Abstract
An examination of electroencephalographic and magnetoencephalographic studies demonstrates how age-related changes in brain neural function temporally constrain their use as diagnostic markers. A first example shows that, given maturational changes in the resting-state peak alpha frequency in typically developing children but not in children who have autism spectrum disorder (ASD), group differences in alpha-band activity characterize only a subset of children who have ASD. A second example, auditory encoding processes in schizophrenia, shows that the complication of normal age-related brain changes on detecting and interpreting group differences in neural activity is not specific to children. MRI studies reporting group differences in the rate of brain maturation demonstrate that a group difference in brain maturation may be a concern for all diagnostic brain markers. Attention to brain maturation is needed whether one takes a DSM-5 or a Research Domain Criteria approach to research. For example, although there is interest in cross-diagnostic studies comparing brain measures in ASD and schizophrenia, such studies are difficult given that measures are obtained in one group well after and in the other much closer to the onset of symptoms. In addition, given differences in brain activity among infants, toddlers, children, adolescents, and younger and older adults, creating tasks and research designs that produce interpretable findings across the life span and yet allow for development is difficult at best. To conclude, brain imaging findings show an effect of brain maturation on diagnostic markers separate from (and potentially difficult to distinguish from) effects of disease processes. Available research with large samples already provides direction about the age range(s) when diagnostic markers are most robust and informative.
Keywords: alpha, auditory steady-state response, autism spectrum disorders, maturation, schizophrenia
Characterizing neural abnormalities in psychiatric and neurological disorders is a promising route to understanding neural and behavioral dysfunction, with the need for markers for diagnosis, prognosis, and treatment widely recognized (see National Institute of Mental Health’s ‘Strategic Research Priorities’1 and Research Domain Criteria [RDoC] framework2–5). With respect to our search for diagnostic electrophysiological markers (markers used to detect or confirm the presence of a disease or condition of interest, preferably with considerable specificity), this article focuses on the impact of typical brain maturation on the challenge of identifying clinically useful markers. Examples are provided to show how normal age-related changes in brain function temporally constrain the use of neural measures as diagnostic markers. The first, an examination of resting-state (RS) brain activity in children, shows the effect of normal brain maturation on identifying a maker that differentiates typically developing children (TDC) from children who have autism spectrum disorder (ASD). The second, an examination of auditory encoding processes in adults with schizophrenia (SCZ), shows that the influence of age-related brain changes on diagnostic markers is not specific to children. Finally, structural and functional MRI studies observing control and ASD or SCZ group differences in brain maturation are presented to show the generality of this problem to other brain measures.
Presented findings show that, with large enough samples, the data point to the age range(s) when neural imaging diagnostic markers are most robust. The article concludes with a discussion of the impact of typical maturation on clinical neuroimaging research and models of neural dysfunction.
Resting-state Measures in ASD
Significant research findings dating to the 1940s demonstrate changes in RS neural activity from birth through adulthood. Commonly reported are an age-related decrease in delta and theta activity (~1 to 8 Hz) and an age-related increase in alpha, beta, and gamma activity (~8 to 50 Hz).6–12 As an example, examining 1416 subjects aged 6 to 39 years, Matsuura et al.13 showed that RS electroencephalographic (EEG) rhythms did not reach adult levels until very late adolescence. Regional differences in the maturation of RS brain rhythms were also observed. In particular, although alpha activity reached adult levels in occipital areas by 10 to 13 years of age, adult alpha levels were not observed in frontal and central areas until 22 to 25 years. Theta-band activity also did not reach adult levels until approximately 23 years in occipital regions versus 27 years in frontal and central regions.
Given the above, a potential problem is that maturational changes in RS brain activity in TDC6–8,10,12–18 mean that an RS marker identified in one pediatric patient cohort may not generalize to a pediatric patient cohort outside the studied age range (or even to all within the studied cohort). This would be especially true if the control and patient groups showed different rates of maturation. Research examining RS alpha rhythms in ASD provides support for this concern. In the eyes-closed RS, 8–13-Hz alpha oscillations are the dominant rhythm, most prominent in parieto-occipital regions.19–23 A well-established finding is that posterior RS alpha activity changes as a function of age. Notable in children is an age-related increase in the frequency at which RS alpha oscillations show maximum power, often referred to as the peak alpha frequency (PAF). Indeed, among examined quantitative EEG and magnetoencephalographic (MEG) parameters, the PAF is considered a robust signature of brain maturation,24,25 with many studies showing an alpha peak at ~6 Hz in young children (5 to 7 years old) and with an adult profile of a 10–12 Hz PAF not observed until at least 15 years.16,26–28
PAF is of clinical interest because it is one of the most heritable brain measures.29,30 As an example, in a large sample of 16-year-old twins, Smit et al.31 obtained a heritability estimate of 0.81 for PAF, with all non-genetic variance attributed to measurement unreliability rather than unique environmental factors. PAF is also of clinical interest given studies showing that it is associated with working memory and speed of information processing.32–34 Studies also indicate that the brain generates its own temporal structure via neural activity, with alpha rhythms providing the timing for communication within and between brain regions (e.g., Klimesch35). Given that alpha oscillations and the circuits associated with alpha oscillations provide a scaffold for basic and more complex brain processes, an examination of alpha rhythms in pediatric psychiatric and neurological disorders may be revealing.
To date, three cross-sectional studies have reported TDC/ASD differences in PAF maturation. Edgar et al.20 applied distributed source localization to RS MEG data to identify brain regions showing group differences in alpha activity (47 TDC and 41 children with ASD). PAF was examined at the calcarine region (the region with the greatest concentration of alpha generators) and at right central sulcus and parieto-occipital regions (where group differences in alpha power were observed). At both regions, analyses showed a positive relation between age and PAF in TDC and a non-significant negative relation in ASD. Dickinson et al.,36 using EEG and examining sensor RS activity in participants 2- to 12-years-old, also observed an age-related increase in PAF in TDC (n = 38) but not children with ASD (n = 59).
In the largest study to date, Edgar et al.37 obtained RS MEG data from 141 TDC (aged 6.13–17.70 years) and 204 children who had ASD (aged 6.07–17.93 years). A neural source model with 15 regional sources projected the raw MEG surface data into the brain source space. PAF was identified in each participant from the source showing the largest amplitude alpha activity (7–13 Hz). Given sex differences in PAF in TDC (females > males) and the relatively few females in both groups, group comparisons were conducted with male TDC (n = 121) and ASD subjects (n = 183).
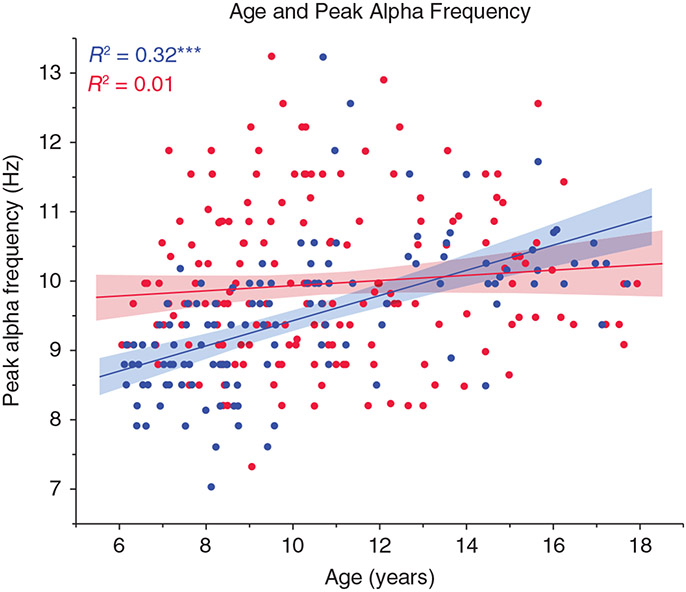
Figure 1 shows associations between age (x-axis) and RS PAF (y-axis). As indicated in the top left of Figure 1, regressions showed a significant Group Age × PAF slope difference, with an age-related increase in PAF in TDC (R2 = 0.32) but not ASD (R2 = 0.01). Analyses examining male children aged below or above 10 years (median split) indicated that, given no age-related PAF change in male ASD subjects, the younger male children with ASD (aged 6 to 10 years) had a higher PAF than the younger male TDC (Cohen’s d = 1.05). In contrast, the older male children with ASD (aged 10 to 18 years) tended to have a lower PAF than the older male TDC (although this group difference was not significant in this sample).
Fig.1.
Scatterplots showing associations between age (x-axis) and peak alpha frequency (y-axis) for typically developing children (TDC; blue, n = 121) and those with autism spectrum disorder (ASD; red, n = 183). As detailed in the Results, a significant interaction term indicated group slope differences. ***P < 0.001. Reproduced from Edgar et al.37 with permission.
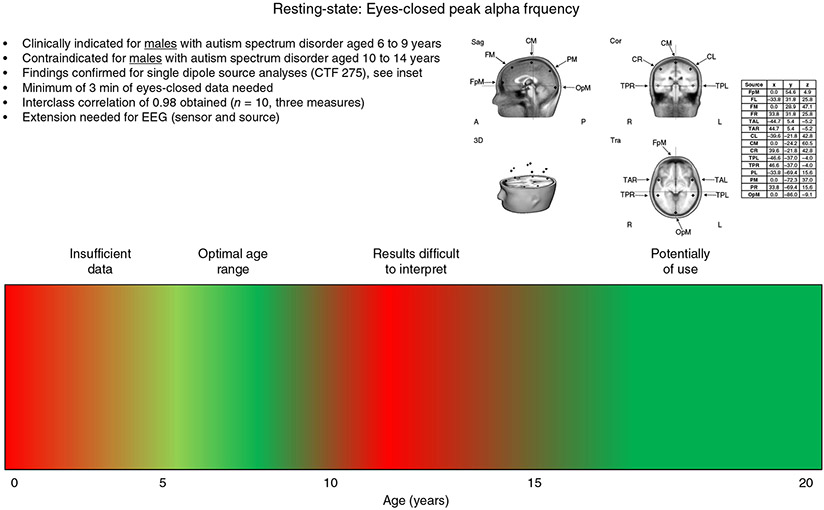
If the above findings were replicated and the field were to pursue PAF frequency as a marker of ASD, allowing this brain measure to be incorporated into clinical practice, something like an RS PAF indication label shown in Figure 2 might be created. The label notes that the PAF measure is clinically indicated for males who have ASD aged 6 to 9 years (shown in green). PAF is contraindicated (uninformative at this time) for males who have ASD aged 10 to 14 years (shown in red). The label also notes that this measure may be of use in males aged 15 years and older who have ASD, although additional studies in older individuals are needed.
Fig.2.
A peak alpha frequency clinical indication label for autism spectrum disorder. Activity examined at the following brain regions: FpM, fronto-polar midline; FL, frontal left; FM, frontal midline; FR, frontal right; TAL, temporal anterior left; TAR, temporal anterior right; CL, central left; CM, central midline; CR, central right; TPL, temporal posterior left; TPR, temporal posterior right; PL, parietal left; PM, parietal midline; PR, parietal right; OpM, occipito-polar midline.
The red at the left of Figure 2 indicates that RS infant and toddler PAF studies are not yet available. Although it is difficult to obtain eyes-closed RS data in infants, of note are findings from Stroganova et al.,38 who identified an infant analog of the child, adolescent, and adult alpha rhythms based on assessment of spatial distribution (largest over primary parieto-occipital cortex) and functional reactivity to visual input by obtaining RS measures in lighted and darkened conditions (see also Mulholland39). Specifically, Stroganova et al.38 found that total darkness is associated with highly synchronous 5.5- to 8-Hz oscillations over the occipital cortex in infants aged 4 to 12 months and that these oscillations are attenuated when the lights are turned on. Further work in this area is of interest.
In the Figure 2 indication label, we might also note the following: (i) findings to date are based on MEG source space analyses (in the label showing the 15-dipole source model used); (ii) if possible, the reliability of the measures should be considered (shown here as being very high and based on a sample of 10 subjects); and (iii) research is needed to determine whether similar group-difference findings are observed using EEG (source and sensor analyses). Finally, it is noted that, given possible sex differences in the maturation of RS PAF, there may be a need for sex-specific brain markers (see also discussion of PAF differences in male and female children in Edgar et al.37).
The following section demonstrates that normal age-related changes in neural activity are also of concern in adults.
Auditory Encoding Measures in SCZ
Over the last 10 years there has been increased interest in clinical trials seeking to use neural measures to demonstrate biological effects of psychological and biological treatments. As an example, there is considerable research examining gamma-band activity (30 to 50 Hz) in SCZ, with the hypothesis that gamma abnormalities are due, in part, to inhibitory interneuron dysfunction, and with clinical trials examining the effectiveness of cognitive therapy, physical exercise, or pharmacology for normalizing gamma activity.40,41
Two auditory encoding measures frequently of interest are considered here: poststimulus transient low-frequency activity and 40-Hz steady-state activity. In a review of studies examining the early transient 100-ms component of the auditory event-related potential, Rosburg et al.42 concluded that auditory encoding abnormalities in SCZ are a robust finding, and studies examining the time-frequency profile of the early transient activity (50-ms and 100-ms activity) have repeatedly demonstrated decreased lower-frequency activity in adults with SCZ,43–46 with Johannesen et al.47 and Jansen et al.48 concluding that the small response observed in SCZ reflects a diminished capacity to ‘gate in’ the relevant signal.
Researchers have also used 40-Hz auditory driving stimuli to examine auditory cortex dysfunction in SCZ. Assessment of 40-Hz (gamma-band) activity is of interest given hypothesized abnormalities in pyramidal cells and inhibitory interneuron networks in the superficial cortical layers in SCZ (for reviews, see Gandal et al.49 and Uhlhaas et al.41). Many studies have reported 40-Hz auditory steady-state abnormalities in SCZ. Although group differences in 40-Hz auditory steady-state activity are most often examined as a function of the strength of the 40-Hz response or the trial-to-trial similarity of the 40-Hz response,50–62 other studies have examined group differences in the timing of the 40-Hz response.55,63 To better understand normal and abnormal 40-Hz auditory steady-state activity, studies have also examined the relation between background brain activity and the 40-Hz auditory steady-state response,64 and cross-frequency associations, assessed via phase-amplitude coupling.65,66
Using 40-Hz auditory steady-state stimuli and relatively long interstimulus intervals (e.g., greater than 1 s) allows examination of both early transient and steady-state activity.67,68 As left and right superior temporal gyrus (STG) regions are the primary generators of the early transient responses69–76 as well as the 40-Hz steady-state response,77,78 some studies have used source localization to directly assess the left and right STG cortical microcircuits involved in auditory encoding. As an example, using a 40-Hz steady-state task with a relatively long interstimulus interval, Edgar et al.79 showed multiple disruptions in STG auditory areas in SCZ, including STG poststimulus low-frequency abnormalities (4–16 Hz) as well as 40-Hz steady-state abnormalities.
A growing literature indicates that the utility of 40-Hz steady-state activity as a biomarker depends on the age of the control and patient sample. Edgar et al.79 showed an association between age and 40-Hz steady-state activity in the left but not right hemisphere in control adults but not adults with SCZ, with a smaller left-hemisphere 40-Hz steady-state response in older compared to younger control adults (age accounting for over 20% of the variance in 40-Hz steady-state activity).
Building on the above study, Edgar et al.45 sought to identify EEG and MEG analysis approaches that best differentiated controls and patients with SCZ. Given an age-related decrease in left 40-Hz steady-state activity in controls but not adults with SCZ, larger control and SCZ 40-Hz group differences were hypothesized in the younger participants compared to their older counterparts. The meta-analysis of 40-Hz steady-state studies of SCZ by Thune et al.80 also provided support for this hypothesis, where a trend for an effect of age on group differences was observed (P = 0.09), with controls versus SCZ effect sizes of 0.52 for patients older than 39.8 years and 0.77 for patients younger than 39.8 years. In addition to examining 40-Hz steady-state activity, left and right auditory cortex poststimulus low-frequency activity was also examined. Given that the primary/secondary auditory cortexes in the left and right STG are primary generators of early transient and 40-Hz steady-state responses, Edgar et al. also hypothesized that the construct-valid STG source measures would better differentiate groups than EEG sensor measures and that larger group-difference effects would be observed in source space than in sensor space.
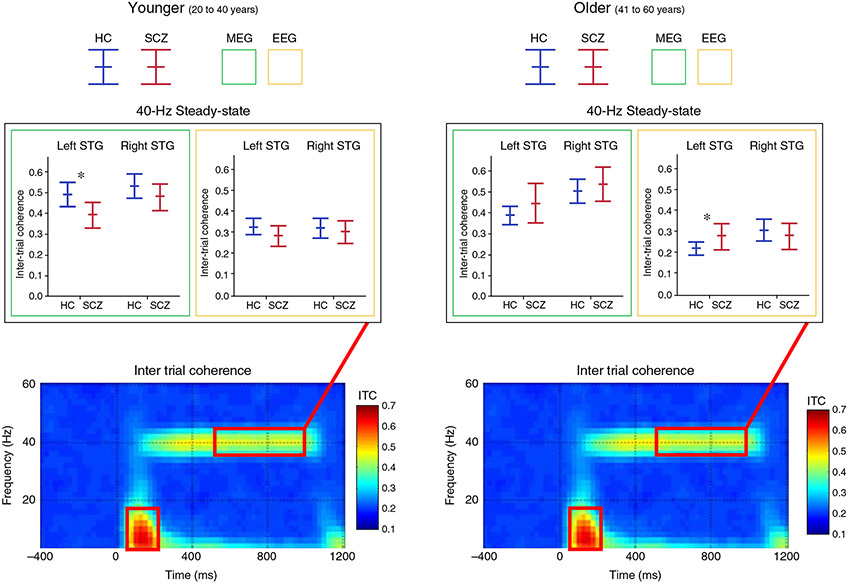
As shown in Figure 3, given an age-related decrease in left STG 40-Hz steady-state activity in adult controls, the expected greater 40-Hz steady-state activity in controls compared to patients was observed only in the left hemisphere, and only in younger participants (20 to 40 years old), as shown with the asterisk for the left MEG source measure. The 40-Hz steady-state group differences were difficult to observe using EEG in source or sensor space, with the only significant EEG group difference finding indicating greater left 40-Hz steady-state activity in older patients than controls, likely due to the age-related change in 40-Hz activity in controls but not patients. It is of note, however, that this somewhat unexpected group difference finding – greater 40-Hz activity in older patients than controls – has been reported in EEG studies examining older participants. In particular, studies examining controls and individuals with SCZ with a mean age in the late 30s59 and early 40s52,81 have reported only trend-level group differences or no group differences. Both Hamm et al.51 and Kim et al.,81 reporting auditory steady-state activity in control and SCZ participants with a mean age of ~40 years, observed greater steady-state activity in SCZ than in controls, with the greater 40-Hz steady-state response in SCZ perhaps due to the age-related decrease in 40-Hz steady-state activity present in controls. In contrast, studies examining first-episode and early onset SCZ have reported control versus SCZ 40-Hz steady-state group differences.60,82
Fig.3.
Intertrial coherence time-frequency plots (grand average of controls shown) with box plots (magnetoencephalography [MEG] = green border, electroencephalography [EEG] = yellow border) showing the mean and 95% confidence intervals for each group (healthy controls [HC] = blue, schizophrenia (SCZ) patients = red), and for younger (left) and older (right) participants. Findings shown for left and right superior temporal gyrus (STG) 40-Hz steady-state activity (38–42-Hz activity averaged from 500 to 1000 ms). In the box plots, significant group differences are indicated. *P < 0.05. Reproduced from Edgar et al.45 with permission.
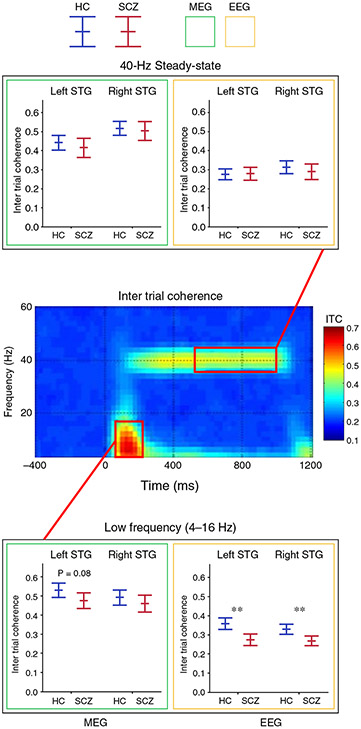
A comparison of the 40-Hz and low-frequency group difference findings is informative, with the Edgar et al.45 results showing that control and SCZ auditory encoding low-frequency group-difference effects were observed in the full sample and also generally comparable across modality and analysis strategies. As shown on the bottom panel of Figure 4, in the full sample, poststimulus low-frequency group differences were observed in the left for MEG and bilaterally for EEG. In addition, for the low-frequency activity, similar effect sizes were observed across source and sensor (e.g., Cz, sensor principal component analysis) methods.
Fig.4.
Intertrial coherence time-frequency plots (grand average of controls shown) with box plots (magnetoencephalography [MEG] = green border, electroencephalography [EEG] = yellow border) showing the mean and 95% confidence intervals for each group (healthy controls (HC) = blue, schizophrenia (SCZ) patients = red), and for the total sample (younger and older). Findings shown for left and right superior temporal gyrus (STG) poststimulus low-frequency activity (4–16-Hz activity averaged from 50 to 200 ms) and 40-Hz steady-state activity (38–42-Hz activity averaged from 500 to 1000 ms). In the box plots, significant group differences are indicated. *P < 0.05, **P < 0.001, trends below P = 0.10 reported with P-value.
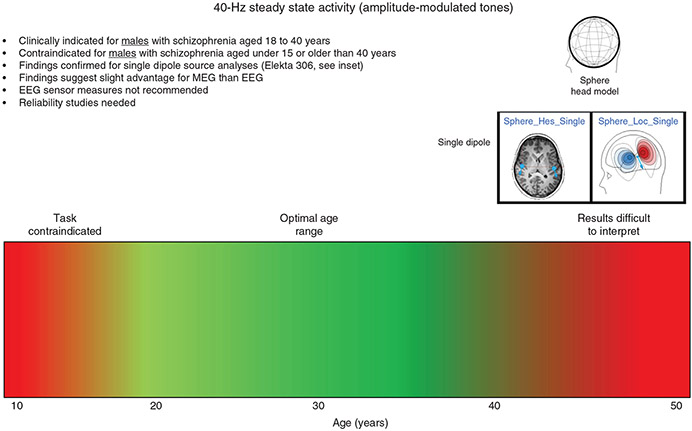
Thus, the findings from Edgar et al.45 indicated that, with regard to identifying group differences in auditory encoding, the ‘optimal’ approach depends on the measure of interest. To this end, a clinical label for 40-Hz steady-state activity might look something like that shown in Figure 5, with the color bar indicating that research shows that 40-Hz steady-state tasks are most useful for the ages shown in green. The red area to the left indicates that previous research has shown that 40-Hz steady-state tasks may not provide brain responses with sufficient signal-to-noise in child and young adolescent populations (discussed further in the Discussion section). Text in the indication label notes that there is some evidence that 40-Hz steady-state group differences are more easily identified using MEG than EEG, also noting that 40-Hz steady-state measures obtained at EEG sensors are contraindicated. So, using source measures, the 40-Hz steady-state task looks to be of use in differentiating controls and adults with SCZ from ages 18 to 40 years.
Fig.5.
A 40-Hz steady-state gamma activity clinical indication label for schizophrenia. EEG, electroencephalography; MEG, magnetoencephalography.
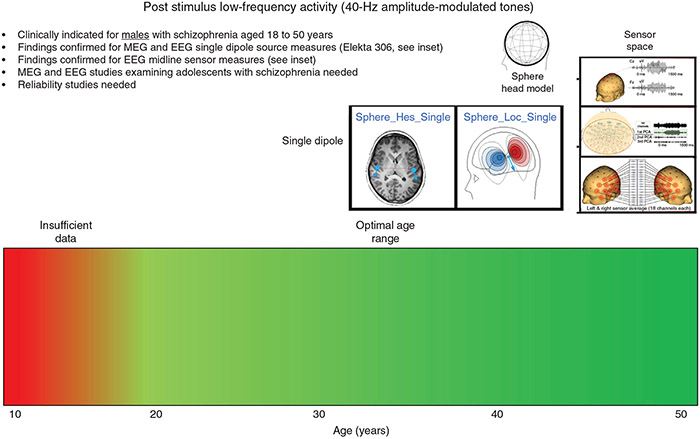
In contrast, a label for auditory poststimulus low-frequency activity might look something like that shown in Figure 6. The color bar indicates that poststimulus low-frequency group differences are robustly observed in controls and adults with SCZ from ages 20 to 50 years, and the text in the label notes that poststimulus low-frequency group differences have been robustly observed using MEG and EEG, and with EEG at the source or sensor level. For both the 40-Hz steady-state and low-frequency activity, additional studies are needed to determine the reliability of the measures.
Fig.6.
A 40-Hz steady-state poststimulus low-frequency activity clinical indication label for schizophrenia. EEG, electroencephalography; MEG, magnetoencephalography; PCA, principal component analysis.
Beyond Electrophysiology
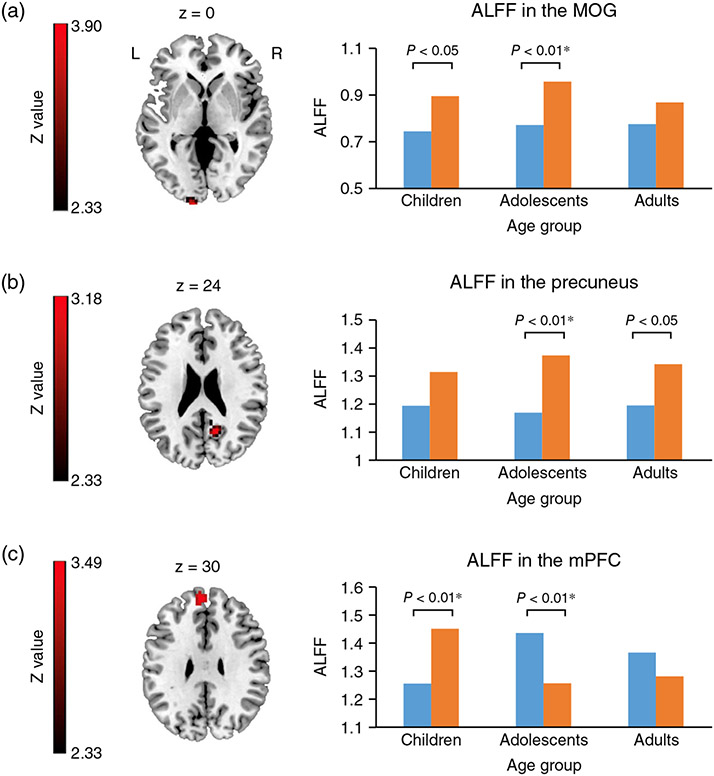
Although this paper focuses on electrophysiology (EEG and MEG), structural and functional MRI studies examining control and ASD or SCZ group differences also often report group differences in the rate of brain maturation.83–89 For example, Ouyang et al.90. found that in TDC and children with ASD aged 2 to 7 years, the pattern of diffusion group differences changed as a function of age. Sheffield et al.91 observed significant differences in the maturation of functional brain networks in controls and individuals with SCZ, with their figure 1 suggesting that the direction of group differences in the cingulo-opercular network changed in younger versus older subjects. Van Haren et al.92 showed differences in the trajectory of gray matter volume change in controls and individuals with SCZ: instead of a curved trajectory found in controls, patients with SCZ showed a linear decrease over time. As a result of different maturation rates, the pattern of gray-matter group differences is expected to change as a function of the age. As a final example, in an fMRI study examining RS activity in controls and individuals with ASD, Guo et al.93 showed that the pattern of group differences changed as a function of age in some but not all brain regions. In particular, as shown in Figure 7 (from Guo et al.93), whereas the group-difference effect was in the same direction for children (<11 years), adolescents (11–18 years), and adults (>18 years) at the middle occipital gyrus and precuneus regions, the group-difference pattern reversed for children and adolescents at the medial prefrontal cortex. This was due to a quadratic age-related change in medial prefrontal cortex RS activity in controls but not in ASD subjects. The above studies suggest that a group difference in brain maturation is a concern for all brain diagnostic markers.
Fig.7.
(a) Significant main effect of diagnosis on the amplitude of low-frequency fluctuations (ALFF) in the left middle occipital gyrus. (b) Significant main effect of diagnosis on ALFF in the right precuneus. (c) Significant Diagnosis × Age interaction effect in the medial prefrontal cortex (mPFC). *Indicates Bonferroni corrected. (■) Autism spectrum disorder. (■) Typical controls. MOG, middle occipital gyrus. Reproduced from Guo et al.93 with permission.
Discussion
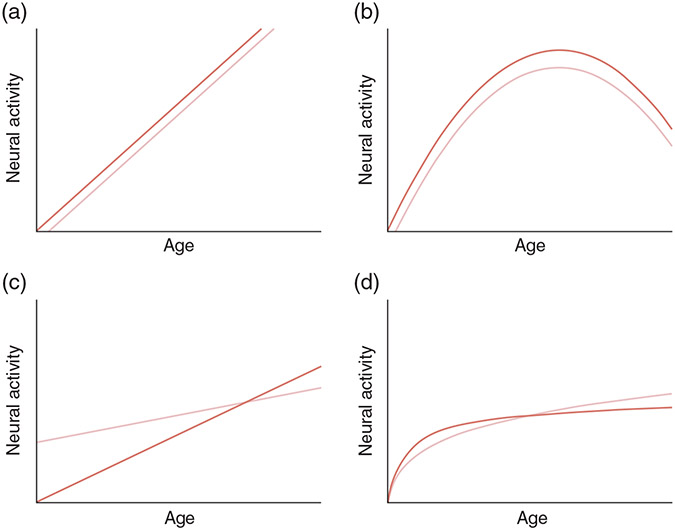
If diagnostic markers are to be of general use in clinical practice, then maturation rates between the control and case groups must be similar (or, at a minimum, known). The upper two panels of Figure 8 show where control and case group-difference effects are maintained given similar linear (Fig. 8a) or quadratic (Fig. 8b) changes in brain activity across the life span in two groups. Different rates of maturation, however, result in electrophysiological markers specific to certain ages (Fig. 8c,d). The ASD and SCZ examples presented above show that maturational changes in brain function in a control group (pediatric as well as adult populations) result in diagnostic markers of use only for specific ages.
Fig.8.
(a,b) Control and case group-difference effects are maintained given similar (a) linear or (b) quadratic changes in brain activity across the life span in the two groups. (c,d) Different rates of maturation, however, result in electrophysiological markers specific to certain ages.
Figures 2,5, and 6 use color coding to indicate when a diagnostic marker is of most use. As more studies are conducted, these figures can be refined to report effect sizes at specific ages. For some diagnostic markers, a group-difference effect-size asymptote surrounded by steep slopes might be observed, indicating a very restricted use of the diagnostic marker. Such a pattern might be observed in studies examining brain areas that show relatively rapid development during infancy, such as primary/secondary somatosensory and visual cortex.94,95 For other measures, an asymptote surrounded by shallow slopes might be observed, indicating a more general use of the diagnostic marker, such as auditory poststimulus low-frequency activity in SCZ. Studies examining maturational changes in brain activity might also indicate for some measures relatively abrupt changes in neural activity, and thus non-symmetric slopes around the asymptote.96 As an example of non-linear change in neural activity, during a Gestalt perception task, Uhlhaas et al.97 observed increased neural synchrony (gamma power) from early childhood to early adolescence, followed by an unexpected decrease during late adolescence, and then a pronounced increase in neural synchrony in adults. Such information is needed to guide future studies, recruiting within an age range most likely to show group differences, as well indicating when a diagnostic marker is of clinical use.
As an extension of the above, although for many decades there has been interest in obtaining large samples to quantitatively identify individuals with abnormal brain activity via a normative data base and regression methods (e.g., see Szava et al.4 and John et al.98), an age-related change in brain activity in one group but not the other creates problems. In particular, a regression approach will not identify individuals with disease who are at the ages where the groups do not differ on the brain measure. For example, using PAF in ASD, regression methods would not identify an 11-year-old male child who has ASD, given that at age 11, a typically developing male child and a male child who has ASD will tend to have a similar PAF. This is not to say that the alpha rhythms in the 11-year-old child who has ASD should be considered normal (especially if a longitudinal study were to show an abnormally high PAF in a 6-year-old who had ASD and with the PAF remaining unchanged in 11-year-olds) but that purely cross-sectional analyses, thus not considering development, will be unable to ‘detect’ this abnormality.
The maturation of neural brain circuits occurs in parallel with the maturation of brain structure, with brain development beginning soon after conception, and with establishing synchronization of neural activity within and between brain regions a fundamental aspect of brain development (e.g., see Uhlhaas et al.41). As an example, electrophysiological studies examining primary sensory neural activity in infants have hypothesized that changes in neural activity during early development are due to maturation of brain structure, such as age-related increases in myelin or increased synaptic efficiency.99,100 Throughout child, adolescent, and adult brain development, among many factors, establishing an efficient cortical network likely involves maturational changes to white matter,101,102 GABAergic interneurons,103,104 and the maturation of gap-junctions between neurons.105
Etiological and biological models of pathology that do not consider maturation risk being simplistic or even wrong. The previously described 40-Hz auditory steady-state SCZ findings perhaps provide an example. Whereas less 40-Hz auditory steady-state activity in SCZ than in controls is often taken as evidence of inhibitory interneuron and pyramidal cell dysfunction in SCZ,49 more 40-Hz auditory steady-state activity in SCZ has been hypothesized to indicate NMDA receptor dysfunction.51,81 These conclusions, based in part on animal models of 40-Hz activity,106,107 are potentially problematic as the temporal stability of 40-Hz group-difference findings are often not considered. The Edgar et al.45 findings suggest that the 40-Hz SCZ/control group difference in older participants is due to an age-related decrease of 40-Hz activity in controls. It is anticipated that models that consider time will have greater explanatory power than models that ignore maturation (see Vaidyanathan et al.108 for a recent discussion regarding experimental design in model generation).
An understanding of normal brain maturation is needed whether one takes a DSM-5 or an RDoC approach to research. What differentiates these approaches is primarily the level of analysis, with a DSM-5 approach focusing on diagnosis and an RDoC approach focusing on smaller units of analysis that may cut across DSM-5 diagnostic categories, such as psychological concepts and biological phenomena associated with disease.3,4 Although a DSM-5 approach considers maturation (e.g., diagnoses such as ASD require childhood onset), the RDoC approach is better positioned to accommodate maturational change in brain activity. Discussing the RDoC initiative, Lake et al.109 noted that an RDoC approach allows that, ‘The time course of the development of features of the disorder may vary considerably, and important psychological and biological changes may occur (and may be targets of preemptive intervention) before the clinical presentation is conventionally diagnosable’ (p. 171).
Given maturational changes in brain activity, seeking to identify electrophysiology markers across DSM-5 diagnoses via an RDoC approach is likely difficult, especially given variability in the age at which symptoms first present. Consideration of gamma-band activity provides an example. As detailed in Foss-Feig et al.,110 given the excitation and inhibition (E/I) imbalance proposed as underlying neural and behavioral dysfunction in ASD and SCZ, cross-diagnostic studies examining E/I imbalance are of interest. As previously described, in adults with SCZ the 40-Hz auditory steady-state exam is frequently used to examine the integrity of inhibitory interneuron and pyramidal cell circuits (and thus E/I balance). This task, however, is unlikely to be of use in studies examining children who have ASD, as the 40-Hz auditory steady-state response is often not observed in children and young adolescents.111,112 As an example, Edgar et al.113 showed that a robust 40-Hz auditory steady-state response was often not observed until mid to late adolescence, with a majority of the children in that study having 40-Hz auditory steady-state magnitudes too small to be distinguished from noise; thus the control and ASD between-group 40-Hz steady-state group comparisons were not informative.
Given the above limitations, studies seeking to use the 40-Hz steady-state procedure to evaluate similarities in auditory E/I imbalance in SCZ and ASD might examine older adolescents with ASD and adolescents with prodromal evidence of SCZ to obtain robust 40-Hz auditory steady-state responses in both groups. This approach, however, has limitations given that brain activity is examined in one group well after the onset of symptoms (and DSM-5 diagnosis) and in the other near the onset of symptoms (but prior to formal DSM-5 diagnosis). RDoC studies applying a trait-based approach – examining traits that inform a clinical diagnosis and that are detectable at a subclinical level114–119 – are of interest, although these would still have the problem of identifying an appropriate task for a given age. Given significant differences in brain activity among infants, toddlers, children, adolescents, and younger and older adults, creating tasks and a research design that produce interpretable findings across the life span and yet allow for development is difficult at best. Nevertheless, life span biomarker research is feasible with adequate understanding of the effects of maturation on the electrophysiological measure(s) of interest. As an example, Chen et al.94 showed that investigation of the maturation of auditory responses from infancy to adulthood suggests that at least some auditory event-related responses can be reliably tracked across the life span (see figure 3 in Chen et al.94).
Interest in maturation inevitably leads to longitudinal studies. With respect to clinical studies, a central question is whether longitudinal measures examining rate-of-change rather than single time-point measures will better predict symptom severity and outcome. Diagnostic and prognostic neural markers that change as a function of maturation have advantages. For example, measures that change in typical development necessarily show the potential to change and thus are of interest in studies examining the effect of treatment on these measures (exercise, cognitive behavioral, transcranial direct current stimulation [tDCS], or pharmacological). First, for a given brain region and brain measure, studies are needed to determine an average change value (and SD) across a fixed time period. It is hypothesized that neural measures will show variability in size similar to that obtained for developmental behavior observed ‘by eye.’ As an example, although on average children learn to walk at ~12 months, it is within normal range to start walking at 18 months. Not walking by 2 years, however, is of clinical concern. Similarly, if from 6 to 8 years the latency of a brain response has not decreased by ~9 ms in females and ~6 ms in males, this may prompt clinical concern.
Although the focus has been on neural activity, as previously noted, the examined issues are general and extend to other imaging modalities, such as structural and functional MRI. This is because brain structure also shows maturational change. Gray matter cortical thickness shows an interesting pattern: whereas in adults cortical thickness decreases as a function of age, cortical thickness increases in infants. As an example, peak cortical thickness in somatosensory cortex is not obtained until 7 to 10 years,120 and only during early adolescence, when dendritic branching stops and neural pruning begins, does cortical thickness start to decrease.121,122 Age-related changes are also observed for white matter, with a well-documented age-related increase in fractional anisotropy due to the myelination of infant white matter.123–125
Finally, and as an extension of the above, strong associations between age and brain measures limit our ability to detect structure–function associations distinct from shared but not causally associated maturation. Whereas larger samples would provide better estimates of the shared structure–function variance after accounting for age, larger samples do not necessarily allow examination of ‘true’ structure–function associations without the ‘confound’ of age. Large samples with a small age range (e.g., 6 to 7 years) are of interest, likely providing better estimates of structure–function by reducing the influence of maturation by restricting age.
To conclude, brain imaging findings show an effect of brain maturation on diagnostic markers separate from (and potentially difficult to distinguish from) effects of disease processes. Available research with large samples already provides direction about the age range(s) when diagnostic markers are most robust and informative.
Acknowledgments
This study was supported in part by the National Institutes of Health (NIH) grant R01MH107506 (to J.C.E.), National Institute of Child Health and Human Development grant R01HD093776 (to J.C.-E.), NIH grant R21MH098204 (to J.C.E.), and NIH grant R21 NS090192 (to J.C.E.). The author thanks Professor Gregory A. Miller for comments and suggestions on early versions of this manuscript.
Footnotes
Disclosure statement
Dr Edgar declares intellectual property relating to the potential use of electrophysiological markers for treatment planning in clinical ASD.
References
- 1.National Institute of Mental Health, Strategic research priorities, 2019. [Cited 11 September 2019.] Available from URL: https://www.nimh.nih.gov/about/strategic-planning-reports/strategic-research-priorities/index.shtml
- 2.Kozak MJ, Cuthbert BN. The NIMH Research Domain Criteria initiative: Background, issues, and pragmatics. Psychophysiology 2016; 53: 286–297. [DOI] [PubMed] [Google Scholar]
- 3.Miller GA, Rockstroh BS, Hamilton HK, Yee CM. Psychophysiology as a core strategy in RDoC. Psychophysiology 2016; 53: 410–414. [DOI] [PubMed] [Google Scholar]
- 4.Yee CM, Javitt DC, Miller GA. Replacing DSM categorical analyses with dimensional analyses in psychiatry research: The Research Domain Criteria initiative. JAMA Psychiatry 2015; 72: 1159–1160. [DOI] [PubMed] [Google Scholar]
- 5.Sahin M, Jones SR, Sweeney JA et al. Discovering translational bio-markers in neurodevelopmental disorders. Nat. Rev. Drug Discov 2018; 18: 235–236. 10.1038/d41573-018-00010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbs FA, Knott JR. Growth of the electrical activity of the cortex. Electroencephalogr. Clin. Neurophysiol 1949; 1: 223–229. [PubMed] [Google Scholar]
- 7.Corbin HP, Bickford RG. Studies of the electroencephalogram of normal children: Comparison of viscal and automatic frequency analyses. Electroencephalogr. Clin. Neurophysiol 1955; 7: 15–28. [DOI] [PubMed] [Google Scholar]
- 8.Matousek M, Petersen I. Automatic evaluation of EEG background activity by means of age-dependent EEG quotients. Electroencephalogr. Clin. Neurophysiol 1973; 35: 603–612. [DOI] [PubMed] [Google Scholar]
- 9.Eeg-Olofsson O. The development of the electroencephalogram in normal adolescents from the age of 16 through 21 years. Neuropadiatrie 1971; 3: 11–45. [DOI] [PubMed] [Google Scholar]
- 10.Gasser T, Verleger R, Bacher P, Sroka L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalogr. Clin. Neurophysiol 1988; 69: 91–99. [DOI] [PubMed] [Google Scholar]
- 11.Edgar JC, Khan SY, Blaskey L et al. Neuromagnetic noise predicts evoked-response delays and core language deficits in autism spectrum disorders. J. Autism Dev. Disord 2015; 45: 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisch B. Fisch and Spehlmann‘s EEG Primer: Basic Principles of Digital and Analog EEG, 3rd edn. Elsevier, Amsterdam, 1999. [Google Scholar]
- 13.Matsuura M, Yamamoto K, Fukuzawa H et al. Age development and sex differences of various EEG elements in healthy children and adults: Quantification by a computerized wave form recognition method. Electroencephalogr. Clin. Neurophysiol 1985; 60: 394–406. [DOI] [PubMed] [Google Scholar]
- 14.Eeg-Olofsson O, Petersen I, Sellden U. The development of the electroencephalogram in normal children from the age of 1 through 15 years. Paroxysmal activity. Neuropadiatrie 1971; 2: 375–404. [DOI] [PubMed] [Google Scholar]
- 15.Niedermeyer E, Lopes da Silva FH. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, 5th edn. Lippincott Williams & Wilkins, Philadelphia, 2005. [Google Scholar]
- 16.Cragg L, Kovacevic N, McIntosh AR et al. Maturation of EEG power spectra in early adolescence: A longitudinal study. Dev. Sci 2011; 14: 935–943. [DOI] [PubMed] [Google Scholar]
- 17.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: Development of the normal child. Clin. Neurophysiol 2001; 112: 806–814. [DOI] [PubMed] [Google Scholar]
- 18.Katada A, Ozaki H, Suzuki H, Suhara K. Developmental characteristics of normal and mentally retarded children’s EEGs, Electroencephalogr. Clin. Neurophysiol 1981; 52: 192–201. [DOI] [PubMed] [Google Scholar]
- 19.Haegens S, Cousijn H, Wallis G, Harrison PJ, Nobre AC. Inter- and intra-individual variability in alpha peak frequency. NeuroImage 2014; 92: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgar JC, Heiken K, Chen YH et al. Resting-state alpha in autism spectrum disorder and alpha associations with thalamic volume. J. Autism Dev. Disord 2015; 45: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang MX, Huang CW, Robb A et al. MEG source imaging method using fast L1 minimum-norm and its applications to signals with brain noise and human resting-state source amplitude images. NeuroImage 2014; 84: 585–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salmelin R, Hari R. Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalogr. Clin. Neurophysiol 1994; 91: 237–248. [DOI] [PubMed] [Google Scholar]
- 23.Berger H. Über das elektrenkephalogramm des menschen. Arch. Psychiatr. Nervenkr 1929; 87: 527–570. [Google Scholar]
- 24.Szava S, Valdes P, Biscay R et al. High resolution quantitative EEG analysis. Brain Topogr. 1994; 6: 211–219. [DOI] [PubMed] [Google Scholar]
- 25.Valdes P, Valdes M, Carballo JA et al. QEEG in a public health system. Brain Topogr. 1992; 4: 259–266. [DOI] [PubMed] [Google Scholar]
- 26.Miskovic V, Ma X, Chou CA et al. Developmental changes in spontaneous electrocortical activity and network organization from early to late childhood. Neuroimage 2015; 118: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somsen RJ, van’t Klooster BJ, van der Molen MW, van Leeuwen HM, Licht R. Growth spurts in brain maturation during middle childhood as indexed by EEG power spectra. Biol. Psychol 1997; 44: 187–209. [DOI] [PubMed] [Google Scholar]
- 28.Petersen I, Eeg-Olofsson O. The development of the electroencephalogram in normal children from the age of 1 through 15 years. Nonparoxysmal activity. Neuropadiatrie 1971; 2: 247–304. [DOI] [PubMed] [Google Scholar]
- 29.Van Baal GC, De Geus EJ, Boomsma DI. Genetic architecture of EEG power spectra in early life. Electroencephalogr. Clin. Neurophysiol 1996; 98: 502–514. [DOI] [PubMed] [Google Scholar]
- 30.van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: A review and a meta-analysis. Biol. Psychol 2002; 61: 111–138. [DOI] [PubMed] [Google Scholar]
- 31.Smit DJ, Posthuma D, Boomsma DI, Geus EJ. Heritability of background EEG across the power spectrum. Psychophysiology 2005; 42: 691–697. [DOI] [PubMed] [Google Scholar]
- 32.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Brain Res. Rev 1999; 29: 169–195. [DOI] [PubMed] [Google Scholar]
- 33.Klimesch W. EEG-alpha rhythms and memory processes. Int. J. Psychophysiol 1997; 26: 319–340. [DOI] [PubMed] [Google Scholar]
- 34.Klimesch W, Doppelmayr M, Schimke H, Pachinger T. Alpha frequency, reaction time, and the speed of processing information. J. Clin. Neurophysiol 1996; 13: 511–518. [DOI] [PubMed] [Google Scholar]
- 35.Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci 2012; 16: 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickinson A, DiStefano C, Senturk D, Jeste SS. Peak alpha frequency is a neural marker of cognitive function across the autism spectrum. Eur. J. Neurosci 2018; 47: 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edgar JC, Dipiero M, McBride E et al. Abnormal maturation of the resting-state peak alpha frequency in children with autism spectrum disorder. Hum. Brain Mapp 2019; 40: 3288–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stroganova TA, Orekhova EV, Posikera IN. EEG alpha rhythm in infants. Clin. Neurophysiol 1999; 110: 997–1012. [DOI] [PubMed] [Google Scholar]
- 39.Mulholland T. Human EEG, behavioral stillness and biofeedback. Int. J. Psychophysiol 1995; 19: 263–279. [DOI] [PubMed] [Google Scholar]
- 40.Popova P, Rockstroh B, Miller GA, Wienbruch C, Carolus AM, Popov T. The impact of cognitive training on spontaneous gamma oscillations in schizophrenia. Psychophysiology 2018; 55: e13083. [DOI] [PubMed] [Google Scholar]
- 41.Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn. Sci 2010; 14: 72–80. [DOI] [PubMed] [Google Scholar]
- 42.Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia: A critical review. Psychiatry Res. 2008; 161: 259–274. [DOI] [PubMed] [Google Scholar]
- 43.Blumenfeld LD, Clementz BA. Response to the first stimulus determines reduced auditory evoked response suppression in schizophrenia: Single trials analysis using MEG. Clin. Neurophysiol 2001; 112: 1650–1659. [DOI] [PubMed] [Google Scholar]
- 44.Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp. Brain Res 2001; 139: 377–390. [DOI] [PubMed] [Google Scholar]
- 45.Edgar JC, Fisk CL IV, Chen YH et al. Identifying auditory cortex encoding abnormalities in schizophrenia: The utility of low-frequency versus 40 Hz steady-state measures. Psychophysiology 2018; 55: e13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edgar JC, Hanlon FM, Huang MX et al. Superior temporal gyrus spectral abnormalities in schizophrenia. Psychophysiology 2008; 45: 812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johannesen JK, Kieffaber PD, O’Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophr. Res 2005; 78: 269–284. [DOI] [PubMed] [Google Scholar]
- 48.Jansen BH, Hegde A, Boutros NN. Contribution of different EEG frequencies to auditory evoked potential abnormalities in schizophrenia. Clin. Neurophysiol 2004; 115: 523–533. [DOI] [PubMed] [Google Scholar]
- 49.Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: Towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 2012; 62: 1504–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brenner CA, Sporns O, Lysaker PH, O’Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am. J. Psychiatry 2003; 160: 2238–2240. [DOI] [PubMed] [Google Scholar]
- 51.Hamm JP, Gilmore CS, Clementz BA. Augmented gamma band auditory steady-state responses: Support for NMDA hypofunction in schizophrenia. Schizophr. Res 2012; 138: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong LE, Summerfelt A, McMahon R et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr. Res 2004; 70: 293–302. [DOI] [PubMed] [Google Scholar]
- 53.Koenig T, van Swam C, Dierks T, Hubl D. Is gamma band EEG synchronization reduced during auditory driving in schizophrenia patients with auditory verbal hallucinations? Schizophr. Res 2012; 141: 266–270. [DOI] [PubMed] [Google Scholar]
- 54.Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O’Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. NeuroImage 2009; 47: 1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwon JS, O’Donnell BF, Wallenstein GV et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch. Gen. Psychiatry 1999; 56: 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lenz D, Fischer S, Schadow J, Bogerts B, Herrmann CS. Altered evoked gamma-band responses as a neurophysiological marker of schizophrenia? Int. J. Psychophysiol 2011; 79: 25–31. [DOI] [PubMed] [Google Scholar]
- 57.Light GA, Hsu JL, Hsieh MH et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol. Psychiatry 2006; 60: 1231–1240. [DOI] [PubMed] [Google Scholar]
- 58.Maharajh K, Teale P, Rojas DC, Reite ML. Fluctuation of gamma-band phase synchronization within the auditory cortex in schizophrenia. Clin. Neurophysiol 2010; 121: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rass O, Forsyth JK, Krishnan GP et al. Auditory steady state response in the schizophrenia, first-degree relatives, and schizotypal personality disorder. Schizophr. Res 2012; 136: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009; 10: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. NeuroImage 2008; 42: 1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuchimoto R, Kanba S, Hirano S et al. Reduced high and low frequency gamma synchronization in patients with chronic schizophrenia. Schizophr. Res 2011; 133: 99–105. [DOI] [PubMed] [Google Scholar]
- 63.Roach BJ, Ford JM, Mathalon DH. Gamma band phase delay in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019; 4: 131–139. [DOI] [PubMed] [Google Scholar]
- 64.Hirano Y, Oribe N, Kanba S, Onitsuka T, Nestor PG, Spencer KM. Spontaneous gamma activity in schizophrenia. JAMA Psychiatry 2015; 72: 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirano S, Nakhnikian A, Hirano Y et al. Phase-amplitude coupling of the electroencephalogram in the auditory cortex in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018; 3: 69–76. [DOI] [PubMed] [Google Scholar]
- 66.Kirihara K, Rissling AJ, Swerdlow NR, Braff DL, Light GA. Hierarchical organization of gamma and theta oscillatory dynamics in schizophrenia. Biol. Psychiatry 2012; 71: 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobson GP, Fitzgerald MB. Auditory evoked gamma band potential in normal subjects. J. Am. Acad. Audiol 1997; 8: 44–52. [PubMed] [Google Scholar]
- 68.Pantev C. Evoked and induced gamma-band activity of the human cortex. Brain Topogr. 1995; 7: 321–330. [DOI] [PubMed] [Google Scholar]
- 69.Hari R. The neuromagnetic method in the study of the human auditory cortex. In: Grandori F, Hoke M, Romani GL (eds). Advances in Audiology: Auditory Evoked Magnetic Fields and Potentials. Karger, Basel, 1990; 222–282. [Google Scholar]
- 70.Hari R, Aittoniemi K, Jarvinen ML, Katila T, Varpula T. Auditory evoked transient and sustained magnetic fields of the human brain. Localization of neural generators. Exp. Brain Res 1980; 40: 237–240. [DOI] [PubMed] [Google Scholar]
- 71.Huotilainen M, Winkler I, Alho K et al. Combined mapping of human auditory EEG and MEG responses. Electroencephalogr. Clin. Neurophysiol 1998; 108: 370–379. [DOI] [PubMed] [Google Scholar]
- 72.Makela JP, Hamalainen M, Hari R, McEvoy L. Whole-head mapping of middle-latency auditory evoked magnetic fields. Electroencephalogr. Clin. Neurophysiol 1994; 92: 414–421. [DOI] [PubMed] [Google Scholar]
- 73.Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology 1987; 24: 375–25. [DOI] [PubMed] [Google Scholar]
- 74.Pelizzone M, Hari R, Makela JP, Huttunen J, Ahlfors S, Hamalainen M. Cortical origin of middle-latency auditory evoked responses in man. Neurosci. Lett 1987; 82: 303–307. [DOI] [PubMed] [Google Scholar]
- 75.Reite M, Teale P, Zimmerman J, Davis K, Whalen J. Source location of a 50 msec latency auditory evoked field component. Electroencephalogr. Clin. Neurophysiol 1988; 70: 490–498. [DOI] [PubMed] [Google Scholar]
- 76.Yvert B, Crouzeix A, Bertrand O, Seither-Preisler A, Pantev C. Multiple supratemporal sources of magnetic and electric auditory evoked middle latency components in humans. Cereb. Cortex 2001; 11: 411–423. [DOI] [PubMed] [Google Scholar]
- 77.Herdman AT, Wollbrink A, Chau W, Ishii R, Ross B, Pantev C. Determination of activation areas in the human auditory cortex by means of synthetic aperture magnetometry. NeuroImage 2003; 20: 995–1005. [DOI] [PubMed] [Google Scholar]
- 78.Ross B, Picton TW, Pantev C. Temporal integration in the human auditory cortex as represented by the development of the steady-state magnetic field. Hear. Res 2002; 165: 68–84. [DOI] [PubMed] [Google Scholar]
- 79.Edgar JCC, H Y, Lanza M et al. Cortical thickness as a contributor to abnormal oscillations in schizophrenia? Neuroimage Clin. 2014; 4: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thune H, Recasens M, Uhlhaas PJ. The 40-Hz auditory steady-state response in patients with schizophrenia: A meta-analysis. JAMA Psychiatry 2016; 73: 1145–1153. [DOI] [PubMed] [Google Scholar]
- 81.Kim S, Jang SK, Kim DW et al. Cortical volume and 40-Hz auditory-steady-state responses in patients with schizophrenia and healthy controls. Neuroimage Clin. 2019; 22: 101732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson TW, Hernandez OO, Asherin RM, Teale PD, Reite ML, Rojas DC. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb. Cortex 2008; 18: 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Res. 2011; 1380: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. NeuroImage 2002; 16: 1038–1051. [DOI] [PubMed] [Google Scholar]
- 85.Schumann CM, Bloss CS, Barnes CC et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J. Neurosci 2010; 30: 4419–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zielinski BA, Prigge MB, Nielsen JA et al. Longitudinal changes in cortical thickness in autism and typical development. Brain 2014; 137: 1799–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ecker C, Bookheimer SY, Murphy DG. Neuroimaging in autism spectrum disorder: Brain structure and function across the lifespan. Lancet Neurol. 2015; 14: 1121–1134. [DOI] [PubMed] [Google Scholar]
- 88.Wiggins JL, Peltier SJ, Ashinoff S et al. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain Res. 2011; 1380: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front. Hum. Neurosci 2013; 7: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ouyang M, Cheng H, Mishra V et al. Atypical age-dependent effects of autism on white matter microstructure in children of 2-7 years. Hum. Brain Mapp 2016; 37: 819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sheffield JM, Repovs G, Harms MP et al. Evidence for accelerated decline of functional brain network efficiency in schizophrenia. Schizophr. Bull 2016; 42: 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Haren NE, Hulshoff Pol HE, Schnack HG et al. Progressive brain volume loss in schizophrenia over the course of the illness: Evidence of maturational abnormalities in early adulthood. Biol. Psychiatry 2008; 63: 106–113. [DOI] [PubMed] [Google Scholar]
- 93.Guo X, Chen H, Long Z, Duan X, Zhang Y, Chen H. Atypical developmental trajectory of local spontaneous brain activity in autism spectrum disorder. Sci. Rep 2017; 7: 39822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen YH, Saby J, Kuschner E, Gaetz W, Edgar JC, Roberts TPL. Magnetoencephalography and the infant brain. NeuroImage 2019; 189: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ouyang M, Dubois J, Yu Q, Mukherjee P, Huang H. Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. NeuroImage 2019; 185: 836–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kurth S, Ringli M, Lebourgeois MK et al. Mapping the electrophysiological marker of sleep depth reveals skill maturation in children and adolescents. NeuroImage 2012; 63: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc. Natl. Acad. Sci. U. S. A 2009; 106: 9866–9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.John ER, Ahn H, Prichep L, Trepetin M, Brown D, Kaye H. Developmental equations for the electroencephalogram. Science 1980; 210: 1255–1258. [DOI] [PubMed] [Google Scholar]
- 99.Goodin DS, Squires KC, Henderson BH, Starr A. Age-related variations in evoked potentials to auditory stimuli in normal human subjects. Electroencephalogr. Clin. Neurophysiol 1978; 44: 447–58. [DOI] [PubMed] [Google Scholar]
- 100.Eggermont JJ. On the rate of maturation of sensory evoked potentials. Electroencephalogr. Clin. Neurophysiol 1988; 70: 293–305. [DOI] [PubMed] [Google Scholar]
- 101.Berman JI, Chudnovskaya D, Blaskey L et al. Relationship between M100 auditory evoked response and auditory radiation microstructure in 16p11.2 deletion and duplication carriers. AJNR Am. J. Neuroradiol 2016; 37: 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roberts TP, Khan SY, Blaskey L et al. Developmental correlation of diffusion anisotropy with auditory-evoked response. Neuroreport 2009; 20: 1586–1591. [DOI] [PubMed] [Google Scholar]
- 103.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 1995; 378: 75–78. [DOI] [PubMed] [Google Scholar]
- 104.Wang XJ, Buzsaki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J. Neurosci 1996; 16: 6402–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Montoro RJ, Yuste R. Gap junctions in developing neocortex: A review. Brain Res. Brain Res. Rev 2004; 47: 216–226. [DOI] [PubMed] [Google Scholar]
- 106.Lambert NA, Wilson WA. Temporally distinct mechanisms of use-dependent depression at inhibitory synapses in the rat hippocampus in vitro. J. Neurophysiol 1994; 72: 121–130. [DOI] [PubMed] [Google Scholar]
- 107.Carlen M, Meletis K, Siegle JH et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol. Psychiatry 2012; 17: 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vaidyanathan U, Vrieze SI, Iacono WG. The power of theory, research design, and transdisciplinary integration in moving psychopathology forward. Psychol. Inq 2015; 26: 209–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lake JI, Yee CM, Miller GA. Misunderstanding RDoC. Z. Psychol 2017; 225: 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Foss-Feig JH, Adkinson BD, Ji JL et al. Searching for cross-diagnostic convergence: Neural mechanisms governing excitation and inhibition balance in schizophrenia and autism spectrum disorders. Biol. Psychiatry 2017; 81: 848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rojas DC, Maharajh K, Teale PD et al. Development of the 40Hz steady state auditory evoked magnetic field from ages 5 to 52. Clin. Neurophysiol 2006; 117: 110–117. [DOI] [PubMed] [Google Scholar]
- 112.Cho RY, Walker CP, Polizzotto NR et al. Development of sensory gamma oscillations and cross-frequency coupling from childhood to early adulthood. Cereb. Cortex 2015; 25: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Edgar JC, Fisk CL IV, Liu S et al. Translating adult electrophysiology findings to younger patient populations: Difficulty measuring 40-Hz auditory steady-state responses in typically developing children and children with autism spectrum disorder. Dev. Neurosci 2016; 38: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): Evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord 2001; 31: 5–17. [DOI] [PubMed] [Google Scholar]
- 115.Wheelwright S, Auyeung B, Allison C, Baron-Cohen S. Defining the broader, medium and narrow autism phenotype among parents using the autism spectrum quotient (AQ). Mol. Autism 2010; 1: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoekstra RA, Bartels M, Verweij CJ, Boomsma DI. Heritability of autistic traits in the general population. Arch. Pediatr. Adolesc. Med 2007; 161: 372–377. [DOI] [PubMed] [Google Scholar]
- 117.Posserud MB, Lundervold AJ, Gillberg C. Autistic features in a total population of 7-9-year-old children assessed by the ASSQ (Autism Spectrum Screening Questionnaire). J. Child Psychol. Psychiatry 2006; 47: 167–175. [DOI] [PubMed] [Google Scholar]
- 118.Miller GA, Crocker LD, Spielberg JM, Infantolino ZP, Heller W. Issues in localization of brain function: The case of lateralized frontal cortex in cognition, emotion, and psychopathology. Front. integr. Neurosci 2013; 7: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Silton RL, Heller W, Engels AS et al. Depression and anxious apprehension distinguish frontocingulate cortical activity during top-down attentional control. J. Abnorm. Psychol 2011; 120: 272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shaw P, Kabani NJ, Lerch JP et al. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci 2008; 28: 3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gogtay N, Giedd JN, Lusk L et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A 2004; 101: 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci 2004; 1021: 77–85. [DOI] [PubMed] [Google Scholar]
- 123.Partridge SC, Mukherjee P, Henry RG et al. Diffusion tensor imaging: Serial quantitation of white matter tract maturity in premature newborns. NeuroImage 2004; 22: 1302–1314. [DOI] [PubMed] [Google Scholar]
- 124.Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin. Fetal Neonatal Med 2006; 11: 489–497. [DOI] [PubMed] [Google Scholar]
- 125.Dubois J, Dehaene-Lambertz G, Perrin M et al. Asynchrony of the early maturation of white matter bundles in healthy infants: Quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum. Brain Mapp 2008; 29: 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]