Background.
This report contains recommendations from 1 of 7 domains of the International Donation and Transplantation Legislative and Policy Forum (the Forum). The purpose is to provide expert guidance on the structure and function of Organ and Tissue Donation and Transplantation (OTDT) systems. The intended audience is OTDT stakeholders working to establish or improve existing systems.
Methods.
The Forum was initiated by Transplant Québec and co-hosted by the Canadian Donation and Transplantation Program partnered with multiple national and international donation and transplantation organizations. This domain group included administrative, clinical, and academic experts in OTDT systems and 3 patient, family, and donor partners. We identified topic areas and recommendations through consensus, using the nominal group technique. Selected topics were informed by narrative literature reviews and vetted by the Forum’s scientific committee. We presented these recommendations publicly, with delegate feedback being incorporated into the final report.
Results.
This report has 33 recommendations grouped into 10 topic areas. Topic areas include the need for public and professional education, processes to assure timely referral of patients who are potential donors, and processes to ensure that standards are properly enforced.
Conclusions.
The recommendations encompass the multiple roles organ donation organizations play in the donation and transplantation process. We recognize the diversity of local conditions but believe that they could be adapted and applied by organ donation organizations across the world to accomplish their fundamental objectives of assuring that everyone who desires to become an organ donor is given that opportunity in a safe, equitable, and transparent manner.
Organ donation is a tremendously generous act, and every year hundreds of thousands of lives across the world are saved or radically improved through the gift of organ donation.1 Transplantation is the only treatment available to prolong life for some patients with end-stage organ failure. For many more, their lives are enhanced through the gift of tissue donation. However, the chronic shortage of organs available for transplant means that thousands of lives are lost each year.1,2 More needs to be done to improve donor awareness and infrastructure to achieve the international target of ethical self-sufficiency in each country that engages in, or aspires to, an Organ and Tissue Donation and Transplantation (OTDT) system.3-5
Achieving self-sufficiency requires designing, implementing, and maintaining an OTDT system. National and international consensus documents have described aspects of the roles that an OTDT system should perform.6-10 These and other documents provide a foundation for stakeholders to create or improve their OTDT systems.
This document builds on that foundation by specifying the markers and elements of a safe, fair, and transparent OTDT system that maximizes the potential for the number of lives saved through the gift of deceased organ donation.
Two factors motivated the recommendations in this report. First, new technologies and practices are continuously being proposed and integrated into OTDT systems, sometimes in ways that strain existing OTDT logistic or regulatory infrastructure. This could include the ethical and logistic challenges of donation after medical assistance in dying (also known as voluntary euthanasia)11,12 or the rapidly expanding area of ex vivo organ support technology.13,14
The other factor was the integration of these recommendations in the International Donation and Transplantation Legislative and Policy Forum (the Forum; Weiss et al).15 The goal of the Forum was to create aspirational recommendations that draw upon international knowledge of OTDT stakeholders from diverse professional expertise, deeply informed by the experience of patient, family, and donor partners.
This report aims to inform those who plan to establish organ donation and transplantation capability or improve an existing system. It will inform those working at a local, regional, or national level to identify and prioritize improvements while recognizing the vast international variability in local culture, legal infrastructure, resources, and other factors that could impact the uptake of an individual recommendation.
MATERIALS AND METHODS
The process for developing these recommendations has been published (Weiss et al).15 In summary, we applied nominal group technique consensus building in a series of virtual conferences between February and September 2021.16 Topic areas for recommendations were identified through consensus, and the subsequent work for each topic was informed by narrative literature reviews and vetted by the scientific committee of the Forum. Participants were selected on the basis of expertise in deceased organ donation administration, governance, and management, while emphasizing geographic and professional expertise diversity.
(SDC, Appendix I http://links.lww.com/TXD/A490) lists participants and their affiliations. Contributors included: 9 members who held current or past leadership positions in organ donation organizations (ODOs); 3 patient, family, or donor partners; 1 transplant surgeon; 2 intensive care specialist consultants; 1 nurse; and 2 policy development experts. Members were recruited from Canada, Australia, the United Kingdom, the United States, and Germany. All contributors participated in all stages of the discussion and drafting process and are listed as authors.
Patient, family, and donor partners contributed to all aspects of the Forum, including each group and the planning and scientific committees. All participants completed conflict of interest forms, and none had conflicts with any for-profit entities. The recommendations were presented at a hybrid in-person and virtual Forum held in Montréal, Canada, in October of 2021. Feedback from that Forum was incorporated into this final version of the recommendations. Recordings of the Forum sessions are available at https://forumtransplantquebec.ca/en.
Although many of the markers of good practice described in the document can be applied to both living and tissue donation, the focus of this group was on deceased organ donation. The Forum included separate working groups for living donation and tissue. Please refer to separate publications from the Forum for more detailed recommendations specific to those practices.
RECOMMENDATIONS
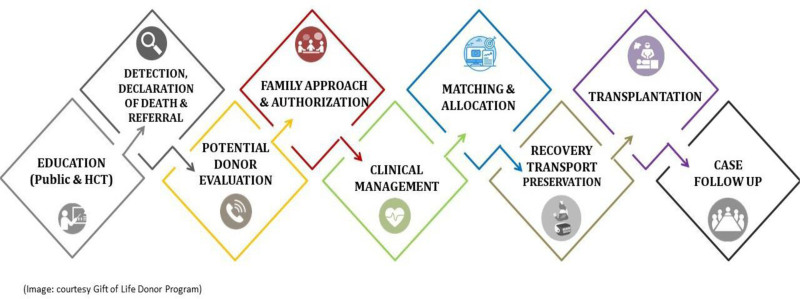
The recommendations in this report follow the organ donation and transplantation care pathway, which is demonstrated in Figure 1. This pathway includes all the points where an ODO or other agencies may influence the outcome of a potential donation and transplantation event.
FIGURE 1.
The donation and transplantation care pathway.
Recommendations on Organ Donation Supporting Infrastructure
Recommendations
Recommendation 1: Establish a clear pathway for the organ and tissue donation and transplantation processes, with clear roles and accountabilities for actions to be taken at all stages and supporting governance to monitor adherence to best practice.
Recommendation 2: Establish detailed legislation, guidance, information, and support to clarify the donation pathway and actions required at each stage, tailored to different audiences (eg, public, patients, families, critical care teams, ODOs, etc), as well as monitoring systems to ensure alignment with innovation and best national/ international practice.
Recommendation 3: Establish open and transparent systems to support clinical and public confidence in OTDT.
Recommendation 4: Establish methods and processes for timely, standardized data collection and sharing to inform improvements in successful clinical outcomes, safety, and performance.
Recommendation 5: Invest in research and innovation at all stages of the care pathway, including continuous improvement of practices and processes.
Recommendation 6: Provide the necessary resources to support all stages of the care pathway to effectively operate at any time, on any day of the year.
An effective donation system relies on a range of supporting infrastructure measures, which cover several stages of the care pathway. The components of this infrastructure are described in multiple publications and the published structures of high-performing OTDT systems.
Markers of Best Practices in Supporting Infrastructure
Professional Education
Standardized training17 and education programs are provided to all healthcare professionals involved in the OTDT care pathway to inform the clinical service and ensure adherence to current best practices.
Roles, Accountability, Legal Authority, Governance
Clarity is provided at all stages of the care pathway about roles, responsibilities, and accountability at the local, regional, and national levels, with a clear legal basis.6,8,18 When setting roles, consideration is given to potential conflicts of interest (eg, transplant surgeons are not responsible for confirming brain death or withdrawing life-sustaining measures).18-21
Accountability includes safety, quality assurance, quality improvement, and regulators. Some aspects are foundational responsibilities of all systems, including traceability of organs and patients to limit abuses such as organ trafficking.4 In contrast, others are required for systems hoping to improve practice, such as potential donor audits.22 Accountability is inexorably linked to data collection and reporting, as discussed below.
Frameworks and Best Practice Guidelines
Clinical, legal, and ethical guidelines are publicly available to inform clinical decision-making.23-25 Given that donation is a low-frequency, high-impact event, these best practice guidelines provide guidance to clinical stakeholders who may have limited regular interactions with donors. This guidance also informs actions across the jurisdiction of the ODO. Publication of this guidance maintains public and clinical confidence in the system.
Collaboration
Effective working relationships between stakeholders at all stages of the care pathway provide seamless care for the patient, family, and the donor. In most areas, a key role of an ODO is to facilitate close collaboration between clinicians responsible for the identification and maintenance of patients who are potential donors and the organ recovery and transplant teams.26 On a system development level, ODOs also coordinate local, regional, national, and international experts to generate and share new research and best practices.
Resources
Hospitals acknowledge that organ donation and transplantation activity is unpredictable, and commit to providing timely access to the right resources. This includes resources to support the clinical donation process (eg, human resources, beds, theater, equipment, data, guidance, etc), public awareness activity and registries, funding for innovation, preservation at the time of retrieval, rapid clinical or ethics advice for challenging situations, etc.
Data Collection and Reporting
Agreed data sets are available across the care pathway, which drives decision-making, action, and improvements. Although these data can be useful to clinicians when stored locally, the true value of data in driving performance improvement is gained when comparisons can be made between centers and jurisdictions. National data systems such as those in the United Kingdom,27 United States,28 and Canada29 are structured to report timely, accurately, and easily to indicate areas of potential improvement or identify high-performing centers that could share practices more broadly. International databases such as the Global Observatory on Donation and Transplantation1 allow for observations between countries over longer time frames. There are also mechanisms in place to ensure that data can be safely and legally stored. For more information, please refer to Legal Foundations of the Forum (Toews et al).30
Whatever data system is in use, outcome measures are required that carefully define and are consistent with the goals of the ODO, the larger healthcare system, and societal expectations. A recent literature review identified multiple outcome measures that OTDT systems reported when defining quality and recommended that quality should be defined holistically throughout the OTDT pathway.31
Innovation
Opportunities to identify and deliver innovation and research are provided throughout the care pathway to support continuous improvements in care and the number and quality of organs available for transplant and, therefore, the number of lives that can be saved.
As detailed in the Baseline Ethical Principles (Gardiner et al)32 and Research and Innovation domains (Escoto et al),33 innovation is not only appropriate but necessary. Furthermore, innovation benefits from coupling with evaluative procedures to ensure efficacy and adherence to ethical standards.
Public Education and Awareness
Recommendations
Recommendation 7: Establish effective governance of the care pathway, to support public confidence in the system.
Recommendation 8: An organ donor registry (opt-in or opt-out or a hybrid thereof) will enable individuals to formally record their donation decision and informs the donation team before the discussion with the family.* However, although registry supports public awareness, it is not essential.
Recommendation 9: Awareness strategies are essential for educating and informing the public. Providing communications strategies, aligned to national campaigns and tailored to different audiences (eg, faith/ beliefs; cultural; age), will support messaging to different communities. Campaigns that promote individuals sharing their decision with their families are effective, as families will always be approached and are more likely to support donation if they knew their loved ones’ decision.
Public education and awareness programs are an important method to change culture and create broad societal support for organ and tissue donation.34 Although the promotion of donation awareness is a primary objective of public relations, ideal systems are able to respond to public scrutiny, particularly if an event occurs that could negatively impact public confidence in the system. Further details of public outreach regarding intent to donate registries are available in the Consent Models and Emerging Legal Issues domain report (Walton et al).35
Markers of Best Practices in Public Education and Awareness
Transparency, especially for any events that may impact the real or perceived safety or fairness of the donation system, is vital to maintaining public trust. Although rare, scandals involving donation systems arise, and these events can profoundly impact trust in OTDT activity.36 This is in addition to the constant threat of transplant tourism-related ethical violations.37 Transparent, accountable systems will have resources available such that the public can be confident that any violations of safety or ethical principles will be quickly identified and the system modified to respond to underlying factors that could have created the conditions for such a violation.
When developing approaches to public awareness, consideration is given to dispelling myths and misconceptions, which are frequently cited as reasons to not register intent to donate38,39; encouraging people to register their decision and to also inform their family of their organ and tissue donor wishes40; and ensuring that family is aware of the possibility of organ donation discussion as part of end-of-life care, which can decrease feelings of surprise that these discussions can provoke during the shock of the loss of a loved one.41
Tailoring public awareness campaigns to different audiences and considering cultural differences provides the greatest impact and best outcomes. Work is undertaken to identify and address any concerns/myths regarding the donation and transplant process, with community and faith leaders taking an active role in raising awareness at local levels (eg, community events, schools, places of worship, etc).42,43
Campaigns also encourage the public to consider and record their donation decision. This requires collaboration between ODOs and all professions that contribute to end-of-life care planning, including but not limited to palliative care, wills, and personal directive planning.
Potential Donor Identification, Referral, and the Determination of Death
Recommendations
Recommendation 10: Establish systems to identify and support the early referral of potential donors.
Recommendation 11: Establish a potential donor audit of all deaths, which monitors adherence to best practices and identifies missed opportunities, with continuous improvement supported through feedback to hospital clinicians.
Recommendation 12: Ensure that determination of death using neurological or circulatory criteria accords with established national/regional/jurisdictional professional standards, complies with legal frameworks, and is reliably performed so that health professionals and the public have trust and confidence in the process.
Recommendation 13: The healthcare professional(s) who perform(s) the death determination of a specific patient cannot be involved in allocation, recovery, and transplantation procedures of the donated organs from that donor.
As outlined in the Legal Foundations domain (Toews et al),30 OTDT legislation includes mandatory referral to donation services for all people who may imminently become potential deceased organ donors. However, confirmation of compliance with such legislation depends on systems and processes in place to ensure that all potential deceased donors are identified and referred.
Markers of Best Practices in Donor Identification and Referral
A referral occurs sufficiently early to ensure that there is time to facilitate donation, which includes obtaining consent, undertaking donor assessment, and coordinating the logistics of donation.22,4439,40,41,42,43,44,45,46 In the absence of an absolute medical contraindication for donation (eg, metastatic cancer), referral leads to the approach to the family of the patient who is a potential donor, as described in detail below.
Early notification to donation services—usually the ODO—of all potential donors once there is medical consensus that death is likely enables the following:
Assessment of donation suitability in all instances with the timely identification of potential organ donors and avoidance of missed donation opportunities.
Provision of advice about medical suitability for organ, eye, and tissue donation.
Planning for family communication about end-of-life choices, including involvement of donation specialist staff to ensure that family communication about donation is undertaken according to best practices.
Provision of expert advice about donor medical management that can be important in preserving donor suitability and optimizing donation and transplantation outcomes.47
Reliable mechanisms for the routine, timely notification of donation services are in place (eg, “clinical trigger” criteria). Collaboration between donation services and hospitals are essential to ensure consistent, transparent, and timely referral practices within intensive care units and emergency departments.
Compliance with these referral policies is audited through a recurring retrospective medical record audit of all deaths.48 Regular and timely feedback to hospital clinicians in the form of case reviews of “missed donors” or late referrals, along with statistical reporting using key performance indicators, supports learning and adherence to best practice.48 The exact format of the feedback to be given is adapted using current best practices regarding audit and feedback strategies adapted to local conditions.49,50
For further information on the legal requirements and considerations for referrals, refer to Legal Foundations of the Forum (Toews et al).30
In nearly all OTDT systems, ODO personnel are expressly prohibited from determining death in an individual patient.18-20,51 ODOs, however, often provide guidance in the generation of clinical protocols to confirm the death of a patient who is a potential donor.52,53
In jurisdictions that lack a legally mandated definition of death but instead rely on best medical practice, these documents often become the de facto legal definition of death.
Markers of Best Practices in the Determination of Death
The processes and tests required to diagnose death are based on established criteria and comply with legal frameworks, including in the context of both deceased organ and tissue donation. Recommendations regarding the legal definition of death are included in the Legal Foundations of the Forum (Toews et al).30
The jurisdiction’s legal framework will set the baseline framework for death determination. Legal definitions vary substantially across the world, including explicitly whole brain criteria (eg, the United States and the Uniform Determination of Death Act) or only brain stem (United Kingdom). Whatever the legal definition, however, best practice would be for medical professionals to define the specific clinical or paraclinical examinations and tests that will be used to determine compliance with the law. These tests will change over time as the scientific understanding of brain function expands, and new testing modalities become available, such as the brain health index. Integration of recommendations from existing national and international protocols for the determination of death according to neurological or circulatory criteria, which expert groups have developed, are available to clinicians and inform clinical practice.19,54-56
The process for death determination is reliable, transparent, and performed at the highest standard to ensure health professionals and the public have trust and confidence in the process.57 Clinicians responsible for death determination are familiar with the processes and have received appropriate and ongoing training.
Roles and accountability are clear to maintain confidence in the donation system and remove any perceived conflicts of interest. The healthcare individual who undertakes the procedures to determine death and make decisions about withdrawal of life-sustaining treatment is not involved in the decisions regarding the allocation and transplantation of any donated organs from the potential donor or the surgical retrieval of any donated organs.
The processes and tests required to diagnose death are based on established criteria and comply with legal frameworks, including in the context of deceased tissue donation.
Potential Donor Evaluation
Recommendations
Recommendation 14: Critical care, organ donation, and transplant teams collaborate to explore the potential donor’s medical history, evaluate the risk of disease transmission, and to determine whether organs are safe for transplantation.
Recommendation 15: Privacy laws and regulations allow for the exchange of patient information within the critical care and organ donation teams and administrators before consent for donation is obtained to enable potential donor evaluation.
Markers of Best Practices in Potential Donor Evaluation
Guidance regarding contraindications to donation is available to ensure the safety and viability of organ donations. 9,58-60 This framework includes guidance on assessing for risk of disease transmission and determining individual organ functional suitability for transplantation.61 Initial assessment of the donation potential against this guidance is undertaken before approaching families. This ensures that families are not inappropriately offered donation where there exist absolute contraindications to donation.9 In such circumstances, it may be helpful to raise the topic of donation with families to explain why it is not possible, particularly where the person had conveyed willingness to be a donor through a register or other means.
Once initial interest to pursue donation is confirmed by the patient or family, a thorough health, lifestyle, and travel history of the donor is obtained to help assess any risks. This includes obtaining past medical records and speaking to family. Further, a clinical examination is performed, and laboratory and imaging tests are undertaken for safety, suitability, and matching purposes.
Critical care, organ donation, and transplant teams collaborate, with advice from specialist experts where appropriate regarding specific disease risks, to explore the potential donor’s medical history and to identify any potential issues with the safety or efficacy of any donated organs.
Consultation with donation services regarding donation feasibility and medical suitability reduces the effect of clinician bias or lack of knowledge regarding particular forms of donation, which has been reported in surveys to be a leading cause of physician nonreferral.56,60,62
Family Approach and Authorization
Recommendations
Recommendation 16: The physician caring for the patient informs the family of the grave prognosis and/or declaration of death by neurological criteria (brain death), before approaching for organ donation.
Recommendation 17: Organ donation is not raised at the same time as the discussion of the patient’s prognosis, and the request is ideally “decoupled” from the news of the patient’s demise.63
Recommendation 18: Before approaching a family, the patient’s donor registry status is checked. If the patient is on the registry as an intended organ donor, the conversation informs the family of the individual’s decision to donate, rather than ask permission.
Recommendation 19: Families are approached by someone with the training and skills in the donation discussion, adhering to their jurisdiction’s agreed-upon guidelines and best practices.64
Recommendation 20: Discussion with the family needs to include the options for organ and tissue donation and research to maximize the potential benefit from every donation.
Recommendation 21: Families are given sufficient information, support, and time to reach a decision.
The families play a vital role in the donation process, and where there are no absolute contraindications, best practice is that families are always approached to discuss the option of donation.65-66
Markers of Best Practices in Approaching the Family of Potential Donors
The family is always involved in the donation discussion.67-72 There are clear roles, accountability, and processes in place to ensure that families of potential donors are approached in a timely way by professionals with the necessary training and insights.73 Healthcare professionals responsible for approaching families are provided with regular training and resources (eg, video, coaching, role-plays) and best practice guidance.74
To support them in their decision, the family is given the time needed to enable them to understand that their loved one is unable to survive their injury and that any further treatment is futile.72,75-78 In very rare cases, a patient is conscious and will be able to give first-person consent for organ donation.79 The family is supported with information and assistance in making a decision, without coercion, that is aligned with their and the potential donor’s wishes and beliefs and for the benefit of potential recipients and medical research.
The donation discussion includes an exploration of all the donation options available, including organ and tissue donation. Where organs may not be suitable for transplantation, the option of donation for research purposes is offered.80-82
No compensation is offered to families to secure authorization for donation.83,84
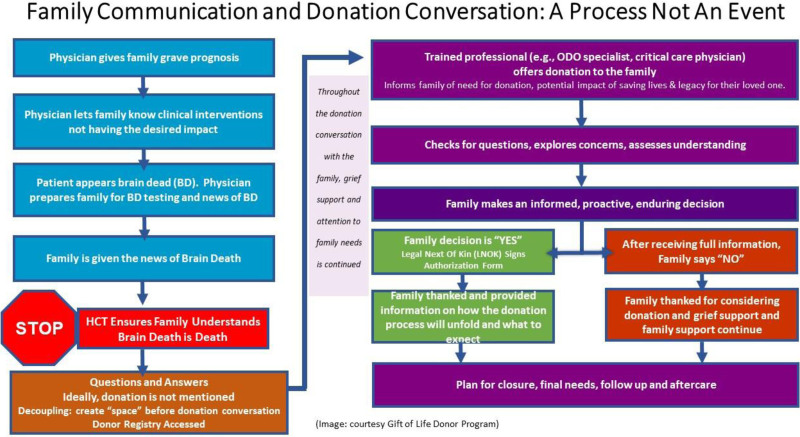
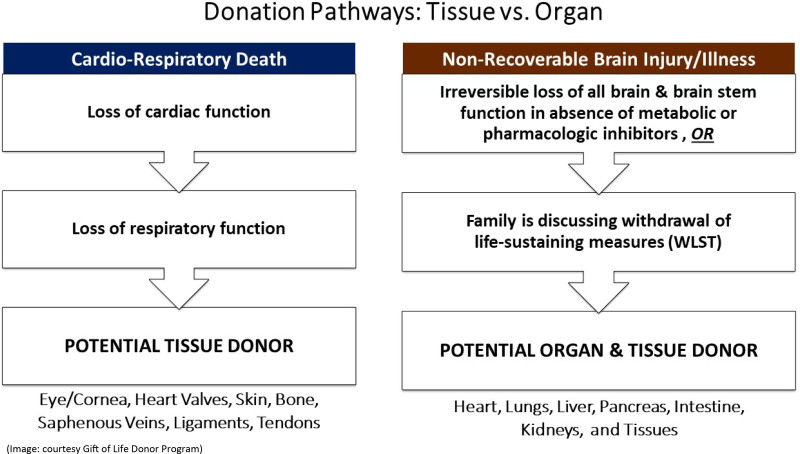
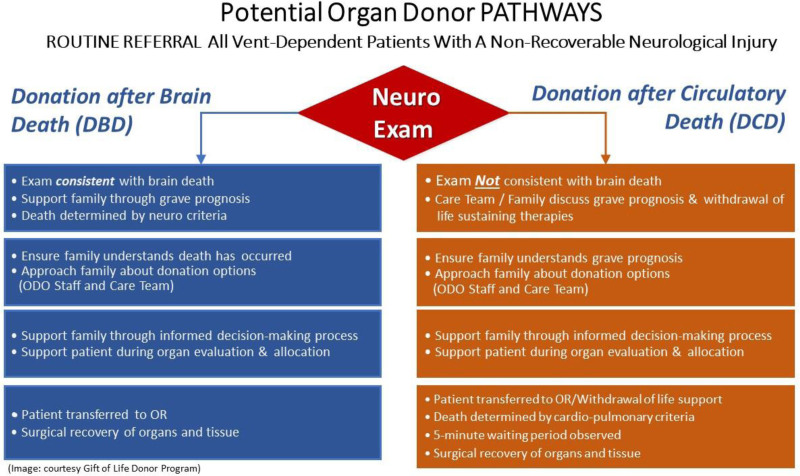
Figures 2 to 4 outline potential guides and pathway for family communication in the US context, which is included in the donation after circulatory death, neurological determination of death, and tissue donation scenarios.
FIGURE 2.
Family communication and donation conversation. ODO, organ donation organization.
FIGURE 4.
Donation pathways: tissue vs organs.
FIGURE 3.
Potential organ donor pathways. ODO, organ donation organization; OR, operating room.
Clinical Management of the Donor
Recommendations
Recommendation 22: Establish national or regional guidelines with clear algorithms to manage the potential organ donor in critical care (after death determination or decision for withdrawal of life-sustaining measures and consent for donation).
Recommendation 23: Access to guidance/advice on individual cases 24 h a day, 7 d a week, regarding donor management will support optimizing donation potential. Maximizing organ function requires specialized knowledge and resources, and the hospital and/or ODOs support this process.
Effective donor management will stabilize the potential donor and optimize the number and quality of organs for transplantation.85
Markers of Best Practice in Donor Management
Care for potential donors is provided at the same level as the care given to any other patient and rapid treatments for supporting successful donation are available as appropriate.
National clinical, legal, and ethical guidelines exist for the medical management of neurologically deceased potential donors and donation after a circulatory determination of death from the time of consent for donation to the transfer of the potential donor to the retrieval team.85 To ensure that local Donor Management guidelines are current and evidence based, there are regular reviews and benchmarking against national and international practices, guidelines, and policies.86
If the legal and medical system support organ donation after medical assistance in dying, then the organ donation system also requires guidelines to support the management of those potential donors.11,79
Matching and Allocation of Organs
Recommendations
Recommendation 24: Provide clear rules and guidance regarding the safe, fair, and equitable allocation of donated organs and tissues, including roles, responsibilities, accountabilities, and governance structures for each action.
Recommendation 25: Ensure timely access to diagnostic services to support donor assessment and inform the organ offering and allocation processes.
Recommendation 26: Establish legislation and guidance to support the secure sharing of data between organ donation, recovery, and transplant teams to support prompt decision-making.
Effective matching and allocation systems87,88 are vital for ensuring that organs are transplanted in a safe, equitable manner89 and reduce the risk of a further transplant being required in the future. Allocation balances multiple competing priorities, including medical efficacy, equity, and respect for human rights.
Placing the responsibility for the offering and allocation of organs and the development of supporting guidance with ODOs removes the risk of bias at individual transplant centers. Keeping the guidance and policies under regular review87,89 and published with open access helps to maintain public and clinical confidence in the system and helps ensure that they are in line with best clinical practice and provide fair opportunities for those on the transplant waiting list.
The increasing use of artificial intelligence will have an impact on offering and allocation processes in the future. Careful monitoring of these new systems will support identification of where improvements could be made, particularly regarding where there may be evidence of potential bias in the programming.
Markers of Best Practice in Matching and Allocation of Organs
Guidance is available to inform the clinical decisions regarding the matching and allocation of organs, taking clinical and ethical considerations, and ensure a safe, efficient, effective, and equitable process.5 This also includes clear guidelines, roles, and accountabilities in place to support the referral and assessment of patients for transplantation. Patients considered suitable for transplantation are placed on a national waiting list, which is accessed during the allocation process.
The allocation system should minimize bias and maximize equity of access and organ utility. The ODO is responsible for organ offers, and processes for allocation should be transparent, equitable, and audited for quality assurance. There are clear roles, responsibilities, and accountability defined in legislation, where appropriate, along with the matching and allocation care pathway. Regular assessments against best practices highlight any areas for improvement, with regular reports being publicly available.
Before transplantation, patients and their caregivers are provided with information to help them consider, in discussion with their clinicians, the levels of risk that they would be willing to accept regarding offered organs (eg, organs from donors at increased risk of blood-borne viruses such as HIV and hepatitis C). These decisions are captured as part of the patient record and shared securely as appropriate to inform the offering and allocation processes.90
The infrastructure and resources support the timely matching and allocation—for example, through the availability of laboratory services for tissue typing and automated algorithms. To maximize transplant opportunities from all potential consented donors, systems are needed to track organs from donors who are at increased risk for transmitting disease, and the patient is made aware of the risks and the opportunity to receiving such an organ.
The data supporting medical efficacy are constantly evolving and requires close attention by multidisciplinary teams. The role of the ODO is to ensure that these changes happen in an open and transparent manner with adequate public input and oversight.
Organ Recovery, Preservation, and Transportation
Recommendations
Recommendation 27: Establish recovery, transport, transplant teams, and hospital services that are available 24 h a day, 7 d a week to support the timely recovery and transplantation of donated organs, and that liaise closely with the donation teams to ensure that organ donation potential can be maximized and any issues/risks are identified, shared, and managed.
Recommendation 28: Machine perfusion may be used to improve organ quality and organ recipient outcomes, as well as to assess organ performance.
Organ recovery only commences after confirmation that the donor has died and that there is consent for donation in place.
Markers of Best Practices for the Organ Retrieval, Preservation, and Transportation
Close cooperation between the ODO, transplant center, retrieval teams, transport teams, and the donor hospital is established, with a central point of command (usually the ODO or donor coordinator) to ensure the timeliness and efficacy of the process.91
There are agreements between the donating hospital and retrieval teams regarding roles and responsibilities. This includes the requirement for donor hospitals to provide operating theaters with appropriate facilities and personnel whenever organ retrieval activity is required, what specialist equipment will be provided by the retrieval teams, timings for the retrieval team to arrive at the donating hospital, the method of preservation to be used, and documentation to be completed and submitted.
Fully trained teams for abdominal and cardiothoracic organ retrieval are available at all times. There is clarity regarding whether transplant teams will retrieve organs themselves or whether national or regional organ retrieval teams are in place to travel to perform the retrieval.92 Clear guidance and protocols are available regarding the clinical retrieval and organ preservation processes against which retrieval teams are trained.93
Transportation of organs is done as quickly as possible, using road, rail, or air travel, as appropriate, with protocols in place regarding packaging, responsibility for transport, and effective tracking mechanisms.94
Alternatives to Deceased Donation Transplantation Quality Assurance
Recommendations
Recommendation 29: The establishment of living donor programs will maximize the potential for transplantation. Active research programs may be developed to identify alternative approaches in the long term (eg, stem-cell therapies, regenerative medicine, xenotransplantation, etc)
Recommendation 30: Short-term and long-term recipient follow-up is essential for the health of the recipient to monitor the efficacy and outcomes of the organ transplant and identify areas for improvement.
The operations of an individual hospital-based transplant program are outside the scope of this report.
However, high functioning OTDT systems integrate living donation programs.95 Although international guidance and recommendations strongly support attempts by jurisdictions to meet their goals of self-sufficiency through deceased donation, the reality of most OTDT systems is that this need cannot be met without supplementation from living donation. Living donation is discussed in detail in a separate publication from the Forum and poses issues related to exploitation and transplant tourism not directly addressed in this report.
High-functioning OTDT systems also integrate ODOs and other agencies responsible for allocation lists, particularly in the context of paired exchanges, and carefully coordinate waiting lists and allocation algorithms to ensure a lack of errors or abuses between the 2 systems.
Alternative sources of transplantable organs are currently being developed and tested, including xenotransplantation or laboratory-grown organs.96,97 These methods will pose new challenges outside the current regulatory scope of most OTDT systems, including questions of equitable access to expensive new methods. Effective OTDT systems anticipate these changes and create regulatory frameworks before implementing these developing technologies.
As mentioned in the section on infrastructure recommendations, a comprehensive data system is a fundamental requirement of OTDT quality assurance. This system benefits from the inclusion of tracking of short-, medium-, and long-term transplant outcomes using agreed-upon metrics that are linked to the donor. Doing so will allow for improvements in posttransplant care and research into donor selection and management that could lead to improved donor management and even expanded donor pools.98
Case Follow-up and Postdonation Family Care
Recommendations
Recommendation 31: Communication of case outcomes to relevant stakeholders, including donor family and donor hospital care teams, enables ongoing improvements in care and supports public and clinical confidence in the system.
Recommendation 32: Establish ongoing support for donor families, transplant recipients, and caregivers, recognizing that caregiver support is a component of the posttransplant outcome and supports improved patient outcomes.
Recommendation 33: Establish policies for communications between donor families and organ recipients communications, including a confidentiality clause, based upon local legal requirements and cultural values.
Case follow-up is essential to ensure the efficacy and safety of the donation and transplantation service. This includes monitoring the outcomes of transplanted organs and providing care and support to the donor family.
Markers of Best Practices in Effective Service for Case Follow-up and Donation Family Care
Stewardship of the gifts of donation is a founding principle that protects and honors donors and their gifts. ODO practices, policies, and guidelines ensure consistency and safety in communication between all parties involved in donation during and after the process, establish trust, and provide a transparent base to advance donation.
ODOs have meaningful and ongoing processes to honor and respect the gifts of donors both at the time of donation and for extended time periods. Events and programs (such as donor family gatherings, forums for families to communicate and public art to honor the legacy of donors) provide ongoing support to donor family members, allow for the legacies of donation to be promoted, and build community among donor families.99
The ODOs have clearly defined processes in place to facilitate anonymous communication between donor families and transplant recipients, provided that both parties are willing. This provides meaningful connection and sense of gratitude and legacy for both recipients and donor families.100
CONCLUSION
Establishing a safe, effective organ donation and transplantation system is complex and requires close collaboration across multiple teams, with the resources in place to ensure that an individual’s wish to be a donor is honored and no opportunity for a safe transplant is missed.
Developing or improving a donation system is complex, takes time, and there are differences in approach, legislation, and requirements among and within countries. This document outlines some key elements of an effective system, any one of which will deliver improvements but when ≥2 elements are combined, the impact on donation and transplantation is strengthened.
Regional or national systems that implement these recommendations do so in a systematic, intentional, and thoughtful manner and measure and evaluate the impacts of the changes in their OTDT system. The outcomes of these evaluations and lessons learned within a region or country are shared honestly and openly with the international OTDT community so that everyone can learn and improve their systems for the benefit of staff, patients, and families. Opportunities for innovation and research, including continuous improvement of practices and processes, are essential for driving improvements in the quality of care, as well as the number and quality of organs that are available for transplant.
The global OTDT community is a small yet dedicated and resourceful group of professionals, and close collaboration is essential to share learning. In drafting this document, the authors have sought to collate expertise about developing a donation system—both for those who are seeking to build or develop a donation system and for those with an existing advanced system to explore where there may be unmet need. The authors hope that this supports further national and international collaboration, using webinars, workshops, and conference so that as many lives as possible can be saved or radically improved through the gift of donation and transplantation.
Appendix 1 (SDC, http://links.lww.com/TXD/A490) shows Committee Members and their affiliations.
Supplementary Material
Footnotes
The authors declare no financial conflicts of interest to disclose.
The Forum is supported financially by Transplant Québec through funding from the government of Québec, the Canadian Donation and Transplantation Research Program, the Legislative Evaluation: Assessment of Deceased Donation Reform program of research, and Canadian Blood Services. The Transplantation Society has provided in-kind logistic support.
C.W., L.B., A.C., S.G., D.H., R.I., G.K., H. M.N., H.O., L.P., and M.J.W. co-drafted, edited, and approved this article. M.E. edited and approved this article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
In this article, we use the term family in reference to the substitute decisionmaker, but family may include the patient themself or whoever else has the legal authority to decide on donation.
Transplant Québec is the provincial organ donation organization for the province of Québec, Canada, and is a government-funded, not-for-profit organization. The Canadian Donation and Transplant Research Program is a Canadian Institute of Health Research-funded national research network in donation and transplantation. Canadian Blood Services is a national, not-for-profit charitable organization that provides national services in the development of donation and transplantation leading practices, system performance measurement, interprovincial organ sharing registries, and public awareness and education, but is not responsible for the management or funding of any Canadian organ donation organizations or transplant programs. The LEADDR research program is a Health Canada-funded research initiative investigating the impact of legislative changes in Organ and Tissue Donation and Transplantation systems.
REFERENCES
- 1.Global Observatory on Donation and Transplantation. Data (charts and tables). GODT. Available at http://www.transplant-observatory.org/data-charts-and-tables/. Accessed April 10, 2022. [DOI] [PubMed]
- 2.Lewis A, Koukoura A, Tsianos GI, et al. Organ donation in the US and Europe: the supply vs demand imbalance. Transplant Rev Orlando Fla. 2021;35:100585. [DOI] [PubMed] [Google Scholar]
- 3.Vanholder R, Domínguez-Gil B, Busic M, et al. Organ donation and transplantation: a multi-stakeholder call to action. Nat Rev Nephrol. 2021;17:554–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller E, Dominguez-Gil B, Martin D. The Declaration of Istanbul on Organ Trafficking and Transplant Tourism (2018 Edition) introduction. Transplantation. 2019;103:217. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO guiding principles on human cell, tissue and organ transplantation. World Health Organization; 2010. Available at https://apps.who.int/iris/handle/10665/341814. Accessed January 26, 2022. [DOI] [PubMed] [Google Scholar]
- 6.The Madrid resolution on organ donation and transplantation: national responsibility in meeting the needs of patients, guided by the WHO principles. Transplantation. 2011;91:S29. [DOI] [PubMed] [Google Scholar]
- 7.Global Alliance of Eye Bank Associations (GAEBA). The Barcelona Principles: an agreement on the use of human donated tissue for ocular transplantation, research and future technologies. Available at https://www.globalalliancepr.org/barcelona-principles. Accessed April 10, 2022. [DOI] [PubMed]
- 8.Institute of Medicine. Institute of Medicine. Organ Donation: Opportunities for Action. Washington, DC: The National Academies Press; 2006. doi:10.17226/11643
- 9.European Directorate for the Quality of Medicines and HealthCare. Guide to the quality and safety of organs for transplantation (7th edition). Available at https://www.edqm.eu/en/guide-quality-and-safety-organs-transplantation. Accessed January 26, 2022.
- 10.Matesanz R, Marazuela R, Domínguez-Gil B, et al. The 40 donors per million population plan: an action plan for improvement of organ donation and transplantation in Spain. Transplant Proc. 2009;41:3453–3456. [DOI] [PubMed] [Google Scholar]
- 11.Bollen J, de Jongh W, Hagenaars J, et al. Organ donation after euthanasia: a Dutch practical manual. Am J Transplant. 2016;16:1967–1972. [DOI] [PubMed] [Google Scholar]
- 12.Ball IM, Healey A, Keenan S, et al. Organ donation after medical assistance in dying—Canada’s first cases. N Engl J Med. 2020;382:576–577. [DOI] [PubMed] [Google Scholar]
- 13.Urbanellis P, Hamar M, Kaths JM, et al. Normothermic ex vivo kidney perfusion improves early DCD graft function compared with hypothermic machine perfusion and static cold storage. Transplantation. 2020;104:947–955. [DOI] [PubMed] [Google Scholar]
- 14.Madan S, Saeed O, Forest SJ, et al. Feasibility and potential impact of heart transplantation from adult donors after circulatory death. J Am Coll Cardiol. 2022;79:148–162. [DOI] [PubMed] [Google Scholar]
- 15.Weiss MJ, Cantarovich M, Chaudhury P, et al. International Donation and Transplantation Legislative and Policy Forum: methods and purpose. Transplant Direct. 2023;9:e1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMillan SS, Kelly F, Sav A, et al. Using the nominal group technique: how to analyse across multiple groups. Health Serv Outcomes Res Methodol. 2014;14:92–108. [Google Scholar]
- 17.Alolod GP, Traino HM, Siminoff LA. Utility and usability of the Rapid Assessment of hospital Procurement barriers in Donation (RAPiD) as a tool for OPO hospital development staff. Prog Transplant. 2016;26:241–248. [DOI] [PubMed] [Google Scholar]
- 18.Council of Europe. NEWSLETTER TRANSPLANT. International figures on donation and transplantation 2019. Available at http://www.ont.es/publicaciones/Documents/NEWSLETTER%202020_baja.pdf. Accessed April 2, 2022.
- 19.Greer DM, Shemie SD, Lewis A, et al. Determination of brain death/death by neurologic criteria: the World Brain Death Project. JAMA. 2020;324:1078–1097. [DOI] [PubMed] [Google Scholar]
- 20.Gries CJ, White DB, Truog RD, et al. An official American Thoracic Society/International Society for Heart and Lung Transplantation/Society of Critical Care Medicine/Association of Organ and Procurement Organizations/United Network of Organ Sharing Statement: ethical and policy considerations in organ donation after circulatory determination of death. Am J Respir Crit Care Med. 2013;188:103–109. [DOI] [PubMed] [Google Scholar]
- 21.President’s Commission for the Study of Ethical, Behavioral Research. Defining death: a report on the medical, legal and ethical issues in the determination of death. 1981. Available at https://scholarworks.iupui.edu/bitstream/handle/1805/707/Definining%20death%20-%201981.pdf?sequence=1&isAllowed=y. Accessed April 13, 2022.
- 22.Domínguez-Gil B, Delmonico FL, Shaheen FAM, et al. The critical pathway for deceased donation: reportable uniformity in the approach to deceased donation. Transpl Int Off J Eur Soc Organ Transplant. 2011;24:373–378. [DOI] [PubMed] [Google Scholar]
- 23.Canadian Blood Services. Data, analytics and reporting systems workshop. Canadian Blood Services. 2013. Available at https://profedu.blood.ca/en/organs-and-tissues/data-reports-and-publications/archived-reports-and-publications. Accessed April 2, 2022.
- 24.Academy of Medical Royal Colleges. An ethical framework for controlled donation after circulatory death. UK Donation Ethics Committee. 2011:66. Available at https://www.aomrc.org.uk/wp-content/uploads/2016/04/Ethical_framework_donation_circulatory_death_1211-3.pdf. Accessed April 14, 2022. [Google Scholar]
- 25.British Transplantation Society, The Intensive Care Society. Organ donation after cirulatory death: report of a conensus meeting. 2010. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/1360/donation-after-circulatory-death-dcd_consensus_2010.pdf. Accessed April 14, 2022.
- 26.Silva V, Tranmer J, Schirmer J, et al. Understanding the influence of inter-professional relational networks within organ donation programs in ontario: a research protocol and preliminary results. Transplantation. 2018;102:S805. [Google Scholar]
- 27.NHS Blood and Transplant. Annual Activity Report. ODT Clinical - NHS Blood and Transplant. Available at https://www.odt.nhs.uk/statistics-and-reports/annual-activity-report. Accessed April 10, 2022.
- 28.OPTN/SRTR 2017 Annual Data Report. Introduction. Am J Transplant. 2019;19:11–18. [DOI] [PubMed] [Google Scholar]
- 29.Canadian Institute for Health Information. Pan-Canadian Organ Donation and Transplantation (ODT) Data and Performance Reporting System Project. Available at https://www.cihi.ca/en/pan-canadian-organ-donation-and-transplantation-odt-data-and-performance-reporting-system-project. Accessed April 10, 2022.
- 30.Toews M, Chandler JA, Pope T, et al. Legislation and policy recommendations on organ and tissue donation and transplantation from an international consensus forum. Transplant Direct. 2023;9:e1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva E Silva V, Schirmer J, Roza BD, et al. Defining quality criteria for success in organ donation programs: a scoping review. Can J Kidney Health Dis. 2021;8:2054358121992921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardiner D, McGee A, Simpson C, et al. Baseline ethical principles and a framework for evaluation of policies: recommendations from an international consensus forum. Transplant Direct. 2023;9:e1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Escoto M, Issa F, Cayouette F, et al. Research and innovation in organ donation: recommendations from an international consensus forum. Transplant Direct. 2023;9:e1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawshaw J, Li AH, Garg AX, et al. Identifying behaviour change techniques within randomized trials of interventions promoting deceased organ donation registration. Br J Health Psychol. 2022;27:822–843. [DOI] [PubMed] [Google Scholar]
- 35.Walton P, Pérez-Blanco A, Beed S, et al. Organ and tissue donation consent model and intent to donate registries: recommendations from an international consensus forum. Transplant Direct. 2023;9:e1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw D, Neuberger J, Murphy P. Lessons from the German Organ Donation Scandal. J Intensive Care Soc. 2013;14:200–201. [Google Scholar]
- 37.AlBugami MM, AlOtaibe FE, Alabadi AM, et al. Transplant tourism following the declaration of Istanbul: poor outcomes and nephrologist dilemma. Nephrol Carlton Vic. 2018;23:1139–1144. [DOI] [PubMed] [Google Scholar]
- 38.Morgan SE, Harrison TR, Afifi WA, et al. In their own words: the reasons why people will (not) sign an organ donor card. Health Commun. 2008;23:23–33. [DOI] [PubMed] [Google Scholar]
- 39.Miller J, Currie S, O’Carroll RE. “If I donate my organs it’s a gift, if you take them it’s theft”: a qualitative study of planned donor decisions under opt-out legislation. BMC Public Health. 2019;19:1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li AH, Lo M, Crawshaw JE, et al. Interventions for increasing solid organ donor registration. Cochrane Database Syst Rev. 2021;4:CD10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Groot J, van Hoek M, Hoedemaekers C, et al. Request for organ donation without donor registration: a qualitative study of the perspectives of bereaved relatives. BMC Med Ethics. 2016;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali A, Ahmed T, Ayub A, et al. Organ donation and transplant: the Islamic perspective. Clin Transplant. 2020;34:e13832. [DOI] [PubMed] [Google Scholar]
- 43.El-Dassouki N, Wong D, Toews DM, et al. Barriers to accessing kidney transplantation among populations marginalized by race and ethnicity in Canada: a scoping review part 2—East Asian, South Asian, and African, Caribbean, and Black Canadians. Can J Kidney Health Dis. 2021;8:2054358121996834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krmpotic K, Payne C, Isenor C, et al. Delayed referral results in missed opportunities for organ donation after circulatory death. Crit Care Med. 2017;45:989–992. [DOI] [PubMed] [Google Scholar]
- 45.Domínguez-Gil B, Coll E, Pont T, et al. Prácticas clínicas al final de la vida en pacientes con daño cerebral catastrófico en España: implicaciones para la donación de órganos. Med Intensiva. 2017;41:162–173. [DOI] [PubMed] [Google Scholar]
- 46.Ehrle R. Timely referral of potential organ donors. Crit Care Nurse. 2006;26:88–93. [PubMed] [Google Scholar]
- 47.Transplant Quebec. Identification and eligibility: identifying a potential organ donor. Available at https://www.transplantquebec.ca/en/identification-and-eligibility. Accessed April 3, 2022.
- 48.Zavalkoff S, Shemie SD, Grimshaw JM, et al. Potential organ donor identification and system accountability: expert guidance from a Canadian consensus conference. Can J Anaesth. 2019;66:432–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pereira VC, Silva SN, Carvalho VKS, et al. Strategies for the implementation of clinical practice guidelines in public health: an overview of systematic reviews. Health Res Policy Syst. 2022;20:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012:CD000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.President's Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. Defining Death: Medical, Legal and Ethical Issues in the Determination of Death. 1981. Available at https://scholarworks.iupui.edu/bitstream/handle/1805/707/Definining%20death%20-%201981.pdf?sequence=1&isAllowed=y. Accessed April 13, 2022.
- 52.Academy of Medical Royal Colleges. A code of practice for the diagnosis and confirmation of death. 2008. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/1338/aomrc-death-2008.pdf. Accessed April 14, 2022.
- 53.Royal College of Paediatrics and Child Health. The diagnosis of death by neurological criteria in infants less than two months old. 2015. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/1354/neurological-death-dnc-guide-final.pdf. Accessed April 14, 2022.
- 54.Shemie SD, Hornby L, Baker A, et al. International guideline development for the determination of death. Intensive Care Med. 2014;40:788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shemie SD, Lee D, Sharpe M, et al. Brain blood flow in the neurological determination of death: Canadian expert report. Can J Neurol Sci J Can Sci Neurol. 2008;35:140–145. [DOI] [PubMed] [Google Scholar]
- 56.Shemie SD, Baker AJ, Knoll G, et al. Donation after cardiocirculatory death in Canada. Can Med Assoc J. 2006;175:S1–S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dhanani S, Hornby L, van Beinum A, et al. Resumption of cardiac activity after withdrawal of life-sustaining measures. N Engl J Med. 2021;384:345–352. [DOI] [PubMed] [Google Scholar]
- 58.The Transplantation Society of Australia and New Zealand. Clinical guidelines for organ transplantation from deceased donors. 2021. Available at https://tsanz.com.au/storage/documents/TSANZ_Clinical_Guidelines_Version-18_Final.pdf. Accessed April 2, 2022
- 59.Edwards J, Mulvania P, Robertson V, et al. Maximizing organ donation opportunities through donation after cardiac death. Crit Care Nurse. 2006;26:101–115. [PubMed] [Google Scholar]
- 60.Weiss MJ, Hornby L, Rochwerg B, et al. Canadian guidelines for controlled pediatric donation after circulatory determination of death—summary report. Pediatr Crit Care Med. 2017;18:1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.British Transplantation Society. Transplantation from deceased donors after circulatory death. Available at https://bts.org.uk/wp-content/uploads/2016/09/15_BTS_Donors_DCD-1.pdf. Accessed April 14, 2022.
- 62.Weiss MJ, English SW, D’Aragon F, et al. Survey of Canadian intensivists on physician non-referral and family override of deceased organ donation. Can J Anaesth J Can Anesth. 2020;67:313–323. [DOI] [PubMed] [Google Scholar]
- 63.Siminoff LA, Mercer MB, Arnold R. Families’ understanding of brain death. Prog Transplant Aliso Viejo Calif. 2003;13:218–224. [DOI] [PubMed] [Google Scholar]
- 64.Siminoff LA, Gardiner HM, Alolod GP, et al. Using online communication skills training to increase organ donation authorization. Prog Transplant. 2020;30:212–219. [DOI] [PubMed] [Google Scholar]
- 65.Siminoff LA, Alolod GP, Wilson-Genderson M, et al. A comparison of request process and outcomes in donation after cardiac death and donation after brain death: results from a national study. Am J Transplant. 2017;17:1278–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Traino HM, Siminoff LA. Attitudes and acceptance of First Person Authorization: a national comparison of donor and nondonor families. J Trauma Acute Care Surg. 2013;74:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siminoff LA, Molisani AJ, Traino HM. A comparison of the request process and outcomes in adult and pediatric organ donation. Pediatrics. 2015;136:e108–e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siminoff LA, Agyemang AA, Traino HM. Consent to organ donation: a review. Prog Transplant Aliso Viejo Calif. 2013;23:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mulvania P, Mehakovic E, Wise C, et al. Successful international collaboration improves family donation conversations resulting in increased organ donation. Transplant Proc. 2014;46:2058–2065. [DOI] [PubMed] [Google Scholar]
- 70.Shafer TJ. Improving relatives’ consent to organ donation. BMJ. 2009;338:b701–b701. [DOI] [PubMed] [Google Scholar]
- 71.Siminoff LA, Alolod GP, Gardiner HM, et al. Comparison of the content and quality of organ donation discussions with African American families who authorize and refuse donation. J Racial Ethn Health Disparities. 2021;8:485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandler JA, Connors M, Holland G, et al. “Effective” requesting: a scoping review of the literature on asking families to consent to organ and tissue donation. Transplantation 2017;101:S1–S16. [DOI] [PubMed] [Google Scholar]
- 73.Noyes J, Mclaughlin L, Morgan K, et al. Process evaluation of specialist nurse implementation of a soft opt-out organ donation system in Wales. BMC Health Serv Res. 2019;19:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siminoff LA, Marshall HM, Dumenci L, et al. Communicating effectively about donation: an educational intervention to increase consent to donation. Prog Transplant Aliso Viejo Calif. 2009;19:35–43. [DOI] [PubMed] [Google Scholar]
- 75.Jacoby LH, Breitkopf CR, Pease EA. A qualitative examination of the needs of families faced with the option of organ donation. Dimens Crit Care Nurs DCCN. 2005;24:183–189. [DOI] [PubMed] [Google Scholar]
- 76.Siminoff L, Mercer MB, Graham G, et al. The reasons families donate organs for transplantation: implications for policy and practice. J Trauma. 2007;62:969–978. [DOI] [PubMed] [Google Scholar]
- 77.Rodrigue JR, Cornell DL, Howard RJ. The instability of organ donation decisions by next-of-kin and factors that predict it. Am J Transplant. 2008;8:2661–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siminoff L, Lawrence R, Zhang AD. What is it and does it really help increase consent to organ donation? Prog Transplant. 2002;12:52–60. [DOI] [PubMed] [Google Scholar]
- 79.Downar J, Shemie SD, Gillrie C, et al. Deceased organ and tissue donation after medical assistance in dying and other conscious and competent donors: guidance for policy. CMAJ. 2019;191:E604–E613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simpkin AL, Robertson LC, Barber VS, et al. Modifiable factors influencing relatives’ decision to offer organ donation: systematic review. BMJ. 2009;338:b991–b991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siminoff LA, Gordon N, Hewlett J, et al. Factors influencing families’ consent for donation of solid organs for transplantation. JAMA. 2001;286:71–77. [DOI] [PubMed] [Google Scholar]
- 82.Siminoff LA, Traino HM, Genderson MW. Communicating effectively about organ donation: a randomized trial of a behavioral communication intervention to improve discussions about donation. Transplant Direct. 2015;1:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Timar J, Bleil M, Daly T, et al. Successful strategies to increase organ donation: the Gift of Life Donor Program Philadelphia model. Indian J Thorac Cardiovasc Surg 2021;37:380–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Delmonico FL, Arnold R, Scheper-Hughes N, et al. Ethical incentives—not payment—for organ donation. N Engl J Med. 2002;346:2002–2005. [DOI] [PubMed] [Google Scholar]
- 85.Ball IM, Hornby L, Rochwerg B, et al. Management of the neurologically deceased organ donor: a Canadian clinical practice guideline. CMAJ Can Med Assoc J J Assoc Medicale Can. 2020;192:E361–E369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. [DOI] [PubMed] [Google Scholar]
- 87.Johnson RJ, Bradbury LL, Martin K, et al. Organ donation and transplantation in the UK—the last decade: a report from the UK National Transplant Registry. Transplantation. 2014;97:S1–S27. [DOI] [PubMed] [Google Scholar]
- 88.Braun F, Rahmel A. Amendments to the transplantation act and impact on the donor situation in Germany. Chir Z Alle Geb Oper Medizen 2020;91:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doby BL, Hanner K, Johnson S, et al. Results of a data-driven performance improvement initiative in organ donation. Am J Transplant. 2021;21:2555–2562. [DOI] [PubMed] [Google Scholar]
- 90.Group TC increased risk donor working. Guidance on the use of increased infectious risk donors for organ transplantation. Transplantation. 2014;98:365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Logemann F. Organ donation—management from donor identification to organ procurement. AINS. 2020;55:467–484. [DOI] [PubMed] [Google Scholar]
- 92.The American College of Surgeons. Donor organ recovery at standalone facility increases suitable organs for transplant. American College of Surgeons. Available at http://www.facs.org/media/press-releases/jacs/donor0316. Accessed April 10, 2022.
- 93.NHS Blood and Transplant. National standards for organ retrieval from deceased donors. 2021. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/23234/mpd1043.pdf. Accessed April 14, 2022.
- 94.Beaulieu L, Beaupre G, Lavoie A, et al. Organizational framework for organ donation and tissue donation services. 2015. Available at https://www.transplantquebec.ca/sites/default/files/quebec_organizational_framework_for_organ_donation_and_tissue.pdf. Accessed April 2, 2022
- 95.Tong A, Chapman JR, Wong G, et al. Public awareness and attitudes to living organ donation: systematic review and integrative synthesis. Transplantation. 2013;96:429–437. [DOI] [PubMed] [Google Scholar]
- 96.Shah AM, Han JJ. First successful porcine to human heart transplantation performed in the United States. Artif Organs. 2022;46:543–545. [DOI] [PubMed] [Google Scholar]
- 97.Pig-to-human transplants take a leap toward reality. Nat Med. 2022;28:423–423. [DOI] [PubMed] [Google Scholar]
- 98.NHS Blood and Transplant. Standard operating procedure for transplanting centre following a cumulative sum (CUSUM) signal. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/22871/sop5963.pdf. Accessed April 14, 2022.
- 99.Wind T, Jansen N, Flodén A, et al. An inventory of deceased donor family care and contact between donor families and recipients in 15 European Countries. Transpl Int Off J Eur Soc Organ Transplant. 2021;35:10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dicks SG, Northam H, van Haren FM, et al. An exploration of the relationship between families of deceased organ donors and transplant recipients: a systematic review and qualitative synthesis. Health Psychol Open. 2018;5:2055102918782172. [DOI] [PMC free article] [PubMed] [Google Scholar]