0166-6851(96)02791-0. [DOI] [PubMed] [Google Scholar]
- 31.Sim B K L, Chitnis C E, Wasniowska K, Hadley T J, Miller L H. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 32.Sim B K L, Orlandi P A, Haynes J D, Klotz F W, Carter J M, Camus D, Zegans M E, Chulay J D. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol. 1990;111:1877–1884. doi: 10.1083/jcb.111.5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 34.Taylor R R, Allen S J, Greenwood B M, Riley E M. IgG3 antibodies to Plasmodium falciparum merozoites surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg. 1998;58:406–413. doi: 10.4269/ajtmh.1998.58.406. [DOI] [PubMed] [Google Scholar]
- 35.Taylor R R, Smith D B, Robinson J V, McBride J S, Riley E M. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect Immun. 1995;63:4382–4388. doi: 10.1128/iai.63.11.4382-4388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ware L A, Kain K C, Sim B K L, Haynes J D, Baird J K, Lanar D E. Two alleles of the 175-kilodalton Plasmodium falciparum erythrocyte binding antigen. Mol Biochem Parasitol. 1993;60:105–109. doi: 10.1016/0166-6851(93)90033-t. [DOI] [PubMed] [Google Scholar]