Abstract
Background
Non-pharmaceutical interventions affected the circulation of and illness due to endemic respiratory pathogens during the COVID-19 pandemic. We investigated the incidence of admissions to hospital for overall and specific pathogen-associated lower respiratory tract infection (LRTI) during the COVID-19 pandemic compared with incidence in the pre-pandemic period.
Methods
In this observational study, we analysed surveillance data for children younger than 5 years from two public hospitals in Soweto, South Africa, for all-cause LRTI, respiratory syncytial virus (RSV), influenza, human metapneumovirus, and Bordetella pertussis from Jan 1, 2015 to Dec 31, 2022. Data were obtained from an electronic database that includes information for all admissions to the general paediatric wards at the two hospitals, automatically identified by a computer program. We excluded children admitted to hospital with incidental SARS-CoV-2 infection or COVID-19 without LRTI diagnosis. Incidence during COVID-19 pandemic years (2020, 2021, and 2022) were compared with pre-pandemic rates (2015–19).
Findings
Overall, there were 42 068 all-cause hospital admissions, including 18 303 all-cause LRTI hospital admissions, from Jan 1, 2015, to Dec 31, 2022, 17 822 (42·4%) of whom were female, 23 893 (57·0%) were male, and 353 (0·8%) had missing data. All-cause LRTI incidence risk ratio (IRR) was 30% lower in 2020 (IRR 0·70, 95% CI 0·67–0·74) and 13% lower in 2021 (0·87, 0·83–0·91), but 16% higher in 2022 (1·16, 1·11–1·21) compared with the pre-pandemic period. Furthermore, compared with the pre-pandemic period, incidence of RSV-associated LRTI (0·52, 0·45–0·58), influenza-associated LRTI (0·05, 0·02–0·11), and pulmonary tuberculosis (0·52, 0·41–0·65) were lower in 2020, with similar trends observed for human-metapneumovirus-associated LRTI, pertussis, and invasive pneumococcal disease (IPD). Compared with the pre-pandemic period, by 2022, RSV-associated LRTI incidence was similar (1·04, 0·95–1·14) and influenza-associated LRTI showed a non-significant increase (1·14, 0·92–1·39), whereas incidence remained lower for tuberculosis (0·79, 0·65–0·94) and IPD (0·51, 0·24–0·99). In 2022, the incidence of COVID-19-associated LRTI hospital admission (65 per 100 000 children younger than 5 years) was lower than pre-pandemic RSV-associated LRTI (0·23, 0·19–0·27) but higher than pre-pandemic influenza-associated LRTI (1·19, 0·97–1·45), although the difference was not significant. All-cause LRTI death in 2022 (57 per 100 000 children younger than 5 years) was 28% higher than in the pre-pandemic period (1·28, 1·03–1·58).
Interpretation
The higher incidence of all-cause LRTI admissions to hospital in 2022 compared with the pre-pandemic period is partly due to ongoing COVID-19 admission to hospital, and could worsen if other endemic respiratory pathogens revert to pre-pandemic incidence. Interventions, including the introduction of vaccines for people who are pregnant that aim to prevent RSV and possibly COVID-19 in young children, are warranted.
Funding
The Bill & Melinda Gates Foundation.
Introduction
Global attempts to reduce transmission of SARS-CoV-2, the cause of the COVID-19 pandemic, included several public health measures that are collectively referred to as non-pharmaceutical interventions. These non-pharmaceutical interventions included regulations regarding restricted travel, restricted social and public engagements, school closures, physical distancing, emphasis on hand hygiene, and wearing face masks.1 The implementation of non-pharmaceutical interventions during the COVID-19 pandemic affected the transmission of other seasonal respiratory pathogens, including respiratory syncytial virus (RSV), human metapneumovirus, influenza virus, and Bordetella pertussis. 2, 3, 4, 5, 6, 7, 8, 9, 10 For some viruses, such as RSV and influenza, disruption to the historical seasonal pattern was noted in 2020 and 2021.10 Furthermore, declines in invasive bacterial disease due to organisms that colonise the upper airways, including invasive pneumococcal disease (IPD), were reported during the first year of the pandemic.11
Research in context.
Evidence before this study
Mobility restrictions and other non-pharmaceutical interventions were variably implemented globally early in the COVID-19 pandemic to attempt to mitigate the spread of SARS-CoV-2. The implementation of non-pharmaceutical interventions also resulted in disruption of the seasonality of illness and a reduced burden of illness due to other endemic respiratory pathogens. The reduction or removal of non-pharmaceutical interventions during 2021 and 2022 coincided with a resurgence of illness due to some respiratory viruses, including increased incidence of admission to hospital for lower respiratory tract infections (LRTIs) associated with respiratory syncytial virus (RSV), mainly in high-income countries.
A systematic review of PubMed from database inception to Sept 12, 2022, of children aged 18 years or younger admitted to hospital with RSV during the COVID-19 pandemic identified 18 studies from Europe (n=7), Asia (n=5), Oceania (n=4), North America (n=1), and South America (n=1) that reported changes in hospital admissions. Search terms were “respiratory syncytial viruses” AND “COVID-19” OR “SARS-CoV-2” and there were no language restrictions. We conducted a similar search of PubMed from database inception to Feb 13, 2023, which identified 56 studies from Asia (n=26), Europe (n=18), Oceania (n=5), North America (n=4), and South America (n=3). Only studies that included children younger than 5 years were included. Most of these studies reported a decline in detection rates of respiratory viruses in 2020, then an increase in detection rates of respiratory viruses in 2021 as non-pharmaceutical interventions were reduced or removed. Most of the studies did not report incidence of respiratory viral infections and none reported changes to hospital admissions for tuberculosis or pertussis infections. There was a single observational study from Africa, published in 2020, that assessed tuberculosis during the COVID-19 pandemic and a single observational study reporting data up to December, 2022, from Denmark that showed an out of season RSV epidemic.
Added value of this study
This study shows significant reductions in incidence of admission to hospital for all-cause LRTI and RSV-associated LRTI (A and B) in 2020 only, and for influenza (A and B), human metapneumovirus, invasive pneumococcal disease (IPD), and pertussis during the COVID-19 pandemic (2020–22) compared with the 5-year period (2015–19) preceding the pandemic. These reductions were followed by a 16% increase in incidence of admission to hospital for all-cause LRTI in children younger than 5 years in 2022 compared with the pre-pandemic period. By 2022, when most non-pharmaceutical interventions were reduced or removed in South Africa, seasonality and incidence of RSV-associated LRTI and influenza-associated LRTI were similar to the pre-pandemic period. By contrast, incidence of human-metapneumovirus-associated LRTI, IPD, pertussis, and tuberculosis in 2022 remained lower than the pre-pandemic period. The ongoing hospital admissions for COVID-19-associated LRTI in 2022 contributed to the higher overall burden of all-cause LRTI in 2022. Furthermore, the incidence of COVID-19-associated LRTI hospital admissions in 2022 was 0·2 times lower than the pre-pandemic incidence of RSV-associated LRTI hospital admissions, but 1·2 times higher than the pre-pandemic incidence of influenza-associated LRTI hospital admissions. The in-hospital incidence of all-cause-LRTI death in 2022 was 28% higher than the pre-pandemic incidence.
Implications of all the available evidence
Contrary to previous studies from predominantly high-income countries, we report that the seasonality and incidence of RSV-associated LRTI in 2022 were similar to the pre-pandemic period (2015–19). Nevertheless, of concern are the ongoing admissions to hospital for COVID-19-associated LRTI in an area in which more than 90% of the population have acquired SARS-CoV-2-infection-induced immunity. The ongoing burden of COVID-19-associated LRTI contributed to an overall higher incidence of all-cause LRTI hospital admissions in children younger than 5 years in 2022 compared with the pre-pandemic period. The increased rate of all-cause LRTI in 2022 transpired despite reduced incidence of IPD, tuberculosis, pertussis, and human-metapneumovirus-associated LRTI, which could rebound to pre-pandemic incidence in the near future. Consequently, as SARS-CoV-2 becomes epidemic and possibly endemic, LRTI-associated morbidity and mortality in children younger than 5 years might be higher than the pre-pandemic period in the absence of new interventions against COVID-19 and other respiratory pathogens.
After extensive infection-induced or vaccine-induced immunity against SARS-CoV-2, most countries reduced or removed non-pharmaceutical interventions during 2022.1 South Africa has fluctuated between different intensities of non-pharmaceutical interventions since March 27, 2020, but was at its lowest level (level 1) of restrictions when the omicron BA.1 lineage variant emerged in mid-November, 2021. All non-pharmaceutical interventions were removed by June, 2022, after the omicron BA.1 wave subsided, during which the decoupling of SARS-CoV-2 infection and severe COVID-19 was observed.12 A community-based SARS-CoV-2 serosurvey conducted in Gauteng, South Africa, between March and April, 2022, recorded seropositivity of 90·9% in the study population and 84·1% in children younger than 12 years who had not received a COVID-19 vaccine.13 The removal of COVID-19-related restrictions was temporally associated with a resurgence of seasonal respiratory virus epidemics, such as RSV, from September, 2022, onward in Europe and the USA. Reports from Europe and the USA indicate that the RSV epidemic of 2022–23 has resulted in an unusually high burden of severe respiratory illness.14
We aimed to analyse all-cause and pathogen-specific trends of lower respiratory tract infection (LRTI) hospital admissions in children younger than 5 years in South Africa, a low-middle-income country. We report the incidence of hospital admissions for all-cause LRTI and LRTI associated with RSV A or B, influenza A or B, human metapneumovirus, and pertussis during each of the COVID-19 pandemic years (2020, 2021, and 2022) compared with the pre-pandemic period (2015–19). Furthermore, we aimed to evaluate incidence of admission to hospital during the COVID-19 pandemic (2020–22) compared with the pre-pandemic period (2015–19) for all-cause illness, IPD, tuberculosis, neonatal sepsis, in-hospital all-cause death, and LRTI-associated death. We also compared the annual incidence of COVID-19-associated LRTI with pre-pandemic rates of RSV-associated LRTI and influenza-associated LRTI.
Methods
Study design and data sources
In this observational study, we used surveillance data from Jan 1, 2015, to Dec 31, 2022, from two hospitals in Soweto (Johannesburg, South Africa), which serve a population of 1·9 million, including 190 000 children younger than 5 years.15 Details of the study setting and hospitals are provided in the appendix (p 1). Between March 27, 2020, and April 5, 2022, the South African Government imposed societal regulations in a 5-level, tiered approach (appendix pp 1–2).
Data were obtained from the electronic Paediatric Discharge Summary Database, which includes information for all admissions to the general paediatric wards at the two public hospitals in Soweto. Data were colleacted for this database by MCN, FS, VB, NS, CV, DPM, ML, MN, and CO. Details of the Paediatric Discharge Summary Database, which is managed by the Vaccines and Infectious Diseases Analytics Research Unit of the University of the Witwatersrand (Wits-VIDA), are provided in the appendix (pp 2–3). All admissions to general hospital wards of children younger than 5 years were included in the study, automatically identified by R version 4.1. The International Classification of Diseases version 10 (ICD-10) codes were then used to identify hospital admissions for any LRTI, pneumonia, bronchiolitis, IPD, pulmonary tuberculosis, COVID-19, and neonatal sepsis (appendix p 2).
Testing for respiratory viruses and pertussis was done after written informed consent was obtained from the parent or legal guardian of a subset of children admitted for LRTI. Need for consent was waived during the period when all children admitted to the hospitals were tested for SARS-CoV-2 (March, 2020, to October, 2022).
The observational studies, of which this study is part, were approved by the Human Research Ethics Committee at the University of the Witwatersrand (140904, M200168, 131109, and M1911156).
Procedures
Standard of care for children younger than 5 years with suspected invasive bacterial infection included collection of blood for culture. Testing for Mycobacterium tuberculosis with GeneXpert MTB/Rif (Cepheid, Sunnyvale, CA, USA) assay or culture was done at the discretion of attending doctors and tested by the National Health Laboratory Service (NHLS). The ICD-10 diagnoses for pulmonary tuberculosis and neonatal sepsis were based on the diagnosis of the clinician, not necessarily culture-confirmed illness. Diagnosis of IPD was based on the culture of Streptococcus pneumoniae from a usually sterile site, mainly from blood or cerebrospinal fluid.
From March 24, 2020, to July 3, 2022, all children admitted to the paediatric wards at the two public hospitals in Soweto were tested for SARS-CoV-2 via a nucleic acid amplification test (NAAT) as part of the standard of care at Wits-VIDA or the NHLS, irrespective of the reason for admission. We restricted our analysis of COVID-19 to children younger than 5 years with an LRTI diagnosis, which would have made them eligible for enrolment into the LRTI surveillance, and who had a reactive SARS-CoV-2 NAAT. Consequently, we excluded children admitted to hospital with incidental SARS-CoV-2 infection or COVID-19 without LRTI diagnosis. Testing for RSV A or B, influenza A or B, human metapneumovirus, and pertussis was done by Wits-VIDA as part of ongoing LRTI surveillance (appendix p 3). Details of the NAAT and sampling methods are reported in the appendix (pp 3–4). Briefly, children younger than 5 years admitted to hospital for LRTI were systematically investigated for the targeted respiratory pathogens throughout the surveillance period, albeit with some variability in the sampling framework. Depending on the surveillance programme objectives at the time, laboratory testing of children admitted to hospital for LRTI varied between testing every day and testing on alternate days with or without testing of children admitted to hospital during weekends and public holidays. All children admitted with LRTI (or suspected sepsis in children younger than 3 months) were approached for testing on days when testing was done. Furthermore, due to non-specific signs with which LRTI can manifest in infants younger than 3 months, testing for respiratory viruses was done in this age group if there was clinical suspicion of infection; this age group was included in the LRTI category for this analysis. Notable interruptions of testing during the COVID-19 period to redirect resources to assist with SARS-CoV-2 testing included no testing for human metapneumovirus and pertussis from March 24 to Oct 20, 2020. Testing for RSV and influenza was interrupted from Sept 4 to Oct 20, 2020, during which time the National Institute for Infectious Diseases (NICD) in South Africa reported low positivity for RSV and no identification of influenza.16 From Oct 21, 2020, testing for the full panel of pathogens resumed.
Any child admitted to hospital with LRTI in whom RSV A or B, influenza A or B, or human metapneumovirus was identified was considered to have a virus-associated LRTI, as the presence of these viruses in the nasopharynx of children in hospital was strongly attributed to causality in the multicentre Pneumonia Etiology Research for Child Health (PERCH) study.17 Only children with a diagnosis of LRTI (or suspected infection in children <3 months) in whom testing for respiratory pathogens was done were included in the analysis to estimate the number of pathogen-specific admissions to hospital. For each pathogen, the total number of LRTI hospital admissions was multiplied by the percentage of positive NAAT results to estimate the monthly number of pathogen-specific hospital admissions by age group and month. Further detail is provided in the appendix (p 4).
A sensitivity analysis that includes admissions to the short-stay ward is discussed in the appendix (pp 5, 7).
Statistical analysis
We assessed the incidence of all-cause and pathogen-specific LRTI admissions to hospital before and during the COVID-19 pandemic as the primary outcome. The exposure was timing of hospital admission (ie, pre-pandemic or during the pandemic); age was a potential confounder. Incidence of pertussis-associated LRTI was limited to children younger than 12 months, as the incidence was extremely low in older children. Analysis for bronchiolitis was limited to children younger than 24 months. Hospital admissions that occurred within 14 days of a previous discharge were excluded from analyses. The annual Gauteng Department of Health population estimates for the subdistricts in the catchment area of the hospitals were used as denominators for incidence calculations (appendix pp 4–5). Incidences of all-cause admission to hospital, all-cause death, all-cause LRTI, all-cause-LRTI-associated death, RSV-associated LRTI, influenza-associated LRTI, human-metapneumovirus-associated LRTI, pulmonary tuberculosis, IPD, and all-cause pneumonia were assessed in children younger than 5 years.
Although the sampling framework for testing for respiratory organisms varied during the study period in terms of the proportion of LRTI cases tested per month, we assumed those changes were independent of the positivity rate and thus the positivity rate could be applied without bias. Yearly and monthly incidence risk (IR) per 100 000 children younger than 5 years (or younger than a specific age group if analyses were stratified by age) were calculated as the number of admissions to hospital divided by the yearly population estimates and multiplied by 100 000. Exact 95% CIs were calculated for all IRs.18 Due to interruption in testing for human metapneumovirus and pertussis in 2020, incidence risk estimates for human-metapneumovirus-associated and pertussis-associated LRTI were not calculated for 2020. A positivity rate of zero was assumed for RSV and influenza between Sept 4 and Oct 20, 2020.
Overall, age-specific and disease-specific incidence between 2015 and 2019 were calculated as the total number of admissions to hospital during the period divided by the sum of the yearly population estimates for the 5 years, expressed per 100 000 children younger than 5 years (or younger than a specific age group). IR ratios (IRRs) for clinical syndromes and pathogen-specific LRTIs were calculated to compare the per-year and per-month incidences during the pandemic period (2020, 2021, and 2022) and compared with the pooled pre-pandemic incidence. 95% CIs for IRR were calculated via the method described by Lehmann and Romano.19 Analyses were stratified by age group (<3 months, 3 to <12 months, 12 to <24 months, and 24 to <60 months) and conducted in R version 4.1.
Records with a missing hospital admission date or date of birth were excluded from analysis (appendix p 3).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Overall, there were 42 068 all-cause hospital admissions from Jan 1, 2015, to Dec 31, 2022 (figure A ; appendix pp 8, 11, 16).
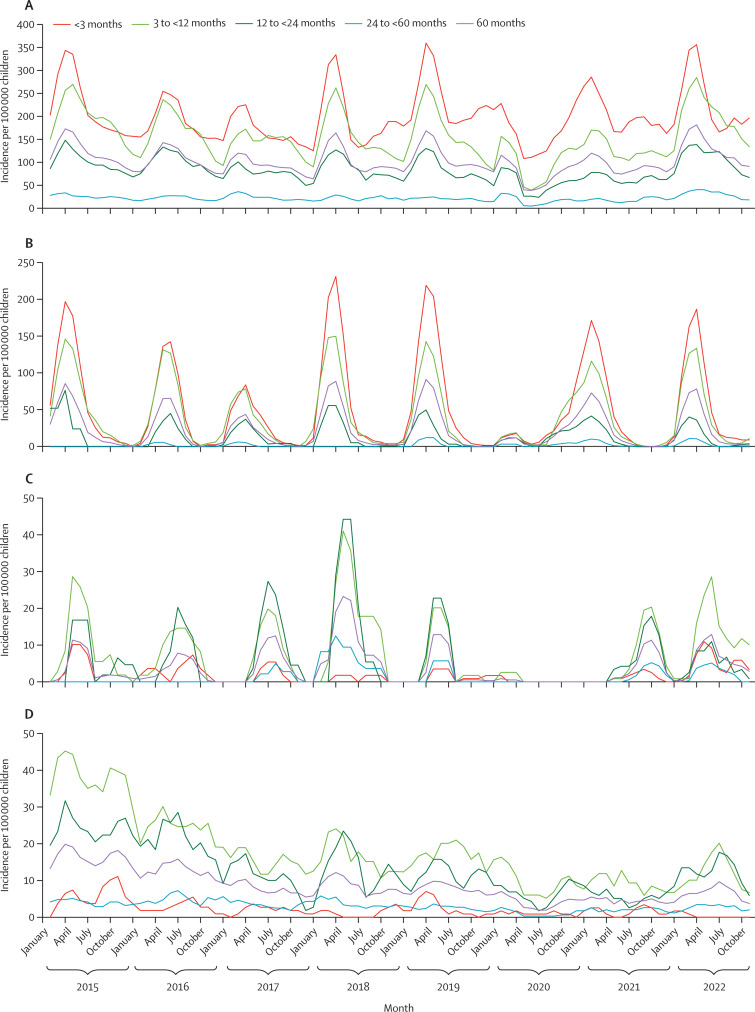
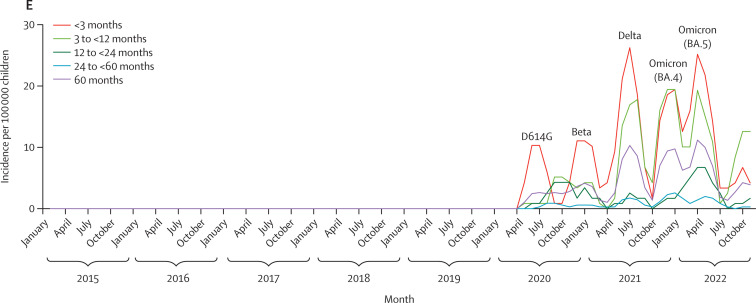
Figure.
Monthly incidences in children younger than 5 years
(A) Monthly incidence of all-cause LRTI. (B) Monthly incidence of RSV-associated LRTI. (C) Monthly incidence of influenza-associated LRTI. (D) Monthly incidence of pulmonary tuberculosis. (E) Monthly incidence of COVID-19-associated LRTI. LRTI=lower respiratory tract infection. RSV=respiratory syncytial virus.
Compared with the pre-pandemic period overall incidence of 2929 per 100 000 children younger than 5 years, the incidence for all-cause admission to hospital was 16% lower in 2020 (IRR 0·84, 95% CI 0·81–0·87) and 13% lower in 2021 (0·87, 0·84–0·89). By 2022, incidence of all-cause admission to hospital was similar to the pre-pandemic period (1·02, 0·99–1·04; table 1 ).
Table 1.
Incidence of primary and secondary outcomes in children younger than 5 years
|
Pre-pandemic (2015–19)* |
2020 |
2021 |
2022 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IR (95% CI) | n | IR (95% CI) | IRR (95% CI) | n | IR (95% CI) | IRR (95% CI) | n | IR (95% CI) | IRR (95% CI) | |
| All-cause admission to hospital | 26 605 | 2929 (2894–2964) | 4694 | 2462 (2392–2533) | 0·84 (0·81–0·87) | 4918 | 2534 (2463–2605) | 0·87 (0·84–0·89) | 5851 | 2975 (2899–3052) | 1·02 (0·99–1·04) |
| All-cause death | 912 | 100 (94–107) | 185 | 97 (84–112) | 0·97 (0·82–1·13) | 210 | 108 (94–124) | 1·08 (0·92–1·25) | 228 | 116 (101–132) | 1·15 (0·99–1·34) |
| All-cause LRTI† | 11 548 | 1271 (1248–1295) | 1704 | 894 (852–937) | 0·70 (0·67–0·74) | 2152 | 1109 (1062–1157) | 0·87 (0·83–0·91) | 2899 | 1474 (1421–1529) | 1·16 (1·11–1·21) |
| All-cause-LRTI death | 404 | 44 (40–49) | 63 | 33 (25–42) | 0·74 (0·56–0·97) | 91 | 47 (38–58) | 1·05 (0·83–1·33) | 112 | 57 (47–69) | 1·28 (1·03–1·58) |
| RSV-associated LRTI (A or B) | 2595 | 286 (275–297) | 281 | 147 (131–166) | 0·52 (0·45–0·58) | 528 | 272 (249–296) | 0·95 (0·87–1·05) | 583 | 296 (273–321) | 1·04 (0·95–1·14) |
| Influenza-associated LRTI (A or B) | 492 | 54 (49–59) | 5 | 3 (1–6) | 0·05 (0·02–0·11) | 81 | 42 (33–52) | 0·77 (0·6–0·98) | 121 | 62 (51–74) | 1·14 (0·92–1·39) |
| Human-metapneumovirus-associated LRTI | 421 | 46 (42–51) | TND | TND | TND | 68 | 35 (27–44) | 0·76 (0·58–0·98) | 71 | 36 (28–46) | 0·78 (0·60–1·00) |
| Bordetella-pertussis-associated LRTI‡ | 190 | 103 (89–118) | TND | TND | TND | 0 | 0 (0–9) | 0 (0·0–0·09) | 28 | 70 (47–102) | 0·69 (0·44–1·03) |
| Pulmonary tuberculosis§ | 526 | 95 (87–104) | 94 | 49 (40–60) | 0·52 (0·41–0·65) | 102 | 53 (43–64) | 0·55 (0·44–0·68) | 147 | 75 (63–88) | 0·79 (0·65–0·94) |
| Invasive pneumococcal disease | 90 | 10 (8–12) | 11 | 6 (3–10) | 0·58 (0·28–1·09) | 10 | 5 (2–9) | 0·52 (0·24–1·00) | 10 | 5 (2–9) | 0·51 (0·24–0·99) |
Analysis stratified by age group is reported in the appendix (pp 8–12). IR=incidence risk. IRR=incidence risk ratio. LTRI=lower respiratory tract infection. TND=testing not done. RSV=respiratory syncytial virus.
Yearly incidence rates for 2015–19 are reported in the appendix (p 8).
<3 months age group for LRTI includes diagnosis of suspected sepsis.
As pertussis was only diagnosed in children aged <12 months, analysis was restricted to the <12 months age group for pertussis.
Incidence rates in the first column represent overall incidence during 2017–19 due to the decline in tuberculosis incidence rates before 2017.
The overall annual incidence of all-cause in-hospital death was 100 per 100 000 children younger than 5 years in the pre-pandemic period, with no difference observed in 2020 (0·97, 0·82–1·13). Compared with the pre-pandemic period, the incidence of all-cause death was increasing, but not significantly, in 2021 and 2022 (1·08, 0·92–1·25), and in 2022 (1·15, 0·99–1·34; table 1; appendix p 16). The age-group stratified analysis for all-cause admission to hospital and death, including comparisons during the pandemic years and the pre-pandemic period, are reported in the appendix (pp 8–11). During the study period, children younger than 12 months had a higher incidence of hospital admission than older children aged 12–60 months, and mortality rates were highest in children younger than 3 months and aged 3 months to younger than 12 months. Similar trends were observed for the age-group stratified analysis compared with the overall analysis in children younger than 5 years.
Overall, 18 303 (43%) of 42 608 admissions to hospital were all-cause LRTI, including suspected sepsis in infants younger than 3 months. The annual incidence of all-cause LRTI was similar from 2015 to 2019, with minimal yearly fluctuations (appendix pp 8, 16). The incidence of all-cause LRTI was 30% lower in 2020 (IRR 0·70, 95% CI 0·67–0·74) and 13% lower in 2021 (0·87, 0·83–0·91), but 16% higher in 2022 (1·16, 1·11–1·21), compared with overall pre-pandemic incidence (1271 per 100 000 children younger than 5 years). The higher incidence of all-cause LRTI in 2022 compared with the pre-pandemic period was evident across all age groups, with the highest increase in children aged 24 months to younger than 60 months (1·31, 1·18–1·47) and lowest increase in children aged younger than 3 months (1·11, 1·04–1·19; appendix p 11). Further analyses stratified by age group and clinically diagnosed syndromes of bronchiolitis and pneumonia are reported in the appendix (pp 5–6, 11). Compared with the pre-pandemic overall incidence of LRTI-associated deaths (44 per 100 000 children younger than 5 years), the incidence was 26% lower in 2020 (0·74, 0·56–0·97), similar in 2021 (1·05, 0·83–1·33), but 28% higher in 2022 (1·28, 1·03–1·58; table 1; appendix p 16). The increased incidence of LRTI-associated deaths in 2022 compared with the pre-pandemic period was evident for all stratified age groups, but not statistically significant (appendix p 11).
The percentages of children younger than 5 years admitted to hospital for LRTI who were tested for respiratory pathogens were similar between 2015 and 2019 (overall 33·4–39·9%), whereas they were higher in 2020 (43·0%), 2021 (78·6%), and 2022 (72·9%; appendix p 12). During the pre-pandemic period, the seasonality of RSV-associated LRTI was predominantly from February to July each year, peaking around mid-March. In 2020, the epidemic of RSV-associated LRTI commenced in February but subsided rapidly after the implementation of level-5 non-pharmaceutical interventions on March 24 (appendix p 16). There was, however, an unseasonal re-emergence of RSV in August, 2020, which continued until June, 2021. In 2022, the seasonality of RSV-associated LRTI was similar to the pre-pandemic period. Details of the strains of RSV circulating from 2015 to 2022 are included in the appendix (pp 6–7, 13).
During the pre-pandemic period, there was some yearly variability in the incidence of RSV-associated LRTI (range 179–344 per 100 000 children younger than 5 years; figure B; appendix p 8). The incidence of RSV-associated LRTI was 48% lower in 2020 (IRR 0·52, 0·45–0·58), but similar in 2021 (0·95, 0·87–1·05) and 2022 (1·04, 0·95–1·14) compared with the pre-pandemic incidence of 286 per 100 000 children younger than 5 years (table 1). Relative to the pre-pandemic period, a higher IRR of RSV-associated LRTI was evident in children aged 24 months to younger than 60 months in each of the pandemic years (IRR range 1·88–2·39). However, this age group had the lowest incidence of RSV-associated LRTI throughout the study (appendix p 14).
In the pre-pandemic period, the seasonality of influenza-associated LRTI was April to September each year, usually peaking in June (figure C). The overall incidence was 54 per 100 000 children younger than 5 years during the pre-pandemic period, including an unusually high incidence in 2018 (195 per 100 000; appendix p 8). In 2020, the incidence of influenza-associated LRTI was 95% lower (0·05, 0·02–0·11) than the pre-pandemic period. In 2021, influenza-associated LRTI was unseasonal compared with the pre-pandemic period, with cases identified between August and December and peaking in October (figure C). The incidence of influenza-associated LRTI was also lower in 2021 (0·77, 0·60–0·98) than in the pre-pandemic period. In 2022, the influenza-associated LRTI seasonality pattern was similar, but non-significant; however, the incidence was significantly increased in children younger than 3 months (2·45, 1·35–4·32) and aged 3 months to younger than 12 months (1·38, 1·00–1·87; appendix p 14).
Pre-pandemic, incidence of human-metapneumovirus-associated LRTI was 44 per 100 000 children younger than 5 years, and was generally highest in children aged 3 months to younger than 12 months (94 per 100 000 children younger than 12 months; table 1; appendix pp 14, 17). Testing for human metapneumovirus was disrupted in 2020. Incidences of human-metapneumovirus-associated LRTI did not differ significantly in 2021 (0·78, 0·60–1·02) and 2022 (0·79, 0·60–1·02) compared with the pre-pandemic period (table 1).
Almost all pertussis occurred in children younger than 12 months during the pre-pandemic period, with some yearly variability ranging from incidence of 46 per 100 000 children younger than 12 months in 2017 to 176 per 100 000 children younger than 12 months in 2018 (appendix pp 8, 17). No testing was done for pertussis in 2020. The incidence of pertussis was 0 in 2021, and incidence did not differ significantly in 2022 compared with the pre-pandemic period (0·69, 0·44–1·03; table 1).
Overall, the incidence of pulmonary tuberculosis decreased between 2015 (193 per 100 000 children younger than 5 years) and 2019 (95 per 100 000; figure D; appendix p 8). Consequently, we used values from 2017 to 2019 to estimate the overall pre-pandemic incidence (95 per 100 000 children younger than 5 years, 95% CI 87–104; table 1). Compared with the pre-pandemic period, the incidence of tuberculosis was lower in 2020 (IRR 0·52, 95% CI 0·41–0·65), 2021 (0·55, 0·44–0·68), and 2022 (0·79, 0·65–0·94). This pattern was also seen in the age-stratified analysis, except for in children older than 12 months in 2022, who had similar incidence to the pre-pandemic period (appendix p 14).
Pre-pandemic, the overall incidence of IPD was stable, and higher in infants younger than 12 months than in children older than 12 months (table 1; appendix pp 8, 17). The incidence of IPD was 42% lower in 2020 (IRR 0·58, 0·28–1·09), 48% in 2021 (0·52, 0·24–1·00), and 49% in 2022 (0·51, 0·24–0·99) compared with the incidence of 10 per 100 000 children younger than 5 years in the pre-pandemic period (table 1; figure D), although estimates had a relatively wide range and some of the changes were not significant.
COVID-19-associated LRTI in children younger than 5 years manifested as five waves, including two waves in 2020 (dominated by wild-type SARS-CoV-2 and the beta variant B1.351) and two waves in 2021 (dominated by the delta variant B.1.617.2 in July and omicron BA.1 in December; figure E).20 The overall incidence of COVID-19-associated LRTI was 19 per 100 000 children younger than 5 years in 2020, 65 per 100 000 in 2021, and 65 per 100 000 in 2022. The incidence of COVID-19-associated LRTI admission to hospital generally decreased with increasing age in each of the three pandemic years (table 2 ).
Table 2.
Total n and IR of COVID-19-associated LRTI admissions to hospital per 100 000 children younger than 5 years; IRR comparing incidence of COVID-19-associated LRTI admissions to hospital with incidence of RSV and influenza during the pre-pandemic period (2015–19); and IRR for yearly comparisons of incidence of COVID-19-associated LRTI admissions to hospital with incidence of RSV and influenza in 2020, 2021, and 2022 by age group
|
COVID-19 |
IRR (95% CI) |
|||||
|---|---|---|---|---|---|---|
| n | IR (95% CI) | Yearly incidence of COVID-19 compared with incidence of RSV | Incidence of COVID-19 compared with pre-pandemic incidence of RSV | Yearly incidence of COVID-19 compared with incidence of influenza | Incidence of COVID-19 compared with pre-pandemic incidence of influenza | |
| 2020 | ||||||
| <3 months | 17 | 44 (26–70) | 0·18 (0·1–0·31) | 0·07 (0·04–0·11) | 8·50 (2·02–75·85) | 2·14 (1·13–3·88) |
| 3 to <12 months | 7 | 18 (7–37) | 0·07 (0·03–0·15) | 0·03 (0·01–0·07) | 2·33 (0·53–13·98) | 0·18 (0·07–0·38) |
| 12 to <24 months | 8 | 21 (9–41) | 0·15 (0·06–0·31) | 0·12 (0·05–0·24) | NC | 0·23 (0·10–0·47) |
| 24 to <60 months | 4 | 4 (1–9) | 0·12 (0·03–0·33) | 0·22 (0·06–0·59) | NC | 0·18 (0·05–0·48) |
| <60 months | 36 | 19 (13–26) | 0·13 (0·09–0·18) | 0·07 (0·05–0·09) | 7·20 (2·82–23·51) | 0·35 (0·24–0·49) |
| 2021 | ||||||
| <3 months | 62 | 157 (121–202) | 0·25 (0·18–0·32) | 0·24 (0·18–0·31) | 12·40 (5·04–39·53) | 7·67 (5·04–11·81) |
| 3 to <12 months | 47 | 119 (88–159) | 0·27 (0·19–0·37) | 0·23 (0·17–0·31) | 1·62 (1–2·67) | 1·19 (0·84–1·64) |
| 12 to <24 months | 6 | 15 (6–33) | 0·11 (0·04–0·24) | 0·09 (0·03–0·2) | 0·23 (0·08–0·57) | 0·17 (0·06–0·38) |
| 24 to <60 months | 12 | 10 (5–18) | 0·27 (0·13–0·53) | 0·65 (0·32–1·20) | 0·57 (0·26–1·22) | 0·53 (0·27–0·97) |
| <60 months | 127 | 65 (55–78) | 0·24 (0·20–0·29) | 0·23 (0·19–0·27) | 1·57 (1·18–2·10) | 1·21 (0·99–1·47) |
| 2022 | ||||||
| <3 months | 50 | 126 (93–166) | 0·18 (0·13–0·25) | 0·19 (0·14–0·25) | 2·50 (1·46–4·43) | 6·14 (3·94–9·61) |
| 3 to <12 months | 48 | 121 (89–160) | 0·23 (0·16–0·32) | 0·23 (0·17–0·31) | 0·87 (0·58–1·31) | 1·20 (0·86–1·66) |
| 12 to <24 months | 14 | 35 (19–59) | 0·25 (0·13–0·45) | 0·21 (0·11–0·35) | 0·7 (0·33–1·46) | 0·40 (0·21–0·69) |
| 24 to <60 months | 15 | 13 (7–21) | 0·35 (0·18–0·64) | 0·80 (0·43–1·40) | 0·58 (0·28–1·13) | 0·66 (0·36–1·13) |
| <60 months | 127 | 65 (54–77) | 0·22 (0·18–0·26) | 0·23 (0·19–0·27) | 1·05 (0·81–1·36) | 1·19 (0·97–1·45) |
IR=incidence risk. IRR=incidence risk ratio. LTRI=lower respiratory tract infection. NC=not calculable. RSV=respiratory syncytial virus.
Despite the lower incidence of RSV-associated LRTI in 2020 compared with the pre-pandemic period, the incidence of COVID-19-associated LRTI was 87% lower than the incidence of RSV-associated LRTI in 2020 (0·13, 0·09–0·18). The incidence of COVID-19-associated LRTI remained 76% (0·24, 0·20–0·29) lower than the incidence of RSV-associated LRTI in 2021 and 78% (0·22, 0·18–0·26) lower in 2022, when RSV-associated LRTI incidence was similar to the pre-pandemic rate (table 2; appendix p 19). Similarly, the incidence of COVID-19-associated LRTI was consistently lower than the pre-pandemic incidence of RSV-associated LRTI in 2020 (0·07, 0·05–0·09), 2021 (0·23, 0·19–0·27), and 2022 (0·23, 0·19–0·27; table 2; appendix p 19). The lower IRR of COVID-19-associated LRTI compared with pre-pandemic RSV-associated LRTI was evident across all age groups except for children aged 24 months to younger than 60 months in 2021 (0·65, 0·32–1·20) and 2022 (0·80, 0·43–1·40; table 2).
During the pandemic years, COVID-19-associated LRTI incidence was higher than influenza-associated LRTI in 2020 (7·2, 2·82–23·51) and 2021 (1·57, 1·18–2·10), particularly in children younger than 3 months (8·50, 2·02–75·85 in 2020; 12·40, 5·04–39·50 in 2021; table 2; appendix p 20). Compared with the pre-pandemic incidence of influenza-associated LRTI, the incidence of COVID-19-associated LRTI was lower in 2020 (0·35, 0·24–0·49; table 2; appendix p 20). The incidence of COVID-19-associated LRTI appeared to be higher than the pre-pandemic incidence of influenza-associated LRTI in 2021 (1·21, 0·99–1·47) and 2022 (1·19, 0·97–1·45), although non-significantly, particularly in children younger than 3 months (7·67, 5·04–11·81 in 2021; 6·14, 3·94–9·61 in 2022; table 2).
The incidence of admission to hospital for suspected sepsis in children younger than 1 month in each of the pandemic years was similar to the pre-pandemic incidence (1251 per 100 000 children younger than 1 month; appendix pp 8, 11, 17).
Discussion
Our study showed a temporal association between the implementation of non-pharmaceutical interventions to mitigate spread of SARS-CoV-2 and a disruption in seasonality and reduced incidence of all-cause LRTI and LRTI admissions to hospital associated with RSV and influenza during 2020. Furthermore, we observed a reduction in the incidence of pulmonary tuberculosis (48% reduction) and probably S pneumoniae (based on a 42% IPD reduction) LRTI during 2020 compared with the pre-pandemic period. Although we did not test for pertussis or human metapneumovirus for most of 2020, the lower incidence of pertussis-associated LRTI and human-metapneumovirus-associated LRTI in 2021 compared with the pre-pandemic period suggests that incidence of LRTI associated with these pathogens was also probably lower in 2020. We showed that the incidence of all-cause LRTI, including RSV-associated LRTI, started to revert to pre-pandemic rates in 2021, which was temporally associated with the reduction or removal of non-pharmaceutical interventions. By 2022, the seasonality and incidence of RSV-associated LRTI and influenza-associated LRTI were similar to the pre-pandemic period.
Our observations of RSV-associated LRTI were in contrast to other studies from primarily high-income countries, which reported more severe outbreaks of RSV in 2022 and 2023 compared with pre-pandemic data.14, 21 The unseasonal re-emergence of RSV in 2020 after the reduction or removal of non-pharmaceutical interventions or restrictions, which extended into 2021, potentially led to infection-induced immunity at a population level against some respiratory pathogens, such as RSV. Areas in which non-pharmaceutical interventions or restrictions were more strict and adherence was higher might have consequently had a longer interruption of circulation of respiratory viruses than occurred in our setting. The resulting immunity gap in such settings could have contributed to increased susceptibility and more severe disease after re-emergence of circulation of respiratory viruses in 2022.22
We report that seasonality and burden of influenza-associated LRTI in 2022 was similar to the pre-pandemic period. There was already a smaller reduction (23%) in influenza-associated LRTI incidence in 2021 compared with the pre-pandemic period, in contrast to the 95% lower incidence of influenza-associated LRTI in 2020. The incidence of human-metapneumovirus-associated LRTI and pertussis, however, remained non-significantly lower in 2022 compared with the pre-pandemic period. Furthermore, although there was a rebound in incidence of tuberculosis in 2022, rates remained lower (21% reduction) compared with the pre-pandemic period. M tuberculosis contributed to 9·7% of all severe-acute-LRTI hospital admissions in our setting before the COVID-19 pandemic.23 The lower incidence of admission to hospital for tuberculosis is consistent with the lower rates of tuberculosis notification in South Africa during the COVID-19 pandemic.24 As children younger than 5 years mainly develop primary pulmonary tuberculosis within 1–2 years of infection, our results indicate that non-pharmaceutical interventions probably reduced community transmission of M tuberculosis.25 Ongoing surveillance is warranted to establish whether interventions during the COVID-19 pandemic might have long-term effects on the burden of childhood tuberculosis in high-burden settings, such as South Africa.
By contrast with reports from the UK, where a rebound of IPD that exceeded pre-pandemic incidence was reported in 2021 for children younger than 15 years, the incidence of IPD in our study remained 49% lower in 2022 compared with the pre-pandemic period.26 Furthermore, the persistently lower incidence of IPD occurred despite the incidence of RSV-associated LRTI and influenza-associated LRTI returning to pre-pandemic rates in 2022. In Israel, lower rates of IPD and presumed pneumococcal pneumonia were attributed to reduced susceptibility to pneumococcal disease due to disruptions of circulation of seasonal respiratory viruses.2 Our observations suggest that other factors might also contribute to the lower risk of pneumococcal disease during the pandemic period, possibly including a lower risk of pneumococcal nasopharyngeal colonisation acquisition and lower density of colonisation during the pandemic period.
Our study also aimed to contextualise the burden of COVID-19-associated LRTI in relation to endemic RSV-associated LRTI and influenza-associated LRTI during the COVID-19 pandemic and relative to the pre-pandemic period. The incidence of COVID-19-associated LRTI was consistently lower than the pre-pandemic rate of RSV-associated LRTI in all children younger than 24 months. In 2022, the incidence of COVID-19-associated LRTI was, however, similar to the pre-pandemic incidence of RSV-associated LRTI in children aged 24 months to younger than 60 months, even though this age group had the lowest incidence of RSV-associated LRTI throughout the study. Consequently, consideration is warranted as to whether COVID-19 vaccination should be recommended for children aged 24 months to younger than 60 months if the burden of COVID-19-associated LRTI remains similar or increases in the future, particularly in settings where influenza vaccination is already used in this age group. By April, 2022, despite COVID-19 vaccines not having been rolled out to children younger than 12 years, SARS-CoV-2 seropositivity was 84·1% and has probably increased further.13 Our findings indicate an ongoing burden of COVID-19-associated LRTI hospital admissions in the era of circulation of sublineages of the omicron variant, despite extensive underlying infection-induced immunity.
In 2022, the incidence of COVID-19-associated LRTI was 19% higher than the pre-pandemic incidence of influenza-associated LRTI. In children younger than 3 months, COVID-19-associated LRTI was 6·14-times higher than pre-pandemic influenza-associated LRTI. People who are pregnant are already considered to be a priority group for seasonal influenza immunisation, protecting not only themselves but also their fetuses and young infants against influenza illness.27 A vaccine effectiveness study reported that parental vaccination with two doses of a messenger RNA COVID-19 vaccine reduced the risk of COVID-19 hospital admission in their young infants by 52% (protection varying between 80% for the delta variant to 38% for the omicron variant).28 The advent of the bivalent vaccine incorporating omicron sublineage variants could increase the effectiveness of parental immunisation in protecting young infants in the era of omicron sublineages being dominant.28
Limitations of our study include being unable to address whether changes in health-seeking behaviour, including reluctance to visit health facilities (especially in 2020), could have contributed to the reduced incidence of hospital admissions for LRTI during the COVID-19 pandemic. Several other studies, however, also reported on disruption of circulation and hospital admission for endemic respiratory viruses, such as RSV, influenza, human metapneumovirus, and B pertussis in 2020, which corroborate our findings. 2, 3, 4, 5, 6, 7, 8, 9, 10 Furthermore, we did not observe any changes in incidence of admission to hospital for suspected neonatal sepsis in any of the pandemic years compared with pre-pandemic period. As there is a low threshold for admitting neonates with suspected sepsis, these data indicate that health-seeking behaviour for hospital care might not have been substantially affected, even in 2020. Another limitation of our study was that the percentage of children tested for LRTI varied depending on the prevailing surveillance strategy at the time. Nevertheless, testing for specific respiratory pathogens was done systematically throughout the years and we assumed those changes were independent of the positivity rate, thus the positivity rate could be applied without bias to those children who were not tested during the corresponding time period. Interruption of testing for influenza and RSV in 2020 could have affected our incidence calculations and consequent comparison with the pre-pandemic period for these pathogens. However, surveillance at the NICD in South Africa reported low levels of positivity for RSV and an absence of influenza during the study period when testing was not done by us.16 Furthermore, our study was of a single geographical locality. The disruption of respiratory virus illness in our study in 2020 was similar to that reported nationally in South Africa, suggesting generalisability at least within the country.16
Strengths of our study include a 5-year pre-pandemic period of surveillance, with a paucity of similar surveillance data elsewhere in Africa. Consequently, our study provides the first and probably most comprehensive insights into the effects of the COVID-19 pandemic on the epidemiology of LRTI, and the relative burden of disease compared with influenza and RSV, in a low-middle-income African country.
We observed a 16% higher incidence of all-cause LRTI in 2022 compared with the pre-pandemic period, including in all age-group stratified analyses. The ongoing hospital admissions for COVID-19-associated LRTI in 2022 (incidence 77 per 100 000 children younger than 5 years) is likely to have partly contributed to the increase in all-cause LRTI hospital admissions relative to the pre-pandemic period, despite lower rates of human-metapneumovirus-associated LRTI, pertussis, tuberculosis, IPD, and presumably pneumococcal pneumonia in 2022. Although the COVID-19 pandemic is receding, the burden of disease as SARS-CoV-2 becomes endemic remains to be established, including in countries with high prevalence of SARS-CoV-2 infection-induced immunity. If the incidence of COVID-19-associated LRTI remains at 2022 levels in the future, and if there is a return to pre-pandemic rates of LRTI hospital admissions due to other endemic respiratory pathogens, the overall incidence of childhood LRTI might remain higher than the pre-pandemic period in the absence of any new interventions (eg, against RSV). Moreover, the pathogens contributing to the 16% higher incidence of all-cause LRTI and 28% higher incidence of LRTI-associated deaths in 2022, relative to the pre-pandemic period, warrant ongoing investigation.
Data sharing
All data collected for this Article are available with publication on a collaborative basis from the Vaccines and Infectious Diseases Analytics Research Unit of the University of the Witwatersrand upon request (alane.izu@wits-vida.org), provided a data transfer agreement is approved. Count data by day and age category will be available for the outcomes: all-cause admission to hospital, all-cause death, lower respiratory tract infection (LRTI), LRTI-associated death, COVID-19-associated LRTI, pulmonary tuberculosis, invasive pneumococcal disease, neonatal sepsis, all-cause pneumonia, bronchiolitis, positive respiratory syncytial virus (RSV) A result, negative RSV A result, positive RSV B result, negative RSV B result, positive influenza A result, negative influenza A result, positive influenza B result, negative influenza B result, positive human metapneumovirus result, negative human metapneumovirus result, positive Bordetella pertussis result, and negative B pertussis result. All requests for data sharing will require approval from the Principal Investigator (SAM) and the University of the Witwatersrand Human Ethics Research Committee. Data will be shared for non-commercial purposes only, with the support of one or more investigators as collaborators. Additional documents that are available upon request include the study protocols, upon which the database was constructed, and informed consent forms.
Declaration of interests
SAM has received study-related grant funding to their institution (Vaccines and Infectious Diseases Analytics Research Unit of the University of the Witwatersrand, Johannesburg, South Africa) from the Bill & Melinda Gates Foundation and the Foundation for Influenza Epidemiology. SAM has received non-study-related grant funding from the Gates Foundation, GlaxoSmithKline, Pfizer, Minervax, Novavax, Providence, Gritstone, and ImmunityBio. SAM has received payment from GlaxoSmithKline for lectures and is Chair of the Rotavirus Vaccine Data Safety Monitoring Board for the Program for Appropriate Techonolgy in Health and Chair of the HIV Monocolonal Antibody Data Safety Monitoring Board for the Centre for the AIDS Programme of Research in South Africa. MCN has received non-study grants to their institution from the Gates Foundation, the European and Developing Countries Clinical Trials Partnership, Sanofi, AstraZeneca, and Pfizer. MCN has received payment from Sanofi for expert testimony. DPM has received a grant from the Carnegie Corporation of New York. CO has received payment from Sanofi–Aventis South Africa for presentations and from the Gates Foundation for travel. CV has received payment from Sanofi–Aventis South Africa for presentations and AstraZeneca South Africa for lectures. VB has received payment from Sanofi–Aventis South Africa for presentations. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank Tselane Makgoba (University of the Witwatersrand, Johannesburg, South Africa) for assistance with study team management and coordination. We acknowledge the Vaccines and Infectious Diseases Analytics Research Unit of the University of the Witwatersrand surveillance team and Chris Hani Baragwaneth Academic Hospital paediatric department for their contributions to data collection and management. The surveillance programme is funded through multiple grants from the Bill & Melinda Gates Foundation (INV-033847, OPP1101764, OPP1118349, OPP1114622, PM0045992, EF-2020–11339, and EF-2021–19548). DPM is in part supported by a grant awarded by the Carnegie Corporation of New York.
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published text and institutional affiliations.
Contributors
SAM and AI conceptualised the study design and objectives. FS, VB, NS, ML, MN, MCN, and AI supervised data collection. MCN, FS, VB, NS, CV, DPM, ML, MN, and CO collected the data. AI and VB accessed and verified laboratory results and AI and FS accessed and verified hospital admission data. AI did the statistical analyses. AI and SAM wrote the first draft of the manuscript. ZD did a literature search and wrote the Research in context panel. All authors had full access to all data in this study, reviewed and approved the final manuscript, and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Hale T, Angrist N, Goldszmidt R, et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker) Nat Hum Behav. 2021;5:529–538. doi: 10.1038/s41562-021-01079-8. [DOI] [PubMed] [Google Scholar]
- 2.Danino D, Ben-Shimol S, van der Beek BA, et al. Decline in pneumococcal disease in young children during the coronavirus disease 2019 (COVID-19) pandemic in Israel associated with suppression of seasonal respiratory viruses, despite persistent pneumococcal carriage: a prospective cohort study. Clin Infect Dis. 2022;75:e1154–e1164. doi: 10.1093/cid/ciab1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. Decreased influenza activity during the COVID-19 pandemic—United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1305–1309. doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tempia S, Walaza S, Bhiman JN, et al. Decline of influenza and respiratory syncytial virus detection in facility-based surveillance during the COVID-19 pandemic, South Africa, January to October 2020. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.29.2001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matczak S, Levy C, Fortas C, et al. Association between the COVID-19 pandemic and pertussis derived from multiple nationwide data sources, France, 2013 to 2020. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.25.2100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley DA, Sikazwe CT, Minney-Smith CA, et al. An unusual resurgence of human metapneumovirus in Western Australia following the reduction of non-pharmaceutical interventions to prevent SARS-CoV-2 transmission. Viruses. 2022;14 doi: 10.3390/v14102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley DA, Yeoh DK, Minney-Smith CA, et al. The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019-related public health measures. Clin Infect Dis. 2021;73:e2829–e2830. doi: 10.1093/cid/ciaa1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowling BJ, Ali ST, Ng TWY, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5:e279–e288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delahoy MJ, Ujamaa D, Taylor CA, et al. Comparison of influenza and COVID-19-associated hospitalizations among children younger than 18 years old in the United States: FluSurv-NET (October–April 2017–2021) and COVID-NET (October 2020–September 2021) Clin Infect Dis. 2023;76:450–459. doi: 10.1093/cid/ciac388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Reeves RM, Wang X, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health. 2019;7:e1031–e1045. doi: 10.1016/S2214-109X(19)30264-5. [DOI] [PubMed] [Google Scholar]
- 11.Brueggemann AB, Jansen van Rensburg MJ, Shaw D, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health. 2021;3:e360–e370. doi: 10.1016/S2589-7500(21)00077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhi SA, Kwatra G, Myers JE, et al. Population immunity and COVID-19 severity with omicron variant in South Africa. N Engl J Med. 2022;386:1314–1326. doi: 10.1056/NEJMoa2119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madhi SA, Kwatra G, Myers JE, et al. Sustained low incidence of severe and fatal COVID-19 following widespread infection induced immunity after the omicron (BA.1) dominant in Gauteng, South Africa: an observational study. Viruses. 2023;15:597. doi: 10.3390/v15030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairbank R. RSV wave hammers hospitals—but vaccines and treatments are coming. Nature. 2022 doi: 10.1038/d41586-022-04434-5. published online Dec 15. [DOI] [PubMed] [Google Scholar]
- 15.Adedini S, Sello M, Thaele D, Madhi S. Patterns of healthcare utilisation and barriers affecting access to child healthcare services in low-income urban South African settings. SAJCH. 2020;14:34–39. [Google Scholar]
- 16.National Institute for Communicable Diseases Weekly respiratory pathogens surveillance report. 2023. https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/surveillance-reports/weekly-respiratory-pathogens-surveillance-report-week/
- 17.The Pneumonia Etiology Research for Child Health (PERCH) Study Group Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019;394:757–779. doi: 10.1016/S0140-6736(19)30721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR) Am J Epidemiol. 1990;131:373–375. doi: 10.1093/oxfordjournals.aje.a115507. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann EL, Romano JP. Springer; New York, NY, USA: 2005. Testing statistical hypotheses. [Google Scholar]
- 20.National Institute for Communicable Diseases Tracking SARS-CoV-2 variants. 2023. https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/sars-cov-2-genomic-surveillance-update/
- 21.Munkstrup C, Lomholt FK, Emborg HD, et al. Early and intense epidemic of respiratory syncytial virus (RSV) in Denmark, August to December 2022. Euro Surveill. 2023;28 doi: 10.2807/1560-7917.ES.2023.28.1.2200937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen R, Ashman M, Taha MK, et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now. 2021;51:418–423. doi: 10.1016/j.idnow.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore DP, Baillie VL, Mudau A, et al. The etiology of pneumonia in HIV-uninfected South African children: findings from the Pneumonia Etiology Research for Child Health (PERCH) study. Pediatr Infect Dis J. 2021;40:S59–S68. doi: 10.1097/INF.0000000000002650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebina L, Dube M, Hlongwane K, et al. Trends in paediatric tuberculosis diagnoses in two South African hospitals early in the COVID-19 pandemic. S Afr Med J. 2020;110:1149–1150. doi: 10.7196/SAMJ.2020.v110i12.15386. [DOI] [PubMed] [Google Scholar]
- 25.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:392–402. [PubMed] [Google Scholar]
- 26.Bertran M, Amin-Chowdhury Z, Sheppard CL, et al. Increased incidence of invasive pneumococcal disease among children after COVID-19 pandemic, England. Emerg Infect Dis. 2022;28:1669–1672. doi: 10.3201/eid2808.220304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etti M, Calvert A, Galiza E, et al. Maternal vaccination: a review of current evidence and recommendations. Am J Obstet Gynecol. 2022;226:459–474. doi: 10.1016/j.ajog.2021.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halasa NB, Olson SM, Staat MA, et al. Maternal vaccination and risk of hospitalization for COVID-19 among Infants. N Engl J Med. 2022;387:109–119. doi: 10.1056/NEJMoa2204399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data collected for this Article are available with publication on a collaborative basis from the Vaccines and Infectious Diseases Analytics Research Unit of the University of the Witwatersrand upon request (alane.izu@wits-vida.org), provided a data transfer agreement is approved. Count data by day and age category will be available for the outcomes: all-cause admission to hospital, all-cause death, lower respiratory tract infection (LRTI), LRTI-associated death, COVID-19-associated LRTI, pulmonary tuberculosis, invasive pneumococcal disease, neonatal sepsis, all-cause pneumonia, bronchiolitis, positive respiratory syncytial virus (RSV) A result, negative RSV A result, positive RSV B result, negative RSV B result, positive influenza A result, negative influenza A result, positive influenza B result, negative influenza B result, positive human metapneumovirus result, negative human metapneumovirus result, positive Bordetella pertussis result, and negative B pertussis result. All requests for data sharing will require approval from the Principal Investigator (SAM) and the University of the Witwatersrand Human Ethics Research Committee. Data will be shared for non-commercial purposes only, with the support of one or more investigators as collaborators. Additional documents that are available upon request include the study protocols, upon which the database was constructed, and informed consent forms.