PURPOSE
Although level 1 evidence supports 45-Gy twice-daily radiotherapy as standard for limited-stage small-cell lung cancer, most patients receive higher-dose once-daily regimens in clinical practice. Whether increasing radiotherapy dose improves outcomes remains to be prospectively demonstrated.
METHODS
This phase III trial, CALGB 30610/RTOG 0538 (ClinicalTrials.gov identifier: NCT00632853), was conducted in two stages. In the first stage, patients with limited-stage disease were randomly assigned to receive 45-Gy twice-daily, 70-Gy once-daily, or 61.2-Gy concomitant-boost radiotherapy, starting with either the first or second (of four total) chemotherapy cycles. In the second stage, allocation to the 61.2-Gy arm was discontinued following planned interim toxicity analysis, and the study continued with two remaining arms. The primary end point was overall survival (OS) in the intention-to-treat population.
RESULTS
Trial accrual opened on March 15, 2008, and closed on December 1, 2019. All patients randomly assigned to 45-Gy twice-daily (n = 313) or 70-Gy once-daily radiotherapy (n = 325) are included in this analysis. After a median follow-up of 4.7 years, OS was not improved on the once-daily arm (hazard ratio for death, 0.94; 95% CI, 0.76 to 1.17; P = .594). Median survival is 28.5 months for twice-daily treatment, and 30.1 months for once-daily treatment, with 5-year OS of 29% and 32%, respectively. Treatment was tolerable, and the frequency of severe adverse events, including esophageal and pulmonary toxicity, was similar on both arms.
CONCLUSION
Although 45-Gy twice-daily radiotherapy remains the standard of care, this study provides the most robust information available to help guide the choice of thoracic radiotherapy regimen for patients with limited-stage small-cell lung cancer.
INTRODUCTION
Small-cell lung cancer (SCLC) represents 13% of all lung cancer, and one third of patients present with limited-stage disease.1 Treatment involves cisplatin-based chemotherapy combined with thoracic radiotherapy followed by prophylactic cranial irradiation in patients with responsive disease.2 The standard radiotherapy regimen, 1.5 Gy twice-daily to 45 Gy over 3 weeks, was defined by Intergroup 0096 (INT-0096), a phase III study completed in 1992. Accelerating radiotherapy from 45 Gy once daily in 5 weeks to 45 Gy twice daily in 3 weeks resulted in improved 5-year overall survival (OS) from 16% to 26%.3 Despite this result, the adoption of the twice-daily regimen has been limited, and most patients receive once-daily treatment.4 Factors affecting the implementation of twice-daily radiotherapy include logistical challenges of treating patients twice daily as well as tolerability concerns, as severe acute esophageal toxicity was twice as likely on the twice-daily radiotherapy arm of Intergroup 0096.
CONTEXT
Key Objective
Does administering higher doses of once-daily radiotherapy improve overall survival, compared with standard 45-Gy twice-daily radiotherapy, for patients treated with concurrent chemotherapy and radiotherapy for limited-stage small-cell lung cancer?
Knowledge Generated
Six hundred thirty-eight patients were randomly assigned between 45-Gy twice-daily radiotherapy and 70-Gy once-daily radiotherapy. Although there was no difference in overall survival between the arms, more patients initiating twice-daily treatment completed planned radiotherapy. Most adverse events were similar between arms, although there was a trend toward increased hematologic toxicity and a greater number of grade 5 events in the 70-Gy arm.
Relevance (B.G. Haffty)
Although the study does not definitively define the most ideal dose fractionation schedule in this setting, survival rates were similar in the once-daily and twice-daily regimen. These data aid in the decision-making process regarding radiation scheduling for limited-stage small-cell lung cancer.*
*Relevance section written by JCO Deputy Editor Bruce G. Haffty, MD.
An overarching concern regarding Intergroup 0096 was the relatively low biologically effective radiotherapy dose used in the once-daily cohort. Higher-dose thoracic radiotherapy regimens, including 70-Gy once-daily and 61.2-Gy concomitant-boost radiotherapy, were studied in cooperative group trials with promising outcomes.5,6 The resultant phase III CALGB 30610/RTOG 0538 trial evaluated the efficacy of high-dose radiotherapy in combination with chemotherapy for limited-stage SCLC. We report the final analysis of the trial's primary end point, OS, for patients randomly assigned to 70-Gy once-daily radiotherapy or standard 45-Gy twice-daily radiotherapy.
METHODS
Trial Oversight
The trial was conducted through the National Cancer Institute National Clinical Trials Network and led by the Cancer and Leukemia Group B, now part of the Alliance for Clinical Trials in Oncology. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center and by the study chairperson (J.B.) following Alliance policies. The authors were responsible for design of the trial and the collection, analysis, and interpretation of the data. Each participant signed an institutional review board–approved, Protocol (online only)-specific informed consent document in accordance with federal and institutional guidelines. Alliance Data and Safety Monitoring Board reviewed safety data semiannually.
Patients
Patients age at least 18 years with limited-stage SCLC, Eastern Cooperative Oncology Group performance status 0-2, and measurable disease by RECIST 1.1 were eligible. Regional lymph node involvement, excluding contralateral hilar and supraclavicular nodes, was required. Adequate baseline initial laboratory values were required. Required imaging before enrollment included spiral computed tomography (CT) of the chest and abdomen, fluorodeoxyglucose-positron emission tomography or bone scan, and magnetic resonance imaging or CT of the brain.
Trial Design
In the first stage of the study, enrolled patients were randomly assigned 1:1:1 to receive four cycles of cisplatin (80 mg/m2 intravenous on day 1, once every 3 weeks) and etoposide (100 mg/m2 intravenous on days 1, 2, and 3, once every 3 weeks) with one of three radiotherapy regimens, 45 Gy in 1.5-Gy twice-daily fractions over 3 weeks, 70 Gy in 35-Gy once-daily fractions over 7 weeks, or 61.2-Gy concomitant-boost over 5 weeks. In the second stage, one experimental arm was dropped, and the study continued with two remaining arms with 1:1 allocation. Radiotherapy could start with either the first or second chemotherapy cycle. Prophylactic cranial radiation, 25 Gy in 10 fractions, was recommended for patients with responsive disease. The trial was amended in July 2015 to permit carboplatin (area under the curve of 5 mg per milliliter per minute, day 1 of each cycle) instead of cisplatin at the discretion of the treating physician. Stratification factors for random assignment included sex, radiotherapy start time (with chemotherapy cycle 1 or cycle 2), performance status (0 v 1 v 2), weight loss before study entry (≤ 5% of body weight v > 5%), radiotherapy planning technique (3D-conformal v intensity-modulated), and chemotherapy choice.
End Points and Assessments
The primary end point was OS (time from random assignment to death from any cause). Key secondary end points included investigator-assessed objective response rates (according to RECIST 1.1), progression-free survival (PFS, time from random assignment to disease progression or death from any cause), and treatment-related toxicity in the intention-to-treat population. Adverse events (AEs) were assessed according to National Cancer Institute Common Terminology Criteria for AEs version 4.0. The investigators determined attribution of AEs.
Tumor assessments were conducted at screening, every two cycles of chemotherapy and at least every 3 months for 2 years, then every 6 months for 3 years, yearly for an additional 5 years until disease progression or death, and after progression, every 6 months for survival.
Thoracic Radiotherapy Planning and Quality Assurance
Radiotherapy was directed to the areas of disease involvement on CT and/or fluorodeoxyglucose-positron emission tomography imaging, and the ipsilateral hilum was included in the target volume regardless of clinical involvement. Repeat simulation and adaptation of the radiotherapy plan was allowed for the final 26 Gy in the 70-Gy once-daily cohort. Three-dimensional planning was required and intensity-modulated radiation permitted with appropriate credentialing. Four-dimensional CT simulation was encouraged and a management plan for tumor motion required. Treatment plans were centrally reviewed for adherence to protocol guidelines at the Imaging and Radiation Oncology Core (Lincoln, RI).
Statistical Analysis
The primary objective of the trial is to determine whether the remaining experimental treatment is superior to the control treatment. For sample size calculation, we assumed the median survival for the remaining experimental arm was 29.9 months and for the control arm was 23 months, or a corresponding hazard ratio (HR) of 0.77 in favor of the remaining experimental arm over the control arm under exponential hazards. With at least 483 deaths observed at the final analysis, we had approximately 82% power to test the null hypothesis H0: log(λ1/λ2) = 0 versus the alternative hypothesis HA: log(λ1/λ2) ≤ 0.77 for the comparison of OS between the two arms at a two-sided significance level of .05, where λ1 is the hazard rate of the experimental arm and λ2 is the hazard rate of the control arm. Interim analyses were conducted annually for possibly stopping the trial early for either superiority or inferiority of the remaining experimental arm relative to the control arm. O'Brien-Fleming–like boundaries7 were constructed using Lan-DeMets error spending function.8 Conditional power was used to evaluate the probability of rejecting the null hypothesis at the final analysis, given the observed test statistic at kth interim analysis at HR 1.3 or futility assessments.9 Additional details of the statistical design can be found in the study protocol.
Patients were randomly assigned with equal allocation using stratified permuted block algorithm with stratification factors. Block size of six was used in both stages of the trial.
A total of 731 patients accrued between March 15, 2008, and December 1, 2019. Of them, 638 patients on the remaining experimental arm and the control arm were included in data analysis. On the basis of the interim analysis conducted in October 2020 with 376 events (about 78% information), the early stopping boundaries were not crossed with the critical Z-value of 2.807 for early superiority stopping and an observed Z statistic of 0.3328. As there was very low probability that the planned final analysis would reject the null hypothesis and conclude that the remaining experimental arm is better than the control, Alliance data safety monitoring board recommended a public release of the data. The results reported here were based on the data locked on March 2, 2022. Kruskal-Wallis test was used to check the balance between arms of the continuous variables of baseline patient characteristics and chi-square test for the categorical variables. The primary end point of OS included all 638 randomly assigned patients in the intent-to-treat analysis. OS and PFS were estimated with the Kaplan-Meier method, and between-group comparisons were evaluated by the stratified log-rank test. Cox proportional hazards model was fitted to estimate the HR of treatment effect and its 95% CI with respect to stratification factors. A total of 144 stratums were defined by randomization stratification factors. For the primary analysis, the stratified log-rank test and the stratified Cox PH analysis will only stratify on subgroups defined by performance status (0, 1/2) and chemotherapy choice (entry before the option to use carboplatin, choice of carboplatin among those patients for which this is an option, and choice of cisplatin among those patients for which this is an option). The heterogeneity of treatment effects for OS and PFS across patient subgroups, for example, sex, chemotherapy backbone, weight loss, performance status, and radiotherapy technique, and the HRs and CIs therein were estimated from unstratified Cox PH models fitted to the specific subgroups. The Kruskal-Wallis test was used to compare the continuous variables of patient characteristics and the chi-square test to compare the categorical variables between arms. Miettinen-Nurminen method was used to compare the specific event proportions between both arms. Reported P values were not adjusted for multiple comparisons for other end points and for the group sequential design for the primary end point. A two-sided significance level of 5% was used in all tests for declaring statistical significance. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Management Center. All analyses were conducted using SAS 9.4 software (Cary, NC) or R version 4.0.5 (Vienna, Austria).
RESULTS
Patients
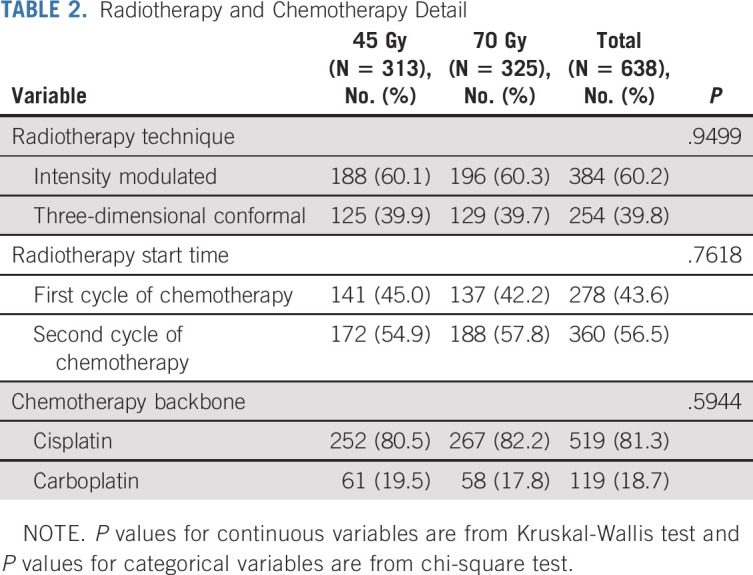
Between March 15, 2008, and December 1, 2019, 731 patients were randomly assigned (Fig 1). A planned interim toxicity analysis resulted in discontinuation of the 61.2-Gy concomitant-boost arm in March 2013,10 leaving 638 patients randomly assigned to 45-Gy twice-daily radiotherapy (n = 313) or 70-Gy once-daily radiotherapy (n = 325) for primary analysis. The distributions of the baseline covariates were comparable between study (Table 1), and treatment details were similar in both cohorts (Table 2).
FIG 1.
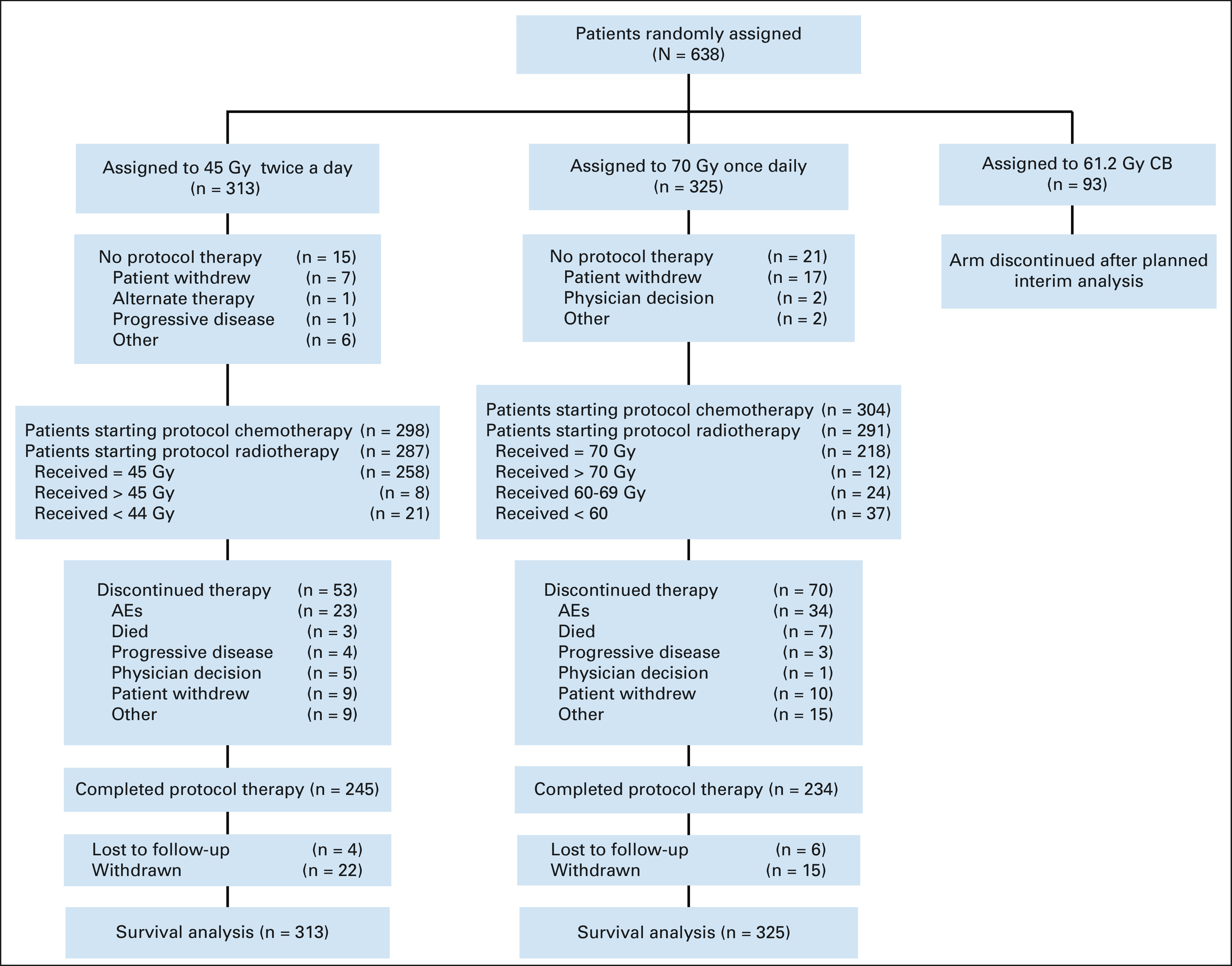
CONSORT diagram. AE, adverse event; CB, concomitant-boost.
TABLE 1.
Baseline Patient Characteristics
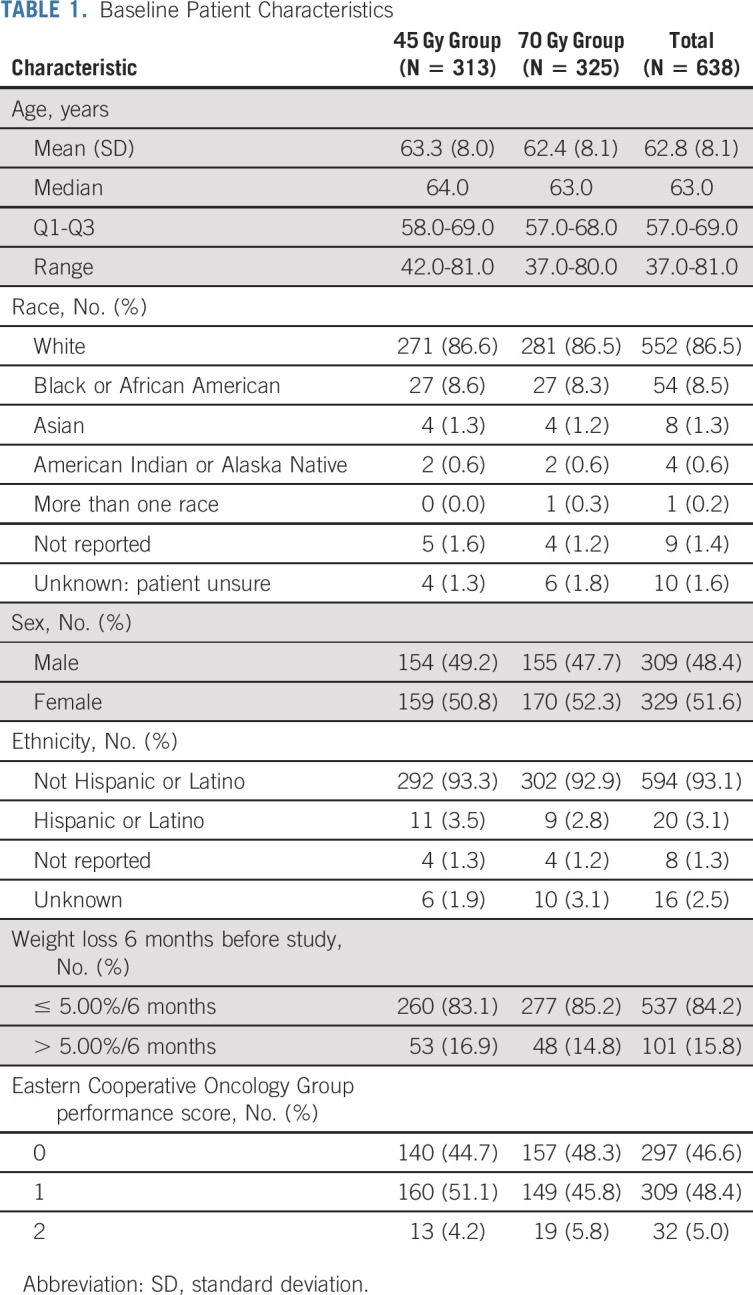
TABLE 2.
Radiotherapy and Chemotherapy Detail
Outcomes
Overall survival.
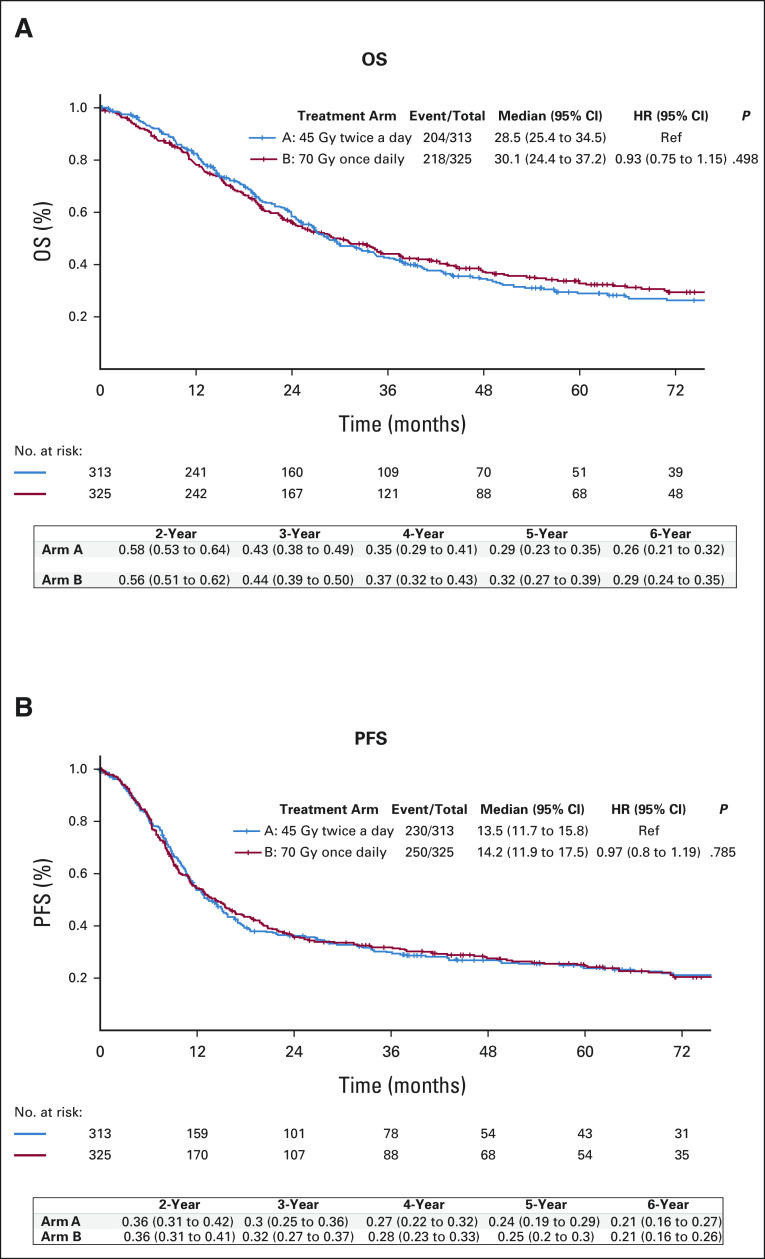
After a median follow-up of 4.7 years (interquartile range, 3.1-7.1 years), 204 patients (65%) in the 45-Gy twice-daily group and 218 patients (67%) in the 70-Gy once-daily group had died. OS did not significantly differ between treatment arms (P = .594). Median OS was 28.5 months (95% CI, 25.4 to 34.5) for 45-Gy twice-daily compared with 30.1 months (95% CI, 24.4 to 37.2) for 70-Gy once-daily radiotherapy. Two-year and 5-year OS were, respectively, 58% (95% CI, 0.53 to 0.64) and 29% (95% CI, 0.24 to 0.35) on the 45-Gy arm, compared with 57% (95% CI, 0.51 to 0.62) and 33% (95% CI, 0.28 to 0.39) on the 70-Gy arm (Fig 2A).
FIG 2.
(A) OS and (B) investigator-assessed PFS in the intention-to treat population. P values are from stratified log-rank test. HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Median OS remained similar between treatment arms in a separate analysis limited to patients who initiated radiotherapy per protocol; 29.4 months (95% CI, 26.5 to 36.8) for twice-daily versus 33.1 months (95% CI, 25.4 to 39.6) for once-daily radiotherapy. Subgroup analysis comparing OS between treatment arms did not reveal a difference among stratification factors.
Treatment response and PFS.
The overall complete and partial response rates were. Respectively, 27.8% and 55.6% on the 45-Gy twice-daily arm and 31.1% and 53.5% on the 70-Gy once-daily arm (P = .7670). Disease progression was observed in a total of 229 patients (73%) in the twice-daily group and 248 patients (76%) in the once-daily group. PFS was similar in both arms (P = .70; Fig 2B).
Safety and tolerability.
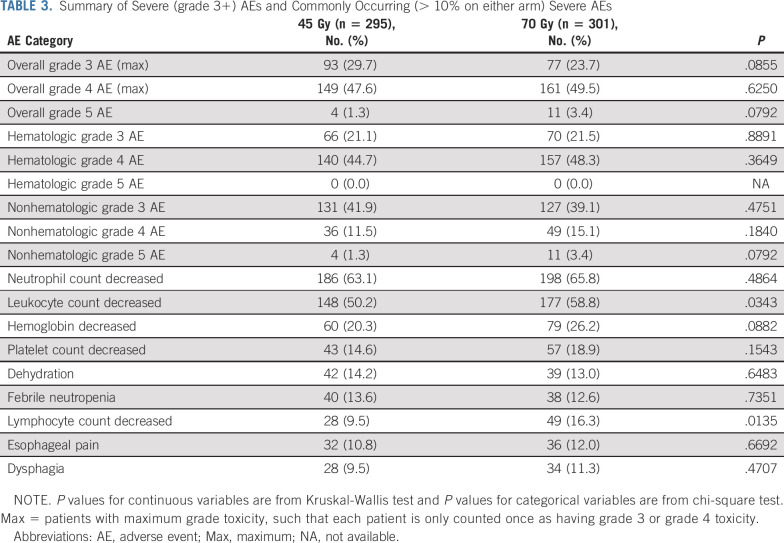
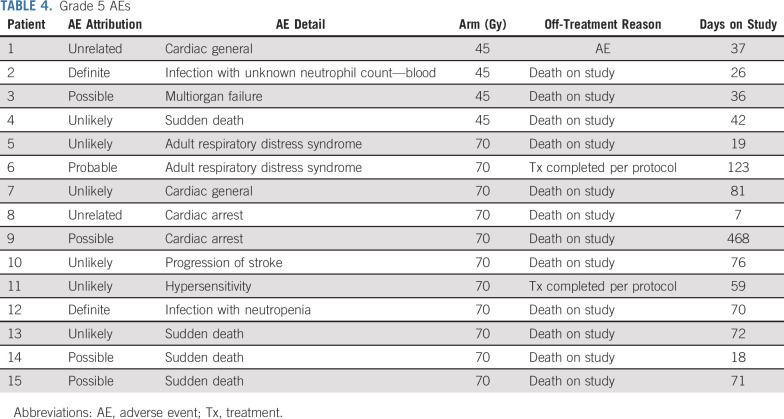
Overall, 295 patients on the 45-Gy twice-daily arm and 301 patients on the 70-Gy once-daily arm could be evaluated for safety. Table 3 details severe (grade 3+) hematologic and nonhematologic AEs as well as relative rates of commonly occurring severe AEs, defined as occurring in at least 10% of patients. Radiation-related toxicity was similar in both arms. Grade 3+ esophageal toxicity was 16% in the 45-Gy twice-daily arm and 17.5% in the 70-Gy once-daily arm. Rates of grade 3 dyspnea were low, 4% (12 patients) on the twice-daily arm and 7% (21 patients) on the once-daily arm, and the only case of grade 4 dyspnea reported was on the twice-daily arm. Patients on the 70-Gy arm had higher rates of leukopenia and lymphopenia, with a trend toward increased anemia. Details of 11 grade 5 AEs on the once-daily radiotherapy arm and four on the twice-daily arm are provided in Table 4.
TABLE 3.
Summary of Severe (grade 3+) AEs and Commonly Occurring (> 10% on either arm) Severe AEs
TABLE 4.
Grade 5 AEs
Of patients starting twice-daily radiotherapy, 92% received at least 45-Gy and 80% completed all four chemotherapy cycles, while 79% of patients starting once-daily radiotherapy received at least 70-Gy (87% received > 60 Gy), and 72% completed four chemotherapy cycles.
DISCUSSION
To our knowledge, CALGB 30610 is the largest study conducted to date for limited-stage SCLC. We hypothesized that raising the nominal radiotherapy dose by more than 50%, from 45-Gy twice-daily to 70-Gy once-daily, would improve tumor control and OS for patients treated with concurrent chemoradiotherapy. Although OS was not better with high-dose radiotherapy, outcomes on both arms of the current trial are reasonably favorable, with median survival in the range of 28-30 months and approximately 30% of patients surviving at least 5 years.11 Both regimens also appeared tolerable, likely due in part to modern radiotherapy planning and integrated quality assurance, with similar overall rates of severe AEs.
This trial was designed at a time when there was substantial enthusiasm for studying high-dose fractionated radiotherapy in the combined-modality treatment of lung cancer. Advances in technology, particularly three-dimensional conformal radiotherapy, led to prospective pilot and phase II trials suggesting that higher radiation doses in the setting of concurrent chemotherapy were safe and effective.5,12-14 Two additional large multi-institutional phase III trials testing radiation dose escalation in lung cancer were initiated at roughly the same time as CALGB 30610. The CONVERT trial compared 66-Gy once-daily with 45-Gy twice-daily radiotherapy in limited-stage SCLC.15 As in our trial, high-dose once-daily radiotherapy did not improve outcomes. Median survival was 25 months with daily radiotherapy and 30 months with twice-daily radiotherapy (HR, 1.18; P = .14). High-dose radiotherapy was also tested in locally advanced non-SCLC. The RTOG-0617 trial showed that increasing once-daily radiotherapy dose with concurrent chemotherapy was detrimental, with a median survival of 20.3 months on the 74-Gy arm and 28.7 months on the standard 60-Gy arm (P = .004).16
The results of the CONVERT and RTOG-0617 trials raised questions about the strategy of using high-dose once-daily radiotherapy with chemotherapy for locally advanced lung cancer. In contrast to those studies, outcomes on the high-dose arm of CALGB-30610 appear similar to standard therapy (HR, 0.94), but CALGB-30610 was not designed as a noninferiority trial and 45-Gy twice-daily radiotherapy remains the standard of care. Regardless, and despite the lack of supporting randomized data, most patients with limited-stage SCLC are treated with once-daily radiotherapy to doses of 60 Gy or higher in clinical practice.4
Although the results on the 70-Gy arm may be interpreted as supporting continued use of high-dose once-daily radiotherapy, both CONVERT and our study could justify increased utilization of 45-Gy twice-daily radiotherapy. More patients on the twice-daily arms of both studies completed radiotherapy, possibly due in part to the timing of radiation-related toxicity, as patients receiving twice-daily treatment may have completed radiotherapy before the maximal onset of acute toxicities such as esophagitis. And although the toxicity profile of once-daily radiotherapy and twice-daily radiotherapy were similar in general, there was an increase in certain hematologic toxicities and a higher numerical rate of grade 5 toxicity on the once-daily arm of both trials.
The optimal once-daily radiotherapy dose is not well defined. Neither CONVERT nor our study included a lower-dose once-daily radiotherapy arm, and there is a lack of randomized data addressing whether increasing the dose of once-daily radiotherapy improves outcomes compared with modest doses. Published guidelines suggest using doses between 60 and 70 Gy, although the low level of evidence for this recommendation is acknowledged.17 Outcomes on the CALGB 30610 70-Gy arm appear more favorable than those on the 66-Gy arm of CONVERT, although it is not valid to compare the results between trials, particularly given the differences in patient selection and treatment detail. Future analysis will assess the relationship between radiotherapy dose and outcomes as > 20% of patients did not complete therapy to 70 Gy.
The failure of high-dose radiotherapy challenges accepted models predicting the biologic efficacy of radiotherapy and demonstrates the need for well-conducted phase III studies.18,19 It also strikes at the heart of long-held beliefs in the radiation oncology community that more treatment equals better outcomes. It may simply be that protracting the treatment course is not effective in the context of chemoradiotherapy for lung cancer, and that incorporating an element of accelerated therapy is critical, particularly for SCLC. Although utilization of modern radiotherapy techniques was expected to permit safe dose escalation, raising the nominal radiotherapy dose beyond a certain level may increase treatment-related toxicity, particularly subacute cardiac effects, as was suggested in the dosimetry analysis from RTOG-0617.20 Nevertheless, a deep belief in dose escalation continues in the radiation oncology community, and national guidelines still include using once-daily doses up to 70 Gy, even with mature outcomes of RTOG 0617 demonstrating worse survival with dose escalation in non-SCLC.21
INT-0096 was one of the few trials, in any disease site, where altering the radiotherapy regimen improved overall patient survival.3 Whether further enhancements in radiotherapy regimens will translate into improvements in survival remains to be seen. An alternative strategy, increasing the dose of twice-daily radiotherapy to 60 Gy, resulted in promising survival in a phase II Scandinavian trial.22 This regimen maintains an element of treatment acceleration, and additional studies are currently being planned.
Although CALGB 30610 was a well-conducted phase III trial, there are several limitations. Slow initial accrual resulted in major amendments including allowing radiotherapy to start with either the first or second cycle of chemotherapy and permitting carboplatin to be used in place of cisplatin. The trial stratified for several potential prognostic factors, but did not control for additional prognostic variables that might affect outcomes such as primary tumor and nodal stage, overall tumor volume, and radiotherapy volumes. Detailed dosimetry analysis and evaluation of patient subsets are pending and may be helpful in hypothesis-generation for future studies.
In conclusion, data from the current study substantially add to, but do not resolve, the debate concerning an optimal radiotherapy regimen for limited-stage SCLC. Although the standard of care remains unchanged, the outcomes from CALGB 30610 provide the most robust information available to help guide the choice of thoracic radiotherapy regimen for patients with limited-stage SCLC.
Jeffrey Bogart
Stock and Other Ownership Interests: Cardan Robotics, Verve Medical
Laurie E. Gaspar
Employment: Banner MD Anderson Colorado
Speakers' Bureau: Cancer Experts Now
Other Relationship: NCI
Open Payments Link: https://openpaymentsdata.cms.gov/physician/275039
John Heymach
Stock and Other Ownership Interests: Cardinal Spine, Bio-Tree
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Spectrum Pharmaceuticals, Guardant Health, Hengrui Pharmaceutical, GlaxoSmithKline, EMD Serono, Takeda, Sanofi/Aventis, Genentech/Roche, Boehringer Ingelheim, Mirati Therapeutics, Janssen, Nexus Health Systems, Pneuma Respiratory, Lilly (Inst)
Speakers' Bureau: IDEOlogy Health, MJH Life Sciences
Research Funding: AstraZeneca (Inst), Spectrum Pharmaceuticals, GlaxoSmithKline
Patents, Royalties, Other Intellectual Property: Licensing agreement between Spectrum and MD Anderson (including myself) regarding intellectual property for treatment of EGFR and HER2 exon 20 mutations
James Bonner
Consulting or Advisory Role: ICON Clinical Research
Saiama Waqar
Research Funding: Pfizer (Inst), Daiichi Sankyo (Inst), Newlink Genetics (Inst), Xcovery (Inst), Vertex (Inst), Bristol Myers Squibb (Inst), Hengrui Therapeutics (Inst), Ignyta (Inst), AstraZeneca (Inst), Verastem (Inst), AbbVie (Inst), Roche (Inst), Lilly (Inst), Genentech (Inst), ARIAD (Inst), Loxo (Inst), Millennium (Inst), Elevation Oncology (Inst), Janssen (Inst), Cullinan Pearl (Inst), Ribon Therapeutics (Inst), Astellas Pharma (Inst)
William Petty
Consulting or Advisory Role: Jazz Pharmaceuticals, Mirati Therapeutics
Research Funding: Merck Sharp & Dohme (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Dava Oncology
Thomas E. Stinchcombe
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: EMD Serono, Janssen Oncology, Turning Point Therapeutics, Sanofi/Aventis, GlaxoSmithKline, Genentech/Roche, Daiichi Sankyo/Astra Zeneca, Takeda, Eisai/H3 Biomedicine, G1 Therapeutics, Spectrum Pharmaceuticals
Research Funding: AstraZeneca (Inst), Takeda (Inst), Regeneron (Inst), Seattle Genetics (Inst), Mirati Therapeutics (Inst), Genentech/Roche (Inst)
Jeffrey D. Bradley
Honoraria: AstraZeneca/MedImmune, Mevion Medical Systems
Consulting or Advisory Role: Varian Medical Systems, Genentech
Research Funding: Varian Medical Systems (Inst)
Everett Vokes
Stock and Other Ownership Interests: Coordination Pharmaceuticals, McKesson
Honoraria: Takeda, Ascendis Pharma
Consulting or Advisory Role: Takeda, Ascendis Pharma, Bristol Myers Squibb/Sanofi, EMD Serono
Research Funding: AbbVie, Bristol Myers Squibb, Celgene, Novartis, Lilly (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/930740
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds on page 2326
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (https://acknowledgments.alliancefound.org).
PRIOR PRESENTATION
Presented in part at the Oral Lung Session, Chicago, IL, June 6, 2021.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under Award Nos. U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA189824, UG1CA189819, UG1CA189858, UG1CA233253, UG1CA233324, UG1CA233327, UG1CA233329, UG1CA233339, and U10CA180868 (NRG Oncology).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Jeffrey Bogart, Xiaofei Wang, Gregory Masters, Ritsuko Komaki, Laurie E. Gaspar, James Bonner, Everett Vokes
Administrative support: Thomas E. Stinchcombe
Provision of study materials or patients: Gregory Masters, Ritsuko Komaki, John Heymach, Saiama Waqar, William Petty, Thomas E. Stinchcombe
Collection and assembly of data: Jeffrey Bogart, Xiaofei Wang, Gregory Masters, Junheng Gao, Charles Kuzma, William Petty, Thomas E. Stinchcombe, Jeffrey D. Bradley, Everett Vokes
Data analysis and interpretation: Jeffrey Bogart, Xiaofei Wang, Gregory Masters, Junheng Gao, Laurie E. Gaspar, John Heymach, Saiama Waqar, William Petty, Thomas E. Stinchcombe, Jeffrey D. Bradley, Everett Vokes
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
High-Dose Once-Daily Thoracic Radiotherapy in Limited-Stage Small-Cell Lung Cancer: CALGB 30610 (Alliance)/RTOG 0538
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jeffrey Bogart
Stock and Other Ownership Interests: Cardan Robotics, Verve Medical
Laurie E. Gaspar
Employment: Banner MD Anderson Colorado
Speakers' Bureau: Cancer Experts Now
Other Relationship: NCI
Open Payments Link: https://openpaymentsdata.cms.gov/physician/275039
John Heymach
Stock and Other Ownership Interests: Cardinal Spine, Bio-Tree
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Spectrum Pharmaceuticals, Guardant Health, Hengrui Pharmaceutical, GlaxoSmithKline, EMD Serono, Takeda, Sanofi/Aventis, Genentech/Roche, Boehringer Ingelheim, Mirati Therapeutics, Janssen, Nexus Health Systems, Pneuma Respiratory, Lilly (Inst)
Speakers' Bureau: IDEOlogy Health, MJH Life Sciences
Research Funding: AstraZeneca (Inst), Spectrum Pharmaceuticals, GlaxoSmithKline
Patents, Royalties, Other Intellectual Property: Licensing agreement between Spectrum and MD Anderson (including myself) regarding intellectual property for treatment of EGFR and HER2 exon 20 mutations
James Bonner
Consulting or Advisory Role: ICON Clinical Research
Saiama Waqar
Research Funding: Pfizer (Inst), Daiichi Sankyo (Inst), Newlink Genetics (Inst), Xcovery (Inst), Vertex (Inst), Bristol Myers Squibb (Inst), Hengrui Therapeutics (Inst), Ignyta (Inst), AstraZeneca (Inst), Verastem (Inst), AbbVie (Inst), Roche (Inst), Lilly (Inst), Genentech (Inst), ARIAD (Inst), Loxo (Inst), Millennium (Inst), Elevation Oncology (Inst), Janssen (Inst), Cullinan Pearl (Inst), Ribon Therapeutics (Inst), Astellas Pharma (Inst)
William Petty
Consulting or Advisory Role: Jazz Pharmaceuticals, Mirati Therapeutics
Research Funding: Merck Sharp & Dohme (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Dava Oncology
Thomas E. Stinchcombe
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: EMD Serono, Janssen Oncology, Turning Point Therapeutics, Sanofi/Aventis, GlaxoSmithKline, Genentech/Roche, Daiichi Sankyo/Astra Zeneca, Takeda, Eisai/H3 Biomedicine, G1 Therapeutics, Spectrum Pharmaceuticals
Research Funding: AstraZeneca (Inst), Takeda (Inst), Regeneron (Inst), Seattle Genetics (Inst), Mirati Therapeutics (Inst), Genentech/Roche (Inst)
Jeffrey D. Bradley
Honoraria: AstraZeneca/MedImmune, Mevion Medical Systems
Consulting or Advisory Role: Varian Medical Systems, Genentech
Research Funding: Varian Medical Systems (Inst)
Everett Vokes
Stock and Other Ownership Interests: Coordination Pharmaceuticals, McKesson
Honoraria: Takeda, Ascendis Pharma
Consulting or Advisory Role: Takeda, Ascendis Pharma, Bristol Myers Squibb/Sanofi, EMD Serono
Research Funding: AbbVie, Bristol Myers Squibb, Celgene, Novartis, Lilly (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/930740
No other potential conflicts of interest were reported.
REFERENCES
- 1.Govindan R, Page N, Morgensztern D, et al. : Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24:4539-4544, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Zelen M: Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3 4:31-42, 1973 [PubMed] [Google Scholar]
- 3.Turrisi AT III, Kim K, Blum R, et al. : Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 340:265-271, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Farrell MJ, Yahya JB, Degnin C, et al. : Radiation dose and fractionation for limited-stage small-cell lung cancer: Survey of US radiation oncologists on practice patterns. Clin Lung Cancer 20:13-19, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Bogart JA, Herndon JE II, Lyss AP, et al. : 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: Analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys 59:460-468, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Komaki R, Paulus R, Ettinger DS, et al. : Phase II study of accelerated high-dose radiotherapy with concurrent chemotherapy for patients with limited small-cell lung cancer: Radiation Therapy Oncology Group protocol 0239. Int J Radiat Oncol Biol Phys 83:e531-e536, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Brien PC, Fleming TR: A multiple testing procedure for clinical trials. Biometrics 35:549-556, 1979 [PubMed] [Google Scholar]
- 8.Lan KG, DeMets DL: Discrete sequential boundaries for clinical trials. Biometrika 70:659-663, 1983 [Google Scholar]
- 9.Jennison C, Turnbull BW: Group Sequential Methods With Applications to Clinical Trials. Boca Raton, FL, Chapman & Hall/CRC, 1999 [Google Scholar]
- 10.Bogart JA, Wang X, Masters GA, et al. : Short Communication: Interim toxicity analysis for patients with limited stage small cell lung cancer (LSCLC) treated on CALGB 30610 (Alliance)/RTOG 0538. Lung Cancer 156:68-71, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaspar LE, McNamara EJ, Gay EG, et al. : Small-cell lung cancer: Prognostic factors and changing treatment over 15 years. Clin Lung Cancer 13:115-122, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Bradley JD, Moughan J, Graham MV, et al. : A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: Phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys 77:367-372, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Socinski MA, Blackstock AW, Bogart JA, et al. : Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol 26:2457-2463, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Machtay M, Bae K, Movsas B, et al. : Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: An analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 82:425-434, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faivre-Finn C, Snee M, Ashcroft L, et al. : Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): An open-label, phase 3, randomised, superiority trial. Lancet Oncol 18:1116-1125, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradley JD, Paulus R, Komaki R, et al. : Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol 16:187-199, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simone CB, Bogart JA, Cabrera AR, et al. : Radiation Therapy for small cell lung cancer: An ASTRO clinical practice guideline. Pract Radiat Oncol 10:158-173, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SP, Leu MY, Smathers JB, et al. : Biologically effective dose distribution based on the linear quadratic model and its clinical relevance. Int J Radiat Oncol Biol Phys 33:375-389, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Fowler JF: 21 Years of biologically effective dose. Br J Radiol 83:554-568, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gore EM, Hu C, Bar-Ad V, et al. : Impact of incidental cardiac radiation on cardiopulmonary toxicity and survival for locally advanced non-small cell lung cancer: Reanalysis of NRG Oncology/RTOG 0617 with centrally contoured cardiac structures. Int J Radiat Oncol Biol Phys 126:S129-S130, 2016 [Google Scholar]
- 21.NCCN Clinical Practice Guidelines in Oncology. Non-small Cell Lung Cancer Version 1.2022. National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf [Google Scholar]
- 22.Gronberg BH, Killingberg KT, Flotten O, et al. : High-dose versus standard-dose twice-daily thoracic radiotherapy for patients with limited stage small-cell lung cancer: An open-label, randomised, phase 2 trial. Lancet Oncol 22:321-331, 2021 [DOI] [PubMed] [Google Scholar]