Abstract
Our understanding of the pathogenesis of large vessel vasculitis (LVV) are mainly achieved by studying the arteries taken from temporal artery biopsy in giant cell arteries (GCA) or surgical or autopsy specimens in Takayasu arteritis (TAK). These artery specimens provide invaluable information about pathological changes in these conditions that GCA and TAK are similar but are distinctly different in immune cell infiltrate and distribution of inflammatory cells in anatomical locations. However, these specimens of established arteritis do not provide information of the arteritis initiation and early events which are impossible to obtain in human artery specimens. Animal models for LVV are needed but not available. Here, several approaches are proposed for experimentation to generate animal models to aid in delineating the interaction of immune reaction with arterial wall components.
Keywords: large vessel vasculitis, animal models, interleukin-1, interferon regulatory factor-4
Introduction
Giant cell arteritis (GCA) and Takayasu arteritis (TAK) are large vessel vasculitis (LVV) affecting the aorta and its branches. LVV are pan-arteritis, that is, the inflammation involves the three layers of artery wall. The typical histologic changes in the affected arteries of LVV are distortion of the media: loss of smooth muscle cells and refilled with areas of inflammatory cells and fibrosis. Inflammatory cells consist of CD4+ and CD8+ T cells, macrophages, natural killer (NK) cells, and plasma cells; granulomatous formation with giant cells is also present. The intima is thickened with inflammation and fibrous changes. Substantial difference is observed between GCA and TAK although similarities in the histologic findings described above. For example, there are more CD8+ T cells, B cells and NK cells infiltrating the media of TAK artery and the adventitia is substantially expanded with fibrosis and pauci-cellular infiltration.[1] These changes in TAK represent histologic findings of advanced and terminal stages of specimens from autopsy or surgically resected arteries.[1,2,3,4]
Elastic artery wall is viewed as an immune privileged site. The media is anatomically barriered by inner and outer elastic layers which rendered the medial layer inaccessible by immune or inflammatory cells.[5] That being the case, how the immune tolerance is breached in LVV is not known. One hypothesis is that inflammation of LVV might be initiated in the vasa vasorum.[6,7] A vasa vasorum is a network of vessels which supplies blood circulation within the adventitia of large artery wall and can be a port for an initial insulting factor to get access to the artery. Indeed, vasculitis and neovascularization in vasa vasorum are found in samples of arteries of TAK patients.[2,3,4,6,8] Inflammatory infiltration starts around vasa vasorum and granuloma formation with giant cells are usually found in the outer layer of media.[7] The similar notion has also been proposed for GCA and is supported by histological findings that newly formed vasa vasorum is presented in inflamed GCA samples[9] and in uninflamed temporal arteries.[10] However, these observations cannot simply be interpreted as the inflammation process begins at vasa vasorum since that it can be the case that inflammation in the media or adventitia extends to vasa vasorum. It is almost impossible to prove or refute this hypothesis in LVV since biopsy of a large artery during the early event is not feasible. Practically, it is extremely challenging to investigate the early events of LVV in humans but this information is critical for design targeted earlier intervention for LVV, especially for TAK. Thereby, appropriate animal models of LVV are needed to be developed.
Human Artery Xenograft Mouse Models
Human artery engraft to immune deficient mice was pioneered by Weyand's group.[11] Temporal artery segments from biopsy samples of patients with GCA were implanted subcutaneously into nonobese diabetic (NOD)-severe combined immunodeficient (SCID) mice.[11] Arteritis is selfsustained after engraft in SCID mice. This human artery-SCID mouse model has been useful to test various therapeutics for GCA.[11,12,13] This model has also provided critical information about how adventitial dendritic cells (DC) are activated in GCA. Normal temporal arteries contain immature DC in the adventitia. After implantation is established in SCID mice, these adventitial resident DC remain to be immature until after they are stimulated by Toll-like receptor (TLR) ligands which are administered systemically.[14] It is likely that the TLR ligands gained access to the adventitial DC via vasa vasorum. Interestingly, adventitial DC in temporal arteries (no GCA) from patients with polymyalgia rheumatica (PMR), when co-implanted with an inflamed temporal artery from GCA, the mature adventitial DC of PMR can recruit T cells that originated from the GCA artery. Thus, the in situ activation of adventitial DC may initiate and maintain T cell response and arterial tissue destruction.[14] These findings, however, may not represent what may have happened in TAK since difference exists in artery status and immune system function between TAK patients (younger) and GCA patients (older).
Recently, Chen and colleagues[15] successfully transplanted temporal artery segments from GCA patients into Nude mice by anastomosis to abdominal aorta. The GCA arteries were subjected for color duplex sonography and enhanced computerized tomography studies. The arteritis of grafted human GCA arteries sustained as demonstrated by the above imaging tests and histological assays. This artery-artery anastomosis is more anatomically physiological and may have a broader application for mechanistic studies. For example, engrafting non-GCA temporal arteries (biopsied from PMR patients) supplemented with peripheral blood mononuclear cells may facilitate studies to investigate triggering factors for development of GCA. Unfortunately, it is impossible to replicate the human artery-mouse chimera experiments to investigate the early events for TAK since arteries without arteritis from younger patients will not be available. Alternative experimental approaches in native arteries of animals must be sought.
Induction of Arteritis via Vasa Vasorum
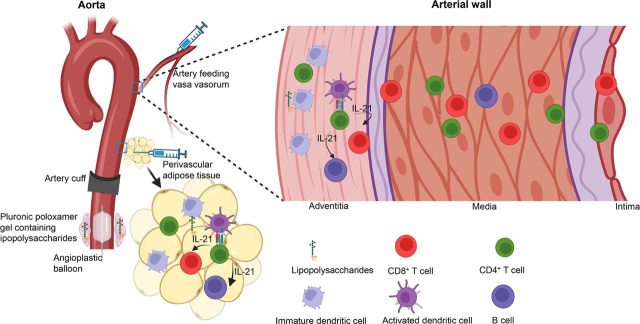
TLR has been implicated in the pathogenesis of LVV.[16,17,18,19] In theory, it is possible to activate adventitial DC in segments of large arteries by systemic administration of TLR ligands in genetic susceptible animals, but such genetically susceptible animal models are not yet identified. Alternatively, TLR ligands can be injected via originating arteries feeding vasa vasorum of certain segments of aorta or main aorta branches (Figure 1), so that a high concentration gradient of TLR ligands can be achieved at the vasa vasorum to adventitial DC since vasa vasorum are end-arteries solely responsible for supplying blood to the local tissue.[20,21] However, this approach is technically challenging, especially in rodents, not only for the small diameter of arteries, but also it is debatable whether the aorta of mice has vasa vasorum. Previous studies identified vasa vasorum in mouse aorta albeit in a very low density,[22,23] but other studies failed to identify such a vasa vasorum network[24] (Cornelia Weyand, MD, PhD, email communication, November 2, 2022). In large animals whose aorta contains vasa vasorum, the originating arteries can be identified with assistance of imaging technology such as intravital microscopy or indocyanine green (ICG) fluorescence in live animals.[25] As early as in 1965, using X-ray microscopy, Clarke has identified that brachiocephalic and intercostal arteries feed ascending and descending aorta respectively, while the lumbar and mesenteric arteries feed the abdominal artery in post-natal humans.[26] It appears feasible to verify these vasa vasorum originating arteries in live animals such as porcine models using ICG fluorescence and perform selective intraarterial injections with TLR ligands to activate adventitial DC (Figure 1).
Figure 1.
Schematic graph depicting approaches to produce animal models of large vessel vasculitis. IL-21, interleukin 21.
Periarterial Adipose Tissue as a Portal to Activate Adventitial DCs
Alternative to selective injection towards vasa vasorum, initiating inflammatory process in periarterial adipose tissue leading to adventitial DC activation would be technically easier and these can be performed in mouse models. It has been recently recognized that perivascular adipose tissue (PVAT) is metabolically active and has a protective role in vessel homeostasis and is viewed as the fourth layer of the large arteries.[27] Involvement of PVAT in atherosclerosis has been recognized.[27,28] It is also speculated that PVAT participate in TAK pathogenesis but direct evidence is lacking.[29] PVAT is anatomically part of the arterial wall and perivascular vessel network is connected. An inflammatory process can be experimentally provoked in PVAT. For instance, TLR ligands can be injected in the PVAT in the selected segments of aorta (Figure 1) to initiate activation of DC in PVAT, and subsequently, adventitial DC can be activated. This approach can be investigated in mice, in particular, in those genetically modified mice, e.g. interferon regulatory factor (IRF)-4 binding protein (IBP) deficient mice and interleukin-1 receptor antagonist (IL-Ra) deficient (Il1rn−/−) mice which develop inflammation in aorta.[30–31]
Arterial Cuffing to Induce Arterial Injury
Experimentally induced arterial injury such as arterial cuffing can induce intimal hyperplasia and inflammation in the media along with inflammation changes in the adventitia.[33] This method has been extensively adopted in genetic modified atherosclerosis prone mice for investigation of inflammation process in the arterial wall.[34,35,36,37] It was proposed that artery cuffing injury may cause obstruction of vasa vasorum leading to regional ischemia in adventitia.[33] Similarly, cuffing a segment of aorta (Figure 1) in IBP deficient or IL-1Ra deficient mice will serve as a model to delineate the early events after artery injury. Isoda et al investigated artery reaction upon cuff injury in IL-1Ra deficient mice (they do not develop aortitis on C57BL/6 background, see below).[38,39] Inflammation appears as early as 3 days after artery injury and this was followed by intima thickening later. There is no inflammation in media noted in this model. These results may suggest that mechanical injury insult is not sufficient to initiate pan-arteritis and other factors such as TLR stimulation is also required. It would be interesting to explore combination of cuff injury with systemic administration of TLR ligands. In this case, mechanical injury initiates inflammation at the adventitia and TLR ligands activate adventitial DC.
Intraluminal Balloon to Induce Arterial Injury
Intraluminal balloon injury triggers inflammation of the artery wall. One of the features of the mechanical injury is neovasculization in vasa vasorum. This has been demonstrated in animal models in mice[40,41] and rats.[42,43] Mouse models have been used in appropriate genetic background for modeling atherosclerosis and this is often complemented with high fat diet.[40,41] However, there is no finding which resembles changes seen in LVV. Like in the artery cuffing model, the balloon injury procedure can be complemented with systemic administration of TLR ligands for activation of adventitial DC. Alternatively, TLR ligands can be applied in a perivascular space contained in pluronic poloxamer gel which is connected with the segment of injured artery (Figure 1). Pluronic poloxamer gel is liquid at 4°C and can rapidly solidify at 37°C when in contact with tissues in vivo. The gel is hydrophilic and degrades rapidly in an aqueous environment. This method has been successfully used to supply monoclonal antibody, ranibizumab locally via vasa vasorum to block vascular endothelial growth factor activity for suppression of fibrosis in the balloon injured artery wall.[42] In mice, balloon injury can be performed in native carotid artery[41] or in aorta in a graft procedure.[40] The latter involves dilation of a segment of aorta and then grafted and ligated with carotid artery in a littermate recipient mouse.[40] Both procedures are technically feasible to perform and reproducible.
Arteritis-Prone Mouse Models for Modification to Produce LVV Models
IL-1Ra blocks function of both IL-1α and IL-1β and is important in maintenance of immune homeostasis. In mice, IL-1Ra deficiency renders mice develop arteritis which can occur spontaneously[30,31,44,45] or upon artery injury.[38,39] Arteritis development is dependent on genetic background. That is, spontaneous arteritis develops in IL-1Ra deficient mice on BALB/c[44,45] or 129/O1a x MF1[30,31] background but not on C57BL/6.[38,39] Spontaneous aortitis can develop as early as 4 weeks of age and the vessel wall inflammation infiltrates include CD4+, CD8+ T cells and monocytes/macrophages throughout adventitia, media and intima along with destruction of media and elastic layers.[30,31,44,45] The pathology of arteritis in these mice is delineated meticulously and the histologic changes resemble those findings in LVV arteries. Interestingly, the development of arteritis in these mice is T cell mediated and dependent on tumor necrosis factor (TNF).[44] Unexpectedly, IL-25 (also called IL-17E) can exacerbate aortitis presumably by inducing IL-1β production by DC and TNF by macrophages.[45] However, the specificity that the T cells react with is not known. Moreover, it is not clear at which anatomical site of the artery wall and how the inflammation initiate. Could adventitial DC become activated due to the unopposed IL-1 activity? On the other hand, arteritis does not develop spontaneously but can be induced by artery wall injury such as artery cuffing in IL-1Ra deficient C57BL/6 mice.[38,39] Thus, they provide a valuable model to investigate the initial insult which triggers arteritis in LVV.
IRF-4 is a crucial regulator for T helper type 17 (Th17) cell differentiation. IBP negatively regulates IRF-4 function. IBP deficient mice develop inflammatory arthritis and inflammation of large vessel wall.[32] The transmural inflammation involves aortic root and medium size arteries of the kidney and the lung. Inflammatory infiltrates include lymphocytes, monocytes, plasma cells, and neutrophils. Multinucleated giant cells are also present. These histological changes resemble those of human LVV. The arthritis and arteritis of IBP deficient mice are accompanied by dysregulated IL-17 and IL-21 production with increased T cell receptor (TCR) response. It is intriguing but interesting that IBP deficient mice carrying transgenic TCR specific to ovalbumin (OVA) peptides cause aortitis. It was suggested that the OVA peptide specific TCR may execute cross reaction to endogenous peptides.[32] But why the inflammation is localized to the aorta. Presumably antigens of the artery wall were released and recognized by the over responsive TCR. The aortitis in these TCR transgenic mice develops in rather old age (12 weeks) which may be considered as a model for GCA. To mimic human TAK, one may design experiments to provoke mechanical artery injury such as artery cuffing or intraluminal balloon injury in younger mice at different ages with and without systemic TLR stimulation.
A fundamental question pertinent to these two mouse models is how the arterial wall immune privilege is breached remains elusive. Nevertheless, findings in these two spontaneous mouse models of aortitis have implications for targeted therapy in GCA and TAK. For example, TNFi inhibitors showed a better therapeutic effect for TAK than that for GCA.[46,47,48,49] Inhibition of Th17 by using ustekinmab is being actively studied in TAK (NCT04882072). Anakinra (the recombinant IL-1Ra) was tried and showed effective in treatment of refractory GCA[50] but IL-1 blockade has not been tried in TAK. Given the robust evidence showing the critical role of IL-1 in developing arteritis in these models and increased IL-1β expression by peripheral blood mononuclear cells of TAK patients in response to TLR4 stimulation,[17] IL-1 is a valid target for treatment of TAK. It is well documented that IL-21 is produced by T follicular helper (Tfh) cells and directly acts on B cells to generate high affinity class-switched antibodies. IL-21 receptor is also expressed by CD8+ T cells implicating IL-21 may act on CD8+ T cells.[51] Tfh cell gene signature has been detected in peripheral blood and aortic infiltrate T cells from patients with TAK.[52,53] A larger number of CD8+ T cells are present in arteritis lesions in TAK than in GCA.[1] It has been shown that in GCA, IL-21 promotes Th1 and Th17 differentiation and represses Foxp3 expression in T regulatory cells.[54] All these imply that IL-21 might play an important role in the pathogenesis of LVV and IL-21 signaling pathway might be a therapeutic target. However, information regarding IL-21 in patients with TAK is lacking.
The integrity of the artery wall immunoprivilege is maintained by active biological processes in addition to anatomical barriers,[5,55] which collectively protect the media of the artery wall from infection or inflammatory attacks. Interferon-γ (IFN-γ) signaling in vascular smooth muscle cells and immune cells is critical for this protection.[5] A defect in IFN-γ signaling can lead to the breach of immunoprivilege. For example, genetic deficiency in the IFN-γ receptor (IFN-γR) renders mice susceptible to chronic infection with γ-herpes virus 68 and murine cytomegalovirus, leading to chronic vasculitis.[5,56] While it has been speculated but not evident that viral infection may trigger GCA and TAK, and IFN-γ signaling deficiency has not been investigated in LVV patients. In Turkish, Northern European descendant, Han Chinese, South Asian, and Italian populations, the IL-12B single nucleotide polymorphism (SNP) rs4379175 is associated with TAK;[57] rs6871626 is associated with TAK in Han Chinese and Japanese patients;[58,59] and rs56167332 is associated with TAK in Turkish and Northern American populations.[60] Moreover, SNP rs6871626 is associated with increased serum levels of IL-12p40 and IL-12p70, which likely drives the increased Th1 cells in TAK patients.[61] These findings suggest that the IL-12/Th1 axis may promote the disease process in TAK. On the other hand, it is possible that in TAK, IFN-γR and/or downstream signaling is defective, and the increased IL-12/Th1 is the result of negative feedback. The defective IFN-γ signaling pathway results in the breach of the arterial wall immunoprivilege upon infection or autoantigen stimulation. Investigating IFN-γ signaling in vascular cells in patients with LVV may provide novel clues towards the early events in LVV.
Concluding Remarks
Insightful understanding of the underlying pathogenesis of LVV is crucial for discovering novel therapeutic targets. However, there is still a significant knowledge gap in understanding the interaction between the immune system and artery wall components, particularly in the early events due to the lack of sequential samples of inflamed arteries in humans. Animal models can provide live and dynamic changes of inflamed arteries for in-depth analysis and in vivo experimentation. Unfortunately, appropriate animal models that closely resemble human LVV have not been described yet, but they are essential. The model of human temporal arteries from GCA patients engrafted to immunodeficient mice has been useful for testing novel therapies. However, this model is not suitable for investigating therapies for TAK due to the scarcity of human artery samples. Atherosclerosis models have been established by applying mechanical injuries to the artery in combination with a high-fat diet in genetically susceptible mice, and this approach is adaptable for creating models for LVV. Multiple innovative concepts have been proposed to inspire further exploration to test the hypothesis that LVV occurs from an injury of various kinds to the artery wall in the context of systemic inflammation, leading to the perpetuation of artery wall inflammation.
Acknowledgements
Dr. Chu's work is supported by an Innovative Research Award from Rheumatology Research Foundation and by a VA Merit Review grant (I01BX005195).
Footnotes
Funding
This article received no external funding.
Author Contributions
Chu CQ drafted and revised the manuscript and approved the final version.
Informed Consent
None declared.
Ethical Statement
None declared.
Conflict of Interest
Cong-Qiu Chu is an Editorial Board Member of the journal. The article was subject to the journal's standard procedures, with peer review handled independently of this member and affiliated research group.
References
- [1].Watanabe R, Berry GJ, Liang DH. et al. Pathogenesis of Giant Cell Arteritis and Takayasu Arteritis-Similarities and Differences. Curr Rheumatol Rep. 2020;22:68. doi: 10.1007/s11926-020-00948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vaideeswar P, Deshpande JR. Pathology of Takayasu arteritis: A brief review. Ann Pediatr Cardiol. 2013;6:52–58. doi: 10.4103/0974-2069.107235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hotchi M. Pathological studies on Takayasu arteritis. Heart Vessels Suppl. 1992;7:11–17. doi: 10.1007/BF01744538. [DOI] [PubMed] [Google Scholar]
- [4].Yoshifuji H. Pathophysiology of large vessel vasculitis and utility of interleukin-6 inhibition therapy. Mod Rheumatol. 2019;29:287–293. doi: 10.1080/14397595.2018.1546358. [DOI] [PubMed] [Google Scholar]
- [5].Dal Canto AJ, Swanson PE, O’Guin AK, Speck SH, Virgin HW. IFN-gamma action in the media of the great elastic arteries, a novel immunoprivileged site. J Clin Invest. 2001;107:R15–R22. doi: 10.1172/JCI11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Numano F. Vasa vasoritis, vasculitis and atherosclerosis. Int J Cardiol. 2000;75(Suppl 1):S1–S8. doi: 10.1016/s0167-5273(00)00196-0. discussion S17–S19. [DOI] [PubMed] [Google Scholar]
- [7].Stone JR, Bruneval P, Angelini A. et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc Pathol. 2015;24:267–278. doi: 10.1016/j.carpath.2015.05.001. [DOI] [PubMed] [Google Scholar]
- [8].Cong XL, Dai SM, Feng X. et al. Takayasu's arteritis: clinical features and outcomes of 125 patients in China. Clin Rheumatol. 2010;29:973–981. doi: 10.1007/s10067-010-1496-1. [DOI] [PubMed] [Google Scholar]
- [9].Kaiser M, Younge B, Björnsson J. et al. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155:765–774. doi: 10.1016/S0002-9440(10)65175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Restuccia G, Cavazza A, Boiardi L. et al. Small-vessel vasculitis surrounding an uninflamed temporal artery and isolated vasa vasorum vasculitis of the temporal artery: two subsets of giant cell arteritis. Arthritis Rheum. 2012;64:549–556. doi: 10.1002/art.33362. [DOI] [PubMed] [Google Scholar]
- [11].Brack A, Rittner HL, Younge BR. et al. Glucocorticoid-mediated repression of cytokine gene transcription in human arteritis-SCID chimeras. J Clin Invest. 1997;99:2842–2850. doi: 10.1172/JCI119477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wen Z, Shen Y, Berry G. et al. The microvascular niche instructs T cells in large vessel vasculitis via the VEGF-Jagged1-Notch pathway. Sci Transl Med. 2017;9:eaal3322. doi: 10.1126/scitranslmed.aal3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang H, Watanabe R, Berry GJ. et al. Inhibition of JAK-STAT Signaling Suppresses Pathogenic Immune Responses in Medium and Large Vessel Vasculitis. Circulation. 2018;137:1934–1948. doi: 10.1161/CIRCULATIONAHA.117.030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ma-Krupa W, Jeon MS, Spoerl S. et al. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–183. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen F, Li Y, Zhou H. et al. Analysis of Development Mechanism of Giant Cell Arteritis in Nude Mouse Model through Color Duplex Sonography and Computerized Tomography Nanocontrast Agent. Biomed Res Int. 2021;2021:6627925. doi: 10.1155/2021/6627925. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [16].Kabeerdoss J, Thomas M, Goel R. et al. High expression of S100 calgranulin genes in peripheral blood mononuclear cells from patients with Takayasu arteritis. Cytokine. 2019;114:61–66. doi: 10.1016/j.cyto.2018.11.033. [DOI] [PubMed] [Google Scholar]
- [17].Kabeerdoss J, Goel R, Mohan H. et al. High expression of pro-inflammatory cytokine genes IL-1β and IL-1R2 upon TLR4 activation in Takayasu arteritis. Rheumatol Int. 2022;42:535–543. doi: 10.1007/s00296-020-04785-0. [DOI] [PubMed] [Google Scholar]
- [18].Tian Y, Huang B, Li J. et al. Identification of the Association Between Toll-Like Receptors and T-Cell Activation in Takayasu's Arteritis. Front Immunol. 2021;12:792901. doi: 10.3389/fimmu.2021.792901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pryshchep O, Ma-Krupa W, Younge BR. et al. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Phillippi JA. On vasa vasorum: A history of advances in understanding the vessels of vessels. Sci Adv. 2022;8:eabl6364. doi: 10.1126/sciadv.abl6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gössl M, Rosol M, Malyar NM. et al. Functional anatomy and hemodynamic characteristics of vasa vasorum in the walls of porcine coronary arteries. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:526–537. doi: 10.1002/ar.a.10060. [DOI] [PubMed] [Google Scholar]
- [22].Mulligan-Kehoe MJ. The vasa vasorum in diseased and nondiseased arteries. Am J Physiol Heart Circ Physiol. 2010;298:H295–H305. doi: 10.1152/ajpheart.00884.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moulton KS, Vakili K, Zurakowski D. et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wolinsky H, Glagov S. Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res. 1967;20:409–421. doi: 10.1161/01.res.20.4.409. [DOI] [PubMed] [Google Scholar]
- [25].Heiliger C, Piecuch J, Frank A. et al. Laparoscopic intraarterial catheterization with selective ICG fluorescence imaging in colorectal surgery. Sci Rep. 2021;11:14753. doi: 10.1038/s41598-021-94244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Clarke JA. An x-ray microscopic study of the postnatal development of the vasa vasorum in the human aorta. J Anat. 1965;99:877–889. [PMC free article] [PubMed] [Google Scholar]
- [27].Hillock-Watling C, Gotlieb AI. The pathobiology of perivascular adipose tissue (PVAT), the fourth layer of the blood vessel wall. Cardiovasc Pathol. 2022;61:107459. doi: 10.1016/j.carpath.2022.107459. [DOI] [PubMed] [Google Scholar]
- [28].Rami AZA, Hamid AA, Anuar NNM. et al. Exploring the Relationship of Perivascular Adipose Tissue Inflammation and the Development of Vascular Pathologies. Mediators Inflamm. 2022;2022:2734321. doi: 10.1155/2022/2734321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Horimatsu T, Kim HW, Weintraub NL. The Role of Perivascular Adipose Tissue in Non-atherosclerotic Vascular Disease. Front Physiol. 2017;8:969. doi: 10.3389/fphys.2017.00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nicklin MJ, Hughes DE, Barton JL. et al. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–312. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shepherd J, Nicklin MJ. Elastic-vessel arteritis in interleukin-1 receptor antagonist-deficient mice involves effector Th1 cells and requires interleukin-1 receptor. Circulation. 2005;111:3135–3140. doi: 10.1161/CIRCULATIONAHA.104.519132. [DOI] [PubMed] [Google Scholar]
- [32].Chen Q, Yang W, Gupta S. et al. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29:899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moroi M, Zhang L, Yasuda T. et al. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J Clin Invest. 1998;101:1225–1232. doi: 10.1172/JCI1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Egashira K, Zhao Q, Kataoka C. et al. Importance of monocyte chemoattractant protein-1 pathway in neointimal hyperplasia after periarterial injury in mice and monkeys. Circ Res. 2002;90:1167–1172. doi: 10.1161/01.res.0000020561.03244.7e. [DOI] [PubMed] [Google Scholar]
- [35].Wu L, Iwai M, Nakagami H. et al. Roles of angiotensin II type 2 receptor stimulation associated with selective angiotensin II type 1 receptor blockade with valsartan in the improvement of inflammation-induced vascular injury. Circulation. 2001;104:2716–2721. doi: 10.1161/hc4601.099404. [DOI] [PubMed] [Google Scholar]
- [36].Zhao Q, Egashira K, Inoue S. et al. Vascular endothelial growth factor is necessary in the development of arteriosclerosis by recruiting/activating monocytes in a rat model of long-term inhibition of nitric oxide synthesis. Circulation. 2002;105:1110–1115. doi: 10.1161/hc0902.104718. [DOI] [PubMed] [Google Scholar]
- [37].Lardenoye JH, Delsing DJ, de Vries MR. et al. Accelerated atherosclerosis by placement of a perivascular cuff and a cholesterol-rich diet in ApoE*3Leiden transgenic mice. Circ Res. 2000;87:248–253. doi: 10.1161/01.res.87.3.248. [DOI] [PubMed] [Google Scholar]
- [38].Isoda K, Shiigai M, Ishigami N. et al. Deficiency of interleukin-1 receptor antagonist promotes neointimal formation after injury. Circulation. 2003;108:516–518. doi: 10.1161/01.CIR.0000085567.18648.21. [DOI] [PubMed] [Google Scholar]
- [39].Isoda K, Akita K, Isobe S. et al. Interleukin-1 receptor antagonist originating from bone marrow derived cells and non-bone marrow-derived cells helps to suppress arterial inflammation and reduce neointimal formation after injury. J Atheroscler Thromb. 2014;21:1208–1218. doi: 10.5551/jat.25668. [DOI] [PubMed] [Google Scholar]
- [40].Ali ZA, Alp NJ, Lupton H. et al. Increased in-stent stenosis in ApoE knockout mice: insights from a novel mouse model of balloon angioplasty and stenting. Arterioscler Thromb Vasc Biol. 2007;27:833–840. doi: 10.1161/01.ATV.0000257135.39571.5b. [DOI] [PubMed] [Google Scholar]
- [41].Matter CM, Ma L, von Lukowicz T. et al. Increased balloon-induced inflammation, proliferation, and neointima formation in apolipoprotein E (ApoE) knockout mice. Stroke. 2006;37:2625–2632. doi: 10.1161/01.STR.0000241068.50156.82. [DOI] [PubMed] [Google Scholar]
- [42].Li XD, Hong MN, Chen J. et al. Adventitial fibroblast-derived vascular endothelial growth factor promotes vasa vasorum-associated neointima formation and macrophage recruitment. Cardiovasc Res. 2020;116:708–720. doi: 10.1093/cvr/cvz159. [DOI] [PubMed] [Google Scholar]
- [43].Bogdanov L, Shishkova D, Mukhamadiyarov R. et al. Excessive Adventitial and Perivascular Vascularisation Correlates with Vascular Inflammation and Intimal Hyperplasia. Int J Mol Sci. 2022;23:12156. doi: 10.3390/ijms232012156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Matsuki T, Isoda K, Horai R. et al. Involvement of tumor necrosis factor-alpha in the development of T cell-dependent aortitis in interleukin-1 receptor antagonist-deficient mice. Circulation. 2005;112:1323–1331. doi: 10.1161/CIRCULATIONAHA.105.564658. [DOI] [PubMed] [Google Scholar]
- [45].Yoshizaki T, Itoh S, Yamaguchi S. et al. IL-25 exacerbates autoimmune aortitis in IL-1 receptor antagonist-deficient mice. Sci Rep. 2019;9:17067. doi: 10.1038/s41598-019-53633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Macaluso F, Marvisi C, Castrignanò P. et al. Comparing treatment options for large vessel vasculitis. Expert Rev Clin Immunol. 2022;18:793–805. doi: 10.1080/1744666X.2022.2092098. [DOI] [PubMed] [Google Scholar]
- [47].Mekinian A, Saadoun D, Vicaut E. et al. Tocilizumab in treatment-naïve patients with Takayasu arteritis: TOCITAKA French prospective multicenter open-labeled trial. Arthritis Res Ther. 2020;22:218. doi: 10.1186/s13075-020-02311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mekinian A, Biard L, Dagna L. et al. Efficacy and safety of TNF-α antagonists and tocilizumab in Takayasu arteritis: multicentre retrospective study of 209 patients. Rheumatology. 2022;61:1376–1384. doi: 10.1093/rheumatology/keab635. [DOI] [PubMed] [Google Scholar]
- [49].Tomelleri A, Campochiaro C, Sartorelli S. et al. Effectiveness and safety of infliximab dose escalation in patients with refractory Takayasu arteritis: A real-life experience from a monocentric cohort. Mod Rheumatol. 2022;32:406–412. doi: 10.1093/mr/roab012. [DOI] [PubMed] [Google Scholar]
- [50].Ly KH, Stirnemann J, Liozon E. et al. Interleukin-1 blockade in refractory giant cell arteritis. Joint Bone Spine. 2014;81:76–78. doi: 10.1016/j.jbspin.2013.06.004. [DOI] [PubMed] [Google Scholar]
- [51].Ren HM, Lukacher AE, Rahman ZSM. et al. New developments implicating IL-21 in autoimmune disease. J Autoimmun. 2021;122:102689. doi: 10.1016/j.jaut.2021.102689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Desbois AC, Régnier P, Quiniou V. et al. Specific Follicular Helper T Cell Signature in Takayasu Arteritis. Arthritis Rheumatol. 2021;73:1233–1243. doi: 10.1002/art.41672. [DOI] [PubMed] [Google Scholar]
- [53].Desbois AC, Ciocan D, Saadoun D. et al. Specific microbiome profile in Takayasu's arteritis and giant cell arteritis. Sci Rep. 2021;11:5926. doi: 10.1038/s41598-021-84725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Terrier B, Geri G, Chaara W. et al. Interleukin-21 modulates Th1 and Th17 responses in giant cell arteritis. Arthritis Rheum. 2012;64:2001–2011. doi: 10.1002/art.34327. [DOI] [PubMed] [Google Scholar]
- [55].Cuffy MC, Silverio AM, Qin L. et al. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J Immunol. 2007;179:5246–5254. doi: 10.4049/jimmunol.179.8.5246. [DOI] [PubMed] [Google Scholar]
- [56].Presti RM, Pollock JL, Dal Canto AJ. et al. Interferon gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J Exp Med. 1998;188:577–588. doi: 10.1084/jem.188.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ortiz-Fernández L, Saruhan-Direskeneli G, Alibaz-Oner F. et al. Identification of susceptibility loci for Takayasu arteritis through a large multi-ancestral genome-wide association study. Am J Hum Genet. 2021;108:84–99. doi: 10.1016/j.ajhg.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Terao C, Yoshifuji H, Kimura A. et al. Two susceptibility loci to Takayasu arteritis reveal a synergistic role of the IL12B and HLA-B regions in a Japanese population. Am J Hum Genet. 2013;93:289–297. doi: 10.1016/j.ajhg.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wen X, Chen S, Li P. et al. Single nucleotide polymorphisms of IL12B are associated with Takayasu arteritis in Chinese Han population. Rheumatol Int. 2017;37:547–555. doi: 10.1007/s00296-016-3648-3. [DOI] [PubMed] [Google Scholar]
- [60].Saruhan-Direskeneli G, Hughes T, Aksu K. et al. Identification of multiple genetic susceptibility loci in Takayasu arteritis. Am J Hum Genet. 2013;93:298–305. doi: 10.1016/j.ajhg.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nakajima T, Yoshifuji H, Shimizu M. et al. A novel susceptibility locus in the IL12B region is associated with the pathophysiology of Takayasu arteritis through IL-12p40 and IL-12p70 production. Arthritis Res Ther. 2017;19:197. doi: 10.1186/s13075-017-1408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]