Abstract
Background:
The effect of intra-operative chemotherapy (IOC) on the long-term survival of patients with colorectal cancer (CRC) remains unclear. In this study, we evaluated the independent effect of intra-operative infusion of 5-fluorouracil in combination with calcium folinate on the survival of CRC patients following radical resection.
Methods:
1820 patients were recruited, and 1263 received IOC and 557 did not. Clinical and demographic data were collected, including overall survival (OS), clinicopathological features, and treatment strategies. Risk factors for IOC-related deaths were identified using multivariate Cox proportional hazards models. A regression model was developed to analyze the independent effects of IOC.
Results:
Proportional hazard regression analysis showed that IOC (hazard ratio [HR]=0.53, 95% confidence intervals [CI] [0.43, 0.65], P < 0.001) was a protective factor for the survival of patients. The mean overall survival time in IOC group was 82.50 (95% CI [80.52, 84.49]) months, and 71.21 (95% CI [67.92, 74.50]) months in non-IOC group. The OS in IOC-treated patients were significantly higher than non-IOC-treated patients (P < 0.001, log-rank test). Further analysis revealed that IOC decreased the risk of death in patients with CRC in a non-adjusted model (HR=0.53, 95% CI [0.43, 0.65], P < 0.001), model 2 (adjusted for age and gender, HR=0.52, 95% CI [0.43, 0.64], P < 0.001), and model 3 (adjusted for all factors, 95% CI 0.71 [0.55, 0.90], P = 0.006). The subgroup analysis showed that the HR for the effect of IOC on survival was lower in patients with stage II (HR = 0.46, 95% CI [0.31, 0.67]) or III disease (HR=0.59, 95% CI [0.45, 0.76]), regardless of pre-operative radiotherapy (HR=0.55, 95% CI [0.45, 0.68]) or pre-operative chemotherapy (HR=0.54, 95% CI [0.44, 0.66]).
Conclusions:
IOC is an independent factor that influences the survival of CRC patients. It improved the OS of patients with stages II and III CRC after radical surgery.
Trial registration:
chictr.org.cn, ChiCTR 2100043775.
Keywords: Colorectal cancer, Intra-operative chemotherapy, Overall survival, Retrospective cohort study, Stage
Introduction
According to the latest data released by the National Cancer Center of the United States, the estimated new cases and deaths of colorectal cancer (CRC) ranked third (both in male and female),[1] while the data from China showed that the estimated new cases of CRC ranked second and the deaths ranked third.[2] A study using the data of 31 provincial-level administrative divisions during 2005 to 2020 in China shows that estimated CRC deaths increased from 111,410 in 2005 to 178,020 in 2020.[3] Xia C et al[4] indicates that there will be approximately 592,232 new CRC cases and 309,114 CRC deaths in China in 2022. In China, CRC rates in the population as a whole have been increasing, but in the United States, they have recently declined. And the proportion of CRC in the total population of China is significantly higher than the averages in other parts of Asia.[1] Major treatments for CRC include surgery, adjuvant chemotherapy, radiotherapy, and other comprehensive treatments. Radical resection (R0 resection) is substantial for reducing the risk of death. However, a study including 5671 patients with CRC undergoing radical resection between 2003 and 2008 in the Dutch Eindhoven Cancer Registry showed that nearly 18% of patients developed metastasis after surgery.[5]
The primary goal of CRC treatment is to prevent post-operative metastasis and prolong long-term survival. The long-term survival of patients with CRC could be improved by optimizing peri-operative treatments. Intra-operative chemotherapy (IOC) is a method of intra-operative treatment and mainly includes intra-arterial chemotherapy,[6] intra-operative intravenous chemotherapy,[7] intra-peritoneal chemotherapy,[8] and peritoneal hyperthermic perfusion chemotherapy.[9] It may be applied as a peri-operative treatment for patients with CRC. In 1990, Hossfeld[10] performed IOC with 5-fluorouracil (5-FU) plus levamisole during CRC surgery but did not observe positive results. In 2019, a retrospective study involving 551 patients with CRC showed that patients who underwent IOC (193/551) and received hydroxycamptothecin for peritoneal irrigation as well as tumor necrosis factor, 5-FU, and calcium folinate via intravenous injection had a more favorable independent survival outcomes than those who did not (hazard ratio [HR]=0.30, 95% confidence intervals [CI] [0.19–0.48], P < 0.001).[11] However, this study had a small sample size, and no regression model based on IOC was developed. Another randomized, multicenter, prospective, phase III IOCCRC trial (NCT01465451) investigated the safety and efficacy of surgical resection plus IOC (n = 341; randomized to three groups: portal vein, intra-luminal, and intra-peritoneal chemotherapies) vs. surgical resection alone (n = 344, patients were randomized to the control group to undergo surgery alone). The results indicated that IOC did not increase the rate of surgical complications in patients with CRC.[12] However, mesenteric vein injection with 5-FU during intravenous chemotherapy may increase the risk of surgery. In addition, rare studies have reported the effect of IOC (5-FU + leucovorin) on the long-term survival of patients with CRC. Therefore, we performed a single-center, retrospective cohort study comprising 1820 patients with CRC and explored the independent effect of IOC on their long-term survival. Our findings may provide preliminary data support for the application of IOC in CRC patients.
Methods
Ethical approval
This retrospective cohort study was approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University (No. 2020kt417), and performed in accordance with the Helsinki Declaration of 1975, as revised in 2000. The requirement for informed consent was waived because of the retrospective design of the study. This study was registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn; ChiCTR 2100043775).
Patients
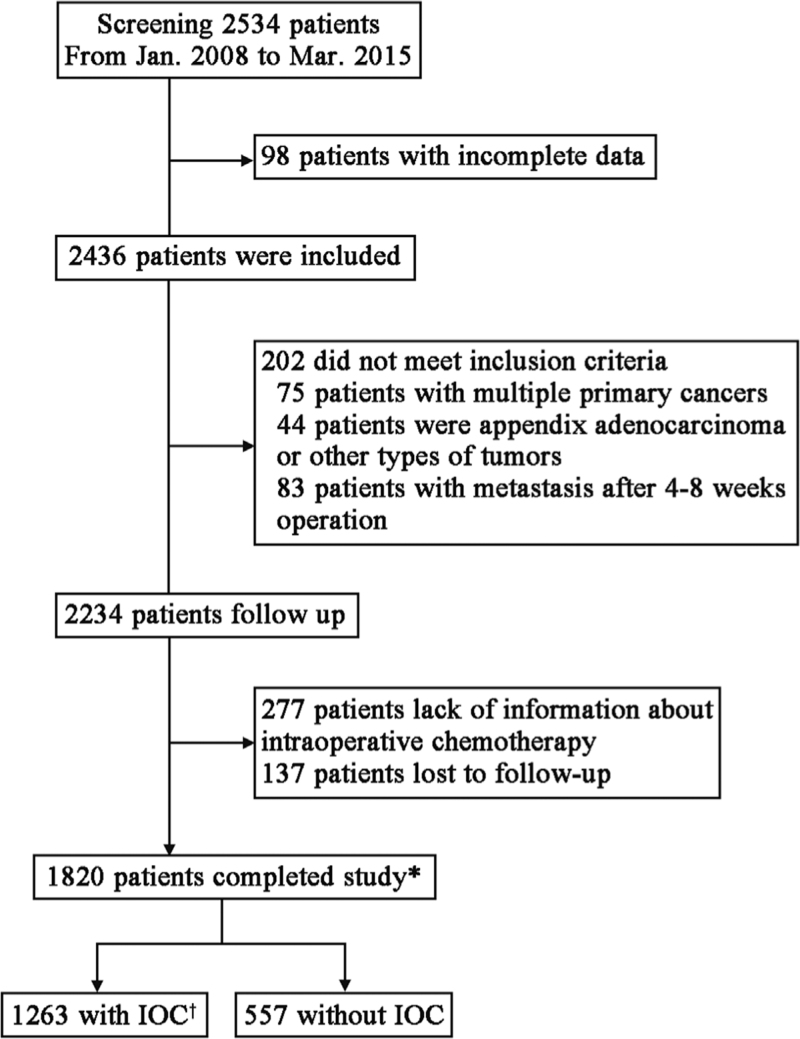
A total of 2534 patients with CRC who underwent radical surgery at the Second General Surgery, The Fourth Hospital of Hebei Medical University were screened between January 2008 and March 2015. Of them, 2234 were eligible for enrollment in the study. After excluding 277 patients without data on IOC and 137 lost to follow up, 1820 patients were finally recruited. Of these patients, 1263 underwent IOC and 557 did not [Figure 1].
Figure 1.
Flow diagram of the screening in patients with CRC administered with and without IOC. ∗Patients who completed the follow up. †For IOC. CRC: Colorectal cancer; IOC: Intra-operative chemotherapy.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) pathological diagnosis of CRC confirmed by histological examination; (2) patients underwent radical surgical resection; (3) patients diagnosed as stage IV with resectable metastases and underwent intra-operative metastasis R0 resection; and (4) patients of all ages. The exclusion criteria were as follows: (1) diagnosis of simultaneous and heterochronous multiple primary cancers, (2) diagnosis of appendix adenocarcinoma or other types of tumors, (3) incomplete data, (4) metastases found after 4 to 8 weeks of follow-up, and (5) lost to follow-up.
Surgical resection
All the patients underwent surgical resection (total mesorectal excision or complete mesocolic excision). Intra-operative resection of metastases was performed by experienced hepatobiliary and thoracic surgeons.
IOC
Patients who underwent IOC received an intravenous infusion of 5-fluorouracil (5-FU 1000 mg + 5% dextrose injection 500 mL) in combination with leucovorin (600 mg, intravenous infusion before 5-FU). The infusion was initiated 30 min after the initial of operation and terminated before the end of the operation. In addition, adverse events (AEs) like skin rashes were monitored.
Other treatments
Pre-operative chemotherapy (PreOC) indicates chemotherapy (oxaliplatin, fluorouracil, or irinotecan) administered to the patients before surgery. Meanwhile, pre-operative radiotherapy (PreOR) refers to radiotherapy administered to patients before surgery, including long and short-term radiotherapies. Post-operative chemotherapy (POC) is the adjuvant chemotherapy with oxaliplatin and fluorouracil administered to patients after radical surgery. The radiotherapy administered to patients according to their post-operative stages is regarded as post-operative radiotherapy (POR). In this cohort, the number of patients who received PreOC, PreOR, POC, and POR was 50, 14, 1085, and 126, respectively [Table 1].
Table 1.
Clinical characteristics of colorectal cancer patients.
| Characteristics | Non-IOC group (n = 557) | IOC group (n = 1263) | Statistics | P values |
| Hospital stay (days) | 16.0 (14.0, 19.0) | 15.0 (13.0, 19.0) | 6.434 | 0.011∗ |
| Age (years) | 62.0 (53.0, 71.0) | 62.0 (54.0, 70.0) | 0.162 | 0.688∗ |
| Gender | 5.219 | 0.022 | ||
| Male | 339 (60.86) | 696 (55.11) | ||
| Female | 218 (39.14) | 567 (44.89) | ||
| Location | 10.846 | 0.028 | ||
| Rectum | 326 (58.53) | 828 (65.56) | ||
| Sigmoid colon | 94 (16.88) | 190 (15.04) | ||
| Descending colon | 15 (2.69) | 39 (3.09) | ||
| Transverse colon | 39 (7.00) | 69 (5.46) | ||
| Ascending colon/ileocecum | 83 (14.90) | 137 (10.85) | ||
| TNM stage | 10.894 | 0.012 | ||
| Stage I | 74 (13.29) | 165 (13.06) | ||
| Stage II | 244 (43.81) | 636 (50.36) | ||
| Stage III | 213 (38.24) | 430 (34.05) | ||
| Stage IV | 26 (4.67) | 32 (2.53) | ||
| T stage | Fisher | <0.001§ | ||
| T1 | 27 (4.85) | 35 (2.77) | ||
| T2 | 56 (10.05) | 153 (12.11) | ||
| T3 | 119 (21.36) | 419 (33.17) | ||
| T4 | 355 (63.74) | 656 (51.95) | ||
| N stage | 9.608 | 0.008 | ||
| N0 | 324 (58.17) | 807 (63.90) | ||
| N1 | 140 (25.13) | 309 (24.47) | ||
| N2 | 93 (16.70) | 147 (11.64) | ||
| M stage | 5.707 | 0.017 | ||
| M0 | 531 (95.33) | 1231 (97.47) | ||
| M1 | 26 (4.67) | 32 (2.53) | ||
| Position | 3.697 | 0.055 | ||
| Left colon and rectum | 445 (79.89) | 1056 (83.61) | ||
| Right colon | 112 (20.11) | 207 (16.39) | ||
| Surgical approach | 19.841 | <0.001 | ||
| Laparoscopic surgery | 139 (24.96) | 449 (35.55) | ||
| Open surgery | 418 (75.04) | 814 (64.45) | ||
| Pathological type | Fisher | 0.684§ | ||
| Tubular adenocarcinoma I–II† | 483 (86.71) | 1124 (88.99) | ||
| Mucinous adenocarcinoma | 48 (8.62) | 96 (7.60) | ||
| Tubular adenocarcinoma III‡ | 19 (3.41) | 29 (2.30) | ||
| Unknown | 7 (1.26) | 14 (1.11) | ||
| Vascular tumor thrombus | 0.704 | 0.401 | ||
| No | 541 (97.13) | 1235 (97.78) | ||
| Yes | 16 (2.87) | 28 (2.22) | ||
| Family history | Fisher | 0.078§ | ||
| No | 526 (94.43) | 1154 (91.37) | ||
| Yes | 31 (5.57) | 106 (8.39) | ||
| Unknown | 0 (0) | 3 (0.24) | ||
| Ascites | 8.348 | 0.015 | ||
| No | 536 (96.23) | 1237 (97.94) | ||
| Yes | 9 (1.62) | 18 (1.43) | ||
| Unknown | 12 (2.15) | 8 (0.63) | ||
| Pre-operative intestinal obstruction | 106.010 | <0.001 | ||
| No | 402 (72.17) | 1147 (90.82) | ||
| Yes | 155 (27.83) | 116 (9.18) | ||
| Local spread | 25.367 | <0.001 | ||
| No | 466 (83.66) | 1157 (91.61) | ||
| Yes | 81 (14.54) | 93 (7.36) | ||
| Unknown | 10 (1.80) | 13 (1.03) | ||
| PreOR | Fisher | <0.001§ | ||
| No | 545 (97.85) | 1252 (99.13) | ||
| Yes | 3 (0.54) | 11 (0.87) | ||
| Unknown | 9 (1.61) | 0 (0) | ||
| PreOC | Fisher | <0.001§ | ||
| No | 510 (91.56) | 1247 (98.73) | ||
| Yes | 34 (6.10) | 16 (1.27) | ||
| Unknown | 13 (2.34) | 0 (0) | ||
| POC | 0.835 | 0.659 | ||
| No | 223 (40.04) | 479 (37.93) | ||
| Yes | 325 (58.35) | 760 (60.17) | ||
| Unknown | 9 (1.61) | 24 (1.90) | ||
| POR | 3.674 | 0.159 | ||
| No | 516 (92.64) | 1140 (90.26) | ||
| Yes | 29 (5.21) | 97 (7.68) | ||
| Unknown | 12 (2.15) | 26 (2.06) |
Data are expressed as median (interquartile range [IQR]) and n (%).
Mann–Whitney U test on comparing two groups.
Moderately differentiated and well-differentiated adenocarcinoma.
Poorly differentiated adenocarcinoma.
P values were calculated using Fisher exact probability method.
IOC: Intra-operative chemotherapy; POC: Post-operative chemotherapy; POR: Post-operative radiotherapy; PreOC: Pre-operative chemotherapy; PreOR: Pre-operative radiotherapy; TNM: Tumor Node Metastasis.
Follow-up
Patients were followed up every three months by telephone calls, outpatient follow-up, or regular review until January 31, 2016, or death. The median follow-up time was (44.0 ± 23.3) months (range: 0.77–98.67 months). The lost to follow-up rate was 6.13% (137/2234).
Data collection
The primary outcome was overall survival (OS), defined as the time from diagnosis till death. Patients who remained survived at the end of the follow-up period were censored. The collected demographic and clinical characteristics of all patients included age, gender, hospital stay (time from hospitalization to discharge), location (rectum, sigmoid colon, descending colon, transverse colon, and ascending colon/ileocecum), position (left colon/rectum, right colon; left colon: sigmoid colon, descending colon; right colon: transverse colon, ascending colon/ileocecum), surgical approach (laparoscopic surgery or open surgery), type of medical record, vascular tumor thrombus, family history, ascites, obstruction, local spread, and treatment strategy [Table 1]. The TNM stage was collected according to the 8th edition of the American Joint Committee on Cancer (AJCC) CRC staging system,[13] including T stage (tumor in situ [Tis], T1, T2, T3, T4), N stage (N0, N1, N2), M stage (M0, M1), and tumor nodal and metastasis (TNM) stage (Stage I: T1–2N0M0; Stage II: T3–4N0M0; Stage III: T1–4N1–2M0; Stage IV: T0–4N0–2M1).
Statistical analysis
Categorical variables were expressed as number and compared using the χ2 test or Fisher's exact probability method. Descriptive variables (age and hospital stay) were biased data, which were presented as median (interquartile range) and compared using the Mann–Whitney U test. Influencing factors were analyzed using Cox proportional hazard regression analysis. The Kaplan–Meier method was used to draw survival curves of patients with CRC after radical resection and the log-rank test was used to compare the OS of patients. Statistical significance was set at P < 0.05. All analyses were performed using Empower (R) (X&Y Solutions, Inc., Boston, MA, USA) and R software (http://www.R-project.org, version 3.5.3).
Results
Clinicopathological features of patients with CRC
A total of 1820 Chinese patients were enrolled in this study. In this cohort, 1263 patients (69.40%) underwent IOC, whereas 557 (30.60%) did not. The age of IOC-treated patients was 62.0 (54.0, 70.0) years, which was not statistically significantly different from that of the non-IOC group (62.0 [53.0, 71.0] years, P = 0.688). The proportion of male patients in the IOC group (696 [55.11%]) was statistically significantly lower than that in the non-IOC group (339 [60.86%]) (P = 0.022). In comparison with IOC group, the non-IOC group had less cases with rectal cancer (IOC vs. non-IOC groups, 828 [65.56%] vs. 326 [58.53%], P = 0.028), at T3 stage (419 [33.17%] vs. 119 [21.36%], P < 0.001), and undergoing laparoscopic surgery (449 [35.55%] vs. 139 [24.96%], P < 0.001) [Table 1]. In contrast, there were more cases in non-IOC group with ascending colon or ileocecum (83 [14.90%] vs. 137 [10.85%], P = 0.028), at T4 stage (355 [63.74%] vs. 656 [51.95%], P < 0.001), with lymph node metastasis (N1–2, 233 [41.83%] vs. 356 [36.11%], P = 0.008), with metastasis (26 [4.67%] vs. 32 [2.53%], P = 0.017), higher incidence of intestinal obstruction (155 [27.83%] vs. 116 [9.18%], P < 0.001), and with local spread (81 [14.54%] vs. 93 [7.36%], P < 0.001) when compared with those in the IOC group [Table 1].
In the IOC group, only 11 (0.87%) patients underwent PreOR. And in the non-IOC group, nine patients with missing information on the PreOR were excluded from the subsequent analysis. The non-IOC group had a lower rate of PreOR (3 [0.54%], P < 0.001) than the IOC group. Meanwhile, the IOC group had a lower rate of PreOC than the non-IOC group (16 [1.27%] vs. 34 [6.10%], P < 0.001; 13 patients with inadequate data in the non-IOC group) [Table 1].
Cox regression analysis on factors influencing the OS of patients with CRC after radical resection
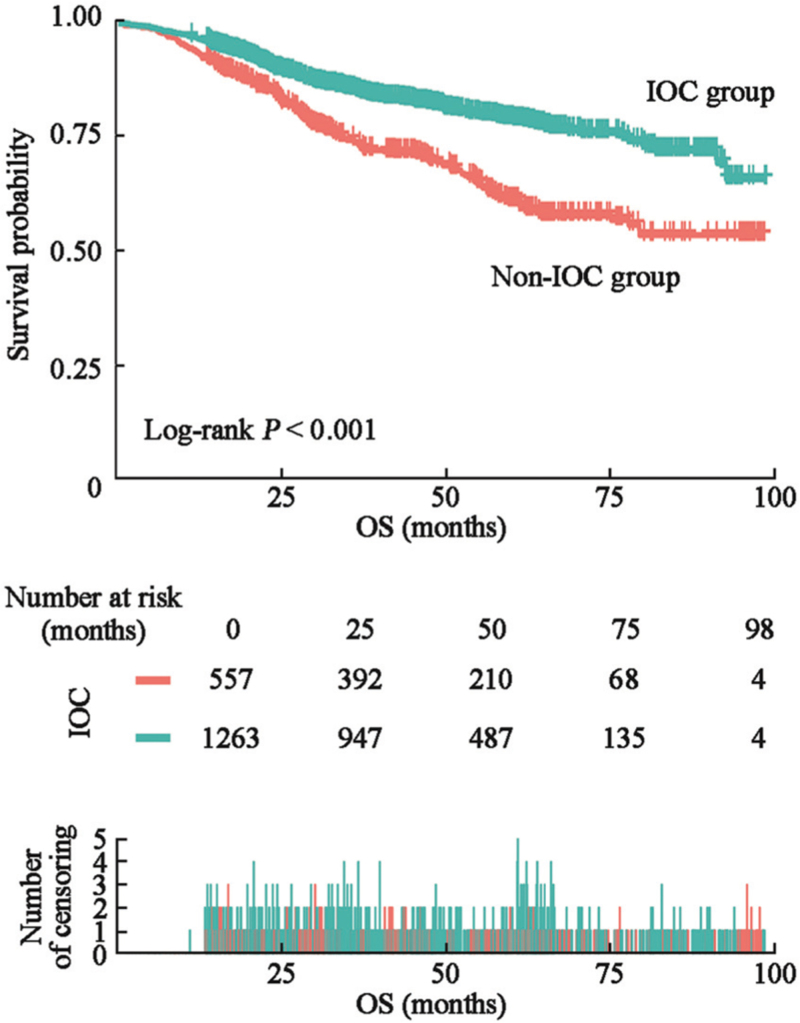
Univariate Cox regression analysis was performed with death as the dependent variable (assigned values: death = 1, survival = 0). The independent variables analyzed include gender, age, tumor position, surgical approach, pathological type, TNM stage, T stage, N stage, M stage, vascular tumor thrombus, family history, ascites, pre-operative intestinal obstruction, PreOR, PreOC, IOC, POC, and POR. The results of the proportional hazard regression analysis revealed TNM stage, N stage, M stage, tumor position, surgical approach, pathological type, vascular tumor thrombus, ascites, local spread, and pre-operative intestinal obstruction as the influencing factors for death in patients with CRC after radical resection (P < 0.05) [Table 2]. Additionally, IOC (HR: 0.53, 95% CI: 0.43, 0.65, P < 0.001) and POR (HR: 1.51, 95% CI: 1.09, 2.10, P = 0.013) were influencing factors for the death in patients with CRC [Table 2]. Survival curves were drawn to investigate the impact of IOC on the OS of patients. The mean overall survival time of IOC group was 82.50 (95% CI, [80.52, 84.49]) months, and 71.21 (95% CI, [67.92, 74.50]) months in non-IOC group. We found that in the IOC group, the 1-year survival rate was 96.91%, the 3-year survival rate was 86.22%, and the 5-year survival rate was 80.14%. And the 1-year survival rate was 94.08%, the 3-year survival rate was 75.50%, and the 5-year survival rate was 63.09% in the non-IOC group. The OS rate in IOC-treated patients was statistically significantly higher than that of non-IOC-treated patients (P < 0.001, log-rank test). The Kaplan–Meier survival curve also demonstrated that the OS rate in the IOC group was statistically significantly higher than that in the non-IOC group [Figure 2].
Table 2.
Univariate Cox proportional-hazard regression analysis of factors on OS in CRC patients.
| Exposures | Survival, HR (95% CI) | P values |
| Gender | ||
| Male | Reference | |
| Female | 1.09 (0.89, 1.34) | 0.391 |
| Age (years) | 1.00 (0.99, 1.01) | 0.825 |
| TNM stage | ||
| Stage I | Reference | |
| Stage II | 1.85 (1.09, 3.14) | 0.022 |
| Stage III | 6.54 (3.94, 10.86) | <0.001 |
| Stage IV | 21.38 (11.89, 38.45) | <0.001 |
| T stage | ||
| T1 | Reference | |
| T2 | 1.16 (0.39, 3.46) | 0.795 |
| T3 | 2.88 (1.06, 7.82) | 0.038 |
| T4 | 4.70 (1.75, 12.62) | 0.002 |
| N stage | ||
| N0 | Reference | |
| N1 | 2.76 (2.15, 3.55) | <0.001 |
| N2 | 6.95 (5.45, 8.86) | <0.001 |
| M stage | ||
| M0 | Reference | |
| M1 | 6.14 (4.36, 8.65) | <0.001 |
| Position | ||
| Left colon and rectum | Reference | |
| Right colon | 1.39 (1.08, 1.78) | 0.010 |
| Surgical approach | ||
| Laparoscopic surgery | Reference | |
| Open surgery | 1.35 (1.07, 1.70) | 0.011 |
| Pathological type | ||
| Tubular adenocarcinoma I–II∗ | Reference | |
| Mucinous adenocarcinoma | 2.03 (1.51, 2.73) | <0.001 |
| Tubular adenocarcinoma III† | 3.52 (2.30, 5.38) | <0.001 |
| Vascular tumor thrombus | ||
| No | Reference | |
| Yes | 1.75 (1.02, 2.98) | 0.041 |
| Family history‡ | ||
| No | Reference | |
| Yes | 0.71 (0.45, 1.11) | 0.135 |
| Ascites§ | ||
| No | Reference | |
| Yes | 2.35 (1.35, 4.08) | 0.003 |
| Local spread|| | ||
| No | Reference | |
| Yes | 4.14 (3.26, 5.26) | <0.001 |
| Pre-operative intestinal obstruction | ||
| No | Reference | |
| Yes | 2.59 (2.07, 3.24) | <0.001 |
| IOC | ||
| No | Reference | |
| Yes | 0.53 (0.43, 0.65) | <0.001 |
| PreOR¶ | ||
| No | Reference | |
| Yes | 1.25 (0.40, 3.88) | 0.704 |
| PreOC∗∗ | ||
| No | Reference | |
| Yes | 0.57 (0.25, 1.28) | 0.173 |
| POC†† | ||
| No | Reference | |
| Yes | 0.97 (0.79, 1.19) | 0.762 |
| POR‡‡ | ||
| No | Reference | |
| Yes | 1.51 (1.09, 2.10) | 0.013 |
Moderately differentiated and well-differentiated adenocarcinoma.
Poorly differentiated adenocarcinoma. In our analysis, these patients (21 cases) with missing data were removed.
Family history group with three cases of data missing.
Ascites group with 20 cases of data missing.
Local spread group with 23 cases of data missing.
PreOR group with nine cases of data missing.
PreOC group with 13 cases of data missing.
POC group with 33 cases of data missing.
POR group with 38 cases of data missing.
CI: Confidence intervals; CRC: Colorectal cancer; HR: Hazard ratio; IOC: Intra-operative chemotherapy; OS: Overall survival; POC: Post-operative chemotherapy; POR: Post-operative radiotherapy; PreOC: Pre-operative chemotherapy; PreOR: Pre-operative radiotherapy; TNM: Tumor Node Metastasis.
Figure 2.
Kaplan–Meier estimates for OS in IOC and non-IOC groups. Red line: non-IOC group. Blue line: IOC group. CRC: Colorectal cancer; IOC: Intra-operative chemotherapy; OS: Overall survival.
Next, the independent effect of IOC on the OS in patients with CRC was examined using Cox multiple regression analysis. An intergroup comparison of the HRs and 95% CI of death was performed using three models [Table 3]. In the non-adjusted model, the IOC group showed a decreased risk of death compared with the non-IOC group (HR: 0.53, 95% CI: 0.43, 0.65, P < 0.001). A reduction in the odds of death (0.53) was observed in the IOC-treated patients. Even after adjusting for age and gender (the most important demographic factors), a reduced risk of death was observed in IOC-treated patients (HR: 0.52, 95% CI: 0.43, 0.64, P < 0.001). After adjustments for age, gender, TNM stage, T stage, N stage, M stage, ascites, surgical approach, local spread, family history, vascular tumor thrombus, pathological type, location, position, pre-operative intestinal obstruction, PreOR, PreOC, POC, and POR, the HR for death in patients treated with IOC was 0.71 (95% CI: 0.55, 0.90, P = 0.006).
Table 3.
Multivariate Cox regression model to analyze the impact of IOC on OS in CRC patients.
| Exposures | HR (95% CI) | ||
| IOC | Non-adjusted∗ | Adjust I∗ | Adjust II† |
| No | Reference | Reference | Reference |
| Yes | 0.53 (0.43, 0.65) | 0.52 (0.43, 0.64) | 0.71 (0.55, 0.90) |
| P-value | <0.001 | <0.001 | 0.006 |
Adjust and adjust I used 1820 samples.
Adjust II used 1709 samples. Adjust I model adjusts for age and gender; adjust II model adjusts for all variables (adjust I + TNM stage, T stage, N stage, M stage, ascites, surgical approach, local spread, family history, vascular tumor thrombus, pathological type, location, position, obstruction, PreOR, PreOC, POC, POR). CI: Confidence intervals; CRC: Colorectal cancer; HR: Hazard ratio; IOC: Intra-operative chemotherapy; OS: Overall survival; POC: Post-operative chemotherapy; POR: Post-operative radiotherapy; PreOC: Pre-operative chemotherapy; PreOR: Pre-operative radiotherapy. TNM: Tumor Node Metastasis.
Subgroup analysis
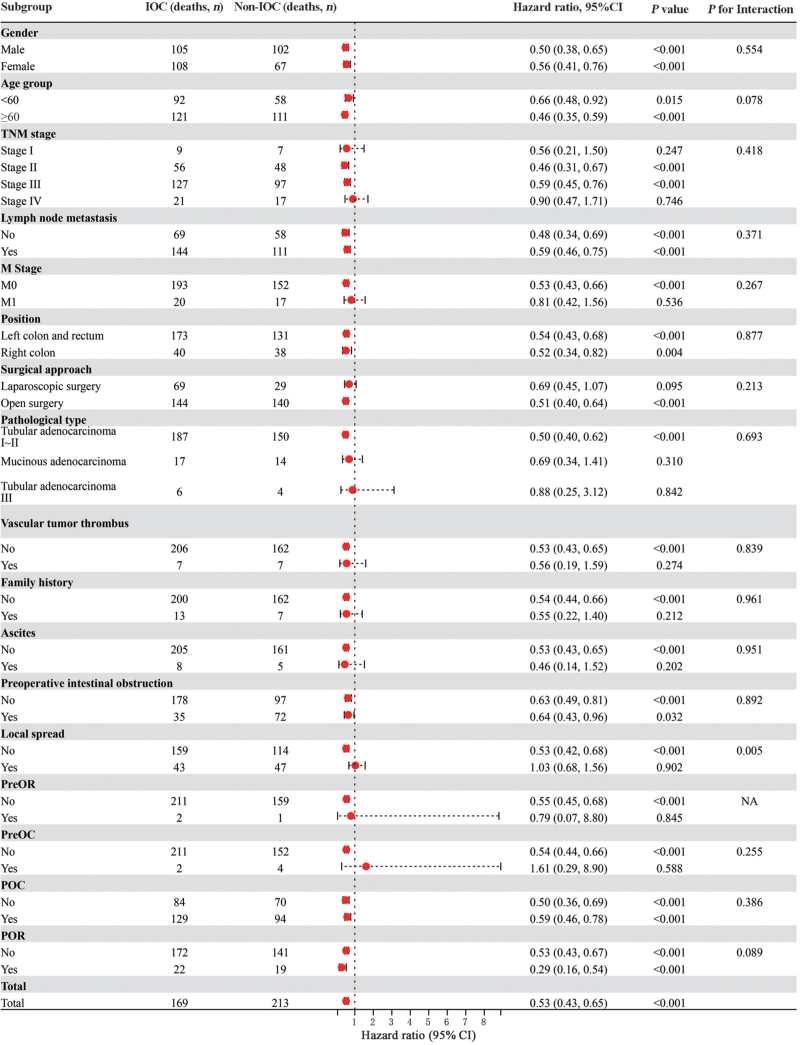
We further analyzed the effect of IOC on the OS of patients with CRC in different subgroups [Figure 3]. The subgroup analysis showed that the effect of IOC on survival was not statistically significantly different in patients with different gender, age (<60 years and ≥60 years), lymph node metastasis, position (left colon and rectum, right colon), or obstruction. The HR for the effect of IOC on the OS of patients with CRC was lower than the non-IOC group in the subgroups at stage II (HR: 0.46, 95% CI [0.31, 0.67]) or III (HR: 0.59, 95% CI [0.45, 0.76]), without metastasis (M0; HR: 0.53, 95% CI [0.43, 0.66]), those treated with open surgery (HR: 0.51, 95% CI [0.40, 0.64]), with non-vascular tumor thrombus (HR: 0.53, 95% CI [0.43, 0.65]), without family history (HR: 0.54, 95% CI [0.44, 0.66]), without ascites (HR: 0.53, 95% CI [0.43, 0.65]), and without local spread (HR: 0.53, 95% CI [0.42, 0.68]). Furthermore, the IOC-treated patients who did not receive PreOR (HR: 0.55, 95% CI [0.45, 0.68]) or PreOC (HR: 0.54, 95% CI [0.44, 0.66]) had a lower risk of death [Figure 3].
Figure 3.
Subgroup analyses for overall survival in patients with IOC vs. without IOC. The hazard ratio for death and corresponding 95% CI for each subgroup were estimated from the unstratified Cox proportional hazards model with treatment as the only covariate. P values were calculated from the Cox model including treatment, subgroup factor, and subgroup factor × treatment interaction term. CI: Confidence intervals; IOC: Intra-operative chemotherapy; OS: Overall survival. CRC: Colorectal cancer; HR: Hazard ratio; POC: Post-operative chemotherapy; POR: Post-operative radiotherapy; PreOC: Pre-operative chemotherapy; PreOR: Pre-operative radiotherapy; TNM: Tumor Node Metastasis; NA: Not available.
IOC-related side effects
In this study, there were two patients reported skin rashes. One reason was antibiotic application, and the other was intra-operative infusion of blood products. Both of the skin rashes disappeared after anti-allergic treatment. There were no IOC-related side effects.
Discussion
Radical surgery is one of the crucial treatment components for CRC, especially total mesorectal excision[14] for rectal cancer and complete mesocolic excision[15] for colon cancer. Moreover, it significantly reduces local recurrence and improves long-term outcomes in patients with CRC. However, radical surgery cannot completely eliminate cancer cells. Therefore, for patients with stage II or III CRC, oxaliplatin and 5-FU are commonly used as adjuvant chemotherapy after radical surgery.[16] Recurrence and metastasis may occur in some patients due to the presence of tumor cells or micrometastases in the circulatory system.[17,18] Our previous study showed that the presence of micrometastatic cells in the bone marrow was significantly associated with poor prognosis in gastric cancer.[19] Preliminary investigations of IOC began as early as 1990.[20] As the liver is the most common metastasis site in CRC, some researchers have attempted to perform perfusion chemotherapy via the hepatic artery to reduce the post-operative liver metastasis occurrence.[21,22] However, a study by Kerr et al[21] showed that the median OS of the intrahepatic arterial infusion group was 14.7 months, whereas that of the intravenous group was 14.8 months (HR: 1.04, 95% CI [0.80–1.33], log-rank test, P = 0.79), indicating intrahepatic arterial infusion cannot significantly improve the the survival of patients with cancer. Investigation on the effect of intra-peritoneal hyperthermic perfusion chemotherapy on locally advanced CRC is currently in progress.[23,24] Recently, a multicenter, open-label, randomized clinical trial (COLORPEC, NCT02231086) investigating the effects of intraperitoneal infusion chemotherapy reported no improvement in the survival of patients without peritoneal metastasis for 18 months; instead a potential increase in the economic and technical burden of patients was noted.[24]
In our study, univariate analysis showed that IOC improved the OS in patients with CRC (HR: 0.53, 95% CI [0.43, 0.65], P < 0.001). IOC is a simple procedure in which intra-operative intravenous infusion of 5-FU and calcium folinate is administered. At present, the studies reporting the effects of intra-operative intravenous chemotherapy are limited, and no high-quality evidence-based medicine is available. Two random clinical trials reported the effect of IOC combined with portal vein injection of fluorouracil on the long-term survival in patients with CRC after radical resection, showing that IOC significantly improve the 5-year survival. However, the sample sizes of both studies were small.[25,26] A randomized controlled study by Chang et al[7] focused on the effects of portal vein chemotherapy plus mFOLFOX6 vs. mFOLFOX6 alone on patients with stage II or III CRC. The results showed reduced distant metastasis incidence and improved disease-free survival in patients administered IOC. In the aforementioned studies, chemotherapeutic drugs were injected into the portal vein through catheter placement during the operation. However, the portal vein through catheter placement has been identified as difficult to operate and long to learn. In 2017, a prospective randomized controlled trial of 696 patients also investigated the effect of IOC, but it mainly focused on the safety of operation without exploring oncological factors.[12] In the current study, we investigated the impact of IOC on the long-term survival in patients with CRC. By dichotomizing patients into IOC and non-IOC groups, we found that the 1- (96.91% vs. 94.08%), 3- (86.22% vs. 75.50%), and 5-year (80.14% vs. 63.09%) survival rates of the IOC group were significantly higher than those of the non-IOC group (P < 0.001). We further constructed three multiple regression models to explore whether other confounding factors could affect the OS of patients with CRC. The results showed that IOC was an independent prognostic factor for CRC after adjusting for all the variables.
Finally, we performed a subgroup analysis to examine the impact of IOC on patients with CRC in different subgroups. The impact of IOC on OS differs among patients with different tumor stages. There was no significant change in the impact of IOC on the OS of patients at stage I or IV, which may be due to the better prognosis of stage I patients and the worse prognosis of stage IV patients. This is also the reason why many studies primarily focused on patients at stages II and III.[7,11] These findings suggest that IOC should not be administered to patients with a pre-operative evaluation of stage I or IV disease to reduce the economic burden and unnecessary chemotherapy-related adverse reactions. In addition, IOC had no significant impact on the OS of patients with local dissemination (HR = 1.03, 95% CI [0.68, 1.56]), which may be related to the presence of local dissemination and potential peritoneal metastasis. Intravenous infusion injection of 5-FU did not show a significant effect; thus, intra-peritoneal hyperthermic perfusion chemotherapy may be a better option for these patients.[9,20,27] The inconsistent results of pre-operative chemoradiotherapy in the subgroup analysis may be explained by the fact that pre-operative treatment significantly suppressed tumor growth in patients. However, the sample size was too small; therefore, the results should be interpreted with caution.
There are some limitations of this study. First, the retrospective nature of this study may have led to biased results. Prospective randomized controlled trials are needed to validate the effects of IOC on prognosis during follow-up. Second, as this was a retrospective study and some patients had missing information related to treatment, a small sample size was available for some analyses. At the same time, only two patients in the IOC group in this study reported rashes, but they were not considered IOC-related AEs. The rate of AE was low, which may be due to the retrospective study with incomplete data recording. Last, the time of IOC could not be clearly defined, which is another disadvantage of a retrospective study.
To summarize, this large-scale retrospective cohort study found that IOC is an independent influencing factor for OS of patients with CRC. Intra-operative intravenous chemotherapy improves the OS of patients with stages II and III CRC after radical surgery. Moreover, future prospective, multicenter, large-scale clinical studies are needed to develop high-quality evidence-based medicine for patients with CRC.
Acknowledgements
Author thank Dr. Junyan Wang for the drawings for this manuscript. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by the Youth Science and Technology Project of Hebei Health Commission (No. 20210029), Hebei Provincial Natural Science Foundation Precision Medicine Joint Project (No. H2020206485), and Hebei Provincial Department of Science and Technology Key Project (No. 206Z7705G).
Conflicts of interest
None.
Footnotes
How to cite this article: Hu X, Zheng Z, Han J, Li B, Guo G, Guo P, Yang Y, Li D, Yan Y, Niu W, Zhou C, Meng Z, Feng J, Yu B, Liu Q, Wang G. Effect of intra-operative chemotherapy with 5-fluorouracil and leucovorin on the survival of patients with colorectal cancer after radical surgery: a retrospective cohort study. Chin Med J 2023;136:830–839. doi: 10.1097/CM9.0000000000002598
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023; 73:17–48. doi:10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Zheng RS, Zhang SW, Zeng HM, Wang SM, Sun KX, Chen R, et al. Cancer incidence and mortality in China, 2016. JNCC 2022; 2:1–9. doi: 10.1016/j.jncc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Yin P, Liu YN, Liu JM, Wang LJ, Qi JL, et al. Mortality and years of life lost of colorectal cancer in China, 2005-2020: findings from the national mortality surveillance system. Chin Med J 2021; 134:1933–1940. doi: 10.1097/CM9.0000000000001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States 2022 profiles, trends, and determinants. Chin Med J 2022; 135:584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Gestel YR, de Hingh IH, van Herk-Sukel MP, van Erning FN, Beerepoot LV, Wijsman JH, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol 2014; 38:448–454. doi: 10.1016/j.canep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010; 28:3687–3694. doi: 10.1200/JCO.2010.28.5643. [DOI] [PubMed] [Google Scholar]
- 7.Chang W, Wei Y, Ren L, Zhong Y, Yu Y, Chen J, et al. Randomized controlled trial of intraportal chemotherapy combined with adjuvant chemotherapy (mFOLFOX6) for stage II and III colon cancer. Ann Surg 2016; 263:434–439. doi: 10.1097/SLA.0000000000001374. [DOI] [PubMed] [Google Scholar]
- 8.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010; 28:63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 9.Kwakman R, Schrama AM, van Olmen JP, Otten RH, de Lange-de Klerk ES, de Cuba EM, et al. Clinicopathological parameters in patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer metastases: a meta-analysis. Ann Surg 2016; 263:1102–1111. doi: 10.1097/SLA.0000000000001593. [DOI] [PubMed] [Google Scholar]
- 10.Hossfeld DK. [Is there a reliable adjuvant (intraoperative) chemotherapy of colorectal cancer?]. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir 1990:175–178. [PubMed] [Google Scholar]
- 11.Liu Z, Zou Y, Rong Y, Shi X, Li C, Li C, et al. Intraoperative chemotherapy with a novel regimen improved the therapeutic outcomes of colorectal cancer. J Cancer 2019; 10:5986–5991. doi: 10.7150/jca.35450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang RX, Lin JZ, Lei J, Chen G, Li LR, Lu ZH, et al. Safety of intraoperative chemotherapy with 5-FU for colorectal cancer patients receiving curative resection: a randomized, multicenter, prospective, phase III IOCCRC trial (IOCCRC). J Cancer Res Clin Oncol 2017; 143:2581–2593. doi: 10.1007/s00432-017-2489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol 2018; 25:1454–1455. doi: 10.1245/s10434-018-6462-1. [DOI] [PubMed] [Google Scholar]
- 14.Pikarsky AJ, Rosenthal R, Weiss EG, Wexner SD. Laparoscopic total mesorectal excision. Surg Endosc 2002; 16:558–562. doi: 10.1007/s00464-001-8250-3. [DOI] [PubMed] [Google Scholar]
- 15.Bertelsen CA, Neuenschwander AU, Jansen JE, Tenma JR, Wilhelmsen M, Kirkegaard-Klitbo A, et al. 5-year outcome after complete mesocolic excision for right-sided colon cancer: a population-based cohort study. Lancet Oncol 2019; 20:1556–1565. doi: 10.1016/S1470-2045(19)30485-1. [DOI] [PubMed] [Google Scholar]
- 16.Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. NCCN Guidelines insights: rectal cancer, version 6.2020. J Natl Compr Canc Netw 2020; 18:806–815. doi: 10.6004/jnccn.2020.0032. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto H, Murata K, Fukunaga M, Ohnishi T, Noura S, Miyake Y, et al. Micrometastasis volume in lymph nodes determines disease recurrence rate of stage II colorectal cancer: a prospective multicenter trial. Clin Cancer Res 2016; 22:3201–3208. doi: 10.1158/1078-0432.CCR-15-2199. [DOI] [PubMed] [Google Scholar]
- 18.Nicastri DG, Doucette JT, Godfrey TE, Hughes SJ. Is occult lymph node disease in colorectal cancer patients clinically significant? A review of the relevant literature. J Mol Diagn 2007; 9:563–571. doi: 10.2353/jmoldx.2007.070032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G, Wang S, Li Y, Yu Y, Song Z, Zhao Q, et al. Clinical study of disseminated tumor cells in bone marrow of patients with gastric cancer. Hepatogastroenterology 2013; 60:273–276. doi: 10.5754/hge12599. [DOI] [PubMed] [Google Scholar]
- 20.Shen P, Stewart JH, Levine EA. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for metastatic colorectal cancer with peritoneal surface disease. Curr Probl Cancer 2009; 33:154–167. doi: 10.1016/j.currproblcancer.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Kerr DJ, McArdle CS, Ledermann J, Taylor I, Sherlock DJ, Schlag PM, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet 2003; 361:368–373. doi: 10.1016/S0140-6736(03)12388-4. [DOI] [PubMed] [Google Scholar]
- 22.Chan R, Kerr D. Hepatic arterial chemotherapy for colorectal cancer liver metastases: a review of advances in 2003. Curr Opin Oncol 2004; 16:378–384. doi: 10.1097/01.cco.0000126577.04727.e9. [DOI] [PubMed] [Google Scholar]
- 23.Goéré D, Glehen O, Quenet F, Guilloit JM, Bereder JM, Lorimier G, et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): a randomised, phase 3 study. Lancet Oncol 2020; 21:1147–1154. doi: 10.1016/S1470-2045(20)30322-3. [DOI] [PubMed] [Google Scholar]
- 24.Klaver CEL, Wisselink DD, Punt CJA, Snaebjornsson P, Crezee J, Aalbers AGJ, et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (CO LOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol 2019; 4:761–770. doi: 10.1016/S2468-1253(19)30239-0. [DOI] [PubMed] [Google Scholar]
- 25.Rougier P, Sahmoud T, Nitti D, Curran D, Doci R, De Waele B, et al. Adjuvant portal-vein infusion of fluorouracil and heparin in colorectal cancer: a randomised trial. European Organisation for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group, the Gruppo Interdisciplinare Valutazione Interventi in Oncologia, and the Japanese Foundation for Cancer Research. Lancet 1998; 351:1677–1681. doi: 10.1016/s0140-6736(97)08169-5. [DOI] [PubMed] [Google Scholar]
- 26.Fielding LP, Hittinger R, Grace RH, Fry JS. Randomised controlled trial of adjuvant chemotherapy by portal-vein perfusion after curative resection for colorectal adenocarcinoma. Lancet 1992; 340:502–506. doi: 10.1016/0140-6736(92)91708-g. [DOI] [PubMed] [Google Scholar]
- 27.Elias D, Honoré C, Dumont F, Ducreux M, Boige V, Malka D, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg 2011; 254:289–293. doi: 10.1097/SLA.0b013e31822638f6. [DOI] [PubMed] [Google Scholar]