Abstract
Background
In Burkina Faso, suspicions have been raised that hospital liquid effluents are a source of microbiological contaminants in surface waters of urban and peri-urban areas. This study aimed to determine the antibiotic residues and the antibiotic resistance phenotype of potential pathogenic bacteria in the hospital liquid effluents discharged into nature by the CHUs Bogodogo, Yalgado Ouédraogo and the WWTS of Kossodo.
Methods
Fifteen samples of liquid effluents discharged into nature were collected. Antibiotic residues were identified by HPLC. A wavelength of 254 nm for the UV detector was set. Antibiotic testing was realized according to CASFM 2019 recommendations.
Results
Three molecules including Amoxicillin, Chloramphenicol and Ceftriaxone were detected in 13 samples. The strains characterized were 06 E. coli, 09 Pseudomonas spp, 05 Staphylococcus aureus and 04 Salmonella spp. Thus, none of the strains was resistant to Imipenem, but they were resistant to Amoxiclav with rates of 83.33% (E. coli), 88.88% (Pseudomonas spp) and 100% (Staphylococcus aureus and Salmonella spp).
Conclusion
Ouagadougou hospital liquid effluents discharged into nature are contaminated with antibiotic residues and potential pathogenic bacteria.
Keywords: wastewater, antibiotics residues, antibiotics resistance, Ouagadougou
Introduction
The emergence of micro-pollutants and pathogenic bacteria in nature is a public health problem that several global health actors are trying to curb.1,2 Indeed, surface waters receive daily from urban discharges generally very loaded with contaminants including resistant pathogenic bacteria, antibiotic residues and metallic trace elements represent potential threats to biodiversity and source of infection for users of the area’s receivers.3,4 Among these sources of pollution, hospital liquid effluents occupy a special place from the point of view of the diversity of antibiotic residues and multi-resistant bacteria that they would harbor.5–7 Indeed, it has been strong to note by several authors around the world, a multitude of residual contaminants of antibiotics, including beta-lactams, aminoglycosides, quinolones, etc., in hospital liquid effluents.6,8–11 In addition, other investigations have reported in hospital liquid effluents multi-resistant and pathogenic bacteria, predominantly E. coli, Pseudomonas, Staphylococcus aureus and Salmonella, which are also the most involved in human and animal pathologies.12–14 Thus, mismanagement of hospital liquid effluents would constitute major sources of pollution with antibiotic residues and developing pathogenic bacteria in receiving areas.15 This could promote high risks of waterborne infections and the dissemination of forms of environmental bacterial multi-resistance to antibiotics in the human and animal community of these areas receiving said effluents.2,7 However, in the southern Saharan zone, this would have serious consequences because the scarcity and the very high cost of drinking water have led to choices to develop market gardening activities, less intensive fishing, watering for livestock around the waters. Wastewater generated by urban activities.6,16 Mismanagement of these effluents would have even more negative impacts in landlocked countries such as Burkina Faso. In fact, in Burkina Faso, water resources are even rarer than in coastal countries. Thus, the use of wastewater from large Burkinabe urban centers such as Ouagadougou, Bobo Dioulasso has become the first option in activities such as market gardening, watering animals in these urban centers and their outskirts.17,18 However, during its routine wastewater analyzes National Office for Water and Sanitation (ONEA), in charge of water management in Burkina Faso, only carries out few parameters including COD, BOD, pH, temperature and conductivity. In addition, these checks are carried out only on a few sites whose liquid effluents from very few hospitals are monitored. On the other hand, several investigations have reported microbial contaminants. The predominant one’s being E. coli, S. aureus and genus Pseudomonas and Salmonella in market gardening products, pipe water and water from dams in the city of Ouagadougou and its peripheral areas.16,18–22 However, suspicions have been raised that microbial contaminants originating from hospital liquid effluents are at the root of the soiling of these vegetables and these surface waters. Investigation’s purpose was to provide microbial and residual contaminants’ first information. In Ouagadougou, microbial and residual contaminants can abound University Hospitals liquid’s discharges and mixed treatment plants generated in nature. Thus, the objective of this study was to determine the antibiotic residues and the phenotype of antibiotic resistance of bacterial isolated in the liquid effluents coming from the CHU (Bogodogo and Yalgado Ouédraogo) and the WWTS of Kossodo at Ouagadougou.
Materials and Methods
Sampling
A total of fifteen liquid effluent samples were collected between October 2019 and October 2020 with the support of the ONEA sampling team from Paspanga on the sites of CHU Yalgado Ouédraogo (CHU-YO), CHU of Bogodogo (CHU-BOG) and of the Kossodo wastewater treatment plant (WWTS-KOS); due to five samples per sampling site. The samples were packaged in one liter bottles before being transported in a cooler to the laboratory in less than two hours. The samples were taken in duplicate. Some were used for the determination of antibiotic residues and the others for the determination of microbiological parameters. The vials whose contents should be used for the dosage of antibiotic residues were washed well beforehand, rinsed with 12% hydrochloric acid and distilled water then rolled up in aluminum foil before being autoclaved. However, the other vials were not acid-treated. Only samples that were sent to the laboratory before two hours were included.
Detection of Antibiotic Residues by UV and Fluorescence HPLC
According to the modified protocol of Mushtak,23 antibiotic residue determination analyzes were performed with an HPLC “Agilent technologie 1260 infinty” mini compartments with UV and flora detection. The HPLC was coupled to a computer which generated the residue detection spectra using the software “CHEMSTATION”.
Mobile and Stationary Phases
Two mobile phases were chosen, including acetonitrile at entry A and a 0.1% acetic acid solution at entry B. The 0.1% acetic acid solution served as blank and eluent. As for the stationary phase, it was a PHENOMENEX column (C18, 5μ, 100A) of dimensions 250×4.60 mm 5μ.
Sample Preparation
A test portion of 50 mL per sample was filtered first through a glass filter with a porosity of 1.6 µm and then a second time through a filter with a porosity of 0.7 µm. The filtrates were brought to an oven at 100°C until complete evaporation. The salts obtained were removed from the oven and left under ambient laboratory conditions until cooling. Thus, 2.5 mL of the 0.1% acetic acid solution was used to elute the salts from each sample. The solutions obtained were well homogenized with a vortex before being brought to the HPLC for the analyses.
Analysis Conditions
The rates of 3% and 97% for the mobile phase have been configured, respectively, for entry entries A and B. Thus, a test sample of 20µL of the solutions to be analyzed has been fixed. Also, a temperature of 40°C for the column and a wavelength of 254 nm for the UV detector were set. In total, 06 antibiotic standards (Table 1) were used to search for the same molecules in the 15 samples.
Table 1.
List of Antibiotic Standards Used
| Molecule Name | Purity | Control Number |
|---|---|---|
| Amoxicillin trihydraty | 87.5% | WS/AT/21/014 |
| Ciprofloxacin hydrochlorid | 97.4% | 197210 |
| Chloramphénicol 200mg | 99.7% | 1107004 |
| Ceftriaxon | 99.8% | 3051912033 |
| Cefixim | 99.56% | 10000421N |
| Tobramycin | 99.7% | 20051045001 |
Enumeration and Characterization of Microbial Contaminants in Liquid Effluents
The microbiological analyzes were carried out following the standards used. Thus, the enumeration of the total mesophilic aerobic flora (FAMT) was carried out using ISO 7218 (2007) standard procedures. Total coliforms and thermo-tolerant coliforms were counted, respectively, with the normative recommendations ISO 9308 (2014) and V08-060 (2009). Preliminary isolation and characterization of E. coli, Salmonella spp, Staphylococcus aureus and Pseudomonas spp. were carried out following, respectively, the normative recommendations ISO 16649–2 (2001), NF ISO 6579 (2002), ISO 6888–3 (2003) and NF 12780 (2002). Strain identities were confirmed by API 20 NE kit (bioMeéreux®, France) for Pseudomonas and API 20E kit (bioMeéreux®, France) for E. coli and Salmonella. As for the presumed Staphylococcus aureus strains, they were confirmed by tests for catalase, DNase, coagulase and Gram.
Antibiotic Resistance Phenotype of Identified Bacteria
The susceptibility of bacterial strains was assessed with nineteen (19) types of antibiotics. The choices of these antibiotic molecules were made on the sole recommendations of CA-SFM 2019. The antibiotics were as follows: Amoxicillin + Clavulanic Acid (20/10 μg), Cefoxitin (30 μg), Ceftazidim (10 μg), Cefepim (30μg), Tobramycin (10μg), Gentamicin (10μg), Nalidixic Acid (30μg), Norfloxacin (10μg), Ciprofloxacin (5μg), Chloramphenicol (30μg), Colistin (50μg), Imipenem (10 μg), Vancomycin (30 μg), Oxacillin (5 μg), Penicillin G (10 μg), Ceftriaxon (30 μg), Kanamycin (30 μg), Tetracyclin (30 μg) and Fosfomycin (200 μg). E. coli strain ATCC 25922 was used for quality control of antibiotics.
Statistical Analyzes
The sphinx V5 software was used to process the bacterial flora count data and the distribution of the residual and microbial components. Means and standard deviations were compared by Student’s t-test with significance p < 0.05. Different samples principal components’ analysis was carried out by the matrix of correlations.
SPSS version.20 software was used to process statistical data on the susceptibility of strains to antibiotics and the correlation between samples. The means of the distributions of the bacterial strains (according to the sampling site) were compared by ANOVA and the cross table was generated using the Pearson correlation test with a significance of P < 0.05. The comparison of the resistance forms in the characterized bacteria was carried out by dendrograms generated on the official website of D-UPGMA (http://genomes.urv.cat/UPGMA/).
Results
Antibiotic Residues in Samples
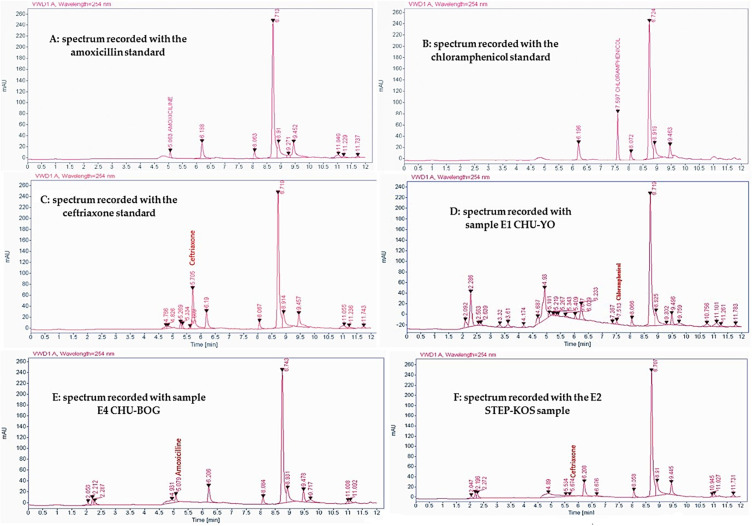
Six molecules of antibiotics (Table 1) were sought in the 15 samples analyzed. Three molecules including amoxicillin, chloramphenicol and ceftriaxone were detected. However, under experimental conditions, amoxicillin, chloramphenicol and ceftriaxone were identified, respectively, at 5.06 min, 7.59 min and 5.70 min with margins of ± 0.05 min (Figure 1). Thus, 13 samples out of the 15 analyzed were contaminated with antibiotic residues. Table 2 shows the distribution of antibiotic residues detected in the different samples. According to this distribution, residual contaminants were predominated by amoxicillin detected in 10 samples, followed by ceftriaxone identified in 03 harbored and chloramphenicol found in 02 contained.
Figure 1.
Antibiotic residue detection spectra.
Note: A: amoxicillin standard spectrum; B: Chloramphenicol standard spectrum; C: Ceftriaxone standard spectrum; D: chloramphenicol detection spectrum in E1 CHU-YO sample; E: Amoxicillin detection spectrum E4 CHU-BOG sample; F: Ceftriaxone detection spectrum E2 STEP-KOS sample.
Table 2.
Distribution of Antibiotic Residues Detected in Samples
| Sites | Samples | Antibiotics | ||
|---|---|---|---|---|
| STEP-KOS | KOS1 | |||
| KOS2 | ||||
| KOS3 | ||||
| KOS4 | ||||
| KOS5 | ||||
| CHU-BOG | BOG1 | |||
| BOG2 | ||||
| BOG3 | ||||
| BOG4 | ||||
| BOG5 | ||||
| CHU-YO | YO1 | |||
| YO2 | ||||
| YO3 | ||||
| YO4 | ||||
| YO5 | ||||
Note: Red: Contaminated by amoxicillin; Blue: Contaminated by Chloramphenicol; Yellow: Contaminated by Ceftriaxon and White: Uncontaminated
Results of Analyzed Liquid Effluents’ Bacterial Flora Count
Table 3 presents the detailed results of the counts and analyzes of the liquid effluents’ the microbial flora from the various sites. The mean values of FAMT were 12.89±1.52x109, 12.46±0.53x109 and 11.82±0.95x109 UFC/mL from WWTS-KOS, CHU-BOG and CHU-YO, respectively. CT were 11.42±1.13x108, 10.34±3.58x108 and 11.49±4.35x108 UFC/mL, respectively. CTT were 12.28±1.01x107, 11.73±1.41x107 and 10.83±2.21x107 UFC/mL, respectively. Statistical analyzes showed no significant difference between the values of each parameter assessed at the three sites (p < 0.05).
Table 3.
Liquid Effluents Detailed Results on the Count and Analysis of the Bacterial Flora
| Sites | Sample Code | FAMT (UFC/mL) | CT (UFC/mL) | CTT (UFC/mL) | |||
|---|---|---|---|---|---|---|---|
| Direct Count (10)9 | Average Generated (10)9 | Direct Count (10)8 | Average Generated (10)8 | Direct Count (10)7 | Average Generated (10)7 | ||
| STEP-KOS | E1 STEP-KOS | 15.27 | 12.89 ±1.52 | 11.53 | 11.42 ±1.13 | 11.26 | 12.28 ±1.01 |
| E2 STEP-KOS | 11.45 | 11.05 | 11.72 | ||||
| E3 STEP-KOS | 13.50 | 13.22 | 12.19 | ||||
| E4 STEP-KOS | 12.32 | 10.96 | 12.30 | ||||
| E5 STEP-KOS | 11.93 | 10.37 | 13.94 | ||||
| CHU-BOG | E1 CHU-BOG | 13.38 | 12.46 ±0.53 | 12.00 | 10.34 ±3.58 | 10.89 | 11.73 ±1.41 |
| E2 CHU-BOG | 12.38 | 12.19 | 10.98 | ||||
| E3 CHU-BOG | 12.19 | 10.98 | 10.59 | ||||
| E4 CHU-BOG | 12.03 | 4.00 | 12.19 | ||||
| E5 CHU-BOG | 12.33 | 12.50 | 14.00 | ||||
| CHU-YO | E1 CHU-YO | 11.36 | 11.82 ±0.95 | 4.00 | 11.49 ±4.35 | 10.89 | 10.83 ±2.21 |
| E2 CHU-YO | 12.30 | 15.09 | 11.95 | ||||
| E3 CHU-YO | 10.52 | 11.88 | 7.00 | ||||
| E4 CHU-YO | 11.88 | 12.90 | 12.03 | ||||
| E5 CHU-YO | 13.06 | 13.60 | 12.30 | ||||
Antibiotic Resistance Phenotype of Identified Bacterial Strains
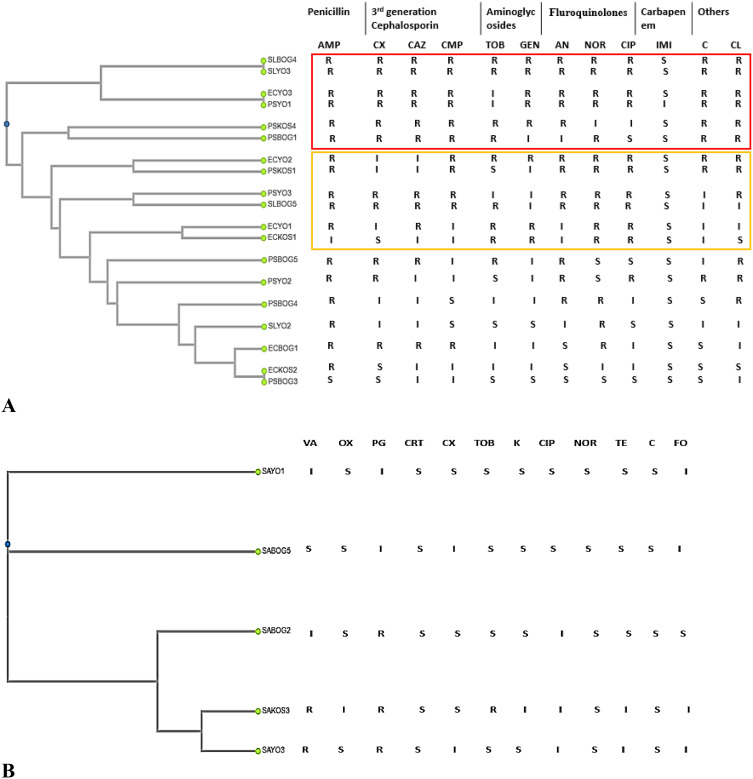
In total, twenty-four (24) strains of E. coli, Pseudomonas spp, Salmonella spp and Staphylococcus aureus were identified in the fifteen (15) samples. These strains were distributed in the liquid effluents of WWTS-KOS (2 E. coli, 2 Pseudomonas spp and 1 Staphylococcus aureus); CHU-BOG (1 E. coli, 4 Pseudomonas spp, 2 Salmonella spp and 2 Staphylococcus aureus) and CHU-YO (3 E. coli, 3 Pseudomonas spp, 2 Salmonella spp and 2 Staphylococcus aureus). These strains showed different resistance phenotypes to antibiotic activities. Figure 2 shows dendrograms that illustrate the results of the susceptibility of the bacterial strains to the various antibiotics tested and the nodes represent the phenotypes of resistance to the closest antibiotics. For further details on strain identity please see additional material. Thus, none of the E. coli, Pseudomonas spp and Salmonella strains was resistant to imipenem but these strains were more resistant to amoxiclav (amoxicillin and clavulanic acid) with respective rates of 83.33%, 88.88% and 100%. On strains of S. aureus, penicillin G was the least effective. The types of resistance did not depend on strain types or sampling site.
Figure 2.
(A) Resistance phenotype of the E. coli, Pseudomonas spp and Salmonella spp strains to the activities of the antibiotics tested and grouping according to the forms of resistance. (B) Resistance phenotype of S. aureus strains to the various antibiotics tested and grouping according to the forms of resistance.  Form of resistance XDR.
Form of resistance XDR.  Form of resistance MDR. S: the antibiotic had an activity on the bacterial strain; R: the antibiotic has no activity on the bacterial strain; I: In this case, antibiotic was also considered to have activity against the bacterial strain.
Form of resistance MDR. S: the antibiotic had an activity on the bacterial strain; R: the antibiotic has no activity on the bacterial strain; I: In this case, antibiotic was also considered to have activity against the bacterial strain.
Abbreviations: ECKOS, E. coli from the Kossodo WWTP; ECYO, E. coli from CHU Yalgado Ouédraogo; ECBOG, E. coli from the University Hospital of Bogodogo; PSKOS, Pseudomonas spp from the Kossodo WWTP; PSYO, Pseudomonas spp from CHU Yalgado Ouédraogo; PSBOG, Pseudomonas spp from the Bogodogo University Hospital; SLKOS, Salmonella spp from the Kossodo WWTP; SLYO, Salmonella spp from CHU Yalgado Ouédraogo; SLBOG, Salmonella spp from the Bogodogo University Hospital; SAKOS, S. aureus from the Kossodo WWTP; SAYO, S. aureus from CHU Yalgado Ouédraogo; SABOG, S. aureus from Bogodogo University Hospital.
Correlation Between Samples According to Their Components
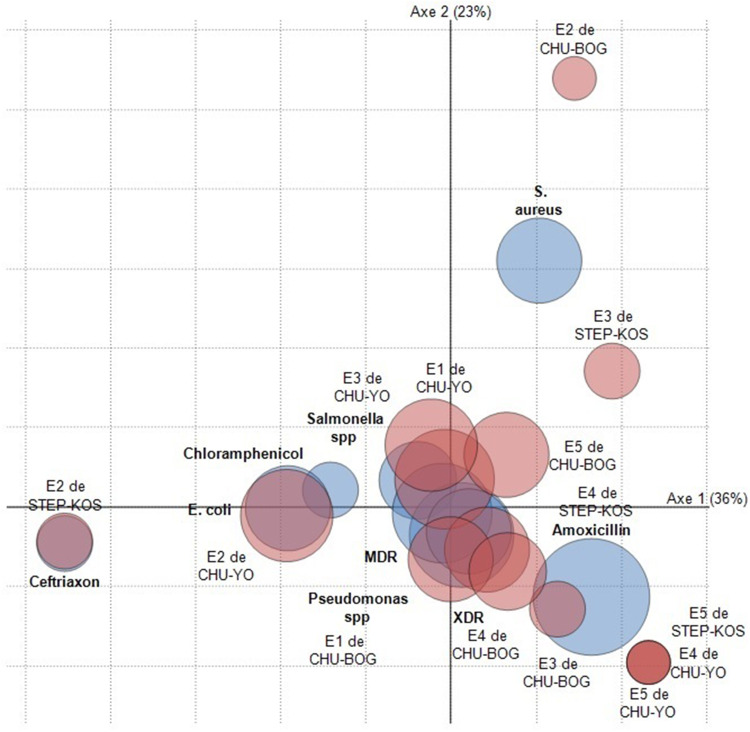
All 15 samples analyzed harbored microbial and residual components (Figure 3). Thus, the statistical analyzes showed high correlation rates between the components of certain samples from the same site; such as the case of samples N°1 and N°3 from the CHU Yalgado Ouédraogo site (E1 and E3 CHU-YO), with a rate of Pearson correlation at 0.798 with a significance of 0.010. Also, strong correlations were observed between samples from different sites; such as the case of sample No.2 from the CHU Bogodogo site (E3 CHU-BOG) with samples No.3 WWTS of Kossodo (E3 WWTS-KOS) with a Pearson’s correlation rate at 0.980 and a significance of 0.000.
Figure 3.
Identified components distribution with their frequencies of appearance in the 15 samples.
Discussion
The search for antibiotic residues in the samples revealed three antibiotic molecules including Amoxicillin, Chloramphenicol and Ceftriaxone. Elsewhere, these molecules and others of the same family have been reported in hospital fluids by several authors.4,24–26 Recently for example, Araba et al highlighted in hospital liquid effluents from Ghana several molecules of Amoxicillin and Chloramphenicol were identified with respective frequencies of 33.30% and 66.70%; then respective average concentrations of 8.76± 0.00 m/L and 1.02±0.39 m/L. These results are explained by the frequent use of these molecules in human therapy and a large part of the doses administered are excreted through the urine or the stool.27,28 In addition, the discharges of certain services such as internal medicine or laboratories for the analysis of pathological products are generally loaded with antibiotic residues which constitute sources of contamination for the common collectors of liquid effluents from hospitals. However, antibiotic residues presence in liquid discharges would constitute emergence and multi-resistant bacteria proliferation risk. Indeed, the non-bactericidal concentration of antibiotic residues in an environment leads to recourse to resistance to antibiotic molecules for the bacteria that colonize this environment.8–28 This would have more impact on the proliferation of multi-resistant bacteria, if microbial hygiene is not respected in the colonized environment.10 Thus, this study was also involved in determining the microbial hygiene of the 15 samples which revealed the presence of strains of Salmonella spp in some samples from the CHU-BOS and CHU-YO sites. However, the Burkinabè standards on the discharge of wastewater into nature fix a total absence of Salmonella in a test sample of 100 mL (DECRET N°2015/1205/PRES/ TRANS/PM/MERH/MEF/MARHASA/MS/MRA/MICA/MME/MIDT/MATD). Elsewhere, subsequent investigations reported contamination of hospital liquid discharges with strains of Salmonella.29–31 Salmonella strains in liquid landfills still pose a potential infection risk to water users in receiving areas.32 In addition, Salmonella strains are among the main stains of porbeagle culture products and responsible for enteric infections in Burkina Faso.20,21 The investigations of this study also showed that the averages of the coliform load varied from 10.83 ± 2.21.107 CFU/mL to 12.28 ± 1.01.107 CFU/mL. These values are much higher than the value of 2000 CFU/100 mL or 20 CFU/mL set by the Burkinabe normative code. The contamination of hospital liquid effluents could be caused by germs originating from patients, accompanying persons or nursing staff. As for thermo-tolerant coliforms, they are potential pathogens and indicators of faecal contamination.33 However, the very high load of thermo-tolerant coliforms in liquid discharges in the environment is a great risk of infection with pathogenic bacteria such as E. coli, Citrobacter, Enterobacter, and Klebsiella for users. To this end, the E. coli strain was characterized at all sites. Notwithstanding that E. coli is part of the commensal flora in humans, but it occupies the first place in enteric and urinary infections in Burkina.34,35 In addition to E. coli (25.00%) and Salmonella spp (16.66%), strains of Pseudomonas spp and S. aureus were characterized with respective prevalence of 37.50% and 20.83% of the 24 bacterial strains identified. Strains of Pseudomonas spp and S. aureus have been reported in hospital liquid effluents by other authors.36,37 S. aureus strains are very famous in skin infections, wounds, and uro-genital. The susceptibility tests of the 24 identified bacterial strains showed that Imipenem was the most effective on E. coli, Pseudomonas spp and Salmonella spp strains and Amoxiclav was the least active on the same strains. Certain authors as Zarfel et al in Australia also reported resistance rates (64.29% to 100%) to penicillins of strains of E. coli identified in liquid effluents discharged into nature. On the other hand, none of their strains was resistant to carbapenem.38 Resistance to beta-lactams is explained by the expression of bla genes which code for beta-lactamases, which are very common in Gram-negative bacilli.10,14 The acquisition of these genes can be facilitated by the transfer of mobile genetic carriers, integrons or plasmids by conjugation between strains of the same species or of different species.11 In addition, of the five S. aureus identified in this study, Penicillin G was less effective with an 80% strain resistance rate, but Oxacillin was active on all strains. Akya et al reported a resistance to Penicillin G with rate of 100% of strains of S. aureus characterized in hospital liquid effluents from Kermanshah in Iran.39 Penicillin G resistance could be explained by the production of penicillinase.40 However, this enzyme has no effect on certain beta-lactams such as Methicillin or Oxacillin. This would justify the activities of Oxacillin on S. aureus strains producing penicillinase.41 Statistical analyzes of strain resistance phenotypes showed tough correlations (Pearson coefficients) of forms of antibiotic resistance between the types of strain identified, on the one hand, and between the sampling sites, on the other hand (Figure 2). Therefore, a form of antibiotic resistance is not linked to a type of bacteria or to the sampling site. Achek et al had the same remarks in S. aureus and coagulase-negative Staphylococcus strains identified in human pathological products and milk in Algeria.42 These would explain the hygiene problems encountered by health systems in Africa in general to control the circulation of pathogenic bacteria and the probable dissemination of the same types of resistance gene between the different bacterial genera.14,43 In this study for example, strains such as SLBOG4 and SLYO3 showed resistance to almost all the antibiotics used except imipenem that allows them to be qualified as XDR bacterial strains. XDR bacterial strains have been staging into hospital liquid effluents by several other authors.10,39 Bacterial multi resistance to antibiotics different families could be explained by the presence in these bacteria of chromosomal or plasma mobile genetic supports; such as integrons, staphylococcal cassette chromosomes that can harbor several resistance genes at the same time. Thus, there are serious concerns about the transmission and spread of these forms of resistance to clinical bacterial strains.2 This would complicate the treatment of bacterial infections before. Especially, in low-income countries like Burkina Faso where the majority of the population already has problems paying medical bills.
Conclusions
This study focused on determining the hygienic quality of liquid effluents directly discharged into the city of Ouagadougou by STEP-KOS, CHU-BOG and CHU-YO. The analyzes revealed contamination of the effluents by residues of amoxicillin, chloramphenicol and ceftriaxone and potential pathogens including E. coli, S. aureus, Pseudomonas spp and Salmonella spp. All the samples presented bacterial floral values higher than those fixed by the Burkinabe environmental code. In addition, penicillins were very weakly active on the characterized bacteria. However, there are concerns about the transmission of MDR and XDR resistance forms to medical strains by those in these effluents.
Acknowledgments
We would like to thank ONEA (Burkina Faso) for supporting us in taking samples and offering the technical platform for bacteriological characterization. To LANAVET (Cameroon), for offering us the technical platform for the search for antibiotic residues in samples. We thank the AFRIDI structure for offering us mobility in Douala, Cameroon.
Funding Statement
This research was funded by AFRIDI structure. The AFRIDI structure financed our stay in Douala (Cameroon) and the payment of a large part of the reagents for the analysis of antibiotic residues.
Declaration of Data
All the detailed data of this manuscript are available from the authors Ouédraogo Ganamé Abasse and Savadogo Aly. For the needs, these data can be provided with the agreement of all the actors of the manuscript to the following Emails: ganamabasse@gmail.com or alysavadogo@gmail.com.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work; Project administration, University Joseph KI-ZERBO and University of Douala.
Disclosure
Mr Ganamé Abasse Ouédraogo reports that this research was funded by AFRIDI structure. The AFRIDI structure financed our stay in Douala (Cameroon) and the payment of a large part of the reagents for the analysis of antibiotic residues, from Projet AFRIDI, outside the submitted work. The authors declare no other conflicts of interest in this work.
References
- 1.WHO/FAO/OIE. Suivi mondial des progrès des pays dans la lutte contre la résistance aux antimicrobiens (AMR) [Global tracking of country progress in tackling antimicrobial resistance (AMR)]; 2018. Available from: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/tripartite-antimicrobial-resistance-country-self-assessment-questionnaire-2018-french.pdf?sfvrsn=a58da30c_18&download=true. Accessed April 22, 2023.
- 2.Chinemerem ND, Ugwu MC, Anie CO, et al. Antibiotic resistance: the challenges and some emerging strategies for tackling a global menace. J Clin Lab Anal. 2022;36:1–10. doi: 10.1002/jcla.24655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emmanuel E, Gisèle M, Perrodin Y. Groundwater contamination by microbiological and chemical substances released from hospital wastewater: health risk assessment for drinking water consumers. Environ Int. 2009;35:718–726. doi: 10.1016/j.envint.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 4.Antonio MRC, Imtiaj A, Foon YL, Dawes L, Ricarda T, Rajapakse J. Removal of micropollutants through a biological wastewater treatment plant in a subtropical climate, Queensland-Australia. J Environ Health Sci Eng. 2016;14:1–10. doi: 10.1186/s40201-016-0257-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberle K, Capdeville M, Berthe T, Petit F, Petit F. Evidence for a complex relationship between antibiotics and antibiotic-resistant Escherichia coli: from medical center patients to a receiving environment. Environ Sci Technol. 2012;46:1859–1868. doi: 10.1021/es203399h [DOI] [PubMed] [Google Scholar]
- 6.Araba B, Anima I, Darko G, Sheringham L. Antibiotic and analgesic residues in the environment – occurrence and ecological risk study from the Sunyani municipality, Ghana. Toxicol Rep. 2022;9:1491–1500. doi: 10.1016/j.toxrep.2022.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alduhaidhawi AHM, AlHuchaimi SN, Al- Mayah TA, et al. Prevalence of CRISPR-cas systems and their possible association with antibiotic resistance in enterococcus faecalis and enterococcus faecium collected from hospital wastewater. Infect Drug Resist. 2022;15:1143–1154. doi: 10.2147/IDR.S358248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voigt AM, Faerber HA, Wilbring G, et al. The occurrence of antimicrobial substances in toilet, sink and shower drainpipes of clinical units: a neglected source of antibiotic residues. Int J Hyg Environ Health. 2019;222:455–467. doi: 10.1016/j.ijheh.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 9.Hernández-García M, Pérez-Viso B, Navarro-San CF, et al. Intestinal co-colonization with different carbapenemase-producing Enterobacterales isolates is not a rare event in an OXA-48 endemic area. EClinicalMedicine. 2019;15:72–79. doi: 10.1016/j.eclinm.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le T, Ng C, Chen H, et al. Occurrences and characterization of antibiotic-resistant bacteria and genetic determinants of hospital Wastewater in a Tropical Country. Antimicrob Agents Chemother. 2016;60:7449–7456. doi: 10.1128/AAC.01556-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain S, Naeem M, Chaudhry MN. Estimation of residual antibiotics in pharmaceutical effluents and their fate in affected areas. Pol J Environ Stud. 2016;25:607–614. doi: 10.15244/pjoes/61229 [DOI] [Google Scholar]
- 12.Hocquet D, Muller A, Bertrand X. What happens in hospitals does not stay in hospitals: antibiotic-resistant bacteria in hospital wastewater systems. J Hosp Infect. 2016;16:195–6701. doi: 10.1016/j.jhin.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 13.Radhakrishna L, Nagarajan P. Isolation and preliminary characterization of bacterial from liquid hospital wastes. Int J Pharmtech Res. 2015;8:308–314. [Google Scholar]
- 14.Moges F, Endris M, Belyhun Y, Worku W. Isolation and characterization of multiple drug resistance bacterial pathogens from waste water in hospital and non-hospital environments, Northwest Ethiopia. BMC Res Notes. 2014;7:1–6. doi: 10.1186/1756-0500-7-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basturk I, Varank G, Murat-hocaoglu S, Yazici-guvenc S. Characterization and treatment of medical laboratory Wastewater by Ozonation: optimization of Toxicity removal by central composite design. Ozone Sci Eng. 2020. doi: 10.1080/01919512.2020.1794794 [DOI] [Google Scholar]
- 16.Dao J, Stenchly K, Traoré O, Amoah P, Buerkert A. Effects of water quality and post-harvest handling on microbiological contamination of lettuce at Urban and Peri-Urban Locations of Ouagadougou, Burkina Faso. Food. 2018;7:206. doi: 10.3390/foods7120206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youenou B, Hien E, Deredjian A, Brothier E, Favre-Bonté S, Nazaret S. Impact of untreated urban waste on the prevalence and antibiotic resistance profiles of human opportunistic pathogens in agricultural soils from Burkina Faso. Environ Sci Pollut Res. 2016;23:25299–25311. doi: 10.1007/s11356-016-7699-5 [DOI] [PubMed] [Google Scholar]
- 18.Nitiema LW, Savadogo B, Zongo D, et al. Microbial quality of wastewater used in Urban Truck Farming and Health Risks Issues in Developing Countries: case Study of Ouagadougou in Burkina Faso. J Environ Prot. 2013;4:575–584. doi: 10.4236/jep.2013.46067 [DOI] [Google Scholar]
- 19.Somda SN, Bonkoungou IJO, Sambe-ba B, et al. Diversity and antimicrobial drug resistance of non-typhoid Salmonella serotypes isolated in lettuce, irrigation water and clinical samples in Burkina Faso. J Agric Food Inf. 2021;5:10067. doi: 10.1016/j.jafr.2021.100167 [DOI] [Google Scholar]
- 20.Soré S, Sawadogo Y, Bonkoungou JIO, et al. Detection, identification and characterization of extended-spectrum beta- lactamases producing Enterobacteriaceae in wastewater and salads marketed in Ouagadougou, Burkina Faso. Int J Chem Biol Sci. 2020;8:14. [Google Scholar]
- 21.Kagambèga A, Bouda SC, Bako E, Cissé H, Barro N, Haukka K. Diversity and antimicrobial resistance of Salmonella strains isolated from different sources in Burkina Faso. Afr J Microbiol Res. 2017;11:1495–1504. doi: 10.5897/AJMR2017.8698 [DOI] [Google Scholar]
- 22.Traoré O, Nyholm O, Siitonen A, Bonkoungou IJO, Traoré AS, Barro N. Prevalence and diversity of Salmonella enterica in water, fish and lettuce in Ouagadougou, Burkina Faso. BMC Microbiol. 2015;15:1–7. doi: 10.1186/s12866-015-0484-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Ouqaili MTS, Musleh MH, Al-Kubaisi SMA. Depending on HPLC and PCR, detection of aflatoxin B1 extracted from Aspergillus flavus strains and it’s cytotoxic effect on AFB treated-hematopoietic stem cells obtained from human umbilical cord. Asian J Pharm. 2018;12:S1048–S1054. [Google Scholar]
- 24.Alexy R, Ku T, Ku K. Assessment of degradation of 18 antibiotics in the closed bottle test. Chemosphere. 2004;57:505–512. doi: 10.1016/j.chemosphere.2004.06.024 [DOI] [PubMed] [Google Scholar]
- 25.Längin A, Alexy R, König A, Kümmerer K. Deactivation and transformation products in biodegradability testing of ß-lactams amoxicillin and Piperacillin. Chemosphere. 2009;75:347–354. doi: 10.1016/j.chemosphere.2008.12.032 [DOI] [PubMed] [Google Scholar]
- 26.Heidari M, Kazemipour M, Bina B, et al. A qualitative survey of five antibiotics in a water treatment plant in central plateau of Iran. J Environ Public Health. 2013;2013:1–9. doi: 10.1155/2013/351528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verlicchi P, Galletti A, Petrovic M, Barceló D. Hospital effluents as a source of emerging pollutants: an overview of micropollutants and sustainable treatment options. J Hydrol. 2010;389:416–428. doi: 10.1016/j.jhydrol.2010.06.005 [DOI] [Google Scholar]
- 28.Sosa-Hernández JE, Rodas-Zuluaga LI, Lopez-Pacheco IY, et al. Sources of antibiotics pollutants in the aquatic environment under SARS-CoV-2 pandemic situation. Case Stud. J Environ Chem Eng. 2021;4:100127. doi: 10.1016/j.cscee.2021.100127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatt P, Mathur N, Anuradha S, Pareek H, Bhatnagar P. Evaluation of factors influencing the environmental spread of pathogens by Wastewater Treatment Plants. Water Air Soil Pollut. 2020;231:1–4. doi: 10.1007/s11270-020-04807-4 [DOI] [Google Scholar]
- 30.Bírošová L, Lépesová K, Grabic R, Mackuľak T. Non-antimicrobial pharmaceuticals can affect the development of antibiotic resistance in hospital wastewater. Environ Sci Poll Res. 2020;27:13501–13511. doi: 10.1007/s11356-020-07950-x [DOI] [PubMed] [Google Scholar]
- 31.Kemprai L, Hussain P, Kader NA, et al. A study on some aspects of bacteriological qualities of hospital wastewater in and around Guwahati city. Pharm Innov J. 2021;10:1179–1183. [Google Scholar]
- 32.Asfaw T. Review on hospital wastewater as a source of emerging drug resistance pathogens. J Res Environ Sci Toxicol. 2018;7:47–52. doi: 10.14303/jrest.2018.020 [DOI] [Google Scholar]
- 33.Abdel-mohsein HS, Feng M, Fukuda Y, Tada C. Remarkable removal of antibiotic-resistant bacteria during dairy wastewater treatment using hybrid full-scale constructed wetland. Water Air Soil Pollut. 2020;231:397. doi: 10.1007/s11270-020-04775-9 [DOI] [Google Scholar]
- 34.Bonkoungou IJO, Haukka K, Österblad M, et al. Bacterial and viral etiology of childhood diarrhea in Ouagadougou, Burkina Faso. BMC Pediatr. 2013;13:2–7. doi: 10.1186/1471-2431-13-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouédraogo A Prévalence, circulation et caractérisation des bactéries multirésistantes au Burkina Faso. Université de Montpellier; 2016:191. Available from: https://theses.hal.science/tel-01476152/file/2016_OUEDRAOGO_archivage.pdf. Accessed November 03, 2022. [Google Scholar]
- 36.Cai L, Sun J, Yao F, et al. Antimicrobial resistance genes and bacteria detected in hospital sewage may provide valuable information in clinical antimicrobial resistance. Res Squ. 2020;1–16. doi: 10.21203/rs.3.rs-93424/v1 [DOI] [PubMed] [Google Scholar]
- 37.Zagui GS, Andrade LN, Moreira NC, et al. Gram-negative bacteria carrying β-lactamase encoding genes in hospital and urban wastewater in Brazil. Environ Monit Assess. 2020;376:192. doi: 10.1007/s10661-020-08319-w [DOI] [PubMed] [Google Scholar]
- 38.Zarfel G, Lipp M, Gürtl E, Folli B, Baumert R, Kittinger C. Troubled water under the bridge: screening of River Mur water reveals dominance of CTX-M harboring Escherichia coli and for the first time an environmental VIM-1 producer in Austria. Sci Total Environ. 2017;593–594:399–405. doi: 10.1016/j.scitotenv.2017.03.138 [DOI] [PubMed] [Google Scholar]
- 39.Akya A, Chegenelorestani R, Shahvaisi-Zadeh J, Bozorgomid A. Antimicrobial resistance of staphylococcus aureus isolated from hospital wastewater in Kermanshah, Iran. Risk Manag Healthc Policy. 2020;13:1035–1042. doi: 10.2147/RMHP.S261311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolte J, Zhang Y, Wente N, Mahmmod YS, Svennesen L, Krömker V. Comparison of phenotypic and genotypic antimicrobial resistance patterns associated with Staphylococcus aureus mastitis in German and Danish dairy cows. J Dairy Sci. 2020;103:3554–3564. doi: 10.3168/jds.2019-17765 [DOI] [PubMed] [Google Scholar]
- 41.Shittu AO, Okon K, Adesida S, et al. Antibiotic resistance and molecular epidemiology of Staphylococcus aureus in Nigeria. BMC Microbiol. 2011;11:92. doi: 10.1186/1471-2180-11-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Achek R, Hotze H, Cantekin Z, et al. Emerging of antimicrobial resistance in staphylococci isolated from clinical and food samples in Algeria. BMC Res Notes. 2018;11:1–7. doi: 10.1186/s13104-018-3762-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pekana A, Green E. Antimicrobial resistance profiles of staphylococcus aureus isolated from meat carcasses and bovine milk in abattoirs and dairy farms of the Eastern Cape, South Africa. Int J Environ Res Public Health. 2018;15:2223. doi: 10.3390/ijerph15102223 [DOI] [PMC free article] [PubMed] [Google Scholar]