Abstract
The enteric nervous system is a dense network of enteric neurons and glia housed in the gastrointestinal tract. This system is responsible for performing several functions that enable digestion as well as maintaining gut homeostasis through diverse signaling processes including those that arise from interactions with the immune system. Bidirectional communication between enteric neurons and enteric glia has gained increased attention for playing essential roles in enteric nervous system function. Neuronal mediators such as neurotransmitters stimulate enteric glia and subsequent gliotransmission processes refine neuronal signaling during intestinal motor control. In this mini-review, we present and discuss the basis of intercellular signaling between neurons and glia in the enteric nervous system and the relevance of these interactions to gut function.
Keywords: enteric glia, enteric neuron, intercellular signaling, gut motor control, inflammatory bowel disease
Introduction
The enteric nervous system (ENS) is a large, complex neural network located within the walls of the gastrointestinal tract with neural circuitry designed to control moment-to-moment gut functions [1]. Neurons within the enteric plexuses are diverse and display unique patterns of neurochemical coding, electrophysiological properties, patterns of gene expression, and morphology based on their function. Integrative signaling amongst the various subtypes of neurons, their effector cells, the “SIP syncytium”, and hormonal, immune, and extrinsic neural cues is responsible for coordinating gut movements, blood flow, secretory processes and immune functions [2,3]
A relatively new addition to the known cellular mechanisms that control gut functions are the enteric glia. Enteric glia surround enteric neurons and nerve fibers in the gut and influence gut functions through bi-directional neuron-glia signaling, and by direct and indirect interactions with immune cells [4,5]. Most known functions of enteric glia are directed at maintaining homeostasis within enteric circuits and at terminal projections in the mucosa and musculature in health and during pathophysiological insults [6]. Chief among these roles are the actions of enteric glia in enteric synaptic signaling whereby glia fine-tune neuronal communication to optimize ENS control of gut functions and responses to injury and/or infection. This mini-review focuses on the current understanding of how enteric glia influence gut motor function through bi-directional communication with enteric neurons. Therefore, the scope is focused on mechanisms of neuron-glia signaling in the myenteric plexus in health and disease.
1. Enteric nervous system: basic cellular organization and sensory-motor integration
Enteric neural cells are concentrated in two main ganglionated plexuses (Figure 1A). The submucosal plexus is adjacent to the intestinal lamina propria and consists of small ganglia with neurons and glia that control mucosal processes [1]. The myenteric plexus is the largest subdivision of the ENS and is situated between the circular and longitudinal smooth muscle layers (Figure 1A). The myenteric plexus houses various functional types of enteric neurons that work in an orchestrated manner to enable the proper execution of movements during peristalsis and colonic motor complexes [7](Figure 1B). Sensory neurons referred to as “intrinsic primary afferent neurons” are located inside the myenteric ganglia and send projections into the subepithelial space where they are positioned to receive stimuli and integrate them into functional responses via the motor neurocircuitry. When stimulated, sensory neurons activate interneurons that promote spatial and convergent synaptic signaling activating ascending and descending motor neurons (excitatory and inhibitory motor neurons respectively). Nitrergic, purinergic, and vasoactive intestinal peptide activity from inhibitory motor neurons control longitudinal and circular smooth muscle relaxation whereas the cholinergic and tachykinergic activity of excitatory motor neurons promotes smooth muscle contraction [7,8] (Figure 1B). This specialized synaptic network results in creating a spatial pattern of commands from the ENS neurocircuitry for enteric neuromuscular control.
Figure 1.
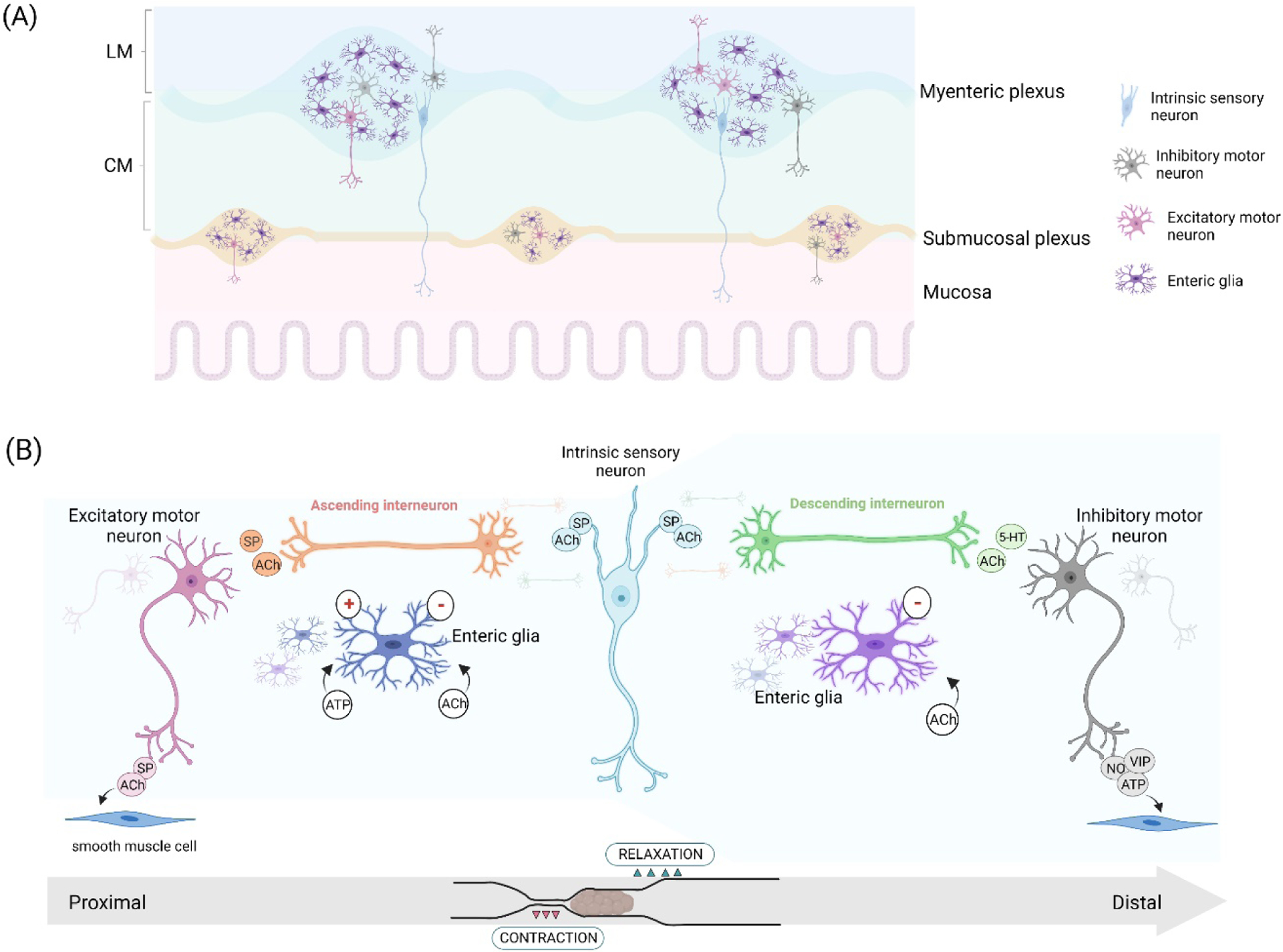
Neural circuitry for controlling intestinal motor activity. (A) The basic structure of the enteric nervous system. Enteric neurons and enteric glia are found widely distributed among gut layers, notably in the nervous plexus. Inside the ganglia, multiple connections are established as well as between ganglia. (B) In the myenteric plexus, a synaptic arrangement spatially signals the contraction and relaxation of circular smooth muscle. Intrinsic sensory neurons activate ascending and descending interneurons, which in turn activate excitatory motor neurons orally and inhibitory motor neurons anally, creating coordinated motor activity for the propulsion of intestinal contents. Enteric glia signaling evoked by acetylcholine and purines reinforces pro-contractile activity and refines gut motility commands. LM=longitudinal muscle; CM=circular muscle. SP=substance P, 5-HT=serotonin, ATP=adenosine triphosphate, ACh=acetylcholine, NO=nitric oxide, VIP=vasoactive intestinal peptide.
2. Neuron–to–glia communication
The neural circuits controlling gut motor function are housed in the myenteric plexus. Within the myenteric ganglia, enteric glia partner with enteric neurons to form the ENS. Neuron cell bodies occupy roughly 40% of the space within enteric ganglia and the remaining 60% is dense neuropil consisting of glia and nerve processes [9]. Potential routes of communication between the two cell types were identified in early work by Giorgio Gabella who described neuro-glial junctions consisting of specialized “synaptoid” contacts between axons and enteric glial cell bodies or processes [10]. Axon-glial contacts were observed in both myenteric and submucosal plexuses, in various species, and were found to be more abundant than neuronal synapses, suggesting conserved functional importance for widespread neuron-to-glia communication [9,11]. Subsequent work identified multiple receptors for neurotransmitters and neuromodulators expressed by enteric glia [12] and showed that activating these receptors with exogenous neurotransmitters could elicit glial activity in the form of intracellular calcium (Ca2+) responses in cell culture and in tissue [13–17]. Final confirmation of the functional nature of neuron-glial contacts was provided by studies that depolarized neurons in intact tissue and in cell culture and observed subsequent responses in enteric glia [18,19]. In addition, triggering neurotransmitter release from endogenous sources by depolarizing neurons with electrical field stimulation (EFS) elicits responses in myenteric glia that require transmitter release from neurons [20,21]. Similarly, enteric glia respond with Ca2+ fluctuations when enteric neurons are activated using photo-stimulation [22]. Under these conditions, specific neuroglial units are observed which suggest spatial specificity in neuron-to-glia communication [22].
Glial excitability is largely encoded by fluctuations in intracellular Ca2+ which can arise from receptor-mediated release from intracellular stores, influx through ionotropic receptors, or combinations of both. Glial Ca2+ responses are considered a primary mechanism used to encode the “language” used by enteric glia. How Ca2+ fluxes are converted into transmitter release dynamics or translated into functional responses is not completely understood. However, it is known that Ca2+ activity underlies glial processing at the single-cell level and at the network level by stimulating cell-cell signaling through mechanisms involving hemichannels [23–25]. Connexin-43 (Cx43) is a component of both hemichannels and gap junctions expressed by enteric glia. Gap junction coupling between enteric glia seems to be sparse but promotes an intercellular route of communication inside the glial network [26,27]. Conversely, extensive hemichannel expression provides a route to exchange glial-derived signals such as ATP between intra- and extracellular spaces and promote another mechanism of communication inside the enteric glia network [23,24,28]. Enteric glia have the potential to release several different types of gliotransmitters, which are neuroactive chemicals released by glia that modulate neuronal activity and neuroglial interactions, such as ATP, GABA, and prostaglandins. Each of these transmitters has well-defined roles in gut physiology and bidirectional neuron-glial signaling likely contributes to their effects on motility [24,25,29,30]. The ability of glia to sense and elicit responses to various neuroactive substances places enteric glia in a key position to integrate and relay signals impacting gut motility [24].
Cholinergic, glutamatergic, serotoninergic, and purinergic neurotransmitter receptors have been described in enteric glia [6]. Not all of them have extensive characterization depicting their pathways and outcomes, but some have been addressed in the last decade. Glial cholinergic receptors and subunits described in the gut include muscarinic M3 and M5 receptors, nicotinic, and its α3 and α7 subunits [13,17,31–33]. This is significant because most excitatory signaling in myenteric circuits is cholinergic and enteric glia can ‘listen’ to cholinergic signaling through these receptor pathways [13]. Indeed, Ca2+ responses in colonic enteric glia can be elicited by muscarine through the M3 receptor [33]. Experiments with Designer Receptors Exclusively Activated by Designed Drugs (DREADD) have shed some light on the specific roles of glial M3 activation in myenteric circuits. Gq-coupled DREADD receptors such as hM3Dq are derived from human M3 muscarinic receptors and expressing hM3Dq in enteric glia (GFAP-hM3Dq) renders them sensitive to clozapine-N-oxide (CNO), a selective activator of hM3Dq that mimics physiological M3 activation. Stimulating glia in this way demonstrates that glial M3 signaling potentiates enteric reflexes ex vivo and that its chronic stimulation alters motility in vivo, both of which suggest a role for enteric glial cholinergic signaling in gut motility [33].
Purinergic signaling is involved in fast neurotransmission in the ENS and in multiple aspects of the circuitry underlying gut motor responses. Enteric glia are sensitive to purines such as ADP, and ADP evokes glial Ca2+ responses through P2Y1 receptors in ex vivo preparations and primarily cultures from mice [25,34]. The extent of purinergic neuron-glia signaling in the myenteric plexus differs depending on the gut region, although most myenteric glia are responsive to P2Y1 agonists in mice [35]. P2Y4, P2X2, and adenosine 2B receptors also contribute to the effects of purines on enteric glia [15,36–38]. Furthermore, purines generated by neurons during synaptic communication and during inflammatory challenges stimulate Ca2+ responses in enteric glia [15,20,22]. Large Ca2+ responses evoked in myenteric neurons by stimulating neuronal P2X7 receptors or by photo-stimulation evoke pannexin-1-dependent ATP release, which recruits a discrete number of glia immediately surrounding the stimulated neuron [22,39]. These functional neuron-glial units play a particularly important role in inflammatory responses where glial stimulation provokes neuron death through purinergic mechanisms [25]
3. Glia-to-neuron signaling in gut motor control
Evidence from studies using glial ablation models or gliotoxins provided strong support for the concept that enteric glia play an important role in regulating enteric neuromotor function [40,41]. Yet how glia might be exerting these effects remained in question. Targeted studies focused on glial signaling mechanisms have begun to illuminate some of the ways in which glia might affect enteric neuromuscular circuits (Figure 2). Among these, glial Cx43 hemichannels have emerged as an important mechanism of gliotransmitter release and glia-to-neuron communication. Animals lacking glial Cx43 display impaired gastrointestinal motor function as reflected by increased colonic time transit and alterations in feces composition [24]. In addition, deleting enteric glial Cx43 creates deficits in neuromuscular contractions and relaxations that underlie motility [24]. Both the mutation and the knock-down of Cx43 in vivo produce intestinal dysfunction, demonstrating that impairments in mechanisms of enteric glial communication alter the functionality of the ENS to signal intestinal reflexes [42]. Interestingly, augmented glial Cx43 during pathophysiology contributes to neuroinflammation and reducing the pro-inflammatory activities of glial Cx43 improves gut motility in conditions such as opioid-driven constipation [43].
Figure 2.
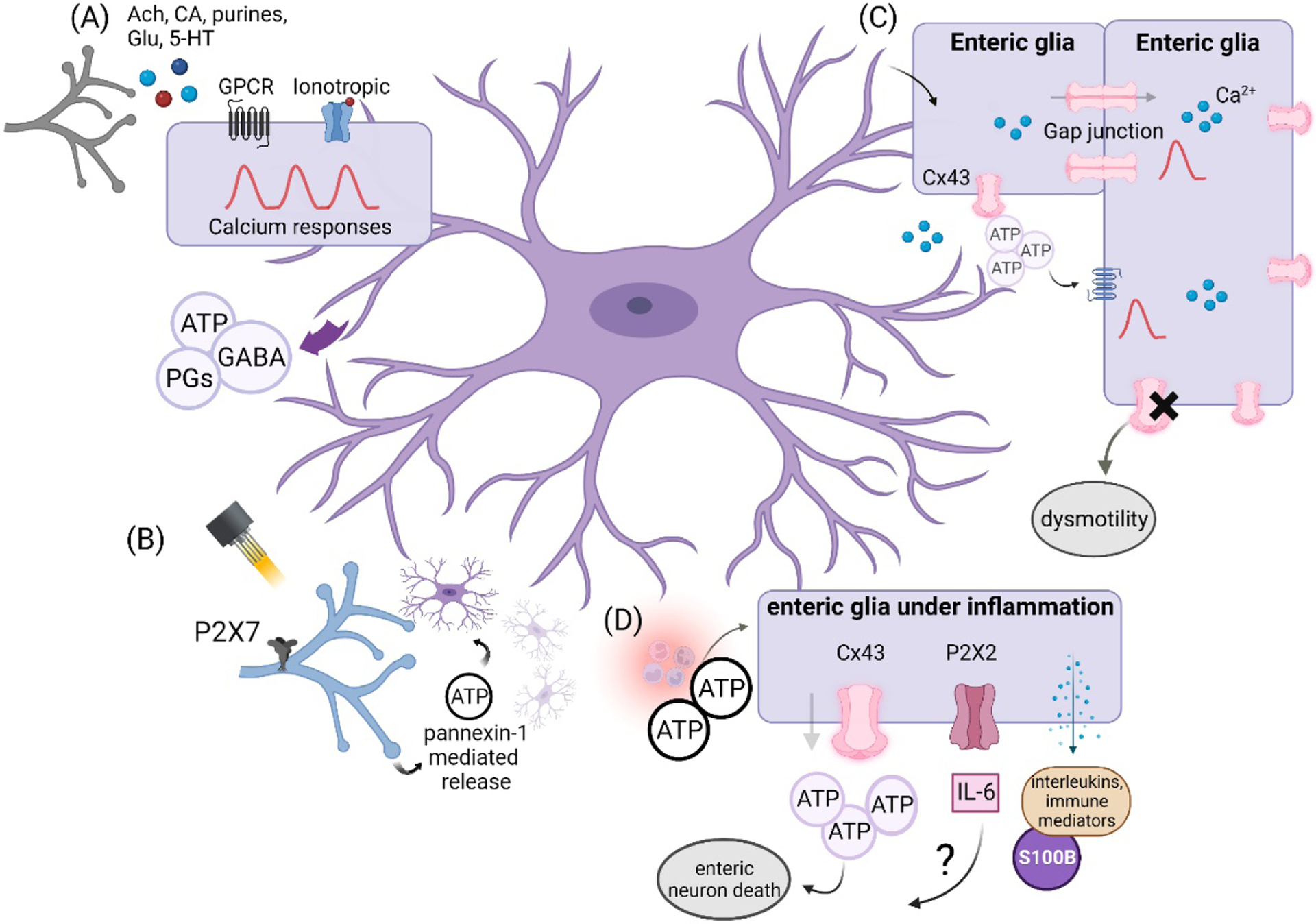
Glial mechanisms for intercellular communication with enteric neurons. (A) Enteric glia express a series of receptors for neurotransmitters that elicit calcium responses and subsequent events leading to gliotransmission. (B) A special unit is found between purinergic neurons and enteric glia, where ATP release via pannexin-1 recruits adjacent enteric glia, a process that probably accounts for pathology or injury. (C) Glial responses can be processed at the cellular and network level by hemichannels containing connexin 43. When Cx43 is absent, gastrointestinal dysfunction can be manifested as dysmotility. (D) During inflammation, the interaction of purines in intercellular communication in the enteric nervous system is evidenced by causing enteric neuron death via ATP release by enteric glia, participation of P2X2 receptors during neuroinflammation, and release of several inflammatory mediators, such as S100B. CA=catecholamines, Glu=glutamate, GPCR=G protein-coupled receptor, PGs=prostaglandins.
Glial Ca2+ responses influence Cx43 hemichannel opening and control the release of various gliotransmitters. Gliotransmitters, in turn, affect colonic motor function by modulating neurotransmission. Direct evidence supporting the role of glial Ca2+ signaling in gut motor function was obtained by stimulating GFAP-hM3Dq expressed in enteric glia. In these studies, specifically activating glial Gq-driven Ca2+ signaling with the DREADD agonist CNO was sufficient to drive neurogenic contractions in the ileum and colon and increased the frequency and amplitude of colonic migrating motor complexes ex vivo and motility in vivo [33,44]. These effects were mediated through neuronal pathways since the effects of glial activity were blocked by limiting neurotransmission with tetrodotoxin [33].
Enteric glial signaling clearly affects gut motor function through actions on enteric neurons, but whether glia influence all neuron subtypes similarly or display some type of specificity in their signaling has remained in question. Evidence shows that strong stimulation of individual enteric neurons evokes Ca2+ responses in a limited number of the surrounding enteric glia [22,45] and that glia are able to discern the activity of adjacent synaptic pathways [46] suggests that there is specificity in neuron-to-glia communication. Recent findings further this concept by showing that subpopulations of enteric glia are functionally devoted to specific pathways in the myenteric plexus [20]. Subpopulations of myenteric glia are functionally committed to ascending and descending neural pathways while many are activated by multipolar intrinsic primary afferent neurons. These pathway-specific glia provide a functional basis for understanding how activating glia influence neuronal activity and motor patterns (Figure 1B). Glia activation by purines reinforces neuronal activity in the ascending neuronal circuitry, while cholinergic glial activation suppresses activity in descending pathways and contributes to cross-inhibition between the ascending and descending circuitry [20]. The net result is the potentiation of pro-contractile excitatory pathways and an overall sharpening of neural signaling to optimize the proper execution of motor activity in the colon (Figure 1B).
4. Enteric glia–neuron signaling during inflammation
Enteric glia are sensitive to pathophysiological insults and react in ways that can cause both gains and losses of functions. These changes can alter neurotransmission in the gut by disrupting normal glial mechanisms that modulate neurotransmission, by changing the repertoire of substances released by glia, and by enacting immune signaling mechanisms with indirect effects on neurons. Enteric glia modify inflammatory responses through interactions with several subtypes of innate immune cells during inflammation [47,48]. Mechanisms of these interactions include glial toll-like receptors, antigen presentation, and the production of both anti- and pro-inflammatory cytokines. The specific roles of glia vary depending on the context and different functional profiles assumed by glia (i.e. reactive gliosis) contribute to modified neuronal signaling and consequent neuroplasticity [4].
Purinergic signaling is prominent during inflammatory responses and glial purinergic signaling contributes to neuroinflammation in the ENS (Figure 2). Elevated purine levels during acute colitis cause enteric neuron death through mechanisms that involve neuronal P2X7 receptors and pannexin-1 channels [39]. Glia play an essential part in this process by detecting elevations in purines through P2Y1 receptor activation, which subsequently drives glial nitric oxide production and ATP release [21,25]. Glial ATP release under these conditions is Cx43-dependent and acts on neuronal P2X7 receptors to promote cell death [25]. Genomic, functional, and anatomical data show that P2X7 receptors are enriched in subsets of enteric neurons in mice, humans, and guinea pigs [39,49–54]. However, a recent study suggests that P2X7 receptors are also expressed by glial and/or macrophages [55].
Tachykinins released from sensory nerve terminals may be also an early trigger that initiates this neuroinflammatory signaling cascade through enteric glia [21]. In addition, ionotropic P2X2 receptors have been implicated in promoting a ‘reactive’ glial phenotype during inflammation and blocking P2X2 receptors reduces IL-6 secretion by enteric glia [37]. How this signaling pathway might impact enteric neurons remains unknown. Given the importance of purinergic signaling by enteric glia during disease and the resulting disruptions in motility and enteric neuroplasticity induced during and after inflammation, it is a relevant topic to be addressed [56,57].
Conclusions
Neuroglial communication is an ongoing process involved in most gastrointestinal functions and is particularly important for motility. Enteric glia are active elements in motor neurocircuits and help to refine and support neuronal signaling. Although the complexities and independence of the ENS have been appreciated for some time, how the ENS adapts and responds to stimuli is still being studied. Potential impairments to intercellular signaling between neurons and enteric glia during inflammatory bowel diseases and diseases of the gut-brain axis reiterate the need to deeply understand the mechanisms of this conversation, thus enabling studies to create therapies that alleviate gastrointestinal symptoms.
Highlights.
Enteric glia partner with enteric neurons in gut motor neurocircuitry
Neurons communicate with glia via neurotransmitters and glial receptors
Glia release gliotransmitters that influence enteric neurotransmission
Glia contribute to neuroinflammation and neuroplasticity in enteric networks during disease
Acknowledgments
Funding:
B.G. receives support from grants R01DK103723 and R01DK120862 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- CNO
clozapine-N-oxide
- Cx43
connexin-43
- DREADD
Designer Receptors Exclusively Activated by Designed Drugs
- EFS
electrical field stimulation
- ENS
enteric nervous system
- GFAPhM3Dq
M3 muscarinic receptors derived from human expressed in enteric glia
- SIP
Smooth muscle cells, interstitial cells of Cajal, and platelet-derived growth factor receptor alpha cells syncytium
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Furness JB, Callaghan BP, Rivera LR, Cho HJ, The enteric nervous system and gastrointestinal innervation: integrated local and central control, Adv Exp Med Biol. 817 (2014) 39–71. 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- [2].Sharkey KA, Mawe GM, The enteric nervous system, Physiol Rev. (2022). 10.1152/physrev.00018.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schneider S, Hashmi SK, Thrasher AJ, Kothakapa DR, Wright CM, Heuckeroth RO, Single Nucleus Sequencing of Human Colon Myenteric Plexus–Associated Visceral Smooth Muscle Cells, Platelet Derived Growth Factor Receptor Alpha Cells, and Interstitial Cells of Cajal, Gastro Hep Adv. 2 (2023) 380–394. 10.1016/j.gastha.2022.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Seguella L, Gulbransen BD, Enteric glial biology, intercellular signalling and roles in gastrointestinal disease, Nat Rev Gastroenterol Hepatol. (2021). 10.1038/s41575-021-00423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Progatzky F, Pachnis V, The role of enteric glia in intestinal immunity, Curr Opin Immunol. 77 (2022) 102183. 10.1016/j.coi.2022.102183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grubisic V, Gulbransen BD, Enteric glia: the most alimentary of all glia, J Physiol. 595 (2017) 557–570. 10.1113/jp271021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Spencer NJ, Costa M, Hibberd TJ, Wood JD, Advances in colonic motor complexes in mice, Am J Physiol-Gastr L. 320 (2021) G12–G29. 10.1152/ajpgi.00317.2020. [DOI] [PubMed] [Google Scholar]
- [8].Dickson EJ, Heredia DJ, McCann CJ, Hennig GW, Smith TK, The mechanisms underlying the generation of the colonic migrating motor complex in both wild-type and nNOS knockout mice, Am J Physiol-Gastr L. 298 (2010) G222–G232. 10.1152/ajpgi.00399.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gabella G, Enteric glia: extent, cohesion, axonal contacts, membrane separations and mitochondria in Auerbach’s ganglia of guinea pigs, Cell Tissue Res. (2022) 1–18. 10.1007/s00441-022-03656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gabella G, Ultrastructure of the nerve plexuses of the mammalian intestine: the enteric glial cells, Neuroscience. 6 (1981) 425–36. http://www.ncbi.nlm.nih.gov/pubmed/7219723. [DOI] [PubMed] [Google Scholar]
- [11].Gabella G, Ultrastructure of the nerve plexuses of the mammalian intestine: The enteric glial cells, Neuroscience. 6 (1981) 425–436. 10.1016/0306-4522(81)90135-4. [DOI] [PubMed] [Google Scholar]
- [12].Grubisic V, Verkhratsky A, Zorec R, Parpura V, Enteric glia regulate gut motility in health and disease, Brain Res Bull. 136 (2018) 109–117. 10.1016/j.brainresbull.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Broadhead MJ, Bayguinov PO, Okamoto T, Heredia DJ, Smith TK, Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine, J Physiol. 590 (2012) 335–50. 10.1113/jphysiol.2011.219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kimball BC, Mulholland MW, Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C-dependent mechanism, J Neurochem. 66 (1996) 604–12. http://www.ncbi.nlm.nih.gov/pubmed/8592130. [DOI] [PubMed] [Google Scholar]
- [15].Gulbransen BD, Sharkey KA, Purinergic neuron-to-glia signaling in the enteric nervous system, Gastroenterology. 136 (2009) 1349–58. 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- [16].Gulbransen BD, Bains JS, Sharkey KA, Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon, J Neurosci. 30 (2010) 6801–9. 10.1523/jneurosci.0603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Boesmans W, Cirillo C, den Abbeel VV, den Haute CV, Depoortere I, Tack J, Berghe PV, Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells, Neurogastroenterol Motil. 25 (2013) e151–60. 10.1111/nmo.12065. [DOI] [PubMed] [Google Scholar]
- [18].Gulbransen BD, Sharkey KA, Purinergic neuron-to-glia signaling in the enteric nervous system., Gastroenterology. 136 (2009) 1349–1358. 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- [19].Gomes P, Chevalier J, Boesmans W, Roosen L, Abbeel VVD, Neunlist M, Tack J, Berghe PV, ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice, Neurogastroenterol Motil. 21 (2009) 870–e62. 10.1111/j.1365-2982.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- [20].Ahmadzai MM, Seguella L, Gulbransen BD, Circuit-specific enteric glia regulate intestinal motor neurocircuits, Proc Natl Acad Sci U S A. 118 (2021). 10.1073/pnas.2025938118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Delvalle NM, Dharshika C, Morales-Soto W, Fried DE, Gaudette L, Gulbransen BD, Communication Between Enteric Neurons, Glia, and Nociceptors Underlies the Effects of Tachykinins on Neuroinflammation, Cell Mol Gastroenterol Hepatol. 6 (2018) 321–344. 10.1016/j.jcmgh.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boesmans W, Hao MM, Fung C, Li Z, den Haute CV, Tack J, Pachnis V, Berghe PV, Structurally defined signaling in neuro-glia units in the enteric nervous system, Glia. 67 (2019) 1167–1178. 10.1002/glia.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang W, Segura BJ, Lin TR, Hu Y, Mulholland MW, Intercellular calcium waves in cultured enteric glia from neonatal guinea pig, Glia. 42 (2003) 252–62. 10.1002/glia.10215. [DOI] [PubMed] [Google Scholar]
- [24].McClain JL, Grubisic V, Fried D, Gomez-Suarez RA, Leinninger GM, Sevigny J, Parpura V, Gulbransen BD, Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice, Gastroenterology. 146 (2014) 497–507 e1. 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Brown IA, McClain JL, Watson RE, Patel BA, Gulbransen BD, Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide, Cell Mol Gastroenterol Hepatol. 2 (2016) 77–91. 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hanani M, Zamir O, Baluk P, Glial cells in the guinea pig myenteric plexus are dye coupled, Brain Res. 497 (1989) 245–249. 10.1016/0006-8993(89)90269-2. [DOI] [PubMed] [Google Scholar]
- [27].Gabella G, Enteric glia: extent, cohesion, axonal contacts, membrane separations and mitochondria in Auerbach’s ganglia of guinea pigs, Cell Tissue Res. 389 (2022) 409–426. 10.1007/s00441-022-03656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS, Connexins: a myriad of functions extending beyond assembly of gap junction channels, Cell Commun Signal. 7 (2009) 4. 10.1186/1478-811x-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Murakami M, Ohta T, Otsuguro K-I, Ito S, Involvement of prostaglandin E2 derived from enteric glial cells in the action of bradykinin in cultured rat myenteric neurons, Neuroscience. 145 (2007) 642–653. 10.1016/j.neuroscience.2006.12.052. [DOI] [PubMed] [Google Scholar]
- [30].Fried DE, Watson RE, Robson SC, Gulbransen BD, Ammonia modifies enteric neuromuscular transmission through glial γ-aminobutyric acid signaling, Am J Physiol-Gastr L. 313 (2017) G570–G580. 10.1152/ajpgi.00154.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].MacEachern SJ, Patel BA, McKay DM, Sharkey KA, Nitric oxide regulation of colonic epithelial ion transport: a novel role for enteric glia in the myenteric plexus, J Physiol. 589 (2011) 3333–48. 10.1113/jphysiol.2011.207902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Costantini TW, Krzyzaniak M, Cheadle GA, Putnam JG, Hageny AM, Lopez N, Eliceiri BP, Bansal V, Coimbra R, Targeting alpha-7 nicotinic acetylcholine receptor in the enteric nervous system: a cholinergic agonist prevents gut barrier failure after severe burn injury, Am J Pathol. 181 (2012) 478–86. 10.1016/j.ajpath.2012.04.005. [DOI] [PubMed] [Google Scholar]
- [33].Delvalle NM, Fried DE, Rivera-Lopez G, Gaudette L, Gulbransen BD, Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility, Am J Physiol Gastrointest Liver Physiol. 315 (2018) G473–G483. 10.1152/ajpgi.00155.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gomes P, Chevalier J, Boesmans W, Roosen L, van den Abbeel V, Neunlist M, Tack J, Berghe PV, ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice, Neurogastroenterol Motil. 21 (2009) 870–e62. 10.1111/j.1365-2982.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- [35].Seguella L, McClain JL, Esposito G, Gulbransen BD, Functional intra- and inter-regional heterogeneity between myenteric glial cells of the colon and duodenum in mice, J Neurosci. (2022) JN-RM-2379–20. 10.1523/jneurosci.2379-20.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Boesmans W, Martens MA, Weltens N, Hao MM, Tack J, Cirillo C, Berghe PV, Imaging neuron-glia interactions in the enteric nervous system, Front Cell Neurosci. 7 (2013) 183. 10.3389/fncel.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schneider R, Leven P, Glowka T, Kuzmanov I, Lysson M, Schneiker B, Miesen A, Baqi Y, Spanier C, Grants I, Mazzotta E, Villalobos-Hernandez E, Kalff JC, Müller CE, Christofi FL, Wehner S, A novel P2X2-dependent purinergic mechanism of enteric gliosis in intestinal inflammation, Embo Mol Med. 13 (2021) e12724. 10.15252/emmm.202012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Grubišić V, Bali V, Fried DE, Eltzschig HK, Robson SC, Mazei-Robison MS, Gulbransen BD, Enteric glial adenosine 2B receptor signaling mediates persistent epithelial barrier dysfunction following acute DSS colitis, Mucosal Immunol. (2022) 1–13. 10.1038/s41385-022-00550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA, Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis, Nat Med. 18 (2012) 600–4. 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Antonioli L, D’Antongiovanni V, Pellegrini C, Fornai M, Benvenuti L, di Carlo A, van den Wijngaard R, Caputi V, Cerantola S, Giron MC, Nemeth ZH, Hasko G, Blandizzi C, Colucci R, Colonic dysmotility associated with high-fat diet-induced obesity: Role of enteric glia, FASEB J. 34 (2020) 5512–5524. 10.1096/fj.201901844r. [DOI] [PubMed] [Google Scholar]
- [41].Rao M, Rastelli D, Dong L, Chiu S, Setlik W, Gershon MD, Corfas G, Enteric Glia Regulate Gastrointestinal Motility but Are Not Required for Maintenance of the Epithelium in Mice, Gastroenterology. 153 (2017) 1068–1081 e7. 10.1053/j.gastro.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Grubisic V, Parpura V, Two modes of enteric gliotransmission differentially affect gut physiology, Glia. 65 (2017) 699–711. 10.1002/glia.23121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bhave S, Gade A, Kang M, Hauser KF, Dewey WL, Akbarali HI, Connexin-purinergic signaling in enteric glia mediates the prolonged effect of morphine on constipation, FASEB J. 31 (2017) 2649–2660. 10.1096/fj.201601068r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McClain JL, Fried DE, Gulbransen BD, Agonist-evoked Ca(2+) signaling in enteric glia drives neural programs that regulate intestinal motility in mice, Cell Mol Gastroenterol Hepatol. 1 (2015) 631–645. 10.1016/j.jcmgh.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA, Activation of neuronal P2X7 receptor–pannexin-1 mediates death of enteric neurons during colitis, Nat Med. 18 (2012) 600–604. 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gulbransen BD, Bains JS, Sharkey KA, Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon., The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 30 (2010) 6801–6809. 10.1523/jneurosci.0603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liu C, Yang J, Enteric Glial Cells in Immunological Disorders of the Gut, Front Cell Neurosci. 16 (2022) 895871. 10.3389/fncel.2022.895871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chow AK, Gulbransen BD, Potential roles of enteric glia in bridging neuroimmune communication in the gut, Am J Physiol Gastrointest Liver Physiol. 312 (2017) G145–G152. 10.1152/ajpgi.00384.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Drokhlyansky E, Smillie CS, Wittenberghe NV, Ericsson M, Griffin GK, Eraslan G, Dionne D, Cuoco MS, Goder-Reiser MN, Sharova T, Kuksenko O, Aguirre AJ, Boland GM, Graham D, Rozenblatt-Rosen O, Xavier RJ, Regev A, The Human and Mouse Enteric Nervous System at Single-Cell Resolution, Cell. 182 (2020) 1606–1622.e23. 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].May-Zhang AA, Tycksen E, Southard-Smith AN, Deal KK, Benthal JT, Buehler DP, Adam M, Simmons AJ, Monaghan JR, Matlock BK, Flaherty DK, Potter SS, Lau KS, Southard-Smith EM, Combinatorial Transcriptional Profiling of Mouse and Human Enteric Neurons Identifies Shared and Disparate Subtypes In Situ, Gastroenterology. 160 (2020) 755–770.e26. 10.1053/j.gastro.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Matheis F, Muller PA, Graves CL, Gabanyi I, Kerner ZJ, Costa-Borges D, Ahrends T, Rosenstiel P, Mucida D, Adrenergic Signaling in Muscularis Macrophages Limits Infection-Induced Neuronal Loss, Cell. 180 (2020) 64–78.e16. 10.1016/j.cell.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, Manno GL, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S, Molecular Architecture of the Mouse Nervous System, Cell. 174 (2018) 999–1014.e22. 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Valdez-Morales E, Guerrero-Alba R, Liñán-Rico A, Espinosa-Luna R, Zarazua-Guzman S, Miranda-Morales M, Montaño LM, Barajas-López C, P2X7 receptors contribute to the currents induced by ATP in guinea pig intestinal myenteric neurons, Eur J Pharmacol. 668 (2011) 366–372. 10.1016/j.ejphar.2011.07.019. [DOI] [PubMed] [Google Scholar]
- [54].Hu H, Gao N, Lin Z, Gao C, Liu S, Ren J, Xia Y, Wood JD, P2X7 receptors in the enteric nervous system of guinea-pig small intestine, J Comp Neurol. 440 (2001) 299–310. 10.1002/cne.1387. [DOI] [PubMed] [Google Scholar]
- [55].Jooss T, Zhang J, Zimmer B, Rezzonico-Jost T, Rissiek B, Pelczar PF, Seehusen F, Koch-Nolte F, Magnus T, Zierler S, Huber S, Schemann M, Grassi F, Nicke A, Macrophages and glia are the dominant P2X7-expressing cell types in the gut nervous system – no evidence for a role of neuronal P2X7 receptors in colitis, Mucosal Immunol. (2023). 10.1016/j.mucimm.2022.11.003. [DOI] [PubMed] [Google Scholar]
- [56].Mawe GM, Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon, J Clin Invest. 125 (2015) 949–955. 10.1172/jci76306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Spear ET, Mawe GM, Enteric neuroplasticity and dysmotility in inflammatory disease: key players and possible therapeutic targets, Am J Physiol Gastrointest Liver Physiol. 317 (2019) G853–G861. 10.1152/ajpgi.00206.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


