Abstract
The influx and death of polymorphonuclear leukocytes within the infected lung are hallmarks of bovine pasteurellosis. Recent reports have shown that the Pasteurella haemolytica leukotoxin (LKT) and other RTX toxins bind β2-integrins on target cells. In this study we demonstrate that exposure of bovine neutrophils to recombinant bovine interleukin-1β upregulates β2-integrins (CD11a/CD18), which in turn enhance the binding and amplify the biological effects of partially purified LKT on these cells. LKT binding and cytotoxicity were inhibited by addition of an anti-integrin antibody (CD11a/CD18). These findings help to clarify the early events that occur in bovine pasteurellosis and support the hypothesis that inflammatory mediators might increase the severity of pasteurellosis by causing upregulation of β2-integrins that serve as an LKT receptor on bovine neutrophils.
Bovine pneumonic pasteurellosis is an acute fibrinonecrotizing pleuropneumonia characterized by an influx of neutrophils into the alveoli and interlobular septa of the lung (1, 25, 39). Some of these infiltrating leukocytes undergo apoptosis in the lung during the infection (17). In severe cases the inflammation extends onto the pleural surface (25, 39). Although Pasteurella haemolytica produces several virulence factors that play a role in the pathogenesis of pasteurellosis, the most important is thought to be a leukotoxin (LKT), whose effects are specific for ruminant leukocytes and platelets (3, 13, 27, 28). The P. haemolytica LKT is a member of a large family of exotoxins produced by gram-negative bacteria, known as RTX toxins, that share similar synthesis and secretion systems and exhibit related biological activities (37). Previously, it has been shown that other RTX toxins bind to β2-integrins on target cells (16). More recently, anti-β2-integrin monoclonal antibodies (MAbs) have been shown to inhibit the cytolysis by P. haemolytica LKT of BL-3 cells (a bovine B lymphoblastoid cell line) and peripheral blood neutrophils (2, 14, 18). Anti-β2-integrin MAbs have also been shown to block binding of LKT to blotted lysates of BL-3 cells and neutrophils (14, 18). The mechanism by which the RTX toxins exert their lethal effect on target cells is only partially understood but is thought to involve changes in selective permeability of the cytoplasmic membrane that result in Ca2+ influx and ATP efflux (23, 34, 36, 37).
The proinflammatory cytokine interleukin-1β (IL-1β), which is produced by alveolar macrophages and other cells, can stimulate migration and functional activation of polymorphonuclear leukocytes (PMNs) in the lung (10). The β2-integrins, which are found on most circulating leukocytes, play an important role in the extravasation of leukocytes during the inflammatory response (11). Studies of other inflammatory stimuli have shown that they can increase surface expression and avidity of β2-integrins on neutrophils (24, 38). In this study, we demonstrate an increase in β2-integrin expression on bovine PMNs following their in vitro incubation with IL-1β. This in turn enhanced the binding and cytotoxicity of partially purified LKT for the PMNs. This effect was diminished by addition of an anti-β2-integrin antibody (CD11a/CD18). These findings suggest that the ability of IL-1β to upregulate β2-integrins could increase their opportunity to act as a receptor for LKT on bovine PMNs and by so doing increase the severity of bovine pasteurellosis.
MATERIALS AND METHODS
Leukocyte preparation.
Leukocyte separation was performed as described previously (6). Briefly, peripheral blood was collected from healthy Holstein donor cows using Vacutainer tubes (Becton-Dickinson, Rutherford, N.J.) that contained sodium citrate (0.38% final volume) as an anticoagulant. The blood was centrifuged (250 × g for 20 min), and the platelet-rich plasma was removed. Neutrophils were obtained from the remaining blood by rapid hypotonic lysis and centrifugation through a Percoll gradient (Pharmacia, Uppsala, Sweden), as described previously (6). The neutrophil pellets were washed twice in Hanks' balanced salt solution (HBSS) and resuspended at 107 cells/ml in HBSS supplemented with 5% fetal bovine serum. These cell suspensions were greater than 95% neutrophils, as determined by evaluation of Diff-Quick-stained cytocentrifuge smears, and greater than 95% viable, as estimated by trypan blue dye exclusion.
IL-1β treatment.
Bovine PMNs (1 × 106 to 2 × 106 per ml in HBSS with 5% fetal bovine serum) were incubated with 50 ng of recombinant bovine IL-1β (generously provided by D. Shuster, American Cyanamid Company, Princeton, N.J.) at 37°C for 15 min. We have previously reported that this treatment stimulates the oxidative burst, degranulation, and adhesiveness of bovine neutrophils (26). Following this period of incubation, the cells were washed with HBSS and incubated with LKT, MAbs, or RGD peptide, as described for the various experiments.
P. haemolytica.
Two strains of P. haemolytica A1 were used in this study. The first of these (D153) was a wild-type strain isolated from a pneumonic bovine lung. The second was an isogenic gene replacement mutant of D153. This mutant, which has a deletion in frame in lktA (corresponding to amino acids 34 through 378), has a rate of growth and production of other known virulence determinants that are unaltered compared with those of the parent strain (unpublished observations).
LKT production and partial purification.
All LKT preparations were produced and partially purified as described previously (6). Briefly, P. haemolytica A1 was inoculated onto blood agar (Remel, Lenexa, Kans.) and incubated overnight at 37°C. The bacteria were washed from the agar surface with 10 ml of brain heart infusion broth containing 0.5% yeast extract (Difco, Detroit, Mich.) and incubated at 37°C for 1 h while rotating (8 rpm) in 15-ml polypropylene tubes. A 10-ml aliquot of this suspension was then used to inoculate 200 ml of brain heart infusion broth–0.5% yeast extract in a 500-ml flask, which was incubated for 2 h at 37°C with shaking (120 rpm). The bacteria were collected by centrifugation (1,600 × g for 15 min), resuspended in 200 ml of RPMI 1640 supplemented with l-glutamine (4.0 mM), and incubated on a shaker apparatus (at 120 rpm) for 4 h at 37°C. The bacteria were pelleted by centrifugation (1,600 × g for 20 min), and the crude LKT-containing supernatant was collected and passed through a 0.45-mm-pore-size bottletop filter (Nalgene, Rochester, N.Y.) to remove any residual bacteria. Aliquots of crude LKT were concentrated with an Amicon ultrafiltration unit equipped with a 62-mm-diameter XM-50 ultrafiltration membrane. The volume was then reduced to 10 to 20 ml over a 1- to 2-h period by applying a transmembrane pressure of 60 lb/in2 with nitrogen gas. The partially purified LKT preparation that remained was then collected and stored as 5-ml aliquots at −70°C. One unit of LKT activity was defined as the dilution causing 50% killing of bovine peripheral blood leukocytes when incubated at 37°C for 1 h, as determined by trypan blue exclusion. Partially purified LKT was stored at −70°C until used in an experiment.
Biotinylation of LKT.
Biotinylated LKT was prepared as described previously (6). Briefly, an 80:1 molar ratio of NHS-LC-biotin (Pierce Chemical, Rockford, Ill.) to partially purified LKT was incubated in an ice bath for 20 min. The mixture was then concentrated to 1 ml in a prechilled Amicon centricon tube (50-kDa cutoff). The reaction was stopped by the addition of crystalline bovine serum albumin (30 mg) and incubated at 4°C for 30 min. Unbound biotin was eliminated by buffer exchange over a Sephadex G-25 column (1 by 25 cm), using phosphate-buffered saline (pH 7.2) as the elution buffer. The LKT eluted in the void volume in 7 to 8 min, as monitored by absorbance at 280 nm. LKT activity was assessed by incubating biotinylated LKT with bovine peripheral leukocytes for 30 min at 37°C, followed by trypan blue exclusion.
MAbs.
A commercial murine immunoglobulin G (IgG) MAb specific for bovine LFA-1 (BAT75A) was purchased as ascites fluid from VMRD (Pullman, Wash.). Although some have described this MAb as specific for CD18 (15), its specificity (CD18 or CD11a) has not been precisely defined (W. C. Davis, personal communication). A murine IgG1 MAb (CA1.4E9) that is specific for canine CD18 and cross-reacts with bovine CD18 was purchased as ascites from Serotec (United Kingdom). For indirect staining of cells, an Fc-specific fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (Sigma, St. Louis, Mo.) was used. Briefly, the PMNs (106 cells) were centrifuged and resuspended in 50 μl of HBSS. The appropriate MAb was added (50-μg/ml final concentration in 50 μl of HBSS), and the cells were incubated at 4°C for 45 min. The FITC-conjugated anti-mouse IgG was then added (1:100 dilution), and the cells were incubated at 4°C for an additional 30 min. Following this, the cells were washed twice with HBSS and assessed by flow cytometry.
Detection of LKT binding.
Flow cytometric analysis of LKT binding to intact PMNs was performed as described previously (6). Briefly, bovine PMNs (106) were incubated with biotinylated LKT (10 to 20 U) for 10 min at 4°C. The cells were washed and resuspended in HBSS. Extra-avidin-FITC (Sigma) was added (in a volume of 4 μl), and the cells were incubated for 30 min at 4°C. The cells were then washed with HBSS, resuspended in 0.3 ml of HBSS, and fixed with 0.4% paraformaldehyde (final concentration). Cells were analyzed by flow cytometry using a Coulter Profile II flow cytometer (10,000 to 30,000 cells were scored for green fluorescence on a log scale).
In some experiments the role of β2-integrins in LKT binding was evaluated using the anti-β2-integrin described above or an RGD peptide (Sigma). The MAbs or RGD peptides (1 mM final concentration) were added to bovine PMNs (106) and incubated at 37°C for 15 min. After incubation, the cells were washed and the biotinylated LKT was added. The cells were then prepared for flow cytometric analysis of LKT binding as described above. An RGES peptide (Arg-Gly-Glu-Ser) (Sigma) that does not bind to β2-integrins (20) was used as a negative control.
Cytotoxicity assay.
2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) was used to evaluate LKT cytotoxicity for bovine PMNs, as previously described (32). Briefly, bovine PMNs (106) were incubated with partially purified LKT (1 to 5 U) for 1 h at 37°C on a rotating platform. The cells were plated into triplicate wells in a 96-well microplate and incubated at 37°C for 1 h. XTT (1 mg/ml) was added (50 μl/well) and incubated at 37°C for 1 h. Absorbance was determined using a microplate reader (MR 600; Dynatech). The percent cytotoxicity was calculated as [1 − (optical density of toxin-incubated cells/optical density of toxin-free cells)] × 100.
Statistical analysis.
Data were analyzed for statistical significance using a repeated-measures analysis of variance, performed by the Instat software program (GraphPad, San Diego, Calif.). The flow cytometry data were analyzed using the WInMDI version 2.8 software.
RESULTS
Recombinant bovine IL-1β upregulates β2-integrins on bovine PMNs.
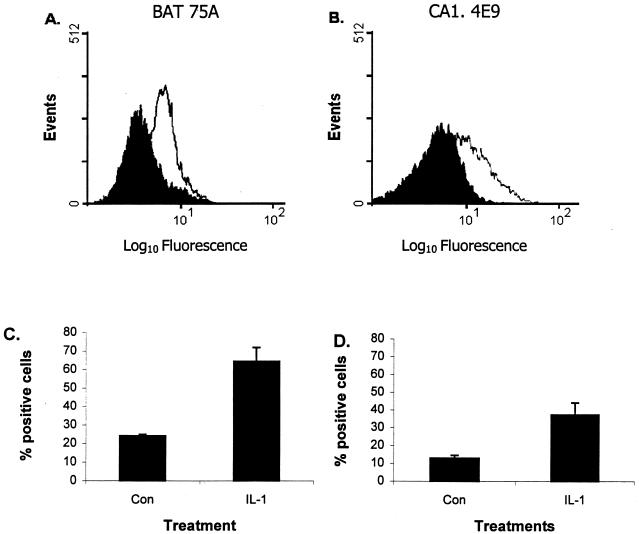
We first examined the expression of β2-integrins on IL-1β-stimulated bovine PMNs by flow cytometry, using two MAbs that bind bovine β2-integrins (BAT75A and CA1.4E9). We observed a significant increase in expression of β2-integrins on bovine PMNs that had been incubated with 50 ng of recombinant bovine IL-1β for 15 min at 37°C (Fig. 1). Similar results were obtained with both MAbs (2.6- and 2.9-fold increases in the number of positive cells using MAbs BAT75A and CA1.4E9, respectively). To the best of our knowledge, this is the first report of enhanced expression of β2-integrins on IL-1-treated bovine neutrophils.
FIG. 1.
Incubation with recombinant bovine IL-1β upregulates β2-integrin expression on bovine PMNs. (A and B) Freshly isolated bovine peripheral blood PMNs (106 cells/ml) were incubated with IL-1β (50 ng) for 15 min at 37°C (open trace) or with medium (solid trace) before incubation (40 min at 4°C) with anti-β2-integrin MAb BAT75A (50-μg/ml final concentration) (A) or CA1.4E9 (50-μg/ml final concentration) (B). The cells were then washed, incubated with an FITC-labeled second antibody, and analyzed by flow cytometry (10,000 cells were scored for green fluorescence). Panels A and B represent a single representative experiment. (C and D) Mean (± standard error of the mean) percent positive cells for five independent experiments (P < 0.01 compared with unstimulated cells [Con]).
Preincubation of bovine PMNs with IL-1β enhances binding of partially purified LKT.
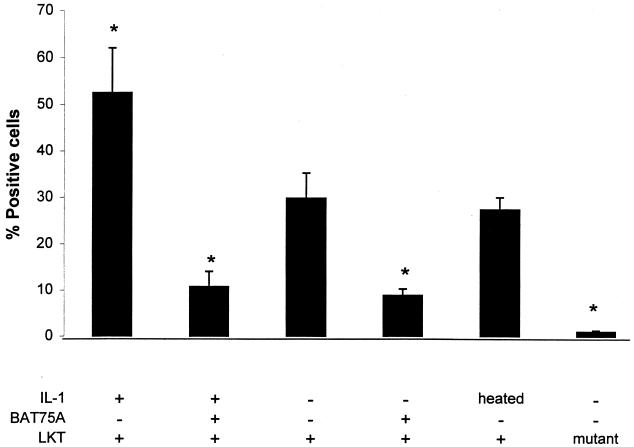
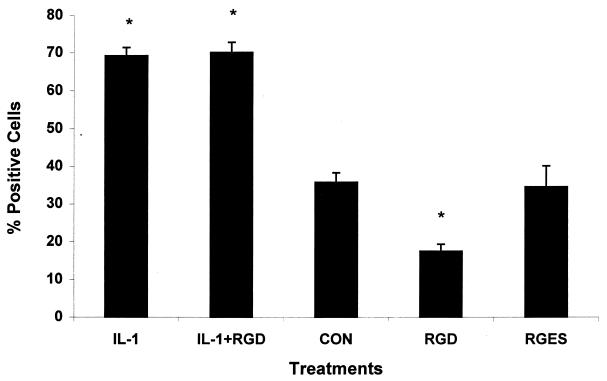
IL-1β-treated and control PMNs were incubated with biotinylated LKT for 10 min at 4°C, as described in Materials and Methods. The cells were then incubated with Extra-avidin-FITC and analyzed by flow cytometry. As shown in Fig. 2, the IL-1β-treated PMNs exhibited a 66% increase in the number of LKT-positive cells compared with control PMNs. A low level of LKT binding was observed when we used a similarly prepared culture filtrate from a P. haemolytica lktA mutant that produces an incomplete LKT that lacks cytolytic activity (Fig. 2). This observation speaks against the likelihood that our assay simply detected binding of lipopolysaccharide (LPS) in the LKT preparations. LKT binding also was not enhanced when the PMNs were preincubated with heat-inactivated IL-1β (100°C for 10 min) (Fig. 2). The binding of LKT to both IL-1β-stimulated and control PMNs was significantly reduced (76 and 68%, respectively) when the PMNs were incubated with anti-β2-integrin MAb BAT75 before exposure to biotinylated LKT. However, inhibition of LKT binding was not observed when we used MAb CA1.4E9, despite its ability to bind to IL-1-stimulated PMNs (Fig. 1B). We also used an RGD peptide that is known to inhibit integrin binding (20) as another measure of the role of β-integrins in LKT binding to PMNs. Although the RGD peptide (1 mM) inhibited LKT binding to control PMNs by 59%, it did not inhibit LKT binding to IL-1β-stimulated PMNs (Fig. 3). As expected, a negative control peptide (RGES) did not inhibit LKT binding to either control or IL-1-stimulated bovine PMNs.
FIG. 2.
Incubation of bovine PMNs with IL-1β enhances LKT binding in a β2-integrin-dependent manner. Freshly isolated bovine PMNs (106 cells/ml) were incubated with recombinant bovine IL-1β (50 ng) or medium for 15 min at 37°C. As an additional control, some PMNs were incubated with heat-inactivated (100°C for 10 min) IL-1β for 15 min at 37°C. Some of the IL-1β-stimulated and control PMNs were incubated (40 min at 4°C) with anti-β2-integrin MAb BAT75A (50-μg/ml final concentration). PMNs were then incubated with biotinylated partially purified P. haemolytica LKT (10 to 20 U) for 10 min on ice. The cells were washed, Extra-avidin-FITC was added, and the cells were incubated for 20 min on ice. The stained cells were washed, fixed with paraformaldehyde, and analyzed by flow cytometry (10,000 cells were scored for green fluorescence). As an additional control, PMNs were incubated with partially purified culture filtrate from an LKT mutant that lacks LKT activity (prepared in the same manner as the wild-type LKT) and stained as indicated above. The data indicate the mean (± standard error of the mean) percent positive cells from five independent experiments. Asterisks indicate statistically significant differences, compared with control PMNs incubated with LKT alone (P < 0.05), determined using the Student-Newman-Keuls multiple-comparison test.
FIG. 3.
RGD peptide inhibits the binding of partially purified LKT to resting, but not IL-1β-stimulated, bovine PMNs. Freshly isolated bovine PMNs (106 cells/ml) were incubated with IL-1β (50 ng) or medium (CON) for 15 min at 37°C. The PMNs were next incubated with RDG or a control peptide (RGES) (1 mM) for 15 min at 37°C. The cells were washed and incubated with biotinylated P. haemolytica LKT (10 to 20 U) for 10 min on ice. Extra-avidin was added, and the cell suspensions were incubated on ice for an additional 20 min. The stained cells were washed and analyzed by flow cytometry (10,000 cells were scored for green fluorescence). The data represent the mean (± standard error of the mean) percent positive cells from four independent experiments. The asterisks indicate significant differences (P < 0.01) compared with control PMNs incubated in medium alone before exposure to LKT (CON), as determined by the Student-Newman-Keuls test.
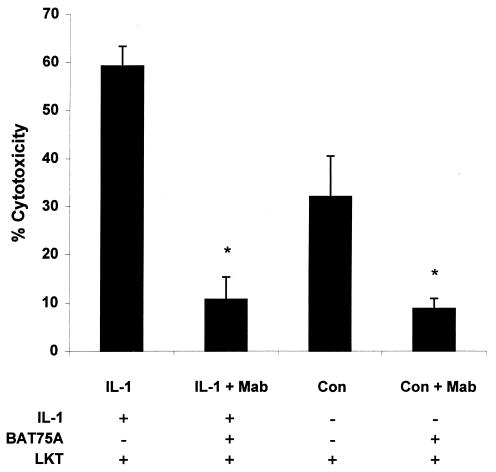
Preincubation with recombinant bovine IL-1β enhances the cytotoxicity of partially purified LKT for bovine PMNs.
We previously demonstrated that biotinylated LKT retains its cytotoxicity for bovine PMNs and that its binding to and cytotoxicity for bovine PMNs can be blocked by an anti-LKT MAb (MM601) (6). In the present study we observed an 85% increase in LKT-mediated cytotoxicity by PMNs stimulated with IL-1β compared with control PMNs (Fig. 4). LKT-mediated cytotoxicity was reduced by 81 and 72%, respectively, when IL-1β-stimulated and control PMNs were incubated with the anti-β2-integrin MAb BAT75A before addition of LKT. Incubation with MAb CA1.4E9 did not reduce LKT-mediated cytotoxicity for either IL-1β-stimulated or control PMNs (data not shown).
FIG. 4.
Incubation of bovine PMNs with IL-1β enhances LKT cytotoxicity in a β2-integrin-dependent manner. Freshly isolated bovine PMNs (106 cells/ml) were incubated with IL-1β (50 ng) or medium (CON) for 15 min at 37°C. This was followed by a 40-min incubation with the anti-β2-integrin MAb BAT75A (50-μg/ml final concentration). Control and IL-1β-treated PMNs were then plated in 96-well plates and incubated with partially purified P. haemolytica LKT (1 to 5 U) for 1 h at 37°C. Cell viability was assessed by XTT reduction, as described previously (32). The data indicate the mean (± standard error of the mean) percent LKT-mediated PMN death from four independent experiments. Asterisks indicate statistically significant differences (P < 0.05) for BAT75A-treated PMNs versus PMNs incubated with LKT alone, using the Student-Newman-Keuls multiple-comparison test.
DISCUSSION
The potential adverse effect of PMNs in the pathogenesis of pneumonic pasteurellosis was demonstrated previously by investigators, who found that depletion of peripheral blood neutrophils reduced the severity of lung damage in cows with experimental pasteurellosis (4, 30). Although the virulence determinants of P. haemolytica are incompletely understood, it is well accepted that the P. haemolytica LKT has an array of biological effects on ruminant PMNs. At low concentrations the LKT stimulates a respiratory burst, degranulation, release of eicosanoids, and secretion of cytokines (7, 9, 12, 19). Exposure of PMNs to LKT for a longer time period, or to higher LKT concentrations, results in cell death by apoptosis or necrosis (33, 34).
The responses of PMNs to inflammation are dependent on both the stimulus and the extracellular environment. β2-Integrins are important for PMN responses to infection and injury (8, 10, 15, 31, 38). In resting PMNs, integrins are maintained in a conformationally inactive state, in which they are unable to bind their respective ligands (24, 38). Activation of PMNs induces an upregulation of integrins on the cell surface and conformational changes that increase avidity for their respective ligands (4, 25). The proinflammatory cytokine IL-1β is one stimulus that can activate β2-integrins on PMNs in vivo (29), and it is responsible in part for the migration of PMNs into the lung (10, 11).
In the present study, we demonstrate that incubation of bovine PMNs with recombinant bovine IL-1β increased their binding and susceptibility to the cytotoxic effects of P. haemolytica LKT. Several lines of evidence suggest that the increased binding and susceptibility were mediated by β2-integrins. First, we observed an upregulation of β2-integrins on the cell surface after IL-1 stimulation, as detected by flow cytometry using two different anti-integrin MAbs. To the best of our knowledge, this is the first report of the upregulation of β2-integrins on IL-1-stimulated bovine neutrophils. Unpublished data from a different laboratory demonstrated a similar increase in CD18 expression on IL-1-stimulated bovine PMNs (M. Kehrli, personal communication). Second, the binding of partially purified LKT was inhibited by addition of an anti-LFA-1 MAb (BAT75A). Third, the LKT-mediated killing of IL-1β-activated and control PMNs was inhibited by preincubation with the BAT75A MAb. This last finding is contrary to that previously reported by Ambagala et al. (2), who did not observe inhibition of LKT-mediated killing of bovine PMNs by BAT75A in vitro. However, a larger amount of LKT was used in the prior study, which could explain the difference between their results and ours.
In the present study, we observed variability in the ability of anti-β2-integrin MAbs and RGD peptides to inhibit LKT binding to and cytotoxicity for bovine PMNs. However, these observations are similar to those of other investigators, who observed that cytotoxicity was influenced by the amount of LKT present, the cell type being used, and the MAb being added (2, 14, 18, 36). Our results are consistent with the pattern reported by other investigators who observed a diminution, but not complete inhibition, of LKT binding and cytotoxicity by anti-β2-integrin MAbs (2, 14, 18, 36). Our work is complementary to the recent report of Jayaseelan et al. (14) demonstrating diminished binding of LKT to lysates of PMNs obtained from β2-integrin-deficient calves. In that study, PMNs from β2-integrin-deficient calves were also partially resistant to the cytotoxicity of LKT (14). The present study provides additional evidence for the importance of β2-integrins in LKT binding. Exposure of neutrophils to a stimulus that upregulates β2-integrins (IL-1β) increases LKT binding and cytotoxicity for bovine PMNs. This is not a trivial observation, because numerous inflammatory mediators will be present in the lung during pasteurellosis. The ability of these mediators to enhance the susceptibility of bovine PMNs to P. haemolytica LKT could be an important early determinant influencing the severity of pulmonary pasteurellosis.
There are several limitations to our study. First, we used a partially purified LKT preparation that does contain LPS (6). However, we have shown previously that binding of this partially purified LKT to bovine PMNs is inhibited by an anti-LKT MAb (6). Likewise, in the present study we detected a low level of binding of a noncytolytic mutant LKT preparation to bovine neutrophils. This mutant LKT has been found by other investigators to bind to membrane-bound bovine leukocyte lysates but to elicit a very weak influx of extracellular Ca2+ (S. Maheswaran, personal communication). This suggests that it has limited ability to bind or activate intact bovine leukocytes. These observations suggest that contaminating endotoxin is not the major component recognized by our LKT binding assay. Although our flow cytometry assay might be somewhat less sensitive than the cell lysate LKT binding methods described by other investigators, our technique has the distinct advantage of using intact cells. This avoids the possibility that the blotted β2-integrins in the cell lysates contain exposed epitopes that would not be accessible to LKT on intact cells. Furthermore, it is only by using intact cells that one can begin to examine regulatory events that alter β2-integrin expression and LKT binding.
Finally, one might consider the biological relevance of the observations that were made in this study. It is well known that infection with bovine herpes virus type 1, or other respiratory viruses, can greatly enhance the susceptibility of cattle to P. haemolytica pneumonia (22, 25). The mechanism for this enhanced susceptibility has eluded investigators. We propose that one possibility is that inflammatory cytokines (i.e., IL-1 and others) that are released during viral infection could enhance the expression of β2-integrins on bovine leukocytes (22). When P. haemolytica then entered the lung, it would encounter leukocytes whose β2-integrins were primed for enhanced binding, and perhaps a biological response, to its LKT. This could trigger the release of additional cytokines, and other inflammatory mediators, in response to LKT (7, 9, 12, 19). This cascade of mediators could then amplify pulmonary inflammation, resulting in the severe fibrinous pneumonia that exemplifies the most severe manifestation of pasteurellosis. In keeping with this possibility, we have obtained preliminary evidence that other inflammatory stimuli (i.e., tumor necrosis factor alpha, LPS, and P. haemolytica LKT itself) also enhance LKT binding to and cytotoxicity for bovine PMNs (unpublished observations). These observations are consistent with previous reports that P. haemolytica LKT and LPS stimulate release of inflammatory cytokines, including IL-1, both in vitro and in vivo (21, 32, 40, 41). Thus, once pasteurellosis is established in the lung, the presence of LKT and LPS would stimulate continued release of IL-1 and other inflammatory mediators that would sustain β2-integrin expression and exacerbate pulmonary inflammation (5, 7, 9, 19, 32, 41). It is also worth noting that stimulation of LFA-1 (the putative LKT receptor) on human neutrophils is reported to enhance apoptosis in vitro (35). It is tempting to speculate that binding of LKT to LFA-1 on bovine neutrophils might be responsible in part for the apoptosis that LKT has been reported to cause (9, 33, 34).
In summary, the results of this study provide evidence that exposure of bovine neutrophils to IL-1β upregulates β2-integrins, which in turn enhance the binding and cytotoxicity of partially purified LKT. If similar events occur in vivo, it might explain in part the intense inflammation that exemplifies bovine pasteurellosis (25). These observations are also consistent with previous reports that neutrophil depletion in cattle can prevent some of the adverse events associated with P. haemolytica infection in the bovine lung (4, 30).
ACKNOWLEDGMENTS
We thank Steve Giles for assistance in preparation of the illustrations.
This work was supported by funds from the Wisconsin Agricultural Experiment Station, the University of Wisconsin—Madison Industrial and Economic Development Research Program, and the University of Wisconsin School of Veterinary Medicine. F. Leite was supported by CAPES-Ministerio da EducaÇão, Brazil.
REFERENCES
- 1.Ackermann M R, Brogden K A, Florance A F, Kehrli M E. Induction of CD18-mediated passage of neutrophils by Pasteurella haemolytica in pulmonary bronchi and bronchioles. Infect Immun. 1999;67:659–663. doi: 10.1128/iai.67.2.659-663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambagala T C, Ambagala A P N, Srikumaran S. The leukotoxin of Pasteurella haemolytica binds to integrins on bovine leukocytes. FEMS Microbiol Lett. 1999;179:161–167. doi: 10.1111/j.1574-6968.1999.tb08722.x. [DOI] [PubMed] [Google Scholar]
- 3.Baluyut C S, Simonson R R, Bemrick W J, Maheswaran S K. Interaction of Pasteurella haemolytica with bovine neutrophils: identification and partial characterization of a cytotoxin. Am J Vet Res. 1981;42:1920–1926. [PubMed] [Google Scholar]
- 4.Breider M A, Walker R D, Hopkins F M, Shultz T W, Bowersock T L. Pulmonary lesions induced by Pasteurella haemolytica in neutrophil sufficient and neutrophil deficient calves. Can J Vet Res. 1988;52:205–209. [PMC free article] [PubMed] [Google Scholar]
- 5.Breider M A, Yang Z. Tissue factor expression in bovine endothelial cells induced by Pasteurella haemolytica lipopolysaccharide and interleukin-1. Vet Pathol. 1994;31:55–60. doi: 10.1177/030098589403100107. [DOI] [PubMed] [Google Scholar]
- 6.Brown F J, Leite F, Czuprynski C J. Binding of Pasteurella haemolytica leukotoxin to bovine leukocytes. Infect Immun. 1997;65:3719–3724. doi: 10.1128/iai.65.9.3719-3724.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinkenbeard K D, Clarke C R, Hague C M, Clinkenbeard P, Srikumaran S, Morton R J. Pasteurella haemolytica leukotoxin-induced synthesis of eicosanoids by bovine neutrophils in vitro. J Leukoc Biol. 1994;56:644–649. doi: 10.1002/jlb.56.5.644. [DOI] [PubMed] [Google Scholar]
- 8.Cronstein B N, Weissmann G. The adhesion molecules of inflammation. Arthritis Rheum. 1993;36:147–157. doi: 10.1002/art.1780360204. [DOI] [PubMed] [Google Scholar]
- 9.Czuprynski C J, Noel E J, Ortiz-Carranza O, Srikumaran S. Activation of bovine neutrophils by partially purified Pasteurella haemolytica leukotoxin. Infect Immun. 1991;59:3126–3133. doi: 10.1128/iai.59.9.3126-3133.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallegri F, Ottonello L. Tissue injury in neutrophilic inflammation. Inflamm Res. 1997;46:382–391. doi: 10.1007/s000110050208. [DOI] [PubMed] [Google Scholar]
- 11.Ding Z M, Babensee J E, Simon S I, Lu H, Perrard J L, Bullard D C, Dail X Y, Bromley S K, Dustin M L, Entman M L, Smith C W, Ballantyme C M. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesin and migration. J Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 12.Henricks P A J, Binkhorst G J, Drijver A A, Nijkamp F P. Pasteurella haemolytica leukotoxin enhances production of leukotriene B4 and 5-hydroxyeicosatetraenoic acid by bovine polymorphonuclear leukocytes. Infect Immun. 1992;60:3238–3243. doi: 10.1128/iai.60.8.3238-3243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himmel M E, Yates M D, Lauerman L H, Squire P G. Purification and partial characterization of a macrophage cytotoxin from Pasteurella haemolytica. Am J Vet Res. 1981;43:764–767. [PubMed] [Google Scholar]
- 14.Jeyaseelan S, Hsuan S L, Kannan M S, Walcheck B K, Wang J F, Kehrli M E, Jr, Lally E T, Sieck G C, Maheswaran S. Lymphocyte function-associated antigen 1 is a receptor for Pasteurella haemolytica leukotoxin in bovine leukocytes. Infect Immun. 2000;68:72–74. doi: 10.1128/iai.68.1.72-79.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishimoto T K, Anderson D C. The role of integrins in inflammation. In: Galin J I, Golstein I M, Snyderman R, editors. Inflammation: basic principles and clinical correlates. New York, N.Y: Raven Press; 1992. pp. 353–406. [Google Scholar]
- 16.Lally E T, Kieba I R, Sato A, Green C L, Rosenbloom J, Korostoff J, Wang J F, Shenker B J, Ortlepp S, Robinson M K, Billings P C. RTX toxins recognize a B2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- 17.Leite F, Malazdrewich C, Yoo H S, Maheswaran S K, Czuprynski C J. Use of TUNEL staining to detect apoptotic cells in the lungs of cattle experimentally infected with Pasteurella haemolytica. Microb Pathog. 1999;27:179–185. doi: 10.1006/mpat.1999.0295. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Clinkenbeard K D, Ritchey J W. Bovine CD18 identified as a species specific receptor for Pasteurella haemolytica leukotoxin. Vet Microbiol. 1999;2:91–97. doi: 10.1016/s0378-1135(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 19.Maheswaran S K, Weiss D J, Kannan M S, Townsend E L, Reddy K R, Whiteley L O, Srikumaran S. Effects of Pasteurella haemolytica A1 leukotoxin on bovine neutrophils: degranulation and generation of oxygen-derived free radicals. Vet Immunol Immunopathol. 1992;33:51–68. doi: 10.1016/0165-2427(92)90034-n. [DOI] [PubMed] [Google Scholar]
- 20.Marcinkiewicz C, Vijay-Kumar S, Mclane M A, Niewiarowski S. Significance of the RDG loop and C-terminal domain of echistatin for recognition of alphaIIb beta3 integrin and expression of ligand-induced binding site. Blood. 1997;90:1565–1575. [PubMed] [Google Scholar]
- 21.Morsey M A, Van-Kessel A G, Popowych Y, Gordon D, Campos M, Babiuk L A. Cytokine profiles following interaction between bovine alveolar macrophages and Pasteurella haemolytica. Microb Pathog. 1999;26:325–331. doi: 10.1006/mpat.1999.0274. [DOI] [PubMed] [Google Scholar]
- 22.Ohmann J B, Babiuk L A, Harland R. Cytokine synergy with viral cytopathic effects and bacterial products during the pathogenesis of respiratory tract infection. Clin Immunol Immunopathol. 1991;60:152–170. doi: 10.1016/0090-1229(91)90060-n. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz-Carranza O, Czuprynski C J. Activation of bovine neutrophils by Pasteurella haemolytica leukotoxin is calcium dependent. J Leukoc Biol. 1992;52:558–564. doi: 10.1002/jlb.52.5.558. [DOI] [PubMed] [Google Scholar]
- 24.Pardi R. Inside-out and outside-in mechanisms in leukocyte adhesion. Fund Clin Immunol. 1994;2:135–146. [Google Scholar]
- 25.Rehmtulla A J, Thomson R G. A review of the lesions in shipping fever of cattle. Can Vet J. 1981;22:1–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Sample A K, Czuprynski C J. Primary and stimulation of bovine neutrophils by recombinant human interleukin-1 alpha and tumor necrosis factor alpha. J Leukoc Biol. 1991;49:107–115. doi: 10.1002/jlb.49.2.107. [DOI] [PubMed] [Google Scholar]
- 27.Shewen P E, Wilkie B N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982;35:91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shewen P E, Wilkie B N. Evidence for the Pasteurella haemolytica cytotoxin as a product of actively growing bacteria. Am J Vet Res. 1985;46:1212–1214. [PubMed] [Google Scholar]
- 29.Shi J, Goodbaud R D, Chegappa M M, Nelson J L, Tokach M D, McVey D S, Blecha F. Influence of interleukin-1 on neutrophil function and resistance to Streptococcus suis in neonatal pigs. J Leukoc Biol. 1994;56:88–94. doi: 10.1002/jlb.56.1.88. [DOI] [PubMed] [Google Scholar]
- 30.Slocombe R F, Malark J, Ingensoll R T, Derksen F J, Robinson N E. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am J Vet Res. 1985;46:2253–2258. [PubMed] [Google Scholar]
- 31.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 32.Stevens P K, Czuprynski C J. Dissociation of cytolysis and monokine release by bovine mononuclear phagocytes incubated with Pasteurella haemolytica partially-purified leukotoxin and lipopolysaccharide. Can J Vet Res. 1995;59:110–117. [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens P K, Czuprynski C J. Pasteurella haemolytica leukotoxin induces bovine leukocytes to undergo morphologic changes consistent with apoptosis in vitro. Infect Immun. 1996;64:2687–2694. doi: 10.1128/iai.64.7.2687-2694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Clinkenbeard K D, Cudd L A, Clarke C R, Clinkenbeard P A. Correlation of Pasteurella haemolytica leukotoxin binding with susceptibility to intoxication of lymphoid cells from various species. Infect Immun. 1999;67:6264–6269. doi: 10.1128/iai.67.12.6264-6269.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walzog B, Jeblonski F, Zakrzewicz A, Gaehtgens P. β2 integrins (CD11/CD18) promote apoptosis of human neutrophils. FASEB J. 1999;11:1177–1186. doi: 10.1096/fasebj.11.13.9367353. [DOI] [PubMed] [Google Scholar]
- 36.Wang J F, Kieba I R, Korostoff J, Guo T L, Yamaguchi N, Rozmiarek H, Billings P C, Shenker B J, Lally E T. Molecular and biochemical mechanisms of Pasteurella haemolytica leukotoxin-induced cell death. Microb Pathog. 1998;25:317–331. doi: 10.1006/mpat.1998.0236. [DOI] [PubMed] [Google Scholar]
- 37.Welch R A. Pore-forming cytolysis of gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 38.Williams M A, Solomkin J S. Integrin-mediated signaling in human neutrophil functioning. J Leukoc Biol. 1999;65:725–735. doi: 10.1002/jlb.65.6.725. [DOI] [PubMed] [Google Scholar]
- 39.Wittum T E, Woollen N E, Perino L J, Littledike E T. Relationship among treatment for respiratory tract disease, pulmonary lesions evident at slaughter and rate of weight gain in feedlot cattle. J Am Vet Med Assoc. 1996;209:814–818. [PubMed] [Google Scholar]
- 40.Yoo H S, Rajagopal B S, Maheswaran S K, Ames T R. Purified Pasteurella haemolytica leukotoxin induces expression of inflammatory cytokines from bovine alveolar macrophages. Microb Pathog. 1995;18:237–252. doi: 10.1016/s0882-4010(05)80001-4. [DOI] [PubMed] [Google Scholar]
- 41.Yoo H S, Maheswaran S K, Srinand S, Ames T R, Suresh M. Increased tumor necrosis factor-α and interleukin-β1 expression in the lungs of calves with experimental pneumonic pasteurellosis. Vet Immunol Imunopathol. 1995;49:15–28. doi: 10.1016/0165-2427(95)05453-d. [DOI] [PubMed] [Google Scholar]