Abstract
Hemoglobin-based oxygen carriers (HBOCs) are used in extreme circumstances to increase hemoglobin concentration and improve oxygen delivery when allogenic red blood cell transfusions are contraindicated or not immediately available. However, HBOC-induced severe pulmonary and systemic vasoconstriction due to peripheral nitric oxide (NO) scavenging has stalled its implementation in clinical practice. We present a case of an 87 year-old patient with acute life-threatening anemia who received HBOC while breathing NO gas. This case shows that inhaled NO allows for the safe use of HBOC infusion by preventing HBOC-induced pulmonary and systemic vasoconstriction.
Keywords: Nitric oxide, Hemoglobin based oxygen carrier (HBOC), Vasoconstriction
1. Introduction
Hemopure, a hemoglobin-based oxygen carrier (HBOC), is a purified, polymerized, and acellular bovine hemoglobin which is a blood substitute for allogenic red blood cell (pRBC) transfusion [1]. HBOC products are potent pulmonary and systemic vasoconstrictors. When infused into the circulation, HBOC is in the form of oxyhemoglobin (Oxy-HBOC), which depletes vascular nitric oxide (NO) in the dioxygenation reaction to form circulating acellular methemoglobin (Met-Hb-HBOC) [2,3]. In previous clinical trials, HBOC showed to increase the risk of myocardial infarction and mortality [4,5]. Due to these safety concerns, the clinical use of HBOC is not approved by the United States Food and drug Administration (FDA) but it is still object of investigation by the United States FDA Center for Biologic Evaluations and Research (CBER) [6].
To prevent pulmonary and systemic vasoconstriction, we administered NO gas to a woman during infusion of HBOC-201 (Hemopure, OPK Biotech, Cambridge, MA) for acute, life-threatening anemia for whom crossmatch compatible pRBC could not be obtained in a timely manner due to the presence of an alloantibody to a high frequency RBC antigen, Jk3 [7].
2. Case Report
An 87-year-old woman with a history of an anti-Jk3 alloantibody, coronary artery disease, hypertension, insulin-dependent diabetes mellitus, paroxysmal atrial fibrillation (on warfarin), and chronic kidney disease stage IIIb presented to the Emergency Department (ED) with one day of right posterior thigh pain and inability to move or feel her right foot. Physical examination revealed a cold right foot with ischemic skin changes and sensory deficits as well as similar findings in the distal parts of her ipsilateral lower leg. She had palpable femoral pulses but no palpable or Doppler signal distally. CT angiogram showed a thrombotic occlusion of the right common femoral artery (CFA) at the level of the femoral neck with no reconstitution of flow distally. Vital signs were normal as well as the mental status. Laboratory results obtained in the ED were unremarkable and the hemoglobin concentration was 11.9 g/dL. At the time of hospital admission the patient was hemodynamically stable with normal hemoglobin levels, and the surgeon opted for a conservative approach by revascularization of her lower right limb. Thus, the patient was taken emergently to the operating room.
A right CFA embolectomy with stenting of the right popliteal artery and four-compartment fasciotomies were performed. In order to correct an elevated INR preoperatively, 4 units of fresh frozen plasma were transfused to achieve an INR of 2.0. At the end of the procedure, her estimated blood loss was 600 mL with a positive fluid balance of 1.8 L. Her subsequent laboratory results were significant for a hemoglobin of 7.0 g/dL (Fig. 1). After extubation, the patient was transferred to the surgical intensive care unit with a continuous infusion of phenylephrine (0.33 μg·kg−1·min−1) to maintain a mean arterial pressure > 70 mm Hg.
Fig. 1.
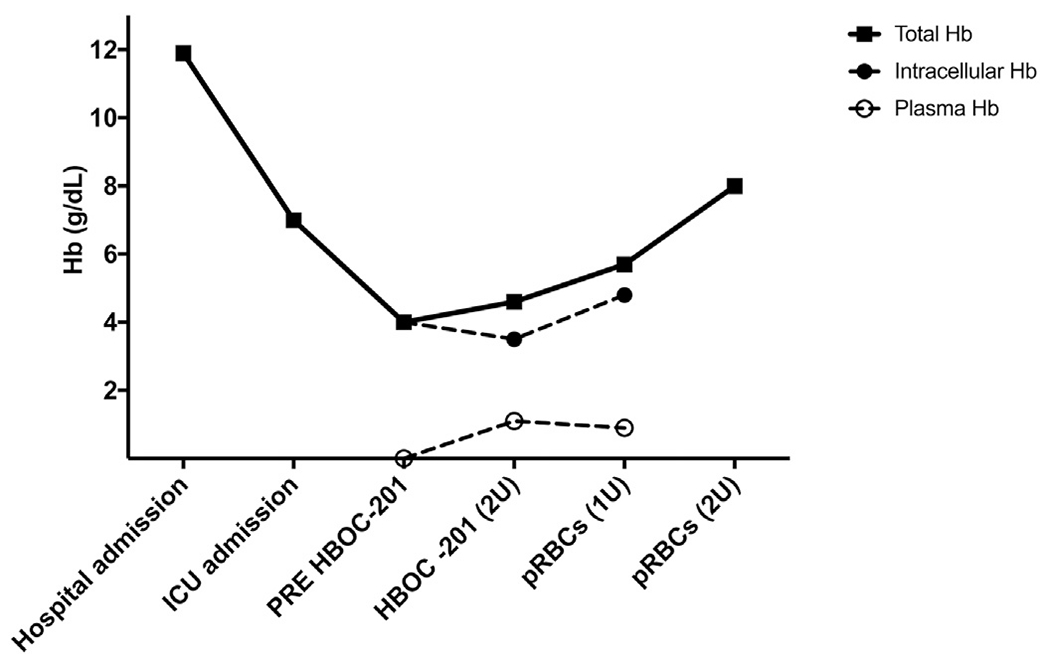
Total hemoglobin, intracellular hemoglobin and plasma hemoglobin concentration variations before and after HBOC-201 and pRBCs transfusions. Concentration of HBOC-201 is measured in plasma. Intracellular hemoglobin is measured as the difference between total hemoglobin and plasma hemoglobin. During HBOC infusion, toxicity and inactivation of infused HBOC-201 related to NO inhalation were assessed by measuring percentage of total metHb and plasma metHb after centrifugation. After infusion of 2 units of HBOC-201, total metHb was 7% and plasma metHb was 28%. These findings suggest that NO inhalation before and during HBOC-201 infusion preserved the oxygen-carrying capacity of 72% of the infused HBOC. HBOC-201: hemoglobin-based oxygen carrier-201 (hemopure); pRBCs: packed red blood cells.
Upon arrival to the surgical ICU, there was evidence of continuous bleeding from the fasciotomy sites. The patient was arousable to command but she became increasingly hemodynamically unstable requiring support with norepinephrine at 0.25 μg·kg−1·min−1. Given the state of hemorrhagic shock refractory to fluid resuscitation while requiring high dose of norepinephrine, pRBC transfusion would ordinarily have been indicated. However, she was known to have a RBC alloantibody with anti-Jk3 specificity, which is known to be hemolytic. The Hematology and Blood Bank Services were consulted and they recommended optimization of her coagulation profile with FFP, vitamin K, and aminocaproic acid given the lack of an immediate supply of pRBCs lacking the Jk3 antigen which is an extraordinarily rare phenotype in most populations.
Towards the end of post-operative day 1, the patient developed a worsening lactic acidosis (lactate 5.7 mmol/L), increasing levels of serum troponin (0.09 ng/mL) without evidence of ST elevation on the electrocardiogram. Hemoglobin was 4.0 g/dL (Fig. 1) due to continued bleeding refractory to pro-coagulant therapy. There was evidence of worsening mental status with somnolence but neither alterations of air-way- protective reflexes nor impaired gas exchange requiring tracheal intubation were observed (ABG: PH 7.32, PaO2 278 mm Hg, PCO2 47 mm Hg, at FiO2 100% delivered by high flow nasal cannula 40 L/min). Since transfusion of non-compatible pRBCs would be deleterious and possibly lethal, we obtained the written informed consent of the patient and her family to administer Hemopure as a bridging oxygen-carrying solution until compatible pRBCs could be found, and her permission to report this case in the medical literature. Compassionate use of Hemopure was granted by our institutional review board and the Food and Drug Administration.
To preemptively monitor and attempt to prevent the known adverse effects of Hemopure such as HBOC-mediated pulmonary arterial hypertension, a pulmonary artery catheter was placed and inhalation of NO (Mallinckrodt. INO Therapeutics, Hazelwood, MO, USA) 25 parts-per-million (ppm) by using high flow nasal cannula device (flow: 40 L/min; FiO2: 100%) was started. Subsequently, 2 units of Hemopure (60 g total of HBOC-201) were administered at a rate of 4.3 g/h while NO was increased to 80 ppm for a brief period and subsequently reduced to 20 ppm. The toxicity of Hemopure and NO were assessed by monitoring the patient’s vital signs, laboratory results, and symptoms.
Inhaled NO decreased total pulmonary vascular resistance index (PVRI) from 17.5 WU·m2 to 13 WU·m2 (Fig. 2A), limited the increase of pulmonary artery pressure (PAP) within the nadir of the baseline value (Fig. 2B), and gradually decreased systemic vascular resistance (SVRI) presumably by exerting systemic effects (Fig. 2C) [7]. Finally, cardiac index (CI) slightly improved (Fig. 2D) and mean arterial pressure remained above 65 mm Hg (Fig. 2E) allowing norepinephrine to be weaned. After discontinuation of inhaled NO, the patient remained hemodynamically stable, and neither rebound pulmonary nor systemic vasoconstriction were observed (Fig. 2). Despite ongoing bleeding, the hemoglobin level increased to 4.6 g/dL (Fig. 1). Finally, NO inhalation during HBOC infusion resulted in partial oxidation of HBOC to methemoglobin (metHb was 28% at the end of HBOC infusion, Fig. 1). Because 72% of HBOC was able to carry oxygen, HBOC infusion improved oxygen delivery as indicated by the reduction of lactate levels (from 5.7 mmol/L to 3 mmol/L).
Fig. 2.
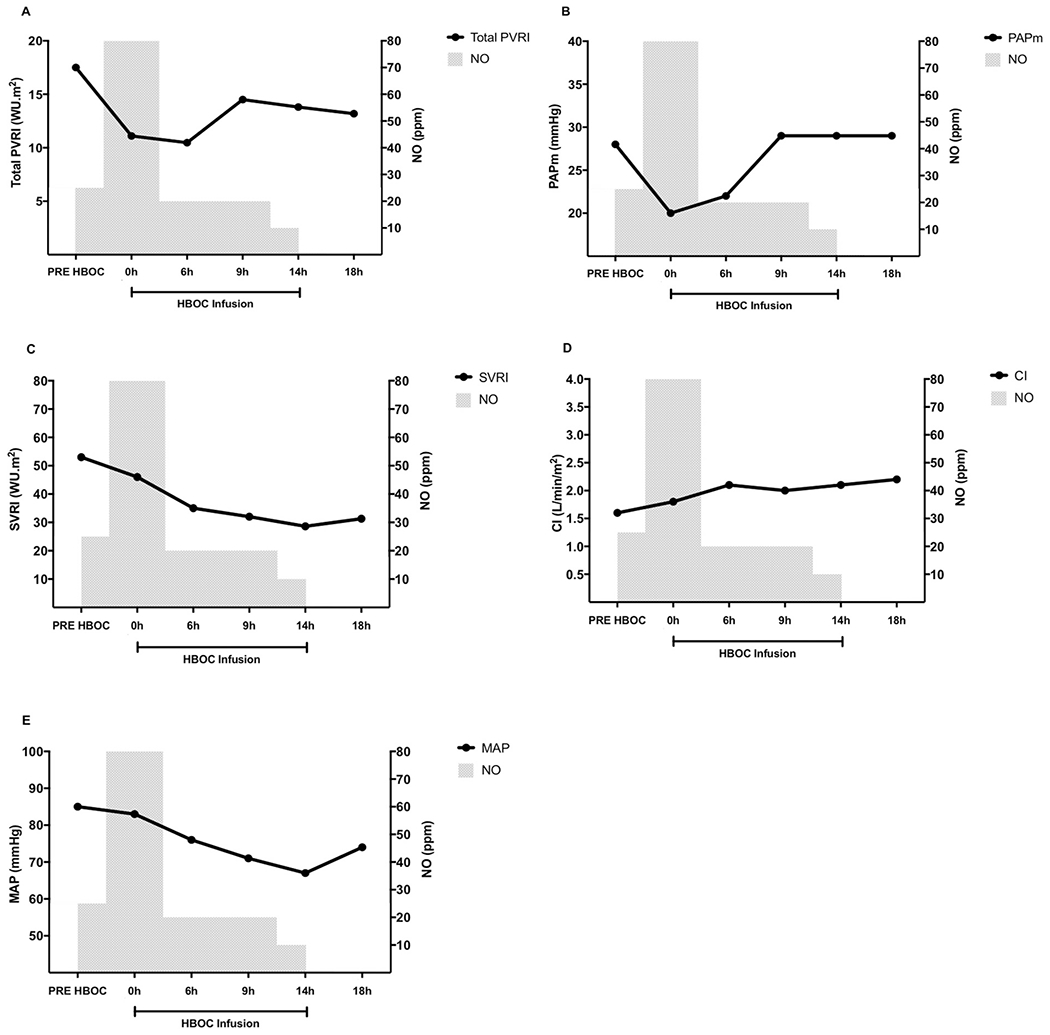
Hemodynamic effects of NO inhalation before and during HBOC infusion. (A) Total pulmonary vascular resistance index (WU·m2) over time. (B) Mean pulmonary arterial pressure (mm Hg) over time. (C) Systemic vascular resistance index (WU·m2) over time. (C) Cardiac index (L/min/m2) over time; (D) Mean arterial pressure (mm Hg) over time.
During and after HBOC infusion, the patient’s mental status and hemodynamics continued to improve and by post-operative day 2, vasopressor support was significantly decreased (norepinephrine from 0.25 μg·kg−1·min−1 to 0.03 μg·kg−1·min−1). She subsequently received 3 units of Jk3 negative pRBCs on post-operative day 2 following which her hemoglobin level rose to 8.0 g/dL (Fig. 1) and she was transferred to the floor on post-operative day 4. Due to a necrotic, multi-bacterial infection at the fasciotomy sites, she underwent an above-the-knee amputation on post-operative day 14. She was then discharged to a rehabilitation facility 12 days later. As of 6 months after discharge, she is still alive and has not had any complication related to the use of HBOC.
3. Discussion
We have described the use of HBOC-201 on a compassionate release basis in an 87 year-old patient with severe, life-threatening anemia in whom inhalation of NO before and during HBOC-201 infusion prevented HBOC-mediated systemic and pulmonary hypertensive crisis. The vasoconstrictor response with HBOC use is caused by the scavenging of endogenous NO by the acellular hemoglobin which results in oxidized hemoglobin or metHb (Fe3+) and nitric oxide metabolite production [2]. NO inhalation before and during HBOC infusion resulted in a decrease of PAP and inhibited HBOC-mediated pulmonary and systemic vasoconstriction.
HBOC-201 or Hemopure is a cell-free, polymerized, bovine hemoglobin containing approximately 13 g/dL of hemoglobin [1]. The basis for its plasma oxygen carrying properties are twofold: firstly, it has a right-shifted oxygen affinity dissociation curve with a P50 of 30 mm Hg allowing for better oxygen unloading in ischemic tissues. Secondly, the oxygen affinity of bovine hemoglobin is regulated by chloride ion at levels which are found in human plasma, rather than by 2,3-diphosphoglycerate as is the case with human hemoglobin [8]. In addition, the lack of RBC surface antigens in Hemopure permits its use in patients like the one in this report who had an anti-Jk3 alloantibody. The Jk (Kidd) glycoprotein is part of the erythrocyte urea transporter system in the erythrocyte cell membrane and expresses three antigens: Jka (Jk1) and Jkb (Jk2) which are co-dominant alleles, and Jk3 which is found on virtually all human erythrocytes. All three of these antigens are absent in the rare Jk-null phenotype. The highest frequency of the Jk-null phenotype is among Polynesians, where it is found in 0.9% of the population. The alloantibodies produced by Jk-null patients react with all RBCs expressing the Jka and/or Jkb antigens (and therefore Jk3 as well) resulting in both acute and delayed transfusion reactions of variable severity [7].
Given the high 30-day mortality rate in patients with hemoglobin levels below 5 g/dL who do not receive pRBC transfusions [9], and the lack of immediately available Jk-null pRBCs at our hospital, the use of HBOCs represented an extreme attempt to improve oxygen delivery without incurring the risk of a hemolytic transfusion reaction.
Persistent safety concerns about diffuse acute vasoconstriction following HBOC administration have impeded its widespread use [5]. The underlying mechanism is that plasma free hemoglobin scavenges endogenous NO in the peripheral vessels and reduces NO peripheral bioavailability [2]. The lack of endogenous NO leads to a dysregulation of the resting tone of the arterioles with coronary and peripheral vasospasm [3].
Preclinical studies have shown that pretreatment with inhaled NO followed by continuous breathing of NO during HBOC-201 infusion prevents the acute pulmonary hypertension and systemic vasoconstriction induced by HBOC-201 infusion [3,10]. Continuous inhalation of NO during infusion of HBOC-201 is essential due to the extreme short halflife of NO in plasma. Inhalation of NO antagonizes HBOC vasoconstriction effects by: (I) Promoting formation of S-nitrosohemoglobin (SNO-Hb) in the lungs which releases NO in the distal part of arterioles due to low oxygen tension in the peripheral tissues [11,12]; and (II) Forming plasma levels of NO metabolites including nitrite, a potent systemic vasodilator [13,14].
Despite the use of HBOC, breathing NO decreased total PVRI and limited the increase of PAP from the baseline levels. These effects cannot be attributable to a reversal of hypoxia-induced vasoconstriction since the patient was hyperoxemic before NO inhalation and maintained high PaO2 levels during HBOC infusion. The prophylactic use of inhaled NO may have also prevented generalized vasoconstriction as indicated by a decrease of SVRI. Neither signs nor symptoms of coronary artery vasospasm were observed during HBOC infusion. In addition, the absence of rebound pulmonary and systemic hypertension suggests that inhalation of NO can be safely limited to the period of infusion of HBOC.
Finally, during inhalation, NO molecules oxidize hemoglobin to its inert form methemoglobin (MetHb) that is unable to bind or release oxygen. MetHb is restored to its usual oxygen-carrying state by an enzyme reducing system present in red cells, NADH-dependent cytochrome b5-metHb reductase. Due to the absence of metHb reductase within HBOC, exposure to a high concentration of NO can result in oxidation of HBOC to metHb thus imparing its ability to carry oxygen [10]. In our patient, due to the persistent bleeding, the infusion of HBOC resulted in only a slight increase in the Hb level (Fig. 1). Twenty ppm NO administration during most of the HBOC infusion increased plasma metHb up to 28%, thus, preserving the oxygen-carrying capacity of 72% of infused HBOC. The use of a combined strategy of NO with HBOC improved cardiac output, arterial oxygen content, lactate clearance, and reduced vasopressor requirement.
4. Conclusion
Clinical scenarios when allogenic RBC transfusions are contraindicated or not accepted by the patient can be challenging. Although hemoglobin based oxygen carrier can restore oxygen carrying capacity of the blood, pulmonary and systemic vasoconstriction prohibits its safe clinical use. In this case, we reported that inhaled NO prevented HBOC-mediated systemic and pulmonary vasoconstriction in a patient with hemorrhagic shock. Strategies, such as breathing NO, may allow safe use of HBOC in emergent situations where pRBC transfusion is not feasible.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- [1].Jahr JS, Moallempour M, Lim JC. HBOC-201, hemoglobin glutamer-250 (bovine), Hemopure ® (Biopure Corporation). Expert Opin Biol Ther 2008;8:1425–33. 10.1517/14712598.8.9.1425. [DOI] [PubMed] [Google Scholar]
- [2].Yu B, Bloch KD, Zapol WM. Hemoglobin-based red blood cell substitutes and nitric oxide. Trends Cardiovasc Med 2009;19:103–7. 10.1016/j.tcm.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yu B, Volpato GP, Chang K, Bloch KD, Zapol WM. Prevention of the pulmonary vasoconstrictor effects of HBOC-201 in awake lambs by continuously breathing nitric oxide. Anesthesiology 2009;110:113–22. 10.1097/ALN.0b013e318190bc4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA 2008;299:2304–12. 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Serruys PW, Vranckx P, Slagboom T, Regar E, Meliga E, de Winter RJ, et al. Haemodynamic effects, safety, and tolerability of haemoglobin-based oxygen carrier-201 in patients undergoing PCI for CAD. EuroIntervention 2008;3:600–9. [DOI] [PubMed] [Google Scholar]
- [6].Alayash AI. Evaluating the safety and efficacy of hemoglobin-based blood substitutes. https://www.fda.gov/BiologicsBloodVaccines/ScienceResearch/BiologicsResearchAreas/ucm127061.htm; 2017.
- [7].Lawicki S, Covin RB, Powers AA. The Kidd (JK) blood group system. Transfus Med Rev 2017;31:165–72. 10.1016/j.tmrv.2016.10.003. [DOI] [PubMed] [Google Scholar]
- [8].Standl T, Freitag M, Burmeister MA, Horn EP, Wilhelm S, Schulte am Esch J. Hemoglobin-based oxygen carrier HBOC-201 provides higher and faster increase in oxygen tension in skeletal muscle of anemic dogs than do stored red blood cells. J Vasc Surg 2003;37:859–65. 10.1067/mva.2003.127. [DOI] [PubMed] [Google Scholar]
- [9].Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion 2002;42:812–8. 10.1046/j.1537-2995.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- [10].Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation 2008;117:1982–90. 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bloch KD, Ichinose F, Roberts JD, Zapol WM. Inhaled NO as a therapeutic agent. Cardiovasc Res 2007;75:339–48. 10.1016/j.cardiores.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McMahon TJ, Doctor A. Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proc Am Thorac Soc 2006;3:153–60. 10.1513/pats.200507-066BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gladwin MT, Raat NJH, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol 2006;291:H2026–35. 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- [14].Cannon RO III, Schechter AN, Panza J a, et al. 22 - effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest 2001;108:279–87. 10.1172/JCI200112761.Introduction. [DOI] [PMC free article] [PubMed] [Google Scholar]


