Abstract
In spite of all the efforts for generating efficient pharmacological treatment options for cancer patients, the unwanted side effect of these substances on the cardiovascular system is becoming a major issue for cancer survivors. The fast pacing oncology field necessitate the quest for more accurate and reliable preclinical screenings. hiPSCs derived cardiomyocytes, endothelial and vascular smooth muscle cells provide unlimited source of physiologically relevant cells that could be used in the screening platforms. Cells derived from hiPSCs can measure drug induced alterations to different aspect of the heart including electrophysiology, contractility and structure. In this review, we will give an overview of the different in vivo and in vitro preclinical drug safety screenings. In following sections, we will focus on hiPSCs-derived cardiomyocytes, endothelial and vascular smooth muscle cells and present the current knowledge of the application of these cells in unicellular cardiotoxicity assays. In the final part, we will focus on cardiac organoids as multi cell type platform and their role in cardiotoxicity screening of the chemotherapeutic drugs.
Keywords: human induced pluripotent stem cells, cardio-oncology, precision medicine, drug Screening, cardiotoxicity, vascular toxicity
Introduction
Thanks to recent advances in oncology field new efficient drugs are being developed to save the lives of millions of people fighting with cancer all over the world. Unfortunately, many of these drugs cause adverse short-term or long-term effect on cardiovascular system such as heart failure, hypertension, arrhythmias, thrombogenesis and vasospasm [1]. This issue is becoming one of the major concerns of the physicians in the filed as the risk of mortality due to cardiovascular abnormalities is becoming higher than the risk of cancer recurrence in many cases [2]. Therefore, there is an urgent need to understand the molecular mechanism of chemotherapy-induced cardiotoxicity and develop safer treatment options. Additionally, cardiotoxicity observed in US Food and Drug Administration (FDA) approved drugs such as some chemotherapeutic agents, including some anthracyclines, tyrosine kinase inhibitors (TKIs) and monoclonal antibodies necessitate the search for more accurate preclinical cardiotoxicity screenings [3].
Preclinical Platforms for Cardiotoxicity Screenings
All drugs need to pass the cardiovascular safety measurement before getting the final approval for releasing to market. Traditionally, animal models have been used to test the cardiotoxicity of the drugs. Zebrafish for instance, provide a low-cost system adoptable to high throughput platforms. For instance, as a result of doxorubicin treatment, a well-known member of anthracyclines chemotherapy drugs, zebrafish heart demonstrated abnormal heart development, pericardial edema, and bradycardia [4]. Although, zebrafish displayed dose-dependent response to doxorubicin treatment, it worth emphasising that the manifestation of cardiotoxicity in zebrafish and patients were completely different. The clinical manifestation of doxorubicin induced cardiotoxicity commonly involves the left ventricular dysfunction and heart failure, which differ vastly from what is observed in zebrafish. Thus, moving to mammalian system would enable us to have model systems closer to human physiology. Based on that notion, mouse models have been widely used in preclinical screening. As well as providing a mammalian model system closer to human compared to zebrafish, mouse models are able to provide mechanistic insight into the cardiotoxicity. For example, HFE knock out mice showed elevated sensitivity to doxorubicin, suggesting a role for iron in cardiotoxicity of doxorubicin [5]. Furthermore, using mouse mutagenesis followed by cardiotoxicity screening after drug treatment, molecular underpinning of the toxicity could be assessed. If as a result of knocking out a gene, animals become immune to cardiotoxicity, this could assist with understanding the mechanism of the cardiotoxicity, eventually leading to development of safer drugs. As an example, mouse models with cardiac specific knock out of TOP2B, exhibited limited cardiotoxicity after doxorubicin treatment [6], suggesting a role for TOP2B in the cardiotoxicity of doxorubicin. In spite of all the advantages of using animal models, they suffer from serious draw backs which limit their application in high throughput safety screenings. Working with animals is usually expensive, time consuming and low throughput. Moreover, animal studies are hampered by the fundamental physiological and electrophysiological differences between human and animal models [7].
To avoid these issues, the closest option to would be to use human heart samples such as the leftover parts of the heart surgery or the failing heart after the transplantation. However, these tissues are very limited, hard to achieve and maintain for long term in culture. Thus, model system more amendable to high throughput screenings are needed.
The alternative approach is to use non-cardiac cells with expression of cardiac ion channels. This system is able to detect the interaction of the drugs to specific ion channels, nonetheless it worth considering that interaction would not necessarily lead to cardiotoxicity. For instance, studies suggest that the interaction with human ether-á-go-go (hERG) related potassium channel is the leading cause of drug induced arrythmia. hERG has a crucial role in the repolarization of the heart, thereby any alteration to its performance could eventually lead to severs ventricular arrythmia [8]. Hence, in most preclinical screening guidelines, assessment for hERG interaction is mandatory. To that end, one option would be to express hERG channel in non-cardiac cell lines and examine the effect of various drugs on the hERG function. However, this assumption is oversimplification of the situation as merely the interaction with hERG channel does not end up with arrythmia. Furthermore, the non-cardiac cells lack the cardiac specific sarcomeric structure and the ion channel complexity present in the cardia cells [9]. This has led to elevation of false-positive outcomes and unnecessary drug attrition. These findings necessitate the need to test the drugs on the cardiovascular cells themselves.
The best model for prediction of cardiotoxicity would be the one that could recapitulate the human heart based on electrophysiology, gene expression and function, is relatively easy to achieve and maintain in culture and is amendable to high throughput screenings. Introduction of the human induced pluripotent stem cells (hiPSCs) to the field has opened new avenues for pharmacological screenings. With recent improvement in culture media compositions [10] and methodologies for mass culture of the cells, hiPSCs now can be expanded and efficiently differentiated into cardiomyocytes in large scale [11]. These in vitro generated cardiomyocytes contract spontaneously, and their gene and protein expression profile are similar to human heart. Moreover, these cells can specifically be differentiated into lineage specific subtypes and be cultured both in 2D and 3D platforms. By having a bank of different hiPSC lines representing the diversity present in the human populations, clinical trials in a dish are possible. All these characteristics make hiPSCs derived cells an attractive option for cardiotoxicity screenings. Figure 1 summarises the preclinical screening platforms. In the following sections we first focus on uni-cell type platforms for cardiotoxicity screenings. Then we move forward to more accurate yet more complex platforms with multiple cell types. Cardiac organoids by representing similar cellular complexity exist in the heart, would be a suitable candidate for drug screening purposes.
Fig. 1.
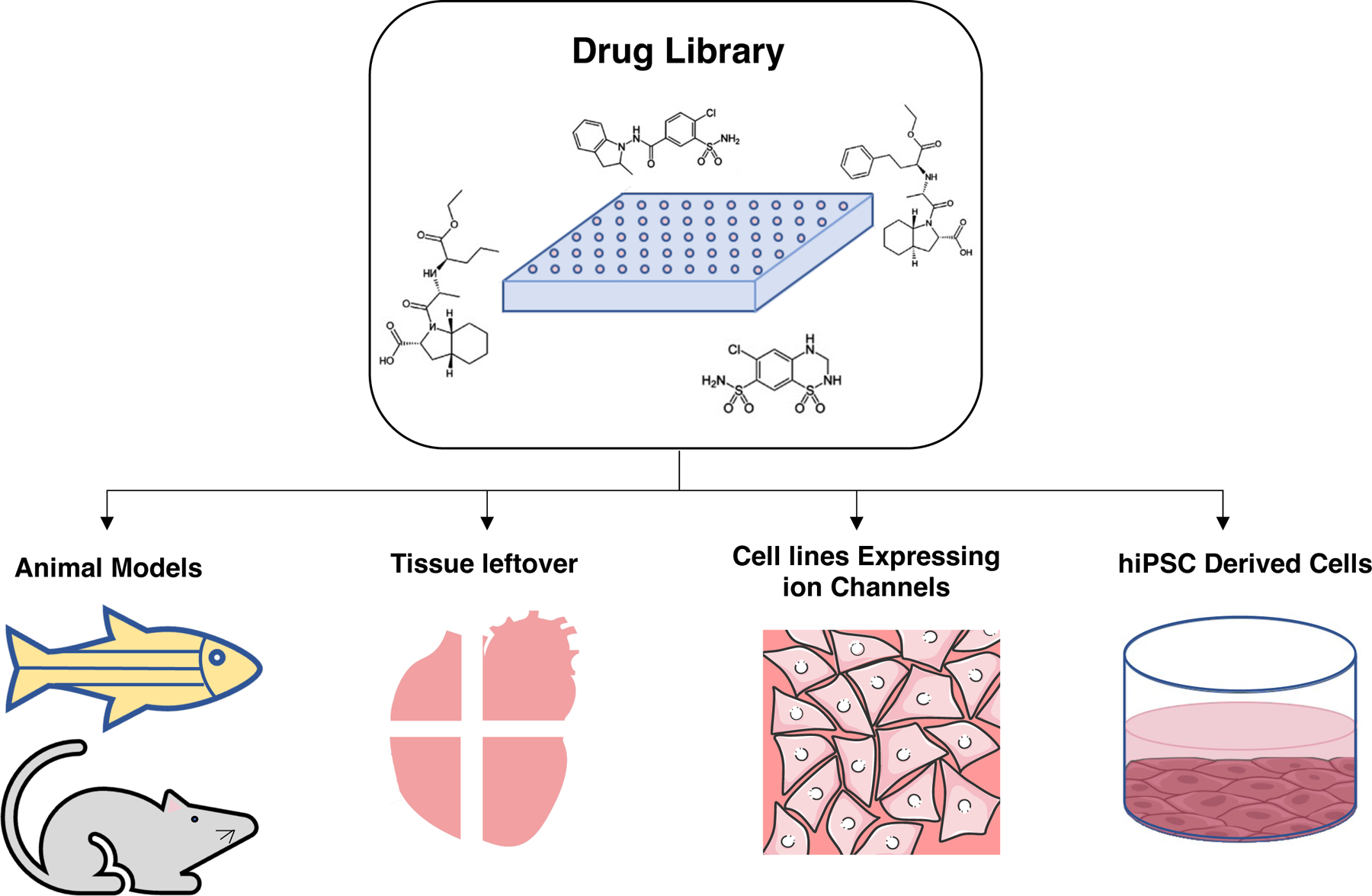
Summary of preclinical platforms used in cardiotoxicity screenings. They include animal models such as mouse and zebrafish, tissue leftovers after heart surgery or the failing heart after the transplantation, human cell lines over expressing cardiac ion channels and hiPSCs derived cardiovascular cells. Among these hiPSCs derived cells by harboring the gene and protein expression profile close to human heart provide the most accurate screening platform
Cardiomyocytes for Drug Induced Cardiotoxicity Screening
The initial introduction of hiPSC-derived cardiomyocytes was in 2D format. 2D adherent cultures are the standard system used for culture and differentiation of the cells. Currently, there are highly efficient chemically defined differentiation protocols that generate cardiomyocytes with high efficacy and reproducibility [12], two critical requirements for the systems to be used in preclinical screening settings.
2D adherent cells have been widely used in cardiotoxicity screenings. The effect of anticancer agents on hiPSCs derived cardiomyocytes could be assessed based on the alteration of gene and protein expression levels, intracellular structure, electrophysiology and contraction. Previously, it has been shown that hiPSCs derived cardiomyocytes could recapitulate the cardiotoxicity side effect of the doxorubicin treatments. The side effect of doxorubicin treatment on cardiomyocytes was similar to clinical trials and it included arrythmia, elevated reactive oxygen species (ROS), apoptosis and sarcomeric disarray [13]. hiPSCs from large cohort could further assist with finding the correlation of genetic backgrounds and the severity of doxorubicin induced toxicity [14]. Analysis of the gene expression post drug treatment could also assist with introduction of biomarkers for early detection of doxorubicin-induced cardiotoxicity [15].
Apart from previously mentioned effect of the anticancer treatments on cardiomyocytes, the cardiotoxicity of compounds might be due to alteration in the metabolism process of the cells. As an example, trastuzumab treatment, a member of monoclonal antibody anticancer treatments, cause decreased small molecule metabolism and glucose uptake [16]. hiPSC derived cardiomyocyte were further used in high throughput screening platforms to measure the safety of small molecule TKIs indicating alteration in metabolic pathways as main causation of cardiotoxicity [17]. As an example, sorafenib caused perturbation in oxidative phosphorylation and increased glycolysis [17]. In another study by Sharma et al. hiPSC derived cardiomyocytes were used for high throughput screening of a library of TKIs [18]. The screening was performed by measuring the cell viability, electrophysiology and contractility of the cells. By summation of the outcome of all the measured parameters, a cardiac safety index was introduced to reflect the level of cardiotoxicity of each compound. This index could be beneficial for simplifying the interpretation of the preclinical studies and facilitate the clinical translations of the results.
Endothelial and VSMCs for Drug Induced Cardiotoxicity Screening
Cardiovascular system consists of a variety of cells types including cardiomyocytes, fibroblasts, endothelial and smooth muscle cells. Majority of the cardiotoxicity platform focus on the cardiomyocytes only, however, toxicity to other components of cardiovascular system should be considered. It is known that a majority of anti-cancer therapies affect the endothelial cells. Nilotinib for example has been shown to affect the viability and angiogenesis function of endothelial cells [19]. As a result, similar screening procedures as mentioned for cardiomyocytes needs to be performed on other cells present in cardiovascular system. For endothelial cells, viability, proliferation and migration assays could be used as an indication of the endothelial health and angiogenesis function. In case of VSMCs, similar assays could be deployed with the difference that opposite to endothelial cells, proliferation and migration here is a pathological signal. This is a marker for cells shifting from contractile state to synthetic which is the phenotype observed in atherosclerosis [20].
Other common side effect of the chemotherapeutic drug is affecting the blood pressure. As a result, developing assays for determination of hypo- and hypertension using the endothelial and VSMCs is crucial. hiPSCs derived endothelial cells are shown to be able to recapitulate the heritable or idiopathic pulmonary arterial hypertension which is manifested as reduced viability and function [21]. VSMCs are also shown to play important role in maintaining the normal blood pressure. Several chemotherapy drugs alter the blood pressure by affecting the vascular stiffness and VSMCs function [22]. All these studies prove the applicability of hiPSC derived endothelial and VSMCs in drug screening assays.
Cardiac Organoids for Drug Induced Cardiotoxicity Screening
In spite of the ability of the unicellular systems to recapitulate the cardiotoxicity of a whole range of anticancer drugs from anthracyclines to TKIs and monoclonal antibodies, still they cannot capture the full picture of the in vivo processes. Heart as an organ is not solely made of cardiomyocytes. Although cardiomyocytes play a crucial role in the function of the heart, the presence, proper function and interaction of other cell types including fibroblasts and endothelial cells are also equally important. Thus, considering the effect of drugs on cardiomyocytes or endothelial cells only can never recapitulate the whole in vivo scenario. As a result, establishment of more physiologically relevant model system are needed. 3D cardiac organoids with their ability to incorporate all the cell types present in the heart would enable us to elucidate the effect of anticancer agents on the crosstalk between multiple cell types. Cardiac organoids are 3D structures generated in different shapes and formats. In this review, we focus on two most common types of cardiac organoids, spheroids and engineered heart tissues. Figure 2 summarises the application of cardiac organoids in cardiotoxicity screenings.
Fig. 2.
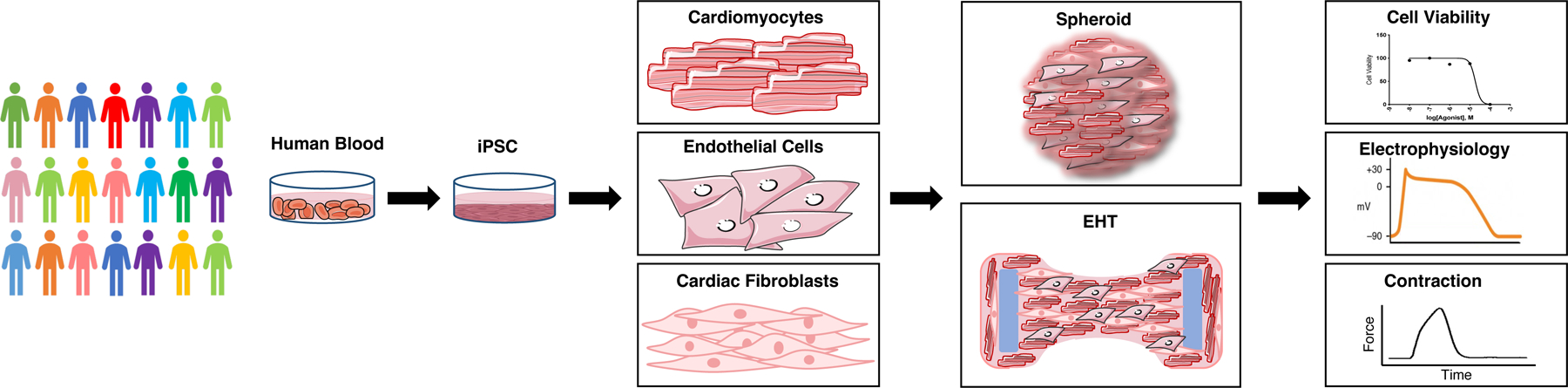
Summary of the flow for application of cardiac organoids in cardiotoxicity screening platfroms. hiPSCs are generated from blood samples of individuals representing the diversity present in human population. These hiPSCs are differentiated into cardiovascular cells including cardiomyocytes, endothelial and cardiac fibroblasts. Then assembled in 3D to form cardiac organoids. Assessing the cell viability, electrophysiology and contractility of cardiac organoids after treatment with drug libraries could provide a metric for cardiotoxicity of different drugs
A. Spheroids
Spheroids are achieved through self-assembly of one or multiple cell types into a ball shape structure. Here, the cells produce their own extracellular matrix (ECM) and generate a microenvironment which resembles the crosstalk of the cells within the organ. Here, however, the ultrastructure of the cells is out of our control. This system could be considered as the simplest version of cardiac organoids, which could be adopted to high throughput screenings. To generate spheroids, cells are cultured in a V-shaped 96-well plates, in a hanging drop or in custom-shaped molds. To mimic the physiological condition cardiomyocytes, fibroblasts and endothelial cells were mixed at ratios of 5:4:0 for developing heart and 3:6:1 for adult hearts [23]. These organoids successfully recapitulated the structural and functional properties of their corresponding heart stages. This simple and reproducible system could be used in large scale screening platforms. In another study, to further improves the model, the fibroblasts were replaced with cardiac fibroblasts. This modification led to the generation of more mature organoids which was evident in their cellular ultrastructure, gene expression, electrophysiological function and metabolome [24].
The spheroids were used in a variety of cardiotoxicity assays for anti-cancer drugs including doxorubicin [25] and sunitinib [26]. In a study by Polonchuk et al. the dose dependency of cardiotoxicity effect of doxorubicin was evaluated using spheroids consist of hiPSC derived cardiomyocytes, endothelial cells and fibroblasts. Cell viability was improved by inhibition of nitric oxide synthase (NOS) suggesting production of peroxynitrous acid as the mechanism of cardiotoxicity [25]. Apart from cell viability, the side effect of anti-cancer treatments on the contraction of the spheroids could be measured. Using video imaging of the spheroids, the cardiotoxicity of several drugs including doxorubicin, verapamil and quinidine has been assessed [27]. This non-invasive approach could be adopted to high throughput screening platforms to facilitate the preclinical screenings of compounds. Furthermore, cardiac spheroids derived from hiPSCs-cardiomyocytes, endothelial and fibroblast cells were deployed to assess the effect of drugs on structure of the cardiomyocytes [26]. Mitochondrial membrane potential, endoplasmic reticulum integrity and cellular viability were measured using high throughput assays to evaluate the effect of 29 FDA approved drugs. 15 of which were known to affect the structure of the cardiomyocytes while for the remaining 14 the cardiotoxicity was independent of structural damage. In vitro cardiac spheroid system was able to detect the toxicity as a result of structural defects at clinically relevant concentrations [26]. All together, these studies support the applicability of hiPSCs derived cardiac spheroids in detecting the cardiotoxicity of anti-cancer reagents by affecting the viability, contraction and structure of the cardiomyocytes.
B. Engineered Heart Tissues
Engineered heart tissues (EHTs) are 3D structures consist of a cellular component, a hydrogel component, a casting device and two rigid posts to generate the mechanical restrain. The cellular component of EHTs can vary from neonatal or adult primary heart samples or hiPSC derived cardiomyocytes. Presence of other cell types, usually a stromal cell, is crucial for proper generation and function of EHTs. As well as mechanical support These cells play produce signals which control the ECM. The most advance version EHTs are consist of cardiomyocytes, endothelial cells and fibroblasts all from hiPSC source. Similarly, several hydrogels used in the formation of EHTs, they include but not limited to Matrigel, fibrinogen, collagen I, and fibrin [28].
In this system, cells are beating against the two rigid posts, which eventually leads to maturation enhancement. Two major hurdles present between EHT systems and high throughput screening platforms are the need to large numbers of cells as well as complex casting and cell assembly procedures, which generally make this system a low throughput one. Recent efforts were focus on making the production of EHTs simplified and more user friendly. Moreover, to catch up with the need to high number of cells for generation of EHTs, Breckwoldt et al. introduced a large scale platform for culture and cardiac differentiation of hiPSCs [29]. Using this system, hiPSCs are cultured and differentiated in suspension in bioreactors, then dissociated and used in EHTs. An alternative approach would be instead of scaling up the culture and differentiation of hiPSCs, miniaturise the EHT platforms. Here, using small casting platforms, the required number of cells also proportionally decreases. Generation of miniaturised EHTs could pave the way for application of this system in drug cardiotoxicity screenings [30].
Limitations and Future Directions
In spite of the advantages of hiPSCs, this system still is far from perfection. One major limitation of using hiPSCs models for drug toxicity screening is the immature nature of the cardiomyocytes derived from hiPSCs. Studies suggest that most differentiation protocols generate immature cardiomyocytes close to fetal cardiomyocytes [31]. However, most cancer patients are adults and adult and fetal heart have fundamental differences from gene expression to metabolome and electrophysiology. Study on hiPSCs derived cardiomyocytes at different stages of development suggest that the ROS level in older cardiomyocytes was elevated after doxorubicin treatment compared to immature cardiomyocytes due to higher mitochondrial content in more mature cells [32]. This raise the flag of applicability of the data generated from the immature cells to adult patients.
Apart from developmental state of the cells derived from hiPSCs, there are some aspects of drug induced cardiotoxicity that are very difficult to recapitulate and measure using in vitro system. Specially, in cases where the side effect of drugs are accumulations of small changes in multiple cell types. Here, the actual toxicity could only be measured using complex systems with multiple cell types.
Predisposition to cardiovascular abnormalities may also exacerbate the side effect of the chemotherapy agents [33]. Currently, it is not a routine approach to test the cardiotoxicity of the drugs on predisposed cells, however, there is an urgent need in the field. Recently, there are reports of generation of organoids models of the diseases. For instance, non-genetic model of myocardial infarction has been developed using cardiac organoids [34]. Introduction of the organoid models of cardiovascular diseases to the field of cardio-oncology would assist with generating safer treatment options for cancer patients with predisposition to cardiovascular defects.
Finally, apart from all the previously mentioned cell types, immune cells are also present in the heart and interaction of chemotherapeutic drugs with them can cause cardiotoxicity. The cardiotoxicity related to immune cells can range from myocarditis and pericarditis to edema. Thus far, animal models have been widely used for this purpose, however, development of in vitro human based system could provide more insight into the field. Generally, immune related cardiotoxicity is caused by activation of immune system against the host. hiPSCs have successfully modelled autoimmune disease [35] and introduction of them to cardiotoxicity field could shed light on the mechanism of this type of cardiotoxicities.
References
- 1.Sorrentino MF, et al. , 5-fluorouracil induced cardiotoxicity: review of the literature. Cardiol J, 2012. 19(5): p. 453–8. [DOI] [PubMed] [Google Scholar]
- 2.Carver JR, et al. , American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol, 2007. 25(25): p. 3991–4008. [DOI] [PubMed] [Google Scholar]
- 3.Moslehi JJ and Deininger M, Tyrosine Kinase Inhibitor-Associated Cardiovascular Toxicity in Chronic Myeloid Leukemia. J Clin Oncol, 2015. 33(35): p. 4210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Y, et al. , Cardiotoxicity evaluation of anthracyclines in zebrafish (Danio rerio). J Appl Toxicol, 2015. 35(3): p. 241–52. [DOI] [PubMed] [Google Scholar]
- 5.Miranda CJ, et al. , Hfe deficiency increases susceptibility to cardiotoxicity and exacerbates changes in iron metabolism induced by doxorubicin. Blood, 2003. 102(7): p. 2574–80. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, et al. , Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med, 2012. 18(11): p. 1639–42. [DOI] [PubMed] [Google Scholar]
- 7.Mercola M, Colas A, and Willems E, Induced pluripotent stem cells in cardiovascular drug discovery. Circ Res, 2013. 112(3): p. 534–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Rosario ME, Weachter R, and Flaker GC, Drug-induced QT prolongation and sudden death. Mo Med, 2010. 107(1): p. 53–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann P and Warner B, Are hERG channel inhibition and QT interval prolongation all there is in drug-induced torsadogenesis? A review of emerging trends. J Pharmacol Toxicol Methods, 2006. 53(2): p. 87–105. [DOI] [PubMed] [Google Scholar]
- 10.Fonoudi H, et al. , Generating a Cost-Effective, Weekend-Free Chemically Defined Human Induced Pluripotent Stem Cell (hiPSC) Culture Medium. Curr Protoc Stem Cell Biol, 2020. 53(1): p. e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonoudi H, et al. , A Universal and Robust Integrated Platform for the Scalable Production of Human Cardiomyocytes From Pluripotent Stem Cells. Stem Cells Transl Med, 2015. 4(12): p. 1482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burridge PW, et al. , Chemically defined generation of human cardiomyocytes. Nat Methods, 2014. 11(8): p. 855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burridge PW, et al. , Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med, 2016. 22(5): p. 547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knowles DA, et al. , Determining the genetic basis of anthracycline-cardiotoxicity by molecular response QTL mapping in induced cardiomyocytes. Elife, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maillet A, et al. , Modeling Doxorubicin-Induced Cardiotoxicity in Human Pluripotent Stem Cell Derived-Cardiomyocytes. Sci Rep, 2016. 6: p. 25333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Necela BM, et al. , The antineoplastic drug, trastuzumab, dysregulates metabolism in iPSC-derived cardiomyocytes. Clin Transl Med, 2017. 6(1): p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, et al. , Adaptation of Human iPSC-Derived Cardiomyocytes to Tyrosine Kinase Inhibitors Reduces Acute Cardiotoxicity via Metabolic Reprogramming. Cell Syst, 2019. 8(5): p. 412–426.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma A, et al. , Use of human induced pluripotent stem cell-derived cardiomyocytes to assess drug cardiotoxicity. Nat Protoc, 2018. 13(12): p. 3018–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadzijusufovic E, et al. , Nilotinib-induced vasculopathy: identification of vascular endothelial cells as a primary target site. Leukemia, 2017. 31(11): p. 2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, et al. , A Human Pluripotent Stem Cell-Based Screen for Smooth Muscle Cell Differentiation and Maturation Identifies Inhibitors of Intimal Hyperplasia. Stem Cell Reports, 2019. 12(6): p. 1269–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sa S, et al. , Induced Pluripotent Stem Cell Model of Pulmonary Arterial Hypertension Reveals Novel Gene Expression and Patient Specificity. Am J Respir Crit Care Med, 2017. 195(7): p. 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safar ME, Arterial stiffness as a risk factor for clinical hypertension. Nat Rev Cardiol, 2018. 15(2): p. 97–105. [DOI] [PubMed] [Google Scholar]
- 23.Richards DJ, et al. , Inspiration from heart development: Biomimetic development of functional human cardiac organoids. Biomaterials, 2017. 142: p. 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giacomelli E, et al. , Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell, 2020. 26(6): p. 862–879 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polonchuk L, et al. , Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci Rep, 2017. 7(1): p. 7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Archer CR, et al. , Characterization and Validation of a Human 3D Cardiac Microtissue for the Assessment of Changes in Cardiac Pathology. Sci Rep, 2018. 8(1): p. 10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergstrom G, et al. , Stem cell derived in vivo-like human cardiac bodies in a microfluidic device for toxicity testing by beating frequency imaging. Lab Chip, 2015. 15(15): p. 3242–9. [DOI] [PubMed] [Google Scholar]
- 28.Mills RJ and Hudson JE, Bioengineering adult human heart tissue: How close are we? APL Bioeng, 2019. 3(1): p. 010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breckwoldt K, et al. , Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat Protoc, 2017. 12(6): p. 1177–1197. [DOI] [PubMed] [Google Scholar]
- 30.Mills RJ, et al. , Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci U S A, 2017. 114(40): p. E8372–E8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, El-Sherif N, and Boutjdir M, Unitary current analysis of L-type Ca2+ channels in human fetal ventricular myocytes. J Cardiovasc Electrophysiol, 1999. 10(5): p. 692–700. [DOI] [PubMed] [Google Scholar]
- 32.Cui N, et al. , Doxorubicin-induced cardiotoxicity is maturation dependent due to the shift from topoisomerase IIα to IIβ in human stem cell derived cardiomyocytes. J Cell Mol Med, 2019. 23(7): p. 4627–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta LS, et al. , Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation, 2018. 137(8): p. e30–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards DJ, et al. , Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat Biomed Eng, 2020. 4(4): p. 446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas CA, et al. , Modeling of TREX1-Dependent Autoimmune Disease using Human Stem Cells Highlights L1 Accumulation as a Source of Neuroinflammation. Cell Stem Cell, 2017. 21(3): p. 319–331.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]


