Abstract
Purpose
The progression of an abnormal inflammatory response plays a crucial role in the lung function decline of chronic obstructive pulmonary disease (COPD) patients. Compared to serum biomarkers, inflammatory biomarkers in induced sputum would be a more reliable reflection of inflammatory processes in the airways.
Patients and Methods
A total of 102 COPD participants were divided into a mild-to-moderate group (FEV1%pred≥ 50%, n=57) and a severe-to-very-severe group (FEV1%pred<50%, n=45). We measured a series of inflammatory biomarkers in induced sputum and analyzed their association with lung function and SGRQ in COPD patients. To evaluate the relationship between inflammatory biomarkers and the inflammatory phenotype, we also analyzed the correlation between biomarkers and airway eosinophilic phenotype.
Results
We found increased mRNA levels of MMP9, LTB4R, and A1AR and decreased levels of CC16 mRNA in induced sputum in the severe-to-very-severe group. After adjustment for age, sex and other biomarkers, CC16 mRNA expression was positively associated with FEV1%pred (r=0.516, p=0.004) and negatively correlated with SGRQ scores (r=−0.3538, p=0.043). As previously known, decreased CC16 was related to the migration and aggregation of eosinophils in airway. It was also found that CC16 had a moderate negative correlation with the eosinophilic inflammation in airway (r=−0.363, p=0.045) in our COPD patients.
Conclusion
Low CC16 mRNA expression levels in induced sputum were associated with low FEV1%pred and a high SGRQ score in COPD patients. Sputum CC16 as a potential biomarker for predicting COPD severity in clinical practice might attribute to the involvement of CC16 in airway eosinophilic inflammation.
Keywords: COPD, induced sputum, airway inflammation, biomarkers, lung function, CC16
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous lung condition with abnormalities of the airways and/or alveoli leading to progressive and persistent airflow limitation.1,2 As one of the leading causes of mortality and morbidity worldwide, COPD imposes serious economic and social burdens.3–5 Lung function is often assessed by measuring forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC).6 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) is the most common standard for assessing the severity and progression of COPD according to the percentage of the predicted FEV1 value (FEV1%pred).2 However, the heterogeneity of COPD causes great challenges in identification of the inflammatory phenotype, individualized treatment and management by lung function alone. Thus, inflammatory biomarkers may identify specific phenotypes and endotypes of COPD and enable individualized treatment for specific patients. However, serum and sputum inflammatory biomarkers in COPD patients have not been studied extensively, and the implementation of biomarkers has been limited.7
Abnormal inflammatory responses in the lungs are usually caused by noxious particles or gases, leading to the progressive destruction of the structure and function of the lungs.8 The various inflammatory cytokines and proteases that are produced and activated by inflammatory cells, such as neutrophils and eosinophils, accumulate in airways and participate in the chronic inflammation of COPD.8–10 Previous studies have shown that Club cell secretory protein-16 (CC16), which is derived from lung parenchymal cells, is associated with airflow limitation in COPD.11 Matrix metalloproteinase 9 (MMP-9) mediates the accumulation and infiltration of inflammatory cells in the lung.12 Moreover, Leukotriene B4 (LTB4) is a chemotactic agent and activating factor for granulocytes and is one of the most important leukotrienes in the onset of acute inflammatory responses.13,14 The LTB4-LTB4R axis has been documented to be an active participant in the development of various inflammatory diseases.15 A1 Adenosine Receptor (A1AR) is one of adenosine receptors. It can modulate inflammation via adenosine. The hypothesis of potential role played by A1AR in chronic lung diseases was proposed before.16 Recepteur d’Origine Nantais (RON) is a receptor tyrosine kinase of the MET receptor family that is canonically involved in mediating growth, promoting wound healing and inflammatory signaling.17 Type II IFNs (IFN-γ) is a pro-inflammatory cytokine that regulate early hematopoiesis.18 It also has antiviral activity and can affect innate immunity.18,19 MicroRNAs (miRNAs) are small noncoding RNAs of approximately 22 nucleotide (nt) that are involved in the negative post-transcriptional gene regulation via mRNA degradation or inhibition of their translation.20–22 Recently, the dysregulation of microRNAs (miRNAs), such as microRNA-155 (miR-155) and microRNA-21 (miR-21), was found to be involved in the inflammatory response or fibrosis in pulmonary diseases.23 Systemic inflammation markers can be assessed in the peripheral blood of COPD patients; however, this method does not seem to be a sufficiently accurate method of reflecting the inflammatory processes within the airways.9,24 Thus, a reliable measurement of local airway inflammation in COPD should be based on samples obtained from the location of sustained inflammation. Induced sputum has been widely acknowledged to be a noninvasive and repeatable sampling method to evaluate the patterns of inflammatory cells and the concentrations of various inflammatory mediators, which would assess local airway inflammation more accurately.25–27
The aim of this study was to determine whether different inflammatory biomarkers are associated with lung function and inflammatory phenotypes in COPD patients and which inflammatory biomarkers play a vital role. Based on previous studies, we measured the expression of several inflammatory biomarkers in the induced sputum of COPD patients.
Materials and Methods
Study Design and Population
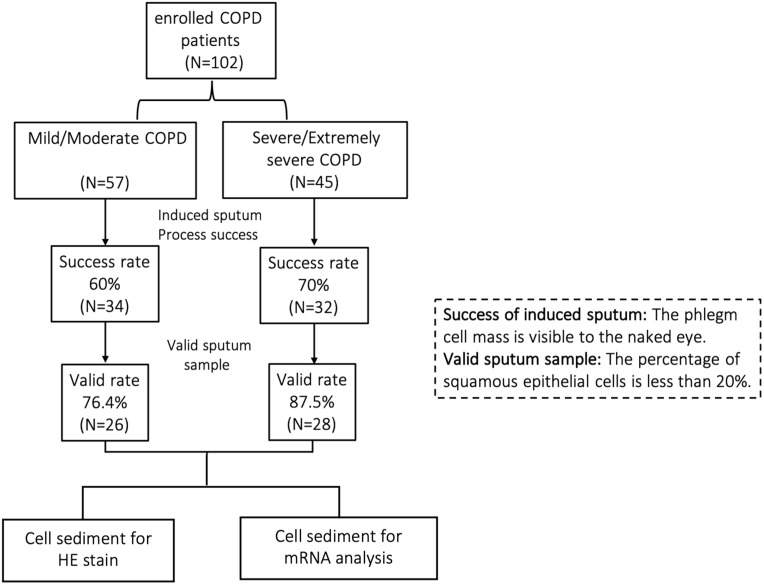
The study was approved by the Hospital Medical Ethics Committee. A total of 102 COPD patients were recruited from Zhongshan Hospital of Fudan University (a tertiary teaching hospital) from 2020 to 2021 (shown in Figure 1). The inclusion criteria: 1) COPD diagnosed by pulmonary physician according to GOLD guideline; 2) stable COPD; 3) COPD patients with qualified induced sputum. Exclusion criteria included any of the following: 1) induced sputum failed (defined as invisible phlegm cell mass by the naked eyes); 2) invalid sputum sample (squamous epithelial cells≥20%). Patient characteristics, clinical examinations, treatments and spirometry results were collected. We also adopted the St George’s Respiratory Questionnaire (SGRQ), modified Medical Research Council (mMRC) scale and COPD Assessment Test (CAT) to evaluate the progression of COPD. The study was carried out in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Figure 1.
A flow chart of subject enrollment, induced sputum collection and assessment and specimen measurement.
Definitions
COPD was diagnosed when FEV1/FVC was <70% according to the GOLD criteria.6 According to FEV1%pred,6 participants with COPD were divided into a mild-to-moderate group (FEV1%pred≥ 50%, n=57) and a severe-to-very-severe group (FEV1%pred<50%, n=45). Participants who met any of the following criteria during the previous year were recorded as having experienced acute exacerbations: 1) hospitalization ≥ once per year; and 2) ambulatory treatment ≥ twice per year. Based on the percentages of neutrophils and eosinophils in induced sputum, the inflammation in the induced sputum sample was classified into four phenotypes, namely, the neutrophilic phenotype, eosinophilic phenotype, mixed granulocyte phenotype and paucigranulocytic phenotype.28 The neutrophilic phenotype was defined as a sample with ≥61% neutrophils and <3% eosinophils; the eosinophilic phenotype was defined as a sample with ≥3% eosinophils and <61% neutrophils; the mixed phenotype was defined as a sample with ≥61% neutrophils and ≥3% eosinophils; and the paucigranulocytic phenotype was defined as a sample with < 60% neutrophils and < 3% eosinophils.29,30
Spirometry
Spirometry was performed by a trained respiratory technician according to the American Thoracic Society recommendations.31 FVC and FEV1 were measured, and FEV1%pred and FEV1%FVC were calculated.
Fraction Exhaled Nitric Oxide (FeNO)
FeNO was evaluated with an NO analyzer (FeNo Expair, Medisoft, Sorinnes, Belgium). In the seated position, patients exhaled all the air from their lungs and then inhaled as deeply as possible for 5 seconds. Then, they exhaled slowly for 10 seconds. The flow rate was maintained at 50 mL/s during the detection period. The FeNO test was repeated 3 times, and the mean value was calculated. This process was performed in strict accordance with the operating instructions.
Sputum Induction
Patients with COPD inhaled 200 μg of salbutamol, and sputum was induced after the inhalation of 4.5% NaCl for 5 min.29,32,33 If insufficient sputum was collected, the previous process was repeated 3 times.34 All sputum plugs with visibly greater solidity were carefully selected and processed immediately.27 Sputum plugs greater than 0.02 g were processed as follows. The sputum plugs were diluted 4 times with a mixture of 0.1% dithiothreitol (DTT) and phosphate-buffered saline (PBS), which was then shaken for 10 min.29,30 Then, a double volume of PBS was added, and the mixture was vortexed briefly. After filtration through two layers of sterile gauze, the sputum was centrifuged for 10 min at 1800 × g. The sediment was collected and stored at −80°C before biomarker analysis.
Induced Sputum Cell Count
The cytospin slides were stained with May-Grunwald-Giemsa stain to determine the differential cell count, and a slide with ≥400 cells was identified as an acceptable sample. The induced sputum was acceptable if it contained fewer than 20% squamous epithelial cells.29
Total RNA Extraction and qRT‒PCR
Based on previous studies, we evaluated the association of lung function with the following inflammatory biomarkers: A1 adenosine receptor (A1AR), CC16, interferon gamma (INFγ), leukotriene B4 receptor (LTB4R), MMP9, RON tyrosine kinase receptor (RON), miR-155, and miR-21.23,35–37 Total RNA from induced sputum was extracted using TRIzol reagent (Sigma-Aldrich, MO, USA) following the manufacturer’s protocol and reverse transcribed into cDNA using a PrimeScript RT reagent kit (Takara Bio, Shiga, Japan) as previously described.38 qRT-PCR analysis was performed with SYBR Premix Ex Taq (Takara Bio) in an Applied Biosystems 7500 Real-Time PCR System using the following three-step cycling programs according to the instructions. The relative expression of target genes was normalized to that of GAPDH, and the expression levels of microRNAs were normalized to that of U6 using the 2-ΔΔCt method. The primers were as follows: GAPDH (forward primer 5’-GCGAGATCCCTCCAAAATCAA-3’, reverse primer 5’-GTTCACACCCATGACGAACAT-3’); IFNγ (forward primer 5’-GAGTGTGGA GACCATCAAGGAA-3’, reverse primer 5’-TGCGTTGGACATTCAAGTCAG-3’); MMP9 (forward primer 5’-TCATCTTCCAAGGCCAATCC-3’, reverse primer 5’-GCAGAAGCCGAAGAGCTTGT-3’); CC16 (forward primer 5’-GGTCACACTGGCTCTCTGCT-3’, reverse primer 5’-CATGGCAGCCTCATAACTG G-3’); LTB4R (forward primer 5’-ACCTGGCCGTATTGCTCACT-3’, reverse primer 5’-GCTGGCGTACATGCTGACTC-3’); RON (forward primer 5’-ATGAATGTGCGTCCAGAACA-3’, reverse primer 5’-CAGGTCCAGCCCAAGAACTA-3’); A1AR (forward primer 5’-GATCCTCTCCTTCGTGGTG-3’, reverse primer 5’-CCCACACAAAGAAGTTGAAG-3’); miR-155 (forward primer 5’-CGCGTTAATGCTAATCGTGATAGGGGT); miR-21 (forward primer 5’-GCGCGTAG CTTATCAGACTGATGTTGA).
Statistical Analysis
Continuous data with a normal distribution are expressed as the mean ± standard deviation (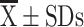 ), and a t-test was used for comparison. Continuous data without a normal distribution were analyzed by the Mann–Whitney U-test, and the results are expressed as medians and quartiles [M (P25, P75)]. Pearson’s correlation was used for the correlation analyses. Partial correlation coefficients were computed between the mRNA expression of all inflammatory biomarkers and FEV1%pred with adjustment for age, sex and other confounding factors. Partial correlation coefficients were calculated between airway inflammation marker mRNA expression and eosinophilic phenotype after removing the effects of age and cough. All data analyses were performed using SPSS 25. Statistical significance was indicated by a p value<0.05.
), and a t-test was used for comparison. Continuous data without a normal distribution were analyzed by the Mann–Whitney U-test, and the results are expressed as medians and quartiles [M (P25, P75)]. Pearson’s correlation was used for the correlation analyses. Partial correlation coefficients were computed between the mRNA expression of all inflammatory biomarkers and FEV1%pred with adjustment for age, sex and other confounding factors. Partial correlation coefficients were calculated between airway inflammation marker mRNA expression and eosinophilic phenotype after removing the effects of age and cough. All data analyses were performed using SPSS 25. Statistical significance was indicated by a p value<0.05.
Results
Characteristics of the Study Population
Data on spirometry were available for 102 participants, and the characteristics of the study population are presented in Table 1. There were fewer women in the severe-to-very-severe group (p=0.039) than in the mild-to-moderate group. Neither symptoms nor complications differed between the two groups. A higher SGRQ score was found in the severe-to-very-severe group than in the mild-to-moderate group (35.88±18.43 vs 25.85±17.08, p=0.006). With regard to treatments, more patients with inhaled corticosteroids (ICS) + long-acting β-agonist (LABA) + long-acting muscarinic antagonist (LAMA) (p=0.024) were found in the severe-to-very-severe group, while the opposite result was found for monobronchodilator therapy (p=0.022). No significant difference was found in the treatments with dual bronchodilator therapy and ICS+LABA/ICS+LAMA therapy. There were no differences in acute exacerbations or FeNO between the two groups.
Table 1.
Demographic and Clinical Characteristics of Participants
| Enrolled Patients | p | Patients with Success Samples | p | |||
|---|---|---|---|---|---|---|
| Mild-to-Moderate Group (n=57) | Severe-to-Very-Severe Group (n=45) | Mild-to-Moderate Group (n=26) | Severe-to-Very-Severe Group (n=28) | |||
| Sex (male) (N, %) | 42 (73.7) | 41 (91.10) | 0.039 | 20 (76.92) | 24 (85.71) | 0.494 |
Age (years) (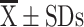 ) ) |
65.42±7.29 | 66.78±5.94 | 0.997 | 66.65±7.62 | 66.54±4.74 | 0.946 |
| Symptoms (N, %) | ||||||
| Cough | 30 (52.60) | 22 (48.90) | 0.842 | 13 (50.00) | 15 (53.57) | 1.000 |
| Expectoration | 37 (64.90) | 34 (75.60) | 0.283 | 17 (65.38) | 24 (85.71) | 0.114 |
| Polypnea | 38 (66.70) | 33 (73.3) | 0.520 | 18 (69.23) | 20 (71.43) | 1.000 |
| Previous history (N, %) | ||||||
| Hypertension | 20 (35.10) | 16 (35.60) | 1.000 | 8 (30.77) | 13 (46.43) | 0.275 |
| Diabetes | 8 (14.00) | 4 (8.90) | 0.542 | 4 (15.38) | 3 (10.71) | 0.699 |
| Arrhythmia | 6 (10.50) | 4 (8.90) | 0.335 | 2 (7.69) | 4 (14.29) | 0.670 |
| Cardiac Dysfunction | 2 (3.50) | 0 (0.00) | 0.502 | 0 (0.00) | 0 (0.00) | / |
| Coronary Artery Disease | 5 (8.80) | 3 (6.70) | 1.000 | 3 (11.54) | 2 (7.14) | 0.663 |
| Hyperlipidemia | 3 (5.30) | 1 (2.20) | 0.628 | 1 (3.85) | 1 (3.57) | 1.000 |
| Other complications | 15 (26.30) | 9 (20.00) | 0.490 | 7 (26.92) | 6 (21.43) | 0.754 |
SGRQ (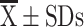 ) ) |
25.85±17.08 | 35.88±18.43 | 0.006 | 25.23±17.53 | 40.00±17.00 | 0.003 |
| mMRC (N, %) | 0.084 | 0.081 | ||||
| 0 | 19 (38.80) | 7 (18.40) | 10 (38.46) | 4 (14.29) | ||
| 1 | 18 (36.70) | 23 (60.50) | 8 (30.77) | 15 (53.57) | ||
| 2 | 8 (16.30) | 7 (18.40) | 5 (19.23) | 6 (21.43) | ||
| 3 | 4 (8.20) | 1 (2.60) | 2 (7.69) | 0 (0.00) | ||
CAT (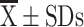 ) ) |
17.50±5.40 | 19.71±6.45 | 0.119 | 18.11±5.29 | 19.46±7.13 | 0.494 |
| FeNO [M (P25, P75)] | 26 (17, 39) | 22.5 (15.75, 35) | 0.270 | 24 (17, 36.75) | 19 (15, 28) | 0.183 |
| Treatments (N, %) | ||||||
| Mono Bronchodilator | 19 (33.3) | 6 (13.3) | 0.022 | 9 (34.62) | 6 (21.43) | 0.366 |
| Dual Bronchodilator | 4 (7.0) | 8 (17.80) | 0.125 | 2 (7.69) | 4 (14.29) | 0.670 |
| ICS+LABA/ICS+LAMA | 14 (24.60) | 8 (17.80) | 0.473 | 5 (19.23) | 5 (17.86) | 1.000 |
| ICS+LABA+LAMA | 16 (28.10) | 23 (51.10) | 0.024 | 7 (26.92) | 13 (46.43) | 0.167 |
| No Inhaled Medication | 4 (7.00) | 0 (00.00) | 0.128 | 3 (11.54) | 0 (0.00) | 0.105 |
| Acute Exacerbation (over the past year) (N, %) | 10 (17.50) | 14 (31.10) | 0.158 | 7 (26.92) | 8 (28.57) | 1.000 |
Notes: Continuous data with a normal distribution are expressed as 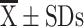 , while continuous data without a normal distribution are expressed as M (P25, P75).
, while continuous data without a normal distribution are expressed as M (P25, P75).
Abbreviations: N, number; 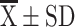 , mean ± standard deviation; M, median; SGRQ, St George’s Respiratory Questionnaire; mMRC, modified British Medical Research Council; CAT, COPD Assessment Test; FeNO, Fraction exhaled nitric oxide; ICS, inhaled corticosteroids; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist.
, mean ± standard deviation; M, median; SGRQ, St George’s Respiratory Questionnaire; mMRC, modified British Medical Research Council; CAT, COPD Assessment Test; FeNO, Fraction exhaled nitric oxide; ICS, inhaled corticosteroids; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist.
Induced Sputum Inflammatory Cell Proportions and Inflammatory Phenotype in COPD Patients
A total of 54 sputum samples were included in the cell component analysis after evaluation (shown in Figure 1). The cellular compositions of the induced sputum samples from COPD patients are shown in Table 2. The eosinophil percentage (p=0.048) was significantly higher in the severe-to-very-severe group than in the mild-to-moderate group, and no significant differences were found in the neutrophil percentage (p=0.311), lymphocyte percentage (p=0.545) or monocyte percentage (p=0.073). Four inflammatory phenotypes were assessed based on the proportions of sputum neutrophils and eosinophils. A more mixed granulocyte phenotype was found in the severe-to-very-severe group (p=0.038). The percentages of the neutrophilic phenotype (p=0.580), eosinophilic phenotype (p=1.000) and paucigranulocytic phenotype (p=0.095) were similar in the two groups.
Table 2.
Cell Phenotypes and mRNA of Inflammatory Biomarkers in Sputum of COPD Patients
| Mild-to-Moderate Group (n=26) | Severe-to-Very-Severe Group (n=28) | p | |
|---|---|---|---|
| Inflammatory cells (%) [M (P25, P75)] | |||
| Neutrophils | 70.68±23.17 | 76.66±19.85 | 0.311 |
| Eosinophils | 1.03 (0.32,2.03) | 2.19 (0.77,6.97) | 0.048* |
| Lymphocytes | 4.04 (1.43,8.462) | 2.23 (1.27,7.74) | 0.545 |
| Macrophage | 10.22 (0.54,23.27) | 2.01 (0.00,9.12) | 0.073 |
| Airway inflammatory phenotype (N, %) | |||
| Neutrophilic phenotype | 12 (46.20) | 10 (35.70) | 0.580 |
| Eosinophilic phenotype | 2 (7.70) | 3 (10.70) | 1.000 |
| Mix Granulocyte phenotype | 4 (15.40) | 12 (42.90) | 0.038* |
| Paucigranuocytic phenotype | 8 (30.80) | 3 (10.70) | 0.095 |
mRNA of sputum inflammatory biomarkers (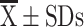 ) ) |
|||
| CC16 | 2.37±0.66 | 0.59±0.13 | 0.013* |
| MMP9 | 1.32±0.19 | 3.30±0.88 | 0.040* |
| A1AR | 1.52±0.24 | 2.65±0.49 | 0.027* |
| RON | 1.88±0.36 | 3.90±1.21 | 0.068 |
| IFNγ | 1.61±0.25 | 2.10±0.74 | 0.810 |
| LTB4R | 1.53±0.27 | 3.36±0.90 | 0.028* |
| miR-21 | 1.15±0.16 | 1.70±0.24 | 0.408 |
| miR-155 | 21.52±10.43 | 1.37±0.75 | 0.066 |
Notes: Continuous data with a normal distribution are expressed as 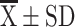 , while continuous data without a normal distribution are expressed as M (P25, P75). *p < 0.05.
, while continuous data without a normal distribution are expressed as M (P25, P75). *p < 0.05.
Abbreviations: N, number; M±SD, mean ± standard deviation; CC16, Club cell secretory protein-16; MMP9, Matrix metalloproteinase 9; A1AR, A1 Adenosine Receptor; RON, Recepteur d’Origine Nantais; IFNγ, Type II IFNs; LTB4R, Leukotriene B4 Receptor; miR-155, microRNA-155; miR-21 microRNA-21.
Inflammatory Biomarker Levels in Induced Sputum of COPD Patients
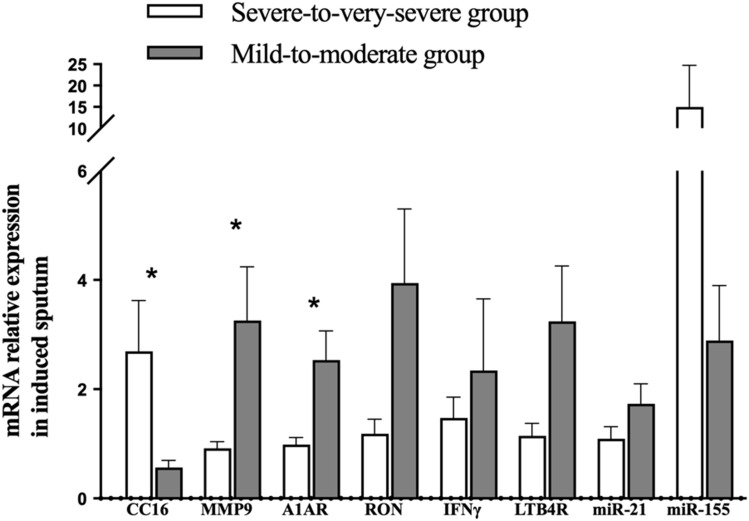
The mRNA expression of inflammatory biomarkers in induced sputum was measured (shown in Figure 2) Compared to the mild-to-moderate group, CC16 mRNA expression (p=0.013) was significantly lower, and the mRNA expression of LTB4R (p=0.028), A1AR (p=0.027) and MMP9 (p=0.040) was significantly higher in the severe-to-very-severe groups. There were no differences in INFγ (p=0.810), RON (p=0.068), miR-155 (p=0.066) or miR-21 (p=0.408) expression between the two groups (Table 2).
Figure 2.
Differences in mRNA expression of CC16, MMP9, A1AR, RON, IFNγ, LTB4R, miR-21, and miR-155 in induced sputum cells between the mild-to-moderate group and the severe-to-very-severe group. Data are expressed as the means ± SEMs. *p<0.05, significant compared to the mild-to-moderate group.
Correlation Between mRNA Expression of Inflammatory Biomarkers in Induced Sputum and Lung Function
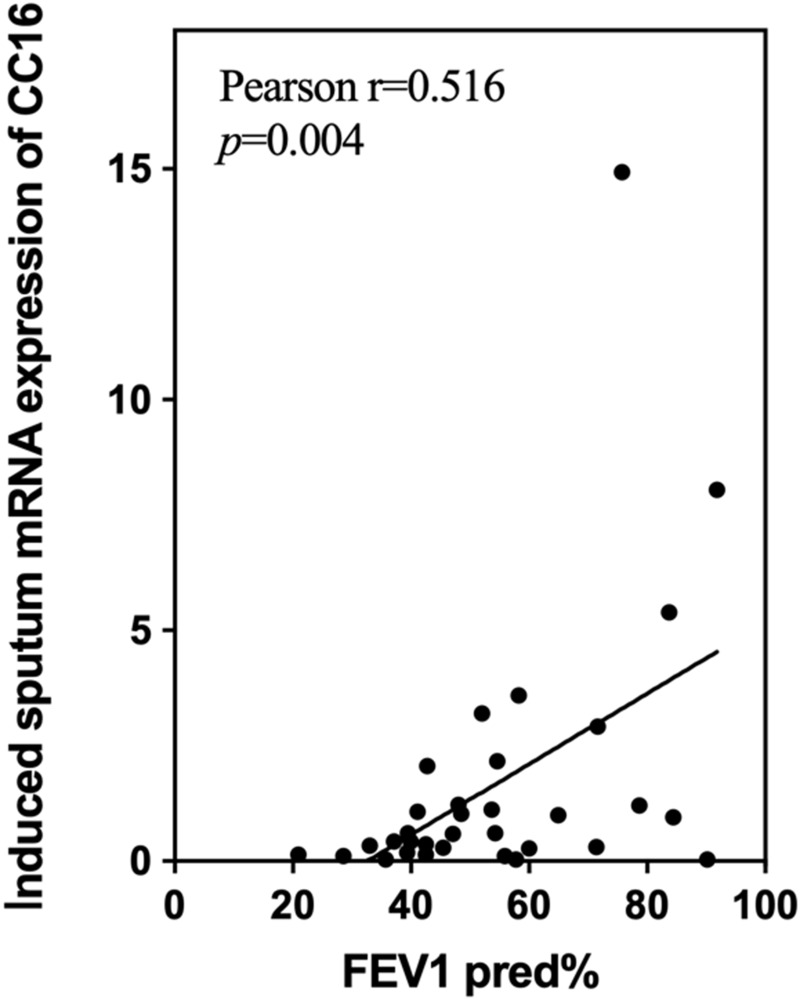
As lung function is believed to be influenced by various factors, we wondered which inflammatory biomarkers in induced sputum would be associated with lung function. Partial correlation coefficients were thus used to assess the correlation between mRNA expression of inflammatory biomarkers and FEV1%pred after removing confounders such as age, sex and other biomarkers. A high positive correlation was observed between CC16 mRNA expression and FEV1%pred (r=0.5160, p=0.004) (Figure 3). Otherwise, no significant associations were found between other induced sputum inflammatory biomarkers and other lung function parameters, such as FEV1/FVC, FVC%pred and DLCO%pred (data not shown).
Figure 3.
Association between CC16 mRNA expression in induced sputum and FEV1%pred. The effects of age, sex and other biomarkers were controlled in a partial correlation analysis.
Correlation Between mRNA Expression of Inflammatory Biomarkers in Induced Sputum and Eosinophilic Phenotype
To assess the correlation between the mRNA expression of inflammatory biomarkers in induced sputum and airway inflammation, we firstly analyzed the correlation between all subjects and eosinophilic phenotype. CC16 (r=−0.388, p=0.008,), MMP9 (r=0.308, p=0.036), IFNγ (r=0.345, p=0.019), age (r=−0.298, p=0.047), cough (r=0.505, p=0.04) were found to be related to eosinophilic phenotype. Therefore, we analyzed the partial correlation coefficients between the mRNA expression of inflammatory biomarkers and eosinophilic phenotype after adjustment for age and cough. The eosinophilic phenotype was defined as a sample with ≥ 3% eosinophils. After adjustment, we found a moderate negative correlation between CC16 mRNA expression and the eosinophilic phenotype (r=−0.3630, p=0.045). However, there were no significant associations between other induced sputum inflammatory biomarkers and eosinophilic phenotype (Table 3). The same analysis was made to explore relationship between mRNA expression of inflammatory biomarkers and other phenotypes. No significant relationship was found to the neutrophilic phenotype, mixed granulocyte phenotype or paucigranulocytic phenotype after adjustment (p>0.05).
Table 3.
Partial Correlation Between Airway Inflammation Marker mRNA Expression in Induced Sputum and the Eosinophilic Phenotype* After Adjustment for Age and Cough
| Coeff | p | |
|---|---|---|
| CC16 | −0.363 | 0.045 |
| MMP9 | 0.152 | |
| A1AR | 0.366 | |
| RON | 0.485 | |
| IFNγ | 0.700 | |
| LTB4R | 0.394 | |
| miR-21 | 0.910 | |
| miR-155 | 0.772 |
Note: *Eosinophilic phenotype: a sample with ≥3% eosinophil.
Correlation Between CC16 mRNA Expression in Induced Sputum and SGRQ Scores
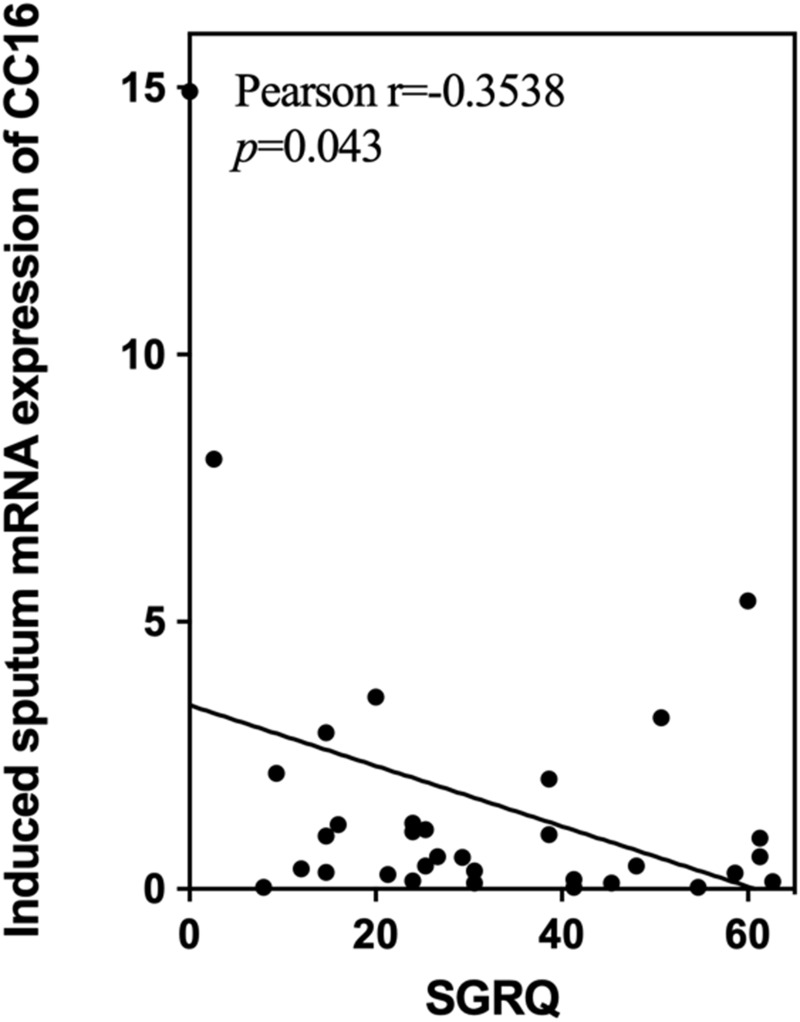
Pearson’s correlation was used to assess the correlation between CC16 mRNA expression in induced sputum and SGRQ scores. There was a negative correlation between CC16 mRNA expression and SGRQ scores (r=−0.3538, p=0.043) (shown in Figure 4).
Figure 4.
Association between mRNA expression of CC16 in induced sputum cells and SGRQ score.
Discussion
In this study, a number of inflammatory cells and inflammatory biomarkers in induced sputum were investigated to determine which could be used as biomarkers to reflect the pulmonary function and inflammatory phenotype of COPD patients. Some of the observations build on previous findings and might provide new sputum inflammatory biomarkers to predict reduced lung function. To our knowledge, this is the first study to evaluate the correlation of mRNA expression of inflammatory biomarkers in induced sputum, rather than in serum or BALF, with the severity of COPD in a Chinese cohort.
The mRNA expression of CC16 in induced sputum was significantly lower in severe-to-very-severe COPD patients, while the mRNA expression of MMP9, A1AR and LTB4R was much higher in those patients (shown in Table 2 and Figure 2). Partial correlation analysis showed a high positive correlation between CC16 mRNA expression and FEV1%pred. This result is consistent with prior studies showing that a reduced serum concentration of CC16 is associated with the severity of COPD.11,39,40 However, few studies have evaluated airway CC16 expression in COPD patients.41–43 Although several studies found decreased airway CC16 expression in severe COPD patients, the expression of airway CC16 was usually estimated by immunohistochemistry.43–46 A similar result was found in a 10-year Chinese longitudinal cohort study conducted by David Chi-Leung Lam et al, who found that the level of CC16 mRNA in endoscopic biopsies of the bronchial epithelium was correlated with the FEV1/FVC ratio.41 Compared to immunohistochemistry and endoscopic biopsies of bronchial epithelium, the induction of sputum production and subsequent detection of CC16 mRNA in our study was less invasive and more practical. Our findings verified that the assessment of induced sputum cells by qRT‒PCR could be a feasible and stable method for the clinical determination of the severity of COPD.
Though differences of MMP9, A1AR and LTB4R between mild-to-moderate group and severe-to-very-severe group, partial correlation analysis showed no relationship between mRNA expressions of MMP9, A1AR and LTB4R and FEV1%pred. MMP9 is known for its ability to, promote neutrophil chemotaxis, mediate inflammation and degrade extracellular matrix proteins, which is associated with exacerbations of COPD.47–49 A strong correlation was observed between increased MMP9 and neutrophil number, rather than eosinophil number.47 A1AR are expressed in all kinds of immune cells and the stimulation of A1AR induces ROS production from activated neutrophils.16 LTB4 was one of the most recognized neutrophil activators which recruits and activates human neutrophils via the LTB4R. Though LTB4 was reported to be chemotactic for eosinophils, it is mostly chemotactic for neutrophils.50 However, there was no significant difference of neutrophil number between groups in our study, which may be the potential cause of low correlation to FEV1%pred. The relatively small sample size may also cause bias.
Although previous data linked serum CC16 with the severity of COPD and the same correlation was shown in induced sputum in our study, evidence supporting the potential causality between CC16 and the severity of COPD is lacking. The mechanism by which CC16 protects the lung from the decline in FEV1%pred and the main cell phenotype of COPD that is protected by CC16 are still unknown.43 Several studies have found that CC16 may modulate inflammatory responses in the lung.7,51 The lack of CC16 substantially exacerbated airway inflammation and alveolar loss,44,45 and exogenous CC16 showed pharmacological properties that could decrease excess airway inflammation and mucus production in ex vivo models.44 However, an opposite view was also held by Zhai52 They believed that lung remodeling is likely a key contributing factor to the altered lung function in mice, which is independent of inflammation, regardless of the level of CC16 detected in mice.
To confirm the link between CC16 and inflammation, we analyzed the association between CC16 and different cell phenotypes in COPD. As we found that the eosinophil percentage was higher in severe-to-very-severe COPD patients (Table 2), the relationship between CC16 mRNA expression and the eosinophilic phenotype was analyzed, and a moderate negative correlation was found (r=−0.3630, p=0.045).
A number of studies have demonstrated that a larger proportion of blood eosinophils is associated with a higher risk of COPD exacerbation.53–55 However, it is unclear whether reduced CC16 expression levels increase the rate of eosinophils or eosinophilic inflammation downregulates CC16 expression or maybe both. CC16 has been demonstrated to provide anti-inflammatory and anti-oxidative effects in various cells.56 In population-based studies, a decrease in circulating CC16 level while T-cells, eosinophils, and mast cells were increased in asthma.56,57 An in vivo study found a significantly higher level of pulmonary eosinophils in CC16-deficient mice.58 The relationship between CC16 and eosinophils may be related to the migration of eosinophils and Th2 modulation. It is reported that CC16 may down-modulate the entry of human eosinophils into the airways during inflammation.59 CC16 could directly or indirectly inhibit the expression of Chitinase-3-like protein 1 (CHI3L1) which is also known as YKL-40 in eosinophilic chronic rhinosinusitis (ECRS), and the reduced levels of eotaxin after anti-CHI3L1 treatment may contribute to the decreased infiltration of eosinophils.60 CC16 has been reported to inhibit Formylated peptide N-formyl-methionine-leucin-phenylalanin (fMLF)-induced migration of human eosinophils.59 Moreover, Li X, etc. found that CC16 mRNA expression levels were negatively correlated with expression levels of Th2 genes, and CC16 could decrease sputum eosinophils through downregulating IL-5 and IL1RL1.61 Though these findings were found in allergic respiratory diseases like asthma and ECRS, it provided possible explanation biologically for the link between CC16 and eosinophilic COPD.
Therefore, eosinophil-mediated inflammation may be the potential cause of the correlation between CC16 mRNA expression and FEV1%pred. The ultimate goal of current research on CC16 is to determine whether CC16 augmentation approaches are a first-in-class disease-modifying therapy for COPD patients.43 Future research may identify COPD patients with both low sputum CC16 levels and the eosinophilic phenotype who are likely to respond to rCC16 therapy.
We also found a negative correlation between CC16 mRNA expression and SGRQ scores. The SGRQ is a 50-item questionnaire developed to assess respiratory health status in patients with obstructive lung diseases and includes three domains: symptoms, activity, and impact.62,63 A higher SGRQ score reflects worse respiratory health status. This finding indirectly supported the relationship between CC16 and FEV1%pred.
The limitations of this study include the lack of data on parameters in the serum of COPD patients; therefore, we were unable to investigate the relationships between and differences in inflammatory biomarker levels in the sputum and the blood. Future studies should be performed to determine the correlations of inflammatory mediators involved in local airway inflammation and systemic inflammation. Second, only the mRNA levels of inflammatory biomarkers in sputum cells were measured. Studies to confirm the protein levels in sputum cells and their associations with pulmonary function parameters are still needed. Furthermore, the sample size was relatively small, which might have introduced some bias. A multicenter study with larger samples is required to validate the effect of CC16 in COPD.
Conclusions
This study demonstrated that the mRNA expression of CC16, MMP9, A1AR and LTB4R in induced sputum was different in mild-to-moderate or severe-to-very-severe COPD patients. From which, CC16 mRNA expression had a high positive correlation with FEV1%pred and a negative correlation with SGRQ scores. The lower expression of CC16 mRNA, the more eosinophil counts in sputum exist. It is speculated that CC16 in induced sputum could be a feasible and stable method for the clinical determination of the severity of COPD and eosinophil-mediated inflammation may be the potential cause of the correlation between CC16 and severity of COPD patients.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82270026 to ZHC), the Shanghai Top-Priority Clinical Key Disciplines Construction Project (2017ZZ02013) and the Shanghai Municipal Key Clinical Specialty (shslczdzk02201).
Ethics Approval and Informed Consent
This study was performed in line with the principles of the Declaration of Helsinki. The institutional review board (IRB) at Zhongshan Hospital, Fudan University, reviewed and approved this study (B2019-309R). All included patients and physicians gave written informed consent prior to participation.
Consent for Publication
Informed consent was obtained from all individual participants included in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95–99. doi: 10.2147/copd.S27480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for chronic obstructinve pulmonary disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 report); 2022.
- 3.López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. doi: 10.1111/resp.12660 [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Du C, Hu F, et al. Management of acute exacerbation of chronic obstructive pulmonary disease under a tiered medical system in China. Ther Adv Respir Dis. 2022;16:17534666221075499. doi: 10.1177/17534666221075499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao R, Liu Z, Zhao Y, et al. Stable Chronic Obstructive Pulmonary Disease (COPD) management under a tiered medical system in China. Int J Chron Obstruct Pulmon Dis. 2022;17:181–194. doi: 10.2147/COPD.S333274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odeyemi YE, Lewis O, Ngwa J, et al. Does low FEV1 in addition to fixed ratio and/or lower limit of normal of FEV1/FVC improve prediction of mortality in COPD? The NHANES-III-linked-mortality Cohort. J Natl Med Assoc. 2019;111(1):94–100. doi: 10.1016/j.jnma.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon JY, Leitao Filho FS, Shahangian K, Takiguchi H, Sin DD. Blood and sputum protein biomarkers for chronic obstructive pulmonary disease (COPD). Expert Rev Proteomics. 2018;15(11):923–935. doi: 10.1080/14789450.2018.1539670 [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Xu J, Meng Y, Adcock IM, Yao X. Role of inflammatory cells in airway remodeling in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3341–3348. doi: 10.2147/copd.S176122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proboszcz M, Mycroft K, Paplinska-Goryca M, et al. Relationship between blood and induced sputum eosinophils, bronchial hyperresponsiveness and reversibility of airway obstruction in mild-to-moderate chronic obstructive pulmonary disease. Copd. 2019;16(5–6):354–361. doi: 10.1080/15412555.2019.1675150 [DOI] [PubMed] [Google Scholar]
- 10.Paplińska-Goryca M, Nejman-Gryz P, Górska K, et al. Expression of inflammatory mediators in induced sputum: comparative study in asthma and COPD. Clinical Research Involving Pulmonary Disorders. Springer; 2016:101–112. [DOI] [PubMed] [Google Scholar]
- 11.Lomas DA, Silverman EK, Edwards LD, et al. Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax. 2008;63(12):1058–1063. doi: 10.1136/thx.2008.102574 [DOI] [PubMed] [Google Scholar]
- 12.Hao W, Li M, Zhang C, Zhang Y, Wang P. Inflammatory mediators in exhaled breath condensate and peripheral blood of healthy donors and stable COPD patients. Immunopharmacol Immunotoxicol. 2019;41(2):224–230. doi: 10.1080/08923973.2019.1609496 [DOI] [PubMed] [Google Scholar]
- 13.Toda A, Yokomizo T, Shimizu T. Leukotriene B4 receptors. Prostaglandins Other Lipid Mediat. 2002;68–69:575–585. doi: 10.1016/s0090-6980(02)00056-4 [DOI] [PubMed] [Google Scholar]
- 14.Wang N, He X, Zhao J, et al. Structural basis of leukotriene B4 receptor 1 activation. Nat Commun. 2022;13(1):1156. doi: 10.1038/s41467-022-28820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang M, Lv J, Jiang Z, et al. Promotion of myofibroblast differentiation and tissue fibrosis by the leukotriene B(4) -leukotriene B(4) receptor axis in systemic sclerosis. Arthritis Rheumatol. 2020;72(6):1013–1025. doi: 10.1002/art.41192 [DOI] [PubMed] [Google Scholar]
- 16.Pasquini S, Contri C, Borea PA, Vincenzi F, Varani K. Adenosine and inflammation: here, there and everywhere. Int J Mol Sci. 2021;22(14):7685. doi: 10.3390/ijms22147685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt BG, Fox LH, Davis JC, et al. An introduction and overview of RON receptor tyrosine kinase signaling. Genes. 2023;14(2):517. doi: 10.3390/genes14020517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paudel S, Ghimire L, Jin L, Jeansonne D, Jeyaseelan S. Regulation of emergency granulopoiesis during infection. Front Immunol. 2022;13:961601. doi: 10.3389/fimmu.2022.961601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langer JA, Cutrone EC, Kotenko S. The class II cytokine receptor (CRF2) family: overview and patterns of receptor-ligand interactions. Cytokine Growth Factor Rev. 2004;15(1):33–48. doi: 10.1016/j.cytogfr.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 20.Abolfathi H, Arabi M, Sheikhpour M. A literature review of microRNA and gene signaling pathways involved in the apoptosis pathway of lung cancer. Respir Res. 2023;24(1):55. doi: 10.1186/s12931-023-02366-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faramin Lashkarian M, Hashemipour N, Niaraki N, et al. MicroRNA-122 in human cancers: from mechanistic to clinical perspectives. Cancer Cell Int. 2023;23(1):29. doi: 10.1186/s12935-023-02868-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomankova T, Petrek M, Kriegova E. Involvement of microRNAs in physiological and pathological processes in the lung. Respir Res. 2010;11(1):159. doi: 10.1186/1465-9921-11-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kara M, Kirkil G, Kalemci S. Differential expression of microRNAs in chronic obstructive pulmonary disease. Adv Clin Exp Med. 2016;25(1):21–26. doi: 10.17219/acem/28343 [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg SR, Kalhan R. Biomarkers in chronic obstructive pulmonary disease. Transl Res. 2012;159(4):228–237. doi: 10.1016/j.trsl.2012.01.019 [DOI] [PubMed] [Google Scholar]
- 25.Tsikrika S, Dimakou K, Papaioannou AI, et al. The role of non-invasive modalities for assessing inflammation in patients with non-cystic fibrosis bronchiectasis. Cytokine. 2017;99:281–286. doi: 10.1016/j.cyto.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Cui B, Wang Q, et al. Biomarkers for respiratory diseases: present applications and future discoveries. Clin Transl Discovery. 2021;1(1):e11. doi: 10.1002/ctd2.11 [DOI] [Google Scholar]
- 27.Zhu T, Li S, Wang J, et al. Induced sputum metabolomic profiles and oxidative stress are associated with chronic obstructive pulmonary disease (COPD) severity: potential use for predictive, preventive, and personalized medicine. EPMA J. 2020;11(4):645–659. doi: 10.1007/s13167-020-00227-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11(1):54–61. doi: 10.1111/j.1440-1843.2006.00784.x [DOI] [PubMed] [Google Scholar]
- 29.Zsoka W, Ildiko H. Induced sputum analysis: step by step educational aims. Breathe. 2013;9:301–306. doi: 10.1183/20734735.042912 [DOI] [Google Scholar]
- 30.Higham A, Cadden P, Southworth T, et al. Leukotriene B4 levels in sputum from asthma patients. ERJ Open Res. 2016;2(4):00088–2015. doi: 10.1183/23120541.00088-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoshino Y, Soma T, Uchida Y, et al. Treatment resistance in severe asthma patients with a combination of high fraction of exhaled nitric oxide and low blood eosinophil counts. Front Pharmacol. 2022;13:836635. doi: 10.3389/fphar.2022.836635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paggiaro PL, Chanez P, Holz O, et al. Sputum induction. Eur Respir J Suppl. 2002;37:3s–8s. doi: 10.1183/09031936.02.00000302 [DOI] [PubMed] [Google Scholar]
- 34.Yin G, Wu X, Wu Y, et al. Evaluating carbon content in airway macrophages as a biomarker of personal exposure to fine particulate matter and its acute respiratory effects. Chemosphere. 2021;283:131179. doi: 10.1016/j.chemosphere.2021.131179 [DOI] [PubMed] [Google Scholar]
- 35.Sun C-X, Young HW, Molina JG, et al. A protective role for the A1 adenosine receptor in adenosine-dependent pulmonary injury. J Clin Invest. 2005;115(1):35–43. doi: 10.1172/jci22656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dahl R, Titlestad I, Lindqvist A, et al. Effects of an oral MMP-9 and −12 inhibitor, AZD1236, on biomarkers in moderate/severe COPD: a randomised controlled trial. Pulm Pharmacol Ther. 2012;25(2):169–177. doi: 10.1016/j.pupt.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 37.Braido F, Riccio AM, Guerra L, et al. Clara cell 16 protein in COPD sputum: a marker of small airways damage? Respir Med. 2007;101(10):2119–2124. doi: 10.1016/j.rmed.2007.05.023 [DOI] [PubMed] [Google Scholar]
- 38.Bi J, Min Z, Yuan H, et al. PI3K inhibitor treatment ameliorates the glucocorticoid insensitivity of PBMCs in severe asthma. Clin Transl Med. 2020;9(1):22. doi: 10.1186/s40169-020-0262-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qaisar R, Karim A, Muhammad T. Circulating biomarkers of handgrip strength and lung function in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:311–321. doi: 10.2147/COPD.S225765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rong B, Fu T, Gao W, et al. Reduced serum concentration of CC16 is associated with severity of chronic obstructive pulmonary disease and contributes to the diagnosis and assessment of the disease. Int J Chron Obstruct Pulmon Dis. 2020;15:461–470. doi: 10.2147/COPD.S230323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam DC, Kwok HH, Yu WC, et al. CC16 levels correlate with cigarette smoke exposure in bronchial epithelial cells and with lung function decline in smokers. BMC Pulm Med. 2018;18(1):47. doi: 10.1186/s12890-018-0607-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerra S, Halonen M, Vasquez MM, et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med. 2015;3(8):613–620. doi: 10.1016/s2213-2600(15)00196-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laucho-Contreras ME, Polverino F, Tesfaigzi Y, et al. Club Cell Protein 16 (CC16) augmentation: a potential disease-modifying approach for Chronic Obstructive Pulmonary Disease (COPD). Expert Opin Ther Targets. 2016;20(7):869–883. doi: 10.1517/14728222.2016.1139084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knabe L, Fort A, Chanez P, Bourdin A. Club cells and CC16: another “smoking gun”? (With potential bullets against COPD). Eur Respir J. 2015;45(6):1519–1520. doi: 10.1183/09031936.00010515 [DOI] [PubMed] [Google Scholar]
- 45.Zhu L, Di PY, Wu R, Pinkerton KE, Chen Y. Repression of CC16 by cigarette smoke (CS) exposure. PLoS One. 2015;10(1):e0116159. doi: 10.1371/journal.pone.0116159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilette C, Godding V, Kiss R, et al. Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(1):185–194. doi: 10.1164/ajrccm.163.1.9912137 [DOI] [PubMed] [Google Scholar]
- 47.Mercer PF, Shute JK, Bhowmik A, et al. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res. 2005;6(1):151. doi: 10.1186/1465-9921-6-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chukowry PS, Spittle DA, Turner AM. Small airways disease, biomarkers and COPD: where are we? Int J Chron Obstruct Pulmon Dis. 2021;16:351–365. doi: 10.2147/COPD.S280157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wells JM, Parker MM, Oster RA, et al. Elevated circulating MMP-9 is linked to increased COPD exacerbation risk in SPIROMICS and COPDGene. JCI Insight. 2018;3(22). doi: 10.1172/jci.insight.123614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Archambault AS, Poirier S, Lefebvre JS, et al. 20-Hydroxy- and 20-carboxy-leukotriene (LT) B(4) downregulate LTB(4) -mediated responses of human neutrophils and eosinophils. J Leukoc Biol. 2019;105(6):1131–1142. doi: 10.1002/JLB.MA0718-306R [DOI] [PubMed] [Google Scholar]
- 51.Laucho-Contreras ME, Polverino F, Gupta K, et al. Protective role for club cell secretory protein-16 (CC16) in the development of COPD. Eur Respir J. 2015;45(6):1544–1556. doi: 10.1183/09031936.00134214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhai J, Insel M, Addison KJ, et al. Club cell secretory protein deficiency leads to altered lung function. Am J Respir Crit Care Med. 2019;199(3):302–312. doi: 10.1164/rccm.201807-1345OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tashkin DP, Wechsler ME. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:335–349. doi: 10.2147/copd.S152291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.David B, Bafadhel M, Koenderman L, De Soyza A. Eosinophilic inflammation in COPD: from an inflammatory marker to a treatable trait. Thorax. 2021;76(2):188–195. doi: 10.1136/thoraxjnl-2020-215167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barnes PJ. Inflammatory endotypes in COPD. Allergy. 2019;74(7):1249–1256. doi: 10.1111/all.13760 [DOI] [PubMed] [Google Scholar]
- 56.Almuntashiri S, Zhu Y, Han Y, et al. Club cell secreted protein CC16: potential applications in prognosis and therapy for pulmonary diseases. J Clin Med. 2020;9(12):4039. doi: 10.3390/jcm9124039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shijubo N, Itoh Y, Yamaguchi T, et al. Clara cell protein-positive epithelial cells are reduced in small airways of asthmatics. Am J Respir Crit Care Med. 1999;160(3):930–933. doi: 10.1164/ajrccm.160.3.9803113 [DOI] [PubMed] [Google Scholar]
- 58.Chen LC, Zhang Z, Myers AC, Huang SK. Cutting edge: altered pulmonary eosinophilic inflammation in mice deficient for Clara cell secretory 10-kDa protein. J Immunol. 2001;167(6):3025–3028. doi: 10.4049/jimmunol.167.6.3025 [DOI] [PubMed] [Google Scholar]
- 59.Johansson S, Andersson K, Wennergren G, Wenneras C, Rudin A. CC16 inhibits the migration of eosinophils towards the formyl peptide fMLF but not towards PGD2. Inflammation. 2009;32(2):65–69. doi: 10.1007/s10753-008-9103-1 [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Long XB, Cao PP, et al. Clara cell 10-kD protein suppresses chitinase 3-like 1 expression associated with eosinophilic chronic rhinosinusitis. Am J Respir Crit Care Med. 2010;181(9):908–916. doi: 10.1164/rccm.200904-0597OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X, Guerra S, Ledford JG, et al. Low CC16 mRNA expression levels in bronchial epithelial cells are associated with asthma severity. Am J Respir Crit Care Med. 2022;206(12):1534–1545. doi: 10.1164/rccm.202206-1230OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones PW. St. George’s respiratory questionnaire: MCID. Copd. 2005;2(1):75–79. doi: 10.1081/copd-200050513 [DOI] [PubMed] [Google Scholar]
- 63.Baldomero AK, Siddiqui M, Lo CY, et al. The relationship between oral health and COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2019;14:881–892. doi: 10.2147/COPD.S194991 [DOI] [PMC free article] [PubMed] [Google Scholar]