Abstract
Background:
Having low-income limits one’s ability to purchase foods that are high in nutritional value (e.g. vegetables and fruits (V/F)). Higher V/F intake is associated with less diet-related chronic disease. Food pharmacy programs are potential solutions to providing V/F to low-income populations with or at-risk for chronic disease.
Aim:
This systematic review aimed to determine the effect of food pharmacy programs, including interventions targeting populations at-risk for chronic disease.
Methods:
We searched Pubmed and Google Scholar databases for studies reporting on food pharmacy interventions and outcomes (hemoglobin A1c, body mass index (BMI), V/F intake, and blood pressure). We calculated pooled mean differences using a random-effects model. Seventeen studies met our inclusion criteria; 13 studies used a pre/post study design, three used a randomized controlled trial, and one was a post-survey only.
Results:
We found that the pooled mean daily servings of V/F (0.77; 95% CI: 0.30 to 1.24) was higher and BMI (−0.40; 95% CI: −0.50 to −0.31) was lower with food pharmacy interventions We did not find any differences in the pooled mean differences for hemoglobin A1c or systolic blood pressure.
Conclusion:
Findings posit that food pharmacy programs delivered to primarily low-income individuals with comorbidities may be a promising solution to improving V/F intake and possibly overall diet in these populations.
Keywords: Food pharmacy, food prescription, fruit and vegetable, meta-analysis
Introduction
Chronic conditions, those lasting longer than four years, impacting individual functionality, and requiring routine medical treatment, are highly prevalent in the United States (US). Approximately 45% of all Americans have at least one chronic disease, and the prevalence continues to increase (Raghupathi and Raghupathi, 2018). Chronic diseases, such as cancer, diabetes, cardiovascular disease, hypertension, and obesity lead to long-term reductions in quality of life and are among the leading causes of death in the US. In addition, chronic diseases are extremely costly to the nation’s economic and healthcare systems, costing the US economy more than a trillion dollars annually (GRAF, 2018) and accounting for almost 75% of aggregate healthcare spending, according to the Centers for Disease Control and Prevention (Centers for Disease Control and Prevention, 2009).
Several risk factors, including inadequate nutrition and physical inactivity, have been cited as factors contributing to the increasing the prevalence of the aforementioned chronic diseases. Food insecurity, defined by the United States Department of Agriculture (USDA) as the lack of reliable access to an adequate supply of nutritious food to support a healthy lifestyle (US Department of Agriculture, 2019), has more recently been associated in a growing body of literature with a higher risk of diet-related chronic disease and poorer disease management (Gregory and Coleman-Jensen, 2017; Laraia, 2013).
A 2017 report by the USDA of 41,854 working-age adults living below 200 percent of the Federal poverty line examined 10 chronic health conditions (asthma, arthritis, cancer, chronic obstructive pulmonary disease, coronary heart disease, diabetes, hepatitis, hypertension, kidney disease and stroke) and the role of food insecurity as a risk factor across the range of household food security categorizations. Lower food security (i.e. food insecurity) was associated with a higher risk of all 10 chronic conditions examined, the number of chronic conditions reported, and self-reported health status (Gregory and Coleman-Jensen, 2017). Adequate and healthy diet plays a noteworthy role in chronic disease risk and management. Increased fruit and vegetable intake is known to reduce the incidence of diet-related chronic diseases (Aune et al., 2017; Harsha et al., 1999; Obarzanek et al., 2001; Rolls et al., 2004). However, for many food insecure individuals and families, this is simply not a possibility or a reality, due to the higher costs and limited access (e.g. limited stores selling healthy food options or lack of transportation) to healthy foods, particularly fresh vegetables and fruits (V/F) (Raghupathi and Raghupathi, 2018).
Food pharmacy or food prescription programs (henceforth referred to as ‘food pharmacy programs’ in this manuscript) have emerged as a novel solution to address health status in populations with chronic disease. Food pharmacy programs vary, but often utilize physicians or other health care professionals to identify food insecure or low-income patients with a diet-related chronic disease, such as cancer, diabetes, heart disease, hypertension, and obesity, and recommend or “prescribe” the individual to the program. While varied, the food pharmacy programs essentially provide supplemental fruits and vegetables and other therapeutic foods, which are high in fiber, vitamins, and minerals (Lundeen et al., 2017), to participants, with the aim of improving chronic disease management. Typically, patients can either redeem their food prescription on-site at the clinic or at a partnering food supplier (e.g. local farmer’s markets, grocery stores, or mobile markets), depending on the program. The cost of the food pharmacy program is often subsidized by grants or non-profit organizations, but the cost per patient typically ranges between $10–$50 per week. Food pharmacy programs range in length between weeks to months. There have been several studies that have aimed to evaluate the effectiveness of food pharmacy interventions, but the design and findings vary, and many studies are underpowered to detect change.
In this systematic review, we aimed to determine the effect of food pharmacy programs in the US on chronic disease risk factors and present the findings of the pooled effects of these interventions, discuss limitations, and offer suggestions for future interventions to reduce the burden of chronic disease for low-income populations with chronic disease.
Methods
Search strategy
We performed a systematic search on PubMed and Google Scholar databases. The search on PubMed used the following search details: “‘fruit and vegetable’ prescription program”. The search on Google Scholar used the following terms: “‘fruit and vegetable’, ‘prescription’ program, health center, low-income, intervention, obesity, hypertension, diabetes.”
Inclusion/exclusion criteria
We included original articles that used a V/F food pharmacy program intervention in people with diet-related chronic disease (e.g. obesity, diabetes, hypertension, or cardiovascular disease). Studies included in our review required an intervention component that either used food checks/coupons redeemable for fresh fruits and vegetables or food boxes containing fresh fruits and vegetables. Studies could also include other intervention components as part of the intervention (e.g. education), but needed to have at least one arm that received a food pharmacy component. All interventions included in this analysis aimed to improve health outcomes or increase fruit and vegetable consumption and were conducted in the US. Any study design was eligible for inclusion, as long as studies had an intervention that met our inclusion criteria. We excluded studies that were not peer reviewed, not in English, that we could not access in their entirety, or that did not report a health outcome. We were particularly interested in studies that included low-income individuals, but because there were limited studies on the topic at large, we did not exclude studies if the study population was not primarily low-income. For dissertation/thesis results, we searched to see if the articles had been published, even under a different title, and we tried to contact the author to see about publication updates. To further enrich the number of studies in our review, we reviewed references of all studies that met our inclusion criteria in the initial systematic search. The searches were performed on December 9, 2020.
Data extraction
For each article, we extracted data on the author, year published, comorbidity/ies of the study population, study design, sample size, intervention, duration of intervention, outcome(s), and results for the outcome(s). We focused on four main outcomes: hemoglobin A1c, body mass index (BMI), daily fruit and vegetable servings, and systolic blood pressure. The mean and standard deviations for continuous data and percent for categorical data, for both comparison groups (e.g. pre/post or intervention/control) and the number in each group were extracted for each study. One study reported mean weekly servings of V/F; therefore, we divided the mean servings by seven to get a value for mean daily servings. For the one study that reported V/F in cups per day, we assumed that a serving was one cup. For studies that had multiple interventions (i.e. education vs. nutrition with education vs. control), we only used the intervention arm that included fruits and vegetables or healthy foods as part of the intervention. If education was part of the nutrition intervention, we were not able to assess the effects of nutrition only. For studies that were missing variability data, we contacted the authors to see if they would provide this information. All results were reviewed by two of the authors (AH and JG). PRISMA (Preferred Reporting Items for Systematic Reviews and MetaAnalyses) guidelines were followed.
Quality of studies
We used the Methodological Index for Non-Randomized Studies (MINORS) instrument (Slim et al., 2003) to determine the quality of the included studies. While this was developed for studies on surgical procedures, we felt that the questions were relevant for the topic we were reviewing and allowed for an objective assessment of study quality. We used items 1–8 and 12 (stated aim, inclusion of consecutive patients, prospective data collection, appropriate endpoints, unbiased study endpoints, appropriate follow-up, low loss to follow-up, calculation of study size, and adequate statistical analyses) for all studies. For randomized control trial studies (RCTs), we also used items 9–11 (adequate control group, contemporary group, and baseline equivalence of groups). For quality assessment, all items were coded as “0” for not reported, “1” for reported but inadequate, and “2” for reported and adequate. Scores were summed (1–8 and 12 for pre/post and post only studies and 1–12 for RCTs) for a maximum overall study quality score of 18 for pre/post and post-only studies and 24 for RCTs and standardized by dividing the calculated score by the maximum score. Higher scores indicated better study quality.
Statistical analysis
We calculated medians and ranges of study characteristics. We calculated pooled effects for studies that reported both means and measures of variability for the main outcomes of interest. To calculate the pooled effect size, we first converted standard deviations to standard errors of the mean. We did not have variability measures for one study, so we imputed the missing values with the average reported standard error of the means for all studies reporting on the respective health outcome (e.g. hemoglobin A1c or fruit and vegetable consumption). Because of unknown variability in our studies, we calculated pooled mean differences for the main outcomes using a random-effects model with the Hartung-Knapp-Sidik-Jonkman method (IntHout et al., 2014). We did a pooled analysis for outcomes that had at least three studies reporting on them. We used the I2 index to assess heterogeneity, and we used Egger’s test to assess publication bias. The interpretation of I2 values were based on Cochrane categorization: 30–60% represents moderate heterogeneity and >75% represents considerable heterogeneity (Higgins et al., 2021). The pooled effect sizes, forest plots, and corresponding statistical tests were generated using the meta package of R. All analyses were conducted using R, version 3.6.1. As a sensitivity analysis, we also calculated pooled estimates for studies that did not include an educational component or adjusted for education in their study design. We used data that were publicly available and that did not include patient-identifiable data. Therefore, we did not require oversight by an Institutional Review Board, in accordance with 45 CFR §46.102(f). The protocol for this systematic review and meta-analysis was not pre-registered with PROSPERO, nor has there been a protocol paper published previously.
Results
The searches produced 20 results in PubMed and 347 results in Google Scholar. We excluded 238 that did not include an intervention of any type, 62 that were not food pharmacy interventions, 18 that were review articles, 18 that did not report health outcomes, seven that reported baseline results only, three that were dissertations with no corresponding peer-reviewed article, and three that did not report quantitative results. We further removed seven articles because they were duplicates of other included articles. We included six articles that we found during a search of the article references. In total, we found 17 articles that met our inclusion criteria (Figure 1).
Figure 1.
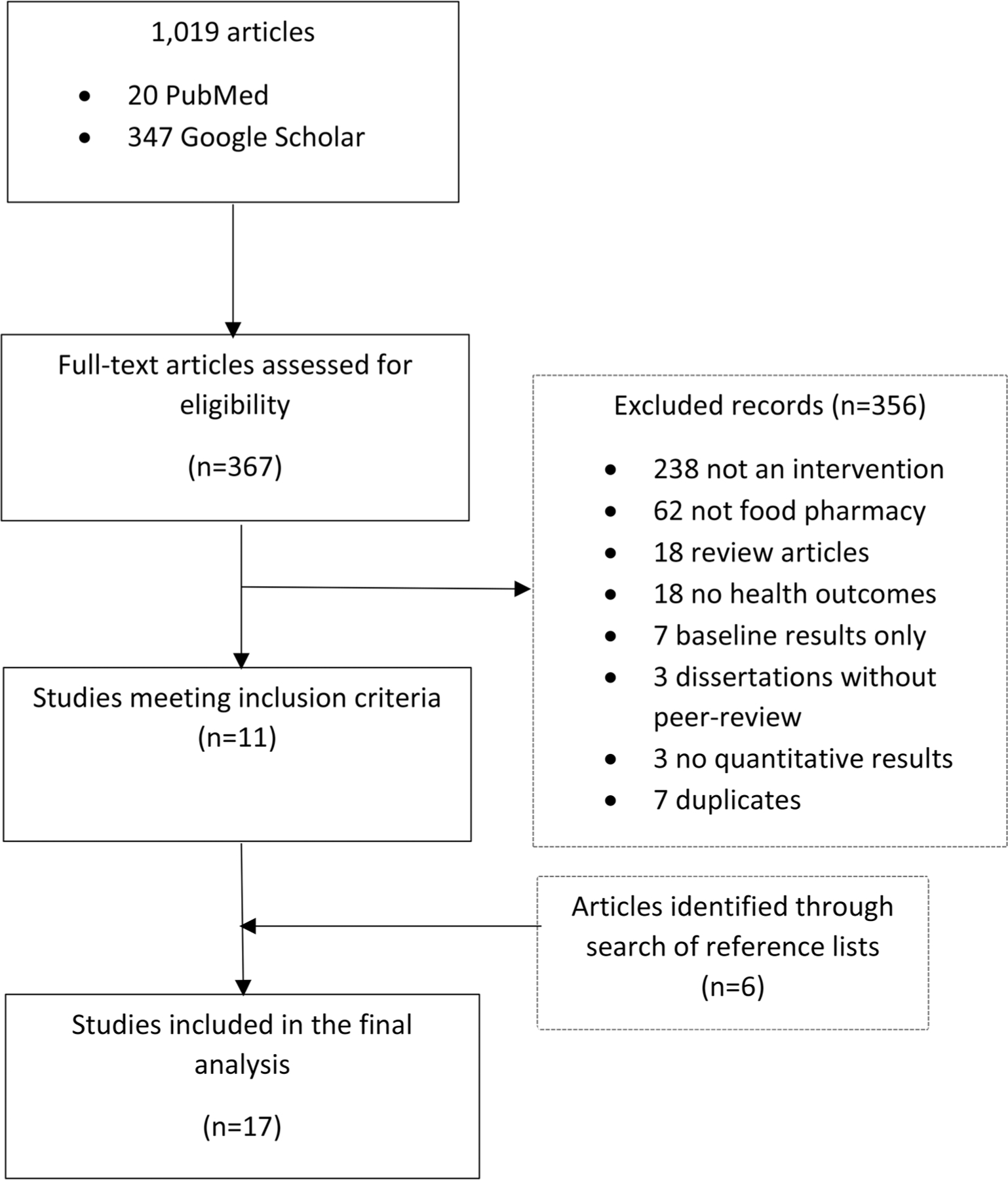
Screening and selection process for intervention studies evaluating food pharmacy programs effect on health outcomes.
For studies that reported respective sociodemographic characteristics (Table 1), the median percentage of study participants who were white was 27% (range = 2–92; n = 12 studies); the median percentage of study participants who were female was 66% (range = 52–84; n = 12 studies); and the median age of study participants was 58 years old (range = 13–72; n = 10 studies). For studies that reported Medicare/Medicaid and Supplemental Nutrition Assistance Program (SNAP) assistance, the median percentage of Medicare/Medicaid recipients was 55% (range = 9–94; n = 5 studies) and the median percentage of SNAP recipients was 54% (range = 50–65; n = 4 studies).
Table 1.
Sociodemographic variables in interventional food pharmacy programs for primarily low-income individuals with at least one comorbidity (n = 12 studies).
| Variable | Number of studies reporting variable information | Median | Range |
|---|---|---|---|
| White, % | 12 | 27 | 2–92 |
| Female, % | 12 | 66 | 52–84 |
| Age, mean | 10 | 58 | 13–72 |
| Medicare/Medicaid recipients, % | 5 | 55 | 9–94 |
| Supplemental Nutrition Assistance Program assistance, % | 4 | 54 | 50–65 |
The characteristics of each study, including the study design, intervention, duration of the intervention, and main health outcomes, are reported in Table 2. Thirteen studies used a pre/post study design, three used an RCT design, and one was a post-survey only. Five studies were targeted towards populations with a combination of comorbidities, five studies were targeted towards populations with only diabetes, one was targeted towards a population with hypertension, five were targeted towards populations that were obese/overweight, and one did not indicate the specific comorbidity of interest, only that chronic disease was high. Eleven interventions provided vouchers for fresh fruits and vegetables, three provided boxes with healthy foods (including V/F), two provided meals, and one provided fruit and vegetable samples. For those that provided vouchers, the value of these ranged between $7 and $40 per week. The interventions for the included studies ranged from six weeks to one year. All studies except for one (n = 16) had a study population where the majority of study participants were low-income.
Table 2.
Characteristics and outcomes variables of interventional food pharmacy programs included in this systematic review (n = 12).
| Author, year | Health condition | Study design | Sample size and population | Intervention | Duration of intervention | Main health outcome(s) | Demographics |
|---|---|---|---|---|---|---|---|
| Forbes et al. (2019) | Chronic illness or metabolic syndrome | Pre/post | 9 adults | $40 voucher/week and nutrition education | 6 weeks | Consumption of one or more salad or dark green vegetables per week increased 25%. Consumption of one or more orange-colored vegetables per week increased by 50%. Consumption of at least one fresh fruit per day increased 25%. |
67% white; 56% female; 67% with annual income of less than $40,000 |
| Emmert-Aronson et al. (2019) | CVD, diabetes, depression | Pre/post | 49 adults | $10 voucher with exercise and nutrition lessons | 16 weeks | V/F consumption increased by 1.24 servings per day (95%: 1.22 to 1.26). BMI decreased by 0.39 kg/m2 (95% CI: −0.49 to −0.29). Systolic blood pressure decreased by 6.74 mm/Hg (95% CI: −6.88 to −6.60) |
28.6% white, 63.3% female; average age was 59.1 years |
| Marcinkevage et al. (2019) | Not specifically stated - counties where “chronic disease are disproportionately high” | Post-survey only | 144 Adults | $10 voucher/week | Up to 6 months | 88.2% of participants reported eating more V/F than before receiving the V/F prescription. 71.5% of participants reported of managing their health condition better. |
No demographic data provided |
| Bryce et al. (2017) | Diabetes | Pre/post | 65 adults | $10 voucher/week | 13 weeks | Hemoglobin A1c decreased 0.71% (absolute; 95% CI: −0.80 to −0.62). Weight difference was 0.7 pounds (p = 0.45). Systolic blood pressure had a mean difference of 0.70 mm/Hg (95% CI: −0.13 to 1.53). |
6.2% white; 70.8% female; 35.4% of participants between 50–59 years; 55.4% were Medicare/Medicaid eligible |
| Cavanagh et al. (2017) | Hypertension, Obesity, diabetes | Pre/post | 54 adults | $7 voucher/week | NI (at least 87 weeks) | BMI decreased by 0.75 kg/m2 (95% CI: −1.30 to −0.20). | 29.6% white; 88% Medicare/Medicaid eligible |
| Trapl et al. (2018) | Hypertension | Pre/post | 224 adults | Four $10 vouchers ($40) and nutrition counseling | 12 weeks | Daily servings of fruit increased by 0.8 (p < 0.001). Dailey servings of vegetables increase by 0.8 (p < 0.001). Weekly days of eating fast food decreased by 0.6 (p< 0.001). |
1.5% white; 71.1% female; average age was 63 years; 49.6% received Supplemental Nutrition Assistance Program assistance |
| Wetherill et al. (2018) | Hypertension, diabetes, hyperlipidemia | Pre/post | 80 adults | Food box with curriculum booklet | NI (7 months) | Systolic blood pressure had a difference of 0.03 mm/Hg (95% CI: −2.76 to 2.81). Daily fiber intake increased 31 grams (p < 0.001). Difference in V/F servings was 0.2 cups (95% CI: −4.11 to 4.51). |
66% female; average age was 51.7 years; 55% received Supplemental Nutrition Assistance Program assistance; 75% had an annual income of less than $15,000 |
| Wagner et al. (2016) | Obesity/overweight | RCT | 54 adults | Fruit and vegetable samples (3 servings/day) and education | 10 weeks | Weekly consumption of V/F increased by 5.1 occurrences (p=0.03). | 92%white; 65%female; average age was 44.7; 33% had an annual income of less than $50,000 |
| Berkowitz et al. (2019) | Diabetes | RCT | 44 adults | 10 medically tailored meals/week | 12 weeks | Healthy Eating Index scores were better by 31 points (out of a 100-point scale; p < 0.0001). Hypoglycemia in prior 3 months decreased 17 percentage points (p=0.03). Hemoglobin A1c levels decreased 0.16 percentage points (95% CI: −0.28 to −0.04). BMI decreased 0.55 kg/m2 (95% CI: −1.00 to −0.10). Systolic blood pressure decreased by 2.96 mm/Hg (95% CI: −4.16 to −1.76). |
54% white; 68.5% female; average age was 58.4 years; 33.2% were Medicare/Medicaid eligible; 65% received Supplemental Nutrition Assistance Program assistance; 80% were food insecure |
| Racine et al. (2012) | Obesity, hypertension, hyperlipidemia | RCT | 298 adults | 7 frozen meals/week that adhered to Dietary Approaches to Stop Hypertension guidelines and nutrition counseling | 52 weeks | BMI increased by 0.70 kg/m2 (95% CI: 0.58 to 0.82). | 62.1% white; 83.9% female; average age was 72.4 years; 9.1% were Medicare/Medicaid eligible; 71.5% were ≤165% of poverty level |
| Freedman et al. (2013) | Diabetes | Pre/post | 41 adults | $50 ($25 after each of 2 surveys) | 6 months | V/F servings increased by 1.57 servings per day (95% CI: 1.31 to 1.83). | 7.3% white; 82.9% female; average age was 63.3 years; 53.7% received Supplemental Nutrition Assistance Program assistance; 90%had an annual income below $30,000 |
| Seligman et al. (2015) | Diabetes | Pre/post | 768 adults | Diabetes appropriate food box | 26 weeks | Hemoglobin A1c values declined 0.15 percentage points (95% CI: −0.18 to −0.12). Daily servings of V/F increased by 0.3 (95% CI: 0.28 to 0.32). |
25% white; 74% female; average age was 56.5 years; 83% were food insecure |
| Ridberg et al. (2019a) | Obesity/overweight | Pre/post | 883 children | $0.50–$1.00/per household member per day and education | 4–6 months | Daily servings of V/F increased by 0.13 (95% CI: 0.05 to 0.21) | 6% white; 54% female; 69% on WIC |
| Ridberg et al. (2019b) | Obesity/overweight | Pre/post | 578 children | $0.50–$1.00/per household member per day and education | 4–6 months | Food insecurity scores decreased by 0.09 points (p < 0.001). | 16.4% white; 52.4% female; 93.6% on Medicare/Medicaid |
| Saxe-Custack et al. (2019) | Obesity/overweight | Pre/post | 108 children | $15/clinic visit | 6 months | Daily servings of V/F increased by 0.19 (p=0.548). | 37.2% white; 55.4% female; average age of 12.9 years |
| Burrington et al. (2020) | Obesity/overweight | Pre/post | 10 children | $15–$25/week, depending on family size | 5 months | Daily servings of V/F increased by 0.8. | |
| Kerr et al. (2020) | Diabetes | Pre/post | 159 adults | 21 weekly servings of V/F | 10 weeks | Hemoglobin A1c values declined 0.1 percentage points (p > 0.05); Systolic blood pressure decreased by 2.4 points (95% CI: 4.56 to 0.28). | 20% white; 52.3% female; average age of 52.5 years |
V/F, vegetables and fruit; BMI, body mass index; NI, not indicated.
We found that the most common health outcomes reported, beginning with the most common reported outcome, were fruit and vegetable consumption, systolic blood pressure, BMI, and hemoglobin A1c. Overall diet quality, fiber intake, food insecurity, and episodes of hypoglycemia were also reported, but were less common. For the overall effects, we found that the pooled mean difference in daily servings of fruits and vegetables was significantly higher with a food pharmacy intervention than in control groups (mean = 0.77; 95% CI: 0.30 to 1.24; Figure 2). The pooled mean difference in BMI was lower among with food pharmacy intervention (mean = −0.40; 95% CI: −0.50 to −0.31). We did not find any differences in the pooled mean differences for hemoglobin A1c (mean = −0.23; 95% CI: −0.77 to 0.32) or systolic blood pressure (mean = −2.34; 95 CI: −6.02 to 1.33). All pooled estimates, except for BMI had considerable and significant heterogeneity (I2 > 75%), but because there were few studies, we were not able to do subgroup or sensitivity analysis. There was no evidence of publication bias with the Egger’s test, where the p-values were 0.18 for systolic blood pressure, 0.69 for fruits and vegetables, 0.54 for hemoglobin A1c, and 0.83 for BMI. Results were similar studies that did not include an educational component (Supplemental figure). Because of fewer studies in the meta-analysis, daily servings of fruits and vegetables was no longer significant, but was in the same direction (mean = 0.71; 95% CI: −0.29 to 1.70).
Figure 2.
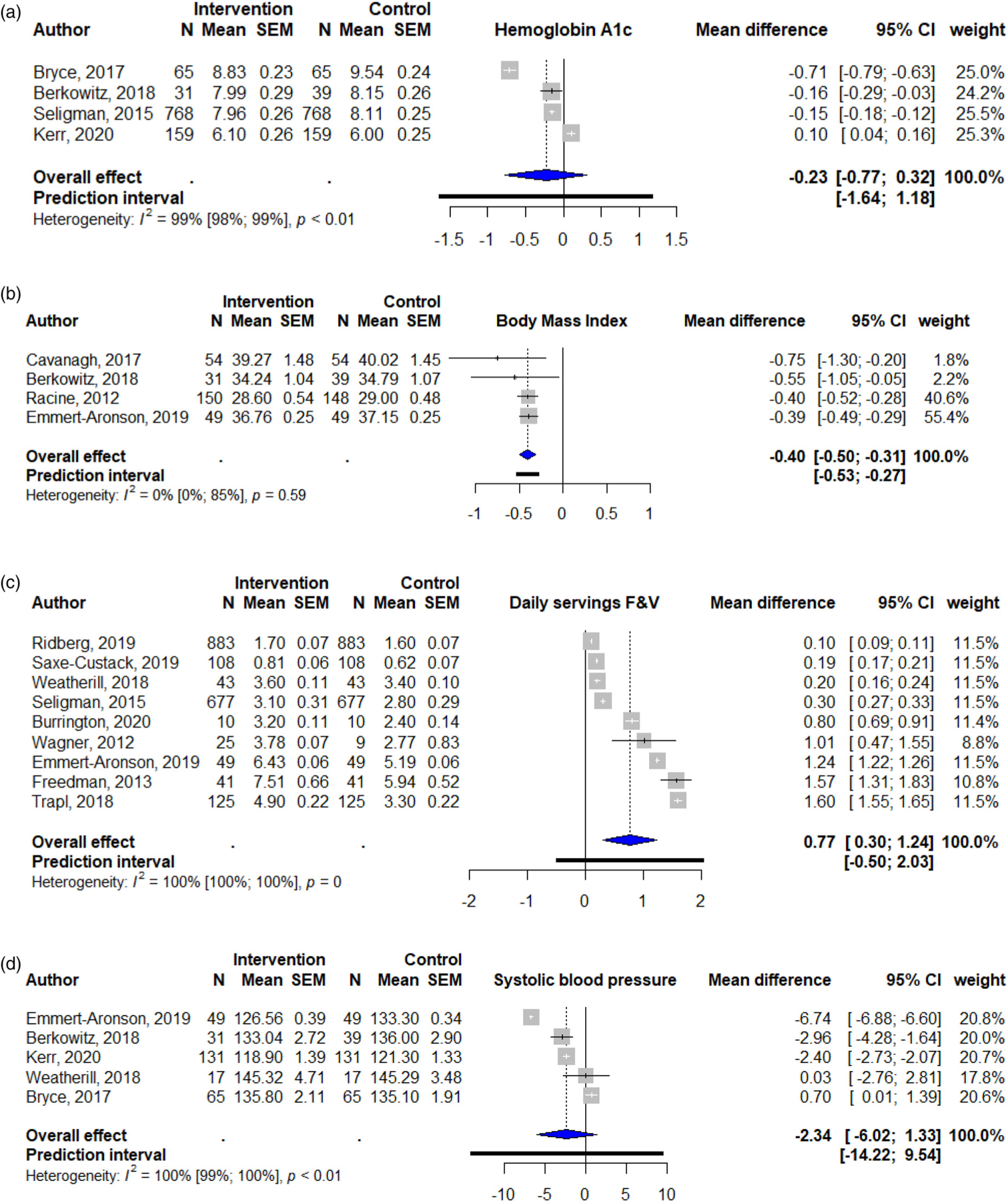
Forest plots of food pharmacy programs’ overall effect on (a) hemoglobin A1c, (b) body mass index, (c) fruit and vegetable consumption (F&V; daily servings), and (d) systolic blood pressure.
F&V, fruit and vegetables.
We found that most studies were of moderate quality, with a median score of 69% (range 50–96%) on the MINORS Index. While most studies were clear in the study aim, very few studies did a formal statistical sample size calculation.
Discussion
In this manuscript, we present a review of the literature on food pharmacy interventions designed to improve diet and reduce chronic disease burden in primarily low-income individuals with comorbidities and to estimate the average effect of these interventions. We found that food pharmacy programs that seek to provide high-quality foods, such as fruits and vegetables, to individuals with comorbidities and that are primarily low-income, may be effective in improving fruit and vegetable intake and possibly overall diet. Of the studies that assessed fruit and vegetable consumption, the studies that had the biggest improvement in fruit and vegetable consumption provided a $10 weekly voucher or fruit and vegetable samples (about three servings per day). Costs for these items seem minimal when compared to costs of managing comorbid conditions (Lee et al., 2019), especially since these costs may be subsidized by programs, such as the recently authorized Produce Prescription Program, (US Department of Agriculture, 2018) which provides funds for state and local agencies to partner with healthcare providers in providing fruits and vegetables to low-income individuals with diet-related conditions. Further, these programs could likely result in long-term cost savings that would exceed the programmatic costs if implemented through a large scale program such as the SNAP (Choi et al., 2017).
Our review is similar to a prior systematic review conducted by Lundeen et al., that aimed to comprehensively describe programs that connected food insecure patients with food resources (Lundeen et al., 2017). While this previous review did include many studies on food pharmacy programs, its aim was to describe ways to connect people with limited food access to food resources, rather than the effect of these programs on health outcomes.
Even though most of the included studies were conducted in populations who were low-income, only two of the studies reported the effects of a food pharmacy intervention on food insecurity. Of these studies, one found that offering 10 medically-tailored meals per week reduced food insecurity in participants with diabetes and food insecurity (Berkowitz et al., 2019), but the other study that offered a healthy food box to participants who were food insecure did not find any differences in food insecurity between pre and post intervention (Wetherill et al., 2018). The results of these studies show that there is equipoise in whether providing food prescription programs, especially ones that offer V/F exclusively, can reduce food insecurity in populations that are low-income and have a comorbidity, and future studies should be conducted to elucidate these effects.
While the median percentage of whites was often low in the studies we included in this review, none of these studies discussed the inclusion of American Indians or Alaska Natives, who are disproportionately affected by chronic diseases, such as obesity and diabetes (Hutchinson and Shin, 2014; Subica et al., 2017), and who have food security rates higher than other racial subgroups (Jernigan et al., 2017). Food pharmacy programs may be particularly beneficial in American Indians who have had a historical reliance on Food Distribution Program on Indian Reservations programs that have often included shelf-stable and processed foods, but are low in fresh V/F (Halpern, 2007). Future research should evaluate the effect of food pharmacy program on these populations.
We did not find that these programs had any detected effect on chronic disease risk factors, such as systolic blood pressure or hemoglobin A1c. These measures may require longer follow-up time to see health improvements, or they may require interventions that are more comprehensive than the addition of fruits and vegetables (e.g. the lowering of calories or sodium, the addition of other healthy foods, or the addition of a physical activity component). The lack of statistically significant effect for these outcomes may also be because many of the studies were small (median sample size was 60) and may not have been powered to detect differences in these outcomes, since only two of the included studies reported adequately reported and carried out a calculation of sample size.
Most of the studies we included had small sample sizes, and the health effects of food pharmacy interventions are still unknown when these programs are implemented on a larger scale. One of the included studies in this review administered fruit and vegetable vouchers to low-income participants throughout the state of Washington (Marcinkevage et al., 2019). Participants reported having improvements in fruit and vegetable consumption, but because the analysis came from questions administered post-intervention only and relied on dietary recall only after the intervention, further studies will need to confirm the directionality of effect. Another ongoing study to improve health in a community with high rates of diabetes and poverty includes a collaborative effort between university researchers, Walgreens, a local Farmer’s market, and six health centers on the south side of Chicago (Goddu et al., 2015). Patients were provided with a prescription for diet type (e.g. low carbohydrates, low fat, high fiber, or low sodium), coupons redeemable for fresh fruits and vegetables at the local Farmer’s market, and a coupon for discounted healthy food ($5 off a purchase of $20 of healthy foods) at any one of the local, participating Walgreen’s stores. The results of this study are still pending, but the partnership of a national retailer with local clinics is an example of a food prescription program being implemented on a large-scale with the potential to benefit thousands of individuals.
While this is a relatively new area of research, we found that many studies used a pre-post study design, and only three of the included studies were RCTs, which provide higher quality evidence of effectiveness. For RCT studies, the results of diet quality and V/F consumption were reflective of the overall positive findings. However, one RCT study found that providing frozen meals that adhere to the Dietary Approaches to Stop Hypertension guidelines led to an increase in BMI, and it is unknown if and how an intervention of fruits and vegetables or vouchers for fruits and vegetables would also affect BMI (Racine et al., 2012). Most of the included studies had small sample sizes, which may lead to unreliable effects, especially since most studies did not do a formal assessment of sample size required to achieve adequate power to detect meaningful differences prior to implementation. We also found that there was variability in follow-up time, which should be both meaningful and adequate to allow for proper assessment of health outcomes. Future studies that use an RCT study design, include adequate sample sizes, and have robust and time-sensitive follow-up data collection events post-intervention should be conducted in order to better assess the benefit of food pharmacy interventions.
A strength of the study is that this is the first meta-analysis to examine the effects of food pharmacy programs on health outcomes. This is particularly noteworthy as food pharmacy programs may provide an inexpensive way to manage chronic health conditions, especially when they result or are affected by food insecurity. Another strength is that we only included RCTs, since they are the gold standard for establishing causality. One of the limitations of this study is that we were limited to the studies published, which may have been affected by publication bias. We did do formal testing of publication bias and did not find evidence of this, but there were not a lot of studies included that reported on each outcome. Another limitation to this analysis is the small number of studies published on this topic. This is a relatively new avenue of research, and we look forward to seeing more studies, especially RCTs powered to detect effect on chronic disease burden, published on this topic. Of note, we did see a lot of heterogeneity, and because of the small number of studies in our analysis, we were not able to do subgroup analysis. Another limitation is that we only used two databases to search for results, which may limit the studies we found. PubMed is commonly used for systematic review searches, but the use of Google Scholar is only recently gaining use in this space due to its increasing coverage of literature. (Halevi et al., 2017) Because of this, most of the potential and usable articles were produced by the Google Scholar search. Finally, with any study examining the effects of diet on health outcomes, there is the potential for recall bias since methods to calculate actual intake are difficult, invasive, and rarely done.
Conclusion
In conclusion, we found that food pharmacy programs have the potential to improve diet quality in individuals who have chronic disease and may have limited access to healthy foods. Larger, randomized studies powered to detect effect on chronic disease burden, with adequate follow-up time are needed to evaluate the effect of these programs on chronic health conditions. Further, more research is needed in how these programs can benefit other populations, such as Native Americans and Alaska Natives who have high rates of food insecurity and chronic disease.
Supplementary Material
Implications for research and practice.
We found food pharmacy programs improve V/F intake and may improve the overall diet in primarily low-income populations with diet-related chronic health conditions. Food pharmacy programs may be a solution in the healthcare setting for people with chronic disease who are trying to improve their diet, and which may be medically cost-saving if implemented through large-scale public nutrition programs. While we found that diet was improved, we did not find that these programs improved measures of health, such as hypertension, A1c, or obesity. The studies that assessed these measures were not adequately powered to research this question and may not have had adequate follow-up time, and therefore further higher-quality, longer-term research needs to be done to be able to answer this question, while addressing current limitations in the literature.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Institute on Minority Health and Health Disparities (R01MD011266).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- Aune D, Giovannucci E, Boffetta P, et al. (2017. Jun 1) Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. International Journal of Epidemiology 46(3): 1029–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz SA, Delahanty LM, Terranova J, et al. (2019) Medically tailored meal delivery for diabetes patients with food insecurity: A randomized cross-over trial. Journal of General Internal Medicine 34(3): 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce R, Guajardo C, Ilarraza D, et al. (2017) Participation in a farmers’ market fruit and vegetable prescription program at a federally qualified health center improves hemoglobin A1C in low income uncontrolled diabetics. Preventive Medicine Reports 7: 176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrington CM, Hohensee TE, Tallman N, et al. (2020) A pilot study of an online produce market combined with a fruit and vegetable prescription program for rural families. Preventive Medicine Reports 17: 101035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh M, Jurkowski J, Bozlak C, et al. (2017) Veggie Rx: An outcome evaluation of a healthy food incentive programme. Public Health Nutrition 20(14): 2636–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2009) The power of prevention: Chronic disease… the public health challenge of the 21st century Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. [Google Scholar]
- Choi SE, Seligman H and Basu S (2017) Cost effectiveness of subsidizing fruit and vegetable purchases through the supplemental nutrition assistance program. American Journal of Preventive Medicine 52(5): e147–e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert-Aronson B, Grill KB, Trivedi Z, et al. (2019) Group medical visits 2.0: The open source wellness behavioral pharmacy model. The Journal of Alternative and Complementary Medicine 25(10): 1026–1034. [DOI] [PubMed] [Google Scholar]
- Forbes JM, Forbes CR, Lehman E, et al. (2019) “Prevention produce”: Integrating medical student mentorship into a fruit and vegetable prescription program for at-risk patients. The Permanente Journal 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DA, Choi SK, Hurley T, et al. (2013) A farmers’ market at a federally qualified health center improves fruit and vegetable intake among low-income diabetics. Preventive Medicine 56(5): 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddu AP, Roberson TS, Raffel KE, et al. (2015) Food Rx: A community-university partnership to prescribe healthy eating on the south side of Chicago. Journal of Prevention & Intervention in the Community 43(2): 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAF HWAM (2018) The costs of chronic disease in the U.S In M. Institute (Ed.). [Google Scholar]
- Gregory CA and Coleman-Jensen A (2017) Food insecurity, chronic disease, and health among working-age adults 235: 1–25. [Google Scholar]
- Halevi G, Moed H and Bar-Ilan J (2017) Suitability of Google scholar as a source of scientific information and as a source of data for scientific evaluation—review of the literature. Journal of Informetrics 11(3): 823–834. [Google Scholar]
- Halpern P (2007) Obesity and American Indians/Alaska Natives In U. S. D. o. H. a. H. Services (Ed.): Office of the Assistant Secretary for Planning and Evaluation. [Google Scholar]
- Harsha DW, Lin PH, Obarzanek E, et al. (1999) Dietary approaches to stop hypertension: A summary of study results. DASH collaborative research group. Journal of the American Dietetic Association 99(8 Suppl): S35–S39. [DOI] [PubMed] [Google Scholar]
- Higgins Jp, Thomas J, Chandler J, et al. (eds) (2021) Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) Cochrane Available from www.training.cochrane.org/handbook. [Google Scholar]
- Hutchinson RN and Shin S (2014) Systematic review of health disparities for cardiovascular diseases and associated factors among American Indian and Alaska native populations. PloS one 9(1): e80973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IntHout J, Ioannidis JP and Borm GF (2014) The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC medical Research Methodology 14(1): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan VBB, Huyser KR, Valdes J, et al. (2017) Food insecurity among American Indians and Alaska natives: A national profile using the current population survey–food security supplement. Journal of Hunger & Environmental Nutrition 12(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D, Barua S, Glantz N, et al. (2020) Farming for life: Impact of medical prescriptions for fresh vegetables on cardiometabolic health for adults with or at risk of type 2 diabetes in a predominantly Mexican-American population. BMJ Nutr Prev Health 3(2): 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraia BA (2013) Food insecurity and chronic disease. Advances in Nutrition (Bethesda, Md.) 4(2): 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Mozaffarian D, Sy S, et al. (2019) Cost-effectiveness of financial incentives for improving diet and health through medicare and medicaid: A microsimulation study. PLoS Medicine 16: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeen EA, Siegel KR, Calhoun H, et al. (2017) Clinical-community partnerships to identify patients with food insecurity and address food needs. Preventing Chronic Disease 14: E113–E113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkevage J, Auvinen A and Nambuthiri S (2019) Washington state’s fruit and vegetable prescription program: Improving affordability of healthy foods for low-income patients. Preventing Chronic Disease 16: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obarzanek E, Sacks FM, Vollmer WM, et al. (2001) Effects on blood lipids of a blood pressure-lowering diet: The dietary approaches to stop hypertension (DASH) trial. The American Journal of Clinical Nutrition 74(1): 80–89. [DOI] [PubMed] [Google Scholar]
- Racine EF, Lyerly J, Troyer JL, et al. (2012) The influence of home-delivered dietary approaches to stop hypertension meals on body mass index, energy intake, and percent of energy needs consumed among older adults with hypertension and/or hyperlipidemia. Journal of the Academy of Nutrition and Dietetics 112(11): 1755–1762. [DOI] [PubMed] [Google Scholar]
- Raghupathi W and Raghupathi V (2018) An empirical study of chronic diseases in the United States: A visual analytics approach to public health. International Journal of Environmental Research and Public Health 15(3): 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridberg RA, Bell JF, Merritt KE, et al. (2019a) Effect of a fruit and vegetable prescription program on children’s fruit and vegetable consumption. Preventing Chronic Disease 16: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridberg RA, Bell JF, Merritt KE, et al. (2019b) A pediatric fruit and vegetable prescription program increases food security in low-income households. Journal of Nutrition Education and Behavior 51(2): 224–230 e221. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Ello-Martin JA and Tohill BC (2004) What can intervention studies tell us about the relationship between fruit and vegetable consumption and weight management? Nutrition Reviews 62(1): 1–17. [DOI] [PubMed] [Google Scholar]
- Saxe-Custack A, LaChance J and Hanna-Attisha M (2019) Child consumption of whole fruit and fruit juice following six months of exposure to a pediatric fruit and vegetable prescription program. Nutrients 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman HK, Lyles C, Marshall MB, et al. (2015) A pilot food bank intervention featuring diabetes-appropriate food improved glycemic control among clients in three states. Health Affairs 34(11): 1956–1963. [DOI] [PubMed] [Google Scholar]
- Slim K, Nini E, Forestier D, et al. (2003) Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ Journal of Surgery 73(9): 712–716. [DOI] [PubMed] [Google Scholar]
- Subica AM, Agarwal N, Sullivan JG, et al. (2017) Obesity and associated health disparities among understudied multiracial, pacific islander, and American Indian adults. Obesity 25(12): 2128–2136. [DOI] [PubMed] [Google Scholar]
- Trapl ES, Smith S, Joshi K, et al. (2018) Peer reviewed: Dietary impact of produce prescriptions for patients with hypertension. Preventing Chronic Disease 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Agriculture (2018) Agriculture Improvement Act fo 2018: Highlights and Implications Retrieved from. https://www.ers.usda.gov/agriculture-improvement-act-of-2018-highlights-and-implications/local-and-regional-foods/ (last accessed October 12, 2020)
- US Department of Agriculture (2019) Definitions of Food Security Retrieved from. https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/definitions-of-food-security.aspx
- Wagner MG, Rhee Y, Honrath K, et al. (2016) Nutrition education effective in increasing fruit and vegetable consumption among overweight and obese adults. Appetite 100: 94–101. [DOI] [PubMed] [Google Scholar]
- Wetherill MS, Chancellor McIntosh H, Beachy C, et al. (2018) Design and implementation of a clinic-based food pharmacy for food insecure, uninsured patients to support chronic disease self-management. Journal of Nutrition Education and Behavior 50(9): 947–949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


